Abstract
This study aimed to evaluate the ability of fluoride-releasing restorative materials to remineralize artificially demineralized proximal enamel adjacent to class II restorations. Fifty-four demineralized enamel lesions were created on extracted premolar teeth, and the baseline lesion depth (LD) and mineral density (MD) were measured using micro-CT. The samples were randomly assigned to one of four groups, each in contact with a class II restorative material: Filtek Z350 XT™ (FZ), Cention N® (CN), Fuji II LC® (FJ), or Equia Forte® (EQ). Finally, post 14 days of pH cycling, measurements were taken. SEM, EDX, and Raman Imaging Microscopy were additionally performed. The results showed significant reductions in LD for all fluoride-releasing materials (CN, FJ, EQ). EQ and CN showed a significantly higher percentage change in LD than FZ. The MD of adjacent demineralized enamel increased significantly in all groups. There was no significant difference in the percentage change in MD between groups. SEM-EDX images revealed greater mineral deposition in fluoride-releasing materials than FZ. The Ca/P ratio of demineralized enamel adjacent to CN was equal to that of EQ, while FJ demonstrated the highest ratio. In conclusion, fluoride-releasing materials exhibited a reduction in the LD of adjacent demineralized enamel and demonstrated Ca/P ratios higher than FZ. CN may be an alternative restorative material to remineralize adjacent demineralized enamel.
1. Introduction
Dental caries remains a prevalent problem in both children and adults. The tight proximal surfaces of teeth are considered areas at high risk for the development of caries lesions. Enamel caries or white spot lesions (WSLs) are the first clinical signs of demineralization and start as non-cavitated. The untreated lesion can become a cavitated lesion that promotes biofilm accumulation and food impaction. In general, lesions that extend to dentine require invasive restorative treatment [1]. It is expected that proximal restoration with the ability to promote ion-releasing actions could potentially help repair the non-cavitated adjacent lesion.
The selection of dental materials is an important step in the treatment process and in preventing future caries. Fluoride-containing restorative materials can promote remineralization and reverse early enamel caries lesions in contact with the restoration [2,3]. The remineralization process, which is essential for repairing early enamel caries lesions, is driven by supersaturated calcium and phosphate ions [4]. However, the process is significantly enhanced by the presence of fluoride ions when pH rises. This enhancement can reprecipitate the minerals and form acid-resistant fluorapatite, thereby boosting mechanical strength [5]. Saliva and toothpaste-derived fluoride play a crucial role in promoting remineralization by converting hydroxyapatite to less soluble fluorapatite. Furthermore, fluoride-containing restorative materials, with their unique ability to recharge fluoride from daily fluoride products, offer an innovative approach to caries prevention by continuously releasing fluoride ions [6,7,8].
Glass ionomer cements (GICs) are fluoride-releasing materials that were first introduced in the late 1960s [9]. Many studies have shown their benefits both in vitro and in clinical use [2,3,10]. Resin-modified glass ionomer cement (RMGIC) and highly viscous glass ionomer cement (HVGIC) such as Fuji II LC®, Fuji IX®, and EQUIA Forte® (GC Corporation, Tokyo, Japan) are the most widely used and critically acclaimed fluoride-releasing materials and have been extensively researched in recent studies [3,7,8,11].
The alkasite resin-based composite (Cention N; CN) is an innovative, dual-cured dental restorative material that features aesthetic properties. Its composition includes fluoride and alkaline fillers. This material can be used as an alternative to amalgam in class II posterior restorations [12,13,14]. CN has a good sealing ability with less microleakage compared to conventional GIC and composite restorations [14]. In one study, CN showed the highest flexural strength, followed by resin composites and RMGIC, respectively [15]. Moreover, CN displayed high microhardness properties capable of withstanding masticatory forces [16] and provides proximal tight contact comparable to a resin composite when used in restoring class II cavities [17].
A substantial amount of fluoride ions can be released from the alkasite resin-based composite. A previous study showed that in acidic pH, self-cured CN demonstrated the highest fluoride ion release, followed by light-cured CN and GICs, respectively, over a long-term duration [18]. CN also raises the pH in degradation and releases calcium in addition to fluoride. Fluoride release from restorative materials has been proven to promote the remineralization of adjacent enamel caries lesions [19,20]. However, there are insufficient studies comparing CN to other restorative materials in promoting the remineralization of enamel caries of the adjacent tooth.
Therefore, the aim of this in vitro study was to investigate the ability of fluoride-releasing restorative materials to remineralize artificially demineralized proximal enamel adjacent to the restoration. The null hypothesis was that there would be no statistically significant difference in the lesion depth (LD) and mineral density (MD) of proximal demineralized enamel following contact with CN, RMGIC, HVGIC, or resin composite materials.
2. Materials and Methods
This study used extracted teeth from anonymous human donors. Verbal informed consent was obtained from all donors of extracted teeth prior to the study. The study protocol was granted an exemption regarding written informed consent and approved by the Human Research Ethics Committee of Thammasat University (Science), (HREC-TUSc), COE No.005/2565.
2.1. Sample Size Calculation and Tooth Selection
The sample size was determined based on data from a previous study by Lee et al. 2008 [21]. Twelve artificial proximal enamel caries per group were estimated to provide a power of 0.92 and a type I error of 0.05, with an effect size of 0.59, using G*Power 3.1.9.7 software (University of Dusseldorf, Germany). To account for potential technical errors, the sample size required per group ranged between 13 and 14 lesions. Fifty-four premolar teeth extracted due to orthodontic treatment were cleaned with distilled water to remove debris and stored in 0.1% thymol solution. The following inclusion criteria determined which teeth were included in the study: (1) the absence of restorations, crack lines, or crown anomalies that could affect the enamel surface, and (2) the absence of caries or visible enamel demineralization on the proximal surfaces of premolars, as determined by using a stereomicroscope.
2.2. Formation of Artificially Demineralized Proximal Enamel Lesion
Fifty-four extracted premolar teeth were prepared as adjacent demineralized enamel teeth. The proximal surfaces were grounded flat and polished by water-cooled abrasive paper no. 400, 800, and 1000 grit, respectively, to remove extraneous matter and expose fresh enamel under running water. Then, all teeth were coated with acid-resistant nail varnish (Revlon®, New York, NY, USA) on enamel surfaces, except for the 2 × 3 mm enamel windows at the contact area adjacent to the restoration tooth. The window of each tooth was separately exposed to a demineralizing solution containing 2.2 mM CaCl2, 2.2 mM KH2PO4, and 50 mM acetic acid adjusted to pH 4.4 with 1 M KOH at 37 °C for 96 h to create an artificially demineralized enamel lesion approximately 100–200 µm in depth, with constant circulation was until lesions were induced. The demineralizing solutions were changed daily. Lesion development was assessed using the International Caries Detection and Assessment System (ICDAS) criteria for stage 2 and confirmed by Bitewing radiography. Lesions that were not deeper than the outer 1/2 of enamel were included in this study. After this, each tooth was cut vertically in a buccolingual direction into two pieces to produce a crown of 4 mm in thickness, which is the same size as the phantom. The cutting surface of the dentine slice was polished manually by microfine 4000-grit sandpaper. The polished specimens were examined for cracks and other defects under a stereomicroscope. Finally, all samples were given the numbers 1–54, respectively, and examined for the baseline LD and MD by using Micro-CT SkyScan 1275 (Bruker micro-CT, Kontich, Belgium).
2.3. Preparation of Restoration Tooth and Mounting in Contact
The resin plastic tooth (Frasaco, Tettnang, Germany) was prepared as a restoration tooth. First, a class II cavity of 2 × 3 × 3 mm3 was prepared on the distal proximal surface of the maxillary premolar tooth and then duplicated into 54 resin teeth by using 3D modeling software (Revit 2021, Autodesk, Inc., San Francisco, CA, USA). After this, all 54 cavities were divided and restored with four material groups (Group 1: Filtek Z350XT (FZ); Group 2: Cention N (CN); Group 3: Fuji II LC (FJ); and Group 4: Equia Forte (EQ)) following the manufacturer’s instructions. The materials used in this study are shown in Table 1. All restorations were polished with Dura white stone FL2 Flame CA (Shofu, Kyoto, Japan) by using a low-speed handpiece. Following this, each restoration was left at room temperature for 30 min and stored in artificial saliva at 37 °C, pH 6.75, with constant circulation. Fifty-four samples of artificially demineralized proximal enamel were randomly assigned by computer generation into 4 groups according to the restorative materials. Finally, a proximal enamel caries tooth was paired with a restored resin tooth and mounted in silicone blocks to imitate maximum natural proximal contact.

Table 1.
Restorative materials and their compositions used in this study.
2.4. pH Cycling Model
All experiment groups were subjected to pH cycling for 14 days to stimulate a high caries challenge with a method modified from previous studies [22] in the following manner: The paired tooth specimens were separately mounted in a 12-well plate. Each specimen was immersed in 5 mL of a demineralizing solution (pH 4.4) used for artificial caries formation for 0.5 h at 37 °C three times a day. After each immersion, specimens were rinsed with deionized water for 60 s. After that, they were immersed in a remineralizing solution (artificial saliva: 2 g of methyl-p-hydroxybenzoate, 0.625 g of KCL, 0.059 g of MgCl2·H2O, 0.125 g of CaCl2, 0.804 of K2HPO4, 0.326 of KH2PO4, and 10 g of sodium carboxymethyl cellulose/1000 mL deionized water at pH 6.75) for 2 periods of 3 h and overnight in a plate container placed in a shaking incubator (Stuart SI500, Bibby Scientific Ltd., Cambridge, UK) at 35 rpm and 37 °C. All groups were also placed into fluoridated toothpaste slurry (Fluocaril®; 1480 ppm F) at a concentration of 1:3 for 2 min twice a day, and after this, they were rinsed with deionized water for 20 s. All solutions were freshly prepared and changed every day. After pH cycling, all proximal demineralized enamel samples were scanned again using micro-CT. The study flowchart is presented in Figure 1.
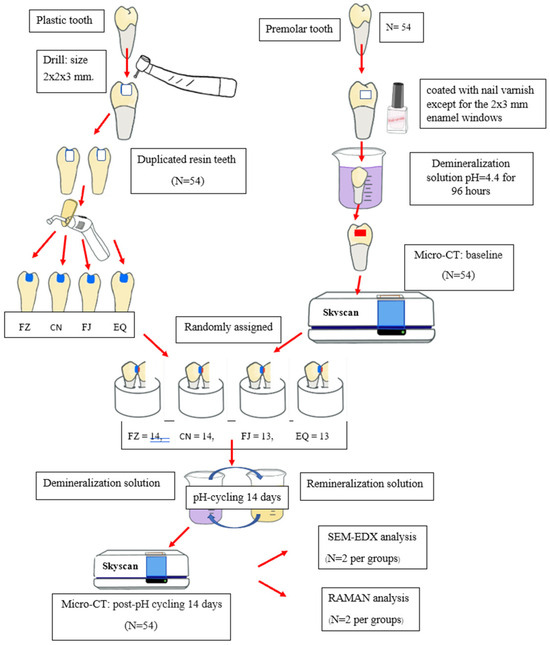
Figure 1.
Flowchart of the study procedures.
2.5. Micro-CT Scanning
The teeth were scanned with micro-CT SkyScan 1275. Micro-CT scanners were used for all X-ray imaging to assess the change in the LD and MD. Digital sectional images included an acceleration voltage of 90 kV, a source current of 70 mA, and an isometric voxel size of 10 µm. The samples were scanned at a standard resolution (1024 × 1024 pixels). A 1 mm aluminum filter was used to reduce beam hardening. The hydroxyapatite phantom (0.25 and 0.75 gHA/cm3) was used to calibrate the MD. Each sample was scanned 2 times, using 360°-round scanning. Teeth were mounted in a holder with a silicone jig and placed in the scanner to minimize the field of view (FOV). The silicone jig was used to fix the position of the tooth when scanned for two periods. All samples were scanned in the same position using the same procedure, and calculations were made by one examiner. Cross-sections were reconstructed by using the NRecon program (version 2.0.0.5). Ring artifacts and beam hardening were routinely corrected during reconstruction to improve image quality. Many imaging errors could be corrected by white and dark flat-field correction (images taken without the sample in the field of view). LD and MD were analyzed by using the CT An program (version 1.20.8.0). The LD was investigated and calculated from the deepest point. The average MDs of each proximal enamel caries lesion were calculated from five different lesion levels (the lesion’s deepest level and two levels over and underneath, respectively). The specific distance between each level was selected at 30 µm (Figure 2A). The lesion area of interest 3000 × 200 µm2 in size from the outer to the inner demineralized enamel surface was equal in every sample measurement (Figure 2B). In addition, the deepest point of the LD was reinvestigated prior to measuring MD to confirm that the MD was determined from the actual lesion depth. The intra-examiner reliability based on all LDs reinvestigated, computed using the intraclass correlation coefficient, was 0.98. LD and MD were evaluated at two different time points: baseline and after 14 days of pH cycling (post pH cycling).
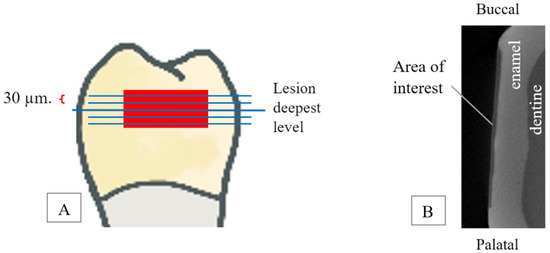
Figure 2.
(A). Five different lesion levels (lesion’s deepest level and two levels over and underneath, respectively): each level was measured every 30 μm. (B). Lesion area of interest size is 3000*200 µm2 from outer to inner surface.
2.6. Scanning Electron Microscope (SEM) and Energy-Dispersive X-Ray Microscope (EDX)
After 14 days of pH cycling, two samples from each group were randomly selected, vacuum-dried, placed on a carbon sheet, and attached to aluminum stubs. The samples were then coated with gold (Q150R ES, Quorum Technologies Limited, West Sussex, UK) using a sputtering condition of 23 mA for 45 s. The surface characteristics of the treated samples were examined using a JEOL JSM-7800F (Tokyo, Japan) scanning electron microscope (SEM) with an acceleration voltage of 1 kV at 5000× magnification. The elemental composition of the surface was further assessed using an energy-dispersive X-ray microscope (EDX, X-Max20, Oxford Instruments, Oxford, UK). The calcium (Ca) and phosphorus (P) concentrations and the Ca/P ratio of the enamel samples were determined.
2.7. Raman Imaging Microscope
To determine the remineralization effect, Raman spectra of the enamel surface were assessed using a Raman Imaging Microscope (DXR3xi, Thermo Fisher Scientific, Loughborough, UK). Two samples from each group were analyzed. The demineralized enamel surface was excited at 532 nm with a He-Ne laser through a microscope objective (10×). Surface mapping spectra were obtained in the region of 800–1700 every 1.0 µm over an area of approximately 50 × 50 µm2. Scanning for each point was performed using a 0.1 s exposure time and 10 scans. The spectra were normalized over the full spectral range, and chemical maps were generated using OMNIC software version 9.2.98 (Thermo Fisher Scientific, Loughborough, UK).
2.8. Statistical Analysis
All data were analyzed by using SPSS 26 software for Windows (SPSS Inc., Chicago, IL, USA). The distribution of normality and homogeneity of variance of the data were determined by using the Kolmogorov–Smirnov test and Levene’s test, respectively. The differences in the LD and MD of adjacent demineralized proximal enamel among the restorative material groups were analyzed by using a one-way analysis of variance (ANOVA). A paired t-test was used to determine the differences in LD and MD between baseline and post-pH cycling within the restorative material group. The differences in the percentage change in LD and MD among the restorative groups were analyzed by using the Kruskal–Wallis test, followed by Dunn’s post hoc test. The significance level was set at α = 0.05.
3. Results
3.1. Lesion Depth
Due to artificially demineralized proximal enamel formation, demineralized enamel lesions measuring approximately 100–200 µm in depth were included in this study. The average LDs at baseline and post pH cycling are shown in Table 2. There was no significant difference in the mean baseline LD between the four material groups (p = 0.80). After 14 days of pH cycling, the mean LD of proximal demineralized enamel decreased in all material groups. The mean difference in LD between baseline and post pH cycling was −6.143 ± 14.04, −18.43 ± 9.09, −20.15 ± 22.99, and −25.46 ± 19.66 µm for FZ, CN, FJ, and EQ, respectively. The paired t-test demonstrated that there were statistically significant differences in LD between baseline and post pH cycling in the CN, FJ, and EQ groups (p < 0.01), while FZ did not show a significant difference.

Table 2.
The lesion depth (LD) and mineral density (MD) of adjacent demineralized proximal enamel at baseline and post pH cycling.
Illustrations of the baseline and post pH cycling LDs are presented in Figure 3. The mean percentage reduction in LD is summarized in Table 3. The greatest percentage reduction in LD was seen in EQ (mean rank = 19.46), whereas FZ demonstrated the lowest percentage reduction in LD (mean rank = 41).
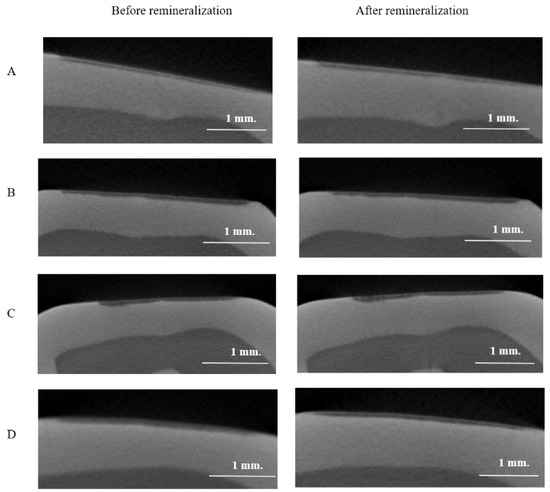
Figure 3.
Micro-CT images of the lesion depth of the artificially demineralized proximal enamel lesion adjacent to the four groups of restorative material at baseline and post 14 days of pH cycling. (A) FZ; (B) CN; (C) FJ; (D) EQ.

Table 3.
The percentage change in the lesion depth and mineral density of adjacent demineralized enamel.
There was a significant difference in the percentage change in LD between groups (p = 0.002). Post hoc pairwise comparisons using Dunn’s test revealed significant differences between CN and FZ (p = 0.011) and EQ and FZ (p = 0.002) (Figure 4). Although CN demonstrated a lower percentage change in LD than the EQ and FJ groups, there were no significant differences between them (p > 0.05).
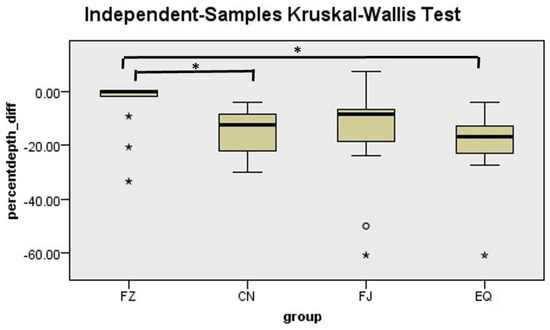
Figure 4.
Mean rank of the percentage change in lesion depth. * Indicates statistically significant difference between groups.
3.2. Mineral Density
At baseline, there was no significant difference in the mean MD of demineralized enamel lesions between the four groups of materials (p = 0.7), as shown in Table 2. Post pH cycling, there was a statistically significant increase in the mean MD of all material groups compared to their baselines (p < 0.01). The mean differences in MD between baseline and post pH cycling were 52.19 ± 46.03, 61.55 ± 79.01, 103.45 ± 108.99, and 101.06 ± 110.36 for FZ, CN, FJ, and EQ, respectively.
FJ exhibited the highest percentage increase in the MD of demineralized enamel lesions (mean rank = 32.15), followed by EQ, CN, and FZ, respectively. However, a Kruskal–Wallis test showed no significant differences in the mean rank of MD among the four groups of restorative materials (p = 0.53) (Table 3).
3.3. Scanning Electron Microscope (SEM) and Energy-Dispersive X-Ray Microscope (EDX)
After 14 days of pH cycling, SEM images demonstrated the precipitation of calcium on the demineralized enamel surface adjacent to all restorative material groups (Figure 5). The roughest surface was seen in FJ, followed by EQ, CN, and FZ, respectively. The Ca/P ratio results obtained from the EDX analysis of each group are as follows: The remineralization of the enamel caries surface after contact with FJ resulted in the highest Ca/P ratio, as shown in Table 4. Additionally, the Ca/P ratio for CN and EQ was equal. The Ca/P ratios for FZ, CN, FJ, and EQ were 0.75, 1.32, 2.17, and 1.32, respectively.
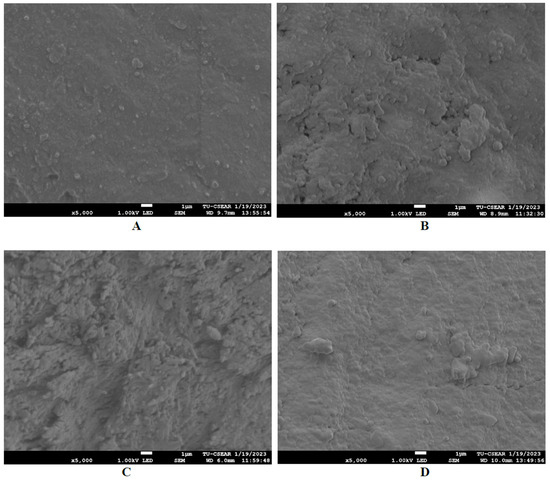
Figure 5.
SEM images of the demineralized enamel lesion surface at proximal contact with the four groups of restorative material after remineralization at a magnification of 5000× (accelerating voltage of 1 kV; working distance of 9 mm). (A) FZ; (B) CN; (C) FJ; (D) EQ.

Table 4.
The percentage weight of Ca and P of the four groups of restorative materials.
3.4. Raman Imaging Microscope
The peak of the Raman spectra representing the phosphate group (960 cm−1) of hydroxyapatite (HA) was detected in all groups (Figure 6). However, the highest intensity of the peak representing the phosphate group (960 cm−1) was detected in CN, followed by FJ, EQ, and FZ, respectively.
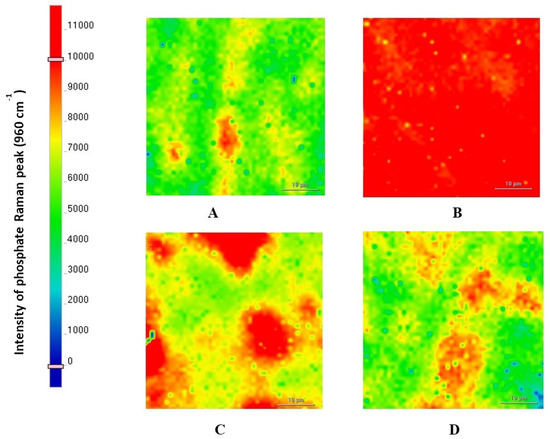
Figure 6.
SEM images of the enamel carious lesion surface at proximal contact with the four groups of restorative material after remineralization at a magnification of 5000× (accelerating voltage of 1 kV; working distance of 9 mm). (A) FZ; (B) CN; (C) FJ; (D) EQ.
4. Discussion
Dental caries is a process of alternating demineralization and remineralization that results directly from acid challenge from dietary cycles and remineralization from oral saliva. The present in vitro study aimed to mimic the behavior of the oral environment in daily life. pH cycling was utilized as it can provide a sufficient amount of calcium and phosphate ions for remineralization [23], and fluoride-containing toothpaste was regularly used to encourage enamel remineralization. Each day, the restorative materials and the artificially demineralized proximal enamel were subjected to a pH cycling regimen consisting of three dietary cycles, exposure to artificial saliva, and immersion twice in fluoride toothpaste.
Fluoride-releasing restorative materials have excellent potential in dental caries prevention. The placement of a proximal restoration in the treatment of dental caries changes the local environment from cariogenic to non-cariogenic, not only for the treated tooth but also for the proximal surface of the adjacent tooth. To enable the exchange of fluoride with the HA of enamel, prolonged fluoride release and close restorative contact with the tooth surfaces are required [24]. In this study, mounting teeth in proximal tight contact emulated a maximum natural contact area. Uncoated GICs were used because the interproximal area cannot be coated in clinical situations, and a study showed that uncoated GICs released more fluoride than coated ones [25]. Furthermore, light-cured CN was used in this study, even though the ability of light-curing alkasite restorative material to release ions has been shown to slightly decrease due to photopolymerization [18]. The present study found that all the materials can reduce the LD of adjacent demineralized proximal enamel, and only fluoride-releasing materials provided a significant reduction in LD compared to their baselines. EQ exhibited a significantly higher percentage reduction in LD than FZ. This result corresponds to previous studies using polarized-light photomicrographs that showed that GICs decreased adjacent enamel LD more than a resin composite [26,27,28]. Moreover, our findings demonstrated a comparable percentage reduction in LD between CN and GICs (EQ, FJ). Additionally, CN exhibited a significantly higher percentage reduction in LD compared to FZ. These results support the findings of Kuphasuk et al. and Albelasy et al., who reported that CN and GICs significantly inhibit the demineralization of enamel caries adjacent to restorations and more effectively reduce lesion depth compared to resin composites [29,30]. This effect may be due to CN’s ability to release fluoride and calcium ions, which assist in the remineralization process [31]. A previous study indicated that CN can continuously release these ions for up to 28 days, at concentrations 300–400 times higher than those found in resin composites [32]. However, FZ also showed a reduced LD. These may be explained by the fluoride toothpaste and artificial saliva we used, containing adequate fluoride, calcium, and phosphate ions that play an important role in enhancing the remineralization process.
The baseline MD of adjacent demineralized proximal enamel in our study was approximately 1500 mgHA/cm3, which is comparable to the values reported by a previously published study [33]. After pH cycling, the MD of these demineralized enamel samples increased significantly in all material groups when compared to their baselines. These results imply that the pH cycling conducted in this study represents a remineralization model, which is also confirmed by our LD results. Although not statistically significant, a greater increase in the percentage change in MD was observed in the fluoride-releasing material groups compared to FZ. In contrast, a previous long-term study demonstrated that GICs had a significantly greater MD change than resin composites [21]. Therefore, a longer experimental period would be suggested.
The null hypothesis of this study was rejected in terms of LD because there was a significant difference in the percentage change in LD of the adjacent demineralized proximal enamel surface between EQ and FZ and CN and FZ, investigated by using micro-CT. This could be attributed to the presence of soluble fluoride in which ion exchange can readily occur not only on the materials’ surface but also possibly at a short distance from the materials. Several studies have proved the ability of enamel adjacent to glass ionomer and alkasite restoration to uptake fluoride and resist demineralization [19,20,28,29,34]. Furthermore, our study found that CN demonstrated a lesser trend in assisting enamel caries remineralization compared to GICs. These results may be supported by several comparative studies evaluating ion release and the recharge of ion-leaching restorative materials, which discovered that GICs released fluoride ions at a higher rate than CN [35,36,37]. On the other hand, a recent study by Theerarath and Sriarj in 2022 reported that CN increased the surface microhardness recovery of adjacent enamel caries more than EQ and FZ, respectively [15]. This difference from our results may be because they used a self-cured mode of CN which releases more fluoride ions than the light-cured mode we used. Concurrently, CN provided the highest calcium ion release and the greatest phosphate ion recharge compared to other ion-leaching restorative materials [29,35].
In this study, SEM-EDX was performed on some specimens to confirm our primary outcome. The SEM images illustrated mineral precipitation on the surfaces of adjacent demineralized enamel. The levels of Ca and P ions on demineralized enamel surfaces reflect the restorative materials’ ability to remineralize and promote hydroxyapatite formation on adjacent demineralized enamel lesions. Therefore, the percentage weight of Ca and P ions, along with the Ca/P ratio derived from EDX analysis, supported our MD results. Specifically, the demineralized enamel adjacent to FJ exhibited greater remineralization compared to EQ, CN, and FZ. The higher Ca/P ratio, indicating a greater propensity for mineral uptake in the adjacent demineralized proximal enamel and a reduced risk of secondary caries, has potential implications for the selection of proximal restorative materials. This is particularly important for patients with a high caries risk. Additionally, we observed a similar Ca/P ratio between EQ and CN, which aligns with a previous study [19]. However, the Ca/P ratio for both EQ and CN in our study was lower, which may be attributed to differences in the composition of remineralization and demineralization solutions or the curing method of CN used in our study.
The results from Raman mapping may indicate that CN encouraged a high level of fluorapatite precipitation on the adjacent demineralized enamel. This could be attributed to the fact that CN released both fluoride and calcium, which were essential for promoting suitable conditions of fluorapatite precipitation [35,38]. The release of calcium from CN was also at much higher levels than from FJ and EQ [36,39]. The high mineralizing potential of CN was also confirmed by previous studies [40,41,42]. Furthermore, the Raman spectra exhibited a prominent peak at 960 cm−1, indicating the presence of the phosphate group. This phosphate is likely derived from the remineralization solution.
The limitations of this study include the use of acetic acid to create proximal demineralized enamel lesions, resulting in erosive-like lesions that differ from natural carious lesions and do not typically occur in interproximal areas. Furthermore, this study employed an in vitro design that cannot fully replicate the actual clinical environment of the oral cavity. Consequently, this model may have limited clinical relevance. Additionally, the MD investigation focused only on the deepest part of the demineralized enamel lesion. To enhance the analysis of the remineralization ability of fluoride-releasing restorative materials, it is recommended that the MD of the sound enamel beneath these lesions be investigated.
Our findings found that the materials had a limited impact, which may be attributed to the short duration of the study. Extending the study period and increasing the sample size could potentially reveal more pronounced effects and strengthen the validity of our findings. In addition, we suggest conducting randomized clinical trials involving patients at high caries risk to further confirm our findings.
5. Conclusions
After 14 days of pH cycling, fluoride-releasing materials (CN, FJ, and EQ) exhibited a reduction in the LD of adjacent demineralized enamel and demonstrated Ca/P ratios higher than FZ. The findings of this in vitro study may have an impact on clinicians’ selection of restorative materials for their patients at high caries risk, and it is possible to use fluoride-releasing materials primarily for class II cavity restorations to arrest the adjacent demineralized proximal enamel in primary and permanent teeth. CN may be an alternative restorative material to remineralize adjacent demineralized enamel.
Author Contributions
O.P.: Methodology, validation, formal analysis, investigation, resources, writing—original draft preparation, P.P. and T.S.: Conceptualization, methodology, data curation, writing (review and editing), supervision, project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by a postgraduate research project grant from the Faculty of Dentistry, Thammasat University.
Institutional Review Board Statement
This study used extracted teeth from anonymous human donors. Verbal informed consent was obtained from all donors of extracted teeth prior to the study. The study protocol was granted an exemption regarding written informed consent and approved by the Human Research Ethics Committee of Thammasat University (Science), (HREC-TUSc), COE No.005/2565. Approved date; 13 February 2022.
Informed Consent Statement
Informed consent was obtained from all donors of the teeth involved in the study.
Data Availability Statement
The data generated or analyzed during this study consist mainly of images, the most representative of which are included in this article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- Gao, S.S.; Zhang, S.; Mei, M.L.; Lo, E.C.; Chu, C.H. Caries remineralisation and arresting effect in children by professionally applied fluoride treatment—A systematic review. BMC Oral. Health 2016, 16, 12. [Google Scholar] [CrossRef]
- Tedesco, T.K.; Bonifacio, C.C.; Calvo, A.F.; Gimenez, T.; Braga, M.M.; Raggio, D.P. Caries lesion prevention and arrestment in approximal surfaces in contact with glass ionomer cement restorations—A systematic review and meta-analysis. Int. J. Paediatr. Dent. 2016, 26, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Trairatvorakul, C.; Itsaraviriyakul, S.; Wiboonchan, W. Effect of glass-ionomer cement on the progression of proximal caries. J. Dent. Res. 2011, 90, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Philip, N. State of the Art Enamel Remineralization Systems: The Next Frontier in Caries Management. Caries Res. 2019, 53, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Guntermann, L.; Rohrbach, A.; Schäfer, E.; Dammaschke, T. Remineralization and protection from demineralization: Effects of a hydroxyapatite-containing, a fluoride-containing and a fluoride-and hydroxyapatite-free toothpaste on human enamel in vitro. Head Face Med. 2022, 18, 26. [Google Scholar] [CrossRef]
- Wang, Y.; Mei, L.; Gong, L.; Li, J.; He, S.; Ji, Y.; Sun, W. Remineralization of early enamel caries lesions using different bioactive elements containing toothpastes: An in vitro study. Technol. Health Care 2016, 24, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Wongphattarakul, S.; Kuson, R.; Sastraruji, T.; Suttiat, K. Fluoride Release and Rechargeability of Poly(lactic acid) Composites with Glass Ionomer Cement. Polymers 2023, 15, 4041. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, A.M.; Elshehawy, T.M.; Ibrahim, H.M. Fluoride release and recharge behavior of bioactive glass ionomer cements using ion chromatography. Egypt. Dent. J. 2019, 65, 399–406. [Google Scholar] [CrossRef]
- Smith, D.C. Development of glass-ionomer cement systems. Biomat 1998, 19, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Krämer, N.; Schmidt, M.; Lücker, S.; Domann, E.; Frankenberger, R. Glass ionomer cement inhibits secondary caries in an in vitro biofilm model. Clin. Oral. Investig. 2018, 22, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Qvist, V.; Poulsen, A.; Teglers, P.T.; Mjör, I.A. Fluorides leaching from restorative materials and the effect on adjacent teeth. Int. Dent. J. 2010, 60, 156–160. [Google Scholar] [PubMed]
- Roulet, J.; Hussein, H.; Abdulhameed, N.; Shen, C. In vitro wear of two bioactive composites and a glass ionomer cement. DZZ Int. 2019, 1, 24–30. [Google Scholar]
- Minocha, A.; Sharma, V.; Gupta, A.; Sharma, N. Comparative evaluation of Cention N and Amalgam in Class II posterior restorations. Univ. J. Dent. Sci. 2021, 7. [Google Scholar] [CrossRef]
- Mazumdar, P.; Das, A.; Das, U.K. Comparative evaluation of microleakage of three different direct restorative materials (silver amalgam, glass ionomer cement, Cention N), in class II restorations using stereomicroscope: An in vitro study. Indian J. Dent. Res. 2019, 30, 277. [Google Scholar] [PubMed]
- Chole, D.; Shah, H.K.; Kundoor, S.; Bakle, S.; Gandhi, N.; Hatte, N. In vitro comparison of flexural strength of cention-n, bulkFill composites, light-cure nanocomposites and resin-modified glass ionomer cement. J. Dent. Med. Sci. 2018, 17, 79. [Google Scholar]
- Mazumdar, P.; Das, A.; Guha, C. Comparative evaluation of hardness of different restorative materials (restorative gic, cention N, nanohybrid composite resin and silver amalgam)—An in vitro study. Int. J. Adv. Res. 2018, 6, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Deepak, S.; Nivedhitha, M. Proximal contact tightness between two different restorative materials—An in vitro study. J. Adv. Pharm. Educ. Res. 2017, 7, 153–156. [Google Scholar]
- Gupta, N.; Jaiswal, S.; Nikhil, V.; Gupta, S.; Jha, P.; Bansal, P. Comparison of fluoride ion release and alkalizing potential of a new bulk-fill alkasite. J. Conserv. Dent. 2019, 22, 296. [Google Scholar] [CrossRef] [PubMed]
- Theerarath, T.; Sriarj, W. An alkasite restorative material effectively remineralized artificial interproximal enamel caries in vitro. Clin. Oral. Investig. 2022, 26, 4437–4445. [Google Scholar] [CrossRef] [PubMed]
- Phyo, W.M.; Saket, D.; da Fonseca, M.A.; Auychai, P.; Sriarj, W. In vitro remineralization of adjacent interproximal enamel carious lesions in primary molars using a bioactive bulk-fill composite. BMC Oral. Health. 2024, 24, 37. [Google Scholar] [CrossRef]
- Lee, H.S.; Berg, J.H.; García-Godoy, F.; Jang, K.T. Long-term evaluation of the remineralization of interproximal caries-like lesions adjacent to glass-ionomer restorations: A micro-CT study. Am. J. Dent. 2008, 21, 129–132. [Google Scholar] [PubMed]
- Rana, R.; Itthagarun, A.; King, N.M. Effects of dentifrices on artificial caries like lesions: An in vitro pH cycling study. Int. Dent. J. 2007, 57, 243–248. [Google Scholar] [CrossRef]
- Ten Cate, J.; Arends, J. Remineralization of artificial enamel lesions in vitro: III. A study of the deposition mechanism. Caries Res. 1980, 14, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Featherstone, J.; Glena, R.; Shariati, M.; Shields, C. Dependence of in vitro demineralization of apatite and remineralization of dental enamel on fluoride concentration. J. Dent. Res. 1990, 69, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Brzović-Rajić, V.; Miletić, I.; Gurgan, S.; Peroš, K.; Verzak, Ž.; Ivanišević-Malčić, A. Fluoride release from glass ionomer with nano filled coat and varnish. Acta Stomatol. Croat. 2018, 52, 307. [Google Scholar] [CrossRef] [PubMed]
- Donly, K.J.; Segura, A.; Wefel, J.S.; Hogan, M.M. Evaluating the effects of fluoride-releasing dental materials on adjacent interproximal caries. J. Am. Dent. Assoc. 1999, 130, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Baliga, M.S.; Bhat, S.S. Effect of fluorides from various restorative materials on remineralization of adjacent tooth: An in vitro study. J. Indian Soc. Pedod. Prev. Dent. 2010, 28, 84–90. [Google Scholar] [CrossRef]
- Jang, K.-T.; Garcia-Godoy, F.; Donly, K.J.; Segura, A. Remineralizing effects of glass ionomer restorations on adjacent interproximal caries. ASDC J. Dent. Child. 2001, 68, 125–128+142. [Google Scholar] [PubMed]
- Kuphasuk, S.; Kunawarote, S. In vitro Caries Inhibition in Enamel Adjacent to Ion-releasing Resin Composite: Original articles. CM Dent. J. 2022, 43, 50–61. [Google Scholar]
- Albelasy, E.H.; Chen, R.; Fok, A.; Montasser, M.; Hamama, H.H.; Mahmoud, S.H.; Abdelrehim, T.; Chew, H.P. Inhibition of Caries around Restoration by Ion-Releasing Restorative Materials: An In Vitro Optical Coherence Tomography and Micro-Computed Tomography Evaluation. Materials 2023, 16, 5558. [Google Scholar] [CrossRef] [PubMed]
- Todd, J.C. Scientific Documentation: Cention N; Ivoclar-Vivadent Press: Schaan, Liechtenstein, 2016; pp. 1–58. [Google Scholar]
- Wiriyasatiankun, P.; Sakoolnamarka, R.; Thanyasrisung, P. The impact of an alkasite restorative material on the pH of Streptococcus mutans biofilm and dentin remineralization: An in vitro study. BMC Oral. Health 2022, 22, 334. [Google Scholar] [CrossRef]
- Nantanee, R.; Santiwong, B.; Trairatvorakul, C.; Hamba, H.; Tagami, J. Silver diamine fluoride and glass ionomer differentially remineralize early caries lesions, in situ. Clin. Oral. Investig. 2016, 20, 1151–1157. [Google Scholar] [CrossRef]
- Retief, D.; Bradley, E.; Denton, J.; Switzer, P. Enamel and cementum fluoride uptake from a glass ionomer cement. Caries Res. 1984, 18, 250–257. [Google Scholar] [CrossRef]
- Ruengrungsom, C.; Burrow, M.F.; Parashos, P.; Palamara, J.E. Evaluation of F, Ca, and P release and microhardness of eleven ion-leaching restorative materials and the recharge efficacy using a new Ca/P containing fluoride varnish. J. Dent. 2020, 102, 103474. [Google Scholar] [CrossRef]
- Kim, M.J.; Lee, M.J.; Kim, K.M.; Yang, S.Y.; Seo, J.Y.; Choi, S.H.; Kwon, J.S. Enamel demineralization resistance and remineralization by various fluoride-releasing dental restorative materials. Materials 2021, 14, 4554. [Google Scholar] [CrossRef] [PubMed]
- Panpisut, P.; Toneluck, A. Monomer conversion, dimensional stability, biaxial flexural strength, and fluoride release of resin-based restorative material containing alkaline fillers. Dent. Mater. J. 2020, 39, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.; Kang, Y.; Dunn, K.; Kugel, G. Hydroxyapatite Formation of Dental Restorative Materials. J. Dent. Sci. 2020, 5, 000263. [Google Scholar] [CrossRef]
- Di Lauro, A.; Di Duca, F.; Montuori, P.; Dal Piva, A.M.O.; Tribst, J.P.M.; Borges, A.L.S.; Ausiello, P. Fluoride and Calcium Release from Alkasite and Glass Ionomer Restorative Dental Materials: In Vitro Study. J. Funct. Biomater. 2023, 14, 109. [Google Scholar] [CrossRef] [PubMed]
- Feiz, A.; Nicoo, M.A.; Parastesh, A.; Jafari, N.; Sarfaraz, D. Comparison of antibacterial activity and fluoride release in tooth-colored restorative materials: Resin-modified glass ionomer, zirconomer, giomer, and cention N. Dent. Res. J. 2022, 19, 104. [Google Scholar]
- Singbal, K.; Shan, M.K.W.; Dutta, S.; Kacharaju, K.R. Cention N Compared to Other Contemporary Tooth-Colored Restorative Materials in Terms of Fluoride Ion Releasing Efficacy: Validation of a Novel Caries-Prevention-Initiative by the Ministry of Health, Malaysia. Biomed. Pharmacol. J. 2022, 15, 669–676. [Google Scholar] [CrossRef]
- Donly, K.J.; Liu, J.A. Dentin and enamel demineralization inhibition at restoration margins of Vitremer, Z 100 and Cention N. Am. J. Dent. 2018, 31, 166–168. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).