Impact of Seed Priming Technologies on the Agronomical Characteristics of Lathyrus sativus L. Commercial and Local Variety Under Normal and Saline Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Design
2.2. Seed Treatments
2.3. Establishment Experiment
Phases of the Experiment
2.4. Statistical Analysis
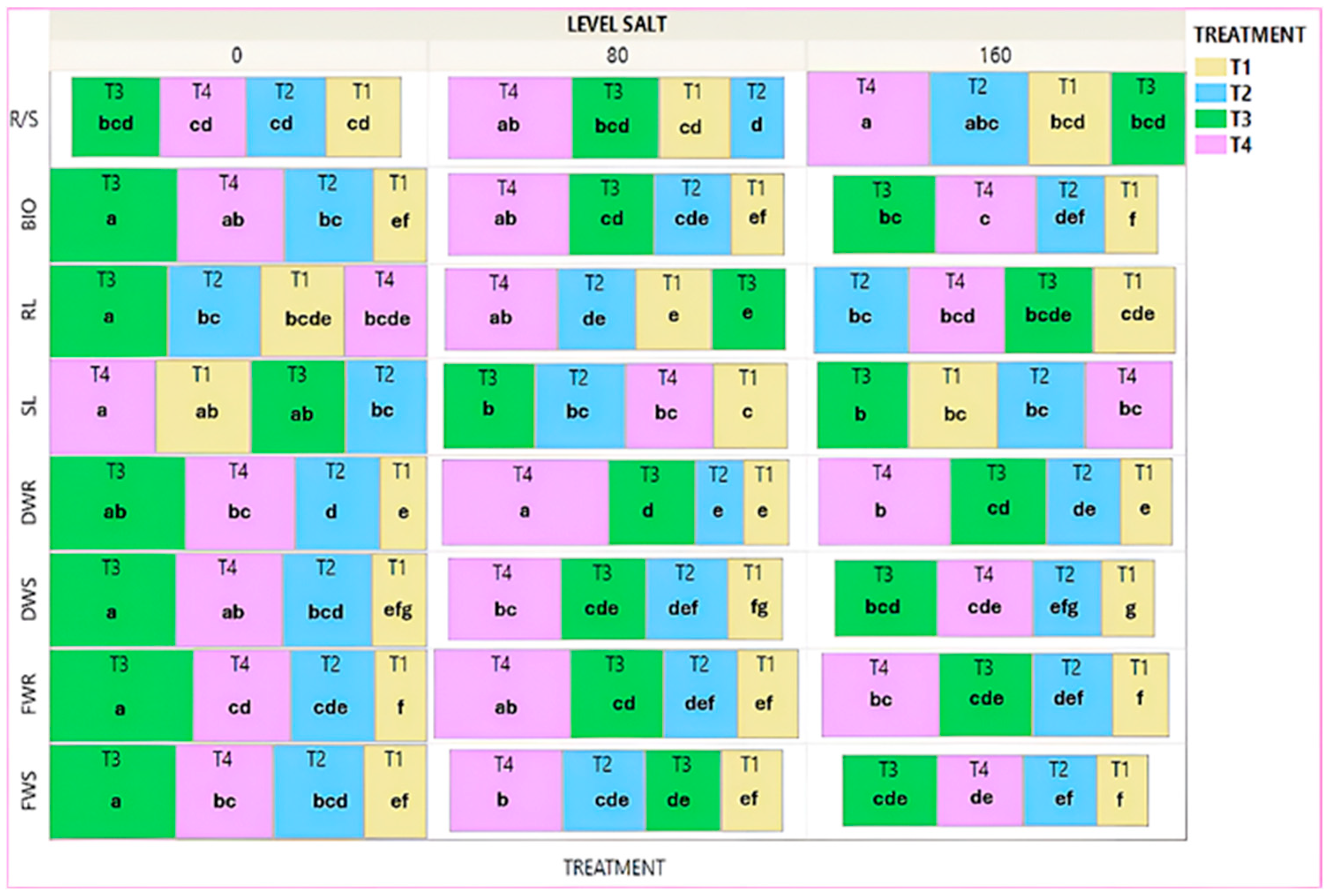
3. Results
3.1. Initial Developmental Stage
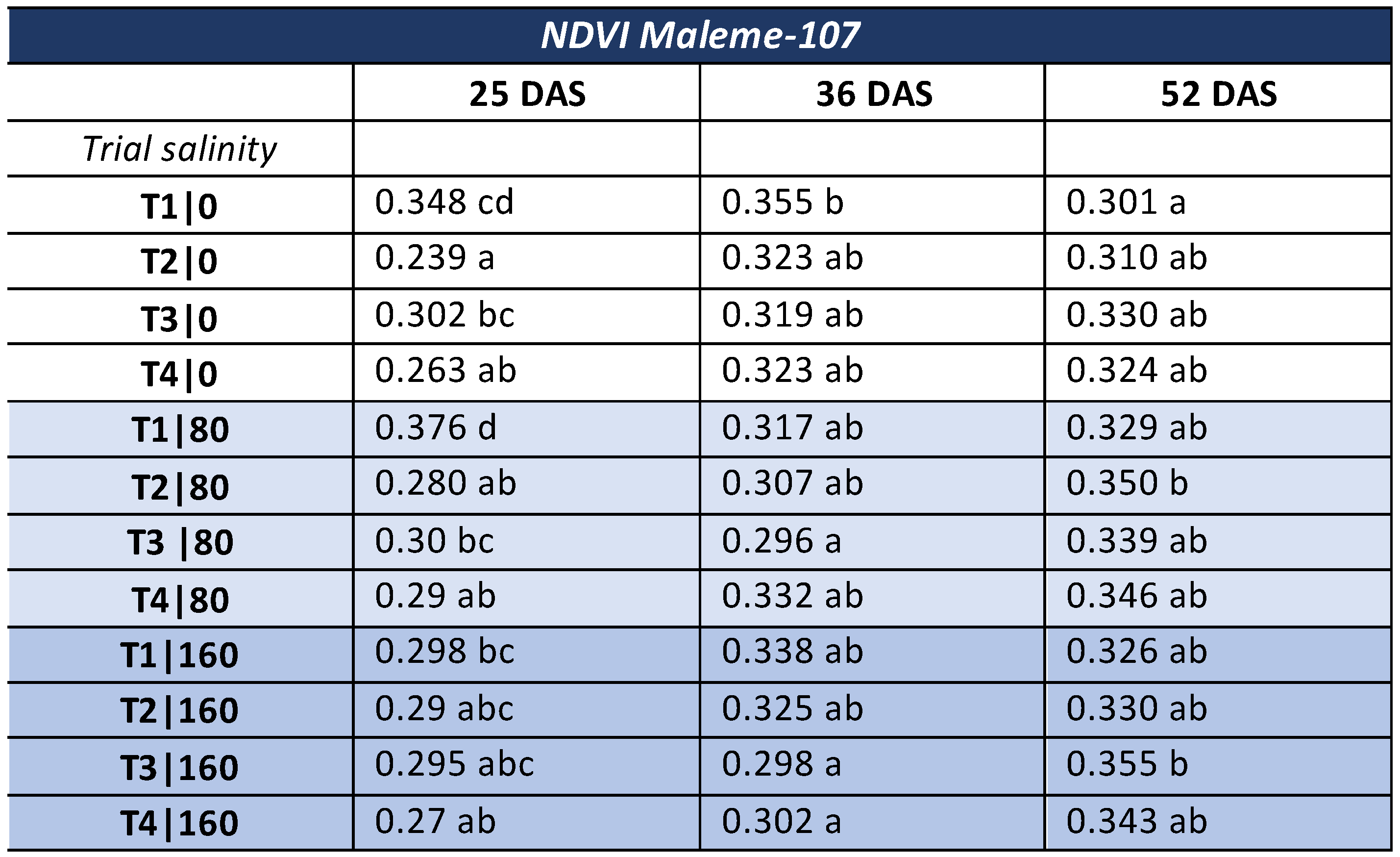
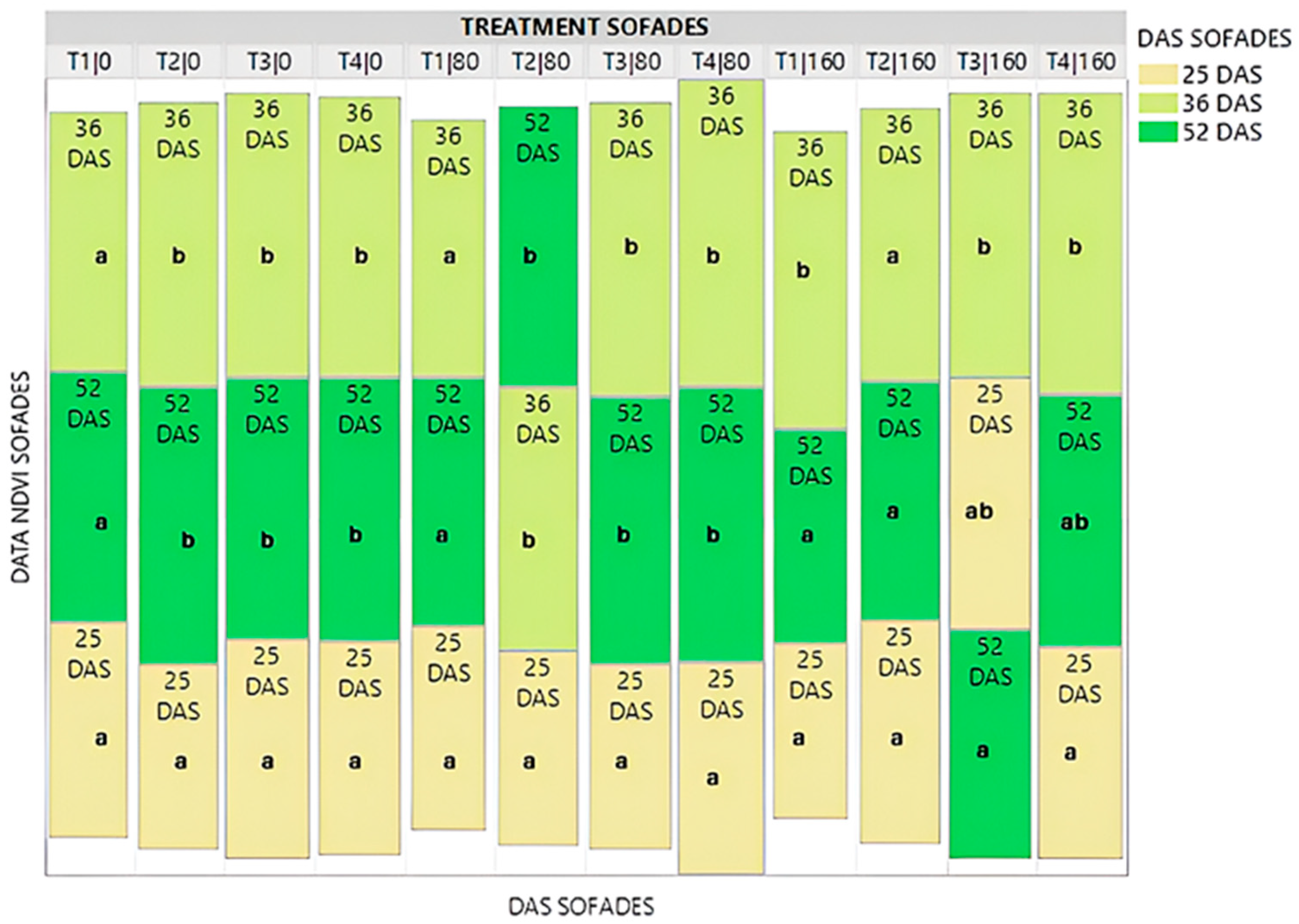
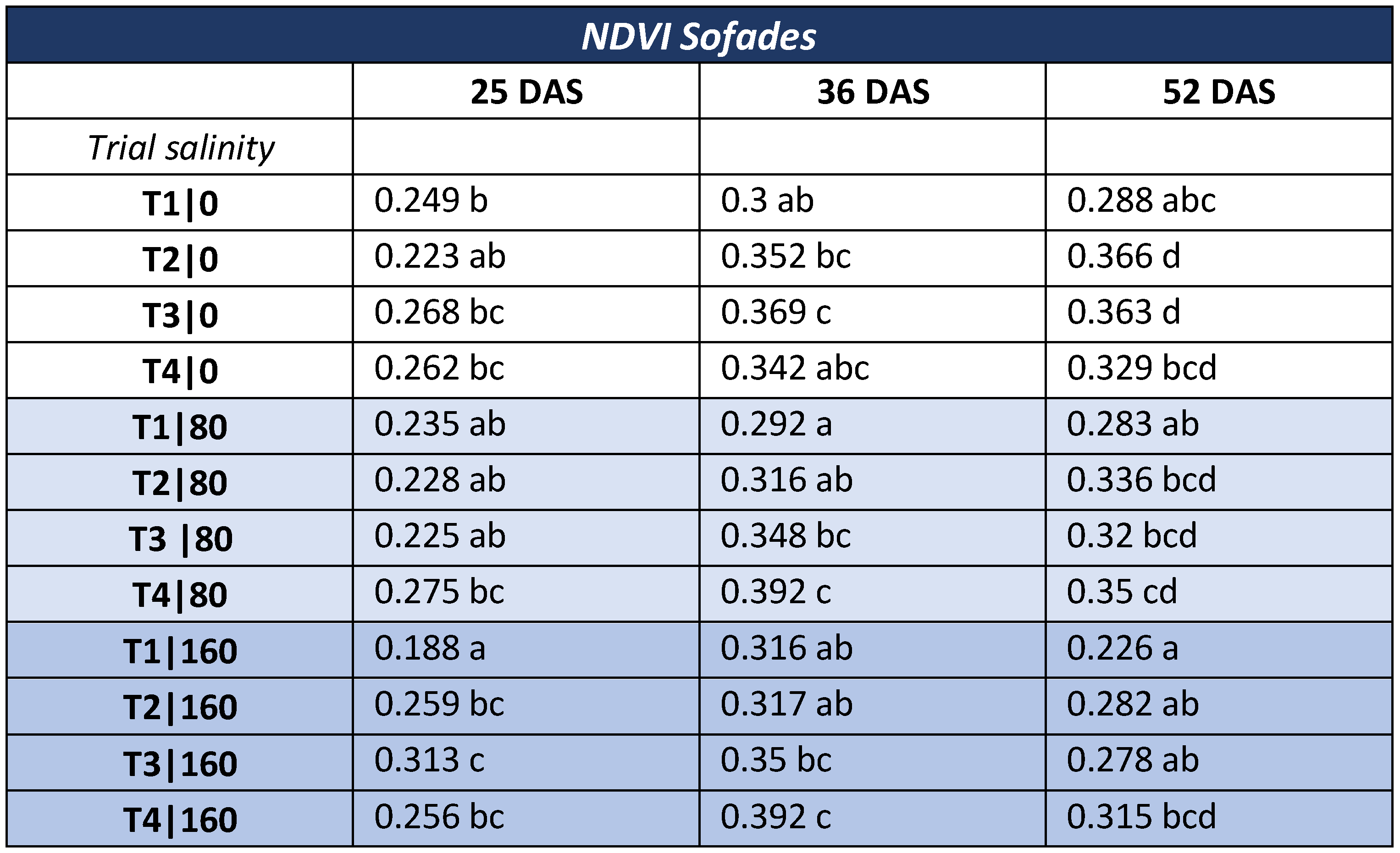
3.2. NDVI—Commercial Variety
3.3. NDVI—Local Variety
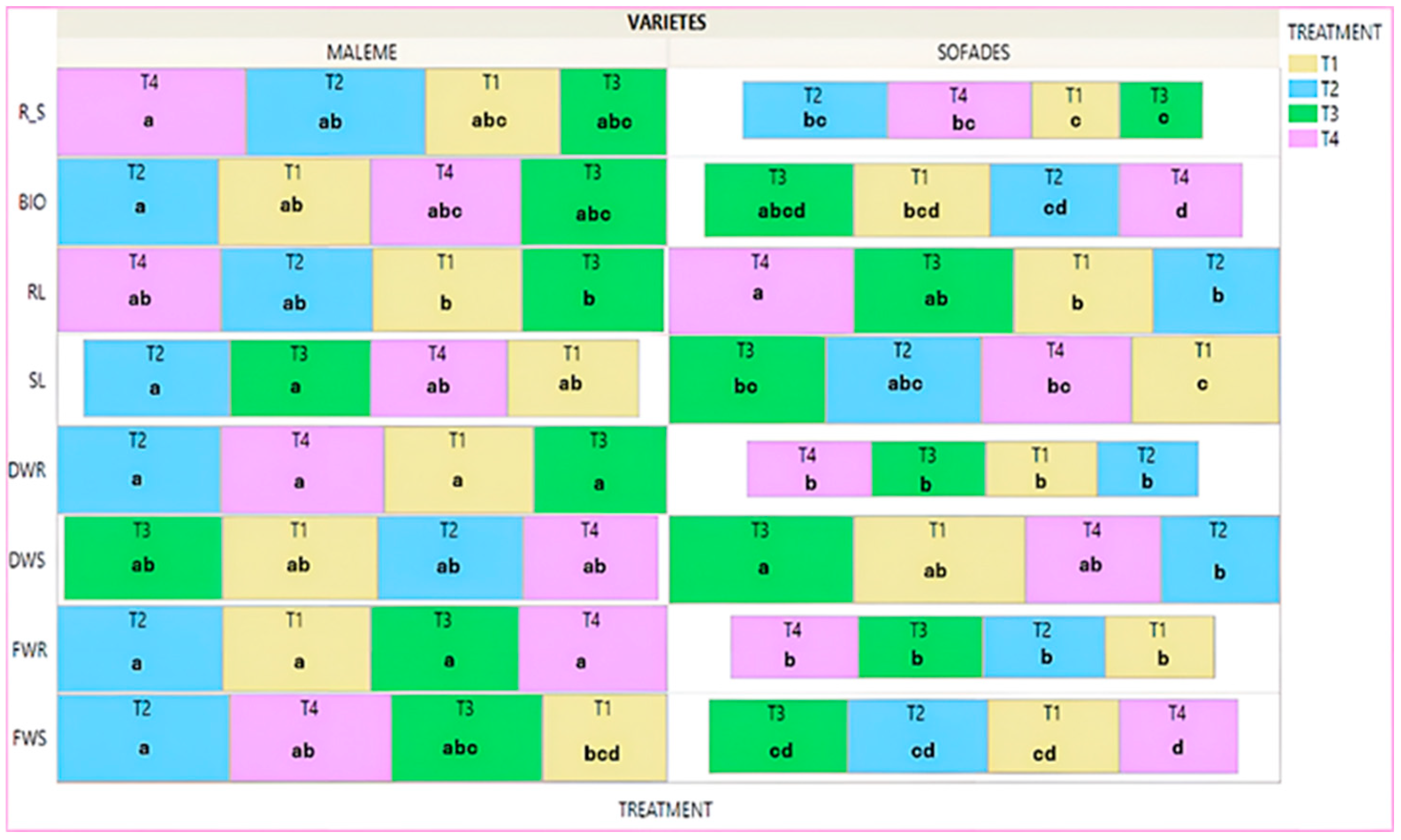
3.4. Advanced Stage of Development—Controls
3.5. Salinity—Commercial Variety
3.6. Salinity—Local Variety
4. Discussion
- Early developmental stage
- Advanced developmental stage—controls
- NDVI
- Salinity Stress
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, M.W.; Grusak, M.A.; Pinto, E.; Gomes, A.; Ferreira, H.; Balázs, B.; Centofanti, T.; Ntatsi, G.; Savvas, D.; Karkanis, A. The biology of legumes and their agronomic, economic, and social impact. In The Plant Family Fabaceae: Biology and Physiological Responses to Environmental Stresses; Springer Nature: Singapore, 2020; pp. 3–25. [Google Scholar] [CrossRef]
- Pickson, R.B.; Gui, P.; Chen, A.; Boateng, E. Climate change and food security nexus in Asia: A regional comparison. Ecol. Inform. 2023, 76, 102038. [Google Scholar] [CrossRef]
- Mirón, I.J.; Linares, C.; Díaz, J. The influence of climate change on food production and food safety. Environ. Res. 2023, 216, 114674. [Google Scholar] [CrossRef]
- Lee, C.-C.; Zeng, M.; Luo, K. How does climate change affect food security? Evidence from China. Environ. Impact Assess. Rev. 2024, 104, 107324. [Google Scholar] [CrossRef]
- Aguilera, E.; Díaz-Gaona, C.; García-Laureano, R.; Reyes-Palomo, C.; Guzmán, G.I.; Ortolani, L.; Sánchez-Rodríguez, M.; Rodríguez-Estévez, V. Agroecology for adaptation to climate change and resource depletion in the Mediterranean region. A review. Agric. Syst. 2020, 181, 102809. [Google Scholar] [CrossRef]
- Araújo, S.S.; Beebe, S.; Crespi, M.; Delbreil, B.; González, E.M.; Gruber, V.; Lejeune-Henaut, I.; Link, W.; Monteros, M.J.; Prats, E. Abiotic stress responses in legumes: Strategies used to cope with environmental challenges. Crit. Rev. Plant Sci. 2015, 34, 237–280. [Google Scholar] [CrossRef]
- Terzopoulos, P.; Bebeli, P. Phenotypic diversity in Greek tomato (Solanum lycopersicum L.) landraces. Sci. Hortic. 2010, 126, 138–144. [Google Scholar] [CrossRef]
- Cooper, H.D.; Spillane, C.; Hodgkin, T. Broadening the Genetic Base of Crops: An Overview; CABI Publishing, CAB International: Oxon, UK, 2001; pp. 1–23. [Google Scholar] [CrossRef]
- Lazaridi, E.; Kapazoglou, A.; Gerakari, M.; Kleftogianni, K.; Passa, K.; Sarri, E.; Papasotiropoulos, V.; Tani, E.; Bebeli, P.J. Crop landraces and indigenous varieties: A valuable source of genes for plant breeding. Plants 2024, 13, 758. [Google Scholar] [CrossRef] [PubMed]
- Antolín, M.C.; Toledo, M.; Pascual, I.; Irigoyen, J.J.; Goicoechea, N. The exploitation of local Vitis vinifera L. biodiversity as a valuable tool to cope with climate change maintaining berry quality. Plants 2020, 10, 71. [Google Scholar] [CrossRef]
- Ribaut, J.-M.; Ragot, M. Modernising breeding for orphan crops: Tools, methodologies, and beyond. Planta 2019, 250, 971–977. [Google Scholar] [CrossRef]
- Lambein, F.; Travella, S.; Kuo, Y.-H.; Van Montagu, M.; Heijde, M. Grass pea (Lathyrus sativus L.): Orphan crop, nutraceutical or just plain food? Planta 2019, 250, 821–838. [Google Scholar] [CrossRef] [PubMed]
- Rubiales, D.; Emeran, A.A.; Flores, F. Adaptation of grass pea (Lathyrus sativus) to Mediterranean environments. Agronomy 2020, 10, 1295. [Google Scholar] [CrossRef]
- Patto, M.V.; Skiba, B.; Pang, E.; Ochatt, S.; Lambein, F.; Rubiales, D. Lathyrus improvement for resistance against biotic and abiotic stresses: From classical breeding to marker assisted selection. Euphytica 2006, 147, 133–147. [Google Scholar] [CrossRef]
- Goufa, M.; Makeroufas, E.; Gerakari, M.; Sarri, E.; Ragkos, A.; Bebeli, P.J.; Balestrazzi, A.; Tani, E. Understanding the Potential to Increase Adoption of Orphan Crops: The Case of Lathyrus spp. Cultivation in Greece. Agronomy 2024, 14, 108. [Google Scholar] [CrossRef]
- Gonçalves, L.; Rubiales, D.; Bronze, M.R.; Vaz Patto, M.C. Grass pea (Lathyrus sativus L.)—A sustainable and resilient answer to climate challenges. Agronomy 2022, 12, 1324. [Google Scholar] [CrossRef]
- Chettri, P.; Atta, K.; Pal, A. Comparative Physiology of Drought and Salinity Stress in Grass Pea (Lathyrus sativus L.) Seedlings. Int. J. Environ. Clim. Chang. 2021, 11, 111–119. [Google Scholar] [CrossRef]
- Tokarz, B.; Wójtowicz, T.; Makowski, W.; Jędrzejczyk, R.J.; Tokarz, K.M. What is the difference between the response of grass pea (Lathyrus sativus L.) to salinity and drought stress?—A physiological study. Agronomy 2020, 10, 833. [Google Scholar] [CrossRef]
- Khosravi, Z.; Pourmohammad, A.; ALILOO, A.A.; Shahabivand, S.; Hassanpouraghdam, M.B.; Topcu, H. In vitro salinity stress mediates grass pea genotypes’ (Lathyrus sativus L.) responses. Turk. J. Agric. For. 2022, 46, 340–351. [Google Scholar] [CrossRef]
- Arslan, M.; Aydınoğlu, B. Effect of salinity (NaCl) stress on germination and seedling growth characteristics in grass pea (Lathyrus sativus L.). Germination Seedl. 2018, 7, 49–54. [Google Scholar]
- Güleç Şen, K.; Başaran, U.; Çopur Doğrusöz, M.; Gülümser, E.; Mut, H. Growth and Biochemical Responses of Grass Pea (Lathyrus sativus L.) Genotypes Under Salt (NaCl) Stress Generated by Irrigation Water, and Changes in Soil pH and EC. Gesunde Pflanz. 2023, 75, 667–675. [Google Scholar] [CrossRef]
- Verma, S.R.; Solanki, H.A. Alleviating Salinity Stress During Seed Germinating by Priming Techniques: A Review. Int. J. Sci. Res. Sci. Technol. 2021, 8, 198–202. [Google Scholar] [CrossRef]
- Stavropoulou, A. About the action of metabolites of plant growth-promoting rhizobacteria Bacillus subtilis on plant salt tolerance (I). Arch. Phytopathol. Plant Prot. 2011, 44, 1867–1882. [Google Scholar] [CrossRef]
- Siddika, A.; Rashid, A.A.; Khan, S.N.; Khatun, A.; Karim, M.M.; Prasad, P.V.; Hasanuzzaman, M. Harnessing plant growth-promoting rhizobacteria, Bacillus subtilis and B. aryabhattai to combat salt stress in rice: A study on the regulation of antioxidant defense, ion homeostasis, and photosynthetic parameters. Front. Plant Sci. 2024, 15, 1419764. [Google Scholar] [CrossRef] [PubMed]
- Omafuvbe, B. Effect of salt on the fermentation of soybean (Glycine max) into padawan using Bacillus subtilis as starter culture. Afr. J. Biotechnol. 2006, 5, 1001–1005. [Google Scholar]
- Nadeem, M.; Li, J.; Yahya, M.; Wang, M.; Ali, A.; Cheng, A.; Wang, X.; Ma, C. Grain legumes and fear of salt stress: Focus on mechanisms and management strategies. Int. J. Mol. Sci. 2019, 20, 799. [Google Scholar] [CrossRef]
- Lastochkina, O. Bacillus subtilis-mediated abiotic stress tolerance in plants. In Bacilli and Agrobiotechnology: Phytostimulation and Biocontrol; Springer: Berlin/Heidelberg, Germany, 2019; Volume 2, pp. 97–133. [Google Scholar] [CrossRef]
- Kaschuk, G.; Auler, A.C.; Vieira, C.E.; Dakora, F.D.; Jaiswal, S.K.; da Cruz, S.P. Coinoculation impact on plant growth promotion: A review and meta-analysis on coinoculation of rhizobia and plant growth-promoting bacilli in grain legumes. Braz. J. Microbiol. 2022, 53, 2027–2037. [Google Scholar] [CrossRef]
- Ibarra-Villarreal, A.L.; Gándara-Ledezma, A.; Godoy-Flores, A.D.; Herrera-Sepúlveda, A.; Díaz-Rodríguez, A.M.; Parra-Cota, F.I.; de los Santos-Villalobos, S. Salt-tolerant Bacillus species as a promising strategy to mitigate the salinity stress in wheat (Triticum turgidum subsp. durum). J. Arid. Environ. 2021, 186, 104399. [Google Scholar] [CrossRef]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef]
- Ferreira, N.C.; Mazzuchelli, R.d.C.L.; Pacheco, A.C.; Araujo, F.F.d.; Antunes, J.E.L.; Araujo, A.S.F.d. Bacillus subtilis improves maize tolerance to salinity. Ciência Rural. 2018, 48, e20170910. [Google Scholar] [CrossRef]
- Damalas, C.A.; Koutroubas, S.D.; Fotiadis, S. Hydro-priming effects on seed germination and field performance of faba bean in spring sowing. Agriculture 2019, 9, 201. [Google Scholar] [CrossRef]
- Abdelhamid, M.T.; El-Masry, R.R.; Darwish, D.S.; Abdalla, M.M.; Oba, S.; Ragab, R.; EL Sabagh, A.; El Kholy, M.H.; Omer, E. Mechanisms of seed priming involved in salt stress amelioratio. In Priming and Pretreatment of Seeds and Seedlings: Implication in Plant Stress Tolerance and Enhancing Productivity in Crop Plants; Springer: Berlin/Heidelberg, Germany, 2019; pp. 219–251. [Google Scholar] [CrossRef]
- Paparella, S.; Araújo, S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed priming: State of the art and new perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Komala, N.; Sumalatha, G.; Gurumurthy, R.; Surendra, P. Seed quality enhancement techniques. J. Pharmacogn. Phytochem. 2018, 7, 3124–3128. [Google Scholar]
- Forni, C.; Borromeo, I. The utilization of seed priming as a tool to overcome salt and drought stresses: Is still a long way to go? Seeds 2023, 2, 406–420. [Google Scholar] [CrossRef]
- Dutta, P. Seed priming: New vistas and contemporary perspectives. In Advances in Seed Priming; Springer: Berlin/Heidelberg, Germany, 2018; pp. 3–22. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Gaurav, A.K.; Srivastava, S.; Verma, J.P. Plant growth-promoting bacteria: Biological tools for the mitigation of salinity stress in plants. Front. Microbiol. 2020, 11, 1216. [Google Scholar] [CrossRef]
- Pagano, A.; Macovei, A.; Balestrazzi, A. Molecular dynamics of seed priming at the crossroads between basic and applied research. Plant Cell Rep. 2023, 42, 657–688. [Google Scholar] [CrossRef]
- Devika, O.S.; Singh, S.; Sarkar, D.; Barnwal, P.; Suman, J.; Rakshit, A. Seed priming: A potential supplement in integrated resource management under fragile intensive ecosystems. Front. Sustain. Food Syst. 2021, 5, 654001. [Google Scholar] [CrossRef]
- Upadhyay, S.; Singh, D. Effect of salt-tolerant plant growth-promoting rhizobacteria on wheat plants and soil health in a saline environment. Plant Biol. 2015, 17, 288–293. [Google Scholar] [CrossRef]
- Swetha, L.N.; Dayal, A. Effect of Seed Biopriming on Germination & Seedling Attributes in Chickpea (Cicer arietinum L.). Int. J. Environ. Clim. Chang. 2024, 14, 431–440. [Google Scholar] [CrossRef]
- Neshat, M.; Abbasi, A.; Hosseinzadeh, A.; Sarikhani, M.R.; Dadashi Chavan, D.; Rasoulnia, A. Plant growth promoting bacteria (PGPR) induce antioxidant tolerance against salinity stress through biochemical and physiological mechanisms. Physiol. Mol. Biol. Plants 2022, 28, 347–361. [Google Scholar] [CrossRef]
- Lastochkina, O.; Aliniaeifard, S.; Garshina, D.; Garipova, S.; Pusenkova, L.; Allagulova, C.; Fedorova, K.; Baymiev, A.; Koryakov, I.; Sobhani, M. Seed priming with endophytic Bacillus subtilis strain-specifically improves growth of Phaseolus vulgaris plants under normal and salinity conditions and exerts anti-stress effect through induced lignin deposition in roots and decreased oxidative and osmotic damages. J. Plant Physiol. 2021, 263, 153462. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Zhao, X.Q.; Javed, M.T.; Khan, K.S.; Bano, A.; Shen, R.F.; Masood, S. Bacillus pumilus enhances tolerance in rice (Oryza sativa L.) to combined stresses of NaCl and high boron due to limited uptake of Na+. Environ. Exp. Bot. 2016, 124, 120–129. [Google Scholar] [CrossRef]
- Haroon, U.; Munis, M.F.H.; Liaquat, F.; Khizar, M.; Elahi, M.; Chaudhary, H.J. Biofilm formation and flocculation potential analysis of halotolerant Bacillus tequilensis and its inoculation in soil to mitigate salinity stress of chickpea. Physiol. Mol. Biol. Plants 2023, 29, 277–288. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Alaraidh, I.A.; Alsahli, A.A.; Alamri, S.A.; Ali, H.M.; Alayafi, A.A. Bacillus firmus (SW5) augments salt tolerance in soybean (Glycine max L.) by moduLating root system architecture, antioxidant defense systems and stress-responsive genes expression. Plant Physiol. Biochem. 2018, 132, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Desoky, E.-S.M.; Saad, A.M.; El-Saadony, M.T.; Merwad, A.-R.M.; Rady, M.M. Plant growth-promoting rhizobacteria: Potential improvement in antioxidant defense system and suppression of oxidative stress for alleviating salinity stress in Triticum aestivum (L.) plants. Biocatal. Agric. Biotechnol. 2020, 30, 101878. [Google Scholar] [CrossRef]
- Biswas, S.; Seal, P.; Majumder, B.; Biswas, A.K. Efficacy of seed priming strategies for enhancing salinity tolerance in plants: An overview of the progress and achievements. Plant Stress 2023, 9, 100186. [Google Scholar] [CrossRef]
- Nautiyal, C.S.; Srivastava, S.; Chauhan, P.S.; Seem, K.; Mishra, A.; Sopory, S.K. Plant growth-promoting bacteria Bacillus amyloliquefaciens NBRISN13 modulates gene expression profile of leaf and rhizosphere community in rice during salt stress. Plant Physiol. Biochem. 2013, 66, 1–9. [Google Scholar] [CrossRef]
- Martínez-Álvarez, J.C.; Castro-Martínez, C.; Sánchez-Peña, P.; Gutiérrez-Dorado, R.; Maldonado-Mendoza, I.E. Development of a powder formulation based on Bacillus cereus sensu lato strain B25 spores for biological control of Fusarium verticillioides in maize plants. World J. Microbiol. Biotechnol. 2016, 32, 1–10. [Google Scholar] [CrossRef]
- Lobo, C.B.; Tomás, M.S.J.; Viruel, E.; Ferrero, M.A.; Lucca, M.E. Development of low-cost formulations of plant growth-promoting bacteria to be used as inoculants in beneficial agricultural technologies. Microbiol. Res. 2019, 219, 12–25. [Google Scholar] [CrossRef]
- Hashmi, I.; Paul, C.; Al-Dourobi, A.; Sandoz, F.; Deschamps, P.; Junier, T.; Junier, P.; Bindschedler, S. Comparison of the plant growth-promotion performance of a consortium of Bacilli inoculated as endospores or as vegetative cells. FEMS Microbiol. Ecol. 2019, 95, fiz147. [Google Scholar] [CrossRef]
- Hatala, J.A. Spatiotemporal Dynamics of Carbon Dioxide and Methane Fluxes from Agricultural and Restored Wetlands in the California Delta. Ph.D. Thesis, University of California, Berkeley, CA, USA, 2013. [Google Scholar]
- Tarchoun, N.; Saadaoui, W.; Hamdi, K.; Falleh, H.; Pavli, O.; Ksouri, R.; Petropoulos, S.A. Seed Priming and Biopriming in Two Squash Landraces (Cucurbita maxima Duchesne) from Tunisia: A Sustainable Strategy to Promote Germination and Alleviate Salt Stress. Plants 2024, 13, 2464. [Google Scholar] [CrossRef] [PubMed]
- Blake, C.; Christensen, M.N.; Kovács, Á.T. Molecular aspects of plant growth promotion and protection by Bacillus subtilis. Mol. Plant-Microbe Interact. 2021, 34, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, M.K. Seed priming: A low-cost technology for resource-poor farmers in improving pulse productivity. In Advances in Seed Priming; Springer: Berlin/Heidelberg, Germany, 2018; pp. 187–208. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Imran, M.; Ahmad, I.; Atif, M.; Alghamdi, S.S. Improving the productivity and profitability of late sown chickpea by seed priming. Int. J. Plant Prod. 2019, 13, 129–139. [Google Scholar] [CrossRef]
- Catiempo, R.L.; Photchanachai, S.; Bayogan, E.R.V.; Wongs-Aree, C. Impact of hydropriming on germination and seedling establishment of sunflower seeds at elevated temperature. Plant Soil Environ. 2021, 67, 491–498. [Google Scholar] [CrossRef]
- Patel, S.; Bhattacharya, C.; Pandhi, N. Screening of Bacillus sp. (OQ654027) Mediated Seed Bio-Priming Enhance Plant-Growth-Promotion for Sustainable Crop Production of Groundnut and Chickpea. Curr. Agric. Res. J. 2023, 11, 865–880. [Google Scholar] [CrossRef]
- Kumari, P.; Meena, M.; Gupta, P.; Dubey, M.K.; Nath, G.; Upadhyay, R. Plant growth promoting rhizobacteria and their biopriming for growth promotion in mung bean (Vigna radiata (L.) R. Wilczek). Biocatal. Agric. Biotechnol. 2018, 16, 163–171. [Google Scholar] [CrossRef]
- Jabborova, D.; Enakiev, Y.; Sulaymanov, K.; Kadirova, D.; Ali, A.; Annapurna, K. Plant growth promoting bacteria Bacillus subtilis promote growth and physiological parameters of Zingiber officinale Roscoe. Plant Sci. Today 2021, 8, 66–71. [Google Scholar] [CrossRef]
- Miljaković, D.; Marinković, J.; Tamindžić, G.; Đorđević, V.; Tintor, B.; Milošević, D.; Ignjatov, M.; Nikolić, Z. Bio-priming of soybean with Bradyrhizobium japonicum and Bacillus megaterium: Strategy to improve seed germination and the initial seedling growth. Plants 2022, 11, 1927. [Google Scholar] [CrossRef]
- Solouki, H.; Kafi, M.; Nabati, J.; Ahmadi, M.J.; Nezami, A.; Ahmady, R.S. Seed biopriming and plant growth-promoting bacteria improve nutrient absorption and dry matter production of fenugreek (Trigonella foenum-graecum) plants. S. Afr. J. Bot. 2023, 162, 296–303. [Google Scholar] [CrossRef]
- Pedrini, S.; Balestrazzi, A.; Madsen, M.D.; Bhalsing, K.; Hardegree, S.P.; Dixon, K.W.; Kildisheva, O.A. Seed enhancement: Getting seeds restoratio-ready. Restoratio Ecol. 2020, 28, S266–S275. [Google Scholar] [CrossRef]
- Nevill, P.G.; Cross, A.T.; Dixon, K.W. Ethical seed sourcing is a key issue in meeting global restoratio targets. Curr. Biol. 2018, 28, R1378–R1379. [Google Scholar] [CrossRef] [PubMed]
- Meissen, J.C.; Galatowitsch, S.M.; Cornett, M.W. Risks of overharvesting seed from native tallgrass prairies. Restoratio Ecol. 2015, 23, 882–891. [Google Scholar] [CrossRef]
- Commission, E. Key Policy Objectives of the CAP 2023-27. Available online: https://agriculture.ec.europa.eu/common-agricultural-policy/cap-overview/cap-2023-27/key-policy-objectives-cap-2023-27_en (accessed on 10 March 2023).
- Roujean, J.-L.; Breon, F.-M. Estimating PAR absorbed by vegetation from bidirectional reflectance measurements. Remote Sens. Environ. 1995, 51, 375–384. [Google Scholar] [CrossRef]
- Price, J.C. Estimating vegetation amount from visible and near infrared reflectances. Remote Sens. Environ. 1992, 41, 29–34. [Google Scholar] [CrossRef]
- Starks, P.; Zhao, D.; Phillips, W.; Coleman, S. Herbage mass, nutritive value and canopy spectral reflectance of bermudagrass pastures. Grass Forage Sci. 2006, 61, 101–111. [Google Scholar] [CrossRef]
- Sadras, V.; Lake, L.; Leonforte, A.; McMurray, L.; Paull, J. Screening field pea for adaptation to water and heat stress: Associations between yield, crop growth rate and seed abortion. Field Crops Res. 2013, 150, 63–73. [Google Scholar] [CrossRef]
- Maphosa, L.; Richards, M.F.; Norton, S.L.; Nguyen, G.N. Breeding for abiotic stress adaptation in chickpea (Cicer arietinum L.): A comprehensive review. Crop Breed. Genet. Genom. 2020, 4, e200015. [Google Scholar] [CrossRef]
- Lake, L.; Sadras, V.O. Screening chickpea for adaptation to water stress: Associations between yield and crop growth rate. Eur. J. Agron. 2016, 81, 86–91. [Google Scholar] [CrossRef]
- Inman, D.; Khosla, R.; Mayfield, T. On-the-go active remote sensing for efficient crop nitrogen management. Sens. Rev. 2005, 25, 209–214. [Google Scholar] [CrossRef]
- Patwa, N.; Pandey, V.; Gupta, O.P.; Yadav, A.; Meena, M.R.; Ram, S.; Singh, G. Unravelling wheat genotypic responses: Insights into salinity stress tolerance in relation to oxidative stress, antioxidant mechanisms, osmolyte accumulation and grain quality parameters. BMC Plant Biol. 2024, 24, 875. [Google Scholar] [CrossRef]
- Goto, K.; Goto, T.; Nmor, J.C.; Minematsu, K.; Gotoh, K. Evaluating salinity damage to crops through satellite data analysis: Application to typhoon affected areas of southern Japan. Nat. Hazards 2015, 75, 2815–2828. [Google Scholar] [CrossRef]
- Duan, Z.; Wang, X.; Sun, L.; Zhou, M.; Luo, Y. An insight into effect of soil salinity on vegetation dynamics in the exposed seafloor of the Aral Sea. Sci. Total Environ. 2024, 951, 175615. [Google Scholar] [CrossRef]
- Brunner, P.; Li, H.; Kinzelbach, W.; Li, W.; Dong, X. Extracting phreatic evaporatio from remotely sensed maps of evapotranspiratio. Water Resour. Res. 2008, 44, W08428. [Google Scholar] [CrossRef]
- Ivushkin, K.; Bartholomeus, H.; Bregt, A.K.; Pulatov, A.; Franceschini, M.H.; Kramer, H.; van Loo, E.N.; Roman, V.J.; Finkers, R. UAV based soil salinity assessment of cropland. Geoderma 2019, 338, 502–512. [Google Scholar] [CrossRef]
- Klimek-Kopyra, A.; Zajac, T.; Oleksy, A.; Kulig, B.; Slizowska, A. The value of different vegetative indices (NDVI, GAI) for the assessment of yield potential of pea (Pisum sativum L.) at different growth stages and under varying management practices. Acta Agrobot. 2018, 71, 1733. [Google Scholar] [CrossRef]
- Kakabouki, I.; Stavropoulos, P.; Roussis, I.; Mavroeidis, A.; Bilalis, D. Contribution of arbuscular mycorrhizal fungi (AMF) in improving the growth and yield performances of flax (Linum usitatissimum L.) to salinity stress. Agronomy 2023, 13, 2416. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004, 166, 3–16. [Google Scholar] [CrossRef]
- Wei, T.-J.; Li, G.; Cui, Y.-R.; Xie, J.; Gao, X.-A.; Teng, X.; Zhao, X.-Y.; Guan, F.-C.; Liang, Z.-W. Variation Characteristics of Root Traits of Different Alfalfa Cultivars under Saline-Alkaline Stress and their Relationship with Soil Environmental Factors. Phyton (0031-9457) 2024, 93, 29–43. [Google Scholar] [CrossRef]
- Wang, Q.H.; Han, W.; Hou, Y.Y.; Feng, L.; Ye, Z.; Gu, H.; Chen, B. Responses of main characters of root system to salt stress among cotton varieties with different salt tolerance. J. Appl. Ecol. 2018, 29, 865–873. [Google Scholar] [CrossRef]
- Thakur, M.; Sharma, P.; Anand, A. Seed priming-induced early vigor in crops: An alternate strategy for abiotic stress tolerance. In Priming and Pretreatment of Seeds and Seedlings: Implication in Plant Stress Tolerance and Enhancing Productivity in Crop Plants; Springer: Berlin/Heidelberg, Germany, 2019; pp. 163–180. [Google Scholar] [CrossRef]
- Seo, D.H.; Seomun, S.; Choi, Y.D.; Jang, G. Root development and stress tolerance in rice: The key to improving stress tolerance without yield penalties. Int. J. Mol. Sci. 2020, 21, 1807. [Google Scholar] [CrossRef]
- El-Hendawy, S.E.; Hu, Y.; Yakout, G.M.; Awad, A.M.; Hafiz, S.E.; Schmidhalter, U. Evaluating salt tolerance of wheat genotypes using multiple parameters. Eur. J. Agron. 2005, 22, 243–253. [Google Scholar] [CrossRef]
- Diya, A.; Beena, R.; Jayalekshmy, V. Physiological, Biochemical and Molecular Mechanisms of Seed Priming: A Review. Legume Res. Int. J. 2024, 47, 159–166. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Ge, J.; Li, R.; Zhang, R.; Zhang, Y.; Huo, Z.; Xu, K.; Wei, H.; Dai, Q. Improved physiological and morphological traits of root synergistically enhanced salinity tolerance in rice under appropriate nitrogen application rate. Front. Plant Sci. 2022, 13, 982637. [Google Scholar] [CrossRef]
- Metwali, E.M.; Abdelmoneim, T.S.; Bakheit, M.A.; Kadasa, N.M. Alleviation of salinity stress in faba bean (Vicia faba L.) plants by inoculation with plant growth promoting rhizobacteria (PGPR). Plant Omics 2015, 8, 449–460. [Google Scholar]
- Han, Q.-Q.; Lü, X.-P.; Bai, J.-P.; Qiao, Y.; Paré, P.W.; Wang, S.-M.; Zhang, J.-L.; Wu, Y.-N.; Pang, X.-P.; Xu, W.-B. Beneficial soil bacterium Bacillus subtilis (GB03) augments salt tolerance of white clover. Front. Plant Sci. 2014, 5, 525. [Google Scholar] [CrossRef] [PubMed]
- Gul, S.; Javed, S.; Azeem, M.; Aftab, A.; Anwaar, N.; Mehmood, T.; Zeshan, B. Application of Bacillus subtilis for the alleviation of salinity stress in different cultivars of Wheat (Tritium aestivum L.). Agronomy 2023, 13, 437. [Google Scholar] [CrossRef]
- Basu, S.; Kumari, S.; Subhadarshini, P.; Rishu, A.K.; Shekhar, S.; Kumar, G. Plant growth promoting rhizobacterium Bacillus sp. BSE01 alleviates salt toxicity in chickpea (Cicer arietinum L.) by conserving ionic, osmotic, redox and hormonal homeostasis. Physiol. Plant. 2023, 175, e14076. [Google Scholar] [CrossRef]
- BiBi, R.; Elahi, N.N.; Danish, S.; Alahmadi, T.A.; Ansari, M.J. Enhancing germination and growth of canola (Brassica napus L.) through hydropriming and NaCl priming. Sci. Rep. 2024, 14, 14026. [Google Scholar] [CrossRef]
- Abd_Allah, E.F.; Alqarawi, A.A.; Hashem, A.; Radhakrishnan, R.; Al-Huqail, A.A.; Al-Otibi, F.O.N.; Malik, J.A.; Alharbi, R.I.; Egamberdieva, D. Endophytic bacterium Bacillus subtilis (BERA 71) improves salt tolerance in chickpea plants by reguLating the plant defense mechanisms. J. Plant Interact. 2018, 13, 37–44. [Google Scholar] [CrossRef]
- Nawaz, A.; Shahbaz, M.; Asadullah; Imran, A.; Marghoob, M.U.; Imtiaz, M.; Mubeen, F. Potential of salt tolerant PGPR in growth and yield augmentation of wheat (Triticum aestivum L.) under saline conditions. Front. Microbiol. 2020, 11, 2019. [Google Scholar] [CrossRef]
- Soofinia, S.; Pourmohammad, A.; Aliloo, A.; Alizadeh, K. The Response of Early-Maturing Grass Pea (Lathyrus sativus) Genotypes to Different Levels of Salinity Stress. J. Crop Breed. 2024, 16, 61–73. [Google Scholar] [CrossRef]
- Ali, M.N.; Yeasmin, L.; Gantait, S.; Goswami, R.; Chakraborty, S. Screening of rice landraces for salinity tolerance at seedling stage through morphological and molecular markers. Physiol. Mol. Biol. Plants 2014, 20, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Al-Farsi, S.M.; Al-Sadi, A.M.; Ullah, A.; Farooq, M. Salt tolerance in alfalfa landraces of omani origin: Morpho-biochemical, mineral, and genetic diversity assessment. J. Soil Sci. Plant Nutr. 2021, 21, 1484–1499. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goufa, M.; Petraki, A.; Katsis, C.; Balestrazzi, A.; Calvio, C.; Kharga, N.; Chachalis, D.; Bebeli, P.J.; Tani, E. Impact of Seed Priming Technologies on the Agronomical Characteristics of Lathyrus sativus L. Commercial and Local Variety Under Normal and Saline Conditions. Appl. Sci. 2025, 15, 1692. https://doi.org/10.3390/app15041692
Goufa M, Petraki A, Katsis C, Balestrazzi A, Calvio C, Kharga N, Chachalis D, Bebeli PJ, Tani E. Impact of Seed Priming Technologies on the Agronomical Characteristics of Lathyrus sativus L. Commercial and Local Variety Under Normal and Saline Conditions. Applied Sciences. 2025; 15(4):1692. https://doi.org/10.3390/app15041692
Chicago/Turabian StyleGoufa, Maria, Angeliki Petraki, Christos Katsis, Alma Balestrazzi, Cinzia Calvio, Nitesh Kharga, Demosthenis Chachalis, Penelope J. Bebeli, and Eleni Tani. 2025. "Impact of Seed Priming Technologies on the Agronomical Characteristics of Lathyrus sativus L. Commercial and Local Variety Under Normal and Saline Conditions" Applied Sciences 15, no. 4: 1692. https://doi.org/10.3390/app15041692
APA StyleGoufa, M., Petraki, A., Katsis, C., Balestrazzi, A., Calvio, C., Kharga, N., Chachalis, D., Bebeli, P. J., & Tani, E. (2025). Impact of Seed Priming Technologies on the Agronomical Characteristics of Lathyrus sativus L. Commercial and Local Variety Under Normal and Saline Conditions. Applied Sciences, 15(4), 1692. https://doi.org/10.3390/app15041692










