1. Introduction
Underwater electric signal sensing [
1,
2] shows great potential for electric-field-sensing-based target detection [
3], positioning [
4], signal transmission [
5] and communications [
6], among others. Nevertheless, underwater equipment such as underwater manipulators, autonomous underwater vehicles and workstations rely heavily on electric power supply [
7,
8]. Therefore, it is of great importance to develop self-powered sensing methods for underwater activities. Based on working principles, the electric current or electric voltage signals generated for sensing can be generally derived from three methods: piezoelectric, triboelectric and piezoionic.
The piezoelectric method is based on the piezoelectric effect, where a voltage difference across the piezoelectric material is generated as it is pressed [
9]. Thus, the mechanical energy is directly converted into electricity. Piezoelectric materials, such as quartz, PZT, ZnO, PVDF and piezoelectric composites [
10,
11], are widely used for electricity generation on land. However, these materials must respond to strain caused by various stimuli, and the high hydrostatic pressure in deep-sea environments can easily deform them, limiting their effectiveness in such conditions.
Underwater electric current generated for sensing based on the triboelectric effect have also been proposed. Compared to land-based triboelectric nanogenerators (TENGs), underwater TENGs should be specially designed to adapt to the complexity of the underwater environment [
12]. To further optimize the underwater performance of TENGs, diverse designs including origami structure [
13], electrode geometry [
14], triboelectric layer innovation [
15], biomimetic structure (seaweed, jellyfish, sea anemones, etc.) [
16,
17,
18] and electric double layer (EDL)-based structure [
19] have been proposed. Nevertheless, as mobile ions in water can neutralize the electrostatic charges of TENGs [
20], waterproof structures including shell, encapsulation, and coatings are necessary to avoid this side effect [
21].
Distinct from the piezoelectric effect or triboelectric effect, using electrons as charge carriers, electric currents can also be generated through the piezoionic effect. Ionic transportation is utilized to produce an electrical output when pressure is applied [
22]. Several underwater applications, such as piezoionic elastomers and TPU film-based tactile sensors, have been developed using the second type of piezoionic process [
23,
24]. Nevertheless, the piezoionic materials have to withstand long-term soaking in an aquatic environment [
25]. Moreover, the mechanical strength is insufficient to work under high hydrostatic pressure conditions, such as in the deep sea [
26].
While the methods of electric current signal-based sensing show some potential in underwater applications, the shortcomings are obvious. Contact electrification (CE) provides a possible solution. The CE of contact and separation mode refers to the charge transfer between the two different materials [
27,
28], where net positive charges accumulate on one material and net negative charges on the other [
29]. For a metal/metal contact pair, electrons are transferred between the two metal surfaces, resulting in a contact potential difference (CPD) between the two contacted metals. From the pioneering investigations of the electron transfer mechanism between metal and metal by Harper [
30] and Lowell [
31], it was revealed that the work function is the minimum work needed to extract the electrons from the Fermi level of a solid carrying a net charge [
32,
33]. Therefore, electrons will flow from the metal with a smaller work function to the metal with a larger work function until the equilibrium of the Fermi level is achieved [
34].
Based on the above-mentioned mechanism, the electric potential difference of the electrodes can be determined via the modulation of the electrode distance by through applying the scanning Kelvin probe (SKP) method [
35]. The suitability of a material is likely correlated with its work function, resulting from the conclusion of the higher work function and the higher resolution of the electrochemical potential [
36]. With the help of this non-destructive method, the work function of a metal is mapped to evaluate the relationship between the metal surface defects and the electrochemical potential [
37]. Kaponig et al. [
38], employing a small gold sphere bouncing on a planar metal electrode, examined the CE of metals and focused on the charging and discharging process when the metal touched and detached, respectively. The charge transfer was found to be limited to the short contact and no charge was accumulated in the following contacts.
It can be seen that the majority of the investigations have been conducted in air or under vacuum. However, the process of the charge transfer between two contacting metals also occurs in underwater conditions. To the best of our knowledge, the only research on metal/metal contact in electrolyte to date is a numerical study of the potential distribution around bimetallic Janus nanoparticles, indicating that the electric field induced by the CE is disrupted beyond the Debye length [
39]. Nevertheless, the fundamental mechanism that underlies the charge (electron) transfer and electricity generation via the CE of metal/metal sheets in underwater conditions is still not completely understood.
In this study, we propose a novel method using bi-metal CE to generate underwater ionic current signals for self-powered sensing. Focus is given to the charge transfer process during the contact and separation between the two metal sheets in water, where one metal is the working electrode, and the ionic current signals are detected far away by the sensing electrode. The effects of metal type, water temperature, ionic concentration and electrode distance on the sensing capability of the ionic current signals were investigated systematically. As a typical application, underwater information transmission via Morse code was demonstrated using the proposed method.
2. Mechanism of Ionic Current Sensing
The system mainly consists of a copper working electrode (for contacting a metal object) and an electrometer connected in series with a copper sensing electrode. Both the working electrode and the sensing electrode, as well as the metal sheet, are immersed in an electrolyte solution. The mechanism of the contact-induced ionic current sensing can be understood as follows. Initially, the working electrode, sensing electrode and the metal sheet are immersed in the electrolyte solution without contact. Generally, a metal electrode is negatively charged in an electrolyte solution. As a result, the cations are attracted onto the electrode surface to form a so-called EDL. Since the materials of the two electrodes are the same, there is a neglected potential difference between them. As a result, there will be no electron flow between them before the working electrode and the metal sheet come into contact.
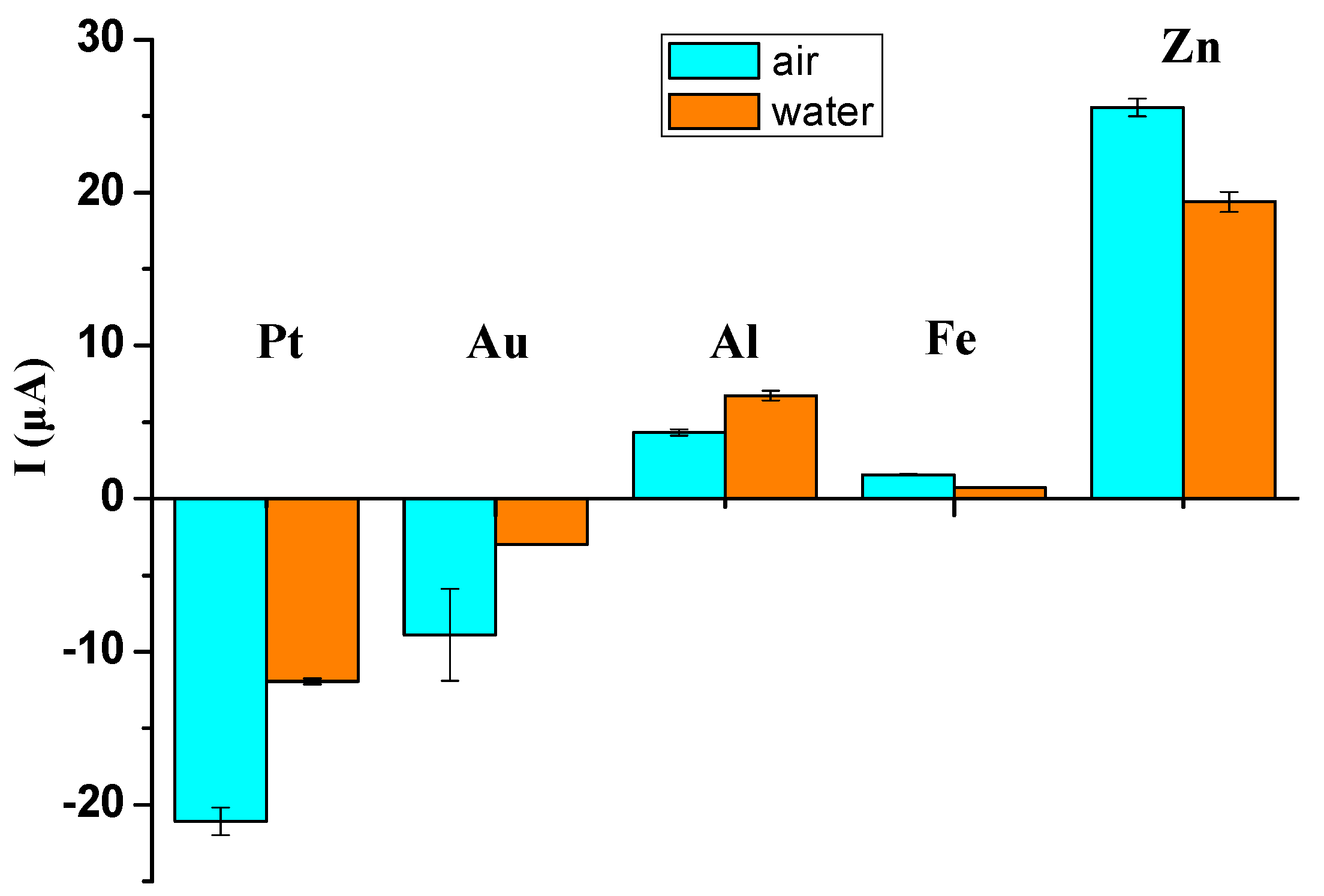
When the copper working electrode and the metal sheet with a higher work function (e.g., gold sheet) are brought into electrical contact (
Figure 1), some electrons will be transferred from the copper working electrode to the gold sheet until their Fermi levels are equal [
33]. This process of electron migration leads to the generation of a contact potential difference (CPD) at the interface of the copper working electrode and the gold sheet. Specifically, the electric potential of the gold sheet is higher than that of the copper working electrode. As electrons move from the copper working electrode to the gold sheet, some electrons will migrate accordingly from the ground to the copper working electrode. For the gold sheet after contact, it becomes the new working electrode (named the gold-composite working electrode) with the copper working electrode working as the function of a copper electric wire. Meanwhile, the electric potential of the sensing electrode is unchanged. Then, the potential difference between the gold-composite working electrode and the sensing electrode is increased to an electric potential difference Δ
V. Accordingly, the cations and the anions in the electrolyte solution are attracted to the gold-composite working electrode/electrolyte solution interface and the sensing electrode/electrolyte solution interface, respectively, forming the EDL capacitors. The accumulated anions on the sensing electrode/electrolyte solution interface will be neutralized by the discharged electrons through the electrometer. According to the connection of the electrometer discussed above, a negative ionic current signal pulse is detected.
Similarly, when the copper working electrode contacts a metal sheet with a lower work function (e.g., aluminum sheet), the aluminum sheet works as a function of the new working electrode (named the aluminum-composite working electrode). The electrons will flow from the aluminum-composite working electrode to the copper working electrode, leading to a lower electric potential of the aluminum-composite working electrode. Then, the anions and the cations are attracted onto the surfaces of the aluminum-composite working electrode and the sensing electrode, respectively. The accumulated cations on the sensing electrode/electrolyte solution interface will attract the electrons to the sensing electrode through the electrometer. Therefore, a positive ionic current signal pulse will be detected.
It should be noted that the above-mentioned contact-induced ionic current is a non-Faradaic current, where only ion absorption and detachment at the electrode/electrolyte solution interface are involved. The contact-induced ionic current sensing process can be electrically modeled with an equivalent circuit. Specifically, the composite working electrode and the sensing electrode can be modeled as the parallel of a resistor
R (
R1,
R2) and an EDL capacitor
C (
C1,
C2), respectively. There is also a water resistor R
s between the two electrodes. In the equivalent circuit, the sum of the voltages,
VR and
Vc, across the water electric resistor and the EDL capacitors, respectively, should be equal to Δ
V [
40].
where
QS is the mobile charges induced in the electric circuit. The ionic current can be expressed as I = d Q
S/dt. Therefore, Equation (1) is rearranged as
If we integrate Equation (2), then
The ionic current
can be expressed by combining Equations (2) and (3).
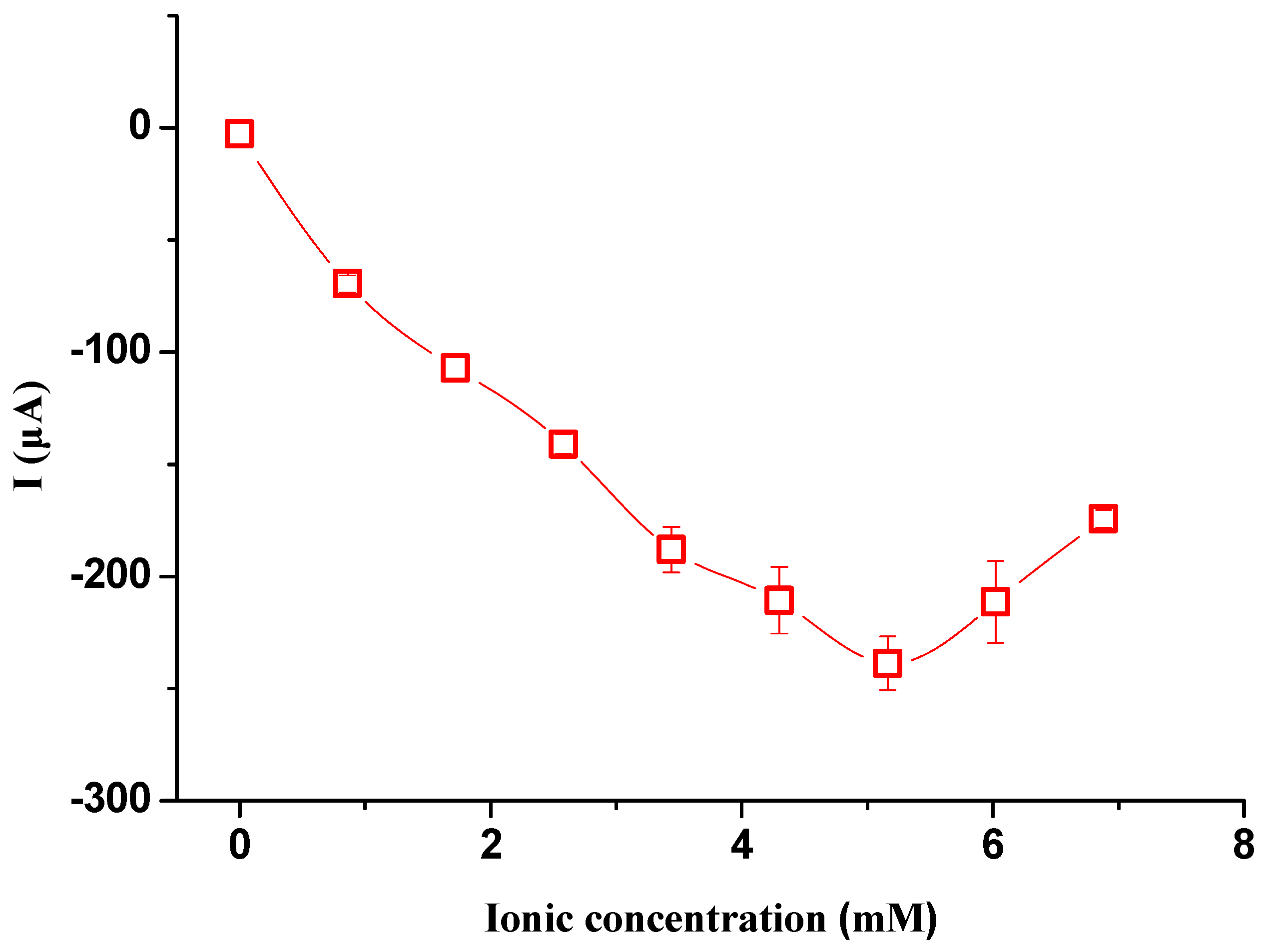
From Equation (4), we can see that the ionic current decays with a time constant τ (τ = RsC) and the ionic current reaches a peak value (Imax = ΔV/Rs) at the moment when CPD is generated. As we only focus on the signal pulse of the ionic current, except where indicated, all the ionic current signals discussed in this study are the peak values of the ionic current. It can be seen that the magnitude of the ionic current increases with the increase in ΔV and decreases with the increase in the electric resistor of water Rs. As Rs decreases with the increase in the ionic concentration of the electrolyte solution in a certain concentration range and increases with the increase in electrode distance, it can be predicted that the ionic current signals increase with the increase in ionic concentration and decrease with the increase in electrode distance.
3. Experimental Setup
3.1. Materials
In the experimental system, a water tank was primed with deionized (DI) water (18.2 ΜΩ) or NaCl solution. DI water was produced using a laboratory water purification system (Smart series, HETAI, Shanghai, China), and the NaCl solution was prepared by diluting a certain amount of NaCl (99.5% purity, Tianjin Zhiyuan, Tianjin, China) in DI water. The copper working electrode and the sensing electrode are both cylindrical with a diameter of 1 mm. The sensing electrode is connected in series with an electrometer (6517B, Keithley, Cleveland, OH, USA). A homemade NI LabVIEW 2018 program was used to record the real-time ionic current signals measured with the electrometer. Gold (Au), platinum (Pt), copper (Cu), aluminum (Al), iron (Fe) and zinc (Zn) were used as the metal sheets (10 mm × 15 mm × 1 mm) in the experiments. None of the metal sheets were further treated regarding the oxidation layers.
3.2. Procedures
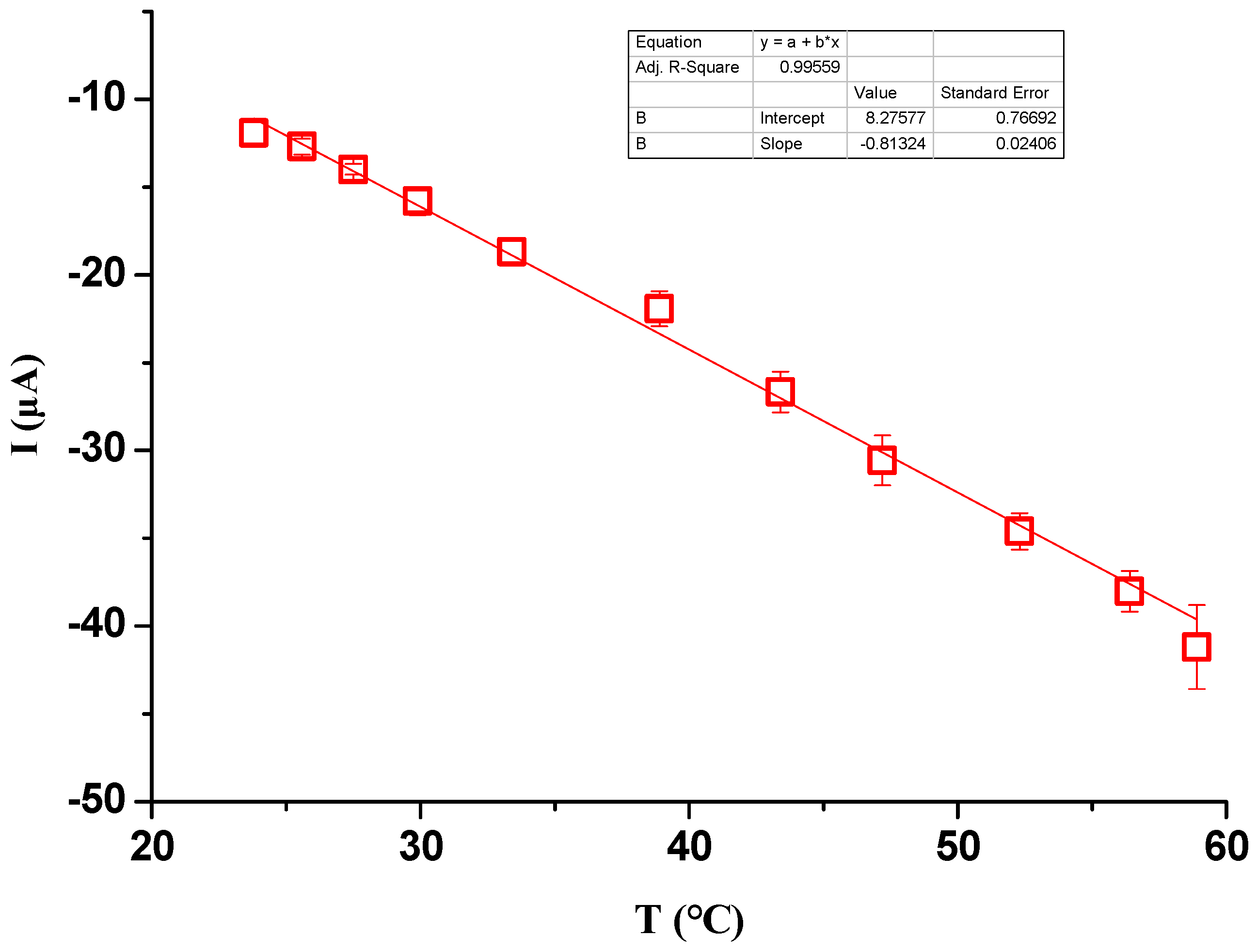
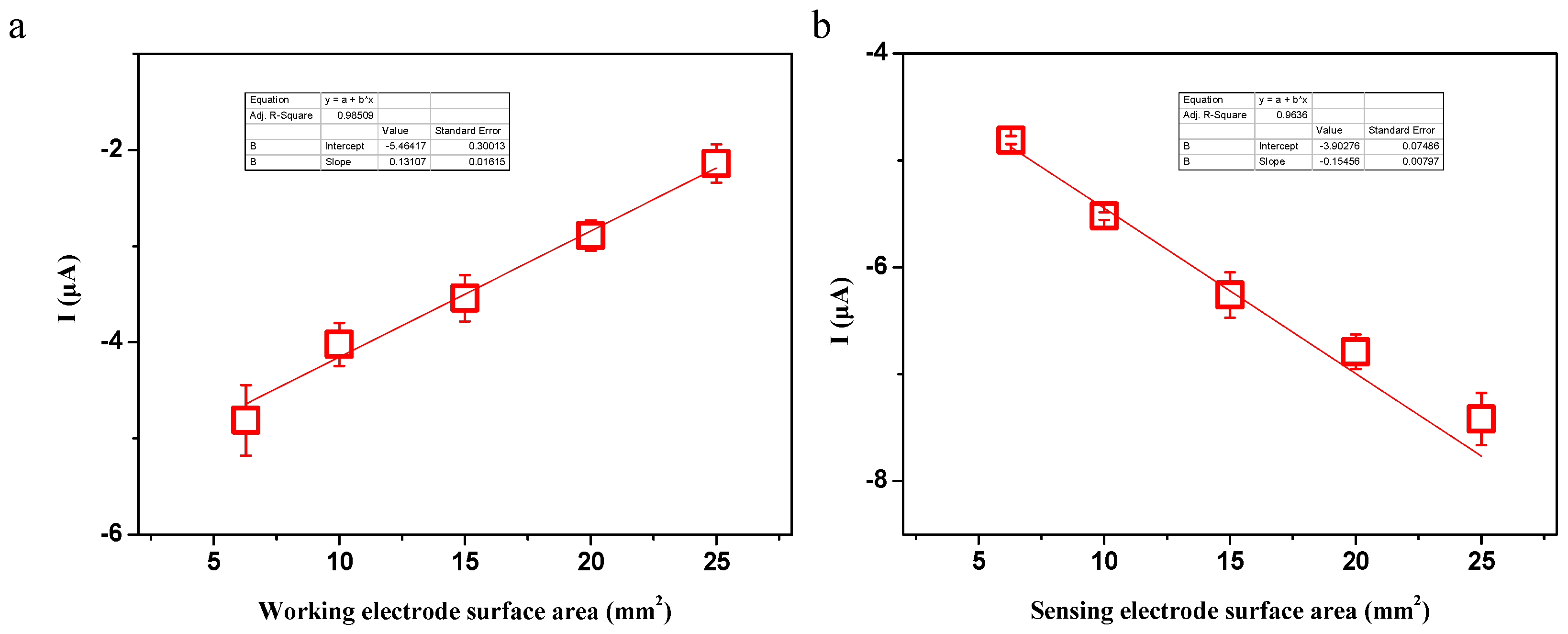
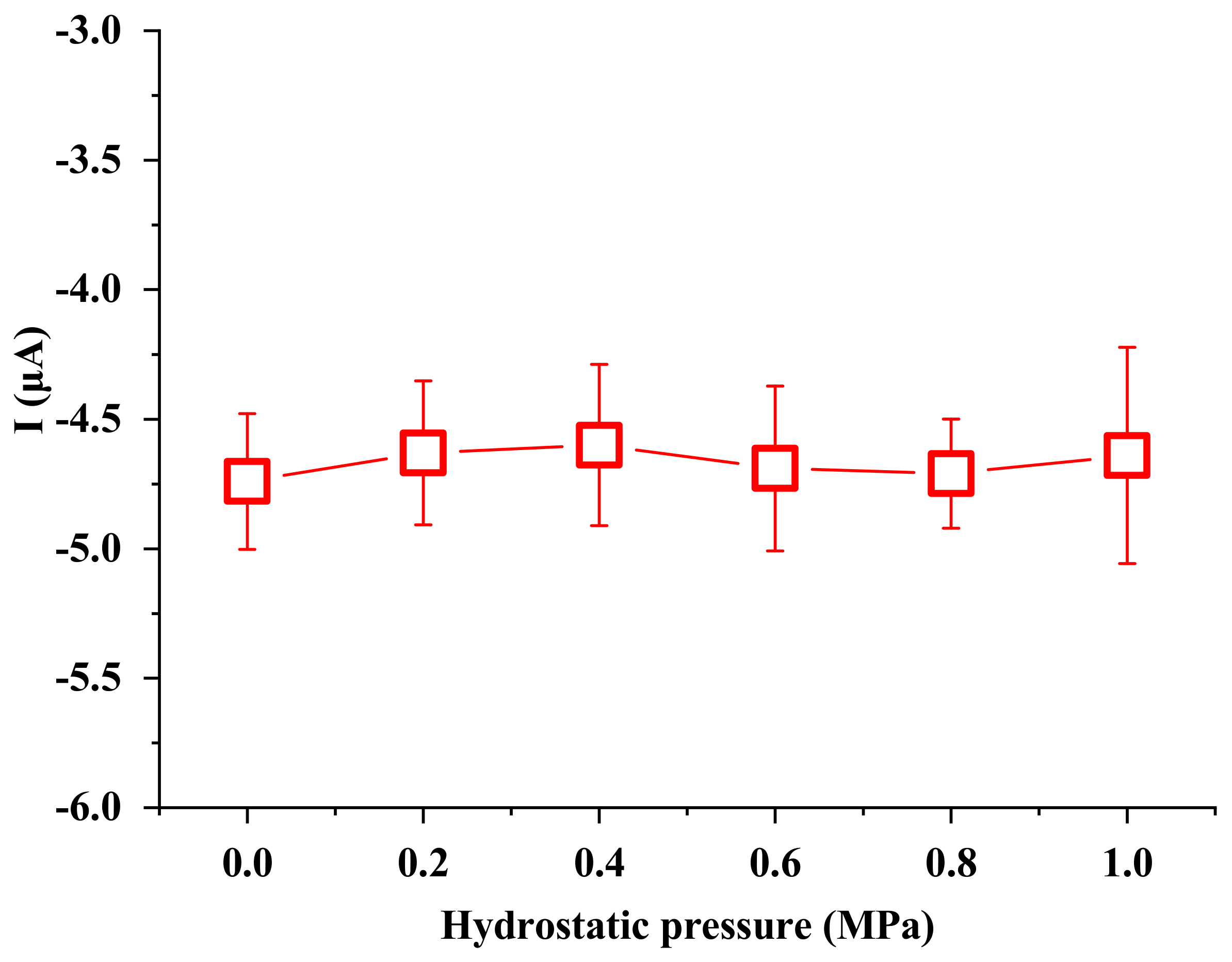
In this study, the effects of ionic concentration, water temperature, electrode surface area, electrode distance and hydrostatic pressure on the ionic current signals were investigated. To start the experiment, the working electrode, the sensing electrode and the metal sheet were immersed in the liquid. Then, the working electrode was manually moved to contact the metal sheet and detached from the metal sheet after the peak value was reached. The above processes were repeated three times, and the ionic current signals were obtained by averaging the three measurements. Ionic concentration ranging from DI water to 7.0 mM NaCl solution was investigated. To check the effect of water temperature on the generated signals, a digital hot plate was used to heat the liquid to a certain temperature. To check the effect of the electrode distance on the generated signals, the working electrode and the metal sheet were inserted into one end of a 60 m long water pool, while the sensing electrode was inserted into the opposite end. Other experiments were conducted under room temperature conditions (23 ± 1 °C).
5. Application of Information Transmission
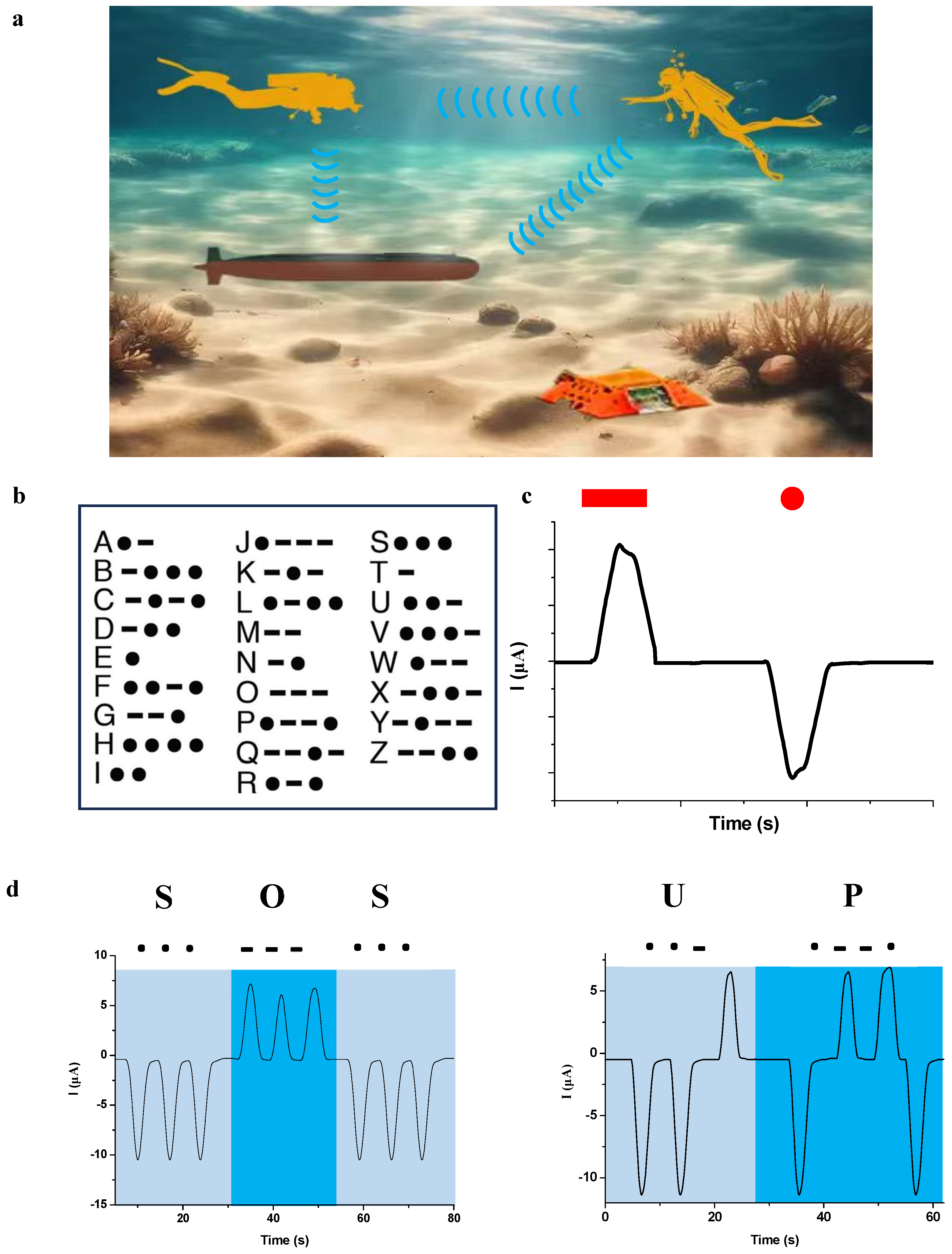
Other than in the civilian field, silent information transmission has received increasing attention in special fields where stealth is a necessity. For example, near-field frogmen need to exchange information with their colleagues, as well as remote-operated vehicles or bases in the sea without being detected (
Figure 9a). As is well known, Morse code is an effective way to transmit information, and it has been used for well over a century. Different Latin alphabets are represented by different configurations of “dot” and “dash” symbols (
Figure 9b). Based on the contact-induced ionic current-generating system and Morse code, we proposed a wireless self-powered device that can silently transmit information in underwater conditions via a modulating decoder to translate the ionic current signals into intelligible letters. The device comprises a signal modulator, an LCD display, a portable lithium battery, a copper working electrode and a copper sensing electrode. In addition, an aluminum sheet and a platinum sheet are secured onto the surface of the gloves. It is therefore possible to generate the ionic current using only three fingers on one hand.
The working principle of the system is as follows. Firstly, we define the positive ionic current pulse generated by contacting the aluminum sheet as the “dash” and the negative ionic current pulse generated by the platinum sheet as the “dot”, as shown in
Figure 9c. For example, the letters S and O are generated by consecutively contacting the platinum sheet and the aluminum sheet three times with the copper working electrode, respectively. Then, messages such as “SOS” and “UP” (
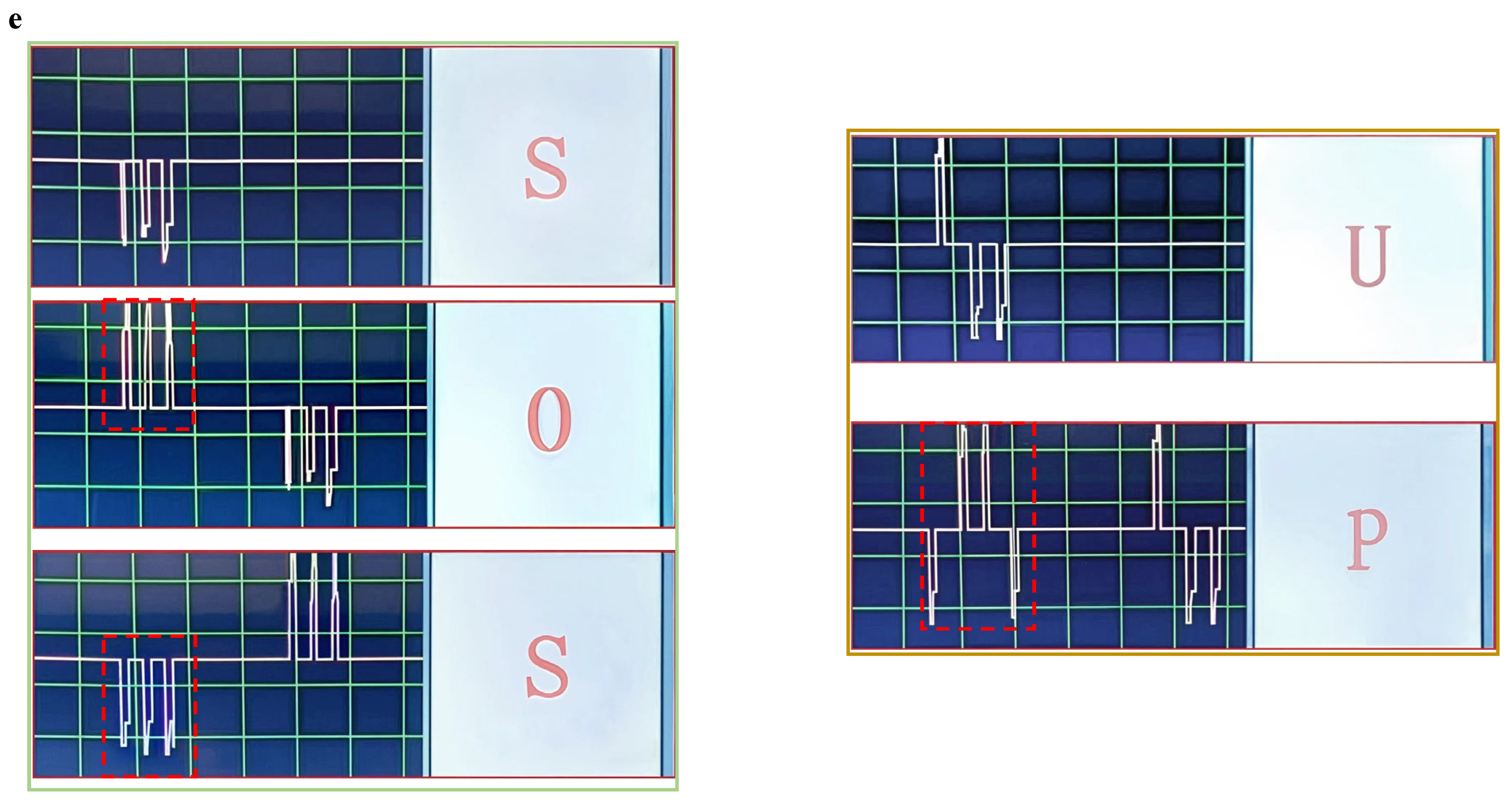
Figure 9d) are expressed by contacting the aluminum or the platinum sheets representing “dash” or “dot” in appropriate sequence and displayed on the screen of the decoder, respectively (
Figure 9e). Other information, such as “GO” and “HELP”, can also be conveyed by simply contacting the two fingers with metal sheets in underwater conditions using the same process. Therefore, silent information transmission can be achieved between vehicles and scuba divers.