Abstract
Hepatocellular carcinoma (HCC) accounts for 90% of primary liver cancers and typically arises in the context of chronic liver disease. With the increasing prevalence of metabolic disorders, metabolic dysfunction-associated steatotic liver disease (MASLD) has become the leading cause of chronic liver disease and the most rapidly increasing cause of HCC. The role of dysfunctional innate and adaptive immune responses in the development and progression of HCC is well-established, prompting numerous trials to evaluate the efficacy of immune checkpoint inhibitors (ICIs) in targeting tumor cells. These trials have yielded promising results, and ICIs, in combination with anti-vascular endothelial growth factor (VEGF) monoclonal antibodies, are now approved as first-line therapy for patients with metastatic or unresectable HCC, irrespective of the underlying liver disease. Notably, MASLD itself is characterized by immune system dysfunction, as metabolic inflammation plays a central role in its onset and progression. However, clinical studies and post-hoc analyses suggest that immunotherapy may be less effective in MASLD-associated HCC compared to viral-related HCC. This emerging evidence raises the question of whether the underlying liver disease influences the therapeutic response to ICIs in HCC. It may be time to consider tailoring therapeutic strategies for HCC based on the specific etiological, histological, and genotypical subgroups.
1. Introduction
The term steatosis refers to the accumulation of fat within cells. When this process occurs in liver cells, it is termed “liver steatosis” or “fatty liver”. Non-alcoholic fatty liver disease (NAFLD) encompasses a wide spectrum of chronic liver diseases characterized by the presence of hepatic steatosis, which can be confirmed either through histological evaluation via liver biopsy (the gold standard) [] or through imaging techniques that document at least 5% steatosis in the hepatic parenchyma []. NAFLD is distinguished from secondary causes of fat accumulation [], such as excessive alcohol consumption [], hereditary disorders (e.g., Wilson’s disease), or the use of drugs known to induce steatosis (e.g., amiodarone, steroids, tamoxifen) [].
The most significant risk factors for NAFLD include metabolic syndrome, obesity, insulin resistance/type 2 diabetes mellitus, and dyslipidemia []. Recently, the term NAFLD has been replaced by metabolic-dysfunction-associated steatotic liver disease (MASLD) to better reflect the association of hepatic steatosis with at least one cardiometabolic risk factor (e.g., obesity, hypertension) [].
NAFLD can progress to non-alcoholic steatohepatitis (NASH), a condition characterized by both steatosis and inflammation, which can lead to fibrosis and, ultimately, cirrhosis. Under the new nomenclature, MASLD may progress to metabolic-dysfunction-associated steatohepatitis (MASH).
The natural history of NAFLD is not fully understood []. The prevailing theory is the “multiple hit” hypothesis, which suggests that genetically predisposed individuals (with genetic variations such as patatin-like phospholipase domain-containing protein 3—PNPLA3, transmembrane 6 superfamily 2—TM6SF2, or membrane-bound O-acyltransferase domain-containing protein 7—MBOAT7 []) are affected by environmental factors (e.g., lifestyle, gut microbiome, dietary habits) that lead to lipid accumulation, increased hepatic de novo lipogenesis, and impaired inhibition of adipose tissue lipolysis. These processes result in hepatic fat accumulation and the creation of a lipotoxic environment, triggering mitochondrial dysfunction, reactive oxygen species (ROS) production, and endoplasmic reticulum stress.
Cellular damage initiates immune cell infiltration, fibrogenesis, and hepatic progenitor cell activation. Additionally, as MASLD [] is closely linked to metabolic disorders, it is often accompanied by adipose tissue dysfunction and increased secretion of adipokines and proinflammatory cytokines. These processes exacerbate oxidative stress and cause DNA damage in liver cells, promoting fibrosis and cirrhosis [,]. Chronic hepatic inflammation may be the driving force behind the progression of MASLD to hepatocellular carcinoma (HCC). However, it is important to emphasize that our understanding of the pathogenesis and progression from MASLD to HCC remains limited. Nevertheless, given that increased hepatic inflammation has been independently associated with a higher risk of HCC—even in individuals with non-cirrhotic MASLD—it is reasonable to attribute a role to MASLD-related inflammation in tumor development. This underscores the critical involvement of inflammatory pathways in the pathogenesis of HCC in patients with MASLD []. As previously mentioned, further research is needed to explore additional mechanisms underlying the progression from MASLD to HCC.
2. Immune System, MASLD, and HCC
2.1. Liver and Immune Response
The liver is a uniquely immunotolerant organ, a feature critical for limiting inflammation caused by constant exposure to antigens through the portal circulation []. Non-parenchymal resident liver cells, including Kupffer cells (KCs), hepatic stellate cells (HSCs), and liver sinusoidal endothelial cells (LSECs), work together to maintain this immunotolerant state. KCs, the liver’s resident macrophages [], are activated during hepatic inflammation, where they upregulate proinflammatory cytokines that contribute to hepatocyte inflammation and HSC activation. However, KCs can also secrete inhibitory molecules, such as IL-10 and prostaglandins, and promote regulatory T cell (Treg) activation [].
Similarly, LSECs and HSCs contribute to Treg activation and accumulation [] and express high levels of PD-L1 [], further supporting the liver’s immunotolerant microenvironment.
2.2. MASH and Immune Response
Immune cells play a central role in the onset and progression of MASLD (Figure 1). In the MASH phase, immune cell-mediated inflammation is a key driver of disease progression []. Numerous studies have highlighted immune dysfunction as a pivotal factor in MASLD pathogenesis [].
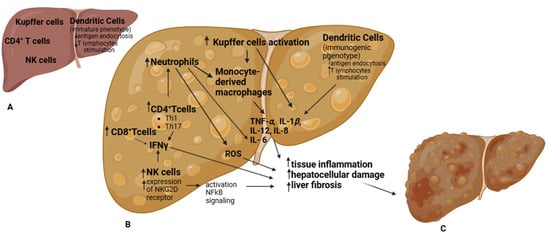
Figure 1.
Immune cells play a central role in the onset and progression from a healthy liver (A) to MASLD (B). Various immune cells from both the innate and adaptive immune systems contribute to this process. In healthy livers, dendritic cells (DCs) predominantly exhibit an immature phenotype, with limited ability to endocytose antigens and stimulate T lymphocytes. However, as intracellular lipid concentrations increase, DCs adopt an immunogenic phenotype, gaining enhanced antigen-presenting capacity and producing proinflammatory cytokines. In MASLD, Kupffer cells (KCs) undergo heightened activation, leading to increased secretion of cytokines and chemokines and the recruitment of circulating monocytes that differentiate into macrophages. These macrophages amplify the proinflammatory response by upregulating chemokine expression, including TNF-α, IL-1β, IL-6, IL-8, and IL-12. Following KC activation, neutrophils are recruited to the liver, where they induce IL-6 secretion, promoting tissue inflammation and fibrosis. Neutrophils also recruit additional monocyte-derived macrophages (Mo-Ms) and enhance reactive oxygen species (ROS) production, further exacerbating liver damage. In MASLD, hepatic natural killer (NK) cells are significantly increased, contributing to fibrosis and, through NF-κB signaling activation, facilitating steatosis and the transition from MASH to HCC. Additionally, NK cells stimulate other immune cells in the liver by increasing IFNγ secretion. In MASLD, some studies suggest that CD4+ T cell polarization toward Th1 and Th17 subsets correlates with increased IFNγ and IL-17A production, further driving MASLD progression. This proinflammatory environment, characterized by increased inflammation, hepatocellular damage, and liver fibrosis, ultimately leads to HCC development (C).
Many innate immune cells are involved in this phenomenon, including Kupffer cells (KCs), monocyte-derived macrophages (Mo-Ms), neutrophils, dendritic cells (DCs), and natural killer (NK) cells. In the liver, macrophages can be categorized into two major subgroups: liver-resident macrophages, or KCs, and Mo-Ms, which are recruited from circulation [,,]. In MASLD, KCs undergo heightened activation, leading to increased cytokine and chemokine secretion and the recruitment of circulating monocytes, which subsequently differentiate into macrophages. In MASLD patients, macrophages exhibit enhanced proinflammatory activity but reduced phagocytic function, along with increased expression of chemokines such as TNF-α, IL-1β, IL-6, IL-8, and IL-12 [,,].
Following KC activation, neutrophils are recruited to the liver via pathogen-associated molecular patterns (PAMPs) [,]. Once in the liver, neutrophils contribute to a proinflammatory environment by intensively secreting IL-6, which promotes tissue inflammation and fibrosis [,], recruiting additional Mo-Ms, and interacting with other antigen-presenting cells (APCs), thereby amplifying the inflammatory cascade []. Furthermore, neutrophil granule proteins (e.g., myeloperoxidase, neutrophil elastase, and proteinase 3) enhance reactive oxygen species (ROS) production [], further exacerbating inflammation, hepatocellular damage, and progression to HCC []. Notably, the neutrophil-to-lymphocyte ratio correlates with MASLD severity and is indicative of a higher risk of advanced cirrhosis [] and HCC [], underscoring the pivotal role of neutrophils in MASLD progression.
Dendritic cells (DCs) also play a distinctive role in MASLD progression. Hepatic DCs are a heterogeneous population that can be further divided into subgroups, including plasmacytoid and classical DCs []. Depending on cellular subtype and environmental cues, DCs can mediate both proinflammatory [] and anti-inflammatory [] responses. In healthy livers, DCs predominantly exhibit an immature phenotype, with limited ability to endocytose antigens and stimulate T lymphocytes. However, during hepatic injury or under conditions of increased intracellular lipid accumulation, DCs adopt an immunogenic phenotype with enhanced antigen-presenting capabilities and increased production of proinflammatory cytokines [].
Hepatic NK cells represent a heterogeneous population that expands in disease states [,,]. In individuals with MASH, the number of NK cells in the liver is markedly increased [], accompanied by elevated expression of the activating receptor NKG2D and its ligands MIC A/B in the liver parenchyma []. This suggests that NK cell activation occurs in MASLD and contributes to disease progression. Notably, NK cell proinflammatory activity is hypothesized to drive MASH [] by exacerbating liver fibrosis [] and activating NF-κB signaling, which facilitates steatosis and the transition from MASH to HCC []. Furthermore, NK cells promote the activation of other immune cells in the liver through increased secretion of IFN-γ.
As previously mentioned, adaptive immunity also plays a role in MASLD development. Adaptive immunity consists of cell-mediated and humoral immunity, primarily mediated by T and B lymphocytes, respectively. The major T lymphocyte subtypes involved in adaptive immunity include CD4+ T cells (further categorized into T helper cells—Th1, Th2, Th17, regulatory T cells—Tregs, etc.), CD8+ T cells, and γδ T cells []. CD4+ T cells are highly versatile immune cells involved in both pro- and anti-inflammatory processes. These cells are classified based on their cytokine production and transcription factor expression, such as Th1 (IFNγ), Th2 (IL-4, GATA3), Th17 (IL-17), and Treg (IL-10/TGFβ, FOXP3) cells [,]. Although reports on hepatic CD4+ T cell presence in MASLD are contradictory—some studies document progressive hepatic accumulation in MASLD [], while others suggest decreased hepatic recruitment during the transition to HCC []—evidence indicates that polarization of CD4+ T cells toward Th1 [] and Th17 [] subsets correlates with increased IFNγ and IL-17A production [,,], contributing to MASLD progression []. Furthermore, Rai et al. [] demonstrated that blocking integrin-mediated hepatic recruitment of CD4+ T cells attenuated liver inflammation and fibrosis in a mouse model of MASLD, providing additional evidence of CD4+ T cells’ essential role in MASLD pathogenesis. Th1 and Th2 cells are implicated in MASH pathogenesis, with an imbalance favoring proinflammatory Th1 responses over anti-inflammatory Th2 responses. Increased accumulation of Th1 cells and elevated systemic and hepatic IFNγ levels have been reported in individuals with MASH []. IFNγ is considered a key pathogenic driver of MASLD progression, as genetic ablation of IFNγ in mice protects against MASH and hepatic fibrosis []. Th17 cells are also major contributors to MASLD pathogenesis, amplifying proinflammatory signals that sustain tissue inflammation, with IL-17A identified as the primary cytokine driving this process [,]. In MASLD, hepatic Th17 cell numbers are increased in mouse models, and IL-17A-producing cells are associated with the transition from steatosis to MASH in humans []. Mechanistically, IL-17 promotes the expression of chemokines (e.g., CXCL1 and CCL2) that facilitate neutrophil and macrophage infiltration and activation, further amplifying tissue inflammation and fibrosis []. Treg cells, which regulate effector T cell activation, act as immune modulators to prevent excessive pathogenic immune responses. However, their role in MASH progression remains unclear. In experimental models of high-fat diet (HFD)-induced MASLD, hepatic Treg cells are reduced [], yet paradoxically, Treg depletion appears to mitigate progression to HCC [], while adoptive Treg transfer in MASH models exacerbates steatosis and liver damage []. Similarly, human studies on Treg cells in MASLD report conflicting findings, with both increased [] and reduced [] intrahepatic Treg cell frequencies and numbers. The contribution of CD8+ T cells to MASLD pathogenesis is context-dependent. CD8+ T cell numbers are elevated in the liver of individuals with MASH [], and inhibition of CD8+ T cell function in animal models reduces hepatic steatosis and inflammation []. Hepatic accumulation of activated CD8+ T cells [] amplifies the proinflammatory environment by increasing IFNγ and TNF production and inducing cytotoxic activity-driven hepatocellular damage. Notably, MASH impairs CD8+ T cell mobility through metabolic and mitochondrial dysfunction [], weakening tumor antigen-specific CD8+ T cell responses [] and ultimately promoting HCC progression. A preclinical study by Pfister et al. [] demonstrated that NASH-related liver damage is associated with elevated levels of activated CD8+ T cells expressing CD69, CD44, and PD-1. The abundance of hepatic CD8+PD-1+ T cells correlates with body mass index, liver damage, and fibrosis severity (F0-F4). Additionally, immunohistochemical analysis revealed that increased PD-L1 expression in hepatocytes and non-parenchymal cells corresponds with NASH severity. Although limited, existing evidence supports a pathogenic role for B cells in MASLD []. Hepatic B cell accumulation has been observed in both humans [] and mice [] with MASLD, accompanied by increased expression of inflammatory mediators such as IL-6 and TNF. However, whether B cell-derived mediators directly drive MASLD pathogenesis or indirectly exacerbate hepatic inflammation by inducing CD4+ T cell differentiation toward Th1/Th17 subsets remains unclear [].
Given the immune system’s central role in MASLD pathogenesis, immunotherapy has emerged as a promising treatment strategy.
Abnormal gene expression related to immune regulation and response is observed in MASLD progression. For example, the mitochondrial antiviral signaling (MAVS) protein regulates interferon expression and inflammatory factor production while also inhibiting lipid accumulation in hepatocytes. MAVS deficiency accelerates hepatic steatosis, and its expression is significantly reduced in MASLD-affected livers []. On the contrary, high levels of mac-2 binding protein (M2BP), a glycoprotein involved in immune regulation, is a predictive biomarker of liver fibrosis progression in MASLD patients []. Another key player, secreted phosphoprotein 1 (SPP1), is implicated in autophagy, inflammation, and hepatocyte fibrosis. Elevated SPP1 levels are associated with increased hepatic lipid synthesis and steatosis [].
Immune signaling alterations and receptor hyperactivation are also hallmarks of MASLD. For instance, the macrophage scavenger receptor 1 (MSR1), predominantly expressed in Kupffer cells, triggers proinflammatory activation via JNK signaling when stimulated by lipids. MSR1 deficiency mitigates MASLD-related metabolic dysregulation, liver injury, and proinflammatory macrophage activity, underscoring its role in lipid-induced inflammation [,]. Additionally, the innate immune signaling pathway STING, which promotes intrahepatic inflammation, may also inhibit lipid degradation, contributing to MASH progression [].
To sum up, chronic steatosis induces auto-aggressive CD8+PD-1+ T cells that, by responding to metabolic stimuli in an antigen-independent way, kill hepatocytes and promote a proinflammatory environment and a pro-tumorigenic environment resulting in chronic liver damage. Therefore, CD8+PD-1+ T cells in a fatty liver are hyperactivated and even exhausted due to chronic dysregulation []. As is well established, advanced NASH stages are associated with a higher risk of developing HCC [].
2.3. Pathogenesis of HCC in MASLD
The mechanisms underlying HCC development in MASLD are complex and not yet fully understood. Lipid accumulation in liver cells and the resulting lipotoxicity create a proinflammatory environment. Chronic hepatocyte regeneration and tissue remodeling, driven by sustained inflammation, contribute to alterations in DNA damage response pathways and hepatocellular genomic instability, ultimately increasing the risk of tumorigenesis [,,].
The diverse pathways involved in carcinogenesis also lead to heterogeneity in the types of HCC that develop.
An emerging hypothesis highlights immune system dysregulation as a critical factor in promoting HCC in MASLD, particularly in the context of NASH. Preclinical studies have shown that CD8+ T cells may play a pivotal role in the progression of NASH to HCC.
Pfister et al. [] demonstrated in murine models that depleting CD8+ T cells reduced liver damage and HCC incidence. Similar results were observed with the co-depletion of CD8+ T cells and NK cells. Conversely, anti-PD-1 immunotherapy exacerbated liver damage, increased hepatic CD8+PD-1+ T cell populations, and had minimal impact on CD4+PD-1+ T cells.
Transcriptome analysis of CD8+PD-1+ T cells in NASH-HCC mice revealed elevated expression of both effector and exhaustion markers, including thymocyte selection-associated high mobility group box protein (TOX). TOX, a transcription factor in the high-mobility group box family, plays a critical role in promoting and sustaining CD8+ T cell exhaustion [,]. By reducing PD-1 degradation and facilitating PD-1 translocation to the T cell surface, TOX exacerbates T cell exhaustion [].
In summary, CD8+PD-1+ T cells appear to drive the transition from NASH to HCC by impairing tumor surveillance and promoting T cell-mediated tissue damage. Moreover, anti-PD-1 immunotherapy, rather than alleviating liver inflammation, may inadvertently support HCC development in this setting.
2.4. HCC and Immune Response
The innate and adaptive immune systems collaborate to provide effective anticancer immune surveillance. When these systems function properly, they detect and eliminate abnormal cells, preventing tumor development. However, dysfunctional interactions between tumors and the immune system can result in immune evasion. This can be related to different mechanisms, including impaired antigen recognition or the creation of an immunosuppressive tumor microenvironment (TME) [] (Figure 2).
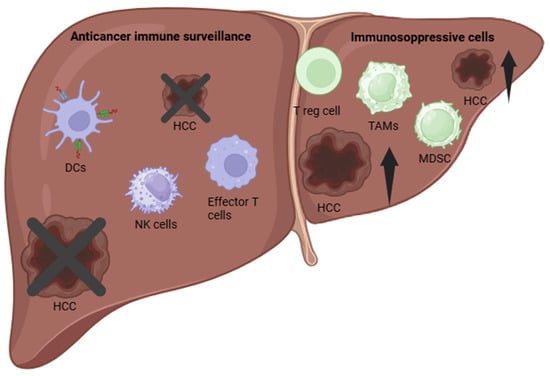
Figure 2.
Immune Tumor Microenvironment (TME) in Hepatocellular Carcinoma (HCC). Dendritic cells (DCs), natural killer (NK) cells, and effector T cells play a crucial role in promoting immune-mediated tumor rejection. In contrast, regulatory T cells (Tregs), M2-polarized tumor-associated macrophages (TAMs), and myeloid-derived suppressor cells (MDSCs) contribute to immunosuppression, facilitating HCC progression.
Particularly, in patients with HCC, both innate and adaptive immune responses are significantly impaired [] due to the following mechanisms: dysfunctional NK cells, accumulation of immunosuppressive cells, immune checkpoint upregulation, and peritumoral environment.
- Dysfunctional NK cells: An increased frequency of NK cells with reduced functionality [].
- Accumulation of immunosuppressive cells: Regulatory immune cells, such as regulatory T cells (Tregs), inhibitory B cells, M2-polarized tumor-associated macrophages (TAMs), and myeloid-derived suppressor cells (MDSCs), aggregate in the tumor microenvironment. These cells support tumor progression by producing vascular endothelial growth factor (VEGF), which promotes vascularization and angiogenesis in HCC []. Additional immunosuppressive cell populations in HCC include the following:
- -
- Regulatory B cells: A subset expressing high levels of PD-1 [];
- -
- T helper 17 (Th17) cells [] CD4+ T cells: Subsets expressing CCR4 and CCR6 [];
- -
- CD14+ dendritic cells (DCs): Cells with elevated CTLA4 and PD-1 expression [];
- -
- Tumor-associated fibroblasts: Cells that inhibit NK cell function [];
- -
- Neutrophils: Cells that recruit macrophages and Tregs, further supporting an immunosuppressive environment [].
- Immune checkpoint upregulation: Enhanced expression of co-inhibitory signals, including immune checkpoint ligands and receptors. HCC evades anti-tumor immune responses by expressing ligands that inhibit immune activity in tumor and stromal cells. The most prominent examples are the following:
- -
- CTLA4: Expressed primarily by activated T cells and Tregs []. CTLA4 inhibits the activation of effector T cells and acts as an effector molecule for Tregs (Figure 3);
- -
- PD-1/PD-L1 axis: PD-1 is expressed by activated T cells, NK cells, Tregs, MDSCs, monocytes, and DCs. Its ligand, PD-L1, is expressed by tumor cells, stromal cells, and myeloid cells, including DCs. The PD-1/PD-L1 interaction inhibits effector cell functions and contributes to T cell dysfunction [] (Figure 4).
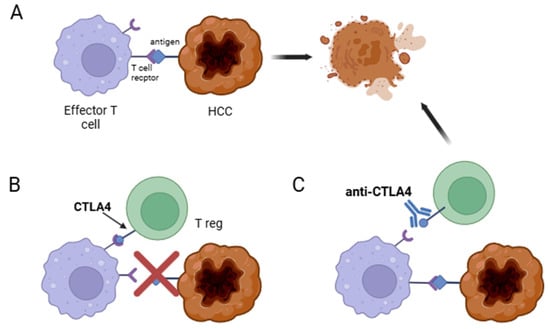 Figure 3. Mechanisms of CTLA4-mediated immune regulation in cancer. (A) Effector T cell recognizes the neoplastic cell and mounts an immune response to kill it. (B) Regulatory T cell (Treg) suppresses the effector T cell through CTLA4 signaling, thereby impairing anticancer immune surveillance. (C) Administration of an anti-CTLA4 antibody (e.g., ipilimumab) blocks CTLA4 activity on Tregs, restoring effector T cell function and enabling the death of the neoplastic cell.
Figure 3. Mechanisms of CTLA4-mediated immune regulation in cancer. (A) Effector T cell recognizes the neoplastic cell and mounts an immune response to kill it. (B) Regulatory T cell (Treg) suppresses the effector T cell through CTLA4 signaling, thereby impairing anticancer immune surveillance. (C) Administration of an anti-CTLA4 antibody (e.g., ipilimumab) blocks CTLA4 activity on Tregs, restoring effector T cell function and enabling the death of the neoplastic cell.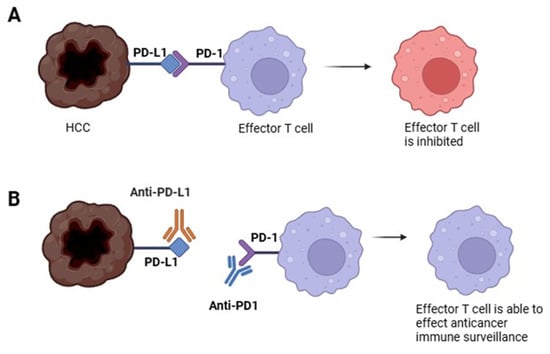 Figure 4. Mechanisms of PD-1/PD-L1 Immune Evasion in Cancer. (A) The neoplastic cell expresses PD-L1, which binds to PD-1 on effector T cells, inhibiting their anticancer activity. (B) An anti-PD-L1 antibody (e.g., atezolizumab) binds to PD-L1 on the neoplastic cell, preventing it from inhibiting the effector T cell. Alternatively, an anti-PD-1 antibody (e.g., nivolumab) binds to PD-1 on the effector T cell, blocking the inhibitory signal from the neoplastic cell and restoring immune function.
Figure 4. Mechanisms of PD-1/PD-L1 Immune Evasion in Cancer. (A) The neoplastic cell expresses PD-L1, which binds to PD-1 on effector T cells, inhibiting their anticancer activity. (B) An anti-PD-L1 antibody (e.g., atezolizumab) binds to PD-L1 on the neoplastic cell, preventing it from inhibiting the effector T cell. Alternatively, an anti-PD-1 antibody (e.g., nivolumab) binds to PD-1 on the effector T cell, blocking the inhibitory signal from the neoplastic cell and restoring immune function.
However, beyond PD-1, PD-L1, and CTLA4, there are other immune checkpoint molecules that can be targeted to stimulate an anti-tumor immune response:
- -
- TIM3: Expressed on CD4+ and CD8+ tumor-infiltrating lymphocytes (TILs) [] and tumor-associated macrophages (TAMs) [] in human HCC tumors; TIM3 negatively regulates T cell effector function []. On Treg cells, its expression enhances suppressive activity []. Additionally, TIM3 is strongly expressed on less differentiated HCC tumors [].
- -
- LAG3: Lymphocyte-activation gene 3 (LAG3) binds MHC class II molecules with high affinity and is upregulated upon T cell activation, providing a negative signal to T cells []. LAG3 expression is significantly higher on tumor-specific CD4+ and CD8+ TILs compared to other immune compartments in patients with HCC []. LAG3 also has a soluble ligand, fibrinogen-like protein 1, which is synthesized by hepatocytes [].
- Immune checkpoint inhibitors (ICIs) are monoclonal antibodies that block the interaction between checkpoint proteins and their ligands, thereby preventing T cell inactivation. ICIs have demonstrated that a properly activated immune response can effectively eliminate tumor cells.
3. Therapeutics in HCC
Treatment options for HCC include surgical, locoregional, orthotopic liver transplantation (OLT), and systemic therapies. The therapeutic decision-making process takes into account both liver function and tumor burden, which are represented in the most widely used and recommended staging system: the Barcelona Liver Cancer Clinic (BCLC) staging system [,].
Systemic treatments are typically offered to patients with BCLC stage C disease, those with BCLC stage B disease who are unsuitable for other treatments, patients with progressive disease following locoregional therapy, or as an adjuvant treatment [].
Currently approved systemic therapies are divided into two main classes: anti-angiogenic targeted therapies and ICIs.
3.1. Anti-Angiogenic Therapies
The anti-angiogenic class includes the following:
- Multi-tyrosine kinase inhibitors (mTKIs). Tyrosine kinases are involved in the activation of a wide range of proteins by phosphorylation. TKIs bind to the active site of tyrosine kinases, preventing phosphorylation and inhibiting downstream signal transduction of a range of growth factors, including the vascular endothelial growth factor receptor (VEGFR) []. Therefore, this class includes VEGF receptor inhibitors like sorafenib, lenvatinib, cabozantinib, and regorafenib. Atezolizumab plus bevacizumab (atezo + bev) or durvalumab plus tremelimumab (durva + treme) are recommended as first-line treatments. However, when contraindications to atezo + bev or durva + treme are present, sorafenib, lenvatinib, or durvalumab may be considered as alternative first-line options. Following first-line treatment with sorafenib or lenvatinib, second-line therapy options include cabozantinib or regorafenib [].
- Monoclonal anti-angiogenic antibodies: Bevacizumab targets vascular endothelial growth factor (VEGF), a family of soluble proteins that regulate angiogenesis in blood and lymphatic vessels. VEGF-A is considered the main pro-angiogenic factor in human malignancies, including HCC [].
For unresectable advanced HCC, mTKIs such as sorafenib, regorafenib, and lenvatinib have been used. However, the therapeutic efficacy of mTKIs was insufficient, leading to numerous randomized trials exploring the use of immunotherapy with ICIs, including anti-programmed death-1 (PD-1) and anti-programmed death-ligand 1 (PD-L1) antibodies [].
In 2017, the FDA approved the anti-PD-1 monoclonal antibody nivolumab for treating HCC after previous sorafenib treatment, based on the findings of the CheckMate-040 study []. In 2018, pembrolizumab, another anti-PD-1 monoclonal antibody, was approved for HCC patients previously treated with sorafenib, based on the KEYNOTE-224 study. However, ICI monotherapy for patients with metastatic or unresectable HCC did not demonstrate a significant survival benefit. This led to the exploration of combination therapies, as a better understanding of the tumor microenvironment (TME) emerged.
3.2. Combination Therapies with ICIs
In the IMbrave150 clinical trial [], patients were randomly assigned to receive the standard dose of atezolizumab (an ICI anti-PD-L1) plus a high dose of the VEGF inhibitor bevacizumab, or sorafenib. The trial showed improved overall survival (OS), confirmed progression-free survival (PFS), and significantly preserved health-related quality of life in the combination treatment arm.
In 2020, the FDA approved the atezolizumab plus bevacizumab combination as first-line therapy for patients with metastatic or unresectable HCC who had not received systemic treatment. In clinical practice, this combination has shown great efficacy, whether or not the patients had previous systemic treatment, significantly improving OS [].
The interaction between VEGF/VEGFR blockade and PD-1/PD-L1 inhibitors works at multiple levels. The reduction and normalization of tumor vasculature are likely only part of the effect, and various lines of research suggest that functional control of innate immune populations, including myeloid-derived suppressor cells (MDSCs), plays a key role []. In this context, bevacizumab has shown the ability to reduce MDSCs in patients with other cancer types, such as lung or colorectal cancer [,]. Additionally, preclinical mouse models of HCC have demonstrated that the superior therapeutic activity of a combination of anti-PD-1 and anti-VEGFR2 antibodies is associated with enhanced M1 and decreased M2 TAM levels, as well as an increased level of infiltrating CD8+ T cells.
A key question remains: which agent would be most beneficial in enhancing the effects of the ICIs–anti-angiogenic combination? Bevacizumab neutralizes VEGF-A, while VEGFR inhibitors block proximal signal transduction from VEGF receptors.
4. MASLD-HCC Treatment
The EASL and AASLD guidelines do not recommend a treatment pathway based solely on the etiology of the underlying liver disease. These guidelines have traditionally focused on patients with viral hepatitis. Notably, the BCLC staging system does not account for non-cirrhotic HCC. Patients with MASLD-HCC (metabolic-associated steatotic liver disease-related HCC) are less likely to have established cirrhosis or clinically significant portal hypertension []. As a result, these patients are less frequently included in surveillance programs and tend to present with more advanced disease at diagnosis, limiting opportunities for curative treatment [].
Furthermore, patients with MASLD-HCC are often affected by additional comorbidities related to metabolic syndrome, complicating treatment decisions.
Increasing evidence suggests that the etiology of the liver disease may influence treatment responses []. However, data specific to MASLD-HCC are limited. For instance, the pivotal SHARP study included only patients with viral hepatitis and alcohol-related liver disease. A recent international cohort study of patients treated with sorafenib did not show significant differences in outcomes between MASLD-HCC and other etiologies, but this study had a small MASLD-HCC subgroup [].
Pre-clinical and clinical data suggest that MASLD-HCC may have a reduced response to ICI therapy. A retrospective cohort study on the therapeutic efficacy of lenvatinib showed similar OS and PFS between MASLD-HCC and other etiology subgroups []. A meta-analysis of eight studies assessing systemic therapies concluded that ICIs offer more benefit in viral HCC than in non-viral HCC. However, the efficacy of tyrosine kinase inhibitors (TKIs) and anti-VEGF agents appeared unaffected by the underlying liver disease etiology []. Notably, none of these studies made further distinctions within the “non-viral HCC” group in terms of MASLD, alcohol, or other chronic liver disease etiologies.
Immunotherapy Outcomes in MASLD-HCC Patients
According to both basic research and clinical data, non-viral HCC, particularly MASLD-HCC, might show limited efficacy to immunotherapy [,]. A meta-analysis of trials (including IMbrave150 [], KEYNOTE-240, and CheckMate-459) showed that ICIs improved OS in patients with viral HCC but not in those with non-viral HCC []. In the CheckMate-459 trial, which compared nivolumab monotherapy with sorafenib as a first-line therapy, nivolumab showed a lower survival benefit in non-viral HCC patients compared to viral HCC patients []. Similarly, in the KEYNOTE-240 study, pembrolizumab extended OS less in non-viral HCC patients than in those with HBV-HCC.
In another meta-analysis, Haber et al. [] found that ICIs were less beneficial in patients with non-viral HCC, while the efficacy of TKIs and anti-VEGF agents remained unaffected by the underlying HCC etiology. Additionally, in the CheckMate-040 trial, which assessed the combination of nivolumab and ipilimumab (an anti-CTLA-4 monoclonal antibody), no significant difference in OS was found among HBV-HCC, HCV-HCC, and non-viral HCC patients []. The loss of anti-tumor CD4+ T cells and the accumulation of exhausted, altered CD8+PD-1+ T cells in NASH may impair tumor immune surveillance and reduce the efficacy of immunotherapy [,].
However, in a recent retrospective multicentric study, Copil et al. [] found that the presence of MASLD did not worsen outcomes in advanced HCC patients treated with atezolizumab and bevacizumab (atezo-beva). Additionally, the post-hoc analysis of the IMbrave150 trial (which tested the combination of atezolizumab and bevacizumab in advanced HCC) did not show significant differences in PFS or OS based on the underlying etiology []. Nevertheless, it is important to note that nearly 70% of the population in the IMbrave150 trial had viral HCC [].
These conflicting results may be due to the lack of clear subgroup distinctions within the non-viral HCC group in some studies or post-hoc analyses, where NASH, alcohol-related HCC, and unknown etiologies are grouped together, complicating data interpretation []. Furthermore, none of these key studies distinguished MASLD-HCC as a separate etiology of liver disease at baseline, with cohorts divided mainly by the presence or absence of viral hepatitis. A small study comparing anti-PD-L1 immunotherapy outcomes in MASLD-HCC versus other etiologies demonstrated reduced OS in the MASLD-HCC group [].
The HIMALYA [] and CARES-310 [] trials did not specifically report outcomes for MASLD-HCC patients, and thus, the role of ICIs in MASLD-HCC requires further investigation [].
Recent studies suggest that combining ICIs with anti-VEGF therapies may improve outcomes in non-viral HCC, including MASLD-HCC. In the GO30140 study, which compared atezolizumab monotherapy with atezolizumab and bevacizumab (atezo-beva combination), PFS was significantly longer in non-viral HCC patients treated with atezo-beva compared to those on monotherapy, indicating that anti-VEGF therapy could complement the limitations of ICIs in monotherapy [].VEGF plays a crucial role in the tumor microenvironment by prompting immune cells to recruit regulatory T cells while reducing the infiltration of cytotoxic T cells (CTLs) []. Vascular endothelial growth factor A (VEGF-A) plays a central role in tumor angiogenesis. However, VEGF-A also supports tumor progression through a dual mechanism: not only by promoting vessel formation but also by acting as an immunosuppressive factor. As previously discussed, CD4+ and CD8+ T lymphocytes can eliminate tumor cells. However, tumors develop various immune evasion strategies, such as the induction of regulatory T cells (Tregs) and T cell exhaustion. VEGF-A appears to contribute to this T cell-mediated immunosuppression. Notably, anti-VEGF therapy has been shown to counteract this immunosuppression through multiple mechanisms, including reducing Treg percentages, enhancing CD4+ and CD8+ T cell infiltration into the tumor, decreasing PD-1 expression on intratumoral CD8+ T cells, and ultimately promoting tumor destruction. Anti-VEGF agents can improve intratumoral infiltration and survival of CTLs by normalizing the vasculature and modulating the immune microenvironment, thus enhancing the response of HCC to ICIs. Recent studies have also highlighted VEGF’s involvement in TOX-dependent T cell exhaustion [], reinforcing the importance of combining anti-VEGF therapies with anti-PD-1/PD-L1 therapies.
As discussed in previous sections, beyond PD-1, PD-L1, and CTLA-4, other immune checkpoint molecules, such as TIM-3 and LAG-3, can also be targeted to stimulate an anti-tumor immune response. To provide a comprehensive overview of the available therapeutic options, we will briefly discuss immunotherapies targeting these molecules. However, it is important to note that research on these targets remains limited, and further human trials are required. These immunotherapies are not yet included in official guidelines and are not widely used in clinical practice.
Targeting TIM-3 has demonstrated anti-tumor efficacy in preclinical studies. Combining TIM-3 inhibitors with other immune checkpoint inhibitors, chemotherapy, or radiotherapy has shown synergistic anti-tumor effects in tumor mouse models. However, additional human trials are necessary to confirm these findings [].
Similarly, anti-LAG-3 immunotherapy has shown promise in this field. LAG-3-based therapies can be classified into three categories: anti-LAG-3 monoclonal antibodies, bispecific LAG-3 antibodies, and LAG-3 immunoglobulin fusion proteins []. However, emerging evidence suggests that targeting LAG-3 alone may not be the most effective therapeutic strategy, as tumor cells can evade anti-tumor immune responses through various molecular mechanisms [].
A recent study in HCC patients demonstrated a correlation between pre-treatment expression levels of LAG-3, CD8, STAT1, and LAG-3+CD8+ cells in tumor tissue and response rates to immunotherapy in individuals with advanced HCC. Notably, LAG-3+CD8+ cells emerged as the most favorable biomarker. The study found that immunohistochemical analysis of LAG-3 and CD8 expression (LAG-3+CD8+ phenotype) was useful in predicting responses to ICIs in treatment-naïve patients with advanced HCC []. Interestingly, only LAG-3+CD8+ cells showed a statistically significant correlation with response to ICIs, regardless of whether the HCC was viral- or non-viral-related. These findings suggest that pre-treatment immunohistochemical assessment of LAG-3 and CD8 levels in the tumor microenvironment may serve as a predictive biomarker for response to ICIs in HCC patients.
5. Steatohepatitic Hepatocellular Carcinoma (SH-HCC)
The introduction of immunotherapy has been a major breakthrough in the treatment of HCC. However, recent data suggest that this is not universally true. According to meta-analyses and reviews of clinical statistics, non-viral HCC—particularly metabolic-associated steatotic liver disease-related HCC (MASLD-HCC)—may demonstrate limited efficacy to immunotherapy, especially ICIs. Nonetheless, these findings remain inconsistent, and the level of evidence is low. This inconsistency arises from the insufficient characterization and subclassification of non-viral HCC groups (e.g., MASLD, autoimmune conditions) in many studies, making it difficult to draw definitive conclusions.
The underlying reasons for the observed reduced efficacy of immunotherapy in MASLD-HCC are unclear. This is particularly puzzling since immune system dysregulation is a common feature of both chronic liver disease and the neoplastic process. One potential approach to addressing this question involves a more detailed analysis of the relationship between the type of chronic liver disease and HCC characteristics. Although current treatment protocols for HCC are largely uniform, regardless of the underlying liver disease, it is plausible that the tumor biology of HCC may vary depending on its etiology. Could there be distinct subtypes of HCC that differ subtly based on their underlying causes?
The 2019 5th edition of the WHO Classification of Digestive System Tumors identifies a subtype of HCC known as steatohepatitic hepatocellular carcinoma (SH-HCC). This subtype, which accounts for 5–20% of cases [], has been linked to various etiologies, including chronic viral hepatitis. Specifically, studies by Yamaoka et al. [] found that SH-HCC was frequently associated with HCV, while its association with HBV infection was significantly lower, consistent with findings by Shibahara et al. []. However, there is growing evidence of an increased incidence of SH-HCC in NAFLD and NASH, suggesting a strong connection to metabolic syndrome [,].
SH-HCC is defined by unique histological features, including large-droplet steatosis in tumor cells, ballooning of malignant hepatocytes, inflammation, Mallory–Denk bodies (eosinophilic inclusions from destroyed intermediate filaments), and pericellular fibrosis [].
From a diagnostic imaging perspective, SH-HCCs are typically smaller than other HCC subtypes and are characterized by intralesional fat in the context of hepatic parenchymal steatosis. On ultrasonography, SH-HCC often appears as a hyperechoic lesion with acoustic shadowing. On MRI, prominent fatty deposits are evident, appearing as intralesional signal loss on opposed-phase images []. On dynamic CT, SH-HCCs are often classified as LR-5 (i.e., definitively HCC) in high-risk patients [], showing hyperenhancement in the arterial phase, washout in the portal venous phase, and hypointensity in the hepatobiliary phase []. However, in unenhanced imaging, SH-HCCs may be challenging to differentiate from the surrounding steatotic liver parenchyma or other fat-containing liver lesions, such as angiomyolipoma or clear-cell HCC.
According to the International Consensus Group for Hepatocellular Neoplasia, intralesional fat is typically found in early-stage HCCs but decreases as tumors progress. Early-stage HCCs rely on a portal venous blood supply, whereas more advanced HCCs shift toward an arterial supply. This transition can cause transient ischemia and hypoxia, potentially inducing fatty metamorphosis in hepatocytes. Intratumoral steatosis is commonly observed in HCCs smaller than 1.5 cm but becomes less frequent and more focal as tumor size and histological grade increase []. However, SH-HCCs are an exception, as they exhibit significant intralesional fat at both early and advanced stages []. This persistence of fat can complicate the diagnosis of SH-HCC, as early-stage HCCs with intratumoral fat might be misclassified [].
Exploring the relationship between SH-HCC and the response to immunotherapy, particularly ICIs, could yield valuable insights. Such research could clarify whether differences in response to immunotherapy are influenced by the underlying hepatic disease. Ultimately, these findings could guide the development of therapeutic strategies tailored to the etiology of HCC, taking into account the chronic liver disease associated with the tumor.
6. Conclusions
Innate and adaptive immune dysfunction have been identified as key mechanisms in the progression of NASH. This altered immune landscape may not only impair the efficacy of ICIs but also exacerbate disease progression. Consequently, it has been suggested that MASLD-HCC may exhibit reduced responsiveness to immunotherapy. However, given the limited evidence and scarcity of dedicated studies, it is essential to first conduct clinical trials specifically designed to investigate the impact of HCC etiology on responses to systemic therapies before dismissing the potential role of immunotherapy in managing NASH-related or non-viral HCC.
In particular, we propose that, given the heterogeneity of HCC subtypes (e.g., SH-HCC), future trials should not only stratify patients based on etiology (e.g., HBV, HCV, MASLD, autoimmune, etc.) but also consider histological and, more importantly, immunohistological characteristics. This approach would help determine whether variations in treatment response across different etiologies are driven by distinct histopathological mechanisms.
Additionally, further research is needed to determine whether the combination of ICIs with anti-VEGF agents represents the most effective strategy for improving therapeutic outcomes in MASLD-related HCC. Future clinical trials should consider stratifying patients based on the etiology of their liver disease to optimize and personalize treatment strategies for individuals with advanced-stage HCC.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pouwels, S.; Sakran, N.; Graham, Y.; Leal, A.; Pintar, T.; Yang, W.; Kassir, R.; Singhal, R.; Mahawar, K.; Ramnarain, D. Non-alcoholic fatty liver disease (NAFLD): A review of pathophysiology, clinical management and effects of weight loss. BMC Endocr. Disord. 2022, 22, 63. [Google Scholar] [CrossRef] [PubMed]
- Phoolchund, A.G.S.; Khakoo, S.I. MASLD and the Development of HCC: Pathogenesis and Therapeutic Challenges. Cancers 2024, 16, 259. [Google Scholar] [CrossRef] [PubMed]
- Mazzolini, G.; Sowa, J.-P.; Atorrasagasti, C.; Kücükoglu, Ö.; Syn, W.-K.; Canbay, A. Significance of Simple Steatosis: An Update on the Clinical and Molecular Evidence. Cells 2020, 9, 2458. [Google Scholar] [CrossRef]
- McCullough, A.J. Pathophysiology of nonalcoholic steatohepatitis. J. Clin. Gastroenterol. 2006, 40 (Suppl. 1), S17–S29. [Google Scholar] [PubMed]
- Satapathy, S.K.; Kuwajima, V.; Nadelson, J.; Atiq, O.; Sanyal, A.J. Drug-induced fatty liver disease: An overview of pathogenesis and management. Ann. Hepatol. 2015, 14, 789–806. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Ann. Hepatol. 2024, 291, 101133. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Dungubat, E.; Kusano, H.; Fukusato, T. Pathology and Pathogenesis of Metabolic Dysfunction-Associated Steatotic Liver Disease-Associated Hepatic Tumors. Biomedicines 2023, 11, 2761. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mantovani, A.; Taverna, A.; Cappelli, D.; Beatrice, G.; Csermely, A.; Sani, E.; Byrne, C.D.; Targher, G. Long-Term Adverse Effect of Liver Stiffness on Glycaemic Control in Type 2 Diabetic Patients with Nonalcoholic Fatty Liver Disease: A Pilot Study. Int. J. Mol. Sci. 2022, 23, 12481. [Google Scholar] [CrossRef] [PubMed]
- Pierantonelli, I.; Svegliati-Baroni, G. Nonalcoholic Fatty Liver Disease: Basic Pathogenetic Mechanisms in the Progression from NAFLD to NASH. Transplantation 2019, 103, e1–e13. [Google Scholar] [CrossRef]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62 (Suppl. 1), S47–S64. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, J.; Xiao, W.; Fan, X. A review of MASLD-related hepatocellular carcinoma: Progress in pathogenesis, early detection, and therapeutic interventions. Front. Med. 2024, 11, 1410668. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Crispe, I.N. The liver as a lymphoid organ. Annu. Rev. Immunol. 2009, 27, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimkhani, M.R.; Mohar, I.; Crispe, I.N. Cross-presentation of antigen by diverse subsets of murine liver cells. Hepatology 2011, 54, 1379–1387. [Google Scholar] [CrossRef]
- Ormandy, L.A.; Hillemann, T.; Wedemeyer, H.; Manns, M.P.; Greten, T.F.; Korangy, F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res. 2005, 65, 2457–2464. [Google Scholar] [CrossRef] [PubMed]
- Dunham, R.M.; Thapa, M.; Velazquez, V.M.; Elrod, E.J.; Denning, T.L.; Pulendran, B.; Grakoui, A. Hepatic stellate cells preferentially induce Foxp3+ regulatory T cells by production of retinoic acid. J. Immunol. 2013, 190, 2009–2016. [Google Scholar] [CrossRef]
- Yu, M.C.; Chen, C.H.; Liang, X.; Wang, L.; Gandhi, C.R.; Fung, J.J.; Lu, L.; Qian, S. Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice. Hepatology 2004, 40, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- Huby, T.; Gautier, E.L. Immune cell-mediated features of non-alcoholic steatohepatitis. Nat. Rev. Immunol. 2021, 22, 429–443. [Google Scholar] [CrossRef]
- Park, S.J.; Garcia Diaz, J.; Um, E.; Hahn, Y.S. Major roles of kupffer cells and macrophages in NAFLD development. Front. Endocrinol. 2023, 14, 1150118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, G.X.; Wei, S.; Yu, C.; Zhao, S.Q.; Yang, W.J.; Feng, Y.H.; Pan, C.; Yang, K.X.; Ma, Y. Activation of Kupffer cells in NAFLD and NASH: Mechanisms and therapeutic interventions. Front. Cell Dev. Biol. 2023, 11, 1199519. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barreby, E.; Chen, P.; Aouadi, M. Macrophage functional diversity in NAFLD—More than inflammation. Nat. Rev. Endocrinol. 2022, 18, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Härtlova, A.; Gierliński, M.; Prescott, A.; Castellvi, J.; Losa, J.H.; Petersen, S.K.; Wenzel, U.A.; Dill, B.D.; Emmerich, C.H.; et al. Triggering MSR1 promotes JNK-mediated inflammation in IL-4-activated macrophages. EMBO J. 2019, 38, e100299. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Qiu, D.; Liang, X.; Huang, Y.; Wang, Y.; Jia, X.; Li, K.; Zhao, J.; Du, C.; Qiu, X.; et al. Lipotoxicity-induced STING1 activation stimulates MTORC1 and restricts hepatic lipophagy. Autophagy 2022, 18, 860–876. [Google Scholar] [CrossRef]
- Asanuma, T.; Ono, M.; Kubota, K.; Hirose, A.; Hayashi, Y.; Saibara, T.; Inanami, O.; Ogawa, Y.; Enzan, H.; Onishi, S.; et al. Super paramagnetic iron oxide MRI shows defective Kupffer cell uptake function in non-alcoholic fatty liver disease. Gut 2010, 59, 258–266. [Google Scholar] [CrossRef]
- Kazankov, K.; Jørgensen, S.M.D.; Thomsen, K.L.; Møller, H.J.; Vilstrup, H.; George, J.; Schuppan, D.; Grønbæk, H. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 145–159. [Google Scholar] [CrossRef] [PubMed]
- McDonald, B.; Jenne, C.N.; Zhuo, L.; Kimata, K.; Kubes, P. Kupffer cells and activation of endothelial TLR4 coordinate neutrophil adhesion within liver sinusoids during endotoxemia. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G797–G806. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Szabo, G. Two Faces of Neutrophils in Liver Disease Development and Progression. Hepatology 2021, 74, 503–512. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paquissi, F.C. Immune Imbalances in Non-Alcoholic Fatty Liver Disease: From General Biomarkers and Neutrophils to Interleukin-17 Axis Activation and New Therapeutic Targets. Front. Immunol. 2016, 7, 490. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giles, D.; Moreno-Fernandez, M.; Divanovic, S. IL-17 Axis Driven Inflammation in Non-Alcoholic Fatty Liver Disease Progression. Curr. Drug Targets 2015, 16, 1315–1323. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, X.; Yang, L.; Chang, N.; Hou, L.; Zhou, X.; Yang, L.; Li, L. Neutrophils undergo switch of apoptosis to NETosis during murine fatty liver injury via S1P receptor 2 signaling. Cell Death Dis. 2020, 11, 379. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van der Windt, D.J.; Sud, V.; Zhang, H.; Varley, P.R.; Goswami, J.; Yazdani, H.O.; Tohme, S.; Loughran, P.; O’Doherty, R.M.; Minervini, M.I.; et al. Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatology 2018, 68, 1347–1360. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alkhouri, N.; Morris-Stiff, G.; Campbell, C.; Lopez, R.; Tamimi, T.A.; Yerian, L.; Zein, N.N.; Feldstein, A.E. Neutrophil to lymphocyte ratio: A new marker for predicting steatohepatitis and fibrosis in patients with nonalcoholic fatty liver disease. Liver Int. 2012, 32, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.E.; Yu, Y.; Luu, H.N.; Wang, R.; Paragomi, P.; Behari, J.; Yuan, J. Neutrophil-lymphocyte ratio in relation to risk of hepatocellular carcinoma in patients with non-alcoholic fatty liver disease. Cancer Med. 2023, 12, 3589–3600. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bernsmeier, C.; Albano, E. Liver dendritic cells and NAFLD evolution: A remaining open issue. J. Hepatol. 2017, 66, 1120–1122. [Google Scholar] [CrossRef] [PubMed]
- Sutti, S.; Locatelli, I.; Bruzzì, S.; Jindal, A.; Vacchiano, M.; Bozzola, C.; Albano, E. CX3CR1-expressing inflammatory dendritic cells contribute to the progression of steatohepatitis. Clin. Sci. 2015, 129, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Henning, J.R.; Graffeo, C.S.; Rehman, A.; Fallon, N.C.; Zambirinis, C.P.; Ochi, A.; Barilla, R.; Jamal, M.; Deutsch, M.; Greco, S.; et al. Dendritic cells limit fibroinflamma-tory injury in nonalcoholic steatohepatitis in mice. Hepatology 2013, 58, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Heymann, F.; Tacke, F. Immunology in the liver—From homeostasis to disease. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 88–110. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Tian, Z. Diversity of tissue-resident NK cells. Semin. Immunol. 2017, 31, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Luci, C.; Vieira, E.; Perchet, T.; Gual, P.; Golub, R. Natural Killer Cells and Type 1 Innate Lymphoid Cells Are New Actors in Non-alcoholic Fatty Liver Disease. Front. Immunol. 2019, 10, 1192. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martínez-Chantar, M.L.; Delgado, T.C.; Beraza, N. Revisiting the Role of Natural Killer Cells in Non-Alcoholic Fatty Liver Disease. Front. Immunol. 2021, 12, 640869. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kahraman, A.; Schlattjan, M.; Kocabayoglu, P.; Yildiz-Meziletoglu, S.; Schlensak, M.; Fingas, C.D.; Wedemeyer, I.; Marquitan, G.; Gieseler, R.K.; Baba, H.A.; et al. Major histocompatibility complex class I-related chains A and B (MIC A/B): A novel role in nonalcoholic steatohepatitis. Hepatology 2010, 51, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.Y.; Park, E.J.; Lee, C.W. Immunological distinctions between nonalcoholic steatohep-atitis and hepatocellular carcinoma. Exp. Mol. Med. 2020, 52, 1209–1219. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Syn, W.K.; Oo, Y.H.; Pereira, T.A.; Karaca, G.F.; Jung, Y.; Omenetti, A.; Witek, R.P.; Choi, S.S.; Guy, C.D.; Fearing, C.M.; et al. Accumulation of natural killer T cells in progressive nonalcoholic fatty liver disease. Hepatology. 2010, 51, 1998–2007. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wolf, M.J.; Adili, A.; Piotrowitz, K.; Abdullah, Z.; Boege, Y.; Stemmer, K.; Ringelhan, M.; Simonavicius, N.; Egger, M.; Wohlleber, D.; et al. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell 2014, 26, 549–564. [Google Scholar] [CrossRef] [PubMed]
- Shuai, Z.; Leung, M.W.; He, X.; Zhang, W.; Yang, G.; Leung, P.S. Eric Gershwin, M. Adaptive immunity in the liver. Cell. Mol. Immunol. 2016, 13, 354–368. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raphael, I.; Nalawade, S.; Eagar, T.N.; Forsthuber, T.G. T cell subsets and their signature cy-tokines in autoimmune and inflammatory diseases. Cytokine 2015, 74, 5–17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saravia, J.; Chapman, N.M.; Chi, H. Helper T cell differentiation. Cell. Mol. Immunol. 2019, 16, 634–643. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moreno-Fernandez, M.E.; Giles, D.A.; Oates, J.R.; Chan, C.C.; Damen, M.S.; Doll, J.R.; Stankiewicz, T.E.; Chen, X.; Chetal, K.; Karns, R.; et al. PKM2-dependent metabolic skewing of hepatic Th17 cells regulates pathogenesis of non-alcoholic fatty liver disease. Cell Metab. 2021, 33, 1187–1204.e9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, C.; Kesarwala, A.H.; Eggert, T.; Medina-Echeverz, J.; Kleiner, D.E.; Jin, P.; Stroncek, D.F.; Terabe, M.; Kapoor, V.; ElGindi, M.; et al. NAFLD causes selec-tive CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature 2016, 531, 253–257. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kremer, M.; Hines, I.N.; Milton, R.J.; Wheeler, M.D. Favored T helper 1 response in a mouse model of hepatosteatosis is associated with enhanced T cell-mediated hepatitis. Hepatology 2006, 44, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Rau, M.; Schilling, A.K.; Meertens, J.; Hering, I.; Weiss, J.; Jurowich, C.; Kudlich, T.; Hermanns, H.M.; Bantel, H.; Beyersdorf, N.; et al. Progression from Nonalcoholic Fatty Liver to Nonalcoholic Steatohepatitis Is Marked by a Higher Frequency of Th17 Cells in the Liver and an Increased Th17/Resting Regulatory T Cell Ratio in Peripheral Blood and in the Liver. J. Immunol. 2016, 196, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Chackelevicius, C.M.; Gambaro, S.E.; Tiribelli, C.; Rosso, N. Th17 involvement in nonalco-holic fatty liver disease progression to non-alcoholic steatohepatitis. World J. Gastroenterol. 2016, 22, 9096–9103. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rolla, S.; Alchera, E.; Imarisio, C.; Bardina, V.; Valente, G.; Cappello, P.; Mombello, C.; Follenzi, A.; Novelli, F.; Carini, R. The balance between IL-17 and IL-22 produced by liv-er-infiltrating T-helper cells critically controls NASH development in mice. Clin. Sci. 2016, 130, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.L.; Teijeiro, A.; Burén, S.; Tummala, K.S.; Yilmaz, M.; Waisman, A.; Theurillat, J.P.; Perna, C.; Djouder, N. Metabolic inflammation-associated IL-17A causes non-alcoholic steatohepatitis and hepatocellular carcinoma. Cancer Cell 2016, 30, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Van Herck, M.A.; Weyler, J.; Kwanten, W.J.; Dirinck, E.L.; De Winter, B.Y.; Francque, S.M.; Vonghia, L. The Differential Roles of T Cells in Non-alcoholic Fatty Liver Disease and Obesity. Front. Immunol. 2019, 10, 82. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rai, R.P.; Liu, Y.; Iyer, S.S.; Liu, S.; Gupta, B.; Desai, C.; Kumar, P.; Smith, T.; Singhi, A.D.; Nusrat, A.; et al. Blocking integrin α4β7-mediated CD4 T cell recruitment to the intestine and liver protects mice from western diet-induced non-alcoholic steatohepatitis. J. Hepatol. 2020, 73, 1013–1022. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferreyra Solari, N.E.; Inzaugarat, M.E.; Baz, P.; De Matteo, E.; Lezama, C.; Galoppo, M.; Ga-loppo, C.; Cherñavsky, A.C. The role of innate cells is coupled to a Th1-polarized immune response in pediatric nonalcoholic steatohepatitis. J. Clin. Immunol. 2012, 32, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.Y.; Takahara, T.; Kawai, K.; Fujino, M.; Sugiyama, T.; Tsuneyama, K.; Tsukada, K.; Nakae, S.; Zhong, L.; Li, X.K. IFN-γ deficiency attenuates hepatic inflammation and fibrosis in a steatohepatitis model induced by a methionine- and choline-deficient high-fat diet. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G891–G899. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Wang, K.; Aoyama, T.; Grivennikov, S.I.; Paik, Y.; Scholten, D.; Cong, M.; Iwaisako, K.; Liu, X.; Zhang, M.; et al. Interleu-kin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology 2012, 143, 765–776.e3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giles, D.A.; Moreno-Fernandez, M.E.; Stankiewicz, T.E.; Cappelletti, M.; Huppert, S.S.; Iwakura, Y.; Dong, C.; Shanmukhappa, S.K.; Divanovic, S. Regulation of Inflammation by IL-17A and IL-17F Modulates Non-Alcoholic Fatty Liver Disease Pathogenesis. PLoS ONE. 2016, 11, e0149783. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tan, Z.; Qian, X.; Jiang, R.; Liu, Q.; Wang, Y.; Chen, C.; Wang, X.; Ryffel, B.; Sun, B. IL-17A plays a critical role in the pathogenesis of liver fibrosis through hepatic stellate cell activation. J. Immunol. 2013, 191, 1835–1844. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Wu, L.; Xie, W.; Shao, Y.; Jiang, J.; Zhao, Z.; Yan, M.; Chen, Z.; Cui, D. The imbalance of Th17/Treg cells is involved in the progression of nonalcoholic fatty liver disease in mice. BMC Immunol. 2017, 18, 33. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, L.; Zhang, H.; Wang, S.; Chen, X.; Su, J. Bone Marrow Adipocytes: A Critical Player in the Bone Marrow Microenvironment. Front. Cell Dev. Biol. 2021, 9, 770705. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dywicki, J.; Buitrago-Molina, L.E.; Noyan, F.; Davalos-Misslitz, A.C.; Hupa-Breier, K.L.; Lieber, M.; Hapke, M.; Schlue, J.; Falk, C.S.; Raha, S.; et al. The Detrimental Role of Regulatory T Cells in Nonalcoholic Steatohepatitis. Hepatol. Commun. 2022, 6, 320–333. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Söderberg, C.; Marmur, J.; Eckes, K.; Glaumann, H.; Sällberg, M.; Frelin, L.; Rosenberg, P.; Stål, P.; Hultcrantz, R. Microvesicular fat, inter cellular adhesion molecule-1 and regulatory T-lymphocytes are of importance for the inflammatory process in livers with non-alcoholic steatohepatitis. APMIS 2011, 119, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, J.; Kirby, M.; Softic, S.; Miles, L.; Salazar-Gonzalez, R.; Shivakumar, P.; Kohli, R. Hepatic natural killer T-cell and CD8+ T-cell signatures in mice with nonalcoholic steatohepatitis. Hepatol. Commun. 2017, 1, 299–310. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wabitsch, S.; McCallen, J.D.; Kamenyeva, O.; Ruf, B.; McVey, J.C.; Kabat, J.; Walz, J.S.; Rotman, Y.; Bauer, K.C.; Craig, A.J.; et al. Metformin treatment rescues CD8+ T-cell response to immune checkpoint inhibitor therapy in mice with NAFLD. J. Hepatol. 2022, 77, 748–760. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McVey, J.C.; Green, B.L.; Ruf, B.; McCallen, J.D.; Wabitsch, S.; Subramanyam, V.; Diggs, L.P.; Heinrich, B.; Greten, T.F.; Ma, C. NAFLD indirectly impairs antigen-specific CD8+ T cell immunity against liver cancer in mice. iScience 2022, 25, 103847. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pfister, D.; Núñez, N.G.; Pinyol, R.; Govaere, O.; Pinter, M.; Szydlowska, M.; Gupta, R.; Qiu, M.; Deczkowska, A.; Weiner, A.; et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature 2021, 592, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Barrow, F.; Khan, S.; Wang, H.; Revelo, X.S. The Emerging Role of B Cells in the Pathogenesis of NAFLD. Hepatology 2021, 74, 2277–2286. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bruzzì, S.; Sutti, S.; Giudici, G.; Burlone, M.E.; Ramavath, N.N.; Toscani, A.; Bozzola, C.; Schneider, P.; Morello, E.; Parola, M.; et al. B2-Lymphocyte responses to oxidative stress-derived antigens contribute to the evolution of nonalcoholic fatty liver disease (NAFLD). Free. Radic. Biol. Med. 2018, 124, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Jiang, W.W.; Li, X.; Qiu, X.Y.; Wu, Z.; Chi, Y.J.; Cong, X.; Liu, Y.L. Role of intrahepatic B cells in non-alcoholic fatty liver disease by secreting pro-inflammatory cytokines and regulating intrahepatic T cells. J. Dig. Dis. 2016, 17, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Chung, H.; Softic, S.; Moreno-Fernandez, M.E.; Divanovic, S. The bidirectional immune crosstalk in metabolic dysfunction-associated steatotic liver disease. Cell Metab. 2023, 35, 1852–1871. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fu, J.; Hu, F.; Ma, T.; Zhao, W.-J.; Tian, H.; Zhang, Y.; Hu, M.; Zhou, J.; Zhang, Y.; Jian, C.; et al. A conventional immune regulator mitochondrial antiviral signaling protein blocks hepatic steatosis by maintaining mitochondrial homeostasis. Hepatology 2022, 75, 403–418. [Google Scholar] [CrossRef]
- Kamada, Y.; Morishita, K.; Koseki, M.; Nishida, M.; Asuka, T.; Naito, Y.; Yamada, M.; Takamatsu, S.; Sakata, Y.; Takehara, T.; et al. Serum Mac-2 Binding Protein Levels Associate with Metabolic Parameters and Predict Liver Fibrosis Progression in Subjects with Fatty Liver Disease: A 7-Year Longitudinal Study. Nutrients 2020, 12, 1770. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Chen, W.; Athavale, D.; Ge, X.; Desert, R.; Das, S.; Han, H.; Nieto, N. Osteopontin Takes Center Stage in Chronic Liver Disease. Hepatology 2021, 73, 1594–1608. [Google Scholar] [CrossRef]
- Govaere, O.; Petersen, S.K.; Martinez-Lopez, N.; Wouters, J.; Van Haele, M.; Mancina, R.M.; Jamialahmadi, O.; Bilkei-Gorzo, O.; Lassen, P.B.; Darlay, R.; et al. Macrophage scavenger receptor 1 mediates lipid-induced inflammation in non-alcoholic fatty liver disease. J. Hepatol. 2022, 76, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Yahoo, N.; Dudek, M.; Knolle, P.; Heikenwälder, M. Role of immune responses in the development of NAFLD-associated liver cancer and prospects for therapeutic modulation. J. Hepatol. 2023, 79, 538–551. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.S.; Taylor, R.J.; Bayliss, S.; Hagström, H.; Nasr, P.; Schattenberg, J.M.; Ishigami, M.; Toyoda, H.; Wong, V.W.-S.; Peleg, N.; et al. Association Between Fibrosis Stage and Outcomes of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Gastroenterology 2020, 158, 1611–1625.e12. [Google Scholar] [CrossRef] [PubMed]
- Sekine, T.; Perez-Potti, A.; Nguyen, S.; Gorin, J.-B.; Wu, V.H.; Gostick, E.; Llewellyn-Lacey, S.; Hammer, Q.; Falck-Jones, S.; Vangeti, S.; et al. TOX is expressed by exhausted and polyfunctional human effector memory CD8+ T cells. Sci. Immunol. 2020, 5, eaba7918. [Google Scholar] [CrossRef]
- Cheng, Y.; Shao, Z.; Chen, L.; Zheng, Q.; Zhang, Q.; Ding, W.; Zhang, M.; Yu, Q.; Gao, D. Role, function and regulation of the thymocyte selection-associated high mobility group box protein in CD8+ T cell exhaustion. Immunol. Lett. 2021, 229, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, Q.; Shen, H.; Xia, A.; Tian, W.; Yu, W.; Sun, B. TOX promotes the exhaustion of antitumor CD8+ T cells by preventing PD1 degradation in hepatocellular carcinoma. J. Hepatol. 2019, 71, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Li, J.; Liu, Y.; Zhou, S.; Wei, X.; Hua, H.; Tang, K.; Zhang, X.; Wang, Y.; Wu, Z.; et al. Roles of immune dysregulation in MASLD. Biomed. Pharmacother. 2024, 170, 116069. [Google Scholar] [CrossRef] [PubMed]
- Sangro, B.; Sarobe, P.; Hervás-Stubbs, S.; Melero, I. Advances in immunotherapy for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 525–543. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Yoshio, S.; Doi, H.; Mori, T.; Matsuda, M.; Kawai, H.; Shimagaki, T.; Yoshikawa, S.; Aoki, Y.; Osawa, Y.; et al. Increased Frequency of Dysfunctional Siglec-7−CD57+PD-1+ Natural Killer Cells in Patients With Non-alcoholic Fatty Liver Disease. Front. Immunol. 2021, 12, 603133. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Veglia, F.; Perego, M.; Gabrilovich, D. Myeloid-derived suppressor cells coming of age. Nat. Immunol. 2018, 19, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Lao, X.; Chen, M.; Liu, R.; Wei, Y.; Ouyang, F.; Chen, D.; Zhao, X.; Zhao, Q.; Li, X.; et al. PD-1hi Identifies a Novel Regulatory B-cell Population in Human Hepatoma That Promotes Disease Progression. Cancer Discov. 2016, 6, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-Y.; Liu, S.; Yang, M. Regulatory T cells and their associated factors in hepatocellular carcinoma development and therapy. World J. Gastroenterol. 2022, 28, 3346–3358. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, F.; Hoechst, B.; Gamrekelashvili, J.; Ormandy, L.A.; Voigtländer, T.; Wedemeyer, H.; Ylaya, K.; Wang, X.W.; Hewitt, S.M.; Manns, M.P.; et al. Human CCR4+CCR6+Th17 cells suppress autologous CD8+ T cell responses. J. Immunol. 2012, 188, 6055–6062. [Google Scholar] [CrossRef]
- Han, Y.; Chen, Z.; Yang, Y.; Jiang, Z.; Gu, Y.; Liu, Y.; Liu, C.; Pan, Z.; Yu, Y.; Jiang, H.; et al. Human CD14+ CTLA-4+ regulatory dendritic cells suppress T-cell response by cytotoxic T-lymphocyte antigen-4-dependent IL-10 and indoleamine-2,3-dioxygenase production in hepatocellular carcinoma. Hepatology 2014, 59, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Affo, S.; Yu, L.-X.; Schwabe, R.F. The Role of Cancer-Associated Fibroblasts and Fibrosis in Liver Cancer. Annu. Rev. Pathol. Mech. Dis. 2017, 12, 153–186. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.-L.; Zhou, Z.-J.; Hu, Z.-Q.; Huang, X.-W.; Wang, Z.; Chen, E.-B.; Fan, J.; Cao, Y.; Dai, Z.; Zhou, J. Tumor-Associated Neutrophils Recruit Macrophages and T-Regulatory Cells to Promote Progression of Hepatocellular Carcinoma and Resistance to Sorafenib. Gastroenterology 2016, 150, 1646–1658.e17. [Google Scholar] [CrossRef]
- Van Coillie, S.; Wiernicki, B.; Xu, J. Molecular and Cellular Functions of CTLA-4. Adv. Exp. Med. Biol. 2020, 1248, 7–32. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar] [PubMed] [PubMed Central]
- Li, H.; Wu, K.; Tao, K.; Chen, L.; Zheng, Q.; Lu, X.; Liu, J.; Shi, L.; Liu, C.; Wang, G.; et al. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology 2012, 56, 1342–1351. [Google Scholar] [CrossRef]
- Yan, W.; Liu, X.; Ma, H.; Zhang, H.; Song, X.; Gao, L.; Liang, X.; Ma, C. Tim-3 fosters HCC development by enhancing TGF-beta-mediated alternative activation of macrophages. Gut 2015, 64, 1593–1604. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Yang, M.; Turner, A.; Xu, C.; Ferris, R.L.; Huang, J.; Kane, L.P.; Lu, B. TIM-3 as a Target for Cancer Immunotherapy and Mechanisms of Action. Int. J. Mol. Sci. 2017, 18, 645. [Google Scholar] [CrossRef]
- Gautron, A.S.; Dominguez-Villar, M.; de Marcken, M.; Hafler, D.A. Enhanced suppressor function of TIM-3+ FoxP3+ regulatory T cells. Eur. J. Immunol. 2014, 44, 2703–2711. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, N.; Li, F.; Zhou, Z.; Sang, J.; Chen, Y.; Han, Q.; Lv, Y.; Liu, Z. Immune checkpoint proteins PD-1 and TIM-3 are both highly expressed in liver tissues and correlate with their gene polymorphisms in patients with HBV-related hepatocellular carcinoma. Medicine 2016, 95, e5749. [Google Scholar] [CrossRef] [PubMed]
- Andrews, L.P.; Marciscano, A.E.; Drake, C.G.; Vignali, D.A.A. LAG3 (CD223) as a cancer immunotherapy target. Immunol. Rev. 2017, 276, 80–96. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Sprengers, D.; Boor, P.P.C.; Doukas, M.; Schutz, H.; Mancham, S.; Pedroza-Gonzalez, A.; Polak, W.G.; de Jonge, J.; Gaspersz, M.; et al. Antibodies Against Immune Checkpoint Molecules Restore Functions of Tumor-Infiltrating T Cells in Hepatocellular Carcinomas. Gastroenterology 2017, 153, 1107–1119.e10. [Google Scholar] [CrossRef]
- Wang, J.; Sanmamed, M.F.; Datar, I.; Su, T.T.; Ji, L.; Sun, J.; Chen, L.; Chen, Y.; Zhu, G.; Yin, W.; et al. Fibrinogen-like Protein 1 Is a Major Immune Inhibitory Ligand of LAG-3. Cell 2019, 176, 334–347.e12. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on the management of hepatocellular carcinoma. J. Hepatol. 2025, 82, 315–374. [Google Scholar] [CrossRef] [PubMed]
- Wai Ling Khoo, T.S.; Rehman, A.; Olynyk, J.K. Tyrosine Kinase Inhibitors in the Treatment of Hepatocellular Carcinoma. In Hepa-Tocellular Carcinoma [Internet]; Tirnitz-Parker, J.E.E., Ed.; Codon Publications: Brisbane, Australia, 2019; Chapter 7. [Google Scholar]
- Gordan, J.D.; Kennedy, E.B.; Abou-Alfa, G.K.; Beal, E.; Finn, R.S.; Gade, T.P.; Goff, L.; Gupta, S.; Guy, J.; Hoang, H.T.; et al. Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline Update. J. Clin. Oncol. 2024, 42, 1830–1850. [Google Scholar] [CrossRef] [PubMed]
- Eso, Y.; Taura, K.; Seno, H. Does immune checkpoint inhibitor exhibit limited efficacy against non-viral hepatocellular carcinoma? A review of clinical trials. Hepatol. Res. 2022, 52, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Sorscher, S. Clarification of the FDA Accelerated Agnostic Approval of Pembrolizumab and the Opportunities Arising From the Required Confirmatory Studies. JAMA Oncol. 2018, 4, 1299–1300. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Chen, M.; Kaseb, A.O.; Kudo, M.; Lee, H.C.; Yopp, A.C.; Zhou, J.; Wang, L.; Wen, X.; Heo, J.; et al. Atezolizumab plus bevacizumab versus active surveillance in patients with resected or ablated high-risk hepatocellular carcinoma (IMbrave050): A randomised, open-label, multicentre, phase 3 trial. Lancet 2023, 402, 1835–1847. [Google Scholar] [CrossRef] [PubMed]
- Piscaglia, F.; Masi, G.; Martinelli, E.; Cabibbo, G.; Di Maio, M.; Gasbarrini, A.; Iavarone, M.; Antonuzzo, L.; Mazzaferro, V.; Ballestrero, A.; et al. Atezolizumab plus bevacizumab as first-line treatment of unresectable hepatocellular carcinoma: Interim analysis results from the phase IIIb AMETHISTA trial. ESMO Open 2025, 10, 104110. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rahma, O.E.; Hodi, F.S. The Intersection between Tumor Angiogenesis and Immune Suppression. Clin. Cancer Res. 2019, 25, 5449–5457. [Google Scholar] [CrossRef]
- Feng, P.H.; Chen, K.; Huang, Y.; Luo, C.; Wu, S.M.; Chen, T.; Lee, C.-N.; Yeh, C.-T.; Chuang, H.-C.; Han, C.-L.; et al. Bevacizumab Reduces S100A9-Positive MDSCs Linked to Intracranial Control in Patients with EGFR-Mutant Lung Adenocarcinoma. J. Thorac. Oncol. 2018, 13, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Limagne, E.; Euvrard, R.; Thibaudin, M.; Rébé, C.; Derangère, V.; Chevriaux, A.; Boidot, R.; Végran, F.; Bonnefoy, N.; Vincent, J.; et al. Accumulation of MDSC and Th17 Cells in Patients with Metastatic Colorectal Cancer Predicts the Efficacy of a FOLFOX-Bevacizumab Drug Treatment Regimen. Cancer Res. 2016, 76, 5241–5252. [Google Scholar] [CrossRef]
- Sanyal, A.; Poklepovic, A.; Moyneur, E.; Barghout, V. Population-based risk factors and resource utilization for HCC: US perspective. Curr. Med. Res. Opin. 2010, 26, 2183–2191. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.R.; Njei, B.; Nguyen, M.H.; Nguyen, A.; Lim, J.K. Survival after treatment with curative intent for hepatocellular carcinoma among patients with vs. without non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2017, 46, 1061–1069. [Google Scholar] [CrossRef]
- Bruix, J.; Raoul, J.-L.; Sherman, M.; Mazzaferro, V.; Bolondi, L.; Craxi, A.; Galle, P.R.; Santoro, A.; Beaugrand, M.; Sangiovanni, A.; et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: Subanalyses of a phase III trial. J. Hepatol. 2012, 57, 821–829. [Google Scholar] [CrossRef]
- Howell, J.; Samani, A.; Mannan, B.; Hajiev, S.; Aval, L.M.; Abdelmalak, R.; Tam, V.C.; Bettinger, D.; Thimme, R.; Taddei, T.H.; et al. Impact of NAFLD on clinical outcomes in hepatocellular carcinoma treated with sorafenib: An international cohort study. Ther. Adv. Gastroenterol. 2022, 15, 17562848221100106. [Google Scholar] [CrossRef]
- Hiraoka, A.; Kumada, T.; Tada, T.; Tani, J.; Kariyama, K.; Fukunishi, S.; Atsukawa, M.; Hirooka, M.; Tsuji, K.; Ishikawa, T.; et al. Efficacy of lenvatinib for unresectable hepatocellular carcinoma based on background liver disease etiology: Multi-center retrospective study. Sci. Rep. 2021, 11, 16663. [Google Scholar] [CrossRef] [PubMed]
- Haber, P.K.; Puigvehí, M.; Castet, F.; Lourdusamy, V.; Montal, R.; Tabrizian, P.; Buckstein, M.; Kim, E.; Villanueva, A.; Schwartz, M.; et al. Evidence-Based Management of Hepatocellular Carcinoma: Systematic Review and Meta-analysis of Randomized Controlled Trials (2002–2020). Gastroenterology 2021, 161, 879–898. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Sanduzzi-Zamparelli, M. Nivolumab and sorafenib in hepatocellular carcinoma: Lessons from the CheckMate 459 study. Lancet Oncol. 2022, 23, 4–6. [Google Scholar] [CrossRef]
- Yau, T.; Kang, Y.K.; Kim, T.Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.M.; Matilla, A.; et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol 2020, 6, e204564. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, B.; Brown, Z.J.; Diggs, L.P.; Vormehr, M.; Ma, C.; Subramanyam, V.; Rosato, U.; Ruf, B.; Walz, J.S.; McVey, J.C.; et al. Steatohepatitis Impairs T-cell–Directed Immunotherapies Against Liver Tumors in Mice. Gastroenterology 2021, 160, 331–345.e6. [Google Scholar] [CrossRef] [PubMed]
- Copil, F.; Campani, C.; Lequoy, M.; Sultanik, P.; Blaise, L.; Wagner, M.; Ganne-Carrié, N.; Ozenne, V.; Thabut, D.; Nault, J.; et al. No correlation between MASLD and poor outcome of Atezolizumab-Bevacizumab therapy in patients with advanced HCC. Liver Int. 2024, 44, 931–943. [Google Scholar] [CrossRef]
- Espinoza, M.; Muquith, M.; Lim, M.; Zhu, H.; Singal, A.G.; Hsiehchen, D. Disease Etiology and Outcomes After Atezolizumab Plus Bevacizumab in Hepatocellular Carcinoma: Post-Hoc Analysis of IMbrave150. Gastroenterology 2023, 165, 286–288.e4. [Google Scholar] [CrossRef]
- Agarwal, P.D.; Lucey, M.R.; Said, A.; Kratz, J. Immunotherapy for HCC: Limitations in patients with NASH. Ann. Hepatol. 2023, 28, 100886. [Google Scholar] [CrossRef]
- Pinter, M.; Pinato, D.J.; Ramadori, P.; Heikenwalder, M. NASH and Hepatocellular Carcinoma: Immunology and Immunotherapy. Clin. Cancer Res. 2023, 29, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Lau, G.; Kudo, M.; Chan, S.L.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.-K.; Van Dao, T.; De Toni, E.N.; et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Évid. 2022, 1, EVIDoa2100070. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Chan, S.L.; Gu, S.; Bai, Y.; Ren, Z.; Lin, X.; Chen, Z.; Jia, W.; Jin, Y.; Guo, Y.; et al. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): A randomised, open-label, international phase 3 study. Lancet 2023, 402, 1133–1146. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Ryoo, B.Y.; Hsu, C.H.; Numata, K.; Stein, S.; Verret, W.; Hack, S.P.; Spahn, J.; Liu, B.; Abdullah, H.; et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): An open-label, multicentre, phase 1b study. Lancet Oncol. 2020, 21, 808–820. [Google Scholar] [CrossRef]
- Bourhis, M.; Palle, J.; Galy-Fauroux, I.; Terme, M. Direct and Indirect Modulation of T Cells by VEGF-A Counteracted by Anti-Angiogenic Treatment. Front. Immunol. 2021, 12, 616837. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.G.; Jang, M.; Kim, Y.; Leem, G.; Kim, K.H.; Lee, H.; Kim, T.-S.; Choi, S.J.; Kim, H.-D.; Han, J.W.; et al. VEGF-A drives TOX-dependent T cell exhaustion in anti–PD-1–resistant microsatellite stable colorectal cancers. Sci. Immunol. 2019, 4, eaay0555. [Google Scholar] [CrossRef]
- Liu, F.; Liu, Y.; Chen, Z. Tim-3 expression and its role in hepatocellular carcinoma. J. Hematol. Oncol. 2018, 11, 126. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, M.; Yuan, F.; Qi, F.; Sun, J.; Rao, Q.; Zhao, Z.; Huang, P.; Fang, T.; Yang, B.; Xia, J. Expression and clinical significance of LAG-3, FGL1, PD-L1 and CD8+T cells in hepatocellular carcinoma using multiplex quantitative analysis. J. Transl. Med. 2020, 18, 306. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sauer, N.; Szlasa, W.; Jonderko, L.; Oślizło, M.; Kunachowicz, D.; Kulbacka, J.; Karłowicz-Bodalska, K. LAG-3 as a Potent Target for Novel Anticancer Therapies of a Wide Range of Tumors. Int. J. Mol. Sci. 2022, 23, 9958. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheung, C.C.L.; Seah, Y.H.J.; Fang, J.; Orpilla, N.H.C.; Lau, M.C.; Lim, C.J.; Lim, X.; Lee, J.N.L.W.; Lim, J.C.T.; Lim, S.; et al. Immunohistochemical scoring of LAG-3 in conjunction with CD8 in the tumor microenvironment predicts response to immunotherapy in hepatocellular carcinoma. Front. Immunol. 2023, 14, 1150985. Available online: https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2023.1150985 (accessed on 1 January 2025). [CrossRef]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A.; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef]
- Yamaoka, K.; Saitoh, S.; Kinowaki, K.; Fujiyama, S.; Kawamura, Y.; Sezaki, H.; Hosaka, T.; Akuta, N.; Kobayashi, M.; Suzuki, F.; et al. Clinicopathological assessment of steatohepatitic hepatocellular carcinoma. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101799. [Google Scholar] [CrossRef] [PubMed]
- Shibahara, J.; Ando, S.; Sakamoto, Y.; Kokudo, N.; Fukayama, M. Hepatocellular carcinoma with steatohepatitic features: A clinicopathological study of Japanese patients. Histopathology 2014, 64, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Olofson, A.M.; Gonzalo, D.H.; Chang, M.; Liu, X. Steatohepatitic Variant of Hepatocellular Carcinoma: A Focused Review. Gastroenterol. Res. 2018, 11, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Salomao, M.; Remotti, H.; Vaughan, R.; Siegel, A.B.; Lefkowitch, J.H.; Moreira, R.K. The steatohepatitic variant of hepatocellular carcinoma and its association with underlying steatohepatitis. Hum. Pathol. 2012, 43, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Salomao, M.; Yu, W.M.; Brown, R.S., Jr.; Emond, J.C.; Lefkowitch, J.H. Steatohepatitic hepatocellular carcinoma (SH-HCC): A distinctive histological variant of HCC in hepatitis C virus-related cirrhosis with associated NAFLD/NASH. Am. J. Surg. Pathol. 2010, 34, 1630–1636. [Google Scholar] [CrossRef] [PubMed]
- Inui, S.; Kondo, H.; Tanahashi, Y.; Fukukura, Y.; Sano, K.; Morisaka, H.; Saito, K.; Kondo, F.; Fukusato, T.; Furui, S.; et al. Steatohepatitic hepatocellular carcinoma: Imaging findings with clinicopathological correlation. Clin. Radiol. 2021, 76, 160.e15–160.e25. [Google Scholar] [CrossRef] [PubMed]
- Cannella, R.; Burgio, M.D.; Beaufrère, A.; Trapani, L.; Paradis, V.; Hobeika, C.; Cauchy, F.; Bouattour, M.; Vilgrain, V.; Sartoris, R.; et al. Imaging features of histological subtypes of hepatocellular carcinoma: Implication for LI-RADS. JHEP Rep. 2021, 3, 100380. [Google Scholar] [CrossRef] [PubMed]
- Low, H.M.; Choi, J.Y.; Tan, C.H. Pathological variants of hepatocellular carcinoma on MRI: Emphasis on histopathologic correlation. Abdom. Imaging 2019, 44, 493–508. [Google Scholar] [CrossRef]
- International Consensus Group for Hepatocellular Neoplasia. Pathologic diagnosis of early hepatocellular carcinoma: A report of the international consensus group for hepatocellular neoplasia. Hepatology 2009, 49, 658–664. [Google Scholar] [CrossRef]
- Campos-Correia, D.; Cruz, J.; Matos, A.P.; Figueiredo, F.; Ramalho, M. Magnetic resonance imaging ancillary features used in Liver Imaging Reporting and Data System: An illustrative review. World J. Radiol. 2018, 10, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Kutami, R.; Nakashima, Y.; Nakashima, O.; Shiota, K.; Kojiro, M. Pathomorphologic study on the mechanism of fatty change in small hepatocellular carcinoma of humans. J. Hepatol. 2000, 33, 282–289. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).