In Vitro Antioxidant Effects of Coenzyme Q10 on Cellular Metabolism in Aged Mesenchymal Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and CoQ10 Treatment
2.2. Viability Assay
2.3. SA-β-Gal Staining
2.4. Mitochondrial Respiration and Energy Metabolism Evaluation
2.5. Antioxidant and Sirtuin Gene Expression Analysis
2.6. Assessment of Fatty Acid Profile
2.7. Statistical Analyses
3. Results
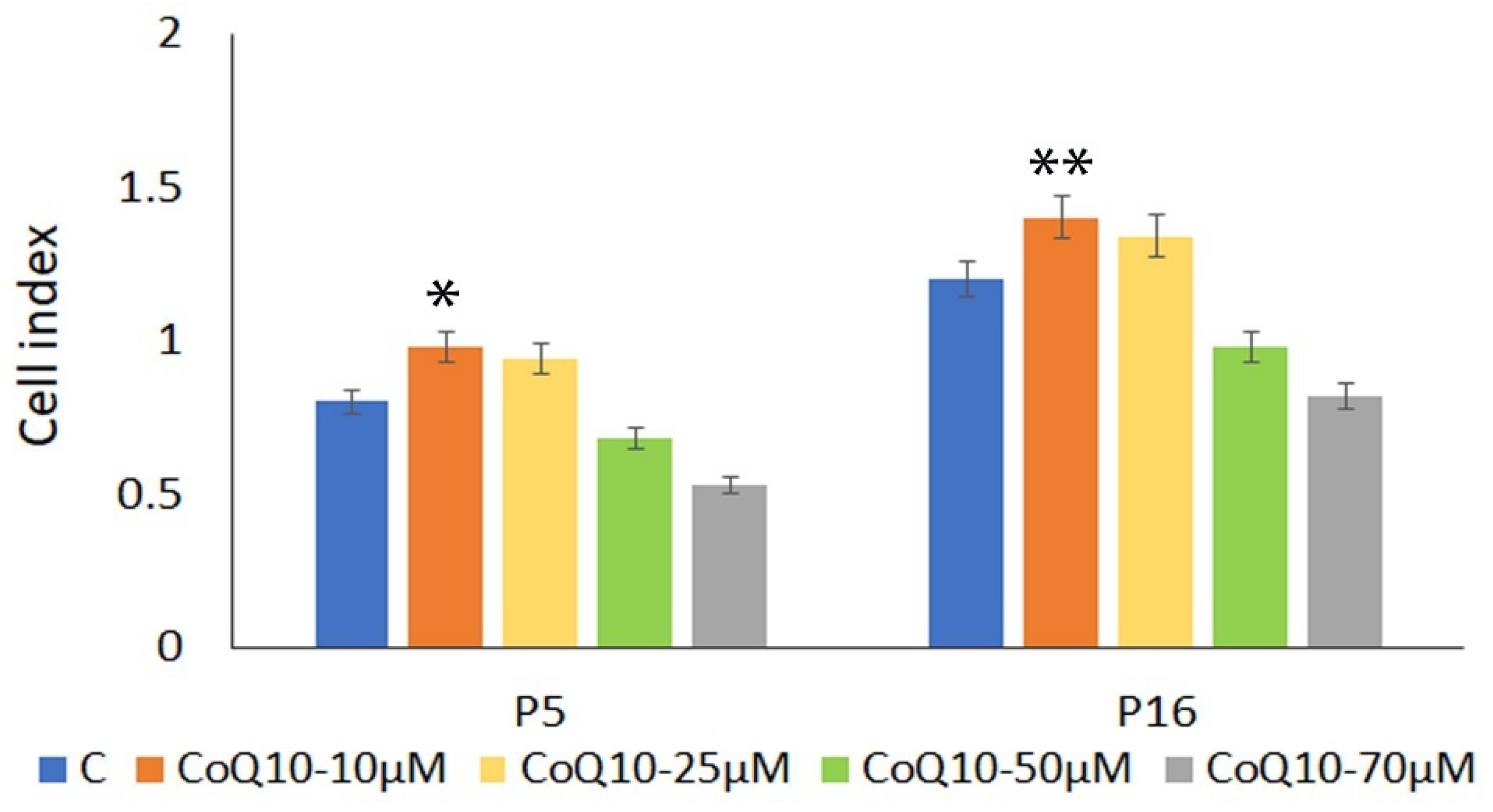
3.1. Cell Viability
3.2. SA-β-Gal Staining
3.3. Mitochondrial Respiration and Changes in Energy Metabolism
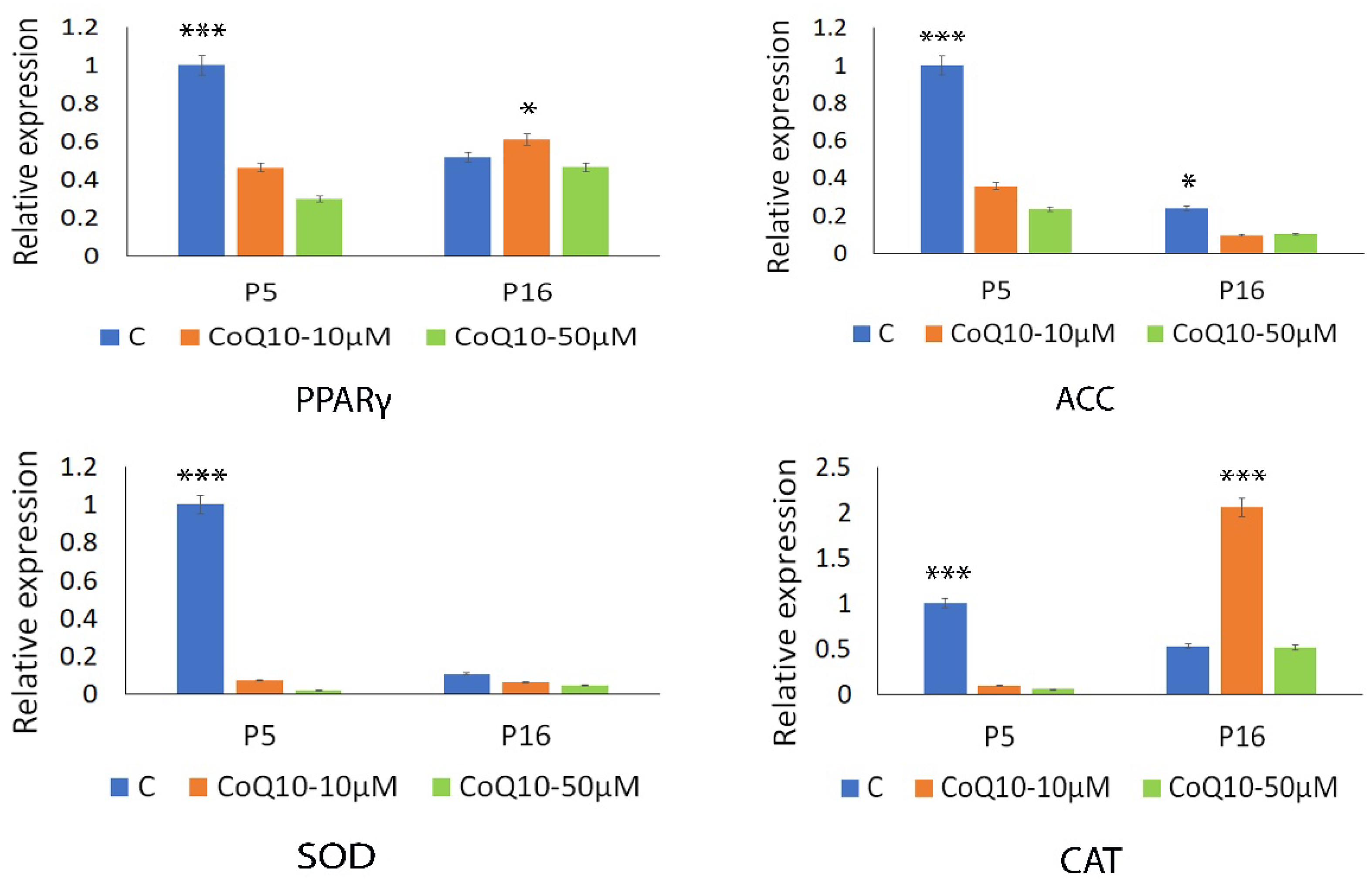
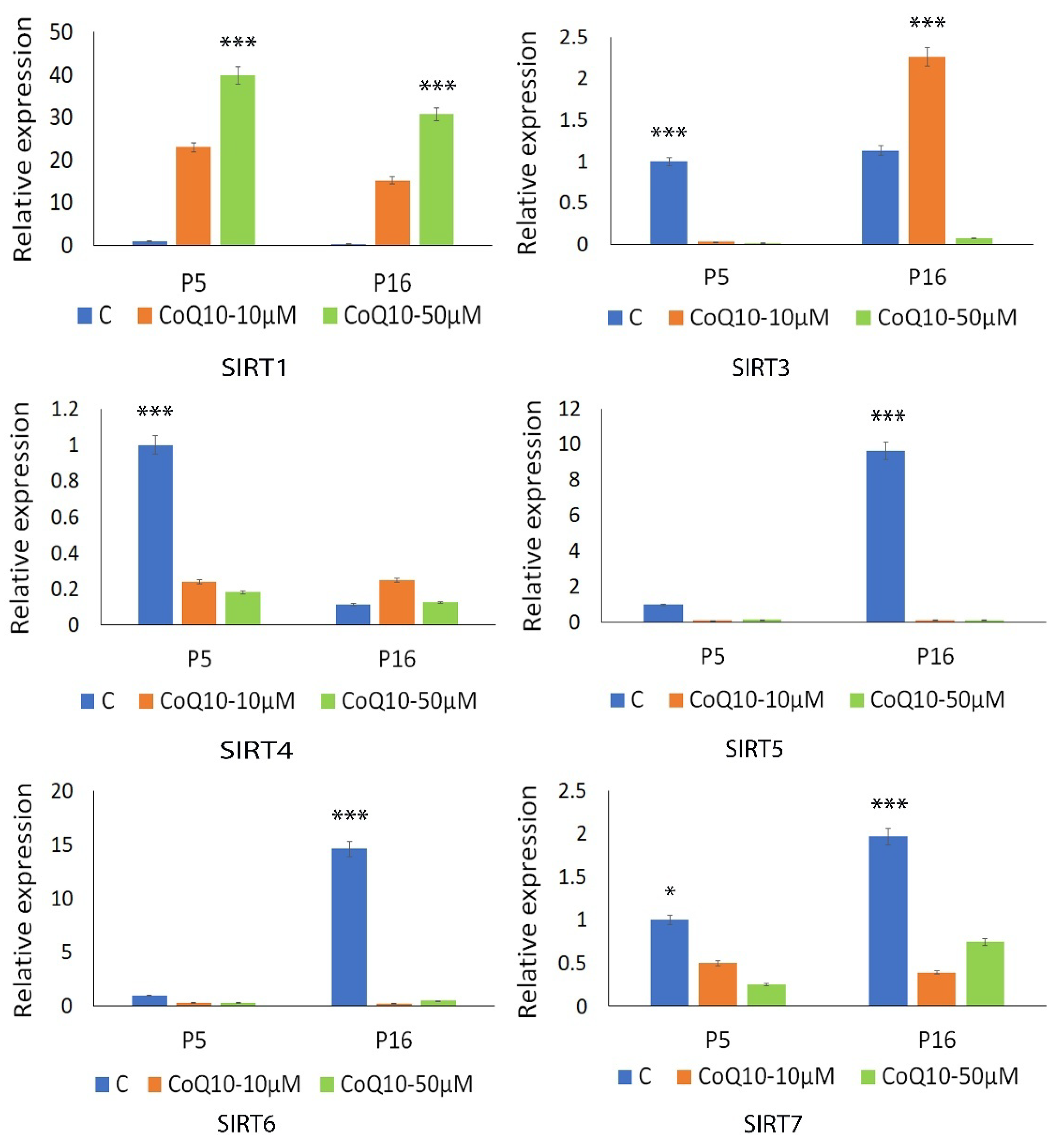
3.4. Analysis of Antioxidant and Sirtuin Gene Expression Response to CoQ10
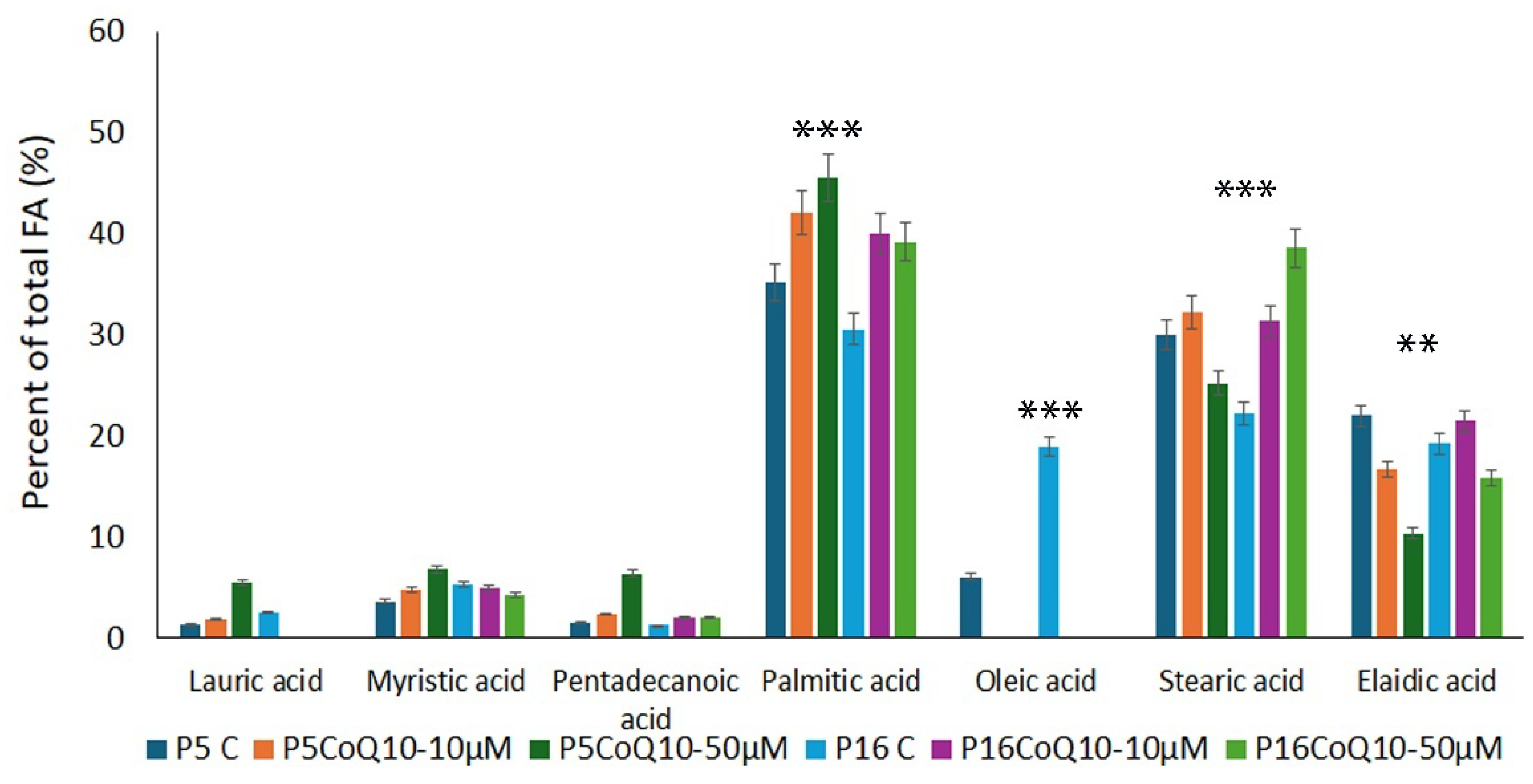
3.5. Assessment of Fatty Acid Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CoQ10 | Coenzyme Q10 |
| MSCs | Mesenchymal Stem Cells |
| GC-MS | Gas Chromatography–Mass Spectrometry |
| qPCR | Quantitative Polymerase Chain Reaction |
| PPARγ | Peroxisome Proliferator-Activated Receptor Gamma |
| CAT | Catalase |
| ROS | Reactive Oxygen Species |
| SRC | Spare Respiratory Capacity |
| DNA | Deoxyribonucleic Acid |
| SHEDs | Stem Cells from Human Exfoliated Deciduous Teeth |
| PBS | Phosphate-Buffered Saline |
| MEM | Minimum Essential Medium |
| FCS | Fetal Calf Serum |
| DMSO | Dimethyl Sulfoxide |
| ETS | Electron Transfer System |
| SA-β-Gal | Senescence-Associated β-Galactosidase |
| ATP | Adenosine Triphosphate |
| FCCP | Carbonyl Cyanide 4-(trifluoromethoxy) Phenylhydrazone |
| cDNA | Complementary DNA |
| RNA | Ribonucleic Acid |
| GMP | Good Manufacturing Practice |
| MTT | (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) |
| NAD+ | Nicotinamide Adenine Dinucleotide Phosphate |
| OXPHOS | Oxidative Phosphorylation |
| SOD | Superoxide Dismutase |
| ACC | Acetyl-CoA |
| SIRT | Sirtuins |
| FAMEs | Fatty Acid Methyl Esters |
| NLRP3 | NOD-, LRR-, and Pyrin Domain-Containing Protein 3 |
| ANT2 | Adenine Nucleotide Translocator 2 |
| GAPDH | Glyceraldehyde-3-Phosphate Dehydrogenase |
| FAs | Fatty Acids |
| PPARs | Peroxisome Proliferator-Activated Receptors |
| PDA | Pentadecanoic Acid |
| AMPK | Adenosine Monophosphate-Activated Protein Kinase |
| mTOR | Mammalian Target of Rapamycin |
References
- Weng, Z.; Wang, Y.; Ouchi, T.; Liu, H.; Qiao, X.; Wu, C.; Zhao, Z.; Li, L.; Li, B. Mesenchymal Stem/Stromal Cell Senescence: Hallmarks, Mechanisms, and Combating Strategies. Stem Cells Transl. Med. 2022, 11, 356–371. [Google Scholar] [CrossRef] [PubMed]
- Fox, I.J.; Daley, G.Q.; Goldman, S.A.; Huard, J.; Kamp, T.J.; Trucco, M. Use of Differentiated Pluripotent Stem Cells in Replacement Therapy for Treating Disease. Science 2014, 345, 1247391. [Google Scholar] [CrossRef] [PubMed]
- Raggi, C.; Berardi, A.C. Mesenchymal Stem Cells, Aging and Regenerative Medicine. Muscles Ligaments Tendons J. 2012, 2, 239–242. [Google Scholar]
- Zhang, D.; Yan, B.; Yu, S.; Zhang, C.; Wang, B.; Wang, Y.; Wang, J.; Yuan, Z.; Zhang, L.; Pan, J. Coenzyme Q10 Inhibits the Aging of Mesenchymal Stem Cells Induced by D-Galactose through Akt/mTOR Signaling. Oxidative Med. Cell. Longev. 2015, 2015, 867293. [Google Scholar] [CrossRef]
- Alt, E.U.; Senst, C.; Murthy, S.N.; Slakey, D.P.; Dupin, C.L.; Chaffin, A.E.; Kadowitz, P.J.; Izadpanah, R. Aging Alters Tissue Resident Mesenchymal Stem Cell Properties. Stem Cell Res. 2012, 8, 215–225. [Google Scholar] [CrossRef]
- Sohal, R.S.; Forster, M.J. Coenzyme Q, Oxidative Stress and Aging. Mitochondrion 2007, 7, S103–S111. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.-F.; Chiao, Y.A.; Marcinek, D.J.; Szeto, H.H.; Rabinovitch, P.S. Mitochondrial Oxidative Stress in Aging and Healthspan. Longev. Healthspan 2014, 3, 6. [Google Scholar] [CrossRef]
- Sohal, R.S.; Dubey, A. Mitochondrial Oxidative Damage, Hydrogen Peroxide Release, and Aging. Free Radic. Biol. Med. 1994, 16, 621–626. [Google Scholar] [CrossRef]
- Ferguson, M.; Mockett, R.J.; Shen, Y.; Orr, W.C.; Sohal, R.S. Age-Associated Decline in Mitochondrial Respiration and Electron Transport in Drosophila Melanogaster. Biochem. J. 2005, 390, 501–511. [Google Scholar] [CrossRef]
- Rauchová, H. Coenzyme Q10 Effects in Neurological Diseases. Physiol. Res. 2021, 70, S683–S714. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, P.; Chen, J.; Yang, C.; Xia, F.; Zhang, J.; Tang, H.; Liu, D.; Gu, L.; Shi, Q.; et al. Dissection of Targeting Molecular Mechanisms of Aristolochic Acid-Induced Nephrotoxicity via a Combined Deconvolution Strategy of Chemoproteomics and Metabolomics. Int. J. Biol. Sci. 2022, 18, 2003–2017. [Google Scholar] [CrossRef]
- Noh, Y.H.; Kim, K.-Y.; Shim, M.S.; Choi, S.-H.; Choi, S.; Ellisman, M.H.; Weinreb, R.N.; Perkins, G.A.; Ju, W.-K. Inhibition of Oxidative Stress by Coenzyme Q10 Increases Mitochondrial Mass and Improves Bioenergetic Function in Optic Nerve Head Astrocytes. Cell Death Dis. 2013, 4, e820. [Google Scholar] [CrossRef] [PubMed]
- Sandhir, R.; Sethi, N.; Aggarwal, A.; Khera, A. Coenzyme Q10 Treatment Ameliorates Cognitive Deficits by Modulating Mitochondrial Functions in Surgically Induced Menopause. Neurochem. Int. 2014, 74, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Liou, S.-W.; Chen, C.-C.; Chen, W.-C.; Hu, F.-R.; Wang, I.-J.; Lin, S.-J. Coenzyme Q10 Reduces Ethanol-Induced Apoptosis in Corneal Fibroblasts. PLoS ONE 2011, 6, e19111. [Google Scholar] [CrossRef]
- Hofman-Bang, C.; Rehnqvist, N.; Swedberg, K.; Wiklund, I.; Aström, H. Coenzyme Q10 as an Adjunctive in the Treatment of Chronic Congestive Heart Failure. The Q10 Study Group. J. Card. Fail. 1995, 1, 101–107. [Google Scholar] [CrossRef]
- Naderi, J.; Somayajulu-Nitu, M.; Mukerji, A.; Sharda, P.; Sikorska, M.; Borowy-Borowski, H.; Antonsson, B.; Pandey, S. Water-Soluble Formulation of Coenzyme Q10 Inhibits Bax-Induced Destabilization of Mitochondria in Mammalian Cells. Apoptosis 2006, 11, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Cleren, C.; Yang, L.; Lorenzo, B.; Calingasan, N.Y.; Schomer, A.; Sireci, A.; Wille, E.J.; Beal, M.F. Therapeutic Effects of Coenzyme Q10 (CoQ10) and Reduced CoQ10 in the MPTP Model of Parkinsonism. J. Neurochem. 2008, 104, 1613–1621. [Google Scholar] [CrossRef]
- Garrido-Maraver, J.; Cordero, M.D.; Oropesa-Avila, M.; Vega, A.F.; de la Mata, M.; Pavon, A.D.; Alcocer-Gomez, E.; Calero, C.P.; Paz, M.V.; Alanis, M.; et al. Clinical Applications of Coenzyme Q10. Front. Biosci. 2014, 19, 619–633. [Google Scholar] [CrossRef]
- Podar, A.S.; Semeniuc, C.A.; Ionescu, S.R.; Socaciu, M.-I.; Fogarasi, M.; Fărcaș, A.C.; Vodnar, D.C.; Socaci, S.A. An Overview of Analytical Methods for Quantitative Determination of Coenzyme Q10 in Foods. Metabolites 2023, 13, 272. [Google Scholar] [CrossRef]
- Zozina, V.I.; Covantev, S.; Goroshko, O.A.; Krasnykh, L.M.; Kukes, V.G. Coenzyme Q10 in Cardiovascular and Metabolic Diseases: Current State of the Problem. Curr. Cardiol. Rev. 2018, 14, 164–174. [Google Scholar] [CrossRef]
- Mancuso, M.; Orsucci, D.; Volpi, L.; Calsolaro, V.; Siciliano, G. Coenzyme Q10 in Neuromuscular and Neurodegenerative Disorders. Curr. Drug Targets 2010, 11, 111–121. [Google Scholar] [CrossRef]
- Sazali, S.; Badrin, S.; Norhayati, M.N.; Idris, N.S. Coenzyme Q10 Supplementation for Prophylaxis in Adult Patients with Migraine-a Meta-Analysis. BMJ Open 2021, 11, e039358. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Kamyab, A.; Hosseini, S.; Ebrahimi, S.; Ashkani-Esfahani, S. Involvement of Coenzyme Q10 in Various Neurodegenerative and Psychiatric Diseases. Biochem. Res. Int. 2023, 2023, 5510874. [Google Scholar] [CrossRef]
- Ivan, A.; Cristea, M.I.; Telea, A.; Oprean, C.; Galuscan, A.; Tatu, C.A.; Paunescu, V. Stem Cells Derived from Human Exfoliated Deciduous Teeth Functional Assessment: Exploring the Changes of Free Fatty Acids Composition during Cultivation. Int. J. Mol. Sci. 2023, 24, 17249. [Google Scholar] [CrossRef]
- Ivan, A.; Lukinich-Gruia, A.T.; Cristea, I.-M.; Pricop, M.-A.; Calma, C.L.; Simina, A.-G.; Tatu, C.A.; Galuscan, A.; Păunescu, V. Quercetin and Mesenchymal Stem Cell Metabolism: A Comparative Analysis of Young and Senescent States. Molecules 2024, 29, 5755. [Google Scholar] [CrossRef]
- Hernández-Pérez, O.R.; Juárez-Navarro, K.J.; Diaz, N.F.; Padilla-Camberos, E.; Beltran-Garcia, M.J.; Cardenas-Castrejon, D.; Corona-Perez, H.; Hernández-Jiménez, C.; Díaz-Martínez, N.E. Biomolecules Resveratrol + Coenzyme Q10 Recover the Cell State of Human Mesenchymal Stem Cells after 1-Methyl-4-Phenylpyridinium-Induced Damage and Improve Proliferation and Neural Differentiation. Front. Neurosci. 2022, 16, 929590. [Google Scholar] [CrossRef]
- Gruia, A.T.; Suciu, M.; Barbu-Tudoran, L.; Azghadi, S.M.R.; Cristea, M.I.; Nica, D.V.; Vaduva, A.; Muntean, D.; Mic, A.A.; Mic, F.A. Mesenchymal Stromal Cells Differentiating to Adipocytes Accumulate Autophagic Vesicles Instead of Functional Lipid Droplets. J. Cell. Physiol. 2016, 231, 863–875. [Google Scholar] [CrossRef]
- Zhang, D.-Y.; Pan, Y.; Zhang, C.; Yan, B.-X.; Yu, S.-S.; Wu, D.-L.; Shi, M.-M.; Shi, K.; Cai, X.-X.; Zhou, S.-S.; et al. Wnt/β-Catenin Signaling Induces the Aging of Mesenchymal Stem Cells through Promoting the ROS Production. Mol. Cell Biochem. 2013, 374, 13–20. [Google Scholar] [CrossRef]
- Borodkina, A.; Shatrova, A.; Abushik, P.; Nikolsky, N.; Burova, E. Interaction between ROS Dependent DNA Damage, Mitochondria and P38 MAPK Underlies Senescence of Human Adult Stem Cells. Aging 2014, 6, 481–495. [Google Scholar] [CrossRef]
- Taniguchi Ishikawa, E.; Gonzalez-Nieto, D.; Ghiaur, G.; Dunn, S.K.; Ficker, A.M.; Murali, B.; Madhu, M.; Gutstein, D.E.; Fishman, G.I.; Barrio, L.C.; et al. Connexin-43 Prevents Hematopoietic Stem Cell Senescence through Transfer of Reactive Oxygen Species to Bone Marrow Stromal Cells. Proc. Natl. Acad. Sci. USA 2012, 109, 9071–9076. [Google Scholar] [CrossRef]
- Stefely, J.A.; Pagliarini, D.J. Biochemistry of Mitochondrial Coenzyme Q Biosynthesis. Trends Biochem. Sci. 2017, 42, 824–843. [Google Scholar] [CrossRef]
- Onur, S.; Niklowitz, P.; Fischer, A.; Metges, C.C.; Grune, T.; Menke, T.; Rimbach, G.; Döring, F. A Comparative Study into Alterations of Coenzyme Q Redox Status in Ageing Pigs, Mice, and Worms. Biofactors 2014, 40, 346–354. [Google Scholar] [CrossRef]
- Casagrande, D.; Waib, P.H.; Jordão Júnior, A.A. Mechanisms of Action and Effects of the Administration of Coenzyme Q10 on Metabolic Syndrome. J. Nutr. Intermed. Metab. 2018, 13, 26–32. [Google Scholar] [CrossRef]
- McCarthy, S.; Somayajulu, M.; Sikorska, M.; Borowy-Borowski, H.; Pandey, S. Paraquat Induces Oxidative Stress and Neuronal Cell Death; Neuroprotection by Water-Soluble Coenzyme Q10. Toxicol. Appl. Pharmacol. 2004, 201, 21–31. [Google Scholar] [CrossRef]
- Binukumar, B.K.; Gupta, N.; Bal, A.; Gill, K.D. Protection of Dichlorvos Induced Oxidative Stress and Nigrostriatal Neuronal Death by Chronic Coenzyme Q10 Pretreatment. Toxicol. Appl. Pharmacol. 2011, 256, 73–82. [Google Scholar] [CrossRef]
- Chokchaiwong, S.; Kuo, Y.-T.; Lin, S.-H.; Hsu, Y.-C.; Hsu, S.-P.; Liu, Y.-T.; Chou, A.-J.; Kao, S.-H. Coenzyme Q10 Serves to Couple Mitochondrial Oxidative Phosphorylation and Fatty Acid β-Oxidation, and Attenuates NLRP3 Inflammasome Activation. Free Radic. Res. 2018, 52, 1445–1455. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Y.; Li, G.; Wang, J.; Yang, E.S. Coenzyme Q10 Attenuates Beta-Amyloid Pathology in the Aged Transgenic Mice with Alzheimer Presenilin 1 Mutation. J. Mol. Neurosci. 2008, 34, 165–171. [Google Scholar] [CrossRef]
- Wada, H.; Goto, H.; Hagiwara, S.-I.; Yamamoto, Y. Redox Status of Coenzyme Q10 Is Associated with Chronological Age. J. Am. Geriatr. Soc. 2007, 55, 1141–1142. [Google Scholar] [CrossRef] [PubMed]
- Limón-Pacheco, J.; Gonsebatt, M.E. The Role of Antioxidants and Antioxidant-Related Enzymes in Protective Responses to Environmentally Induced Oxidative Stress. Mutat. Res. /Genet. Toxicol. Environ. Mutagen. 2009, 674, 137–147. [Google Scholar] [CrossRef]
- Sangsefidi, Z.S.; Yaghoubi, F.; Hajiahmadi, S.; Hosseinzadeh, M. The Effect of Coenzyme Q10 Supplementation on Oxidative Stress: A Systematic Review and Meta-Analysis of Randomized Controlled Clinical Trials. Food Sci. Nutr. 2020, 8, 1766–1776. [Google Scholar] [CrossRef]
- Hargreaves, I.P. Coenzyme Q10 in Mitochondrial and Lysosomal Disorders. J. Clin. Med. 2021, 10, 1970. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; McCarty, M.F.; O’Keefe, J.H. Coenzyme Q10 Deficiency Can Be Expected to Compromise Sirt1 Activity. Open Heart 2022, 9, e001927. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.; Titus, A.S.; Banerjee, K.K.; George, S.; Lin, W.; Deota, S.; Saha, A.K.; Nakamura, K.; Gut, P.; Verdin, E.; et al. SIRT4 Regulates ATP Homeostasis and Mediates a Retrograde Signaling via AMPK. Aging 2013, 5, 835–849. [Google Scholar] [CrossRef]
- Raza, U.; Tang, X.; Liu, Z.; Liu, B. SIRT7: The Seventh Key to Unlocking the Mystery of Aging. Physiol. Rev. 2024, 104, 253–280. [Google Scholar] [CrossRef]
- Currie, E.; Schulze, A.; Zechner, R.; Walther, T.C.; Farese, R.V. Cellular Fatty Acid Metabolism and Cancer. Cell Metab. 2013, 18, 153–161. [Google Scholar] [CrossRef]
- Agmo Hernández, V.; Eriksson, E.K.; Edwards, K. Ubiquinone-10 Alters Mechanical Properties and Increases Stability of Phospholipid Membranes. Biochim. Biophys. Acta 2015, 1848, 2233–2243. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Hossain, S. Fatty Acids: From Membrane Ingredients to Signaling Molecules. In Biochemistry and Health Benefits of Fatty Acids; IntechOpen: London, UK, 2018; ISBN 978-1-78984-873-1. [Google Scholar] [CrossRef]
- Tamalampudi, S.; Fukuda, H. 3.07—Biodiesel. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Academic Press: Burlington, VT, USA, 2011; pp. 63–70. ISBN 978-0-08-088504-9. [Google Scholar]
- Ciesielski, V.; Legrand, P.; Blat, S.; Rioux, V. New Insights on Pentadecanoic Acid with Special Focus on Its Controversial Essentiality: A Mini-Review. Biochimie 2024, 227, 123–129. [Google Scholar] [CrossRef]
- Fu, W.-C.; Li, H.-Y.; Li, T.-T.; Yang, K.; Chen, J.-X.; Wang, S.-J.; Liu, C.-H.; Zhang, W. Pentadecanoic Acid Promotes Basal and Insulin-Stimulated Glucose Uptake in C2C12 Myotubes. Food Nutr. Res. 2021, 65, 4527. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivan, A.; Lukinich-Gruia, A.T.; Cristea, I.-M.; Pricop, M.-A.; Calma, C.L.; Paunescu, A.; Tatu, C.A.; Galuscan, A.; Paunescu, V. In Vitro Antioxidant Effects of Coenzyme Q10 on Cellular Metabolism in Aged Mesenchymal Stem Cells. Appl. Sci. 2025, 15, 2783. https://doi.org/10.3390/app15052783
Ivan A, Lukinich-Gruia AT, Cristea I-M, Pricop M-A, Calma CL, Paunescu A, Tatu CA, Galuscan A, Paunescu V. In Vitro Antioxidant Effects of Coenzyme Q10 on Cellular Metabolism in Aged Mesenchymal Stem Cells. Applied Sciences. 2025; 15(5):2783. https://doi.org/10.3390/app15052783
Chicago/Turabian StyleIvan, Alexandra, Alexandra Teodora Lukinich-Gruia, Iustina-Mirabela Cristea, Maria-Alexandra Pricop, Crenguta Livia Calma, Andreea Paunescu, Calin Adrian Tatu, Atena Galuscan, and Virgil Paunescu. 2025. "In Vitro Antioxidant Effects of Coenzyme Q10 on Cellular Metabolism in Aged Mesenchymal Stem Cells" Applied Sciences 15, no. 5: 2783. https://doi.org/10.3390/app15052783
APA StyleIvan, A., Lukinich-Gruia, A. T., Cristea, I.-M., Pricop, M.-A., Calma, C. L., Paunescu, A., Tatu, C. A., Galuscan, A., & Paunescu, V. (2025). In Vitro Antioxidant Effects of Coenzyme Q10 on Cellular Metabolism in Aged Mesenchymal Stem Cells. Applied Sciences, 15(5), 2783. https://doi.org/10.3390/app15052783






