Electrochemical Surface Modification of Fully Biodegradable Mg-Based Biomaterials as a Sustainable Alternative to Non-Resorbable Bone Implants
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
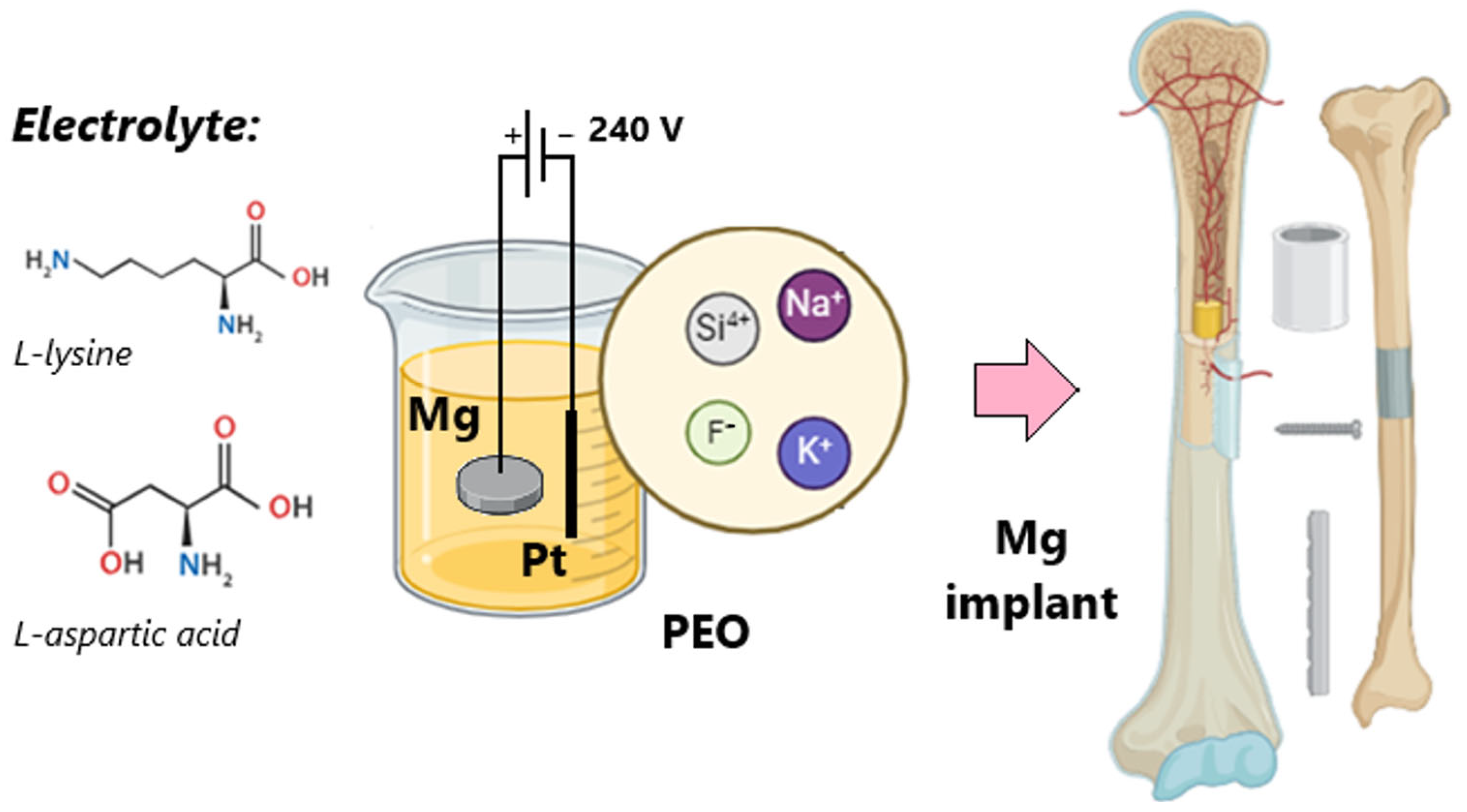
2.2. Methods
Biomaterials Preparation
2.3. Wettability Assessment
2.4. Structure and Morphology
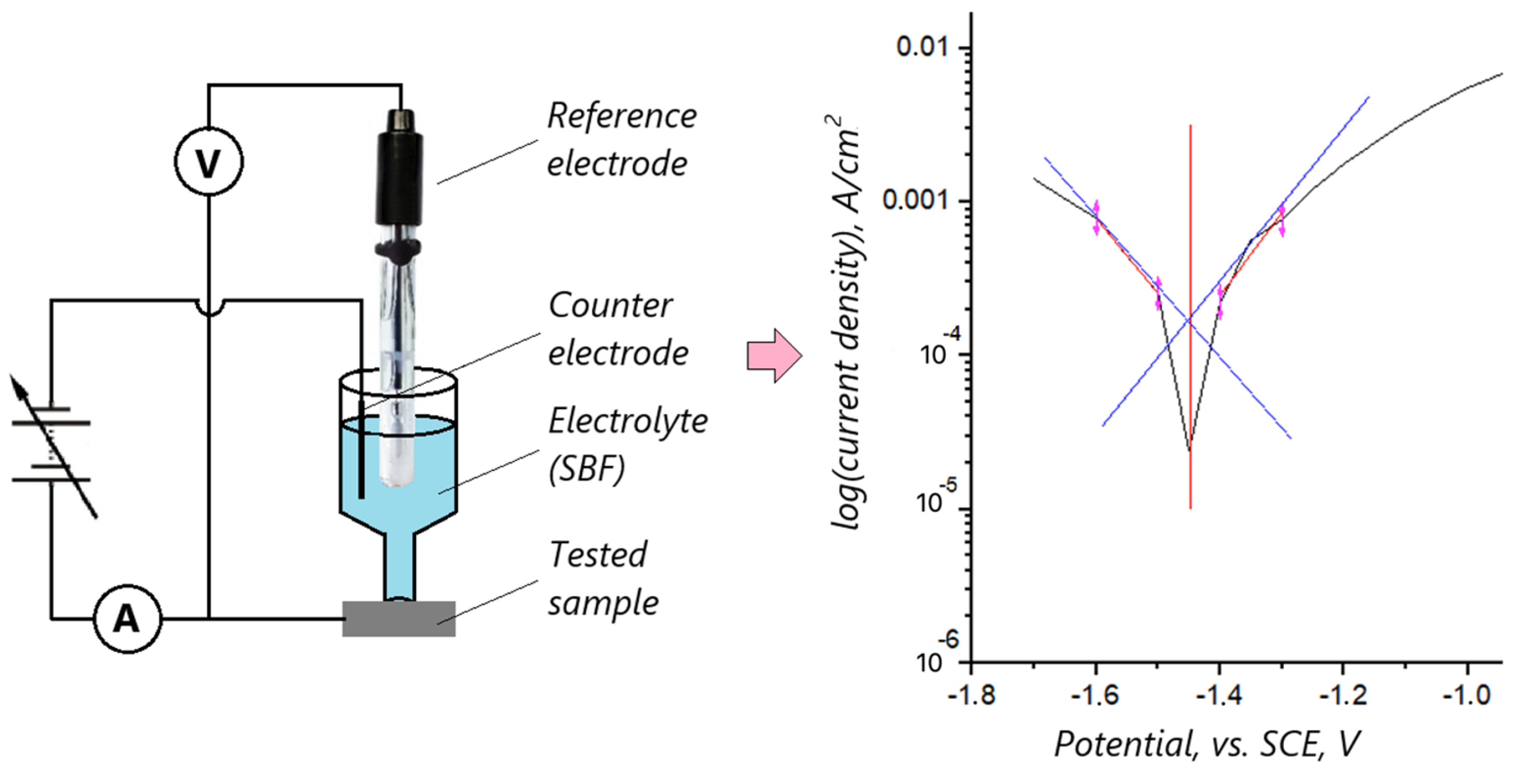
2.5. Biocorrosion Studies
2.6. Cytotoxicity Assessment
3. Results and Discussion
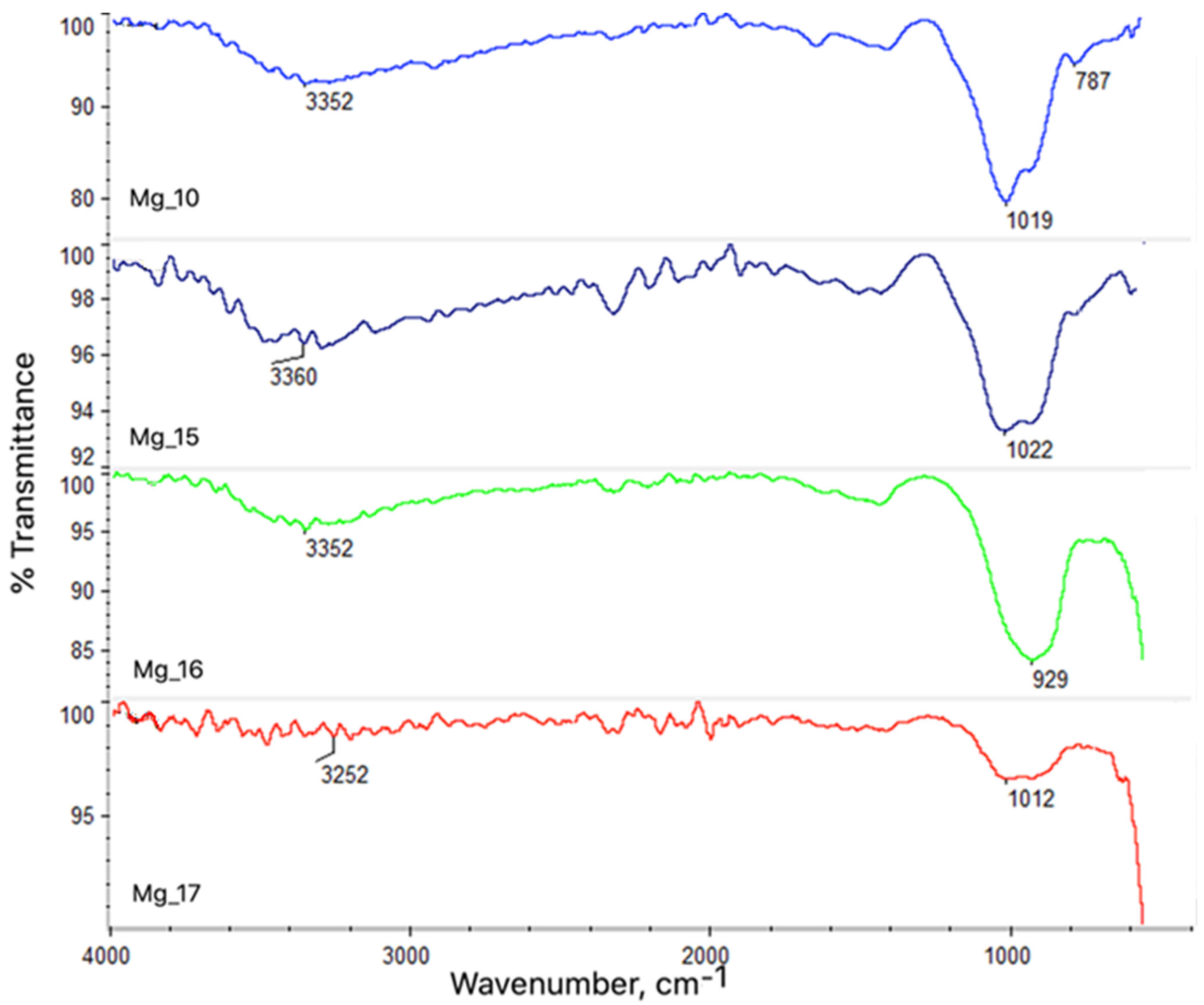
3.1. Analysis of Chemical Structure Using ASA and FT-IR Methods
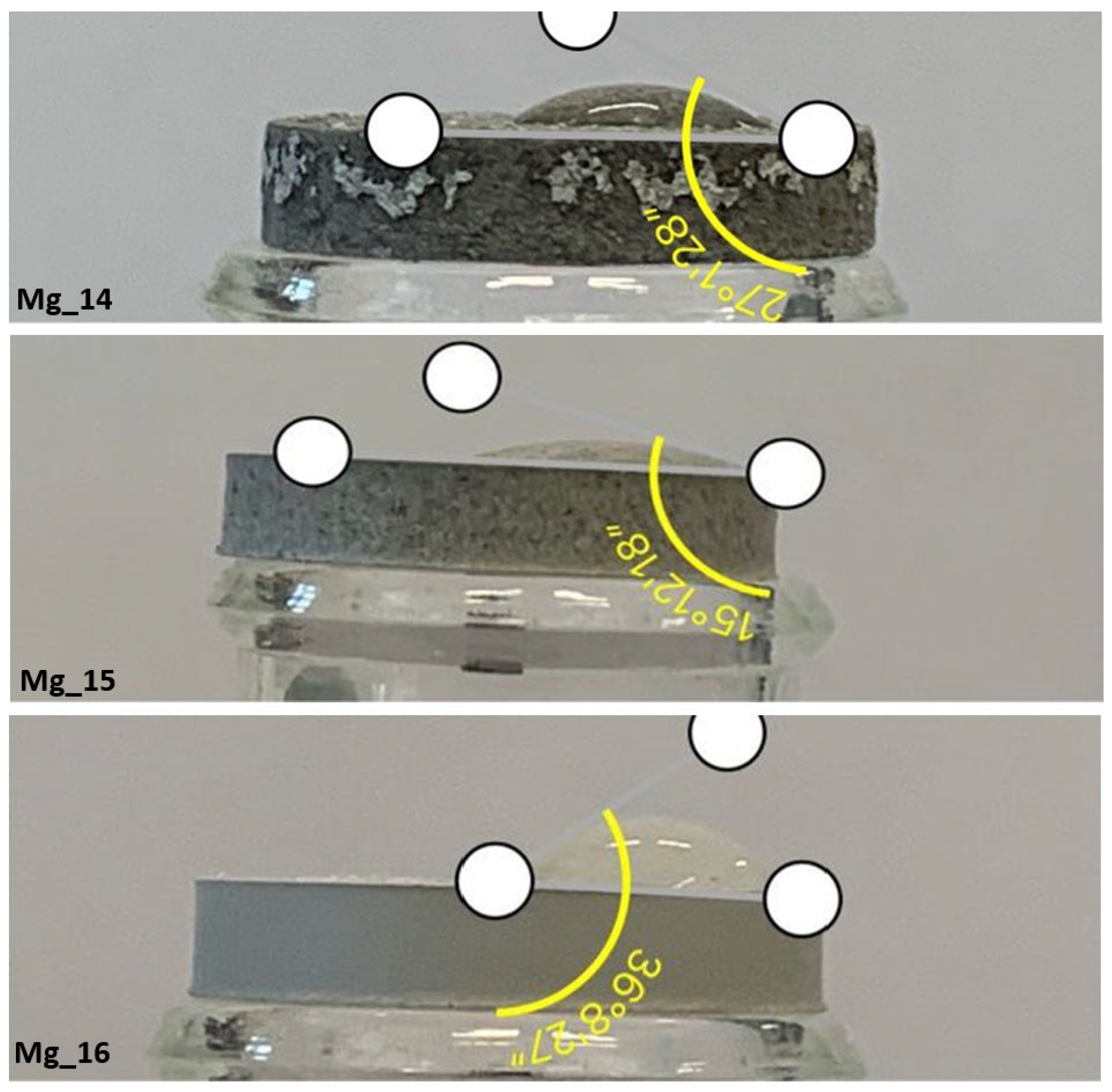
3.2. Wettability Study
3.3. Morphology Study
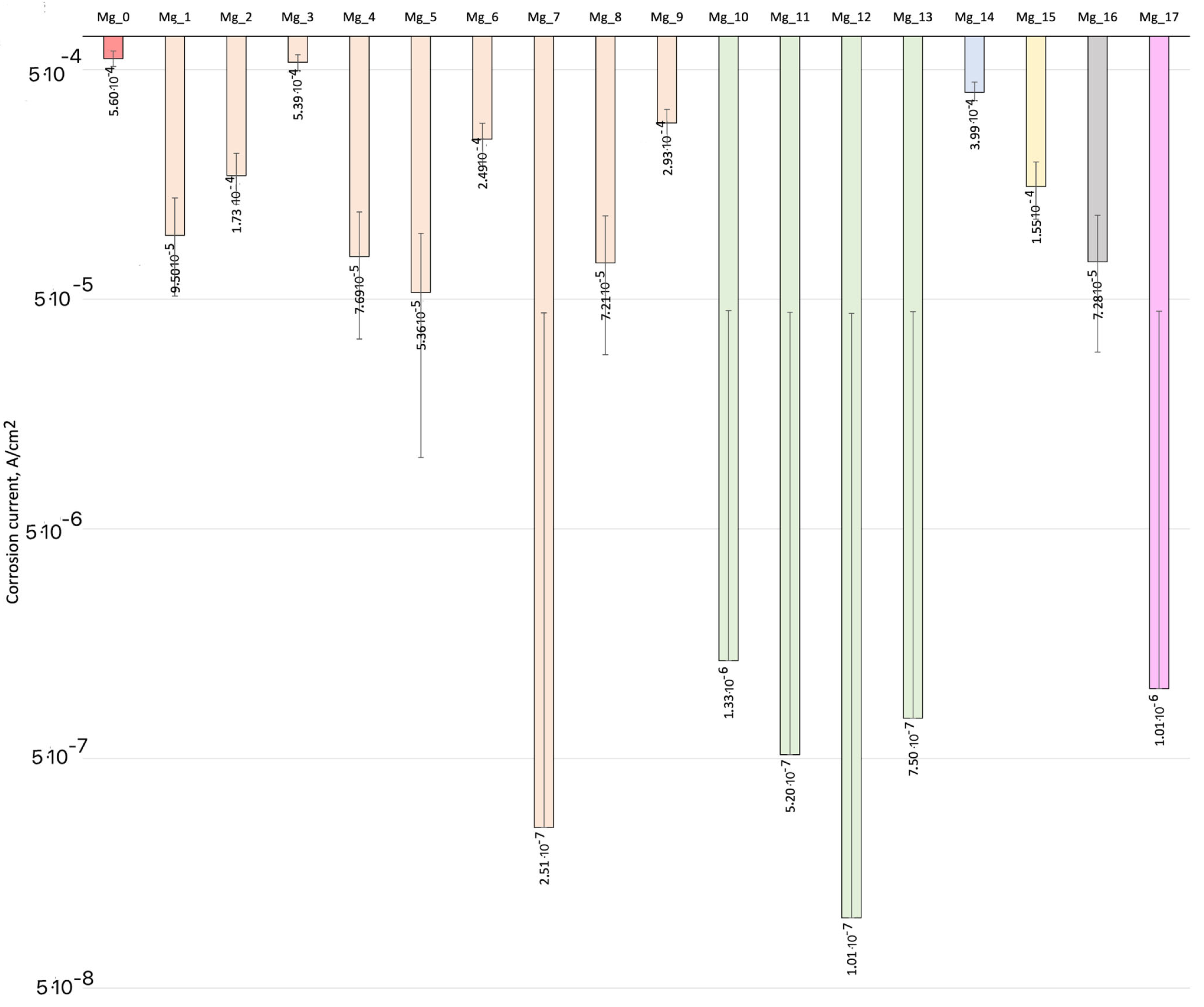
3.4. Biocorrosion Study
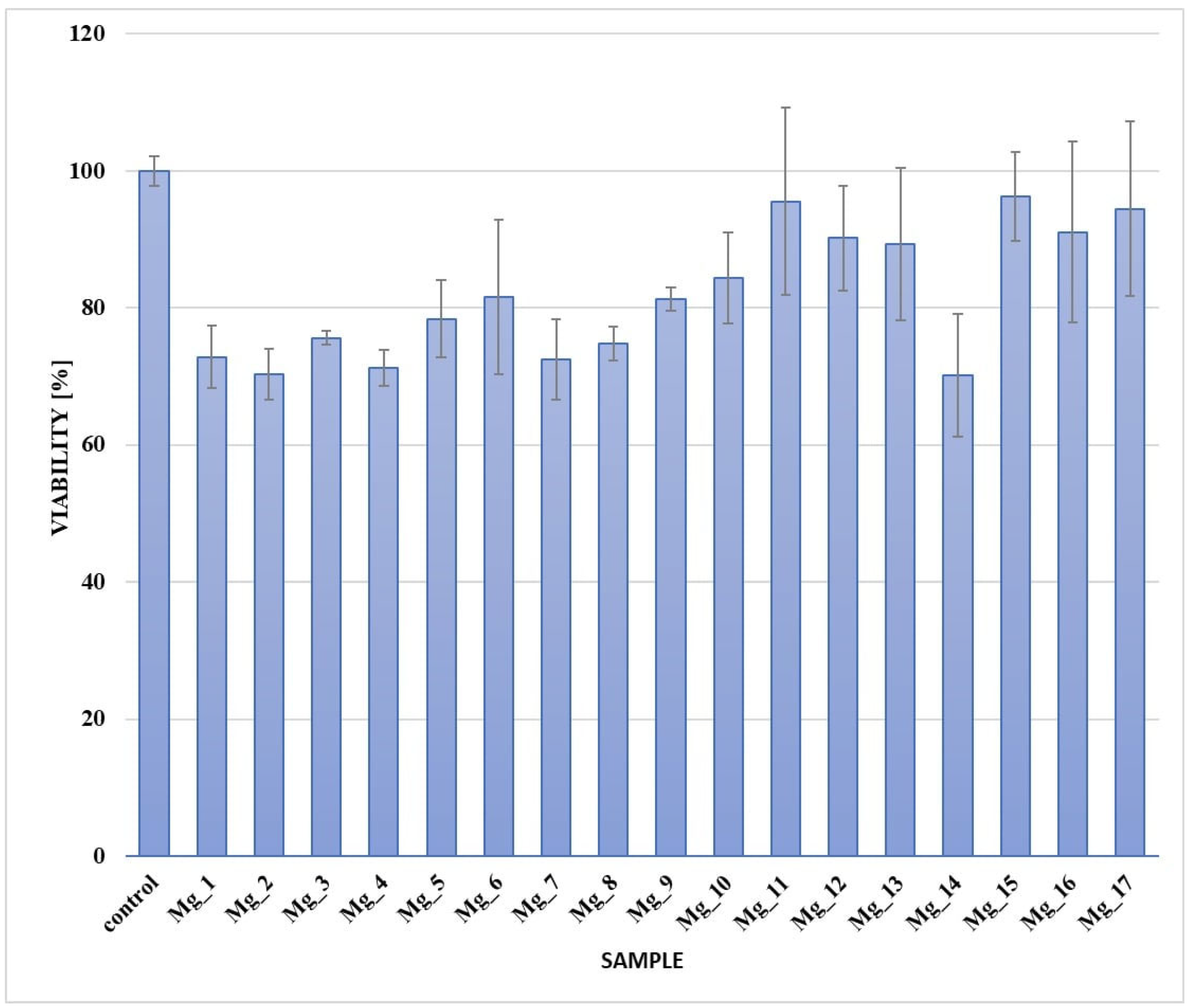
3.5. Cytotoxicity Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Fan, H.; Li, H.; Hua, L.; Du, J.; He, Y.; Jin, Y. Recent Advancements in the Surface Modification of Additively Manufactured Metallic Bone Implants. Addit. Manuf. Front. 2025, 200195. [Google Scholar] [CrossRef]
- Chang, J.Z.-C.; Hsu, J.-T.; Li, M.-J.; Lin, H.-Y.; Sun, J.; Tsou, N.-T.; Sun, J.-S. Optimizing Dental Implant Design: Structure, Strength, and Bone Ingrowth. J. Dent. Sci. 2024, in press. [Google Scholar] [CrossRef]
- Elboraey, M.O.; Alqutaibi, A.Y.; Aboalrejal, A.N.; Borzangy, S.; Zafar, M.S.; Al-Gabri, R.; Alghauli, M.A.; Ramalingam, S. Regenerative Approaches in Alveolar Bone Augmentation for Dental Implant Placement: Techniques, Biomaterials, and Clinical Decision-Making: A Comprehensive Review. J. Dent. 2025, 154, 105612. [Google Scholar] [CrossRef]
- Ouqi, Y.; Wang, J.; Yang, X.; Man, Y. Factors Influencing Labial Bone Resorption After Implant Insertion with Simultaneous Guided Bone Regeneration: Retrospective Cone Beam Computed Tomography Study. Int. J. Oral Maxillofac. Surg. 2024, 54, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-L.; Chen, J.-J.; Chen, C.-S. Using Optimization Approach to Design Dental Implant in Three Types of Bone Quality—A Finite Element Analysis. J. Dent. Sci. 2025, 20, 126–136. [Google Scholar] [CrossRef]
- An, Y.; Zhang, H.; Zhang, S.-A.; Zhang, Y.; Zheng, L.; Chen, X.; Tong, W.; Xu, J.; Qin, L. Degradation Products of Magnesium Implant Synergistically Enhance Bone Regeneration: Unraveling the Roles of Hydrogen Gas and Alkaline Environment. Bioact. Mater. 2025, 46, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Malinovschi, V.; Marin, A.; Ducu, C.; Moga, S.; Craciun, V.; Lungu, C.P.; Cimpoesu, R.; Golgovici, F.; Cristea, D. Preparation and Characterization of Ti5Al16O34 PEO Ceramic Coatings Deposited on CP-Ti in Mixed Aluminate-Phosphate Electrolytes. Ceram. Int. 2024, 50, 40955–40975. [Google Scholar] [CrossRef]
- Fattah-alhosseini, A.; Chaharmahali, R.; Kaseem, M. Corrosion Behavior Amelioration of Ti-Based Alloys by the Hybrid Plasma Electrolytic Oxidation (PEO)/Polymer Coatings: A Review. Hybrid Adv. 2024, 5, 100151. [Google Scholar] [CrossRef]
- Kuroda, P.A.B.; Cardoso, G.C.; Rossi, M.C.; Afonso, C.R.M.; Grandini, C.R. Analyzing PEO Anodization Time to Monitor Coatings Phases, Composition, Morphology, Thickness, and Microhardness During the Growth of TiO2 Pores on the CP-Ti Surface. Mater. Lett. 2024, 363, 136226. [Google Scholar] [CrossRef]
- Nisar, S.S.; Choe, H.-C. Mechanical Hydroxyapatite Coatings on PEO-Treated Ti–6Al–4V Alloy for Enhancing Implant’s Surface Bioactivity. Ceram. Int. 2024, 50, 17703–17719. [Google Scholar] [CrossRef]
- Nadimi, M.; Dehghanian, C. Incorporation of ZnO–ZrO2 Nanoparticles into TiO2 Coatings Obtained by PEO on Ti–6Al–4V Substrate and Evaluation of Its Corrosion Behavior, Microstructural, and Antibacterial Effects Exposed to SBF Solution. Ceram. Int. 2021, 47, 33413–33425. [Google Scholar] [CrossRef]
- Lim, S.-G.; Choe, H.-C. Bioactive Apatite Formation on PEO-Treated Ti-6Al-4V Alloy After 3rd Anodic Titanium Oxidation. Appl. Surf. Sci. 2019, 484, 365–373. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, D.; Yang, Z.; Zhu, Z.; Lu, X.; Ou, B.; Zhang, J.; Jin, S.; Wang, Q.; Yu, K. Structural Characterization and Biological Compatibilities of PEO Coated Ti–Mg Metal Matrix Composites. J. Mater. Res. Technol. 2024, 30, 2911–2921. [Google Scholar] [CrossRef]
- Lim, B.-S.; Lim, S.-G.; Choe, H.-C. Precipitation of Bone-Like Apatite on Plasma Electrolytic Oxidized Ti-6Al-4V Alloy. Thin Solid Film. 2022, 746, 139136. [Google Scholar] [CrossRef]
- Gonzlez, S.; Pellicer, E.; Suriach, S.; Bar, M.D.; Sort, J. Biodegradation and Mechanical Integrity of Magnesium and Magnesium Alloys Suitable for Implants. In Biodegradation–Engineering and Technology; InTech: Rijeka, Croatia, 2013. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Kim, Y.-K.; Jang, Y.-S.; Lee, M.-H. Advantages of Electrochemically Deposited Bioceramic-Coating on Magnesium Implant for Anti-Corrosion and Bone Regeneration. Surf. Interfaces 2025, 59, 105936. [Google Scholar] [CrossRef]
- Tian, P.; Liu, X. Surface Modification of Biodegradable Magnesium and Its Alloys for Biomedical Applications. Regen. Biomater. 2015, 2, 135–151. [Google Scholar] [CrossRef] [PubMed]
- Wydawnictwo Politechniki Poznańskiej. Materiały Inżynierskie w Zastosowaniach Biomedycznych; Wydawnictwo Politechniki Poznańskiej: Poznań, Poland, 2011. [Google Scholar]
- Li, X.; Gao, P.; Wan, P.; Pei, Y.; Shi, L.; Fan, B.; Shen, C.; Xiao, X.; Yang, K.; Guo, Z. Novel Bio-functional Magnesium Coating on Porous Ti6Al4V Orthopaedic Implants: In Vitro and In Vivo Study. Sci. Rep. 2017, 7, 40755. [Google Scholar] [CrossRef]
- Park, J.Y.; Jung, Y.S.; Lee, K.K.; Park, H.J. Behavior of Osteoblast-Like Cells on a β-Tricalcium Phosphate Synthetic Scaffold Coated with Calcium Phosphate and Magnesium. J. Craniofac. Surg. 2016, 27, 898–903. [Google Scholar] [CrossRef]
- Witte, F. The History of Biodegradable Magnesium Implants: A Review. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef]
- Gray, J.E.; Luan, B. Protective Coatings on Magnesium and Its Alloys—A Critical Review. J. Alloys Compd. 2002, 336, 88–99. [Google Scholar] [CrossRef]
- Kainer, K.U.; Srinivasan, P.B.; Blawert, C.; Dietzel, W. Corrosion of Magnesium and Its Alloys; In Shreir’s Corrosion; Elsevier: Oxford, UK, 2010. [Google Scholar]
- Hornberger, H.; Virtanen, S.; Boccaccini, A.R. Biomedical Coatings on Magnesium Alloys—A Review. Acta Biomater. 2012, 8, 2442–2455. [Google Scholar] [CrossRef]
- Saternus, M. Magnez—Technologia, Produkcja, Perspektywy; Rudy Metale: Sacramento, CA, USA, 2008. [Google Scholar]
- Karmańska, A. Magnez—Aktualny Stan Wiedzy. Bromatol. I Chem. Toksykol. Arch. 2015, 4, 677–689. [Google Scholar]
- Zhang, Y.; Xu, J.; Ruan, Y.C.; Yu, M.K.; O’Laughlin, M.; Wise, H.; Chen, D.; Tian, L.; Shi, D.; Wang, J.; et al. Implant-Derived Magnesium Induces Local Neuronal Production of CGRP to Improve Bone-Fracture Healing in Rats. J. Mater. Sci. 2017, 52, 5277–5289. [Google Scholar] [CrossRef] [PubMed]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and Its Alloys as Orthopedic Biomaterials: A Review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef]
- Saris, N.-E.L.; Mervaala, E.; Karppanen, H.; Khawaja, J.A.; Lewenstam, A. Magnesium: An Update on Physiological, Clinical and Analytical Aspects. Clin. Chim. Acta 2000, 294, 1–26. [Google Scholar] [CrossRef]
- Tian, Q.; Lin, J.; Rivera-Castaneda, L.; Tsanhani, A.; Dunn, Z.S.; Rodriguez, A.; Aslani, A.; Liu, H. Nano-to-Submicron Hydroxyapatite Coatings for Magnesium-Based Bioresorbable Implants—Deposition, Characterization, Degradation, Mechanical Properties, and Cytocompatibility. Acta Biomater. 2016, 43, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Barati Darband, G.; Aliofkhazraei, M.; Hamghalam, P.; Valizade, N. Plasma Electrolytic Oxidation of Magnesium and Its Alloys: Mechanism, Properties, and Applications. J. Magnes. Alloys 2017, 5, 74–132. [Google Scholar] [CrossRef]
- Lucas, R.R.; Silva, E.R.R.; Marques, L.F.B.; da Silva, F.J.G.; Abrahão, A.B.R.M.; Vieira, M.d.O.L.; Hein, L.R.d.O.; Botelho, E.C.; Mota, R.P.; Sales-Contini, R.d.C.M. Analysis of Plasma Electrolytic Oxidation Process Parameters for Optimizing Adhesion in Aluminum–Composite Hybrid Structures. Appl. Sci. 2024, 14, 7972. [Google Scholar] [CrossRef]
- Ji, R.; Wang, S.; Zhao, X.; Zou, Y.; Zhang, T.; Qian, X.; Chen, G.; Wang, Y.; Ouyang, J.; Jia, D.; et al. Enhanced Wear Resistance, Corrosion Behavior, and Thermal Management in Magnesium Alloys with PEO Coatings. Surf. Coat. Technol. 2024, 494, 131438. [Google Scholar] [CrossRef]
- Dong, H.; Wang, S.; Xie, D.; Li, Q.; Zhao, D.; Guo, H.; Wu, J.; Jiang, W.; Meng, X.; An, L. Enhancing Understanding of Cooperation Mechanism of Electrolyte Composition on PEO Coating Growth for Magnesium Alloys via Load Characteristics. Surf. Coat. Technol. 2024, 494, 131307. [Google Scholar] [CrossRef]
- Kojouri Naftchali, N.; Mehdinavaz Aghdam, R.; Najjari, A.; Dehghanian, C. Investigating a Nanocomposite Coating of Cerium Oxide/Merwinite via PEO/EPD for Enhanced Biocorrosion Resistance, Bioactivity and Antibacterial Activity of Magnesium-Based Implants. Ceram. Int. 2024, 50, 42766–42779. [Google Scholar] [CrossRef]
- Wu, G.; Li, L.; Chen, X.; Zhu, L.; Wang, Y.; Wen, C.; Yao, J. The Growth Mechanism and Corrosion Resistance of Laser-Assisted Plasma Electrolytic Oxidation (PEO) Composite Coating on AZ31B Magnesium Alloy. J. Magnes. Alloys 2024, in press. [Google Scholar] [CrossRef]
- Nachtsheim, J.; Ma, S.; Burja, J.; Markert, B. In Vitro Evaluation of Stress Corrosion Cracking Susceptibility of PEO-Coated Rare-Earth Magnesium Alloy WE43. Surf. Coat. Technol. 2024, 477, 130391. [Google Scholar] [CrossRef]
- Khalili, V.; Ghaleh, H.; Namdar Asl, H.; Ege, D.; Dikici, B.; Kaseem, M.; Breisch, M.; Frenzel, J.; Eggeler, G. Assessing the Properties of Biodegradable Magnesium Alloy AZ31 Protected by a Polymer Layer on a Plasma Electrolytic Oxidized (PEO) Surface. Surf. Coat. Technol. 2024, 487, 131002. [Google Scholar] [CrossRef]
- Nachtsheim, J.; Ma, S.; Burja, J.; Šetina Batič, B.; Markert, B. Tuning the Long-Term Corrosion Behaviour of Biodegradable WE43 Magnesium Alloy by PEO Coating. Surf. Coat. Technol. 2023, 474, 130115. [Google Scholar] [CrossRef]
- Knap, V.; Blawert, C.; Serdechnova, M.; Pastorek, F.; Kajánek, D.; Obertová, V.; Hadzima, B. Use of NaAlO2 Additions to Extend the Corrosion Resistance of PEO Layer on EV31 Magnesium Alloy. J. Mater. Res. Technol. 2024, 29, 2083–2096. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Nomerovskii, A.D.; Filonina, V.S.; Ustinov, A.Y.; Gnedenkov, S.V. Design of Self-Healing PEO-Based Protective Layers Containing In-Situ Grown LDH Loaded with Inhibitor on the MA8 Magnesium Alloy. J. Magnes. Alloys 2023, 11, 3688–3709. [Google Scholar] [CrossRef]
- Bai, L.-j.; Gao, X.; Luo, Y.; Chen, G.; Wu, X.; Sun, X. In-Situ Preparation of (Ti, Al) Codoped Blue PEO Ceramic Coating on Magnesium Alloy and Chromogenic Mechanism. Surf. Coat. Technol. 2023, 470, 129829. [Google Scholar] [CrossRef]
- Witte, F.; Hort, N.; Vogt, C.; Cohen, S.; Kainer, K.U.; Willumeit, R.; Feyerabend, F. Degradable Biomaterials Based on Magnesium Corrosion. Corros. Sci. 2007, 49, 2781–2793. [Google Scholar] [CrossRef]
- Dobrzański, L. Metaloznawstwo Opisowe Stopów Metali Nieżelaznych; Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 2008. [Google Scholar]
- Przybyłowicz, P.J. Repetytorium z Materiałoznawstwa, Część VI, Metale i Stopy Nieżelazne; Politechnika Świętokrzyska: Kielce, Poland, 1997. [Google Scholar]
- Atrens, A.L.; Liu, M.; Zainal Abidin, N.I. Corrosion Mechanism Applicable to Biodegradable Magnesium Implants. Mater. Sci. Eng. B 2011, 176, 1609–1636. [Google Scholar] [CrossRef]
- Song, G. Control of Biodegradation of Biocompatible Magnesium Alloys. Corros. Sci. 2007, 49, 1696–1701. [Google Scholar] [CrossRef]
- Saji, V.S. Organic Conversion Coatings for Magnesium and Its Alloys. J. Ind. Eng. Chem. 2019, 75, 20–37. [Google Scholar] [CrossRef]
- Wierzchoń, B.J. Ćwiczenia Laboratoryjne z Inżynierii Powierzchni; OWPW: Warszawa, Poland, 1996. [Google Scholar]
- Chang, L. Growth Regularity of Ceramic Coating on Magnesium Alloy by Plasma Electrolytic Oxidation. J. Alloys Compd. 2009, 468, 462–465. [Google Scholar] [CrossRef]
- Simchen, F.; Sieber, M.; Kopp, A.; Lampke, T. Introduction to Plasma Electrolytic Oxidation—An Overview of the Process and Applications. Surf. Coat. Technol. 2011, 205, 1500–1509. [Google Scholar] [CrossRef]
- Vertaľ, J.; Kajánek, D.; Kubásek, J.; Minárik, P. Improving Biodegradable Mg-Zn(-Ca) Alloys by Surface Treatment via Plasma Electrolytic Oxidation. Materials 2025, 18, 747. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Wang, B.; Xia, J.; Fu, K.; Han, J. The Incorporation of Nano-MoSi2 Particles into a Black PEO Coating on Ti Alloy and Its Corrosion Performance. Coatings 2025, 15, 145. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.


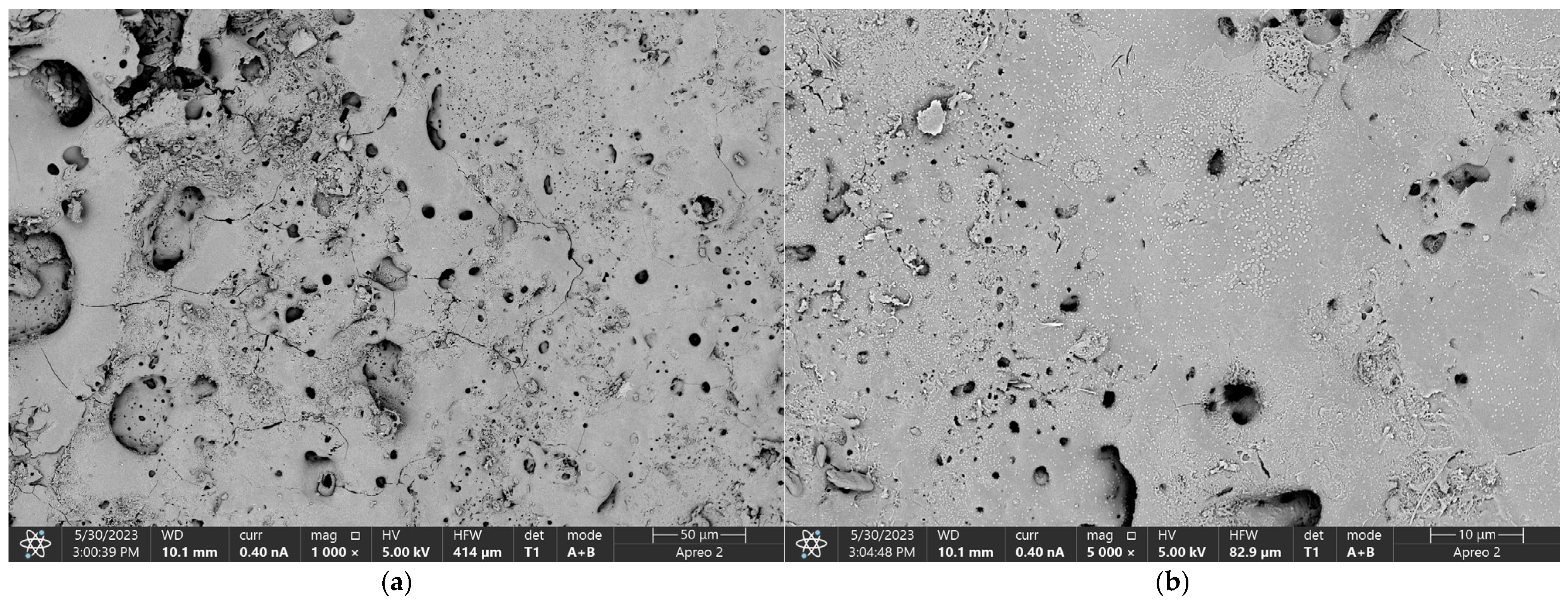


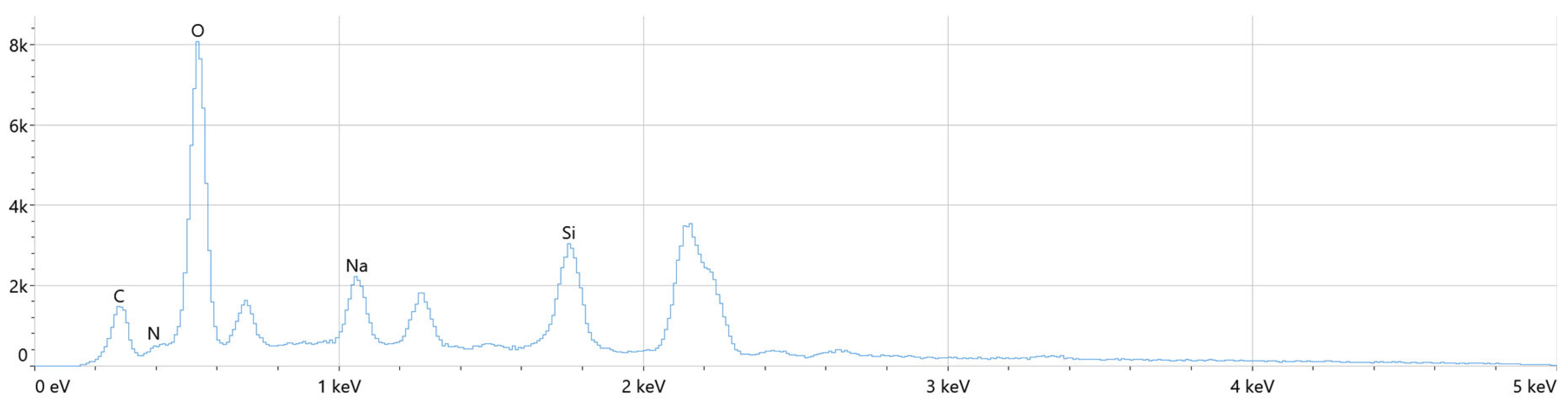


| Sample | Component | Content |
|---|---|---|
| E1 | Na2SiO3•9H2O KOH | 7 g/L 3 g/L |
| E2 | NaOH NH4F Na2SiO3•5H2O | 5 g/L 100 g/L 25 g/L |
| E3 | NaOH, NH4F, Na2SiO3•5H2O Lysine | 100 mL 1 g |
| E4 | NaOH Lysine E2 | 0.5 g 0.1 g/100 mL Refilled to 100 mL |
| E5 | L-aspartic acid | 0.1 g/100 mL |
| E6 | NaOH L-aspartic acid E2 | 0.5 g 0.1 g/100 mL Refilled to 100 mL |
| Mg Sample with Electrolytes | Voltage (V) | Running Time (min) |
|---|---|---|
| Mg_1, E1 | 5 | 15 |
| Mg_2, E1 | 10 | 15 |
| Mg_3, E1 | 25 | 15 |
| Mg_4, E1 | 50 | 15 |
| Mg_5, E1 | 100 | 15 |
| Mg_6, E1 | 125 | 15 |
| Mg_7, E1 | 150 | 15 |
| Mg_8, E1 | 175 | 15 |
| Mg_9, E1 | 200 | 15 |
| Mg_10, E2 | 100 | 1 |
| Mg_11, E2 | 100 240 | 1 2 |
| Mg_12, E2 | 100 240 | 1 4 |
| Mg_13, E2 | 100 240 | 1 6 |
| Mg_14, E3 | 17 | 3 |
| Mg_15, E4 | 100 240 240 | 1 3 2 |
| Mg_16, E5 | 100 240 240 | 1 3 3 |
| Mg_17, E6 | 100 240 | 1 3 |
| Element | Content (%) |
|---|---|
| Zn | 2.4 |
| Mg | 75.4 |
| Al | 22.1 |
| Other | 0.10 |
| Sample | Wetting Angle Value | Wettability Rating |
|---|---|---|
| Mg_01 | 0.0° | Complete wettability |
| Mg_02 | 0.0° | Complete wettability |
| Mg_03 | 0.0° | Complete wettability |
| Mg_04 | 0.0° | Complete wettability |
| Mg_05 | 0.0° | Complete wettability |
| Mg_06 | 0.0° | Complete wettability |
| Mg_07 | 0.0° | Complete wettability |
| Mg_08 | 0.0° | Complete wettability |
| Mg_09 | 0.0° | Complete wettability |
| Mg_10 | 0.0° | Complete wettability |
| Mg_11 | 0.0° | Complete wettability |
| Mg_12 | 0.0° | Complete wettability |
| Mg_13 | 0.0° | Complete wettability |
| Mg_14 | 27.1° | Very good wetting properties |
| Mg_15 | 15.1° | Very good wetting properties |
| Mg_16 | 36.8° | Very good wetting properties |
| Mg_17 | 30.5° | Very good wetting properties |
| Element | Atomic % | Atomic % Error | Weight % | Weight % Error |
|---|---|---|---|---|
| N | 1.3 | 0.3 | 1.0 | 0.2 |
| O | 57.0 | 0.5 | 48.9 | 0.4 |
| Na | 0.9 | 0.2 | 1.1 | 0.2 |
| C | 6.3 | 0.1 | 4.0 | 0.1 |
| Mg | 34.5 | 0.3 | 44.9 | 0.4 |
| Element | Atomic % | Atomic % Error | Weight % | Weight % Error |
|---|---|---|---|---|
| O | 46.1 | 0.4 | 36.6 | 0.3 |
| Na | 9.4 | 0.2 | 10.8 | 0.2 |
| Si | 26.5 | 0.3 | 37.1 | 0.4 |
| C | 10.1 | 0.1 | 6.1 | 0.1 |
| Mg | 7.8 | 0.1 | 9.5 | 0.1 |
| Element | Atomic % | Atomic % Error | Weight % | Weight % Error |
|---|---|---|---|---|
| N | 0.0 | --- | 0.0 | --- |
| O | 46.6 | 0.3 | 36.3 | 0.3 |
| Na | 8.2 | 0.2 | 9.1 | 0.2 |
| Si | 28.1 | 0.3 | 38.5 | 0.3 |
| C | 7.0 | 0.1 | 4.1 | 0.1 |
| Mg | 10.1 | 0.2 | 12.0 | 0.2 |
| Sample | Process Parameters | Corrosion Potential, V | Corrosion Current, A/cm2 | βK | βA |
|---|---|---|---|---|---|
| Mg_0 | Pure implant disk | −1.46 | 5.60 × 10−4 | −4.75 | 5.00 |
| Mg_1 | MgO electrode 1 voltage 5 V | −1.54 | 9.50 × 10−5 | −5.54 | 6.51 |
| Mg_2 | MgO electrode 1 voltage 10 V | −1.45 | 1.73 × 10−4 | −4.90 | 5.40 |
| Mg_3 | MgO electrode 1 voltage 25 V | −1.44 | 5.39 × 10−4 | −1.82 | 2.87 |
| Mg_4 | MgO electrode 1 voltage 50 V | −0.79 | 7.69 × 10−5 | −1.23 | 6.11 |
| Mg_5 | MgO electrode 1 voltage 100 V | −1.64 | 5.36 × 10−5 | −6.02 | 6.66 |
| Mg_6 | MgO electrode 1 voltage 125 V | −1.51 | 2.49 × 10−4 | −4.47 | 4.45 |
| Mg_7 | MgO electrode 1 voltage 150 V | −1.00 | 2.51 × 10−7 | −2.72 | 2.10 |
| Mg_8 | MgO electrode 1 voltage 175 V | −0.78 | 7.21 × 10−5 | −1.87 | 5.64 |
| Mg_9 | MgO electrode 1 voltage 200 V | −1.48 | 2.93 × 10−4 | −2.99 | 5.11 |
| Mg_10 | MgO electrode 2 voltage 240 V 1 min. | −1.40 | 1.33 × 10−6 | −5.26 | 7.32 |
| Mg_11 | MgO electrode 2 voltage 240 V 3 min. | −1.40 | 5.20 × 10−7 | −3.52 | 3.52 |
| Mg_12 | MgO electrode 2 voltage 240 V 5 min. | −1.06 | 1.01 × 10−7 | −1.76 | 5.55 |
| Mg_13 | MgO 240 V 7 min. | −1.09 | 7.50 × 10−7 | −3.09 | 6.10 |
| Mg_14 | MgO electrode 3 17 V 3 min. | −1.55 | 3.99 × 10−4 | −4.96 | 3.74 |
| Mg_15 | MgO electrode 2 240 V 3 min. + Lysine 0.1 g 2 min. | −1.00 | 1.55 × 10−4 | −1.61 | 1.03 |
| Mg_16 | MgO electrode 2 240 V 3 min. + electrode 4 50 V 10 min. | −1.15 | 7.28 × 10−5 | −1.49 | 1.98 |
| Mg_17 | MgO electrode 2 240 V 3 min. + electrode 6 100 V 10 min. | −1.19 | 1.01 × 10−6 | −2.00 | 2.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radwan-Pragłowska, J.; Legutko, K.; Janus, Ł.; Sierkowska-Byczek, A.; Kuźmiak, K.; Radwan-Pragłowska, N. Electrochemical Surface Modification of Fully Biodegradable Mg-Based Biomaterials as a Sustainable Alternative to Non-Resorbable Bone Implants. Appl. Sci. 2025, 15, 2492. https://doi.org/10.3390/app15052492
Radwan-Pragłowska J, Legutko K, Janus Ł, Sierkowska-Byczek A, Kuźmiak K, Radwan-Pragłowska N. Electrochemical Surface Modification of Fully Biodegradable Mg-Based Biomaterials as a Sustainable Alternative to Non-Resorbable Bone Implants. Applied Sciences. 2025; 15(5):2492. https://doi.org/10.3390/app15052492
Chicago/Turabian StyleRadwan-Pragłowska, Julia, Kinga Legutko, Łukasz Janus, Aleksandra Sierkowska-Byczek, Klaudia Kuźmiak, and Natalia Radwan-Pragłowska. 2025. "Electrochemical Surface Modification of Fully Biodegradable Mg-Based Biomaterials as a Sustainable Alternative to Non-Resorbable Bone Implants" Applied Sciences 15, no. 5: 2492. https://doi.org/10.3390/app15052492
APA StyleRadwan-Pragłowska, J., Legutko, K., Janus, Ł., Sierkowska-Byczek, A., Kuźmiak, K., & Radwan-Pragłowska, N. (2025). Electrochemical Surface Modification of Fully Biodegradable Mg-Based Biomaterials as a Sustainable Alternative to Non-Resorbable Bone Implants. Applied Sciences, 15(5), 2492. https://doi.org/10.3390/app15052492







