Abstract
Background: Polycystic ovary syndrome (PCOS) is the most common hyperandrogenic disorder in reproductive age women. Identifying new biomarkers with high specificity and sensitivity is crucial for diagnosing and monitoring the disease. MiRNAs correlate with PCOS-related comorbidities, suggesting their potential as biomarkers. This study examined plasma concentrations of miR-451a, miR-15a-5p, miR-320-5p, miR-28-5p, miR-103a-5p, and miR-194-5p in adolescents with PCOS, healthy controls, and hyperandrogenic controls without PCOS. Correlations between microRNA levels and PCOS features were analyzed. Methods: Data on auxological, physical, metabolic, and hormonal parameters were collected from study groups. MiRNAs were extracted from plasma. Expression levels were measured by qPCR and calculated using the 2−∆∆Ct method. Results: Significant elevations in plasma levels of miR-15a-5p, miR-320-5p, miR-103a-5p, and miR-194-5p were observed in PCOS patients. Optimal threshold values for plasma miR-320-5p and miR-103a-5p effectively differentiated PCOS patients from healthy subjects, achieving 100% specificity and 76% sensitivity. In patients, expression of the four miRNAs showed significant positive correlations with some anthropometric and clinical parameters: miR-320-5p with systolic blood pressure, miR-103a-5p with CRP levels, miR-15a-5p with systolic blood pressure and CRP, and miR-194-5p with weight, waist circumference, and CRP. Conclusions: Among the investigated miRNAs, miR-320-5p and miR-103a-5p exhibited the most favorable diagnostic performance for PCOS.
1. Introduction
Polycystic ovary syndrome (PCOS) is the most common hyperandrogenic disorder affecting reproductive age women, beginning in adolescence and having a huge impact throughout their lifespan on their health status, fertility and psychosocial wellbeing [1,2]. The global prevalence of the disorder, depending on the used diagnostic criteria, is between 5.5% and 11.5% in adult women [3]. In adolescents, the global prevalence is 6.3% based on the latest International Evidence-based Guideline criteria from 2023 and 9.8% based on the Rotterdam Consensus criteria from 2003 [4].
The etiopathogenesis of PCOS is still not fully elucidated. Neuroendocrine disorders leading to elevated LH levels, tissue-selective insulin resistance and hyperinsulinemia, genetic and epigenetic factors (DNA methylation, non-coding RNAs), imbalance between androgens, FSH and anti-mullerian hormone (AMH) are involved, causing a self-perpetuating vicious circle [5,6]. The clinical presentation of PCOS evolves with age from ovulatory dysfunction (menstrual irregularities, anovulation) and hyperandrogenism (hirsutism, severe acne), frequently associated with obesity and metabolic disturbances (insulin resistance, hyperinsulinemia, dyslipidemia), to a mainly metabolic disorder with obesity, insulin resistance, impaired glucose metabolism, dyslipidemia and increased cardiovascular risk [7]. Impaired fertility, obstetric complications and psychological issues are common, and an increased cancer risk has been reported [7,8].
The current criteria for diagnosis of PCOS in adolescents include the presence of clinical and/or biochemical hyperandrogenism (HA) and oligomenorrhea/secondary amenorrhea (OA), after exclusion of other hyperandrogenic endocrine disorders (congenital adrenal hyperplasia, androgen producing tumors, etc.) [1]. These diagnostic criteria are controversial because they overlap with common physiological changes typical of puberty [9]. This creates the prerequisites for both late and inaccurate diagnosis, and overdiagnosis with adverse late consequences in terms of quality of life [10]. Therefore, in recent years, much work has been carried out for the detection of new biomarkers with high specificity and sensitivity, which would help clarifying the pathogenetic mechanism of PCOS, earlier establishing the diagnosis and monitoring the therapy.
MicroRNAs (miRNAs) are endogenous small non-coding single-stranded RNA molecules with 20–25 nucleotides, found in various environments—plasma and serum, ovarian granulosa cells and follicular fluid, seminal fluid, urine, etc. They inhibit the expression of the target messenger RNA and block the post-transcriptional protein translation [11]. MiRNAs regulate a number of processes, growth, cell differentiation and dedifferentiation, apoptosis, hormone biosynthesis, hematopoiesis, etc., and because of their stability are used as biomarkers for different diseases [11]. In the ovaries, miRNAs are involved in the regulation of proliferation and apoptosis of granulosa cells, growth and atresia of follicles, ovulation, luteinization and steroidogenesis. They are also important for the metabolic aspects in the pathogenesis of PCOS [12]. Different studies report many differently expressed miRNAs in PCOS. Some of them correlate with PCOS-related comorbidities—insulin resistance (miR-320, miR-93) and obesity (miR-197-3p, miR-223-3p, miR-877-5p, miR-122-5p). Others are significantly down-regulated (miR-451a, miR-6767-5p, miR-103a-3p, etc.) or up-regulated (miR-21-5p, miR-23a-3p, miR-26a-5p, etc.) in women with PCOS compared to controls, making them suitable for diagnostic biomarkers for PCOS [12,13,14,15].
The aim of the current study was to explore the plasma levels of selected miRNAs in adolescent girls with PCOS, healthy controls and controls with HA, but not fulfilling the diagnostic criteria for PCOS in adolescence (HAC) with evaluation of their suitability as diagnostic biomarkers for PCOS or predictors of PCOS-related comorbidities. As a secondary outcome of the study, we looked for correlations with the typical clinical, hormonal and metabolic characteristics of PCOS.
2. Materials and Methods
2.1. Patients and Control Groups
The participants in the study were 53 adolescent girls, with a mean age of 15.8 (11.9–17.9) years, all followed at one and the same unit and included in subsequent manner according to their clinic visits. They were divided into three groups. The PCOS group consisted of 34 girls, fulfilling the criteria of the International Evidence-based Guideline for PCOS in adolescents—irregular menstrual cycle (<21 or >45 days) and clinical (hirsutism, acne, seborrhea) and/or biochemical HA (10). Other causes of HA were excluded. The healthy control group included 11 BMI- and age-matched girls with regular menstrual cycles and without clinical or biochemical HA. The hyperandrogenic control group (HAC) consisted of 8 girls with clinical and/or biochemical HA but without menstrual disturbances, thus not fulfilling the criteria for PCOS but considered as being at risk for developing PCOS, according to the International Evidence-based Guideline [1]. The exclusion criteria for all participants were age above 18 years, less than 2 years post-menarche, other disorders with OA and/or HA (Cushing syndrome, androgen-producing tumors, non-classical or classical congenital adrenal hyperplasia, hyperprolactinemia, hypo-/hyperthyroidism), use of contraceptive pills or other drugs affecting the hormonal levels in the past 3 months.
All participants underwent a complete clinical examination and auxological assessment with measurement of height, weight and waist circumference (WC) using a standard technique. BMI was calculated with the formula Weight (kg)/Height2 (m). The presence of clinical HA and OA was assessed using a questionnaire and physical examination. A modified version of the Ferriman–Gallwey scale (FGS) with semi-subjective analysis of hair growth in nine androgen-dependent zones was used for assessment of hirsutism, with a score of ≥8 indicating hirsutism. All participants underwent metabolic and hormonal measurements using commercial laboratory kits and assay specifications according to the manufacturer’s recommendations. The hormonal analyses were performed during the early follicular phase of the menstrual cycle in girls with regular menstruation and, when possible, in girls with PCOS. The free androgen index (FAI) was calculated with the formula FAI = (T (nmol/L)/SHBG (nmol/L)) × 100, with FAI > 5 indicating androgen excess [16]. Biochemical HA was defined as testosterone levels above 1.4 nmol/L and/or FAI above 5. For calculation of HOMA-IR (Homeostatic Model Assessment for Insulin Resistance) the formula HOMA-IR = (fasting blood glucose (mmol/l) × fasting insulin (µmol/L))/22.5 was used, with HOMA-IR > 2.5 indicating insulin resistance [17]. Plasma samples were collected from all participants and stored at −80 °C.
We decided to investigate 6 miRNAs—miR-451a, miR-15a-5p, miR-320-5p, miR-28-5p, miR-103a-5p and miR-194-5p—most of which have not yet been studied in PCOS and, apart from miR-451a, miR194-5p and miR-15a-5p, have not been studied in adolescent girls. MiR-451a was found to be significantly down-regulated in PCOS girls, compared to healthy controls, with 100% sensitivity and 100% specificity for diagnosing PCOS [13]. MiR-103a-3p and miR-28-3p were also found significantly down-regulated in patients with PCOS [18]. Mir-320 is reported to be a potential biomarker for disorders of the lipid and glucose metabolism (type 2 diabetes mellitus, hyperlipidemia, atherosclerosis, fatty liver disease), which are often related to PCOS, and is found to be up-regulated in these conditions but down-regulated in PCOS [19,20,21]. MiR-194 was found to be overexpressed in women with PCOS and impaired glucose metabolism and correlated with HOMA-IR [22]. It was also found to be increased in granulosa cells of PCOS women, where it induced apoptosis [23]. We decided to investigate if we would find similar changes in the plasma levels of other members of miR-103, miR-28, miR-320 and miR-194 families. MiR-15a-5p was found to be significantly up-regulated in patients with endometrial cancer (EC) and was proposed as a biomarker for its early detection. It also correlated with androgen levels [24]. MiR-15a-5p was chosen because women with PCOS have a higher risk for EC at a premenopausal age [1]. The study was approved by the Ethics Review Board of the Medical University of Varna (protocol № 107 from 28 October 2021) and conducted according to the Declaration of Helsinki. Written informed consent was obtained by a parent or a legal representative of each participant prior to inclusion in the study.
2.2. miRNA Extraction, cDNA Synthesis and qPCR
The NucleoSpin miRNA Plasma kit (Macherey-Nagel GmbH & Co. KG., Düren, Germany) was employed to extract six circulating miRNAs from 200 µL serum samples. Following the assessment of RNA concentration and purity, cDNA synthesis was carried out on 90 ng of RNA. This process involved the use of stem-loop primers for miR-451a, miR-15a-5p, miR-320-5p, miR-28-5p, miR-103a-5p and miR-194-5p, and U6 reverse primers as the endogenous reference control (Table 1), via the RevertAid First Strand cDNA Synthesis kit (Thermo Fisher Scientific, Waltham, MA, USA). Luna Universal qPCR Master Mix (New England BioLabs, Ipswich, MA, USA) was used for quantitative PCR (qPCR), along with 3 × diluted cDNA templates, miRNA-specific forward primers, a universal reverse primer, and U6 forward and reverse primers (Table 1). Triplicate runs were performed for all qPCR reactions. To mitigate batch effects and control experimental variability, we divided our qPCR reactions into two batches: the first batch included 17 PCOS samples, 6 HC samples, and 4 HAC samples; the second batch included 17 PCOS samples, 5 HC samples, and 4 HAC samples. The 2−∆∆Ct method was employed to determine the comparative expression levels of miRNAs. We performed two separate comparative analyses of miRNA expression: HC vs. PCOS and HAC vs. PCOS. For the first comparison, miRNA fold-change values in PCOS patients were calculated using HC as the reference group. For the second comparison, miRNA fold-change values in PCOS patients were calculated using HAC as the reference group. For conducting qPCR analyses, the QuantStudio 5 Real-Time PCR system (Applied Biosystems, Waltham, MA, USA) was used.

Table 1.
Primer sequences (Sigma Aldrich, Taufkirhen, Germany) of analyzed miRNAs and endogenous reference control.
2.3. Statistical Analysis
Statistical analysis with the software IBM SPSS Statistics v30.0.0.0. and GraphPad Prism v6.01. was performed. Comparisons of the anthropometric, clinical, metabolic and hormonal parameters between the groups were performed using t-tests or one-way ANOVA with Tukey HSD post hoc tests. Quantitative variables were expressed as mean ± standard deviation (SD) and categorical variables as percentages (%) and numbers. Correlations were assessed with the Pearson Correlation Coefficient. Receiver Operating Characteristics (ROC) analysis and Youden index were employed to assess each miRNA’s capacity for differentiating PCOS patients from healthy control and hyperandrogenic control groups. Statistical significance was considered at p < 0.05.
3. Results
3.1. Characteristics of the Studied Groups
The anthropometric parameters and clinical characteristics of the groups are shown in Table 2. No significant differences in age, weight, height, BMI, WC, systolic and diastolic blood pressure (BP) were found between the groups. The prevalence of hirsutism and acne in the PCOS group, as well as FGS, was significantly higher compared to the healthy control group (all p < 0.05). The prevalence of hirsutism, seborrhea and the FGS in the PCOS group compared to the HAC group was also significantly higher (all p < 0.05). No significant differences in the prevalence of the symptoms of HA and FGS were found between the healthy control group and the HAC group.

Table 2.
Anthropometric parameters and clinical characteristics of PCOS patients and control groups. A multigroup comparison was conducted using one-way ANOVA with Tukey HSD post hoc test.
The metabolic and hormonal parameters are shown in Table 3. Apart from significantly higher fasting insulin levels in the PCOS group compared to the HAC group (p = 0.042), no other significant differences in the metabolic parameters were found between the groups. The PCOS group had significantly higher testosterone, androstenedione, AMH and FAI (all p < 0.05) compared to the healthy control group and a significantly lower SHBG compared to the healthy control and HAC groups (all p < 0.05). The HAC group had significantly higher testosterone, androstenedione and FAI compared to the healthy control group (all p < 0.001) but there were no significant differences for these parameters between the PCOS and the HAC groups.

Table 3.
Metabolic and hormonal parameters of PCOS patients and control groups (HC and HAC). A multigroup comparison was conducted using one-way ANOVA with Tukey HSD post hoc tests.
3.2. Comparative Analysis of Plasma Levels of Selected miRNAs in Samples of the Studied Groups
The relative expression levels of the selected miRNAs are shown in Figure 1. Four out of the six analyzed miRNAs displayed significant differential expression in adolescent females with PCOS: miR-320-5p, miR-103a-5p, miR-15a-5p, and miR-194-5p. These four miRNAs exhibited substantial upregulation in the PCOS group relative to the healthy control group (all p < 0.05). Furthermore, miR-15a-5p showed marked upregulation in the PCOS cohort when compared to the HAC group (p = 0.045). The expression patterns of miR-451a and miR-28-5p, the two remaining miRNAs, did not reveal any notable differences across the three study groups.
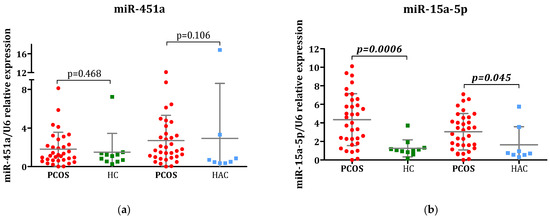
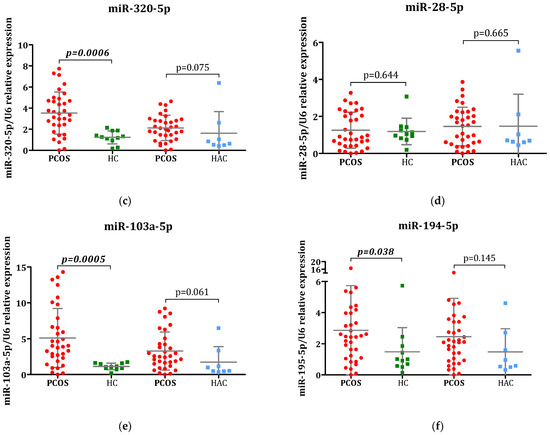
Figure 1.
Comparative analysis of miRNA expression: Evaluation of (a) miR-145a, (b) miR-15a-5p, (c) miR-320-5p, (d) miR-28-5p, (e) miR-103a-5p, and (f) miR-194-5p levels in plasma samples obtained from individuals with polycystic ovary syndrome (PCOS), healthy control (HC), and hyperandrogenic control group (HAC). All miRNA expression levels were determined through qPCR analysis. U6 RNA was utilized as the internal reference control. The 2−∆∆Ct method was applied to calculate comparative expression of miRNAs. Statistical comparisons between PCOS patients and control groups (HA and HAC) were conducted using the Mann–Whitney U test and the results are displayed as mean ± SD.
The sensitivity and specificity of each miRNA as a potential diagnostic marker for PCOS were assessed via ROC curve analyses (Figure 2). MiR-15a-5p, miR-320-5p, miR-103-5p, and miR-194-5p demonstrated statistically significant accuracy as independent diagnostic markers, as miR-15a-5p additionally exhibited the ability to differentiate PCOS from hyperandrogenic controls. Optimal threshold values for plasma miR-320-5p and miR-103a-5p effectively differentiated PCOS patients from healthy subjects, achieving 100% specificity and 76% sensitivity. MiR-194-5p exhibited 65% sensitivity and 81% specificity for diagnosing PCOS. Mir-15a-5p displayed higher specificity in distinguishing PCOS patients from healthy controls (91% specificity, 76% sensitivity) compared to distinguishing PCOS patients from HAC (75% specificity, 82% sensitivity).
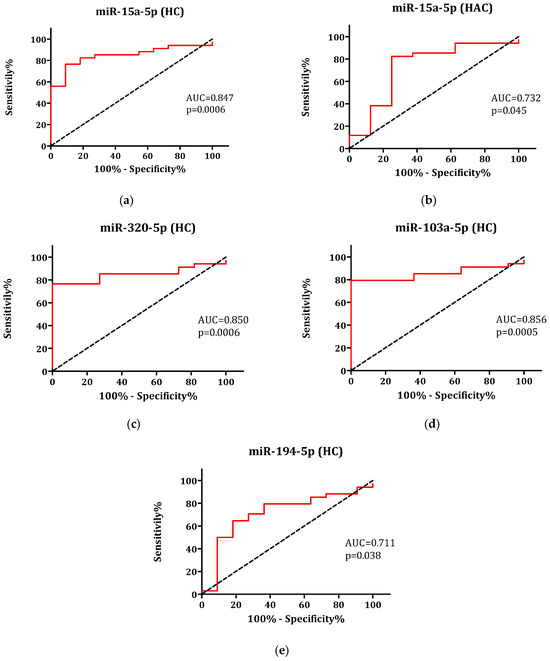
Figure 2.
ROC curves of miR-15a-5p utilized to differentiate patients with polycystic ovary syndrome (PCOS) from (a) healthy control (HC) and (b) hyperandrogenic control (HAC) groups, and ROC curves of (c) miR-320-5p, (d) miR-103a-5p, and (e) miR-194-5p employed to distinguish PCOS patients from HC.
3.3. Analysis of the Correlation Between Plasma Levels of the miRNAs and Clinical Parameters of PCOS Patients
Bivariate Pearson analysis was performed for detecting correlations between the differently expressed miRNAs and the clinical, metabolic and hormonal parameters related to PCOS in each group. Only the significant positive correlations observed in the PCOS group are shown in Table 4. These correlations were not found in the control groups.

Table 4.
Pearson bivariate correlation analysis of dysregulated miRNAs in the PCOS group (N = 34).
4. Discussion
The current study found four differently expressed miRNAs in the PCOS patients. MiR-320a-5p, miR-103a-5p, miR-15a-5p and miR-194-5p were all up-regulated in the PCOS group compared to the healthy control group, and miR-15a-5p was up-regulated in the PCOS group compared to the HAC group also. Plasma miR-320-5p and miR-103a-5p demonstrated the highest specificity and sensitivity for diagnosing PCOS, followed by miR-15a-5p, and these miRNAs could be used as independent diagnostic markers for PCOS patients. Furthermore, the latter miRNA showed a very good sensitivity profile in distinguishing PCOS women not only from healthy individuals but also from female patients with other hyperandrogenic conditions.
In recent years, extensive research on the diagnostic potential of different miRNAs as biomarkers for PCOS or PCOS-related metabolic disorders has been carried out but mainly in reproductive-age adult women. The number of studies concerning adolescents with PCOS is limited. Díaz et al. were the first to conduct a study in adolescents. It included 31 girls with PCOS, without obesity, and 13 controls. MiR-451a, miR-652-3p, miR-106b-5p and miR-206 were significantly down-regulated in PCOS girls compared to controls [13]. The authors found miR-451a to be 100% sensitive and 100% specific, miR-652-3p to be 81% sensitive and 100% specific and miR-106b-5p to be 100% sensitive and 85% specific for diagnosing PCOS. An inverse correlation was found with the degree of androgen excess and HOMA-IR for miR-451a, miR-652-3p, miR-106b-5p and miR-206, and a positive correlation with SHBG levels [13]. In our study, we did not find significant differences in the relative expression levels of miR-451a in the PCOS patients and the controls and could not confirm the described correlations. Though the number and age of the participants in both studies are similar, in our study, most of the participants were overweight/obese (65% of PCOS girls, 59% of healthy controls and 43% of HAC), which probably attributes to the difference in the results.
In a study by Udesen et al., serum mirR-28-3p, miR-103a-3, miR-139-5p and miR-376a-3p were found to be significantly down-regulated in women with PCOS [18]. MiR-28-3p and miR-139-5p correlated with the total testosterone and LH:FSH ratio. MiR-28-3p expressed a specificity of 100% and a sensitivity of 59%. It has been reported to be associated with granulosa cell proliferation [18]. In the present study, we assessed the relative expression of miR-28-5p, which has been reported to be associated with granulosa cell proliferation, and is found to be dysregulated in ovarian and other types of cancers [25]. We did not find significant differences in the relative expression of miR-28-5p in the patients and the control groups.
Mir-320 is involved in glucose and lipid metabolism and is a potential biomarker for associated metabolic disorders [19]. Krentowska et al. in their study found low serum expression levels of miR-320 in women with PCOS, compared to healthy controls. The expression of miR-320 correlated with fasting glucose, insulin levels and HOMA-IR, independently of age, BMI and androgen levels [26]. Rashad et al. found lower serum miR-320 expression levels in PCOS patients compared to controls and negative correlation with fasting serum insulin, HOMA-IR and PCOS phenotype. The sensitivity of serum miR-320 expression levels for the diagnosis of PCOS was 80% and the specificity was 97.5% [27]. On the other hand, Soyman et al. did not find significant differences in the expression levels of miR-320 in 50 women with PCOS or 50 BMI- and age-matched healthy controls [28]. In our study, we assessed the levels of miR-320-5p, a member of the miR-320 family that had not been investigated in PCOS so far. MiR-320-5p (and miR-320) has been described as a tumor suppressor, affecting cell proliferation and migration [29]. In PCOS, a significant proliferation rate and low apoptotic rate of granulosa cells are associated with abnormal folliculogenesis, leading to anovulation [30]. In the current study, miR-320-5p was significantly up-regulated in PCOS patients compared to healthy controls (p = 0.0006). It also showed a trend of overexpression in the PCOS group compared to the HAC group (p = 0.079). In the PCOS group, miR-320-5p correlated with the systolic blood pressure, but we did not find correlations with any of the metabolic parameters, the androgen excess or the PCOS phenotype.
MiR-103 has been detected in human blood and granulosa cells and is involved in steroid synthesis, insulin signaling and folliculogenesis [15,20]. Murri et al. found decreased expression of miR-103 in obese women but increased expression in PCOS patients and a positive correlation with testosterone concentrations [31]. Maffazioli et al. reported decreased expression of miR-103a-3p and a positive correlation with androgen levels in 36 Brazilian women with PCOS [15]. In our study, we investigated miR-103a-5p, which to our knowledge had not been assessed in PCOS patients so far. Like Murri et al., we found significantly increased expression in the PCOS group compared to the healthy controls (p = 0.0005) but not significant differences in the expression compared to the HAC groups (p = 0.061) [32]. In the PCOS group, but not in the control groups, miR-103a-5p showed a positive correlation with CRP and, unlike the mentioned above studies, no correlations with other metabolic parameters, androgens and PCOS phenotype. CRP has been validated as a biomarker of an increased cardiovascular risk, equal to increased LDL cholesterol levels [32], and has been found to be increased in PCOS patients in a number of studies [33], including the current one.
MiR-194 has been found to be up-regulated in granuslosa cells of women with PCOS and in PCOS rat models where it induced apoptosis [23]. Jiang et al. in their study, including 65 PCOS women with impaired glucose metabolism, 65 PCOS women with normal glucose metabolism and 45 healthy women, found that miR-194 was overexpressed in women with PCOS and correlated with HOMA-IR [34]. Similar to these results, we found significant overexpression of another miRNA from the miR-194 family. MiR-194-5p was significantly up-regulated in the PCOS patients compared to the healthy control group and showed 65% sensitivity and 81% specificity for diagnosing PCOS. No significant differences in expression compared to the HAC group were found. In the PCOS group, the expression of miR-194-5p correlated with weight, waist circumference and CRP, parameters that are important for cardiometabolic disturbances. These correlations were not found in the control groups. MiR-194-5p was reported to have a regulatory role in different cancers and was proposed as a biomarker for breast cancer [35], but, to our knowledge, has not been assessed or reported in PCOS patients so far.
Endometrial cancer (EC) is the second most common gynecologic cancer [24]. Premenopausal women with PCOS have a higher risk of endometrial hyperplasia and EC [1,8]. MiR-15a-5p was found to be significantly up-regulated in plasma and tumor tissue of patients with EC and was identified as a promising biomarker for the early detection of EC. It was also associated with testosterone, which is increased in PCOS, and DHEA-S [24]. These findings determined the choice to study miR-15a-5p. It was significantly upregulated in the PCOS group compared to the two control groups (p = 0.006 and p = 0.045, respectively) but displayed higher specificity in distinguishing PCOS patients from healthy controls compared to distinguishing PCOS patients from HAC. In addition, miR-15a-5p showed significant correlation with the systolic blood pressure and CRP in the PCOS group only, but did not show any correlations with other metabolic parameters, androgens and PCOS phenotype.
Finally, we must acknowledge several limitations of the present study. Primarily, our sample size is small, particularly in the control groups. To address the potential impact of this imbalance on our predictive modeling, we employed standard binary classification models, evaluating each miRNA individually as a potential biomarker. Nevertheless, these results require further validation in larger cohorts.
Additionally, direct comparison of our findings with published studies is challenging because our cohort consists of adolescent girls without concomitant cardio-metabolic complications, whereas most existing literature focuses on adult populations with more advanced disease profiles.
5. Conclusions
Four significantly upregulated miRNAs in PCOS adolescent girls were found in the present study. MiR-320-5p and miR-103a-5p exhibited optimal diagnostic performance for PCOS, followed by miR-15a-5p, and these miRNAs could be used as potential independent diagnostic markers for PCOS. MiR-15a-5p also showed a very good sensitivity profile in distinguishing PCOS patients from girls with other hyperandrogenic conditions. The utility of these biomarkers for clinical practice must be validated by further studies involving more patients and healthy controls. The exact role of these miRNAs for the etiopathogenesis of PCOS and their correlation with PCOS-related comorbidities need to be further elucidated.
Author Contributions
Conceptualization, V.M. and M.R.; methodology, M.R.; formal analysis, M.R. and V.M.; investigation, V.M. and M.R.; resources, V.M., S.G. and V.I.; data curation, V.M. and M.R.; writing—original draft preparation, V.M. and M.R.; writing—review and editing, M.R., S.G. and V.I.; visualization, M.R.; supervision, V.I.; project administration, V.M., M.R. and V.I.; funding acquisition, V.I. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Fund “Science”, Medical University of Varna, Bulgaria (Project No 21019, Grant No FS-6/02 February 2022) and the European Union NextGenerationEU program, through the National Recovery and Resilience Plan of the Republic of Bulgaria (Project No BG-RRP-2.004-0009-C02).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the “Commission for Scientific Research Ethics” of Medical University of Varna, Bulgaria (protocol № 107 from 28 October 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author using the following e-mail: vilhelm.mladenov@mu-varna.bg. The data are not publicly available due to their containing information that could compromise the privacy of research participants.
Acknowledgments
We appreciate the support from all participants in the study, nurses from First Pediatric Clinic of the University hospital “Sveta Marina”—Varna, laboratory technicians, fund “Science” staff and all colleagues who contributed to the study.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Abbreviations
The following abbreviations are used in this manuscript:
| PCOS | Polycystic ovary syndrome |
| AMH | Anti-Mullerian hormone |
| HA | Hyperandrogenism |
| OA | Oligomenorrhea/secondary amenorrhea |
| BMI | Body mass index |
| WC | Waist circumference |
| SBP | Systolic blood pressure |
| DBP | Diastolic blood pressure |
| FGS | Ferriman–Gallwey scale |
| SHBG | Sex-hormone binding globulin |
| FAI | Free androgen index |
| 17-OHP | 17-hydroxyprogesterone |
| DHEA-S | Dehydroepiandrosterone sulphate |
| HOMA-IR | Homeostatic model assessment for insulin resistance |
References
- Teede, H.J.; Tay, C.T.; Laven, J.J.E.; Dokras, A.; Moran, L.J.; Piltonen, T.T.; Costello, M.F.; Boivin, J.; Redman, L.M.; Boyle, J.A.; et al. Recommendations from the 2023 international evidence-based guideline for the assessment and management of polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2023, 108, 2447–2469. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, R.J.; Avery, J.C.; Moore, V.M.; Davies, M.J.; Azziz, R.; Stener-Victorin, E.; Moran, L.J.; Robertson, S.A.; Stepto, N.K.; Norman, R.J.; et al. Complex diseases and co-morbidities: Polycystic ovary syndrome and type 2 diabetes mellitus. Endocr. Connect. 2019, 8, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Nankali, A.; Ghanbari, A.; Jafarpour, S.; Ghasemi, H.; Dokaneheifard, S.; Mohammadi, M. Global prevalence of polycystic ovary syndrome in women worldwide: A comprehensive systematic review and meta-analysis. Arch. Gynecol. Obstet. 2024, 310, 1303–1314. [Google Scholar] [CrossRef] [PubMed]
- Neven, A.C.H.; Forslund, M.; Ranashinha, S.; Mousa, A.; Tay, C.T.; Peña, A.; Oberfield, S.; Witchel, S.; Teede, H.; Boyle, J.A. Prevalence and accurate diagnosis of polycystic ovary syndrome in adolescents across world regions: A systematic review and meta-analysis. Eur. J. Endocrinol. 2024, 191, S15–S27. [Google Scholar] [CrossRef]
- Ibáñez, L.; Oberfield, S.E.; Witchel, S.; Auchus, R.J.; Chang, R.J.; Codner, E.; Dabadghao, P.; Darendeliler, F.; Elbarbary, N.S.; Gambineri, A.; et al. An international consortium update: Pathophysiology, diagnosis, and treatment of polycystic ovarian syndrome in adolescence. Horm. Res. Paediatr. 2017, 88, 371–395. [Google Scholar] [CrossRef]
- Witchel, S.F.; Oberfield, S.E.; Peña, A.S. Polycystic ovary syndrome: Pathophysiology, presentation, and treatment with emphasis on adolescent girls. J. Endocr. Soc. 2019, 3, 1545–1573. [Google Scholar] [CrossRef]
- Louwers, Y.V.; Laven, J.S.E. Characteristics of polycystic ovary syndrome throughout life. Ther. Adv. Reprod. Health 2020, 14, 1–9. [Google Scholar] [CrossRef]
- Harris, H.R.; Terry, K.L. Polycystic ovary syndrome and risk of endometrial, ovarian, and breast cancer: A systematic review. Fertil. Res. Pract. 2016, 5, 2–14. [Google Scholar] [CrossRef]
- Ramezani, T.F.; Amiri, M. Polycystic ovary syndrome in adolescents: Challenges in diagnosis and treatment. Int. J. Endocrinol. Metab. 2019, 17, e91554. [Google Scholar] [CrossRef]
- Peña, A.S.; Witchel, S.F.; Hoeger, K.M.; Oberfield, S.E.; Vogiatzi, M.G.; Misso, M.; Garad, R.; Dabadghao, P.; Teede, H. Adolescent polycystic ovary syndrome according to the international evidence-based guideline. BMC Med. 2020, 18, 72. [Google Scholar] [CrossRef]
- Abdalla, M.; Deshmukh, H.; Atkin, S.L.; Sathyapalan, T. miRNAs as a novel clinical biomarker and therapeutic targets in polycystic ovary syndrome (PCOS): A review. Life Sci. 2020, 259, 118174. [Google Scholar] [CrossRef]
- Deswal, R.; Dang, A.S. Dissecting the role of micro-RNAs as a diagnostic marker for polycystic ovary syndrome: A systematic review and meta-analysis. Fertil. Steril. 2020, 113, 661–669.e2. [Google Scholar] [CrossRef] [PubMed]
- Díaz, M.; Bassols, J.; López-Bermejo, A.; de Zegher, F.; Ibáñez, L. Low circulating levels of miR-451a in girls with polycystic ovary syndrome: Different effects of randomized treatments. J. Clin. Endocrinol. Metab. 2020, 105, dgz204. [Google Scholar] [CrossRef] [PubMed]
- Song, D.K.; Sung, Y.A.; Lee, H. The role of serum microRNA-6767-5p as a biomarker for the diagnosis of polycystic ovary syndrome. PLoS ONE 2016, 11, e0163756. [Google Scholar] [CrossRef] [PubMed]
- De Nardo Maffazioli, G.; Baracat, E.C.; Soares, J.M.; Carvalho, K.C.; Maciel, G.A.R. Evaluation of circulating microRNA profiles in Brazilian women with polycystic ovary syndrome: A preliminary study. PLoS ONE 2022, 17, e0275031. [Google Scholar] [CrossRef]
- Deans, R. Polycystic ovary syndrome in adolescence. Med. Sci. 2019, 7, 101. [Google Scholar] [CrossRef]
- Yetim Şahin, A.; Baş, F.; Yetim, Ç.; Uçar, A.; Poyrazoğlu, Ş.; Bundak, R.; Darendeliler, F. Determination of insulin resistance and its relationship with hyperandrogenemia, anti-Müllerian hormone, inhibin A, inhibin B, and insulin-like peptide-3 levels in adolescent girls with polycystic ovary syndrome. Turk. J. Med. Sci. 2019, 49, 1117–1125. [Google Scholar] [CrossRef]
- Udesen, P.B.; Sørensen, A.E.; Svendsen, R.; Frisk, N.L.S.; Hess, A.L.; Aziz, M.; Wissing, M.L.M.; Englund, A.L.M.; Dalgaard, L.T. Circulating miRNAs in women with polycystic ovary syndrome: A longitudinal cohort study. Cells 2023, 12, 983. [Google Scholar] [CrossRef]
- Du, H.; Zhao, Y.; Yin, Z.; Wang, D.W.; Chen, C. The role of miR-320 in glucose and lipid metabolism disorder-associated diseases. Int. J. Biol. Sci. 2021, 17, 402–416. [Google Scholar] [CrossRef]
- Sørensen, A.E.; Wissing, M.L.; Salö, S.; Englund, A.L.M.; Dalgaard, L.T. MicroRNAs related to polycystic ovary syndrome (PCOS). Genes 2014, 5, 684–708. [Google Scholar] [CrossRef]
- Cirillo, F.; Catellani, C.; Lazzeroni, P.; Sartori, C.; Nicoli, A.; Amarri, S.; La Sala, G.B.; Street, M.E. MiRNAs regulating insulin sensitivity are dysregulated in polycystic ovary syndrome (PCOS) ovaries and are associated with markers of inflammation and insulin sensitivity. Front. Endocrinol. 2019, 10, 879. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Xu, P.; Wang, J.; Zhang, C. The role of MiRNA in polycystic ovary syndrome (PCOS). Gene 2019, 706, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.X.; Lin, Y.S.; Li, S.C.; Yao, X.; Cheng, M.; Zhu, L.; Liu, H.Y. microRNA-194 is increased in polycystic ovary syndrome granulosa cell and induce KGN cells apoptosis by direct targeting heparin-binding EGF-like growth factor. Reprod. Biol. Endocrinol. 2021, 19, 170. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, W.; Wang, F.; Yang, S.; Hu, J.; Lu, B.; Pan, Z.; Ma, Y.; Zheng, M.; Zhou, L.; et al. Plasma-derived exosomal miR-15a-5p as a promising diagnostic biomarker for early detection of endometrial carcinoma. Mol. Cancer 2021, 20, 57. [Google Scholar] [CrossRef]
- Meng, L.; Yang, H.; Jin, C.; Quan, S. miR-28-5p suppresses cell proliferation and weakens the progression of polycystic ovary syndrome by targeting prokineticin-1. Mol. Med. Rep. 2019, 20, 2468–2475. [Google Scholar] [CrossRef]
- Krentowska, A.; Ponikwicka-Tyszko, D.; Łebkowska, A.; Adamska, A.; Sztachelska, M.; Milewska, G.; Hryniewicka, J.; Wołczyński, S.; Kowalska, I. Serum expression levels of selected microRNAs and their association with glucose metabolism in young women with polycystic ovary syndrome. Pol. Arch. Intern. Med. 2024, 134, 16637. [Google Scholar] [CrossRef]
- Rashad, N.M.; Ateya, M.A.; Saraya, Y.S.; Elnagar, W.M.; Helal, K.F.; Lashin, M.E.; Abdelrhman, A.A.; Alil, A.E.; Yousef, M.S. Association of miRNA—320 expression level and its target gene endothelin-1 with the susceptibility and clinical features of polycystic ovary syndrome. J. Ovarian Res. 2019, 12, 39. [Google Scholar] [CrossRef]
- Soyman, Z.; Durmus, S.; Ates, S.; Simsek, G.; Sozer, V.; Kundaktepe, B.P.; Kurtulus, D.; Gelisgen, R.; Sal, V.; Uzun, H. Circulating mir-132, mir-146a, mir-222, and mir-320 expression in differential diagnosis of women with polycystic ovary syndrome. Acta Endocrinol. 2022, 18, 13–19. [Google Scholar] [CrossRef]
- Li, W.; Wang, S.; Shan, B.; Cheng, X.; He, H.; Qin, J.; Tang, Y.; Zhao, H.; Tian, M.; Zhang, X.; et al. CircHECTD1 regulates cell proliferation and migration by the miR-320-5p/SLC2A1 axis in glioblastoma multiform. Front. Oncol. 2021, 11, 666391. [Google Scholar] [CrossRef]
- Ruiz-Manriquez, L.M.; Ledesma Pacheco, S.J.; Medina-Gomez, D.; Uriostegui-Pena, A.G.; Estrada-Meza, C.; Bandyopadhyay, A.; Pathak, S.; Banerjee, A.; Chakraborty, S.; Srivastava, A.; et al. A brief review on the regulatory roles of microRNAs in cystic diseases and their use as potential biomarkers. Genes 2022, 13, 191. [Google Scholar] [CrossRef]
- Murri, M.; Insenser, M.; Fernández-Durán, E.; San-Millán, J.L.; Escobar-Morreale, H.F. Effects of polycystic ovary syndrome (PCOS), sex hormones, and obesity on circulating miRNA-21, miRNA-27b, miRNA-103, and miRNA-155 expression. J. Clin. Endocrinol. Metab. 2013, 98, E1835–E1844. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.D.; Budoff, M.J.; Ferdinand, K.; Graham, I.M.; Michos, E.D.; Reddy, T.; Shapiro, M.D.; Toth, P.P. Atherosclerotic cardiovascular disease risk assessment: An american society for preventive cardiology clinical practice statement. Am. J. Prev. Cardiol. 2022, 10, 100335. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, E.; Suchta, K.; Grymowicz, M.; Calik-Ksepka, A.; Smolarczyk, K.; Duszewska, A.M.; Smolarczyk, R.; Meczekalski, B. Chronic low grade inflammation in pathogenesis of PCOS. Int. J. Mol. Sci. 2021, 22, 3789. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Huang, J.; Chen, Y.; Yang, Y.; Li, R.; Li, Y.; Chen, X.; Yang, D. Identification of several circulating microRNAs from a genome-wide circulating microRNA expression profile as potential biomarkers for impaired glucose metabolism in polycystic ovarian syndrome. Endocrine 2016, 53, 280–290. [Google Scholar] [CrossRef]
- Yen, Y.T.; Yang, J.C.; Chang, J.B.; Tsai, S.C. Down-regulation of miR-194-5p for predicting metastasis in breast cancer cells. Int. J. Mol. Sci. 2021, 23, 325. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).