Physical, Compressive Strength, and Microstructural Characteristics of Alkali-Activated Engineered Composites Incorporating MgO, MWCNTs, and rGO
Abstract
Featured Application
Abstract
1. Introduction
2. Experimental Program, Materials, Mix Designs, Mix Preparation, and Test Methods
2.1. Materials
2.2. Mix Proportions
2.3. Mixing, Dispersion of MWCNT/rGO, Casting, and Curing of Specimens
2.4. Test Methods
3. Results and Discussion
3.1. Physical Characteristics: Slump Flow, Flow Time, Flow Velocity and Density
3.2. Hardened Characteristics: Compressive Strength and UPV
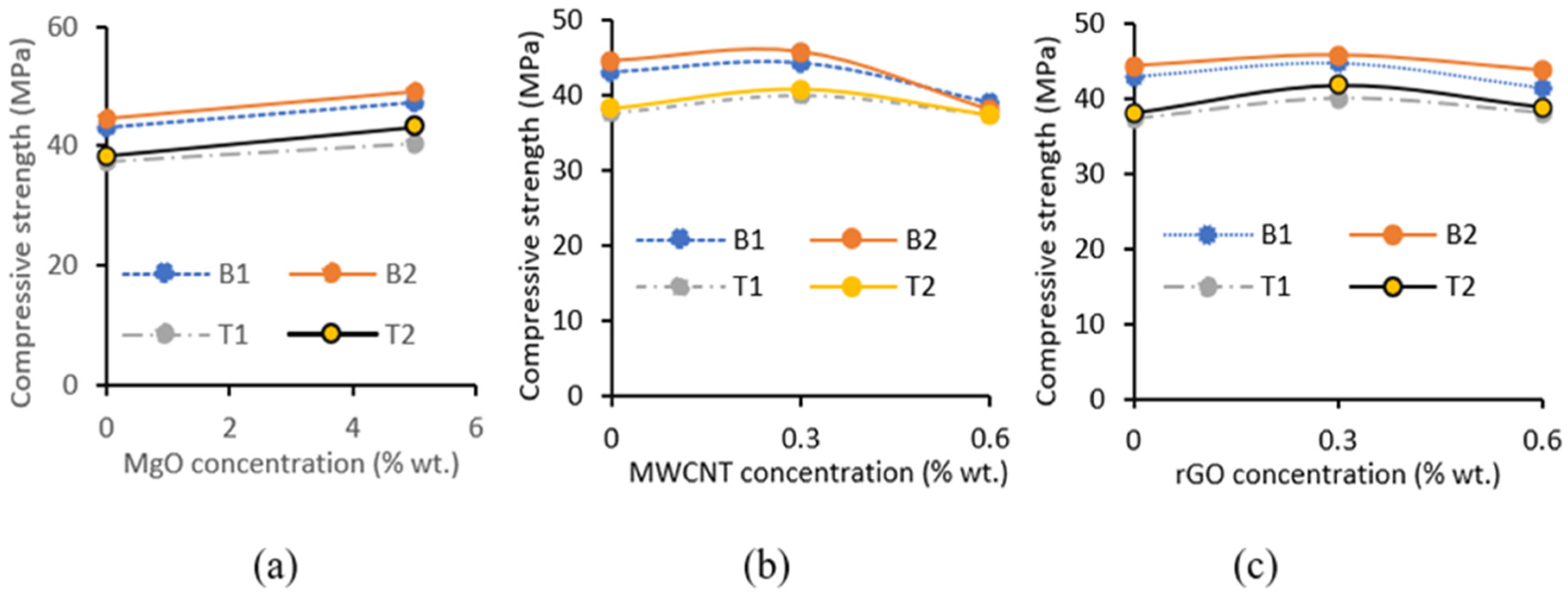
3.2.1. Influence of MgO, MWCNT and rGO on Compressive Strength Development
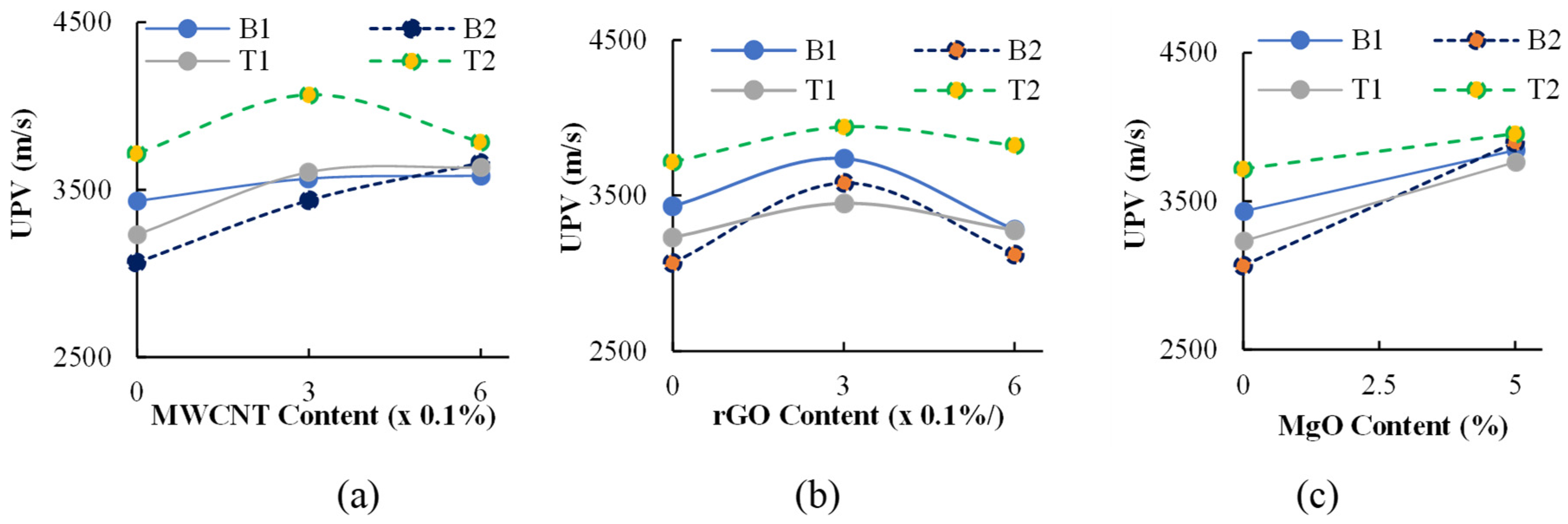
3.2.2. Influence of MgO, MWCNT, and rGO on 28-Day UPV
3.3. Microstructural Characteristics
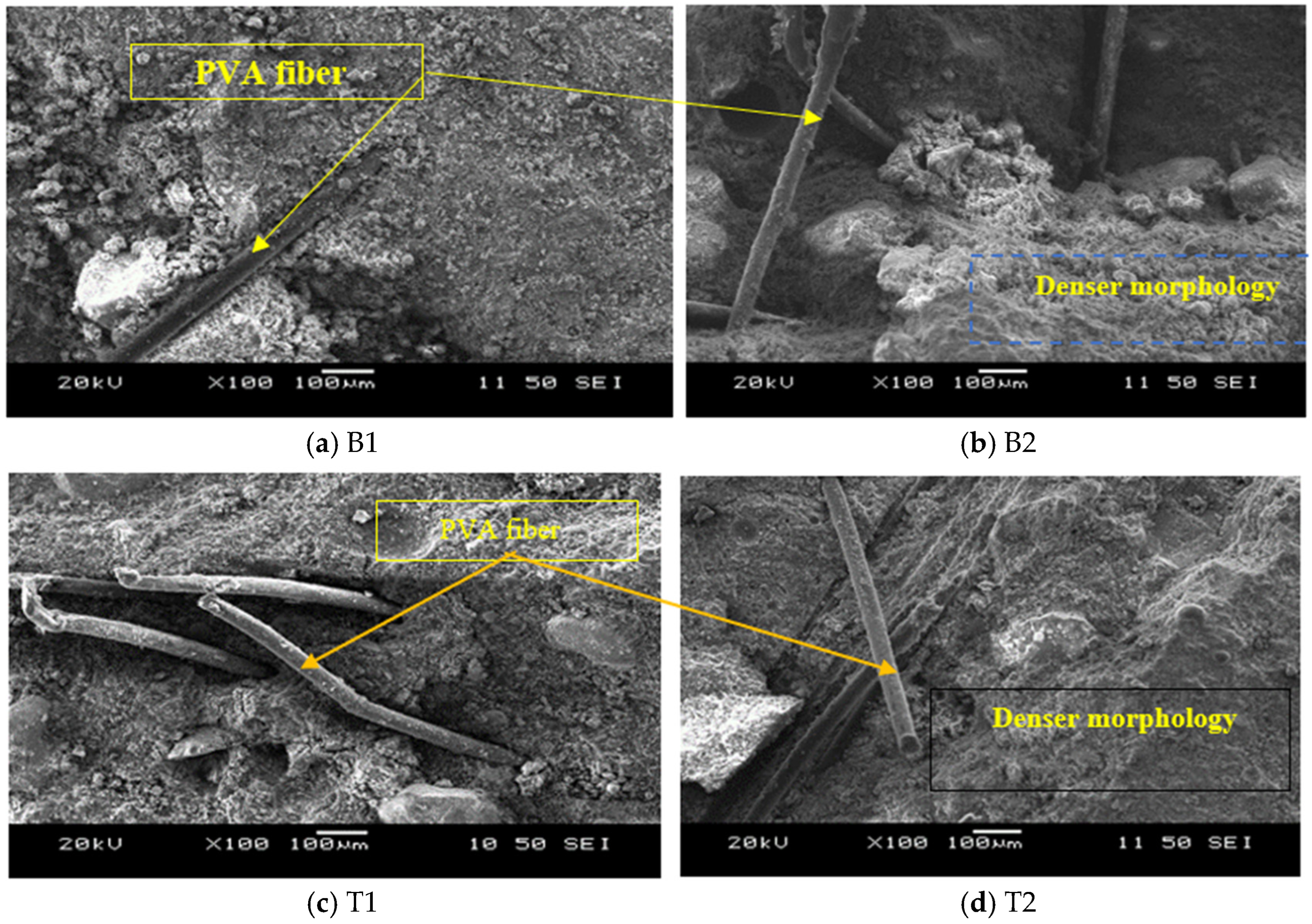
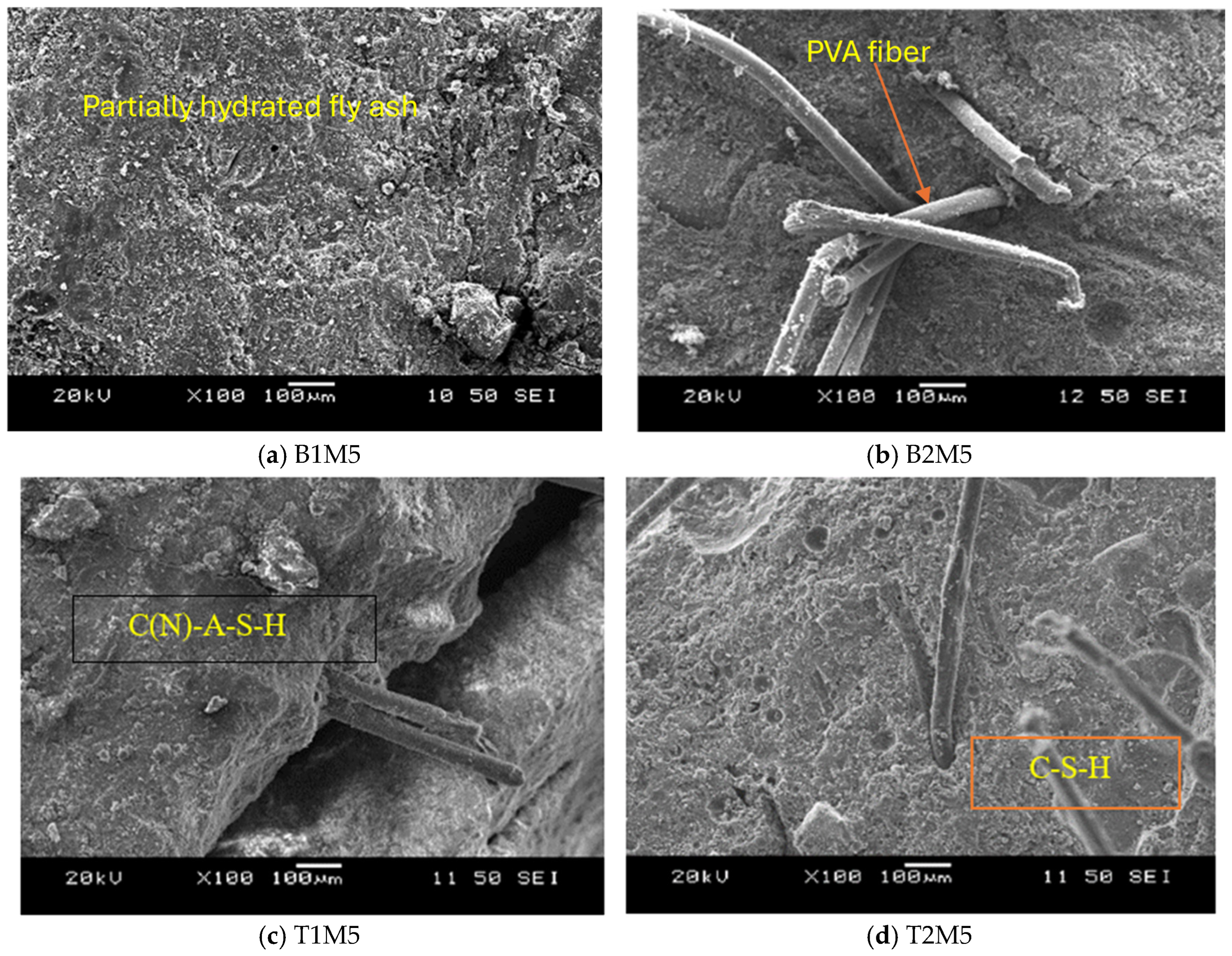
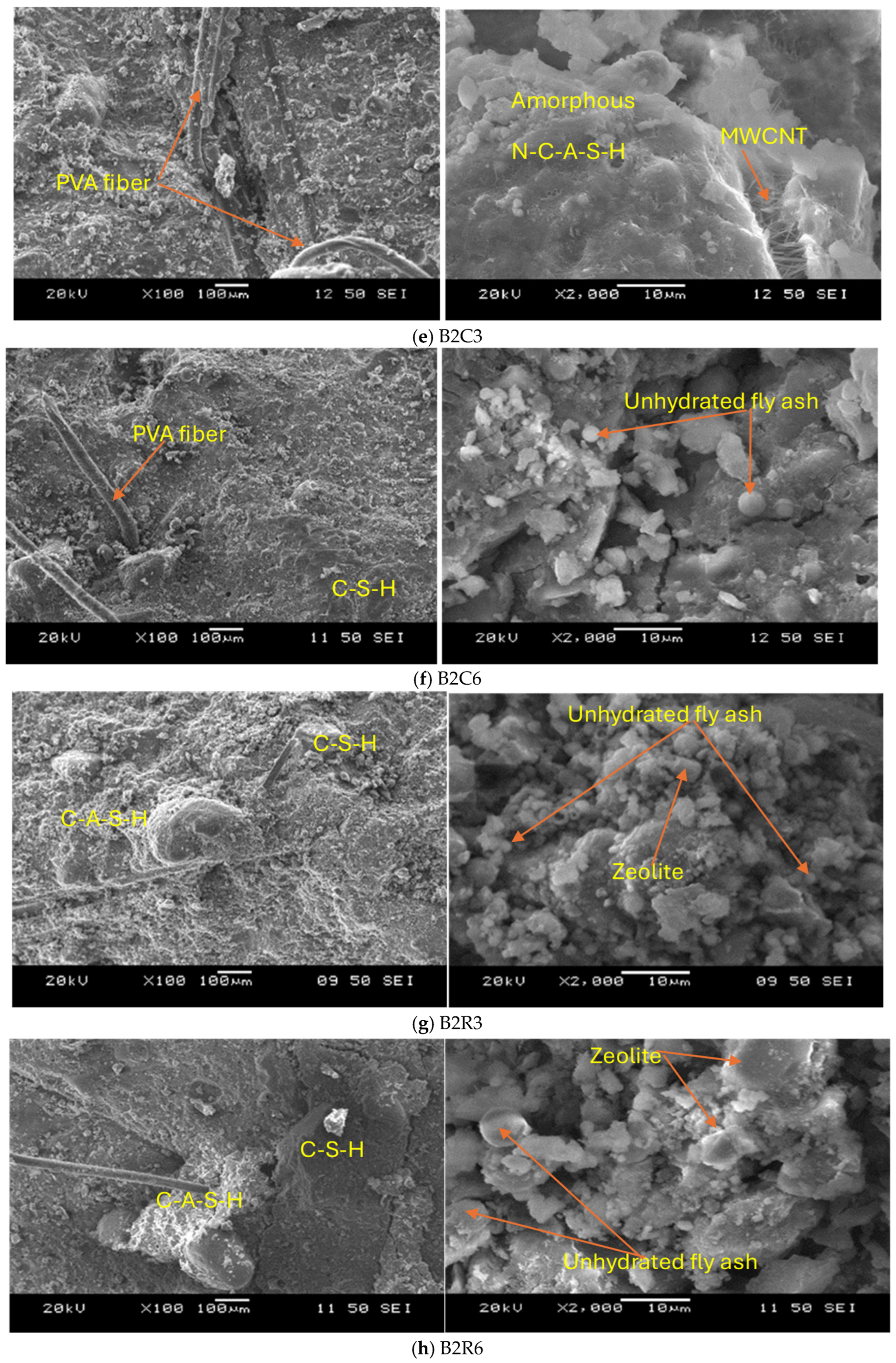
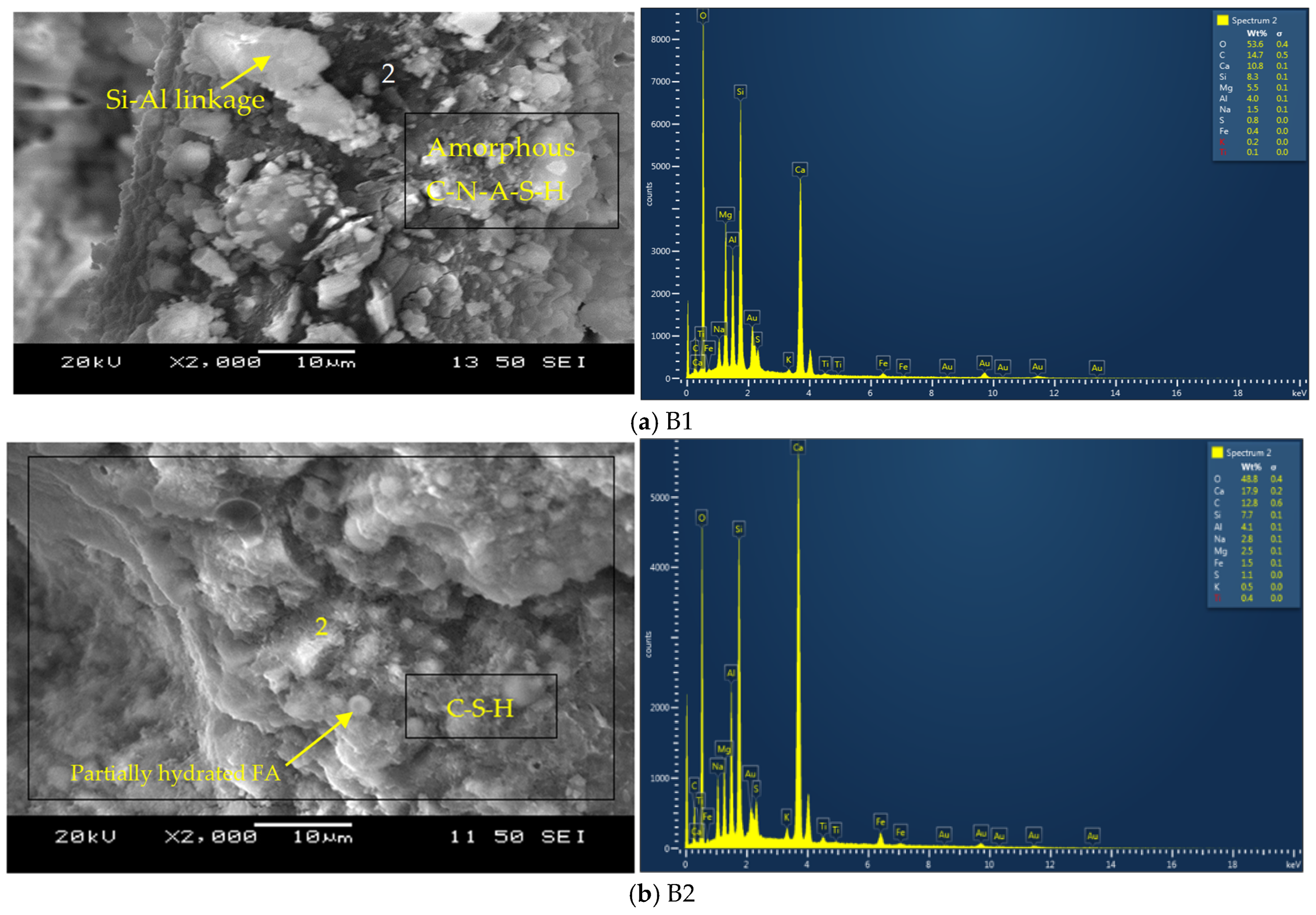
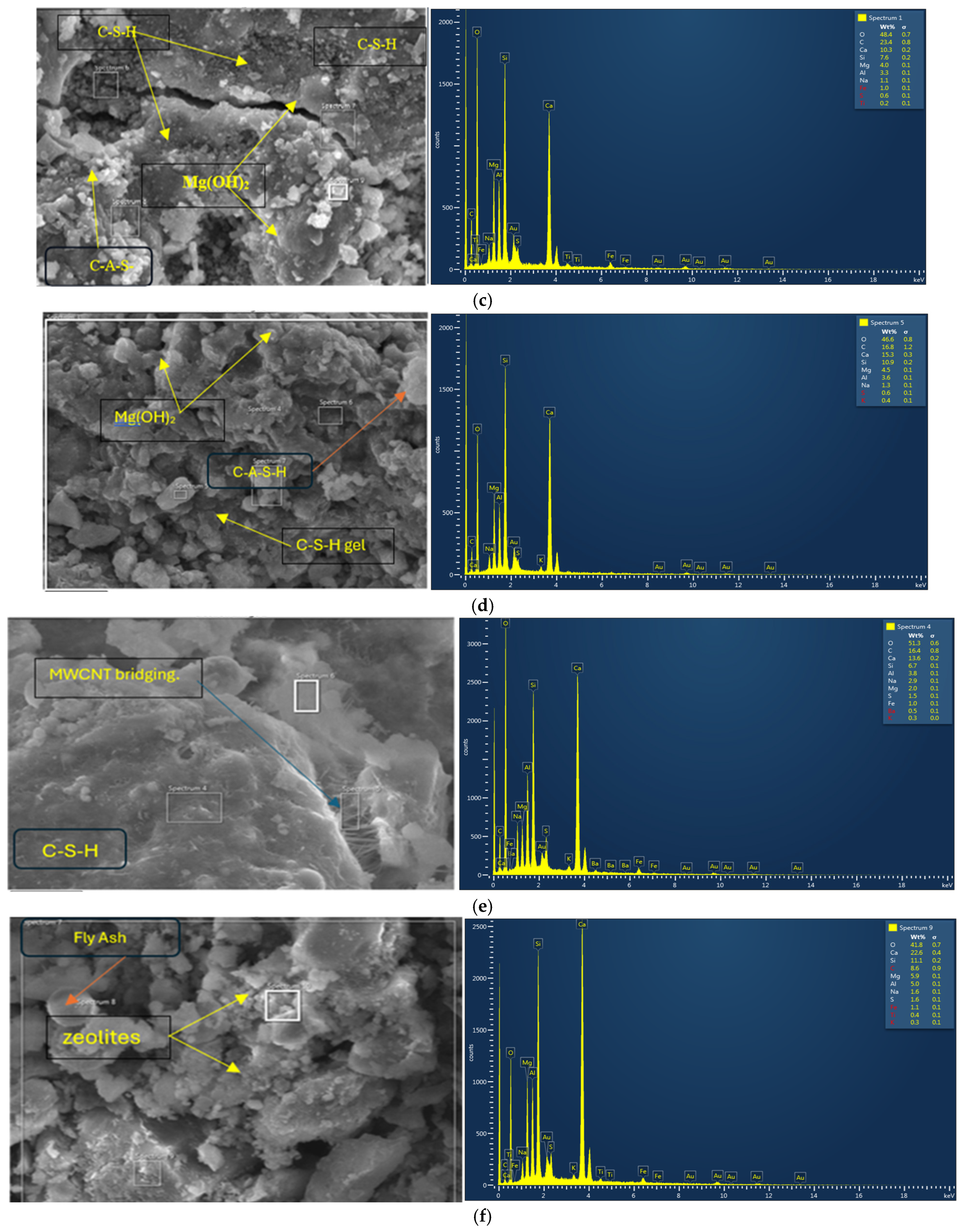
3.3.1. SEM/EDS Analyses
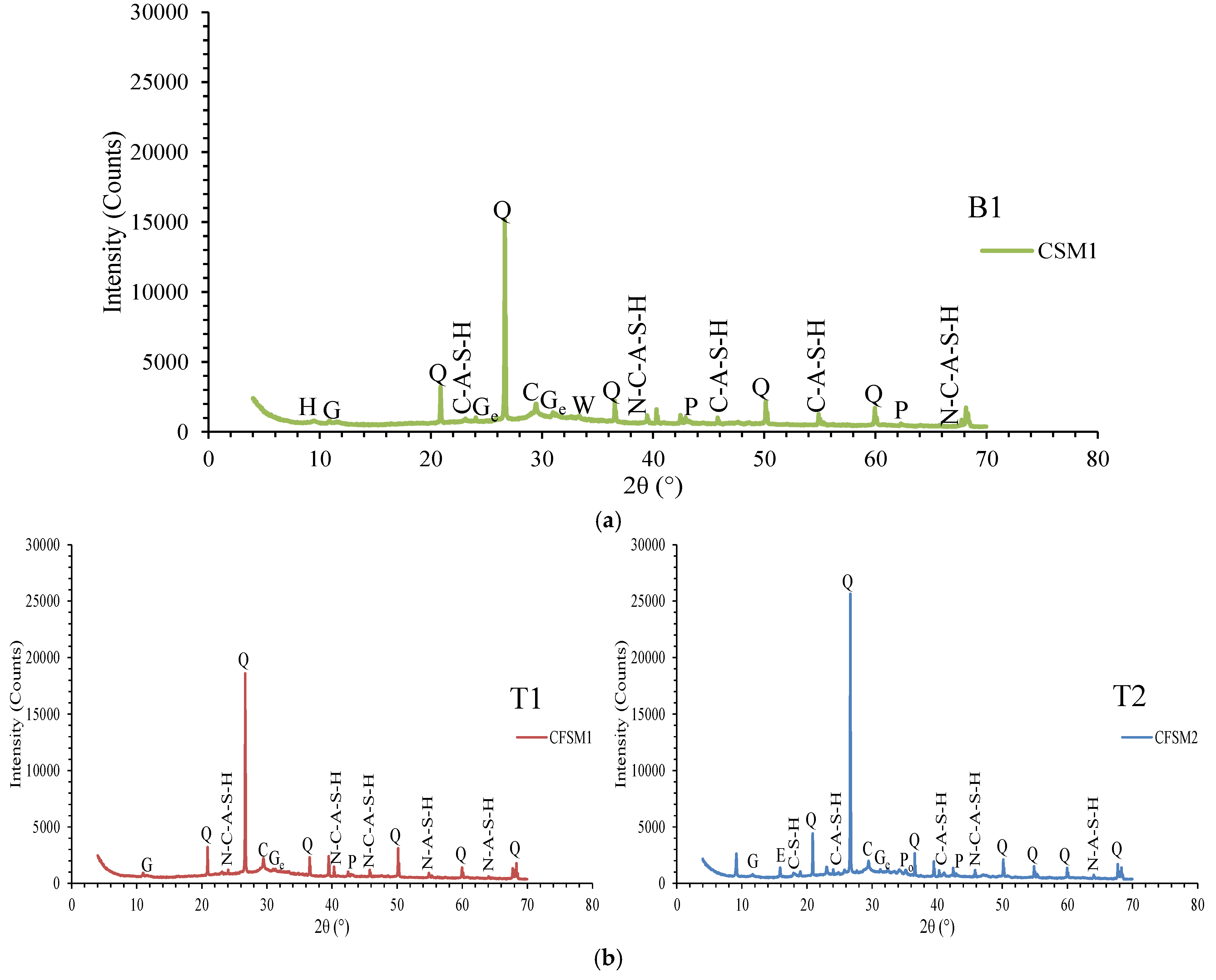
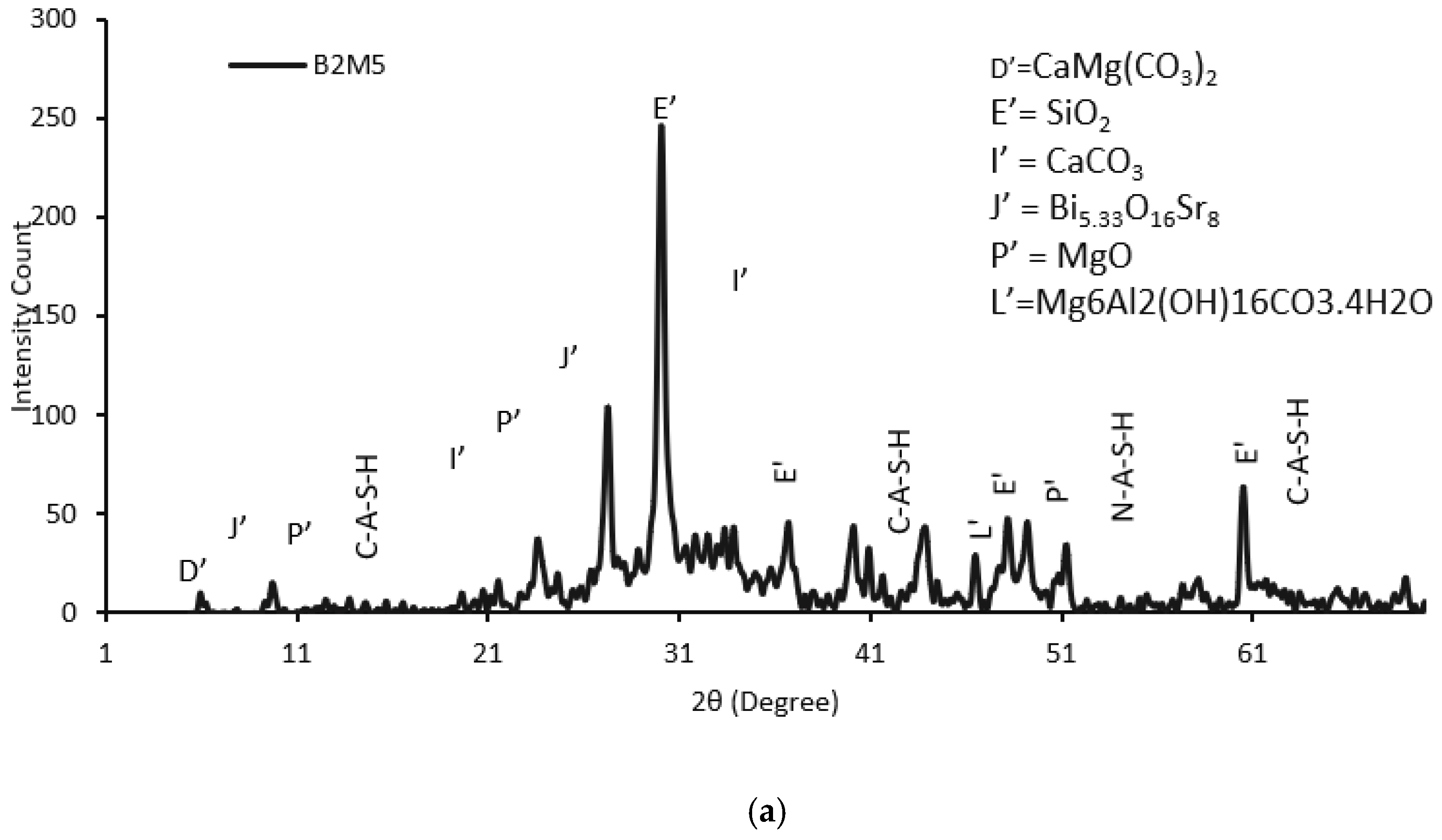
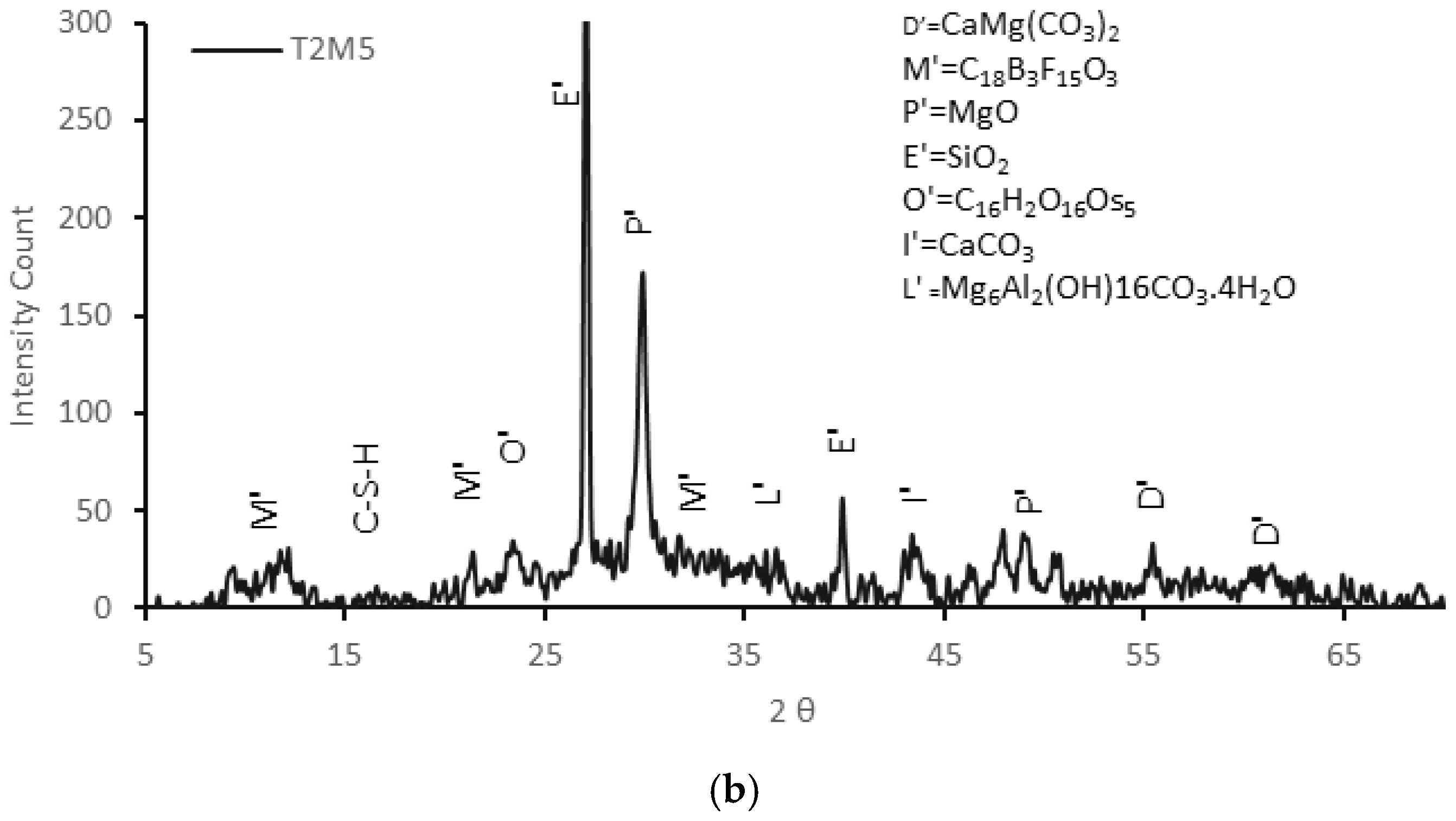
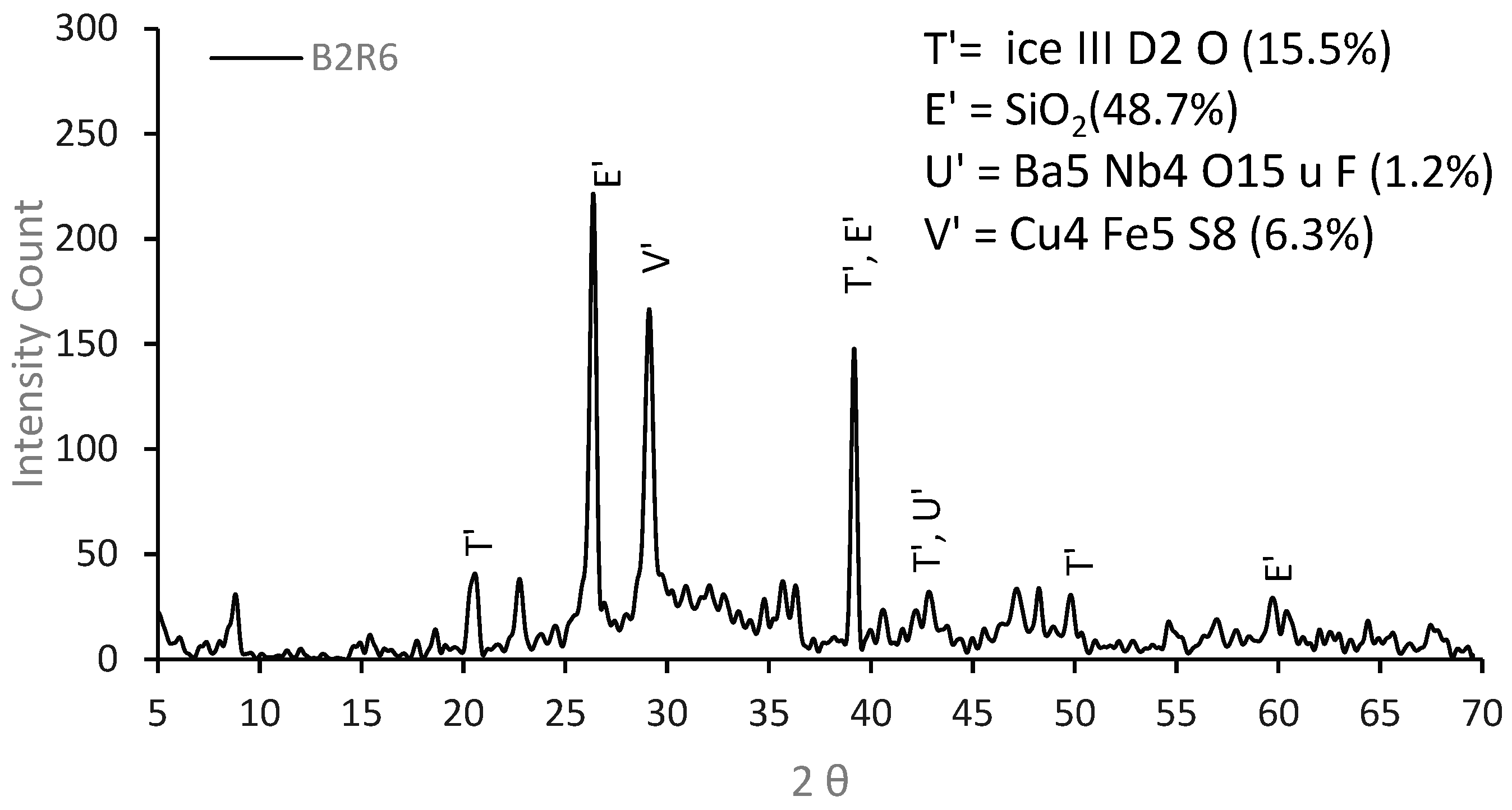
3.3.2. XRD Analysis
3.3.3. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
4. Conclusions
- The AAEC mixes satisfied the EFNARC [77] criteria for self-consolidating ability. The initial and final setting times ranged from 190 min to 311 min and from 230 min to 369 min, respectively;
- The ternary (fly ash C ‘FA-C’ + FA-F + ground granulated blast furnace slag ‘GGBFS’) mixes exhibited higher slump flows than their binary (FA-C + GGBFS) counterparts due to having a lower FA-C/GGBFS content, while the activator/reagent 2 (calcium hydroxide: sodium sulfate = 2.5:1) mixes resulted in higher slump flows due to having lower silica ratio than the mixes that used reagent 1 (calcium hydroxide: sodium metasilicate = 1:2.5);
- The slump flow of the AAECs decreased with an increase in the MWCNT/rGO content, with a few exceptions depending on mix/reagent types, while the addition of MgO showed a less significant decrease. The higher surface area or water affinity of MgO/MWCNTs and higher agglomeration tendency of MWCNTs/rGO were considered contributing factors;
- All AAECs achieved a 28-day compressive strength ranging from 26.0 MPa to 48.5 MPa > 18 MPa and satisfied the criteria for structural concrete. Control binary AAECs obtained higher 28-day compressive strengths than their ternary counterparts, due to the dominant formation of C-A-S-H/C-S-H compared to the dominant formation of amorphous N-C-A-S-H/N-A-S-H in their ternary counterparts. Binary mix B2 with reagent 2 exhibited a 22% higher 28-day compressive strength than its ternary counterpart T2 due to having higher FA-C and GGBFS contents;
- The AAECs with reagent 2 generally produced a higher compressive strength compared to their reagent 1 counterparts due to the formation of additional C-S-H gel (in addition to C-A-S-H/N-C-A-S-H or C-A-S-H/C-S-H) and more compact microstructures, as per XRD-SEM analysis, which showed the presence of sharper crystalline peaks of quartz and calcite;
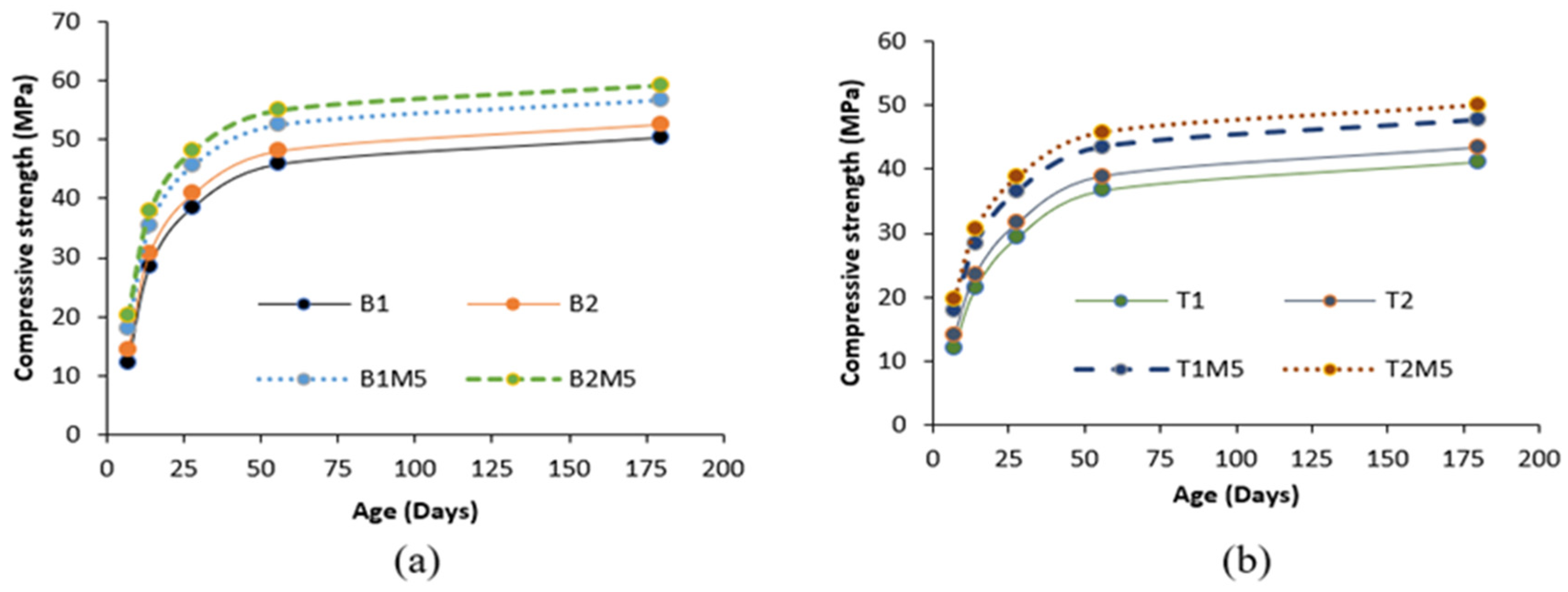
- The addition of 5% MgO increased the 28-day compressive strength (by 19~25%), and UPV exhibited potential self-healing capability due to the formation of well-dispersed fine Mg(OH)2, hydrotalcite, and M-S-H, which compensated for volume shrinkage and refined the matrix pore size, as confirmed from XRD-SEM/EDS;
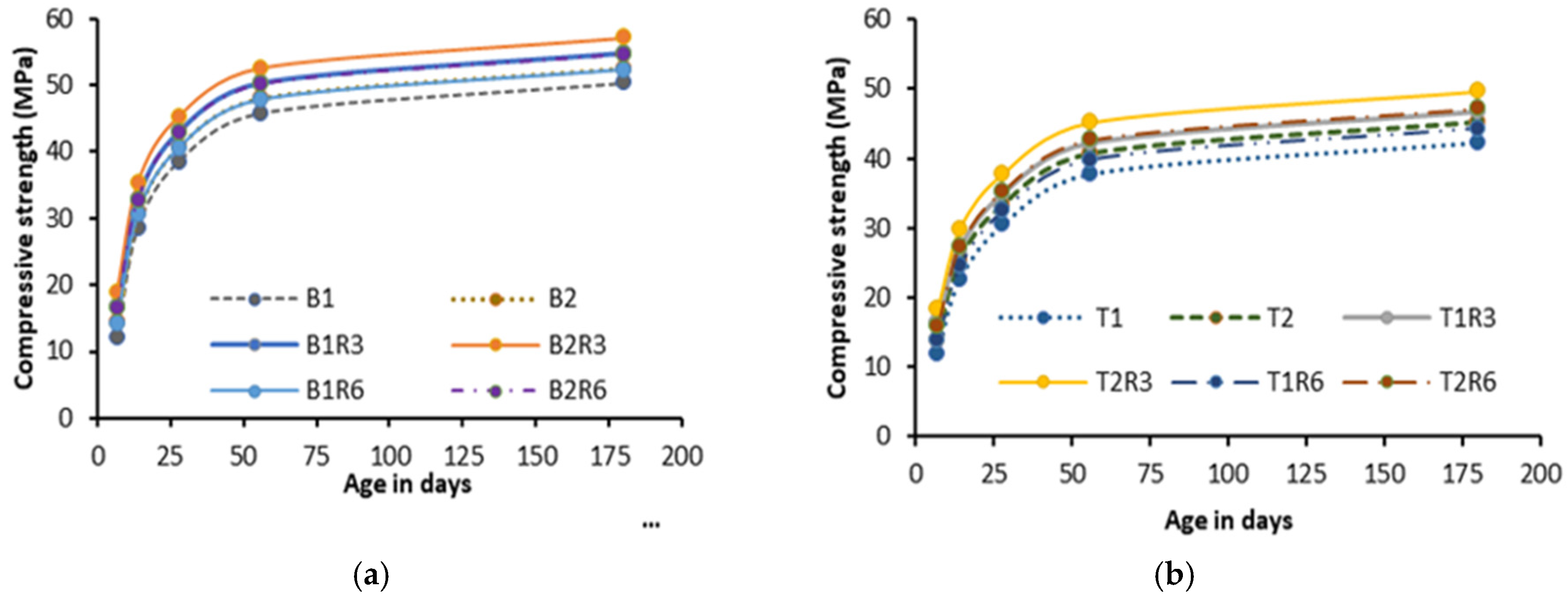
- The 28-day compressive strength increased with the increase in MWCNT/rGO content up to 0.3%, indicating it as an optimum dosage. The decrease in strength at higher dosages (>0.3% wt) of MWCNTs/rGO can be compensated for/reversed by using MgO-MWCNT/rGO combinations that show beneficial effects of MgO addition;
- The addition of 0.3% MWCNTs and rGO increased the compressive strength by 9% and 14.8%, respectively, showing a higher effectiveness of rGO in improving strength. The increase in compressive strength and UPV was attributed to the uniform dispersion and good MWCNT-matrix bond bridging cracks (with no newly formed MWCNT-induced reaction products), while the formation of additional zeolites caused matrix densification with the addition of rGO, as confirmed from the SEM/EDS analysis. Proper dispersion of the MWCNTs/rGO is important to avoid agglomeration and negative effects on the workability, mechanical, and micro-structural properties;
- Fourier transform infrared spectrometer (FTIR) analysis suggested the formation of an aluminosilicate network in AAECs with a higher concentration of Si-O-Si bonds, indicating a more stable structure. A lower % of transmittance at 3380 cm−1 in binary AAECs with reagent 2 and 0.6% MWCNTs (B2C6), compared to their counterpart with 5% MgO (B2M5), confirmed a weaker bond and lower compressive strength even though MWCNTs can act as nucleation sites and accelerate polymerization;
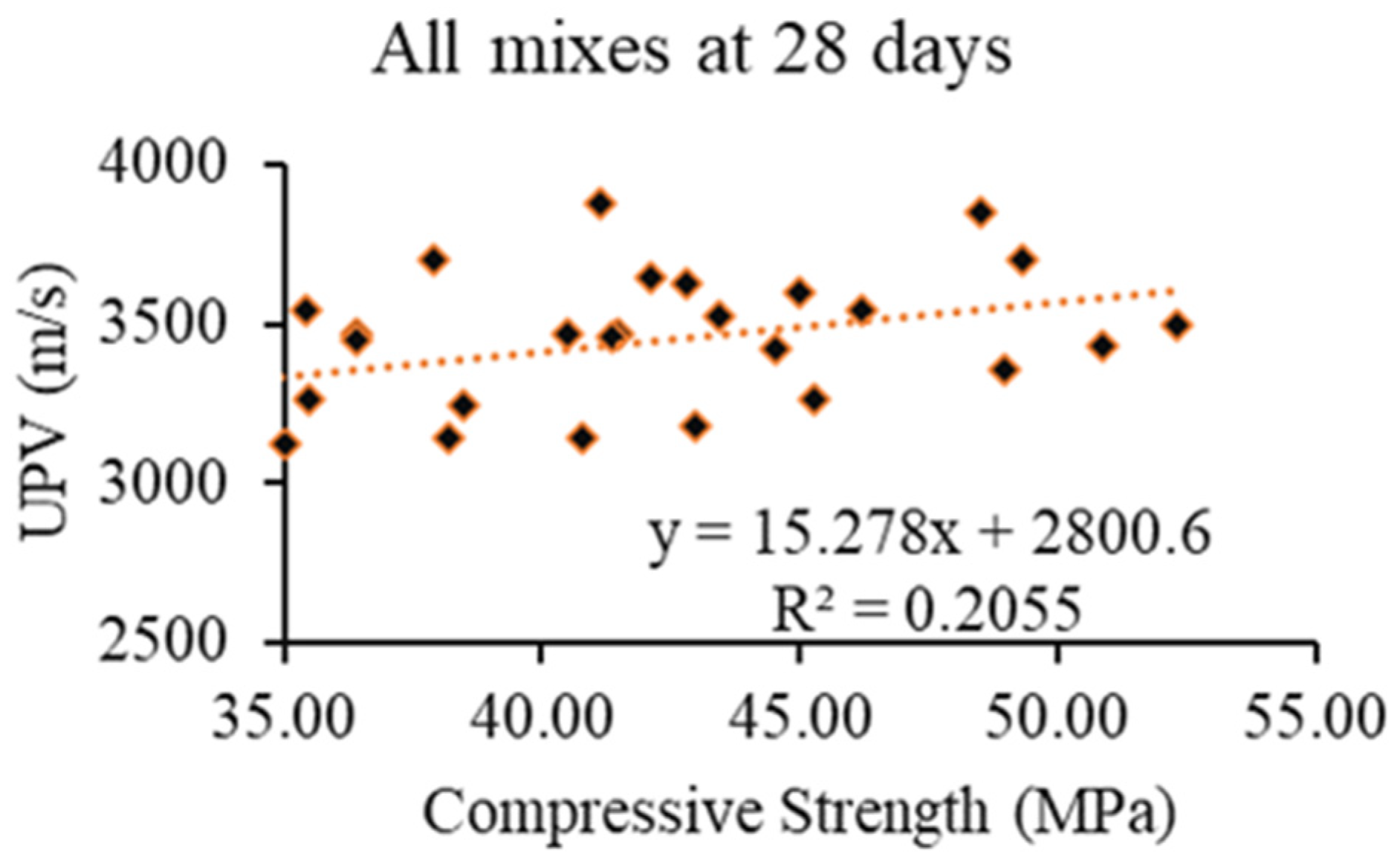
- The UPV values ranged from 3067 m/s to 4068 m/s, showing an increase with the addition MWCNTs/rGO/MgO, with the highest values (19% higher) being observed for MWCNT-incorporated AAECs. This is an indication of a higher capacity to transmit ultrasonic waves, better conductivity, and a better self-sensing ability. A linear correlation revealed an increase in compressive strength with an increase in the UPV content of AAEC mixes at 28 days;
- This research demonstrated the viability of producing ambient-cured powder-based AAECs, as more green and sustainable alternatives to conventional ECCs, that incorporate carbon-nano materials and MgO additives, as they have satisfactory workability, mechanical, and microstructural characteristics. The developed multi-functional AAECs have the potential to be used for structural applications and to produce high-performance resilient durable bridge-building infrastructures with self-healing/sensing and 3-D printing potentials.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hossain, K.M.A.; Sood, D. The Strength and Fracture Characteristics of One-Part Strain-Hardening Green Alkali-Activated Engineered Composites. Materials 2023, 16, 5077. [Google Scholar] [CrossRef]
- Huseien, G.F.; Shah, K.W. Durability and life cycle evaluation of self-compacting concrete containing fly ash as GBFS replacement with alkali activation. Constr. Build. Mater. 2020, 235, 117458. [Google Scholar] [CrossRef]
- Naqi, A.; Jang, J.G. Recent Progress in Green Cement Technology Utilizing Low-Carbon Emission Fuels and Raw Materials: A Review. Sustainability 2019, 11, 537. [Google Scholar] [CrossRef]
- Nematollahi, B.; Sanjayan, J.; Shaikh, F.U.A. Tensile Strain Hardening Behavior of PVA Fiber-Reinforced Engineered Geopolymer Composite. J. Mater. Civ. Eng. 2015, 27, 0001242. [Google Scholar] [CrossRef]
- Samantasinghar, S.; Singh, S. Effects of curing environment on strength and microstructure of alkali-activated fly ash-slag binder. Constr. Build. Mater. 2020, 235, 117481. [Google Scholar] [CrossRef]
- Shah, S.F.A.; Chen, B.; Oderji, S.Y.; Haque, M.A.; Ahmad, M.R. Improvement of early strength of fly ash-slag based one-part alkali activated mortar. Constr. Build. Mater. 2020, 246, 118533. [Google Scholar] [CrossRef]
- Davidovits, P.J. 30 Years of Successes and Failures in Geopolymer Applications. In Proceedings of the Geopolymer 2002 Conference, Melbourne, VIC, Australia, 28–29 October 2002; pp. 1–16. [Google Scholar]
- Huseien, G.F.; Shah, K.W. Performance evaluation of alkali-activated mortars containing industrial wastes as surface repair materials. J. Build. Eng. 2020, 30, 101234. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Mid-infrared spectroscopic studies of alkali-activated fly ash structure. Microporous Mesoporous Mater. 2005, 86, 207–214. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X. Self-healing of concrete cracks by use of bacteria-containing low alkali cementitious material. Constr. Build. Mater. 2018, 167, 1–14. [Google Scholar] [CrossRef]
- Mehta, A.; Siddique, R.; Ozbakkaloglu, T.; Uddin Ahmed Shaikh, F.; Belarbi, R. Fly ash and ground granulated blast furnace slag-based alkali-activated concrete: Mechanical, transport and microstructural properties. Constr. Build. Mater. 2020, 257, 119548. [Google Scholar] [CrossRef]
- Khan, M. Evidence on the functional form of inflation and output growth variability relationship in European economies. Int. Econ. 2016, 146, 119548. [Google Scholar] [CrossRef]
- Khan, M.Z.N.; Shaikh, F.; Uddin, A.; Hao, Y.; Hao, H. Synthesis of high strength ambient cured geopolymer composite by using low calcium fly ash. Constr. Build. Mater. 2016, 125, 809–820. [Google Scholar] [CrossRef]
- Herbert, S.J.; Sakthieswaran, N.; Shiny, B.G. Review on geopolymer concrete with different additives. Int. J. Eng. Res. 2015, 1, 21–31. [Google Scholar]
- Shah, S.P.; Konsta-Gdoutos, M.S.; Metaxa, Z.S.; Mondal, P. Nanoscale Modification of Cementitious Materials. In Nanotechnology in Construction 3: Proceedings of the NICOM3; Springer: Berlin, Germany, 2009; pp. 125–130. [Google Scholar] [CrossRef]
- Guo, X.; Shi, H.; Dick, W.A. Compressive strength and microstructural characteristics of class C fly ash geopolymer. Cem. Concr. Compos. 2010, 32, 142–147. [Google Scholar] [CrossRef]
- Saafi, M.; Tang, L.; Fung, J.; Rahman, M.; Liggat, J. Enhanced properties of graphene/fly ash geopolymeric composite cement. Cem. Concr. Res. 2015, 67, 292–299. [Google Scholar] [CrossRef]
- Zhang, C.; Khorshidi, H.; Najafi, E.; Ghasemi, M. Fresh, mechanical and microstructural properties of alkali-activated composites incorporating nanomaterials: A comprehensive review. J. Clean. Prod. 2023, 384, 135390. [Google Scholar] [CrossRef]
- Abdollahnejad, Z.; Dalvand, A.; Mastali, M.; Luukkonen, T.; Illikainen, M. Effects of waste ground glass and lime on the crystallinity and strength of geopolymers. Mag. Concr. Res. 2019, 71, 1218–1231. [Google Scholar] [CrossRef]
- Mastali, M.; Kinnunen, P.; Dalvand, A.; Mohammadi Firouz, R.; Illikainen, M. Drying shrinkage in alkali-activated binders–A critical review. Constr. Build. Mater. 2018, 190, 533–550. [Google Scholar] [CrossRef]
- Kheradmand, M.; Abdollahnejad, Z.; Pacheco-Torgal, F. Shrinkage Performance of Fly Ash Alkali-activated Cement Based Binder Mortars. KSCE J. Civ. Eng. 2018, 22, 1854–1864. [Google Scholar] [CrossRef]
- Sherir, M.A.A.; Hossain, K.M.A.; Lachemi, M. Self-healing and expansion characteristics of cementitious composites with high volume fly ash and MgO-type expansive agent. Constr. Build. Mater. 2016, 127, 80–92. [Google Scholar] [CrossRef]
- MacKenzie, K.J.D.; Bolton, M.J. Electrical and mechanical properties of aluminosilicate inorganic polymer composites with carbon nanotubes. J. Mater. Sci. 2009, 44, 2851–2857. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, D. Multifunctional structural supercapacitor based on graphene and geopolymer. Electrochim. Acta 2017, 224, 105–112. [Google Scholar] [CrossRef]
- Wang, S.; Li, V. Engineered Cementitious Composites with High-volume Fly Ash. ACI Mat. J. 2007, 104, 233–241. [Google Scholar]
- Bhutta, A.; Borges, P.H.R.; Zanotti, C.; Farooq, M.; Banthia, N. Flexural behavior of geopolymer composites reinforced with steel and polypropylene macro fibers. Cem. Concr. Compos. 2017, 80, 31–40. [Google Scholar] [CrossRef]
- Nematollahi, B.; Sanjayan, J.; Shaikh, F.U.A. Matrix design of strain hardening fiber reinforced engineered geopolymer composite. Compos. Part B Eng. 2016, 89, 253–265. [Google Scholar] [CrossRef]
- Nguyen, H.; Carvelli, V.; Adesanya, E.; Kinnunen, P.; Illikainen, M. High performance cementitious composite from alkali-activated ladle slag reinforced with polypropylene fibers. Cem. Concr. Compos. 2018, 90, 150–160. [Google Scholar] [CrossRef]
- Ranjbar, N.; Mehrali, M.; Mehrali, M.; Alengaram, U.J.; Jumaat, M.Z. High tensile strength fly ash based geopolymer composite using copper coated micro steel fiber. Constr. Build. Mater. 2016, 112, 629–638. [Google Scholar] [CrossRef]
- Shaikh, F.U.A. Deflection hardening behaviour of short fibre reinforced fly ash based geopolymer composites. Mater. Des. 2013, 50, 674–682. [Google Scholar] [CrossRef]
- Yu, J.; Chen, Y.; Leung, C.K.Y. Mechanical performance of Strain-Hardening Cementitious Composites (SHCC) with hybrid polyvinyl alcohol and steel fibers. Compos. Struct. 2019, 226, 111198. [Google Scholar] [CrossRef]
- Alrefaei, Y.; Dai, J.-G. Tensile behavior and microstructure of hybrid fiber ambient cured one-part engineered geopolymer composites. Constr. Build. Mater. 2018, 184, 419–431. [Google Scholar] [CrossRef]
- Bahari, A.; Sadeghi-Nik, A.; Roodbari, M.; Sadeghi-Nik, A.; Mirshafiei, E. Experimental and theoretical studies of ordinary Portland cement composites contains nano LSCO perovskite with Fokker-Planck and chemical reaction equations. Constr. Build. Mater. 2018, 163, 247–255. [Google Scholar] [CrossRef]
- Sadeghi-Nik, A.; Berenjian, J.; Bahari, A.; Safaei, A.S.; Dehestani, M. Modification of microstructure and mechanical properties of cement by nanoparticles through a sustainable development approach. Constr. Build. Mater. 2017, 155, 880–891. [Google Scholar] [CrossRef]
- Abbasi, S.M.; Ahmadi, H.; Khalaj, G.; Ghasemi, B. Microstructure and mechanical properties of a metakaolinite-based geopolymer nanocomposite reinforced with carbon nanotubes. Ceram. Int. 2016, 42, 15171–15176. [Google Scholar] [CrossRef]
- Nazar, S.; Yang, J.; Khan, M.S.; Quazi, M.F.; Li, H.; Rahim, A. Investigating the effects of MWCNTs on sustainable alkali-activated mortar: Physical, rheological, and mechanical analysis. Mater. Today Sustain. 2023, 24, 100519. [Google Scholar] [CrossRef]
- Rovnaník, P.; Šimonová, H.; Topolář, L.; Bayer, P.; Schmid, P.; Keršner, Z. Carbon nanotube reinforced alkali-activated slag mortars. Constr. Build. Mater. 2016, 119, 223–229. [Google Scholar] [CrossRef]
- Khater, H.M.; Abd El Gawaad, H.A. Characterization of alkali activated geopolymer mortar doped with MWCNT. Constr. Build. Mater. 2016, 102, 329–337. [Google Scholar] [CrossRef]
- Li, F.; Yang, Z.; Zheng, A.; Li, S. Properties of modified engineered geopolymer composites incorporating multi-walled carbon Nanotubes(MWCNTs) and granulated blast furnace Slag(GBFS). Ceram. Int. 2021, 47, 14244–14259. [Google Scholar] [CrossRef]
- Rovnaník, P.; Šimonová, H.; Topolář, L.; Schmid, P.; Keršner, Z. Effect of Carbon Nanotubes on the Mechanical Fracture Properties of Fly Ash Geopolymer. Procedia Eng. 2016, 151, 321–328. [Google Scholar] [CrossRef]
- Jittabut, P.; Horpibulsuk, S. Physical and Microstructure Properties of Geopolymer Nanocomposite Reinforced with Carbon Nanotubes. Mater. Today: Proc. 2019, 17, 1682–1692. [Google Scholar] [CrossRef]
- Yuan, J.; He, P.; Jia, D.; Fu, S.; Zhang, Y.; Liu, X.; Cai, D.; Yang, Z.; Duan, X.; Wang, S.; et al. In situ processing of MWCNTs/leucite composites through geopolymer precursor. J. Eur. Ceram. Soc. 2017, 37, 2219–2226. [Google Scholar] [CrossRef]
- Hilding, J.; Grulke, E.A.; George Zhang, Z.; Lockwood, F. Dispersion of Carbon Nanotubes in Liquids. J. Dispers. Sci. Technol. 2003, 24, 1–41. [Google Scholar] [CrossRef]
- Schaefer, D.W.; Brown, J.M.; Anderson, D.P.; Zhao, J.; Chokalingam, K.; Tomlin, D.; Ilavsky, J. Structure and dispersion of carbon nanotubes. J. Appl. Crystallogr. 2003, 36, 553–557. [Google Scholar] [CrossRef]
- Vaisman, L.; Wagner, H.D.; Marom, G. The role of surfactants in dispersion of carbon nanotubes. Adv. Colloid Interface Sci. 2006, 128–130, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Revathi, T.; Jeyalakshmi, R. Fly ash–GGBS geopolymer in boron environment: A study on rheology and microstructure by ATR FT-IR and MAS NMR. Constr. Build. Mater. 2021, 267, 120965. [Google Scholar] [CrossRef]
- Zhou, G.-X.; Li, C.; Zhao, Z.; Qi, Y.-Z.; Yang, Z.-H.; Jia, D.-C.; Zhong, J.; Zhou, Y. 3D printing geopolymer nanocomposites: Graphene oxide size effects on a reactive matrix. Carbon 2020, 164, 215–223. [Google Scholar] [CrossRef]
- Ranjbar, N.; Mehrali, M.; Mehrali, M.; Alengaram, U.J.; Jumaat, M.Z. Graphene nanoplatelet-fly ash based geopolymer composites. Cem. Concr. Res. 2015, 76, 222–231. [Google Scholar] [CrossRef]
- Dong, Q.; Wan, L.; Luan, C.; Wang, J.; Du, P. Effect of Graphene Oxide on Properties of Alkali-Activated Slag. Materials 2021, 14, 6107. [Google Scholar] [CrossRef] [PubMed]
- Matalkah, F.; Soroushian, P. Graphene nanoplatelet for enhancement the mechanical properties and durability characteristics of alkali activated binder. Constr. Build. Mater. 2020, 249, 118773. [Google Scholar] [CrossRef]
- Xu, G.; Zhong, J.; Shi, X. Influence of graphene oxide in a chemically activated fly ash. Fuel 2018, 226, 644–657. [Google Scholar] [CrossRef]
- Gao, P.; Lu, X.; Tang, M. Shrinkage and expansive strain of concrete with fly ash and expansive agent. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2009, 24, 150–153. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, W.; Wang, R.; Chen, F.; Jia, X.; Cong, P. Effects of reactive MgO on the reaction process of geopolymer. Materials 2019, 12, 526. [Google Scholar] [CrossRef]
- Abdel-Gawwad, H.A.; Abd El-Aleem, S. Effect of reactive magnesium oxide on properties of alkali-activated slag geopolymer cement pastes. Res. Gate 2015, 59, 37–47. [Google Scholar]
- Haha, M.B.; Lothenbach, B.; Le Saout, G.; Winnefeld, F. Influence of slag chemistry on the hydration of alkali-activated blast-furnace slag—Part I: Effect of MgO. Cem. Concr. Res. 2011, 41, 955–963. [Google Scholar] [CrossRef]
- Shen, W.; Wang, Y.; Zhang, T.; Zhou, M.; Li, J.; Cui, X. Magnesia modification of alkali-activated slag fly ash cement. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2011, 26, 121–125. [Google Scholar] [CrossRef]
- Jin, F.; Gu, K.; Al-Tabbaa, A. Strength and drying shrinkage of reactive MgO modified alkali-activated slag paste. Constr. Build. Mater. 2014, 51, 395–404. [Google Scholar] [CrossRef]
- Ružek, V.; Dostayeva, A.M.; Walter, J.; Grab, T.; Korniejenko, K. Carbon Fiber-Reinforced Geopolymer Composites: A Review. Fibers 2023, 11, 17. [Google Scholar] [CrossRef]
- Hossain, M.A.; Hossain, K.M.A. Rheological, Fresh State, and Strength Characteristics of Alkali-Activated Mortars Incorporating MgO and Carbon Nanoparticles. Materials 2024, 17, 5931. [Google Scholar] [CrossRef] [PubMed]
- Sood, D.; Hossain, K.M.A. Fresh State, Rheological and Microstructural Characteristics of Alkali-Activated Mortars Developed Using Novel Dry Mixing Technique under Ambient Conditions. Appl. Sci. 2021, 11, 8920. [Google Scholar] [CrossRef]
- Sood, D.; Hossain, K.M.A. Strength, Fracture and Durability Characteristics of Ambient Cured Alkali—Activated Mortars Incorporating High Calcium Industrial Wastes and Powdered Reagents. Crystals 2021, 11, 1167. [Google Scholar] [CrossRef]
- Cheaptubes. April 2024. Available online: https://www.cheaptubes.com/product-category/multi-walled-carbon-nanotubes/ (accessed on 22 October 2024).
- Yang, S.; Yue, W.; Huang, D.; Chen, C.; Lin, H.; Yang, X. A facile green strategy for rapid reduction of graphene oxide by metallic zinc. RSC Adv. 2012, 2, 8827. [Google Scholar] [CrossRef]
- Fan, Z.-J.; Kai, W.; Yan, J.; Wei, T.; Zhi, L.-J.; Feng, J.; Ren, Y.; Song, L.-P.; Wei, F. Facile Synthesis of Graphene Nanosheets via Fe Reduction of Exfoliated Graphite Oxide. ACS Nano 2011, 5, 191–198. [Google Scholar] [CrossRef]
- Chen, J.; Akono, A.-T. Influence of multi-walled carbon nanotubes on the fracture response and phase distribution of metakaolin-based potassium geopolymers. J. Mater. Sci. 2021, 56, 19403–19424. [Google Scholar] [CrossRef]
- Luz, G.; Gleize, P.J.P.; Batiston, E.R.; Pelisser, F. Effect of pristine and functionalized carbon nanotubes on microstructural, rheological, and mechanical behaviors of metakaolin-based geopolymer. Cem. Concr. Compos. 2019, 104, 103332. [Google Scholar] [CrossRef]
- Jindal, B.B.; Sharma, R. The effect of nanomaterials on properties of geopolymers derived from industrial by-products: A state-of-the-art review. Constr. Build. Mater. 2020, 252, 119028. [Google Scholar] [CrossRef]
- Wang, W.; Fan, C.; Wang, B.; Zhang, X.; Liu, Z. Workability, rheology, and geopolymerization of fly ash geopolymer: Role of alkali content, modulus, and water–binder ratio. Constr. Build. Mater. 2023, 367, 130357. [Google Scholar] [CrossRef]
- Davoodabadi, M.; Vareli, I.; Liebscher, M.; Tzounis, L.; Sgarzi, M.; Paipetis, A.; Yang, J.; Cuniberti, G.; Mechtcherine, V. Thermoelectric Energy Harvesting from Single-Walled Carbon Nanotube Alkali-Activated Nanocomposites Produced from Industrial Waste Materials. Nanomaterials 2021, 11, 1095. [Google Scholar] [CrossRef]
- Suave, J.; Coelho, L.A.F.; Amico, S.C.; Pezzin, S.H. Effect of sonication on thermo-mechanical properties of epoxy nanocomposites with carboxylated-SWNT. Mater. Sci. Eng. A 2009, 509, 57–62. [Google Scholar] [CrossRef]
- ASTM C1611; Standard Test Method for Slump Flow of Self-Consolidating Concrete. ASTM International: West Conshohocken, PA, USA, 2014; pp. 1–6.
- ASTM C138/C138M; Standard Test Method for Density (Unit Weight), Yield, and Air Content (Gravimetric) of Concrete. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM C1437; Standard Test Method for Flow of Hydraulic Cement Mortar. ASTM International: West Conshohocken, PA, USA, 2015.
- ASTM C109/C109M; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50-mm] Cube Specimens). ASTM International: West Conshohocken, PA, USA, 2016.
- ASTM C597-16; Standard Test Method for Pulse Velocity Through Concrete. ASTM: West Conshohocken, PA, USA, 2016.
- Kastiukas, G.; Ruan, S.; Liang, S.; Zhou, X. Development of precast geopolymer concrete via oven and microwave radiation curing with an environmental assessment. J. Clean. Prod. 2020, 255, 120290. [Google Scholar] [CrossRef]
- EFNARC Specification. Guidelines for Self-Compacting Concrete; Report from EFNARC; Association House: London, UK, 2002; p. 32. [Google Scholar]
- Abbas, S.; Qissab, M. Effect of Multi-Walled Carbon Nanotubes on Mechanical Properties of Concrete. Coll. Eng. J. (NUCEJ) 2016, 91, 6192–6194. [Google Scholar]
- Gado, R.A.; Hebda, M.; Łach, M.; Mikuła, J. Alkali Activation of Waste Clay Bricks: Influence of The Silica Modulus, SiO2/Na2O, H2O/Na2O Molar Ratio, and Liquid/Solid Ratio. Materials 2020, 13, 383. [Google Scholar] [CrossRef] [PubMed]
- Allahverdi, A.; Kani, E.N. Use of construction and demolition waste (CDW) for alkali-activated or geopolymer cements. In Handbook of Recycled Concrete and Demolition Waste; Elsevier: Amsterdam, The Netherlands, 2013; pp. 439–475. [Google Scholar] [CrossRef]
- ACI 318. Building Code Requirements for Structural Concrete; American Concrete Institute: Farmington Hills, MI, USA, 2019. [Google Scholar]
- Kaya, M.; Koksal, F.; Gencel, O.; Munir, M.J.; Kazmi, S.M.S. Influence of micro Fe2O3 and MgO on the physical and mechanical properties of the zeolite and kaolin based geopolymer mortar. J. Build. Eng. 2022, 52, 104443. [Google Scholar] [CrossRef]
- Asil, M.B.; Ranjbar, M.M. Hybrid effect of carbon nanotubes and basalt fibers on mechanical, durability, and microstructure properties of lightweight geopolymer concretes. Constr. Build. Mater. 2022, 357, 129352. [Google Scholar] [CrossRef]
- Ofuyatan, O.; Olowofoyeku, A.; Oluwafemi, J.; Ighalo, J. Predicting the Compressive Strength of Concrete By Ultrasonic Pulse Velocity. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1036, 012053. [Google Scholar] [CrossRef]
- Wei, J.; Yang, Q.; Jiang, Q.; Li, X.; Liu, S.; Li, K.; Wang, Q. Mechanical properties of basalt fiber reinforced ambient-cured lightweight expanded polystyrene geopolymer concrete. J. Build. Eng. 2023, 80, 108072. [Google Scholar] [CrossRef]
- Ryu, G.S.; Lee, Y.B.; Koh, K.T.; Chung, Y.S. The mechanical properties of fly ash-based geopolymer concrete with alkaline activators. Constr. Build. Mater. 2013, 47, 409–418. [Google Scholar] [CrossRef]
- Mo, L.; Deng, M.; Wang, A. Effects of MgO-based expansive additive on compensating the shrinkage of cement paste under non-wet curing conditions. Cem. Concr. Compos. 2012, 34, 377–383. [Google Scholar] [CrossRef]
- Bakharev, T. Geopolymeric materials prepared using Class F fly ash and elevated temperature curing. Cem. Concr. Res. 2005, 35, 1224–1232. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.; Qi, S.; Xu, L.; Shi, G.; Ding, Y.; Yan, X.; Huang, Y.; Geng, J. Graphene Oxide Facilitates Solvent-Free Synthesis of Well-Dispersed, Faceted Zeolite Crystals. Angew. Chem. 2017, 129, 14278–14283. [Google Scholar] [CrossRef]
- Hanjitsuwan, S.; Hunpratub, S.; Thongbai, P.; Maensiri, S.; Sata, V.; Chindaprasirt, P. Effects of NaOH concentrations on physical and electrical properties of high calcium fly ash geopolymer paste. Cem. Concr. Compos. 2014, 45, 9–14. [Google Scholar] [CrossRef]
- Pan, Z.; Tao, Z.; Cao, Y.F.; Wuhrer, R.; Murphy, T. Compressive strength and microstructure of alkali-activated fly ash/slag binders at high temperature. Cem. Concr. Compos. 2018, 86, 9–18. [Google Scholar] [CrossRef]
- Sherir, M.A.A.; Hossain, K.M.A.; Lachemi, M. Permeation and Transport Properties of Self-Healed Cementitious Composite Produced with MgO Expansive Agent. J. Mater. Civ. Eng. 2018, 30, 04018291. [Google Scholar] [CrossRef]
- Jin, F.; Abdollahzadeh, A.; Al-Tabbaa, A. Effect of Different MgO on the Hydration of MgO-Activated Granulated Ground Blastfurnace Slag Paste. 2013. Available online: http://www.claisse.info/Proceedings.htm (accessed on 22 June 2024).
- Zhang, T.; Cheeseman, C.R.; Vandeperre, L.J. Development of low pH cement systems forming magnesium silicate hydrate (M-S-H). Cement Concr. Res. 2011, 41, 439–442. [Google Scholar] [CrossRef]
- Brew, D.R.M.; Glasser, F.P. Synthesis and characterisation of magnesium silicate hydrate gels. Cem. Concr. Res. 2005, 35, 85–98. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Y.; Li, M.; Jiang, J.; Guo, L.; Wang, W.; Zhang, W.; Zhang, Z.; Duan, P. Effects of graphene oxide on microstructure and mechanical properties of graphene oxide-geopolymer composites. Constr. Build. Mater. 2020, 247, 118544. [Google Scholar] [CrossRef]
- Mužek, M.N.; Zelić, J.; Jozić, D. Microstructural characteristics of geopolymers based on alkali-activated fly ash. Chem. Biochem. Eng. Q. 2012, 26, 89–95. [Google Scholar]
- Swanepoel, J.C.; Strydom, C.A. Utilisation of fly ash in a geopolymeric material. Appl. Geochem. 2002, 17, 1143–1148. [Google Scholar] [CrossRef]
- Álvarez-Ayuso, E.; Querol, X.; Plana, F.; Alastuey, A.; Moreno, N.; Izquierdo, M.; Font, O.; Moreno, T.; Diez, S.; Vázquez, E.; et al. Environmental, physical and structural characterisation of geopolymer matrixes synthesised from coal (co-) combustion fly ashes. J. Hazard. Mater. 2008, 154, 175–183. [Google Scholar] [CrossRef]
- Kupwade-Patil, K.; Allouche, E.N. Impact of Alkali Silica Reaction on Fly Ash-Based Geopolymer Concrete. J. Mater. Civ. Eng. 2013, 25, 131–139. [Google Scholar] [CrossRef]
- Mollah, M.Y.A.; Hess, T.R.; Cocke, D.L. Surface and bulk studies of leached and unleached fly ash using XPS, SEM, EDS and FTIR techniques. Cem. Concr. Res. 1994, 24, 109–118. [Google Scholar] [CrossRef]
- Mozgawa, W.; Sitarz, M.; Rokita, M. Spectroscopic studies of different aluminosilicate structures. J. Mol. Struct. 1999, 511–512, 251–257. [Google Scholar] [CrossRef]
- Sitarz, M.; Handke, M.; Mozgawa, W. Identification of silicooxygen rings in SiO2 based on IR spectra. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2000, 56, 1819–1823. [Google Scholar] [CrossRef]
- Reig, L.; Soriano, L.; Borrachero, M.V.; Monzó, J.; Payá, J. Influence of calcium aluminate cement (CAC) on alkaline activation of red clay brick waste (RCBW). Cem. Concr. Compos. 2016, 65, 177–185. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Zhang, W.; Li, Z.; Zhang, Y.; Li, Y.; Ren, Y. Effects of Si/Al ratio on the efflorescence and properties of fly ash-based geopolymer. J. Clean. Prod. 2020, 244, 118852. [Google Scholar] [CrossRef]
- Wan, Q.; Zhang, Y.; Zhang, R. Using mechanical activation of quartz to enhance the compressive strength of metakaolin-based geopolymers. Cem. Concr. Compos. 2020, 111, 103635. [Google Scholar] [CrossRef]
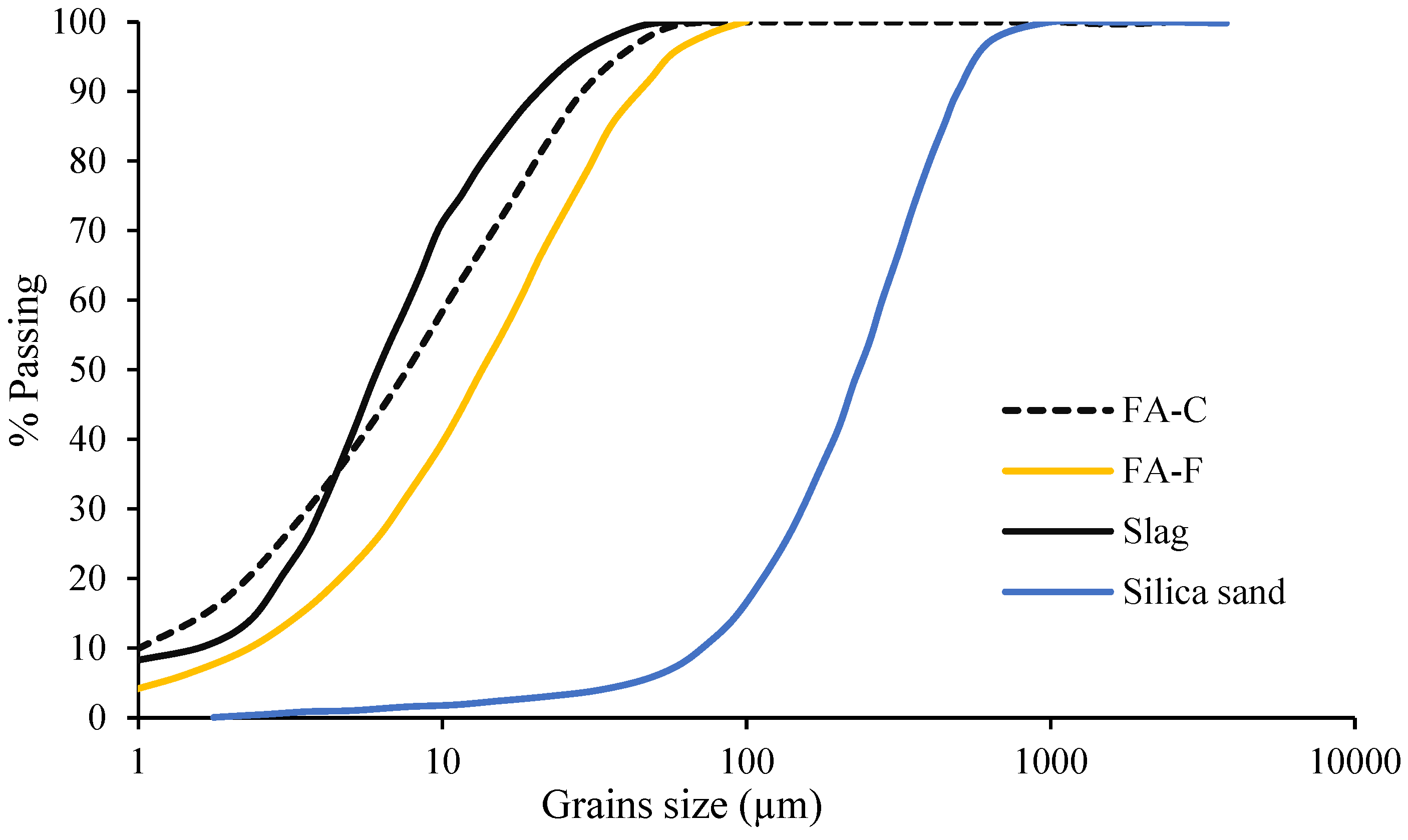








| Chemical Composition (%) | Fly Ash Type C (FA-C) | Fly Ash Type F (FA-F) | Ground Granulated Blast Furnace Slag (GGBFS) | Silica Sand | HRWRA | Magnesium Oxide (MgO) |
|---|---|---|---|---|---|---|
| SiO2 | 36.53 | 55.66 | 35.97 | 99.70 | 2.02 | |
| Al2O3 | 18.26 | 22.09 | 9.18 | 0.14 | 6.124 | |
| Fe2O3 | 5.66 | 4.26 | 0.50 | 0.016 | 0.94 | |
| CaO | 20.97 | 7.97 | 38.61 | 0.01 | 2.40 | |
| MgO | 5.08 | 1.16 | 10.99 | 0.01 | 92.26 | |
| K2O | 0.68 | 1.49 | 0.36 | 0.04 | - | |
| Na2O | 4.04 | 4.10 | 0.28 | 0.01 | - | |
| MnO | 0.03 | 0.03 | 0.25 | 0.00 | - | |
| TiO2 | 1.26 | 0.61 | 0.39 | 0.00 | - | |
| P2O5 | 0.96 | 0.43 | 0.01 | 0.00 | - | |
| L.O.I. | 2.18 | 1.05 | 0.74 | 0.00 | 1.14 | |
| pH | 6.00 | |||||
| Density (g/cm3) | 2.61 | 2.02 | 2.87 | 2.65 | 1.06 | 3.58 |
| Retained on 45µ, % | - | 18.00 | - | 3.00 | ||
| Blaine fineness (m2/kg) | 315.00 | 306.00 | 489.30 | - |
| AAECs Mix ID. | Total SCMs (Binder *) | MgO/MWCNT/rGO | SCMs | Reagent Component Ratio | R./B | Chemical Ratios (SCMs + Reagent) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FA-C | FA-F | GGBFS | SiO2/ Al2O3 | Na2O/ SiO2 | CaO/ SiO2 | Na2O/ Al2O3 | |||||
| Four basic AAEC mixes (with 0% MgO/MWCNT/rGO) | |||||||||||
| B1 | 1 | 0 | 0.55 | 0 | 0.45 | 1:2.5 | 0.09 | 2.62 | 0.09 | 0.84 | 0.23 |
| B2 | 1 | 0 | 0.55 | 0 | 0.45 | 2.5:1 | 0.12 | 2.56 | 0.14 | 1.02 | 0.35 |
| T1 | 1 | 0 | 0.25 | 0.35 | 0.40 | 1:2.5 | 0.09 | 2.75 | 0.08 | 0.59 | 0.22 |
| T2 | 1 | 0 | 0.25 | 0.35 | 0.40 | 2.5:1 | 0.12 | 2.69 | 0.12 | 0.73 | 0.32 |
| Four AAEC mixes with 5% MgO | |||||||||||
| B1M5 | 1 | 0.05 | 0.52 | 0 | 0.43 | 1:2.5 | 0.09 | 2.58 | 0.09 | 0.85 | 0.23 |
| B2M5 | 1 | 0.05 | 0.52 | 0 | 0.43 | 2.5:1 | 0.12 | 2.51 | 0.14 | 1.03 | 0.35 |
| T1M5 | 1 | 0.05 | 0.24 | 0.33 | 0.38 | 1:2.5 | 0.09 | 2.97 | 0.05 | 0.54 | 0.14 |
| T2M5 | 1 | 0.05 | 0.24 | 0.33 | 0.38 | 2.5:1 | 0.12 | 2.97 | 0.05 | 0.54 | 0.14 |
| Eight AAEC mixes with 0.3% and 0.6% MWCNT | |||||||||||
| B1C3 | 1 | 0.003 | 0.55 | 0 | 0.45 | 1:2.5 | 0.09 | 2.62 | 0.09 | 0.84 | 0.23 |
| B2C3 | 1 | 0.003 | 0.55 | 0 | 0.45 | 2.5:1 | 0.12 | 2.56 | 0.14 | 1.02 | 0.35 |
| T1C3 | 1 | 0.003 | 0.25 | 0.35 | 0.40 | 1:2.5 | 0.09 | 2.75 | 0.08 | 0.59 | 0.22 |
| T2C3 | 1 | 0.003 | 0.25 | 0.35 | 0.40 | 2.5:1 | 0.12 | 2.69 | 0.12 | 0.73 | 0.32 |
| B1C6 | 1 | 0.006 | 0.55 | 0 | 0.45 | 1:2.5 | 0.09 | 2.62 | 0.09 | 0.84 | 0.23 |
| B2C6 | 1 | 0.006 | 0.55 | 0 | 0.45 | 2.5:1 | 0.12 | 2.56 | 0.14 | 1.02 | 0.35 |
| T1C6 | 1 | 0.006 | 0.25 | 0.35 | 0.40 | 1:2.5 | 0.09 | 2.75 | 0.08 | 0.59 | 0.22 |
| T2C6 | 1 | 0.006 | 0.25 | 0.35 | 0.40 | 2.5:1 | 0.12 | 2.69 | 0.12 | 0.73 | 0.32 |
| Eight AAEC mixes with 0.3% and 0.6% rGO | |||||||||||
| B1R3 | 1 | 0.003 | 0.55 | 0 | 0.45 | 1:2.5 | 0.09 | 2.62 | 0.09 | 0.84 | 0.23 |
| B2R3 | 1 | 0.003 | 0.55 | 0 | 0.45 | 2.5:1 | 0.12 | 2.56 | 0.14 | 1.02 | 0.35 |
| T1R3 | 1 | 0.003 | 0.25 | 0.35 | 0.40 | 1:2.5 | 0.09 | 2.75 | 0.08 | 0.59 | 0.22 |
| T2R3 | 1 | 0.003 | 0.25 | 0.35 | 0.40 | 2.5:1 | 0.12 | 2.69 | 0.12 | 0.73 | 0.32 |
| B1R6 | 1 | 0.006 | 0.55 | 0 | 0.45 | 1:2.5 | 0.09 | 2.62 | 0.09 | 0.84 | 0.23 |
| B2R6 | 1 | 0.006 | 0.55 | 0 | 0.45 | 2.5:1 | 0.12 | 2.56 | 0.14 | 1.02 | 0.35 |
| T1R6 | 1 | 0.006 | 0.25 | 0.35 | 0.40 | 1:2.5 | 0.09 | 2.75 | 0.08 | 0.59 | 0.22 |
| T2R6 | 1 | 0.006 | 0.25 | 0.35 | 0.40 | 2.5:1 | 0.12 | 2.69 | 0.12 | 0.73 | 0.32 |
| Eight AAEC with 5%MgO and MWCNT or rGO (0.3% or 0.6%)) | |||||||||||
| B2M5C3 | 1 | 0.05/0.003 | 0.52 | 0 | 0.43 | 2.5:1 | 0.12 | 2.56 | 0.14 | 1.02 | 0.35 |
| T2M5C3 | 1 | 0.05/0.003 | 0.24 | 0.33 | 0.38 | 2.5:1 | 0.12 | 2.97 | 0.05 | 0.54 | 0.14 |
| B2M5C6 | 1 | 0.05/0.003 | 0.52 | 0 | 0.43 | 2.5:1 | 0.12 | 2.56 | 0.14 | 1.02 | 0.35 |
| T2M5C6 | 1 | 0.05/0.003 | 0.24 | 0.33 | 0.38 | 2.5:1 | 0.12 | 2.97 | 0.05 | 0.54 | 0.14 |
| B2M5R3 | 1 | 0.05/0.006 | 0.52 | 0 | 0.43 | 2.5:1 | 0.12 | 2.56 | 0.14 | 1.02 | 0.35 |
| T2M5R3 | 1 | 0.05/0.006 | 0.24 | 0.33 | 0.38 | 2.5:1 | 0.12 | 2.97 | 0.05 | 0.54 | 0.14 |
| B2M5R6 | 1 | 0.05/0.006 | 0.52 | 0 | 0.43 | 2.5:1 | 0.12 | 2.56 | 0.14 | 1.02 | 0.35 |
| T2M5R6 | 1 | 0.05/0.006 | 0.24 | 0.33 | 0.38 | 2.5:1 | 0.12 | 2.97 | 0.05 | 0.54 | 0.14 |
| AAECMix ID | Fresh Density (kg/m3) | Slump Flow (mm) | Slump Flow Time (s) | Slump Flow Velocity (mm/s) | * Hard/Dry Density kg/m3 | * 28-Day UPV (m/s) | * 28-Day Compressive Strength (MPa) |
|---|---|---|---|---|---|---|---|
| B1 | 1921 | 700 | 4 | 175.0 | 2000 | 3432 | 38.5 |
| B2 | 1924 | 705 | 5 | 141.0 | 2032 | 3067 | 40.8 |
| T1 | 1930 | 710 | 3 | 236.7 | 2120 | 3231 | 30.5 |
| T2 | 1914 | 750 | 3 | 250.0 | 2064 | 3717 | 33.4 |
| B1M5 | 1924 | 690 | 5 | 138.0 | 2016 | 3845 | 46.2 |
| B2M5 | 1923 | 685 | 5 | 137.0 | 2072 | 3896 | 48.5 |
| T1M5 | 1927 | 700 | 4 | 175.0 | 1984 | 3762 | 38.2 |
| T2M5 | 1933 | 705 | 4 | 176.3 | 2056 | 3954 | 41.1 |
| B1C3 | 1930 | 720 | 4 | 180.0 | 2008 | 3566 | 41.5 |
| B2C3 | 1930 | 660 | 4 | 165.0 | 2080 | 3436 | 44.6 |
| T1C3 | 1903 | 710 | 3 | 236.7 | 2016 | 3605 | 33.2 |
| T2C3 | 1928 | 725 | 3 | 241.7 | 2096 | 4068 | 36.4 |
| B1C6 | 1950 | 700 | 5 | 140.0 | 2044 | 3582 | 35.5 |
| B2C6 | 1950 | 670 | 5 | 134.0 | 2036 | 3659 | 36.4 |
| T1C6 | 1935 | 630 | 4 | 157.5 | 1960 | 3634 | 26.0 |
| T2C6 | 1938 | 710 | 4 | 177.5 | 2168 | 3785 | 28.6 |
| B1R3 | 1939 | 650 | 4 | 162.5 | 2032 | 3738 | 43.0 |
| B2R3 | 1978 | 620 | 4 | 155.0 | 2152 | 3582 | 45.3 |
| T1R3 | 1882 | 625 | 3 | 200.0 | 2060 | 3450 | 35.0 |
| T2R3 | 1911 | 720 | 3 | 240.0 | 2096 | 3943 | 37.9 |
| B1R6 | 1945 | 620 | 4 | 155.0 | 2060 | 3282 | 40.5 |
| B2R6 | 1954 | 660 | 4 | 165.0 | 2060 | 3122 | 42.8 |
| T1R6 | 1953 | 735 | 3 | 245.0 | 2068 | 3278 | 32.5 |
| T2R6 | 1953 | 780 | 2 | 260.0 | 2176 | 3823 | 35.4 |
| AAEC Mix ID | Compressive Strength (MPa) * | Strength Change with Respect to Control (%) (+ve: Increase, −ve: Decrease) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 7 Days | 14 Days | 28 Days | 56 Days | 180 Days | 7 Days | 14 Days | 28 Days | 56 Days | 180 Days | |
| Control AAECs | ||||||||||
| B1 | 14.3 | 28.5 | 38.5 | 45.8 | 50.3 | 0 | 0 | 0 | 0 | 0 |
| B2 | 15.6 | 30.8 | 40.8 | 48.1 | 52.6 | 0 | 0 | 0 | 0 | 0 |
| T1 | 14.9 | 22.5 | 30.5 | 37.8 | 42.3 | 0 | 0 | 0 | 0 | 0 |
| T2 | 15 | 25.4 | 33.4 | 40.7 | 45.2 | 0 | 0 | 0 | 0 | 0 |
| 5% MgO Incorporated AAECs | ||||||||||
| B1M5 | 20.1 | 35.5 | 46.2 | 52.6 | 56.8 | 40.4 | 24.6 | 20 | 14.8 | 12.9 |
| B2M5 | 21.4 | 37.8 | 48.5 | 54.9 | 59.1 | 37.1 | 22.7 | 18.9 | 14.1 | 12.4 |
| T1M5 | 20.7 | 29.5 | 38.2 | 44.6 | 48.8 | 38.9 | 31.1 | 25.2 | 18 | 15.4 |
| T2M5 | 20.7 | 32.4 | 41.1 | 47.5 | 51.7 | 38.6 | 27.6 | 23.1 | 16.7 | 14.4 |
| MWCNT (0.3% and 0.6%) incorporated AAEC | ||||||||||
| B1C3 | 17.9 | 30.5 | 41.5 | 49.4 | 53.9 | 25.1 | 7 | 7.8 | 7.8 | 7.1 |
| B2C3 | 19.4 | 34.6 | 44.6 | 51.9 | 56.4 | 24 | 12.2 | 9.2 | 7.8 | 7.1 |
| T1C3 | 18.4 | 26 | 33.2 | 41.3 | 45.8 | 23.5 | 15.6 | 8.9 | 9.3 | 8.3 |
| T2C3 | 18.5 | 28.9 | 36.4 | 44.2 | 48.7 | 23.4 | 13.8 | 9 | 8.6 | 7.7 |
| B1C6 | 15.3 | 29.5 | 35.5 | 44.2 | 48.4 | 7 | 3.5 | −7.8 | −3.5 | −3.8 |
| B2C6 | 16.6 | 31.8 | 36.4 | 45.3 | 50.3 | 6.4 | 3.2 | −10.8 | −5.8 | −4.4 |
| T1C6 | 15.9 | 20.2 | 26 | 36.2 | 40.4 | 6.7 | −10.2 | −14.8 | −4.2 | −4.5 |
| T2C6 | 16 | 21.1 | 28.6 | 39.1 | 42.7 | 6.7 | −16.9 | −14.4 | −3.9 | −5.5 |
| rGO (0.3% and 0.6%) incorporated AAEC | ||||||||||
| B1R3 | 18.8 | 33 | 43 | 50.3 | 54.8 | 31.5 | 15.8 | 11.7 | 9.8 | 8.9 |
| B2R3 | 20.1 | 35.3 | 45.3 | 52.6 | 57.1 | 28.8 | 14.6 | 11 | 9.4 | 8.6 |
| T1R3 | 19.4 | 27 | 35 | 42.3 | 46.8 | 30.3 | 20 | 14.8 | 11.9 | 10.6 |
| T2R3 | 19.5 | 29.9 | 37.9 | 45.2 | 49.7 | 30.1 | 17.7 | 13.5 | 11.1 | 10 |
| B1R6 | 16.3 | 30.5 | 40.5 | 47.8 | 52.3 | 14 | 7 | 5.2 | 4.4 | 4 |
| B2R6 | 17.6 | 32.8 | 42.8 | 50.1 | 54.6 | 12.8 | 6.5 | 4.9 | 4.2 | 3.8 |
| T1R6 | 16.9 | 24.5 | 32.5 | 39.8 | 44.3 | 13.4 | 8.9 | 6.6 | 5.3 | 4.7 |
| T2R6 | 17 | 27.4 | 35.4 | 42.7 | 47.2 | 13.4 | 7.9 | 6 | 4.9 | 4.4 |
| 5% MgO and MWCNT or rGO (0.3% and 0.6%) incorporated AAECs | ||||||||||
| B2M5C3 | 23.8 | 40.2 | 50.9 | 57.3 | 61.5 | 52.4 | 30.5 | 24.8 | 19.1 | 16.9 |
| T2M5C3 | 23.1 | 34.8 | 43.5 | 49.9 | 54.1 | 54.3 | 36.8 | 30.1 | 22.5 | 19.6 |
| B2M5C6 | 21.6 | 38 | 48.9 | 55.1 | 59.3 | 38.6 | 23.5 | 20 | 14.6 | 12.8 |
| T2M5C6 | 21 | 32.7 | 41.4 | 47.8 | 52 | 40.4 | 28.6 | 23.8 | 17.3 | 15 |
| B2M5R3 | 25.18 | 41.6 | 52.3 | 58.7 | 62.9 | 61.4 | 35.1 | 28.2 | 22 | 19.6 |
| T2M5R3 | 24.61 | 36.27 | 44.97 | 51.37 | 55.57 | 64.5 | 42.8 | 34.6 | 26.2 | 22.9 |
| B2M5R6 | 22.18 | 38.6 | 49.3 | 55.7 | 59.9 | 42.2 | 25.3 | 20.8 | 15.8 | 13.9 |
| T2M5R6 | 21.7 | 33.4 | 42.1 | 48.5 | 52.7 | 45.3 | 31.5 | 26 | 19.2 | 16.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, M.A.; Hossain, K.M.A. Physical, Compressive Strength, and Microstructural Characteristics of Alkali-Activated Engineered Composites Incorporating MgO, MWCNTs, and rGO. Appl. Sci. 2025, 15, 1712. https://doi.org/10.3390/app15041712
Hossain MA, Hossain KMA. Physical, Compressive Strength, and Microstructural Characteristics of Alkali-Activated Engineered Composites Incorporating MgO, MWCNTs, and rGO. Applied Sciences. 2025; 15(4):1712. https://doi.org/10.3390/app15041712
Chicago/Turabian StyleHossain, Mohammad Ali, and Khandaker M. A. Hossain. 2025. "Physical, Compressive Strength, and Microstructural Characteristics of Alkali-Activated Engineered Composites Incorporating MgO, MWCNTs, and rGO" Applied Sciences 15, no. 4: 1712. https://doi.org/10.3390/app15041712
APA StyleHossain, M. A., & Hossain, K. M. A. (2025). Physical, Compressive Strength, and Microstructural Characteristics of Alkali-Activated Engineered Composites Incorporating MgO, MWCNTs, and rGO. Applied Sciences, 15(4), 1712. https://doi.org/10.3390/app15041712






