Abstract
Background: Osseointegrated implants are essential for rehabilitating edentulous patients, but critical bone defects remain challenging. Guided bone regeneration (GBR) with barrier membranes is an effective approach. This study evaluated a 3D printed membrane made from acrylated epoxidized soybean oil (AESO) combined with a xenogeneic graft for GBR in critical-size defects. Methods: Forty-eight male Sprague Dawley rats (150 g) were assigned to four groups: a negative control group (NC, blood clot only), a positive control group (PC, biomaterial without membrane), a negative test group (NT, blood clot with membrane), and a positive test group (PT, biomaterial with membrane). Results: The PT group showed the highest bone volume and superior bone maturation compared to the other groups. Bone quality parameters (Tb.N, Tb.Th) indicated enhanced maturation in the groups using the membrane. A histological analysis confirmed centripetal bone formation. Conclusion: The AESO-based membrane provided mechanical support and controlled resorption, addressing collagen membrane limitations. Its combination with the GTO® graft material enhanced osteoconduction, bone formation, and bone quality, highlighting its potential for complex bone defect reconstructions.
1. Introduction
Osseointegrated implants are vital for rehabilitating lost teeth in edentulous patients [1]. The alveolar process is influenced by factors that lead to bone loss from congenital absence, infections, trauma, or periodontitis. Tooth extraction is a major cause of this [2].
Critical-size bone defects, which do not heal without intervention, cause structural damage and dysfunction, posing a challenge in maxillofacial surgery [3,4]. Ensuring adequate bone volume at the recipient site is crucial for implant success. Guided bone regeneration (GBR) is a widely used technique that excludes non-osteogenic tissues using barrier membranes, promoting osteogenic cell colonization [5,6,7].
Various materials for resorbable and non-resorbable membranes have been developed for clinical use [8]. Collagen-based resorbable membranes are common in horizontal bone augmentation due to their biological properties [9]. Vertical augmentation benefits from titanium-reinforced polytetrafluoroethylene membranes, requiring soft tissue management and a second intervention for material removal [10,11].
A membrane combining the properties of both would expand the clinical applications, reducing discomfort and treatment time. Additive manufacturing plays a significant role in 3D printing polymers [12], using resins with photo-initiators [13]. However, most commercial resins lack biocompatibility and bioabsorbability [14]. Bioplastics from natural materials offer light-curable, biodegradable resins, allowing for the full elimination of material and byproducts, removing the need for second interventions in GBR [15].
Vegetable oils are a promising source for bioplastic production, consisting of a mixture of unsaturated and saturated fatty acids and triglycerides, which contain reactive sites suitable for functionalization, enabling UV-induced polymerization and the processing of bioresins using 3D printing techniques [16,17]. Among these oils, acrylated epoxidized soybean oil (AESO) has been highlighted as a photocurable liquid resin, utilizing its acrylate groups for easy polymerization via free radical reactions. However, due to its high viscosity, AESO cannot be printed alone using SLA-DLP printers [18]. Many studies report combining AESO with polyethylene glycol [19] or polyethylene glycol diacrylate [20], resulting in biocompatible structures with high cell adhesion and proliferation [21].
The feasibility of this study is anchored in the use of animal models, specifically, rat calvaria, which are widely recognized as the gold standard for evaluating bone regeneration in induced critical-size defects [22]. These models allow for the creation of controlled and reproducible defects, facilitating the comparison of different biomaterials and bone regeneration techniques. Moreover, the bone physiology of rat calvaria shares similarities with that of humans, particularly in terms of bone healing behavior, making them ideal for the preclinical testing of novel materials [23].
To the best of our knowledge, this study represents the first comprehensive exploration of a 3D printed customized membrane designed for controlled-resorption structural maintenance and bone formation in a rat calvaria defect model. Therefore, the aim of this study is to evaluate the osteogenic potential, structural integrity, and biodegradability of a novel soybean oil-based 3D printed resin membrane in guided bone regeneration. Specifically, the study seeks to analyze bone volume gain, membrane degradation potential, and histological response in a critical-size calvarial defect model in rats, testing the null hypothesis that the soybean oil-based 3D printed resin membrane does not promote significant differences in bone regeneration compared to the control conditions.
2. Material and Methods
2.1. Ethical Statements
The protocol for the present study was approved by the Ethics Committee of the Faculty of dentistry of Ribeirao Preto at the University of São Paulo-CEUA (protocol no. 2023.1.416.58.9 approved on 27 September 2023) following the Brazilian Council Control for Animal Experimentations (CONCEA). The study was conducted in accordance with the ARRIVE guidelines.
2.2. Sample Size
The sample size calculation was performed using the G*Power v3.1.9.7 software, considering α = 0.05, test power = 0.95, and correlation between measurements = 0.5, similarly to previous studies where critical bone defects of 7 mm were created in rat calvaria and filled with different biomaterials [24]. Considering multiple comparison corrections and the possibility of complications or animal deaths, the sample size for each group was n = 6, totaling 48 animals.
2.3. Study Design
Forty-eight adult male Sprague Dawley rats, with an average weight of 150 g, were used in the experimental procedures. The animals were provided by the Central Animal Facility of the University of São Paulo, where they were housed in shared cages, with a maximum of three animals per cage, in a climate-controlled environment. They received a controlled diet and water ad libitum throughout the experiment. Following a previously published methodology [24], the animals were divided into four groups of twelve animals each (n = 12), based on the treatment administered, with euthanasia scheduled at 4 (n = 6) and 8 (n = 6) weeks (Figure 1), as follows:
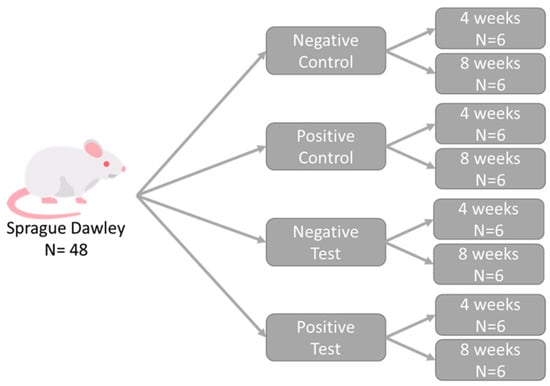
Figure 1.
Schematic representation of the study design. Each group, composed of randomly selected animals, received one type of treatment.
- Negative control group (NC): critical bone defects filled only with blood clot.
- Positive control group (PC): critical bone defects filled with a biomaterial (OsteoBiol GTO®-Tecnoss, Giaveno, Italy) and not covered by a membrane.
- Negative test group (NT): critical bone defects filled only with blood clot and covered by a printed membrane.
- Positive test group (PT): critical bone defects filled with a biomaterial (OsteoBiol GTO®-Tecnoss, Giaveno, Italy) and covered by a printed membrane.
2.4. Randomizations and Allocation Concealment
The randomization of the proposed groups was performed electronically (https://www.randomizer.org) by an author (SPX) not involved in the selection, handling, or surgical procedures. Group allocation was ensured using sealed, opaque, coded envelopes and was revealed to the surgeon (not involved in the study randomization) immediately after the preparation of the critical-size bone defect.
2.5. Printed-Membrane Characteristics
The membrane developed for this study was 3D printed using a photocurable resin composed of natural acrylated epoxidized soybean oil (AESO, Sigma Aldrich, Burlington, MA, USA) blended in the weight ratio of 50:50 with a synthetic biocompatible polymer, namely, polyethylene glycol diacrylate (PEGDA, Mn 700, Sigma Aldrich). After mechanical mixing, 1% by weight of phenylbis (2,4,6-trimethylbenzoyl) phosphine oxide (BAPO Sigma Aldrich) was dissolved in acetone (Sigma Aldrich) at the concentration of 0.1 g/mL and poured into the AESO-PEGDA resin as a radical photo-initiator [18]. The mixture was placed in the dark, under magnetic stirring at RT for 24 h to let the solvent evaporate. Spherical caps having a chord (D) of 10 mm, a thickness of 1 mm, and a height (H) of 3 mm were fabricated using a commercial DLP 3D printer (Elegoo Mars 3, Elegoo inc., Shenzhen, China) working with a wavelength of 405 nm and equipped with a building box of 143 × 90 × 175 mm3. The caps were printed without pores, forming a solid dome-shaped structure designed to fully cover the critical-size bone defect (Figure 2). After 3D printing, the samples were collected with a spatula and washed in an isopropyl alcohol (propan-2-ol, Carlo Erba, Cornaredo, Italy) bath to remove the uncured resin. The samples were post-cured by exposing them to a UV lamp (λ = 405 nm) for 60 min. The samples were immersed in distilled water (dH2O) for 24 h, washed three times in dH2O, and immersed again in sterile dH2O [18].
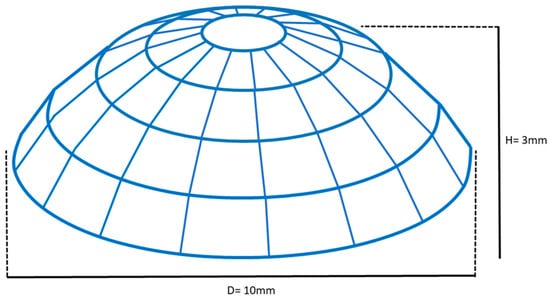
Figure 2.
Schematic drawing of the membrane printed from AESO with dimensions of 1 mm thickness, 10 mm chord, and 3 mm height.
2.6. Biomaterial Characteristics
OsteoBiol GTO® (Tecnoss, Giaveno, Italy) was used for filling the critical-size bone defect in the PC and PT groups. It consists of a mixture of 80% pre-hydrated porcine-derived granules ranging from 600 to 1000 µm and 20% collagen gel. It is composed of type I and III collagen, polyunsaturated fatty acids, and a biocompatible synthetic copolymer diluted in an aqueous solution, making it easily adaptable to the defect site.
2.7. Anesthesia and Medication Procedures
The animals were anesthetized with 10% ketamine hydrochloride (75 mg/kg, intraperitoneal; Agener União, São Paulo, Brazil) and 2% xylazine hydrochloride (6 mg/kg, intraperitoneal; Calier, Juatuba, Minas Gerais, Brazil). All animals received a pre-operative dose of 2% meloxicam (Maxicam 2%, Ourofino, São Paulo, Brazil) and a single dose of an antibiotic solution (1 mL/kg, intraperitoneal; Pentabiotic, Zoetis, São Paulo, Brazil).
2.8. Surgical Procedures
The surgical area was pre-trimmed, and antisepsis of the region was performed by the topical application of a 1% povidone–iodine solution (Riodeíne Tintura, Rioquímica, São José do Rio Preto, São Paulo, Brazil). During the anesthesia period, the animals’ vital signs were monitored, their eyes were lubricated with saline solution, and they were kept covered with wood shavings to prevent hypothermia.
All surgical procedures were performed by a single skilled and experienced surgeon. In each animal, a 15 mm incision was made along the midline of the skull, and the tissue flap was elevated to expose the periosteum. A critical-size bone defect measuring 7 mm in diameter was then created using a trephine bur (Neodent, Paraná, Brazil), following a previously published methodology. Only at this point was the group allocation revealed to the operator. Each experimental group received the proposed treatment. All groups had their sutures performed with a nylon thread (Ethilon 4–0®, Ethicon, São Paulo, São Paulo, Brazil) (Figure 3).
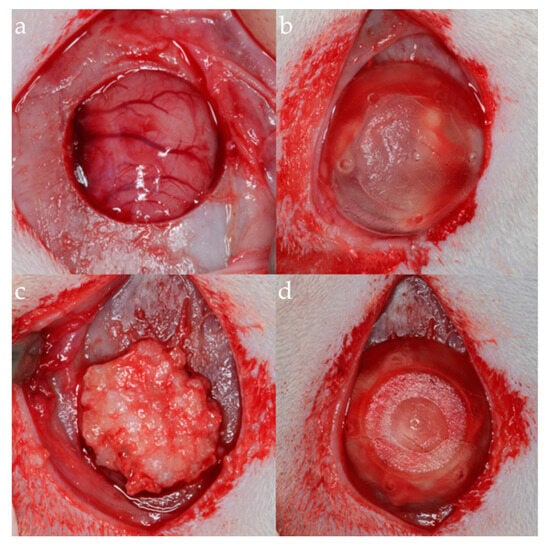
Figure 3.
Surgical photographs of the experimental groups. (a) Negative control group. (b) Positive control group. (c) Negative test group. (d) Positive test group.
2.9. Animal Maintenance
Twenty-four hours post-surgery, the animals received a postoperative dose of 2% meloxicam (Maxicam 2%, Ourofino, São Paulo, Brazil). The rats were housed in shared cages containing a maximum of three animals, lined with wood shavings. The room where they were kept had a split air conditioning system (25 °C) without humidity control and with 15 to 25 air exchanges per hour, and automatic lighting control on a 12/12 h cycle. The animals had ad libitum access to filtered food and water, and the following sanitary barriers were available: autoclave, restrooms, changing rooms, and insect control screens.
Throughout the experiment, the animals were observed daily for biological functions, wound monitoring, and signs of pain and/or infection in the surgical wound area, and appropriate medications were administered as necessary.
2.10. Euthanasia
The animals were anesthetized with 10% ketamine hydrochloride (75 mg/kg, intraperitoneal; Agener União, São Paulo, Brazil) and 2% xylazine hydrochloride (6 mg/kg, intraperitoneal; Calier, Juatuba, Minas Gerais, Brazil) and then placed in a CO2 chamber with a controlled flow of 7 L/min, approximately 20% of the total volume. This flow was maintained for at least 1 min after confirming the clinical death of the animal, checking for signs of respiratory arrest and mucosal cyanosis. Subsequently, biopsies from the surgical sites were collected at 4 and 8 weeks postoperatively (n = 48) and immediately submerged in a 10% paraformaldehyde solution.
2.11. Microtomographic Processing
After fixation, the biopsies underwent microtomographic scanning using the SkyScan 1172-160 system (Bruker-SkyScan, Kontich, Belgium). The following parameters were applied for both two-dimensional tomographic projections and three-dimensional reconstructions: isotropic pixel size of 9.92 µm, voltage of 60 kV, current of 165 µA, aluminum filter diameter of 0.5 mm, exposure time of 596 ms, rotation step of 0.4°, frame averaging of 4, and random movement set to 10.
The projections were reconstructed using the NRecon® 1.6.10 software (Bruker-SkyScan; Kontich, Belgium), and the images were positioned using the DataViewer® 1.5.4.6 software (Bruker-SkyScan, Kontich, Belgium), selecting the axis of interest for the complete visualization of the experimental area.
2.12. Microtomographic Evaluation
After reconstruction, a morphometric analysis was performed using the CT Analyzer software (CTAn, v1.15.4.0, Bruker, Kontich, Antwerp, Belgium) to assess bone volume (BV), new bone formation (NBF-BV/TV), residual graft (RG-BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), and trabecular spacing (Tb.Sp). To ensure accurate data acquisition, distinct threshold ranges were applied for different parameters: a threshold range of 70–100 was utilized to evaluate the newly formed bone, while a range of 100–255 was employed for residual graft assessment.
In CTAn, a task list was created for the analysis of the parameters mentioned above. This process involves the determination of the region of interest (ROI). The collective sum of all ROIs in a contiguous set of cross-sectional images was used to determine the volume of interest (VOI), representing the selected 3D volume.
All micro-CT analyses were performed by a single trained examiner, following calibration with another professional until the agreement between the evaluators in tissue recognition reached K > 0.9, and the examiner was blinded to the experimental groups.
2.13. Histological Processing
After micro-CT processing, the specimens were dehydrated in a series of ascending alcohol concentrations (50–100%) over 6 days, followed by successive immersion in resin solutions (LR White™ HardGrid (London Resin Co Ltd., Berkshire, UK)) with ascending concentration (50–100%). During the resin polymerization, the specimens were kept in an oven at 60 °C for 24 h. Once polymerization was complete, each block was sectioned along a sagittal plane. Sections of approximately 100–150 µm were prepared using a precision cutting/grinding system (Exakt, Apparatebau, Norderstedt, Germany) and ground to obtain slides with a thickness of approximately 50–60 µm. The histological sections were stained with two different stains: toluidine blue and Stevenel’s blue with alizarin red.
2.14. Histological Evaluation
The histologist performed the calibration of the examiner responsible for the histological analyses. An Eclipse Ci optical microscope (Nikon Corporation, Tokyo, Japan) equipped with a digital camera (Digital Sight DS-2Mv, Nikon Corporation, Tokyo, Japan), connected to a computer, was used to capture photomicrographs. The presence of new mineralized bone, remaining xenograft, connective tissue, inflammatory cells, osteoclasts, blood vessels, and bioresin membrane remnants was qualitatively evaluated in 16× and 100× photomicrographs to assess the bone formation pattern near the defect margin.
2.15. Statistical Analysis
New bone formation in the microtomographic analysis was considered the primary variable. Differences between the four treatment groups at both experimental time points were evaluated using one-way analysis of variance (ANOVA). The Shapiro–Wilk test was initially applied to assess the normality of the data for each group and time point. If normality was confirmed, parametric tests were used; otherwise, non-parametric tests were performed. Post-hoc pairwise comparisons were conducted using Tukey’s test for normally distributed data or Dunn’s test for non-normally distributed data, to identify significant differences between groups. Additionally, a Pearson correlation test was performed to assess the relationship between bone volume and new bone formation. Statistical analyses were performed using GraphPad Prism software (v10.0.0 for Windows, GraphPad Software, San Diego, CA, USA, https://www.graphpad.com/ accessed on 1 August 2024). The significance level was set at α = 0.05.
3. Results
All animals included in the experiment recovered from surgery, and no adverse events were observed.
3.1. Micro-CT Results
The three-dimensional reconstructions obtained by micro-CT showed bone formation starting from the edges of the critical-size bone defect towards the center in all groups. Additionally, they revealed bone formation involving the granules of the xenogeneic graft used in the positive control group (PC) and the positive test group (PT), as shown in Figure 4 and Figure 5.
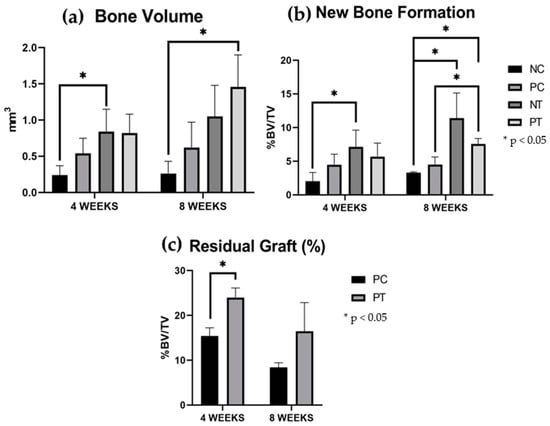
Figure 4.
Micro-CT results. The results are expressed as mean and standard deviation. (a) Bone volume (BV-mm3). (b) New bone formation (%BV/TV, threshold, 70–100). (c) Residual graft (%BV/TV, threshold, 100–255). Vertical bars indicate standard deviation. Horizontal bars indicate statistical differences with values of * p < 0.05.
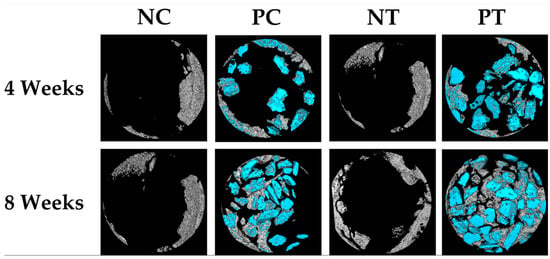
Figure 5.
Three-dimensional reconstructed micro-CT images of negative control group (NC), positive control group (PC), negative test group (NT), and positive test group (PT) samples at 4 weeks and 8 weeks post-surgery. The gray color represents newly formed bone, and the blue color represents the remaining graft granules. These images were obtained using CT Analyzer software.
After 4 weeks, the NT and PT groups exhibited greater bone volume (BV) and new bone formation (NBF) compared to the NC group. Statistically significant differences were observed between the NT and the NC groups. The residual xenogeneic bone graft (RG) was larger in the PT group compared to the PC group, with a significant difference between these groups.
After 8 weeks, the PT group showed the largest BV and NBF among all groups. Significant differences were found between the PT group and the NC and PC groups. The NT group also showed greater BV and NBF compared to the NC group. A positive correlation was observed between bone volume (0.81) and new bone formation (0.80) in the groups that used a membrane (NT and PT). The RG percentages decreased over time but showed no significant differences between groups or time points. The quantitative data are summarized in Table 1 and Figure 4.

Table 1.
Micro-CT results of the NC, PC, NT, and PT groups at 4 and 8 weeks of repair. Bone volume (BV) values. Percentage of newly formed bone (NBF). Percentage of residual graft (RG).
The quality of the newly formed bone was assessed by evaluating three parameters: trabecular number (Tb.N), trabecular separation (Tb.Sp), and trabecular thickness (Tb.Th). The measured values for each parameter are presented in Table 2 and illustrated in Figure 6, Figure 7 and Figure 8. The results are reported as the mean ± standard deviation.

Table 2.
Micro-CT results of the NC, PC, NT, and PT groups at 4 and 8 weeks of repair, about new bone formation. Results about Trabecular number (Tb.N), trabecular separation (Tb.Sp), trabecular thickness (Tb.Th).
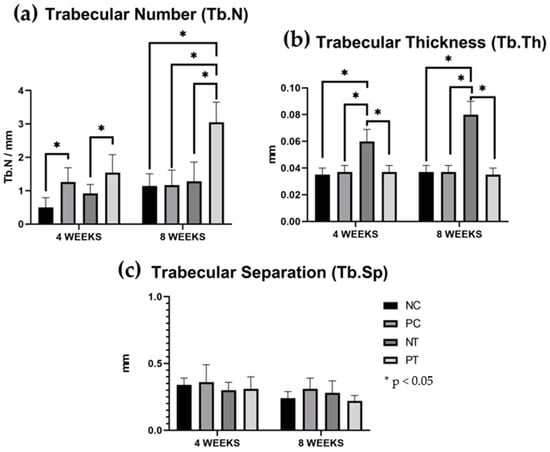
Figure 6.
Quality of the newly formed bone. The results are expressed as mean and standard deviation. (a) Trabecular number (Tb.N). (b) Trabecular thickness (Tb.Th). (c) Trabecular separation (Tb.Sp). Vertical bars indicate standard deviation. Horizontal bars indicate statistical differences with values of * p < 0.05.
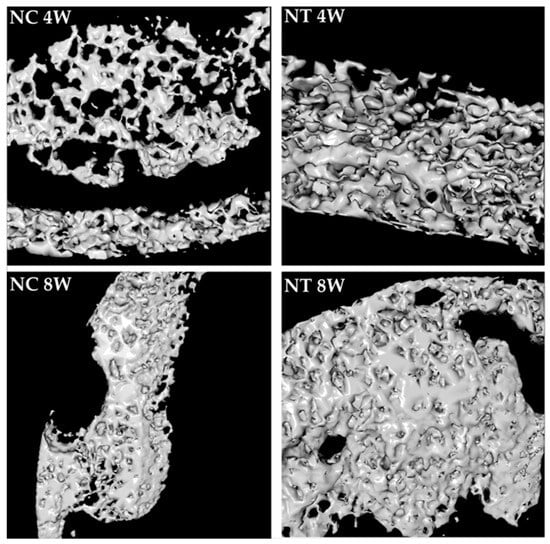
Figure 7.
High-magnification 3D reconstructions of bone defect repair in the negative control group (NC) and negative test group (NT) at 4 and 8 weeks, showing newly formed bone. These images were obtained using CT Analyzer software.
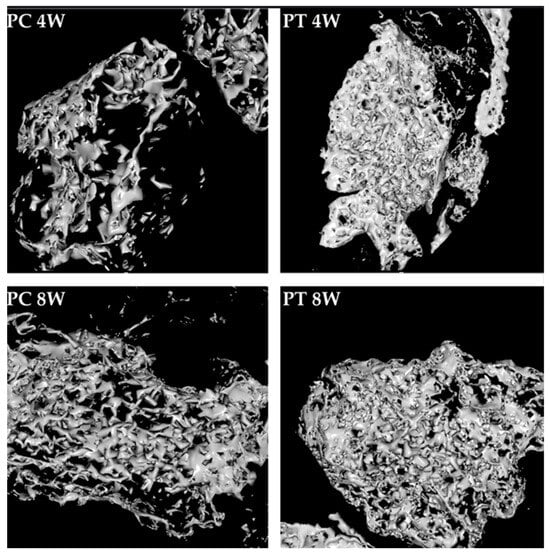
Figure 8.
High-magnification 3D reconstructions of bone defect repair in the positive control group (PC) and positive test group (PT) at 4 and 8 weeks, showing newly formed bone. These images were obtained using CT Analyzer software.
The positive test group demonstrated higher trabecular number (Tb.N) values at both evaluation periods, with 1.54 ± 0.54 at 4 weeks and 3.05 ± 0.60 at 8 weeks, showing a statistically significant difference compared to the negative control and negative test groups in 4 weeks and a statistically difference compared to the other groups in 8 weeks. (p < 0.05). Regarding trabecular thickness (Tb.Th), the negative test group exhibited higher values, with 0.0675 ± 0.0096 at 4 weeks and 0.0875 ± 0.0126 at 8 weeks, also displaying a statistically significant difference compared to the other groups (p > 0.05).
3.2. Histological Observation
The four experimental groups shared a common pattern of new bone formation, observed primarily at the edges of the critical-size bone defect. In all groups, connective tissue rich in fibroblasts and oriented collagen fibers was noted connecting the defect margins. The photomicrographs revealed the presence of bone bridges near the defect edges in some cases, particularly after 8 weeks. This centripetal progression of bone formation was consistent across all groups, as shown in the Figure 9.
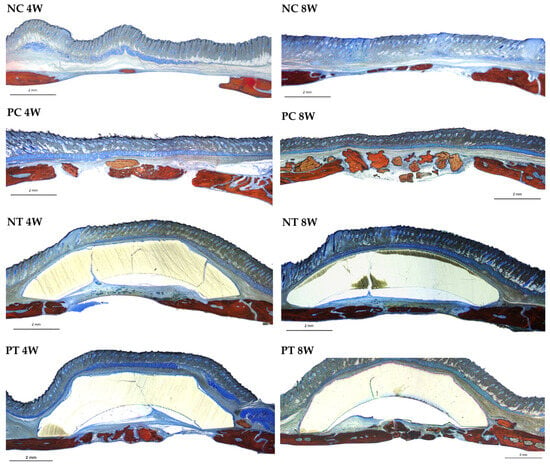
Figure 9.
Photomicrographs at 4 and 8 weeks of repair at 16x magnification. NC (negative control group). PC (positive control group). NT (negative test group). PT (positive test group). Alizarin red and Stevenel’s blue.
The negative control group exhibited bone formation primarily at the edges of the defect. Bone bridges were observed in some sections at 8 weeks. The negative test group similarly showed bone formation confined to the defect margins, with connective tissue connecting the edges. In the negative test group, the membrane remained intact throughout the evaluation periods, with no signs of resorption noted in the histological sections, as shown in the Figure 10.
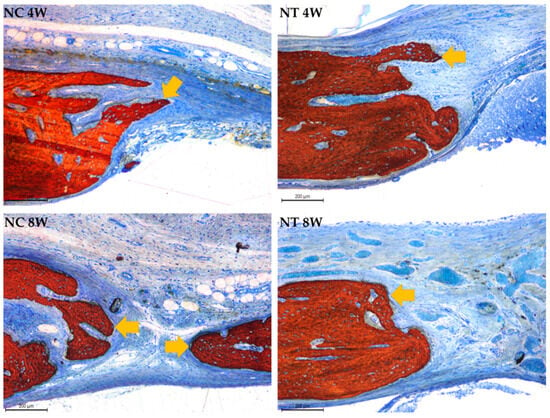
Figure 10.
Photomicrographs at 100× magnification illustrating bone formation originating from the walls of the critical-size bone defect, indicated by yellow arrows, in the negative control (NC) and negative test (NT) groups at 4 and 8 weeks. Stained with Stevenel’s blue and alizarin red.
In the positive control group, newly formed bone surrounded the remaining xenograft granules at both time points, with a reduction in the number of particles observed after 8 weeks. The particulate graft covered the entire defect surface at 4 weeks and was associated with connective tissue containing inflammatory cells. The positive test group also displayed graft particles across the defect, with fewer remaining at 8 weeks. The connective tissue in this group was rich in fibroblasts, and the membrane used remained intact during the observation period, though some photomicrographs suggested possible membrane displacement, as shown in the Figure 11.
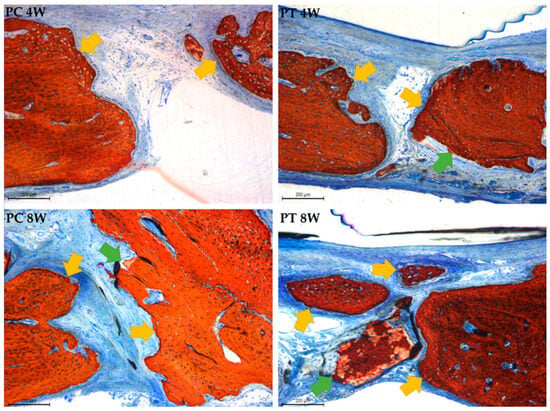
Figure 11.
Photomicrographs at 100× magnification illustrating bone formation originating from the walls of the critical-size bone defect, indicated by yellow arrows, and bone formation over the graft granules, represented by green arrows, in the positive control (PC) and positive test (PT) groups at 4 and 8 weeks. Stevenel’s blue and alizarin red staining.
4. Discussion
This study aimed to evaluate the osteogenic potential, structural integrity, and biodegradability of a novel 3D printed resin membrane based on soybean oil for guided bone regeneration (GBR). We emphasize the critical role of adequate bone volume in the long-term survival of implants and the necessity of an approach that combines the advantages of both resorbable and non-resorbable membranes in GBR procedures to meet these demands.
Regarding the null hypothesis of this study, which postulated that the 3D printed soybean oil-based resin membrane would not promote significant differences in bone regeneration compared to the control conditions, the results indicate that the null hypothesis was rejected. The use of the 3D membrane demonstrated a significant increase in bone volume and superior bone formation outcomes compared to the control groups. Furthermore, the histological analysis revealed a favorable regenerative response, corroborating the osteogenic potential of the membrane and validating its applicability in GBR procedures.
Different types of membranes are employed to address the specific needs of bone defects, whether vertical or horizontal [25,26]. To assess the efficacy of these materials and techniques, preclinical experimental models are essential, particularly critical-size defects in the calvaria of animals [27]. These defects have minimum dimensions that prevent spontaneous bone regeneration throughout the animal’s lifetime [28]. Although the exact size remains debated, studies suggest diameters ranging from 4 mm to 8 mm [29]. In this study, a 7 mm defect was chosen based on previous research within this reference range [24].
In GBR, the use of bone substitute materials, such as autogenous, allogeneic, and xenogeneic grafts, is fundamental in creating a matrix that supports cellular colonization and mesenchymal cell differentiation into osteoblasts, thereby promoting bone formation [30]. Xenogeneic grafts are biocompatible and possess osteoconductive properties. Among them, GTO, the material selected for this study, stands out due to these characteristics and its slow resorption rate, as well as its angiogenic and osteogenic potential demonstrated by Jeanneau et al. [28]. In the present study, a comparative analysis between the NC and the PC groups revealed greater bone volume in the PC group, which received the graft, both at 4 weeks (0.54 ± 0.21 vs. 0.24 ± 0.13) and 8 weeks (0.62 ± 0.35 vs. 0.46 ± 0.17), demonstrating the material’s efficacy in bone regeneration.
Favorable clinical outcomes can be achieved when bone grafts are associated with membranes (barriers) to cover the bone defect [30]. Mechanical separation of the treated area prevents the invasion of undesirable tissues, thereby enhancing regeneration. In the present study, a comparison between the PC and the PT groups showed that using the AESO-based membrane resulted in increased bone volume and new bone formation at 4 weeks (4.48% ± 1.56% vs. 5.68% ± 2.02%) and 8 weeks (4.51% ± 1.11% vs. 7.58% ± 0.79%), demonstrating its efficacy in maintaining the graft.
The combination of GTO® with the AESO-based membrane provided a favorable level of osteoconductivity and supported bone formation. In all evaluated groups, both microtomography and histology revealed a trend of increasing new bone formation between 4 and 8 weeks, accompanied by a decrease in residual graft material, indicating its resorption potential.
The histological images of the AESO-based membrane, compared to traditional collagen membranes, revealed significant advantages, particularly in terms of resorption rate. Experimental studies have shown that lyophilized porcine collagen membranes from various brands undergo rapid resorption within 30 days [29], which may compromise their stability and limit their ability to maintain the necessary space for bone regeneration during the healing process [31]. Although collagen membranes offer bioactive advantages, such as low immunogenicity, attraction of reparative cells, and high vascularization, their rapid degradation, particularly in the case of type I collagen membranes, can impair the maintenance of the bone volume required for complete defect filling [29].
In contrast, the 3D printed membrane developed in this study, with controlled and prolonged absorption beyond 8 weeks, as evaluated in photomicrographs, provides durable mechanical support, which is essential for graft stability and predictability in bone regeneration, resembling for these functions non-resorbable barriers such as expanded polytetrafluoroethylene (e-PTFE) [32]. Thus, the customized membrane not only surpasses collagen membranes in terms of structural stability but also offers greater potential for long-term regenerative outcomes, especially in the presence of defects requiring extended remodeling periods, as described by Mir-Mari et al., 2017 [33].
In a comparative study investigating the use of autogenous grafts with or without a non-resorbable rigid membrane [31], the results for the group that used a graft with a non-resorbable membrane were similar to those in this study. Specifically, the reported mean bone formation was 22% ± 7.9% after 45 days, compared to a mean of 5.68% ± 2.02% at 4 weeks and 16.53% ± 6.43% at 8 weeks in this study, following a similar pattern of centripetal bone formation.
The centripetal bone formation observed in this and other studies may be related to the regeneration initiated at the edges of the defect, where there is a greater availability of osteoprogenitor cells and growth factors, which are enhanced by the use of grafts and membranes. This pattern also reflects the vascularization process, which progresses from the periphery to the center of the defect. Additionally, biomaterial resorption directly influences this dynamic, as slow-resorbing materials such as GTO act as osteoconductive scaffolds, facilitating a gradual bone deposition. Although the biomaterial and membrane support bone regeneration, the progression of bone formation still follows a natural biological dynamic influenced by vascularization and cellular recruitment.
Despite the challenges associated with stabilizing dome-shaped membranes, this configuration offers significant benefits for guided bone regeneration (GBR). Previous studies indicate that the three-dimensional curved conformation improves space maintenance, which is essential for bone formation without soft tissue invasion, while potentially distributing mechanical forces more efficiently at the defect site, minimizing the risk of membrane collapse [34]. The lower bone formation observed in our study may be attributed to a key limitation: the difficulty in achieving stable fixation of the AESO-based membrane over the bone defect. Despite its diameter exceeding that of the defect, the membrane exhibited some degree of mobility, which may have impacted the stability of the bone substitute and the preservation of the necessary space for effective tissue regeneration [35].
Nevertheless, the results indicate that the AESO-based membrane has promising potential for clinical applications in maxillofacial and dental surgeries requiring guided bone regeneration (GBR). Future studies could explore the development of a new three-dimensional membrane configuration for cranial defect applications, leveraging the flexibility offered by personalized printing to adapt to the specific needs of each defect.
5. Conclusions
This study suggests the potential of a customized 3D printed AESO-based membrane for GBR in bone defects, offering prolonged mechanical support and controlled resorption, addressing some limitations of traditional collagen membranes. Although bone formation was lower, the membrane, in combination with the GTO® graft material, appeared to promote osteoconduction and progressive bone formation. After eight weeks, bone volume in the PT group was significantly higher (1.46 ± 0.44) compared to the NC group (0.46 ± 0.17). Similarly, the trabecular number was notably higher in the PT group (3.05 ± 0.60) compared to the NC group (1.14 ± 0.37). However, the lack of resorption of the 3D printed membrane may have impaired the natural bone remodeling process, suggesting that optimizing its resorption profile is crucial for improving its effectiveness.
The 3D printing technology may offer a promising approach for developing customized solutions in complex bone defect treatments. Additional research is needed to refine the resorption characteristics of the membrane and assess its long-term efficacy and safety.
Author Contributions
Conceptualization, M.B., F.N. (Francesca Nanni), S.P.X. and E.R.S.; Methodology, E.P.G., L.G.A., S.P.X. and E.R.S.; Formal analysis, L.G.A. and D.B.; Investigation, E.P.G. and L.G.A.; Resources, D.B.; Writing—original draft, D.B., S.P.X. and E.R.S.; Writing—review and editing, D.B., F.N. (Fabrizio Nicoletti) and L.D.; Supervision, S.P.X. and E.R.S.; Funding acquisition, F.N. (Fabrizio Nicoletti), L.D., M.B. and F.N. (Francesca Nanni). All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Italian Ministry of Education, University and Research (MIUR), PRIN 2020, Project 2020F23HZ7, CUP E85F22000230006, Zero Impact MUltifunctional 3D printed composite materials for biomedical and industrial applications in the neXt generation society (ZIMuX).
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee of the Faculty of Dentistry of Ribeirao Preto at the University of São Paulo-CEUA (protocol no. 2023.1.416.58.9 approved on 27 September 2023).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We acknowledge the contribution of Sebastião Bianco (Faculty of Dentistry of Ribeirão Preto, SP, Brazil) for the histological processing and Adriana Luisa Gonçalves de Almeida for the microtomography processing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Urban, I.A.; Monje, A.; Nevins, M.; Lozada, J.L.; Wang, H.L. Surgical management of significant maxillary anterior vertical ridge defect. Int. J. Periodontics Restor. Dent. 2016, 36, 329–337. [Google Scholar] [CrossRef]
- Esposito, M.; Grusovin, M.G.; Kwan, S.; Worthington, H.V.; Coulthard, P. Interventions for replacing missing teeth: Bone augmentation techniques for dental implant treatment. Cochrane Database Syst. Rev. 2008, CD003607. [Google Scholar] [CrossRef]
- Ashour, A.A.; Zaghloul, M.; Mahmoud, W.E.; Helal, M.E.; Grawish, M.E. Gelfoam haemostatic agent with or without autologous bone marrow-derived stem cells for the regeneration of critical-size mandibular defects in the rabbit. Int. J. Oral Maxillofac. Surg. 2018, 47, 1488–1494. [Google Scholar] [CrossRef] [PubMed]
- Monir, A.; Mukaibo, T.; Abd El-Aal, A.B.M.; Nodai, T.; Munemasa, T.; Kondo, Y.; Masaki, C.; El-Shair, M.A.; Matsuo, K.; Hosokawa, R. Local administration of HMGB-1 promotes bone regeneration on the critical-sized mandibular defects in rabbits. Sci. Rep. 2021, 11, 88195. [Google Scholar] [CrossRef] [PubMed]
- Abou Fadel, R.; Samarani, R.; Chakar, C. Guided bone regeneration in calvarial critical size bony defect using a double-layer resorbable collagen membrane covering a xenograft: A histological and histomorphometric study in rats. Oral Maxillofac. Surg. 2018, 22, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, S.; Schwarz, F.; Cordaro, L.; Derks, J.; Hämmerle, C.H.F.; Heitz-Mayfield, L.J.; Hernández-Alfaro, F.; Meijer, H.J.A.; Naenni, N.; Ortiz-Vigón, A.; et al. Regeneration of alveolar ridge defects. Consensus report of group 4 of the 15th European Workshop on Periodontology on Bone Regeneration. J. Clin. Periodontol. 2019, 46 (Suppl. 21), 277–286. [Google Scholar] [CrossRef] [PubMed]
- Naenni, N.; Lim, H.-C.; Papageorgiou, S.N.; Hämmerle, C.H.F. Efficacy of lateral bone augmentation prior to implant placement: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46 (Suppl 21), 287–306. [Google Scholar] [CrossRef] [PubMed]
- Bottino, M.C.; Thomas, V.; Schmidt, G.; Vohra, Y.K.; Chu, T.-M.G.; Kowolik, M.J.; Janowski, G.M. Recent advances in the development of GTR/GBR membranes for periodontal regeneration—A materials perspective. Dent. Mater. 2012, 28, 703–721. [Google Scholar] [CrossRef]
- Tolstunov, L.; Hamrick, J.F.E.; Broumand, V.; Shilo, D.; Rachmiel, A. Bone Augmentation Techniques for Horizontal and Vertical Alveolar Ridge Deficiency in Oral Implantology. Oral Maxillofac. Surg. Clin. N. Am. 2019, 31, 163–191. [Google Scholar] [CrossRef] [PubMed]
- Urban, I.A.; Montero, E.; Monje, A.; Sanz-Sánchez, I. Effectiveness of vertical ridge augmentation interventions: A systematic review and meta-analysis: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46 (Suppl. 21), 319–339. [Google Scholar] [CrossRef]
- Pang, S.; Wu, D.; Gurlo, A.; Kurreck, J.; Hanaor, D.A.H. Additive manufacturing and performance of bioceramic. scaffolds with different hollow strut geometries. Biofabrication 2023, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef]
- Oskui, S.M.; Diamante, G.; Liao, C.; Shi, W.; Gan, J.; Schlenk, D.; Grover, W.H. Assessing and Reducing the Toxicity of 3D-Printed Parts. Environ. Sci. Technol. Lett. 2016, 3, 1. [Google Scholar] [CrossRef]
- Cywar, R.M.; Rorrer, N.A.; Hoyt, C.B.; Beckham, G.T.; Chen, E.Y.-X. Bio-based polymers with performance-advantaged properties. Nat. Rev. Mater. 2022, 7, 83–103. [Google Scholar] [CrossRef]
- Cherubini, V.; Lamastra, F.R.; Bragaglia, M.; Nanni, F. Waste cooking oils as processing aids for eco-sustainable elastomeric compounding. Prog. Rubber Plast. Recycl. Technol. 2022, 38, 3–20. [Google Scholar] [CrossRef]
- Pelletier, H.; Belgacem, N.; Gandini, A. Acrylated vegetable oils as photocrosslinkable materials. J. Appl. Polym. Sci. 2006, 99, 3218. [Google Scholar] [CrossRef]
- Bragaglia, M.; Sciarretta, F.; Filetici, P.; Lettieri-Barbato, D.; Dassatti, L.; Nicoletti, F.; Sibilia, D.; Aquilano, K.; Nanni, F. Soybean Oil-Based 3D Printed Mesh Designed for Guided Bone Regeneration (GBR) in Oral Surgery. Macromol. Biosci. 2024, 24, 2300458. [Google Scholar] [CrossRef]
- Palucci Rosa, R.; Rosace, G.; Arrigo, R.; Malucelli, J. Preparation and Characterization of 3D-Printed Biobased Composites Containing Micro- or Nanocrystalline Cellulose. Polymers 2022, 14, 1886. [Google Scholar] [CrossRef]
- Sibilia, D.; Amendolea, M.; Sangiovanni, R.; Bragaglia, M.; Nicoletti, F.; Filetici, P.; D’Addona, A.; Nanni, F.; Dassatti, L.; Nocca, G. Biodegradation Study of Biomaterials Composed of Acrylated Epoxidized Soybean Oil: An In Vitro Study. Biomed. Res. Int. 2024, 2024, 7100988. [Google Scholar] [CrossRef] [PubMed]
- Mondal, D.; Haghpanah, Z.; Huxman, C.J.; Tanter, S.; Sun, D.; Gorbet, M.; Willett, T.L. mSLA-based 3D printing of acrylated epoxidized soybean oil-nano-hydroxyapatite composites for bone repair. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 130, 112456. [Google Scholar] [CrossRef]
- Liu, Z.; Knetzer, D.A.; Wang, J.; Chu, F.; Lu, C.; Calvert, P.D. 3D printing acrylated epoxidized soybean oil reinforced with functionalized cellulose by UV curing. J. Appl. Polym. Sci. 2022, 139, 51561. [Google Scholar] [CrossRef]
- Trejo-Iriarte, C.G.; Serrano-Bello, J.; Gutiérrez-Escalona, R.; Mercado-Marques, C.; García-Honduvilla, N.; Buján-Varela, J.; Medina, L.A. Evaluation of bone regeneration in a critical size cortical bone defect in rat mandible using microCT and histological analysis. Arch. Oral Biol. 2019, 101, 165–171. [Google Scholar] [CrossRef]
- Vajgel, A.; Mardas, N.; Farias, B.C.; Petri, A.; Cimões, R.; Donos, N. A systematic review in a critical sized defect model. Clin. Oral Implants Res. 2014, 25, 879–893. [Google Scholar] [CrossRef] [PubMed]
- Puttini, I.; Poli, P.; Maiorana, C.; Vasconcelos, I.; Schmidt, L.; Colombo, L.; Souza, F. Evaluation of Osteoconduction of Biphasic Calcium Phosphate Ceramic in the Calvaria of Rats: Microscopic and Histometric Analysis. J. Funct. Biomater. 2019, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral. Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.S.; Fernandes, M.H. Rodent models in bone-related research: The relevance of calvarial defects in the assessment of bone regeneration strategies. Lab. Anim. 2011, 45, 14–24. [Google Scholar] [CrossRef]
- Basler, T.; Naenni, N.; Schneider, D.; Hämmerle, C.H.F.; Jung, R.E.; Thoma, D.S. Randomized controlled clinical study assessing two membranes for guided bone regeneration of peri-implant bone defects: 3-year results. Clin. Oral. Implant. Res. 2018, 29, 499–507. [Google Scholar] [CrossRef]
- Jeanneau, C.; Catherine, J.H.; Giraud, T.; Lan, R.; About, I. The Added Value of a Collagenated Thermosensitive Bone Substitute as a Scaffold for Bone Regeneration. Materials 2024, 17, 625. [Google Scholar] [CrossRef] [PubMed]
- Hämmerle, C.H.; Jung, R.E. Bone augmentation by means of barrier membranes. Periodontology 2000 2003, 33, 36–53. [Google Scholar] [CrossRef] [PubMed]
- Widmark, G.; Andersson, B.; Ivanoff, C.J. Mandibular bone graft in the anterior maxilla for single-tooth implants. Presentation of surgical method. Int. J. Oral Maxillofac. Surg. 1997, 26, 106–109. [Google Scholar] [CrossRef]
- Meyer, M. Processing of collagen based biomaterials and the resulting materials properties. Biomed. Eng. Online 2019, 18, 24. [Google Scholar] [CrossRef] [PubMed]
- Retzepi, M.; Donos, N. Guided Bone Regeneration: Biological principle and therapeutic applications. Clin. Oral Implants Res. 2010, 21, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Mir-Mari, J.; Benic, G.I.; Valmaseda-Castellón, E.; Hämmerle, C.H.F.; Jung, R.E. Influence of wound closure on the volume stability of particulate and non-particulate GBR materials: An in vitro cone-beam computed tomographic examination. Part II. Clin. Oral Implant. Res. 2017, 28, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Urban, I.A.; Jovanovic, S.A. Vertical ridge augmentation using guided bone regeneration (GBR) with titanium-reinforced ePTFE membranes in combination with autogenous and xenogeneic bone grafts: A retrospective study 12 to 72 months after loading. Clin. Implant. Dent. Relat. Res. 2009, 24, 502–510. [Google Scholar]
- Gómez-Cerezo, N.; Casarrubios, L.; Saiz-Pardo, M.; Ortega, L.; de Pablo, D.; Díaz-Güemes, I.; Fernández-Tomé, B.; Enciso, S.; Sánchez-Margallo, F.M.; Portolés, M.T.; et al. Mesoporous bioactive glass/e-polycaprolactone scaffolds promote bone regeneration in osteoporotic sheep. J. Biomed. Mater. Res. Part A 2019, 109, 748–762. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).