First Appraisal of Effective Microplastics Removal from the Textile Manufacturing Processes
Abstract
1. Introduction
2. Materials and Methods
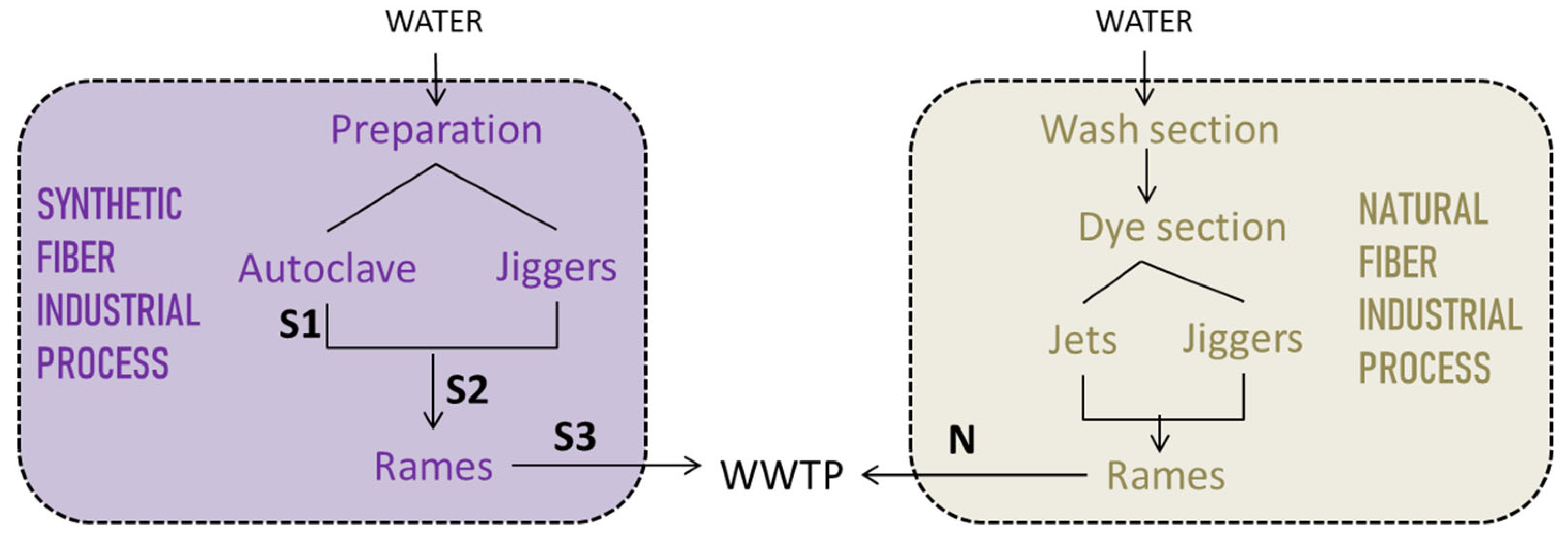
2.1. Liquid Residues
2.2. Adiabatic Sonic Evaporation and Crystallization (ASEC) Technology
2.3. Experiment
2.4. Sample Collection and Analyses
2.4.1. MPs Characterization
2.4.2. Determination of Surfactants and Sulfates
3. Results
3.1. Mass Balance
3.2. By-Product Characterization
3.2.1. Water Intake Characterization
3.2.2. Water Output Characterization
3.2.3. Solid Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, H.; Pu, S.; Liu, S.; Bai, Y.; Mandal, S.; Xing, B. Microplastics in aquatic environments: Toxicity to trigger ecological consequences. Environ. Pollut. 2020, 261, 114089. [Google Scholar] [CrossRef] [PubMed]
- Vivekanand, A.C.; Mohapatra, S.; Tyagi, V.K. Microplastics in aquatic environment: Challenges and perspectives. Chemosphere 2021, 282, 131151. [Google Scholar] [CrossRef]
- Ahmed, A.S.S.; Billah, M.M.; Ali, M.M.; Alam Bhuiyan, K.; Guo, L.; Mohinuzzaman, M.; Hossain, M.B.; Rahman, M.S.; Islam, S.; Yan, M.; et al. Microplastics in aquatic environments: A comprehensive review of toxicity, removal, and remediation strategies. Sci. Total Environ. 2023, 876, 162414. [Google Scholar] [CrossRef] [PubMed]
- HIS Markit. Anon. Natural and Man-Made Fibers Overview. IHS Markit. 2015. Available online: https://ihsmarkit.com/products/fibers-chemical-economics-handbook.html (accessed on 14 May 2024).
- Periyasamy, A.P.; Tehrani-Bangha, A. A review on microplastic emission from textile materials and its reduction techniques. J. Polym. Degrad. Stab. 2022, 199, 109901. [Google Scholar] [CrossRef]
- Cai, Y.; Mitrano, D.M.; Heuberger, M.; Hufenus, R.; Nowack, B. The origin of microplastic fiber in polyester textiles: The textile production process matters. J. Clean. Prod. 2020, 267, 121970. [Google Scholar] [CrossRef]
- Sherrington, C. Plastics in the Marine Environment; Eunomia: Bristol, UK, 2016. [Google Scholar]
- Ellen MacArthur Foundation. A New Textiles Economy: Redesigning Fashion’s Future. 2017. Available online: https://www.ellenmacarthurfoundation.org/a-new-textiles-economy (accessed on 26 January 2021).
- Salam, M.; Zheng, H.; Liu, Y.; Zaib, A.; Rehman, S.A.U.; Riaz, N.; Eliw, M.; Hayat, F.; Li, H.; Wang, F. Effects of micro(nano)plastics on soil nutrient cycling: State of the knowledge. J. Environ. Manag. 2023, 344, 118437. [Google Scholar] [CrossRef]
- Salam, M.; Li, H.; Wang, F.; Zaib, A.; Yang, W.; Li, Q. The impacts of microplastics and biofilms mediated interactions on sedimentary nitrogen cycling: A comprehensive review. Process Safe Environ. Prot. 2024, 184, 332–341. [Google Scholar] [CrossRef]
- Li, X.; Bao, L.; Wei, Y.; Zhao, W.; Wang, F.; Liu, X.; Su, H.; Zhang, R. Occurrence, Bioaccumulation, and Risk Assessment of Microplastics in the Aquatic Environment: A Review. Water 2023, 15, 1768. [Google Scholar] [CrossRef]
- European Union. Council Decision (EU) 2021/764 of 10 May 2021 Establishing the Specific Programme Implementing Horizon Europe—The Framework Programme for Research and Innovation, and Repealing Decision 2013/743/EU (Text with EEA Relevance). 2021. Available online: https://eur-lex.europa.eu/eli/dec/2021/764/oj/eng (accessed on 26 January 2021).
- Uddin, F. Introductory Chapter: Textile Manufacturing Processes. In Textile Manufacturing Processes; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar] [CrossRef]
- Majumdar, A.; Das, A.; Alagirusamy, R.; Kothari, V.K. (Eds.) Process Control in Textile Manufacturing, 1st ed.; Woodhead Publishing: Sawston, UK, 2012; p. 512. ISBN 9780857090270. [Google Scholar]
- Zhou, T.; Song, S.; Min, R.; Liu, X.; Zhang, G. Advances in chemical removal and degradation technologies for microplastics in the aquatic environment: A review. Mar. Pollut. Bull. 2024, 201, 116202. [Google Scholar] [CrossRef]
- Park, H.; Park, B. Review of Microplastic Distribution, Toxicity, Analysis Methods, and Removal Technologies. Water 2021, 13, 2736. [Google Scholar] [CrossRef]
- Mustapha, S.; Tijani, J.; Elabor, R.; Salau, R.; Egbosiuba, T.; Amigun, A.; Shuaib, D.; Sumaila, A.; Fiola, T.; Abubakar, Y.; et al. Technological approaches for removal of microplastics and nanoplastics in the environment. J. Environ. Chem. Eng. 2024, 12, 112084. [Google Scholar] [CrossRef]
- Nasir, M.S.; Tahir, I.; Ali, A.; Ayub, I.; Nasir, A.; Abbas, N.; Sajjad, U.; Hamid, K. Innovative technologies for removal of micro plastic: A review of recent advances. Heliyon 2024, 10, e25883. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Prakash, S.; Kumari, N.; Sharma, D.; Laller, R.; Pundir, A.; Puri, S.; Radha. From Challenges to Opportunities: Exploring Minimum Liquid Discharge and Zero Liquid Discharge Strategies for Wastewater Management and Resource Recovery. In Role of Science and Technology for Sustainable Future; Sobti, R.C., Ed.; Springer: Singapore, 2024. [Google Scholar] [CrossRef]
- Pundir, A.; Thakur, M.S.; Goel, B.; Prakash, S.; Kumari, N.; Sharma, N.; Parameswari, E.; Senapathy, M.; Kumar, S.; Radha; et al. Innovations in textile wastewater management: A review of zero liquid discharge technology. Environ. Sci. Pollut. Res. 2024, 31, 12597–12616. [Google Scholar] [CrossRef] [PubMed]
- Bonnail, E.; Vera, S.; Blasco, J.; DelValls, T.Á. Towards a Cleaner Textile Industry: Using ASEC to Decrease the Water Footprint to Zero Liquid Discharge. Water 2023, 15, 3781. [Google Scholar] [CrossRef]
- Bonnail, E.; Vera, S.; Blasco, J.; Conradi, M.; DelValls, T.Á. Metal pollution and mining in the Iberian Pyrite Belt: New remediation technologies to improve the ecosystem services of the river basins. Water 2023, 15, 1302. [Google Scholar] [CrossRef]
- Bonnail, E.; Vera, S.; DelValls, T.Á. A New Disruptive Technology for Zero-Brine Discharge: Towards a Paradigm Shift. Appl. Sci. 2023, 13, 13092. [Google Scholar] [CrossRef]
- Tang, K.H.D.; Li, R.; Li, Z.; Wang, D. Health risk of human exposure to microplastics: A review. Environ. Chem. Lett. 2024, 22, 1155–1183. [Google Scholar] [CrossRef]
- Carney Almroth, B.M.; Åström, L.; Roslund, S.; Petersson, H.; Johansson, M.; Persson, N.-K. Quantifying shedding of synthetic fibers from textiles; a source of microplastics released into the environment. Environ. Sci. Pollut. Res. 2018, 25, 1191–1199. [Google Scholar] [CrossRef]
- Osman, A.I.; Hosny, M.; Eltaweil, A.S.; Omar, S.; Elgarahy, A.M.; Farghali, M.; Yap, P.-S.; Wu, Y.-S.; Nagandran, S.; Batumalaie, K.; et al. Microplastic sources, formation, toxicity and remediation: A review. Environ. Chem. Lett. 2023, 21, 2129–2169. [Google Scholar] [CrossRef] [PubMed]
- Cesa, F.S.; Turra, A.; Checon, H.H.; Leonardi, B.; Baruque- Ramos, J. Laundering and textile parameters influence fibers release in household washings. Environ. Pollut. 2020, 257, 113553. [Google Scholar] [CrossRef]
- O’Brien, S.; Rauert, C.; Ribeiro, F.; Okoffo, E.D.; Burrows, S.D.; O’Brien, J.W.; Wang, X.; Wright, S.L.; Thomas, K.V. There’s something in the air: A review of sources, prevalence and behaviour of microplastics in the atmosphere. Sci. Total Environ. 2023, 874, 162193. [Google Scholar] [CrossRef] [PubMed]
- Vdovchenko, A.; Resmini, M. Mapping Microplastics in Humans: Analysis of Polymer Types, and Shapes in Food and Drinking Water—A Systematic Review. Int. J. Mol. Sci. 2024, 25, 7074. [Google Scholar] [CrossRef] [PubMed]
- Lang, Y.; O’Neill, S.; Lawler, J. Considerations for the pharmaceutical industry regarding environmental and human health impacts of microplastics. In Influence of Microplastics on Environmental and Human Health; CRC Press: Boca Raton, FL, USA, 2022; pp. 61–78. [Google Scholar]
- Pashaei, R.; Dzingelevičienė, R.; Bradauskaitė, A.; Lajevardipour, A.; Mlynska-Szultka, M.; Dzingelevičius, N.; Raugelė, S.; Razbadauskas, A.; Abbasi, S.; Rees, R.M.; et al. Pharmaceutical and microplastic pollution before and during the COVID-19 pandemic in surface water, wastewater, and groundwater. Water 2022, 14, 3082. [Google Scholar] [CrossRef]
- Zurier, H.S.; Goddard, J.M. Biodegradation of microplastics in food and agriculture. Curr. Opin. Food Sci. 2021, 37, 37–44. [Google Scholar] [CrossRef]
- Akbay, H.E.G.; Akarsu, C.; Isik, Z.; Belibagli, P.; Dizge, N. Investigation of degradation potential of polyethylene microplastics in anaerobic digestion process using cosmetics industry wastewater. Biochem. Eng. J. 2022, 187, 108619. [Google Scholar] [CrossRef]
| Samples | Salt Formation (g/L) | MP Mass (mg) | Retained Ratio | MPs Removal | ||
|---|---|---|---|---|---|---|
| Aqin | Aqout | Solidout | (mg MP/Solid) | (%) | ||
| S1 | 19 | 44 ± 1 | 0.3 ± 0.1 | 95 ± 1.0 | 0.005 | 99.97 |
| S2 | 5.8 | 182 ± 3 | 0.1 ± 0.1 | 0.27 ± 2.0 | 4.65 × 10−5 | 99.99 |
| S3 | 7 | 55 ± 2 | 0.1 | 101 ± 2.0 | 0.014 | 99.99 |
| N | 4 | 69 ± 3 | 0.25 ± 0.1 | 146 ± 2.0 | 0.0365 | 99.99 |
| Compound Input Water | S1in | S2in | S3in | Nin |
|---|---|---|---|---|
Benzene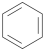 | Polyester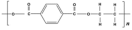 | Polyester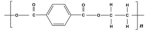 | Low match | Polyester |
Benzoic acid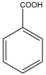 | Polyester | |||
2,4-dymethyl-1-heptene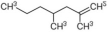 | Polypropylene | PMMA polymer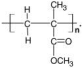 | ||
Styrene/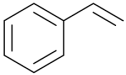 | Polyester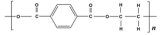 (Low match) | Low match | Presence | |
Cyclopentanone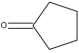 | Nylon-6,6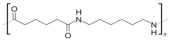 |
| Solid S1out | |||
|---|---|---|---|
| Compound | CAS | Characteristic ions m/z | Match |
| Benzene | 71-43-2 | 50, 51, 77, 78 | 790 |
| Cyclopentanone | 120-92-3 | 41, 55, 84 | 714 |
| 2,4-Dimethyl-1-heptene | 19549-87-2 | 43, 55, 70, 83 | 887 |
| Styrene | 100-42-5 | 51, 78, 104 | 888 |
| Cyclohexane, 1,3,5-triphenyl- | 117-81-7 | 149, 167 | 666 |
| Bis(2-ethylhexyl) phthalate | 117-81-7 | 149, 167 | 665 |
| Solids S2out | |||
| Compound | CAS | Characteristic ions m/z | Match |
| Benzene | 71-43-2 | 50, 51, 77, 78 | 805 |
| Styrene | 100-42-5 | 51, 78, 104 | 834 |
| Bis(2-ethylhexyl) phthalate | 117-81-7 | 149, 167 | 820 |
| 2,4-Dimethyl-1-heptene | 19549-87-2 | 43, 55, 70, 83 | 895 |
| Toluene | 108-88-3 | 91,92 | 708 |
| Solids S3out | |||
| Compound | CAS | Characteristic ions m/z | Match |
| Benzene | 71-43-2 | 50, 51, 77, 78 | 886 |
| Toluene | 108-88-3 | 91, 92 | 773 |
| 2,4-Dimethyl-1-heptene | 19549-87-2 | 43, 55, 70, 83 | 842 |
| Styrene | 100-42-5 | 51, 78, 104 | 843 |
| 1-Eicosene | 01-07-3452 | 43, 55, 69, 71, 83, 97 | 830 |
| Bis(2-ethylhexyl) phthalate | 117-81-7 | 149, 167 | 676 |
| Nout | |||
| Compound | CAS | Characteristic ions m/z | Match |
| Benzene | 71-43-2 | 50, 51, 77, 78 | 798 |
| 2,4-Dimethyl-1-heptene | 19549-87-2 | 43, 55, 70, 83 | 919 |
| Styrene | 100-42-5 | 51, 78, 104 | 709 |
| Benzene, 1-ethyl-3-methyl- | 620-14-4 | 120, 105 | 703 |
| Bis(2-ethylhexyl) phthalate | 117-81-7 | 149, 167 | 913 |
| Sample | Sulfate Ions (mg/L) | Concentration of Surfactants (mg/L) | |
|---|---|---|---|
| MBAS Anionic | CTAB Anionic | ||
| S1in | 8000 | <0.1 | 1.0 |
| S-in | 200 | <0.1 | 1.0 |
| S-in | 400 | <0.1 | 1.0 |
| Nin | 200 | 2.0 | 3.0 |
| S1out | <25 | <0.1 | <0.1 |
| S2out | <25 | <0.1 | <0.1 |
| S3out | <25 | <0.1 | <0.1 |
| Nout | <25 | <0.1 | <0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonnail, E.; Vera, S.; Blasco, J.; DelValls, T.Á. First Appraisal of Effective Microplastics Removal from the Textile Manufacturing Processes. Appl. Sci. 2025, 15, 2630. https://doi.org/10.3390/app15052630
Bonnail E, Vera S, Blasco J, DelValls TÁ. First Appraisal of Effective Microplastics Removal from the Textile Manufacturing Processes. Applied Sciences. 2025; 15(5):2630. https://doi.org/10.3390/app15052630
Chicago/Turabian StyleBonnail, Estefanía, Sebastián Vera, Julián Blasco, and T. Ángel DelValls. 2025. "First Appraisal of Effective Microplastics Removal from the Textile Manufacturing Processes" Applied Sciences 15, no. 5: 2630. https://doi.org/10.3390/app15052630
APA StyleBonnail, E., Vera, S., Blasco, J., & DelValls, T. Á. (2025). First Appraisal of Effective Microplastics Removal from the Textile Manufacturing Processes. Applied Sciences, 15(5), 2630. https://doi.org/10.3390/app15052630






