A Novel 3D-Printed Training Platform for Ossiculoplasty with Objective Performance Evaluation
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
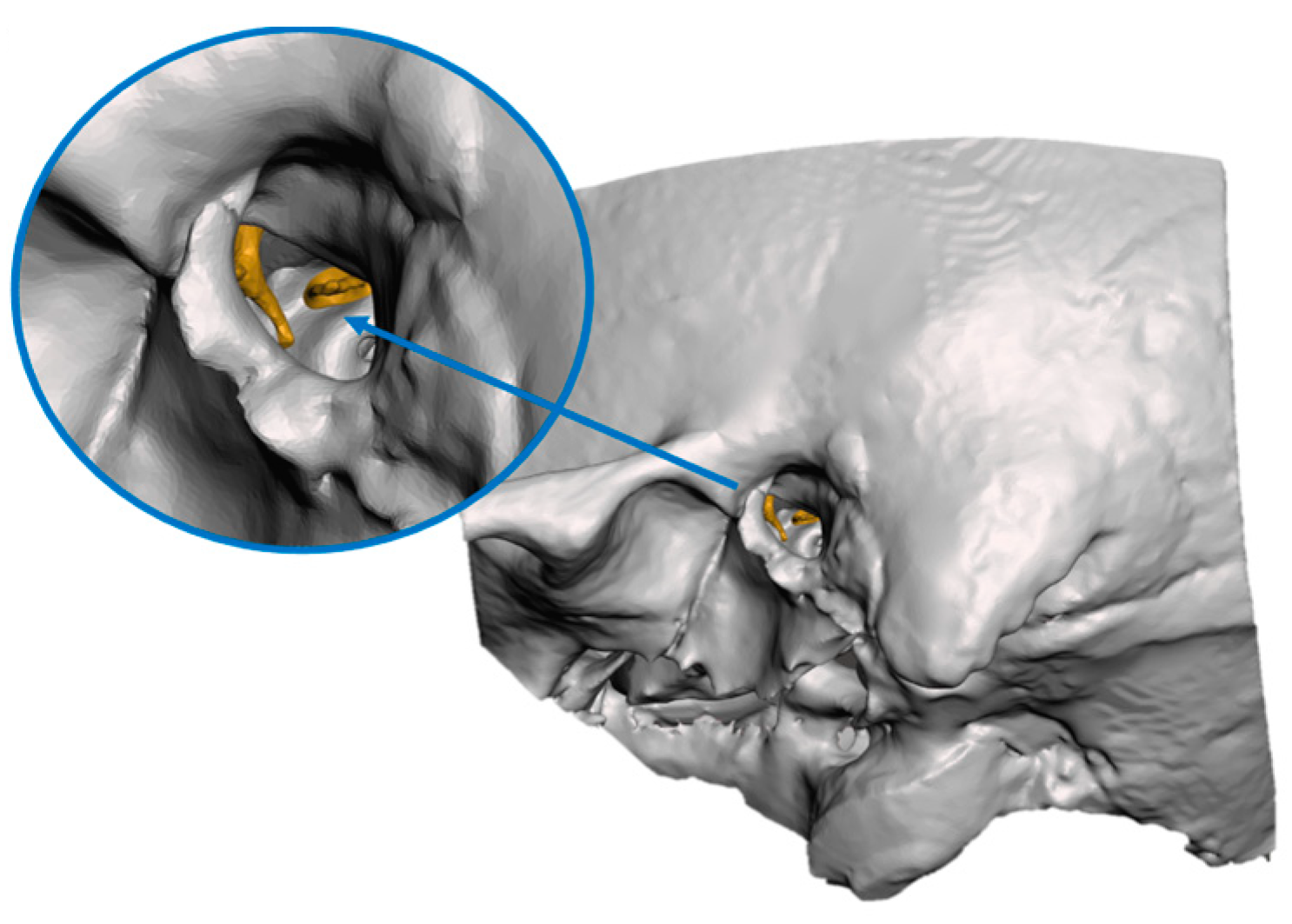
2.1. Image Segmentation and Creation of Virtual Models
2.2. 3D Printing of the Models
2.3. Ossiculoplasty Simulation Hands-On Session
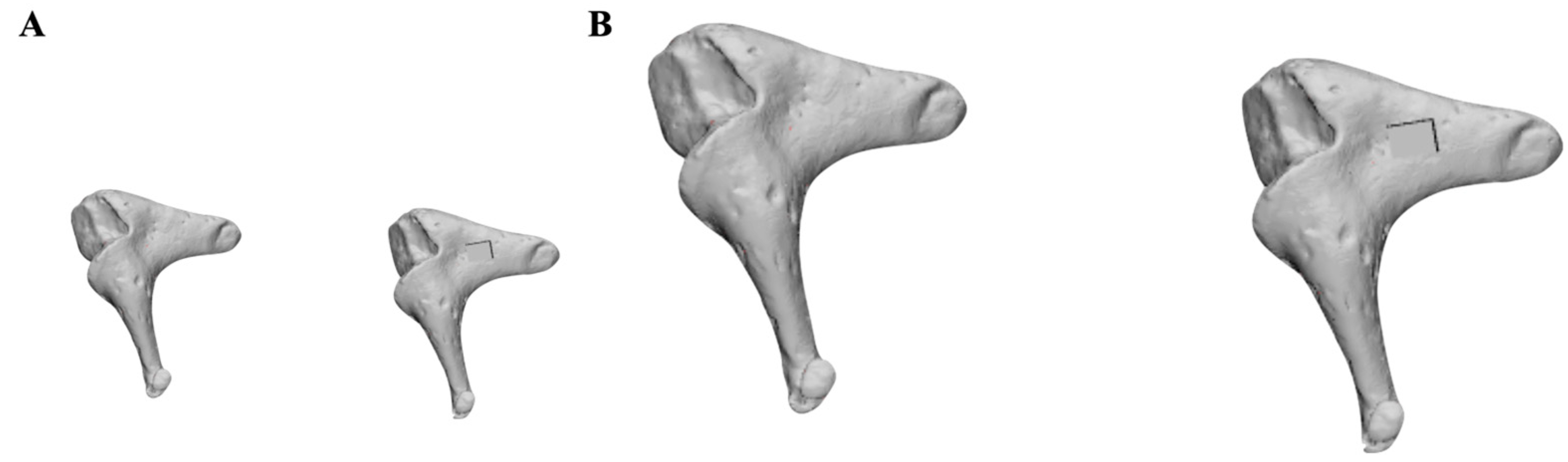
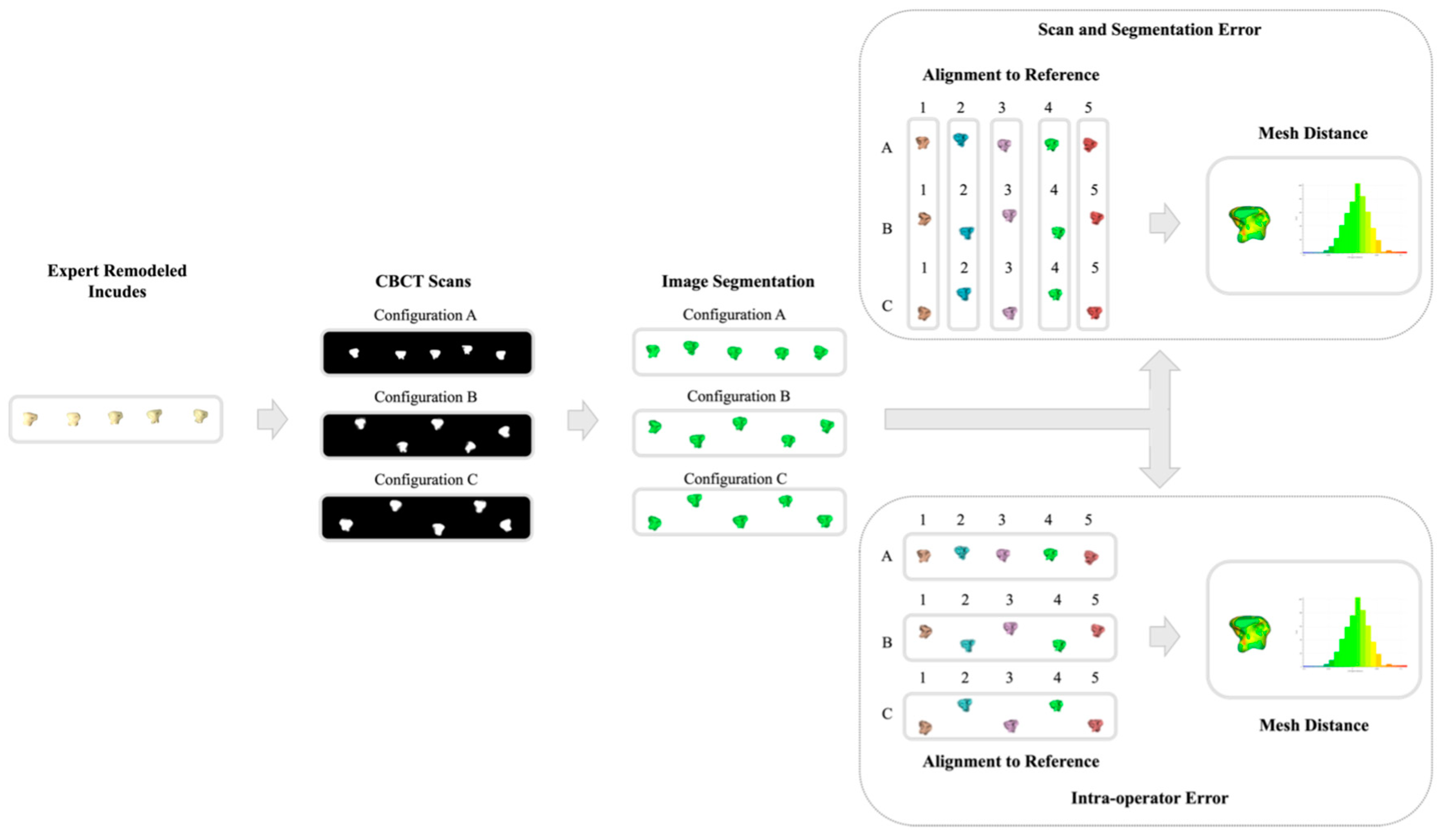
2.4. Error Range Quantification
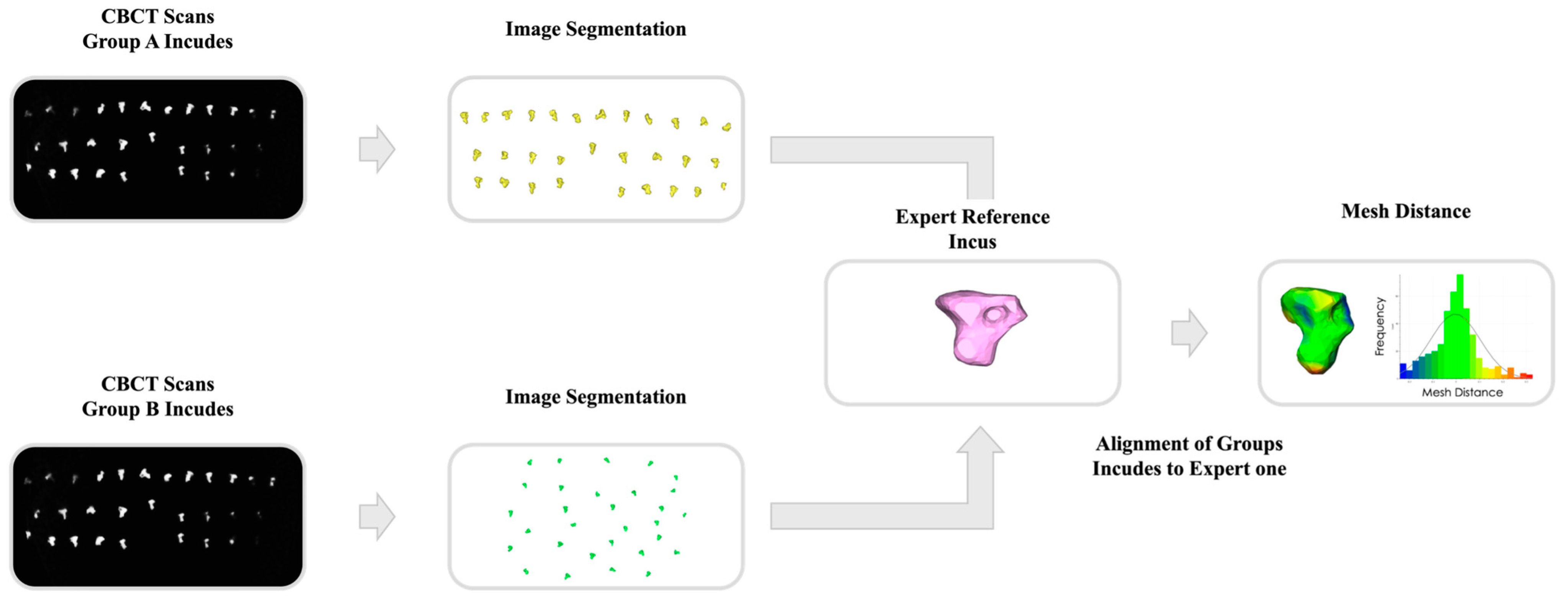
2.5. Quantitative Performance Analysis
3. Results
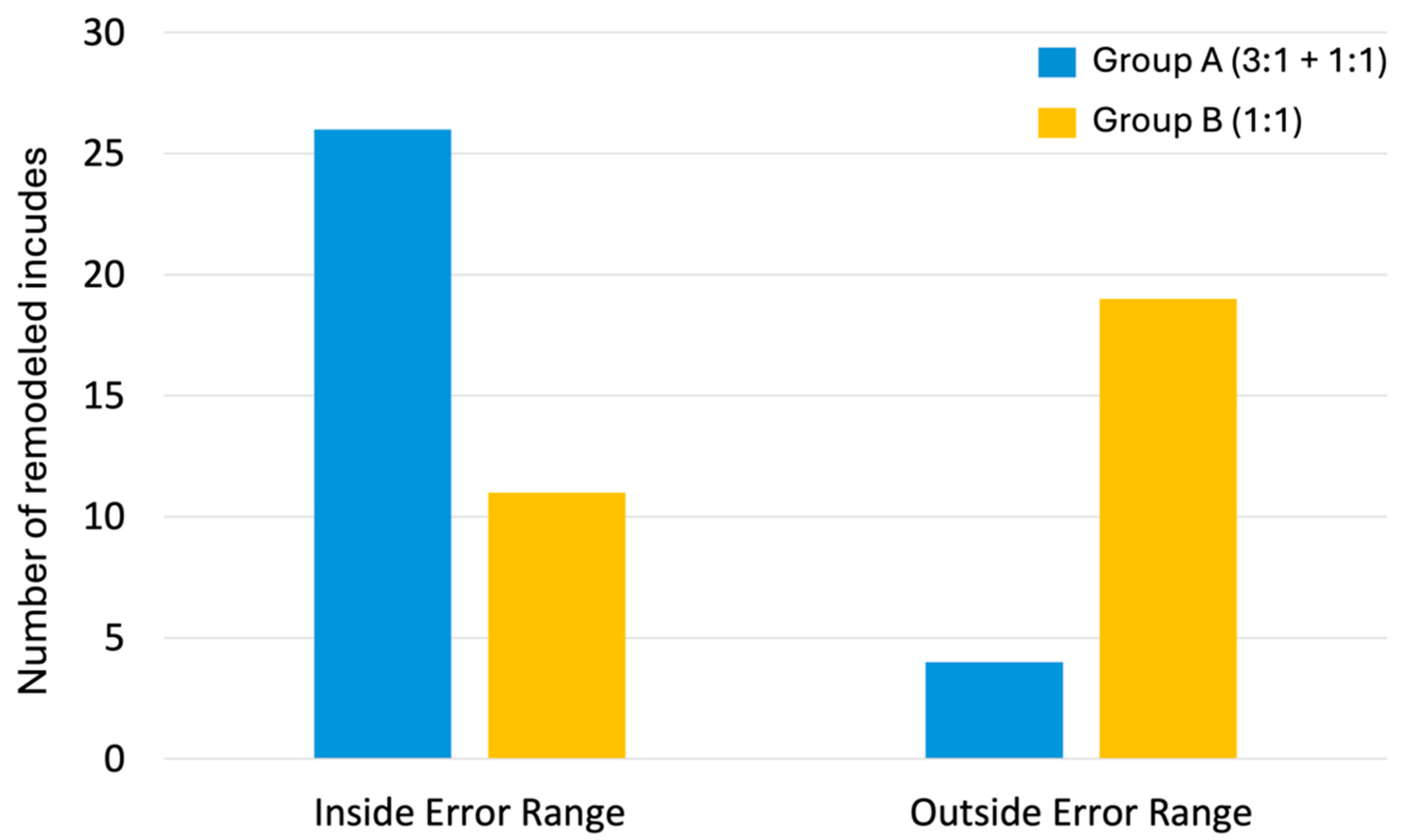
3.1. Error Range
3.2. Quantitative Performance Results
3.3. Qualitative Questionnaires
4. Discussion
Limitations and Future Outlook of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fischer, C.; Lieu, J. Unilateral hearing loss is associated with a negative effect on language scores in adolescents. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 1611–1617. [Google Scholar] [CrossRef] [PubMed]
- Kamrava, B.; Roehm, P.C. Systematic Review of Ossicular Chain Anatomy: Strategic Planning for Development of Novel Middle Ear Prostheses. Otolaryngol.—Head Neck Surg. 2017, 157, 190–200. [Google Scholar] [CrossRef]
- Mudhol, R.S.; Naragund, A.I.; Shruthi, V.S. Ossiculoplasty: Revisited. Indian. J. Otolaryngol. Head Neck Surg. 2013, 65, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Olaison, S.; Berglund, M.; Taj, T.; Knutsson, J.; Westman, E.; Eriksson, P.O.; Bonnard, Å. Hearing Outcomes After Ossiculoplasty With Bone or Titanium Prostheses—A Nationwide Register-Based Study. Clin. Otolaryngol. 2024, 49, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Leahy, J.; Wong, K.; Govindan, A.; Powers, A.; Perez, E.R.; Wanna, G.B.; Cosetti, M.K. Long-term outcomes following pediatric endoscopic titanium ossiculoplasty: A single-institution experience. Int. J. Pediatr. Otorhinolaryngol. 2024, 179, 111938. [Google Scholar] [CrossRef]
- Fink, R.; Molinari, G.; Beckmann, S.; Fernandez, I.J.; Burato, A.; Caversaccio, M.; Presutti, L.; Anschuetz, L. Techniques of Endoscopic Ossiculoplasty. JoVE 2024, 203, 66155. [Google Scholar] [CrossRef] [PubMed]
- Soloperto, D.; Laura, E.; Gazzini, L.; Cerullo, R.; Ferrulli, G.; Nocini, R.; Molteni, G.; Marchioni, D. Exclusive endoscopic ossiculoplasty with autologous material: Step-by-step procedure and functional results. Eur. Arch. Otorhinolaryngol. 2023, 280, 4869–4878. [Google Scholar] [CrossRef]
- Kawatra, R.; Maheshwari, P. A comparative study of surgical outcomes of ossiculoplasty using biomaterials and autologous implants. Bangladesh J. Otorhinolaryngol. 2013, 19, 29–35. [Google Scholar] [CrossRef]
- Alzhrani, F.; Aldueb, R.; Alosaimi, K.; Islam, T.; Almuhawas, F.; Alsanosi, A. Safety of tympanoplasty and ossiculoplasty performed by otorhinolaryngology trainees. J. Laryngol. Otol. 2020, 134, 213–218. [Google Scholar] [CrossRef]
- Fieux, M.; Gavoille, A.; Subtil, F.; Bartier, S.; Tringali, S. Otoskills training during covid-19 pandemic: A before-after study. BMC Med. Educ. 2021, 21, 284. [Google Scholar] [CrossRef] [PubMed]
- Neri, I.; Cercenelli, L.; Marcuccio, M.; Lodi, S.; Koufi, F.; Fazio, A.; Marvi, M.V.; Marcelli, E.; Billi, A.M.; Ruggeri, A.; et al. Dissecting Human Anatomy Learning Process through Anatomical Education with Augmented Reality: AEducAR 2.0, an Updated Interdisciplinary Study. Anat. Sci. Educ. 2024, 17, 693–711. [Google Scholar] [CrossRef] [PubMed]
- Traynor, G.; Shearn, A.I.; Milano, E.G.; Ordonez, M.V.; Velasco Forte, M.N.; Caputo, M.; Schievano, S.; Mustard, H.; Wray, J.; Biglino, G. The use of 3D-Printed Models in Patient Communication: A Scoping Review. J. 3D Print. Med. 2022, 6, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.H.; Kang, J.-W.; Kim, N.; Song, J.-K.; Lee, J.-W.; Lim, T.-H. Myocardial 3-Dimensional Printing for Septal Myectomy Guidance in a Patient With Obstructive Hypertrophic Cardiomyopathy. Circulation 2015, 132, 300–301. [Google Scholar] [CrossRef]
- Mounsef, P.J.; Aita, R.; Skaik, K.; Addab, S.; Hamdy, R.C. Three-dimensional-printing-guided preoperative planning of upper and lower extremity pediatric orthopedic surgeries: A systematic review of surgical outcomes. J. Child. Orthop. 2024, 18, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, X.Y.; Yu, X.F. Application of 3D printing surgical training models in the preoperative assessment of robot-assisted partial nephrectomy. BMC Surg. 2024, 24, 167. [Google Scholar] [CrossRef]
- Molinari, G.; Emiliani, N.; Cercenelli, L.; Bortolani, B.; Gironi, C.; Fernandez, I.J.; Presutti, L.; Marcelli, E. Assessment of a novel patient-specific 3D printed multi-material simulator for endoscopic sinus surgery. Front. Bioeng. Biotechnol. 2022, 10, 974021. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Jiang, H.; Yang, Z.; Li, Y. The current application of 3D printing simulator in surgical training. Front. Med. 2024, 11, 1443024. [Google Scholar] [CrossRef]
- Ock, J.; Choi, Y.; Lee, D.-G.; Chung, J.W.; Kim, N. Educational simulator for mastoidectomy considering mechanical properties using 3D printing and its usability evaluation. Sci. Rep. 2024, 14, 7661. [Google Scholar] [CrossRef]
- Aussedat, C.; Venail, F.; Marx, M.; Boullaud, L.; Bakhos, D. Training in temporal bone drilling. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2022, 139, 140–145. [Google Scholar] [CrossRef]
- Okhovat, S.; Milner, T.D.; Iyer, A. Feasibility of ovine and synthetic temporal bone models for simulation training in endoscopic ear surgery. J. Laryngol. Otol. 2019, 133, 966–973. [Google Scholar] [CrossRef]
- Anschuetz, L.; Bonali, M.; Ghirelli, M.; Mattioli, F.; Villari, D.; Caversaccio, M.; Presutti, L. An Ovine Model for Exclusive Endoscopic Ear Surgery. JAMA Otolaryngol. Head Neck Surg. 2017, 143, 247. [Google Scholar] [CrossRef]
- Zaidi, A.; Khan, M.M.; Parab, S.R. The Goat Model for Exclusive Two Handed Endoscopic Middle Ear Surgery Training: A Novel Technique. Indian. J. Otolaryngol. Head Neck Surg. 2019, 71, 1478–1484. [Google Scholar] [CrossRef]
- Bento, R.F.; Rocha, B.A.; Freitas, E.L.; Balsalobre, F.D.A. Otobone®: Three-dimensional printed Temporal Bone Biomodel for Simulation of Surgical Procedures. Int. Arch. Otorhinolaryngol. 2019, 23, e451–e454. [Google Scholar] [CrossRef]
- Probst, R.; Stump, R.; Mokosch, M.; Röösli, C. Evaluation of an Infant Temporal-Bone Model as Training Tool. Otol. Neurotol. 2018, 39, e448–e452. [Google Scholar] [CrossRef] [PubMed]
- Boillat, M.; Bonnet, A.-S.; Groubatch, F.; Falanga, A.; Gillet, R.; Parietti-Winkler, C. Analysis of the milling response of an artificial temporal bone developed for otologic surgery in comparison with human cadaveric samples. Med. Eng. Phys. 2024, 131, 104220. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, M.S.; Mosegaard, J.; Trier, P. The Visible Ear Simulator: A Public PC Application for GPU-Accelerated Haptic 3D Simulation of Ear Surgery Based on the Visible Ear Data. Otol. Neurotol. 2009, 30, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Varoquier, M.; Hoffmann, C.P.; Perrenot, C.; Tran, N.; Parietti-Winkler, C. Construct, Face, and Content Validation on Voxel-Man® Simulator for Otologic Surgical Training. Int. J. Otolaryngol. 2017, 2017, 2707690. [Google Scholar] [CrossRef] [PubMed]
- Lähde, S.; Hirsi, Y.; Salmi, M.; Mäkitie, A.; Sinkkonen, S.T. Integration of 3D-printed middle ear models and middle ear prostheses in otosurgical training. BMC Med. Educ. 2024, 24, 451. [Google Scholar] [CrossRef] [PubMed]
- Sokołowski, J.; Orłowski, A.; Lachowska, M.; Gosiewska, A.; Biecek, P.; Bartoszewicz, R.; Niemczyk, K. 3D-printed custom ossicular prosthesis—methodology of design and LDV measurements in a cadaver study. Otolaryngol. Pol. 2023, 77, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Kamrava, B.; Gerstenhaber, J.A.; Amin, M.; Har-el, Y.; Roehm, P.C. Preliminary Model for the Design of a Custom Middle Ear Prosthesis. Otol. Neurotol. 2017, 38, 839–845. [Google Scholar] [CrossRef]
- Mukherjee, P.; Cheng, K.; Chung, J.; Grieve, S.M.; Solomon, M.; Wallace, G. Precision Medicine in Ossiculoplasty. Otol. Neurotol. 2021, 42, e177–e185. [Google Scholar] [CrossRef] [PubMed]
- Heikkinen, A.-K.; Lähde, S.; Rissanen, V.; Salmi, M.A.; Aarnisalo, A.; Mäkitie, A.; Sivonen, V.; Sinkkonen, S.T. Feasibility of 3D-printed middle ear prostheses in partial ossicular chain reconstruction. IJB 2024, 9, 727. [Google Scholar] [CrossRef] [PubMed]
- Brumpt, E.; Bertin, E.; Gabrion, X.; Coussens, C.; Tatu, L.; Louvrier, A. Are 3D-printed anatomical models of the ear effective for teaching anatomy? A comparative pilot study versus cadaveric models. Surg. Radiol. Anat. 2024, 46, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Rose, A.S.; Kimbell, J.S.; Webster, C.E.; Harrysson, O.L.A.; Formeister, E.J.; Buchman, C.A. Multi-material 3D Models for Temporal Bone Surgical Simulation. Ann. Otol. Rhinol. Laryngol. 2015, 124, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Kuru, I.; Maier, H.; Müller, M.; Lenarz, T.; Lueth, T.C. A 3D-printed functioning anatomical human middle ear model. Hear. Res. 2016, 340, 204–213. [Google Scholar] [CrossRef]
- Stramiello, J.A.; Wong, S.J.; Good, R.; Tor, A.; Ryan, J.; Carvalho, D. Validation of a three-dimensional printed pediatric middle ear model for endoscopic surgery training. Laryngoscope Investig. Oto 2022, 7, 2133–2138. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emiliani, N.; Molinari, G.; Bortolani, B.; Lotto, C.; Burato, A.; D’Azzeo, R.; Anschuetz, L.; Fernandez, I.J.; Presutti, L.; Molteni, G.; et al. A Novel 3D-Printed Training Platform for Ossiculoplasty with Objective Performance Evaluation. Appl. Sci. 2025, 15, 1763. https://doi.org/10.3390/app15041763
Emiliani N, Molinari G, Bortolani B, Lotto C, Burato A, D’Azzeo R, Anschuetz L, Fernandez IJ, Presutti L, Molteni G, et al. A Novel 3D-Printed Training Platform for Ossiculoplasty with Objective Performance Evaluation. Applied Sciences. 2025; 15(4):1763. https://doi.org/10.3390/app15041763
Chicago/Turabian StyleEmiliani, Nicolas, Giulia Molinari, Barbara Bortolani, Cecilia Lotto, Arianna Burato, Rossana D’Azzeo, Lukas Anschuetz, Ignacio Javier Fernandez, Livio Presutti, Gabriele Molteni, and et al. 2025. "A Novel 3D-Printed Training Platform for Ossiculoplasty with Objective Performance Evaluation" Applied Sciences 15, no. 4: 1763. https://doi.org/10.3390/app15041763
APA StyleEmiliani, N., Molinari, G., Bortolani, B., Lotto, C., Burato, A., D’Azzeo, R., Anschuetz, L., Fernandez, I. J., Presutti, L., Molteni, G., Cercenelli, L., & Marcelli, E. (2025). A Novel 3D-Printed Training Platform for Ossiculoplasty with Objective Performance Evaluation. Applied Sciences, 15(4), 1763. https://doi.org/10.3390/app15041763






