Abstract
One of the main abiotic factors affecting agricultural productivity in semi-arid regions is salinity. Seed priming is a frequently used method to enhance plant growth under saline environments. The aim of this work was to demonstrate the differences in eight agronomical characteristics of two grass pea varieties under two salinity regimes (80 and 160 mM NaCl) when pre-exposed to seed priming (hydropriming, biopriming with Bacillus subtilis and their combination). The two varieties responded well to the priming treatments, with more beneficial effects monitored for the local variety. Evaluating the root characteristics that are most affected by stress, it was found that, at 80 mM NaCl, the combination of biopriming and hydropriming increased the fresh root weight by 36.8% and root length by 70% in the commercial variety, and by 124% and 47%, in the local variety, respectively. At 160 mM NaCl, biopriming increased the fresh root weight by 40.3% and root length by 50.3% in the commercial variety, while in the local variety, the combination of biopriming and hydropriming increased the fresh root weight by 124% and root length by 47%, respectively. Overall, biopriming and the combination of biopriming and hydropriming significantly enhanced plant growth characteristics of the two grass pea genotypes.
1. Introduction
Climate change is currently a serious threat with negative consequences for agricultural systems. High temperatures, drought, and rising sea levels are leading to a decrease in biodiversity and deteriorate soil erosion. Furthermore, one of the biggest threats to global food security is the expansion of agricultural lands with high levels of salt concentration due to climate change. Around the world, soil salinization affects about 6% of the cultivated area and continues to add 1% to 2% of land deterioration per year, resulting in large losses in staple grain crop yields [1]. This creates uncertainty and conditions of food insecurity, urging researchers to improve the design of agricultural systems and adapt agricultural practices and varieties to the new reality to enhance resilience to climate change [2,3,4,5]. Researchers suggest that crop adaptation to the new environmental conditions should be based on agroecology, reconnecting local production with food consumption through the recovery of locally produced products [6] and promoting the use of legumes for maintaining the sustainability of agricultural systems [7]. Biological and environmental factors have shaped the genetic diversity of local populations [8], giving them perfect adaptability to the environment in which they grow [9] and therefore constitute valuable genetic material that can be used to improve the resilience of cultivated species [10,11]. Many studies on plant adaptation to new conditions include crop landraces that were previously underutilized, had limited global economic value, and gained little attention for improvement. Thus, they were called “orphan crops”, such as grass pea (Lathyrus sativus L.) [12,13]. Lathyrus sativus is a legume adapted to Mediterranean cropping systems and even to marginal environments. It is cultivated for human consumption, animal feed and fodder. It is resistant to biotic and abiotic stress, while its high protein content makes it a valuable crop for farmers; therefore, it has gained the attention of researchers [14,15,16,17,18,19]. Research on its tolerance to salinity conditions is ongoing and multifaceted. A study by Khosravi et al. [20] on in vitro cultivation of genotypes from different countries showed high biomass and increased tolerance of calli and young seedlings of Greek genotypes of Lathyrus sativus at 125 mM NaCl. Experiments on pots showed that Lathyrus sativus demonstrated good germination subjected to 150 mM NaCl [21] while others indicate that, for some varieties of grass pea, 120 mM NaCl is a threshold for tolerance to salinity [22]. Several studies also incorporate improved seed treatment technologies before sowing (priming) such as hydropriming, biopriming and specifically the inoculation of plants with the bacterium Bacillus subtilis, which provide significant advantages to plants such as improved germination rates, uniform growth, vigor of young seedlings, resistance to biotic and abiotic stresses [23,24,25,26,27,28,29,30,31,32,33,34] as well as better yield [35,36,37,38]. Several researchers recognize salinity as a serious environmental problem and report that exploiting the properties of Plant Growth Promoting Bacteria (PGPR) could help address the problem as a low-cost biological method with long-term use. These halotolerant bacteria produce specific compounds that aid crops in adjusting to high salts and encourage their growth [39,40]. Despite the advantages of using PGPRs, researchers should study and find the appropriate balance between stimulating seed metabolism and decreasing the undesirable side effects they may cause [41]. A study by Devika et al. [42] evaluated different seed biofortification techniques in terms of crop productivity, resource efficiency, cost–benefit balance, and environmental impacts. Researchers’ experiments on wheat, rice, chickpea, and bean under high salinity conditions showed that inoculation with rhizobacteria of the genus Bacillus sp. increased their growth, yield, and resistance through the induction of defense mechanisms [43,44,45,46,47,48,49,50,51,52]. In the present study, the behaviors of two Lathyrus sativus varieties, an improved commercial Maleme-107 and a local variety Sofades, were investigated under the influence of four seed priming treatments (no-priming, hydropriming, biopriming with Bacillus subtilis, and combined hydropriming/biopriming), in conditions with and without salinity stress to unravel the impact of seed priming to salinity tolerance under controlled conditions.
2. Materials and Methods
2.1. Experimental Materials and Design
To assess the seed priming effect on the capacity of grain legumes to face salt stress, a pot experiment was conducted in a glass-covered greenhouse and Laboratory of Weed Science at the Benaki Phytopathological Institute, which is in Athens, Greece (38°04′55.1″ N and 23°48′43.6″ E). The experiment was set up on 20 October 2023 and was terminated on 12 December 2023. Two Greek varieties of Lathyrus sativus L. (Maleme-107 and Sofades) were tested, which were collected from the Institute of Industrial Forage Crops and Agroland, respectively. The Bacillus subtilis strain NCBI 3610 (ATTC 6051) was supplied by the Bacillus genetic stock center (https://bgsc.org/ (accessed on 4 February 2025)). Spores were obtained by nutrient exhaustion by growing the strain at 37 °C with vigorous agitation in 2× Difco Sporulation Medium (16 g/L Nutrient Broth, 2 g/L KCl, 0.5 g/L MgSO4∙7H2O; 1 mM Ca(NO3)2; 0.1 mM MnCl2, 1 μM FeSO4, 0.1% Glucose; pH 7) for 48 h. Spores were collected by centrifugation at 4000× g 30 min and purification was carried out by 5 washes with 200 mL of sterile cold water over a period of 10 days. Spores were quantified by dilution plating on standard LB plates (10 g/L tryptone, 10 g/L NaCl, 5 g/L yeast extract) before and after 10-min incubation at 80 °C. The correct number of spores was then dried under vacuum (Concentrator Plus, Eppendorf, Hamburg, Germany) and stored at room temperature till use.
The pot experiment was arranged as a completely randomized block design with four priming treatments and three replications per treatment. Daily temperature and relative humidity were monitored using a temperature and humidity smart sensor. The temperature range was between 7.2 °C (min) and 30.3 °C (max). Relative humidity was 70.7% and photoperiod was monitored as 8 h light and 14 h dark.
2.2. Seed Treatments
For seed priming treatment, all steps of preparation were carried out at room temperature (~25 °C). The required quantity of seeds was 25 g per variety. Seeds were divided into four groups for priming: the first group was un-primed (T1); the second group was hydroprimed (T2); the third group was bioprimed (T3)); and the fourth group was hydro-bioprimed (T4). Concerning the treatments that included hydropriming (Τ2 and Τ4), seeds were soaked in a container (880 cm3) with tap water for 8 h. To prevent imbibed seeds from overflowing during priming, the volume of the container soaking was a 1:4 ratio to the volume of water. Periodically, the container was stirred to reduce anoxic conditions. Following hydropriming, seeds were distributed on a layer of absorbing paper and air dried for 24 h, to maximize evaporation and prevent humidity retention. A sticker solution was prepared of 10% w/v sucrose in the required volumes (1.83 mL), enough to carry out the T3 and T4 treatments for the required amounts of seeds.
The number of spores required for seed treatments was calculated in order to achieve an average number of spores per seed within the ranges reported by the current literature [53,54,55]. An amount of 4 × 106 spores per single seed was used for calculations and adjusted based on the average weight and the required number of seeds. Lyophilized Bacillus subtilis spores were transferred to the tube containing the required volume of the sticker. The tube was stirred by vortexing until the pellet was dissolved. Then, the sticker was mixed with seeds in a dry container until the seeds were uniformly coated with sticker solution. Seeds remained in the container 24 h before sowing.
2.3. Establishment Experiment
Grass pea genotypes were sowed in plastic pots of 12 cm in height and 13 cm in diameter (1.59 L). The pots were filled with a mixture of loam soil and perlite in a 1:3 (w/w) ratio, with a pH of 7.0. Each pot had a volume of 1.32 L. Three seeds were sown at 3 cm depth in each pot. Plants were irrigated with tap water once a week. After germination, the number of seedlings was reduced to one per pot to avoid competition among them. One plant from each treatment and each replication for each genotype, when the seedlings were 20 days old, was removed from the pots, and roots were rinsed carefully to remove the attached soil with tap water. Fresh root/shoot weight (FWS/FWR) and shoot length and root length (SL/RL) were measured. Then shoots and roots were oven-dried at 120 °C for 24 h and dry root and shoot weight (DWR/DWS) were measured. The dry biomass (BIO) was also calculated, along with the ratio of root dry weight to shoot dry weight (R/S). After the 21st day, salt stress was induced by watering the plants with solutions containing sodium chloride (NaCl) at concentrations of 0 mM (control), 80 mM (mild stress), and 160 mM (high stress). NaCl was diluted in sterile water and applied at six-day intervals until the completion of the experiment. The electrical conductivity (EC) of each treatment at 25 °C was 0 dS/m 4.68 dS/m and 9.35 dS/m, respectively. To ensure water holding capacity, one irrigation with tap water took place once per week. For every salt application, we irrigated with 100 mL of salt solution and with 100 mL of tap water for control. At 52 days after sowing, (52 DAS), the plants were harvested, and the aforementioned eight agronomic traits were measured.
Phases of the Experiment
The first phase of the experiment focused on evaluating the effects of treatments during the early stages of plant growth (when the 3rd and 4th leaves emerged), prior to the application of any salinity treatments.
In the second phase, the normalized difference vegetation index NDVI was evaluated at three points, (25 DAS, 36 DAS and 52 DAS). Normalized differential vegetation index readings were performed using a Trimble GreenSeeker hand-held instrument. All readings were taken at 10 a.m. to ensure consistency and reduce the effect of fluctuating light conditions. The data obtained are related to relative chlorophyll content, with values closer to 1 indicating higher chlorophyll content [56].
In the third phase, the effect of priming treatments was assessed during the late growth stage (52 DAS), without the addition of salt. Finally, in the fourth phase (52 DAS), the impact of priming treatments on the plants’ agronomic traits was evaluated under two salt levels: 80 mM NaCl and 160 mM NaCl.
2.4. Statistical Analysis
Statistical analyses of the collected data (ANOVA test, Least Significant Difference (LSD) test, Principal Component Analysis (PCA), Treemap) were performed using JMP Pro 14. Regarding Treemap construction, the mean values of each treatment for each attribute are displayed as rectangular squares. The rectangular squares are arranged by line in descending order. To the left of the row, the largest mean values are placed, and to the right, the smallest mean values are placed as rectangles (JMP_rectangles). Different Latin letters within the squares indicate significant differences according to the Least Significant Difference (LSD) test at p < 0.05.
3. Results
3.1. Initial Developmental Stage
The Treemap graph in Figure 1 shows the mean value of the eight agronomic traits measured at the 3–4 leaf stage, before salinity stress imposition (NaCl application). In the commercial variety, the hydropriming treatment (T2) improved the mean values of most of the traits compared to no-priming, such as FWS (38%), FWR (11%), DWR (9.5%), SL (10.2%), BIO (6.2%), the hydropriming–biopriming treatment (T4), and the mean values of the traits RL (9%) and R/S (41.3%), while the biopriming treatment (T3) gave the highest mean value of the trait DWS. Also, the plants without any priming (no-priming treatment (T1)) performed well for most of the studied attributes. Statistically significant differences were found, in FWS, between the no-priming (T1) and hydropriming (T2) treatments. Regarding the local variety, the biopriming treatment (T3) improved the values of the following traits compared to no-priming: FWS(6.1%), DWS (7.7%), SL (5.4%) and BIO (9.5%), the hydropriming–biopriming treatment (T4) improved the following traits: FWR (14.5%), DWR (11.9%), root length (RL) and finally the hydropriming treatment (T2) improved the R/S ratio (64.6%). Statistically significant differences were found in DWS between hydropriming (T2) and biopriming (T3) treatments, and in RL between hydropriming (T2) and hydropriming/biopriming (T4) treatments, and between no-priming (T1) with hydropriming/biopriming (T4) treatments. In conclusion, the commercial variety seems to show higher mean values compared to the local variety in most agronomic traits in the early growth stages, mainly utilizing the hydropriming (T2) and hydropriming/biopriming (T4) treatments (Figures S1 and S2).
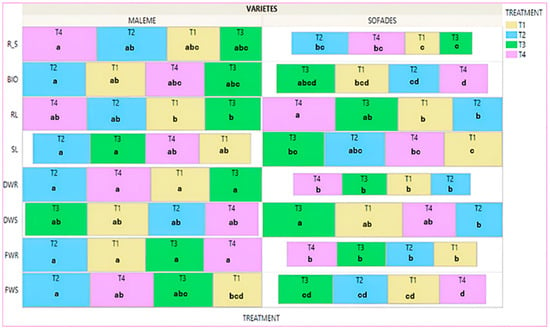
Figure 1.
Treemap plot of the mean values of the eight priming treatments (four for each treatment). The average values for each treatment for each characteristic are displayed as rectangular squares. The rectangular squares are arranged in rows in descending order. The largest average values are placed on the left side of the row, and the smallest average value is placed on the right. Different Latin letters indicate significant differences according to the test of Least Significant Difference (LSD) at p < 0.05. T1: no-priming, T2: hydropriming, T3: biopriming, T4: hydropriming/biopriming, FWR: Fresh Weight Root, DWR: Dry Weight Root, FWS: Fresh Weight Shoot, DWS: Dry Weight Shoot, RL: Root Length, SL: Shoot length, BIO: Biomass, R/S: Dry Weight Root/Dry Weight Shoot.
3.2. NDVI—Commercial Variety
The Treemap graph in Figure 2 shows the mean value of the Normalized Difference Vegetation Index (NDVI) for all treatments at all three salinity levels of the commercial variety. In general, it appeared that under normal irrigation, NDVI continued to increase with plant growth, only in the biopriming treatment (T3). At the salinity level of 80 mM NaCl, the index continued to increase in the hydropriming (T2), biopriming (T3) and hydropriming/biopriming (T4) treatments, while at 160 mM NaCl salinity, the increase in the index continued across all treatments: no-priming (T1), hydropriming (T2), biopriming (T3), and hydropriming/biopriming (T4).
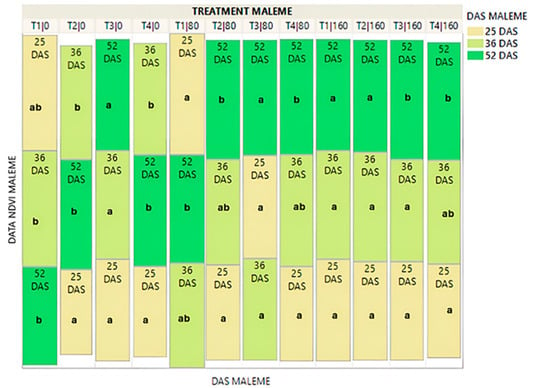
Figure 2.
Treemap plot for the average NDVI values in the commercial variety, at three different time points (25DAS/36DAS/52DAS). The mean values of the NDVI for each DAS per treatment are shown as rectangular squares. The rectangular squares are arranged by column (treatment) in descending order. The highest average NDVI values are placed at the top of the column. Different Latin letters indicate significant differences according to the test of Least Significant Difference (LSD) at p < 0.05 between the three different DAS per treatment. T1|0: no-priming/salinity level 0, T2|0: hydropriming/salinity level 0, T3|0: biopriming/salinity level 0, T4|0: hydropriming–biopriming/salinity level 0, T1|80 mM NaCl: no-priming/salinity level 80 mM NaCl, T2|80 mM: hydropriming/salinity level 80 mM, T3|80: biopriming/salinity level 80 mM, T4|80 mM: hydropriming–biopriming/salinity level 80 mM, Τ1|160 mM: no-priming/salinity level 160 mM, T2|160 mM: hydropriming/salinity level 160 mM, T3|160 mM: biopriming/salinity level 160 mM, T4|160 mM: hydropriming–biopriming/salinity level 160 mM. 25 DAS: 25 days after sowing, 35 DAS: 35 days after sowing, 52 DAS: 52 days after sowing.
In Figure 3, the four priming treatments were compared with each other within each DAS separately for each salinity level to assess their effect on the NDVI. The results showed that at the time point of 25 DAS, the no-priming treatment (T1) showed the highest values of NDVI index with statistically significant differences from the other treatments at 0 and 80 mM NaCl salt levels. At the time point of 52 DAS, hydropriming (T2), biopriming (T3), and hydropriming/biopriming (T4) treatments gave slightly higher values of the NDVI index compared to no-priming (T1) treatment at all salinity levels without statistically significant differences.
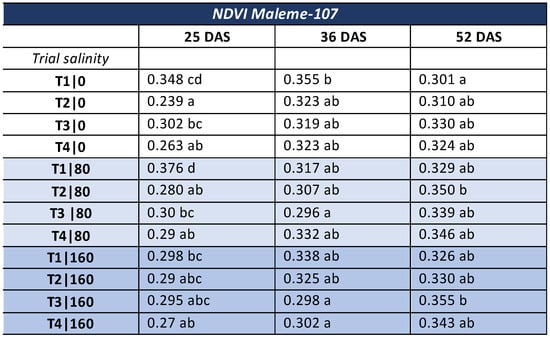
Figure 3.
Mean values of the NDVI of the commercial variety for each treatment per time point (DAS) at the three salinity levels. Different colours highlight different salinity treatments. Different Latin letters indicate significant differences according to the Least Significant Difference (LSD) test at p < 0.05. Τ1|0: no-priming/salinity level 0, T2|0: hydropriming/salinity level 0, T3|0: biopriming/salinity level 0, T4|0: hydropriming–biopriming/salinity level 0, Τ1|80: no-priming/salinity level 80, T2|80: hydropriming/salinity level 80, T3|80: biopriming/salinity level 80, T4|80: hydropriming–biopriming/salinity level 80, Τ1|160: no-priming/salinity level 160, T2|160: hydropriming/salinity level 160, T3|160: biopriming/salinity level 160, T4|160: hydropriming–biopriming/salinity level 160. 25 DAS: 25 days after sowing, 36 DAS: 36 days after sowing, 52 DAS: 45 days after sowing.
3.3. NDVI—Local Variety
The Treemap graph in Figure 4 shows the mean value of the Normalized Difference Vegetation Index (NDVI) for all treatments at all three salinity levels of the local variety. In general, it appeared that from time 36 DAS onwards, NDVI, in almost all treatments, no-priming (T1), hydropriming (T2), biopriming (T3), hydropriming/biopriming (T4), showed a decrease as plant growth progressed, except for treatment T2|80. This result prompted the completion of the salinity application experiment, and the time point of 52 DAS was the starting point for agronomic trait measurements.
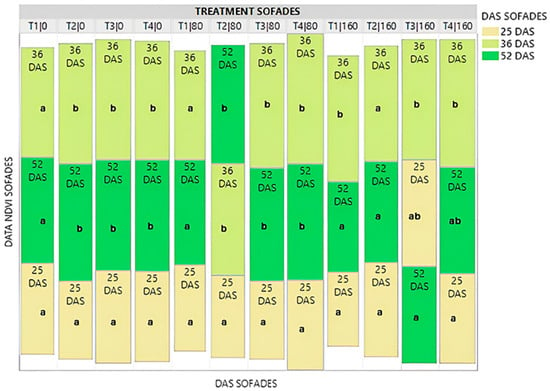
Figure 4.
Treemap plot for the mean NDVI values in the local variety at three different times (25DAS/36DAS/52DAS). The mean values of the NDVI variable for each DAS per treatment are shown as rectangular squares. The rectangular squares are arranged by column (treatment) in descending order. The highest average NDVI values are placed at the top of the column. Different Latin letters indicate significant differences according to the Least Significant Difference (LSD) test at p < 0.05, between the three DAS per treatment.
In Figure 5, the four treatments were compared with each other within each DAS separately for each salinity level to assess their effect on the NDVI. The results showed that hydropriming (T2), biopriming (T3), and hydropriming/biopriming (T4) treatments gave higher NDVI index values compared to the no-priming treatment (T1). The hydropriming/biopriming (T4) treatment at time points 36 DAS and 52 DAS showed statistically significant differences compared to the no-priming (T1) treatment at salinity levels of 80 NaCl and 160 NaCl.

Figure 5.
Mean values of the NDVI of the local variety for each treatment, per time point (DAS), at the three salinity levels. Different colours highlight different salinity treatments. Different Latin letters indicate significant differences according to the Least Significant Difference (LSD) test at p < 0.05. Τ1|0: no-priming/salinity level 0, T2|0: hydropriming/salinity level 0, T3|0: biopriming/salinity level 0, T4|0: hydropriming–biopriming/salinity level 0, Τ1|80: no-priming/salinity level 80, T2|80: hydropriming/salinity level 80, T3|80: biopriming/salinity level 80, T4|80: hydropriming–biopriming/salinity level 80, Τ1|160: no-priming/salinity level 160, T2|160: hydropriming/salinity level 160, T3|160: biopriming/salinity level 160, T4|160: hydropriming–biopriming/salinity level 160. 25 DAS: 25 days after sowing, 36 DAS: 36 days after sowing, 52 DAS: 45 days after sowing.
3.4. Advanced Stage of Development—Controls
The Treemap diagram in Figure 6 shows the mean value of the eight agronomic traits for each treatment of both cultivars measured at the end of the experimental procedure and refers to the plants that did not receive any salinity treatment (controls). In the commercial variety, the biopriming treatment (T3) showed the highest mean values in six of the eight traits: FWS, DWS, FWR, RL, SL and BIO, with statistically significant differences from the no-priming treatment (T1) in three of them, FWS (36.9%), SL (50.3%) and RL (34.6%). The hydropriming–biopriming treatment (T4) also presented high mean values with a statistically significant difference from no-priming treatment (T1), on the RL (29.6%) and on the SL (24.6%). The hydropriming treatment (T2) provided high mean values with statistically significant difference from the no-priming treatment (T1) on SL (31.8%). It is worth noting that the no-priming (T1) treatment gave the highest mean values in DWR and R/S with statistically significant differences from the hydropriming (T2) and biopriming (T3) treatments, respectively. In the local variety the treatments biopriming (T3), hydropriming–biopriming (T4), and hydropriming (T2) gave higher values in almost all agronomic traits compared to the no-priming (T1) treatment with statistically significant differences per trait. Specifically, the biopriming (T3) treatment improved FWS (100.1%), DWS (129.7%), FWR (172%), DWR (187.9%), RL (39.8%) BIO (136.7%). Regarding R/S, the biopriming treatment (T3) also gave the highest mean value compared to the other treatments, albeit with no statistically significant differences. The hydropriming–biopriming treatment (T4) showed statistically significant differences from no-priming treatment (T1) for FWS (51%), FWR (44.2%), and BIO (98.9%). The hydropriming treatment (T2) showed statistically significant differences from the no-priming treatment (T1) for FWR (60.2%). Comparing the priming treatments among the cultivars, the no-priming treatment (T1) gave lower values to the local cultivar with statistically significant differences compared to the commercial cultivar in the traits FWR, DWS, DWR and BIO. On the other hand, SL presented a significantly higher value than the commercial cultivar. The biopriming treatment (T3) gave significantly higher values in the local variety compared to the commercial variety in the traits FWR, DWR, RL and R/S. The hydropriming–biopriming treatment (T4) provided statistically greater values for the local variety compared to the commercial variety in DWS, SL and BIO and significantly lower value for RL. No significant differences were observed in the hydropriming treatment (T2) (Figure S3).
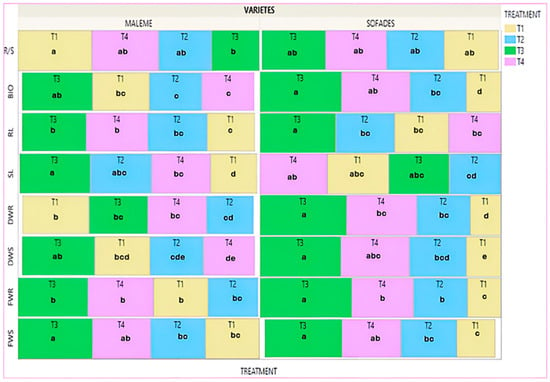
Figure 6.
Treemap plot for the mean value of the treatments, each trait separately, for the two varieties. Different Latin letters within the squares indicate significant differences according to the Least Significant Difference (LSD) test at p < 0.05, among the eight treatments (four for each variety). T1: no-priming, T2: hydropriming, T3: biopriming, T4: hydropriming/biopriming, FWR: Fresh Weight Root, DWR: Dry Weight Root, FWS: Fresh Weight Shoot, DWS: Dry Weight Shoot, RL: Root Length, SL: Shoot length, BIO: Biomass, R/S: Dry Weight Root/Dry Weight Shoot.
3.5. Salinity—Commercial Variety
In the Treemap diagram in Figure 7, the mean values of all treatments of each agronomic trait are given without salt addition and at salt concentrations of 80 mM NaCl and 160 mM NaCl. The results showed that at 80 mM NaCl salinity level, the biopriming treatment (T3) gave the highest mean values for the following traits: FWS (25.7%), SL (33.5%), and BIO (26.2%), with statistically significant differences from the no-priming treatment (T1) only regarding the SL. The biopriming/hydropriming treatment (T4) gave the highest values, with statistically significant differences from the no-priming treatment (T1), FWR (36.9%), RL (70.5%), and R/S (25.3%). The hydropriming treatment (T2) gave the lowest values across all treatments, except for the RL trait, where it showed a statistically significant difference compared to the unprimed (T1) treatment. At 160 mM NaCl, the no-priming (T1) treatment gave the highest values regarding the DWS, SL, and BIO. Subsequently, the biopriming treatment (T3) improved the following traits: FWS (7.7%), as well as FWR (40.4%) and RL (50.4%), with statistically significant differences from the no-priming treatment (T1). Finally, the biopriming/hydropriming treatment (T4) gave the highest values for the traits R/S (37.2%) and DWR (13.2%) with statistically significant differences from the no-priming treatment (T1) only for the root/shoot ratio.
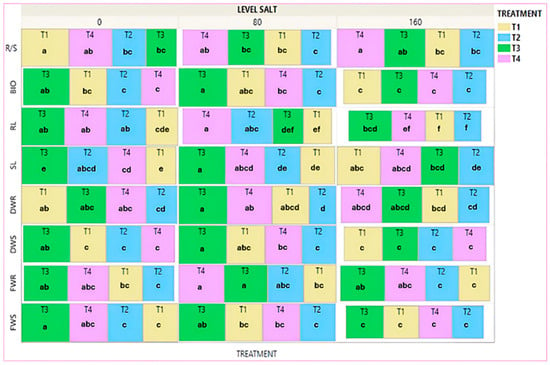
Figure 7.
Treemap plot for the mean value of treatments for each trait separately for the commercial variety, in the salinity tests. Different Latin letters within the squares indicate significant differences according to the Least Significant Difference (LSD) test at p < 0.05, among the 12 treatments (4 for each variety). Τ1: no-priming, Τ2: hydropriming, T3: biopriming, T4: hydropriming/biopriming, FWR: Fresh Weight Root, DWR: Dry Weight Root, FWS: Fresh Weight Shoot, DWS: Dry Weight Shoot, RL: Root Length, SL: Shoot length, BIO: Biomass, R/S: Dry Weight Root/Dry Weight Shoot.
Comparing the treatments among salinity levels, the biopriming treatment (T3) significantly reduced DWS, SL, and BIO. The biopriming/hydropriming treatment (T4) gave reduced values for all traits without statistically significant difference. The hydropriming treatment (T2) gave the lowest values across all treatments at the high salinity level.
Finally, the unprimed (T1) treatment either maintained or increased the mean values for each trait, and for stem length (SL), it exhibited a statistically significant increase in its mean value (Figures S4, S5 and S8).
3.6. Salinity—Local Variety
In the Treemap diagram in Figure 8, the mean values of all treatments are given for each agronomic trait, without salinity imposition and at salt concentrations of 80 mM NaCl and 160 mM NaCl. The results showed that at 80 mM NaCl salinity level, the biopriming/hydropriming treatment (T4) gave the highest mean values for almost all the traits with statistically significant differences from the no-priming treatment (T1) on the traits FWS (80.1%), FWR (124.9%), DWS (104.7%), DWR (260%), RL (47.4%), BIO (123.8%) and R/S (76.7%). The biopriming treatment (T3) gave the highest mean value for SL (24.9%) with statistically significant differences from the no-priming treatment (T1). The hydropriming (T2) treatment gave the lowest values across the treatments, like the no-priming (T1) treatment. At 160 mM NaCl salinity level, the biopriming/hydropriming (T4) treatment gave the highest mean values with statistically significant differences from the no-priming (T1) treatment for FWR (110.6%), DWR (145.7%) and R/S (49.7%). The biopriming treatment (T3) gave the highest mean values with statistically significant differences than the no-priming treatment (T1) for FWS (83.6%), DWS (91.8%), and BIO (90.5%). It also resulted in significantly higher values for FWR (67.5%) and DWR (82.9%). The hydropriming (T2) treatment gave the lowest values along with the no-priming (T1) treatment, except for the root length (RL).
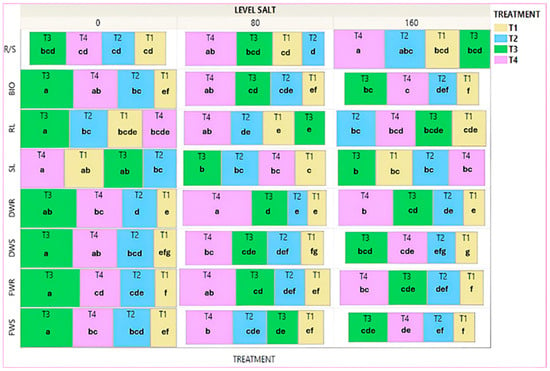
Figure 8.
Treemap graph for the mean value of treatments for each trait separately for the local variety, under salinity treatments. Different Latin letters within the squares indicate significant differences according to the Least Significant Difference (LSD) test at p < 0.05 among the 12 treatments (4 for each variety). T1: no-priming, T2: hydropriming, T3: biopriming, T4: hydropriming/biopriming, FWR: Fresh Weight Root, DWR: Dry Weight Root, FWS: Fresh Weight Shoot, DWS: Dry Weight Shoot, RL: Root Length, SL: Shoot length, BIO: Biomass, R/S: Dry Weight Root/Dry Weight Shoot.
Comparing the treatments between salinity levels, the biopriming treatment (T3) gave increased values at high salinity levels without statistically significant differences, while the biopriming/hydropriming treatment (T4) presented decreased values at high salinity levels with a statistically significant difference in FWR, DWS, and BIO traits. The hydropriming treatment (T2) presented significantly higher values under high salt stress regarding RL. Finally, the no-priming (T1) treatment maintained lower values at a high salinity regime compared to the other treatments except for the SL (Figures S6, S7 and S9).
4. Discussion
The study aimed to determine the behavior of the two varieties under the influence of priming treatments, at an early (2nd-3rd leaf) and advanced developmental stage (52 DAS), without the addition of NaCl, but also under conditions of increased salinity. Our study is the first on grass pea; however, there are studies that focus on individual aspects of this study and provided us with useful information for the analysis and evaluation of our results.
- Early developmental stage
In the early stages of plant growth (2nd-3rd leaf) (Figure 1 and Figures S1 and S2), the commercial variety showed better adaptation than the local variety by taking advantage of the controlled growth conditions. In the commercial variety, even though no statistically significant differences were observed between the treatments, the hydropriming application (T2) had a positive effect, causing an increase in most growth parameters, while the hydropriming/biopriming application (T4) also had a positive effect, increasing root length and R/S ratio. Regarding the local variety, although no significant differences were found between the treatments, the biopriming application (T3) mainly increased characteristics related to the shoot, fresh and dry weight, length and biomass, while the hydropriming–biopriming application (T4) increased root characteristics, such as length and fresh-dry weight. Many studies have demonstrated the positive effect of hydropriming and biopriming in the early stages of growth, with uniform and rapid germination, better establishment and improved growth [35,41,57,58,59]. A study by Farooq. et al. [60] found the positive impact of hydropriming on the dry weight of chickpea plants in a pot experiment, while research by Catiembo et al. [61] showed an increase in the average dry weight and length of young shoots in sunflower seedlings, in a hydropriming experiment after 12–16 h of seed immersion in H2O. Also, a study by Patel et al. [62] revealed that the inoculation of chickpea and groundnut plants with Bacillus sp. in pot experiments increased the number of roots, shoot length and leaves in the early stages of growth.
- Advanced developmental stage—controls
At the end of the experimental process, the results were also analyzed only for the control plants (those that were not subjected to salinity stress), to test the effect of each priming treatment on the agronomic characteristics at an advanced developmental stage. In the commercial variety (Figure 6 and Figure S3), it was found that the plants without treatment (no-priming) performed well in relation to the other treatments regarding fresh and dry root weight, dry shoot weight, biomass and root/shoot ratio, while the biopriming application (T3) significantly improved the fresh shoot weight and shoot and root lengths compared to the no-priming application (T1). Kumari et al. [63] reported similar results on a pot experiment with mung beans (Vigna radiata), inoculated with Bacillus sp., promoting plant growth and improving the characteristics of shoot and root length, fresh and dry weight of root and shoot, leaf area and chlorophyll percentage. Also, in a greenhouse pot experiment with ginger plants after the inoculation with Bacillus subtilis, improved plant growth, increased biomass, plant length, and leaf area were reported [64]. The local variety responded differently since plants without treatment (no-priming application) performed worse in almost all growth parameters compared to other treatments. The application of biopriming (T3) caused a statistically significant improvement compared to the no-priming (T1) treatment in characteristics, fresh and dry root and stem weight, root length and biomass, improving the response of the local variety at the advanced stage of development. Similar results were presented in a study by Miljaković et al. [65] where, during a sand pot experiment on soybean plants, treatment with Bacillus megaterium significantly improved shoot length, root length, dry root weight and seedling robustness index, compared to control. Also, a study by Solouki et al. [66] on fenugreek plants in pots showed that plant biomass, root length and weight, were positively affected by PGPB treatment. Evaluating the two varieties (after the completion of the experiment), it appeared that the commercial variety responded better under controlled greenhouse conditions without salinity treatment, as opposed to the local variety. Evaluating the priming treatments, it appeared that the application of biopriming (T3) improved the growth parameters of both varieties, but was better utilized by the local variety, improving stem and root characteristics. Advances in seed improvement (priming) technologies are expected to contribute to the safe and successful ex situ conservation of genetic material such as seeds of local varieties and minimize the risk of seed depletion of wild relatives from habitat loss, land degradation and climate change. Therefore, it is an alternative solution in the context of the restoration of native vegetation of ecosystems, an action supported by the new CAP 2023–2027 [67,68,69,70].
- NDVI
During the experiment, the Normalized Difference Vegetation Index (NDVI) [71,72], which has been successfully utilized in legumes to predict growth rate, yield and biomass, under field conditions as well as controlled greenhouse conditions, was measured at three time points [73,74,75,76,77]. Overall, it was shown that, in the commercial variety, the NDVI index value continued to increase with plant growth (Figure 2 and Figure 3) without being affected by increasing salinity even at the high salinity level of 160 mM. Although studies linking the NDVI index to changes in vegetation claim that increasing salinity causes a decrease in the index [78,79,80,81], the same does not happen in salt-tolerant plants as shown in a field experiment by Ivushkin et al. [82] where quinoa plants gave a slightly higher NDVI index under saline conditions. When Klimek-Kopyra et al. [83] studied the developmental effects of Rhizobium inoculations on a pea field experiment (Pisum sativum L.), they observed an increase in NDVI value at the maturation stage. The results in the local variety indicated that from the time point of 36 DAS, the NDVI index decreased at all salinity levels, with the only exception of the hydropriming treatment (T2) at a salinity level of 80 mM (Figure 4 and Figure 5). This result prompted the completion of exogenous NaCl application and, at the 45 DAS time point, measurements for agronomic characteristics began. A study by Kakabouki et al. [84] in pots also demonstrated a decrease in NDVI with increasing salinity to 150 mM in flax plants.
- Salinity Stress
The last part of the experimental procedure was related to the behavior of the varieties with priming treatments, under conditions of salinity stress (80 mM NaCl and 160 mM NaCl). The complex physiology of salt tolerance and the differentiation between species make it difficult to identify individual selection criteria for salt tolerance [85]. The traits measured in the study allow for the assessment of the effect of salinity on the developmental functions of plants; thus, they are useful indicators for the evaluation of resistance and possibly for the selection of resistant species or varieties that can thrive in environments with high salinity [1,86,87,88,89,90,91,92]. As previously indicated, the results showed that the commercial variety without any treatment (no-priming application) gave satisfactory values for the measured agronomical traits, especially regarding fresh-dry shoot weight and biomass at both salinity levels (approximately the same values as the other treatments). The results indicate that the commercial variety benefited from biopriming (T3) and biopriming/hydropriming treatments (T4), with the choice of treatment depending on the stress level. These treatments helped enhance root and shoot characteristics that were deficient and mitigated the negative effects of salinity (Figure 7 and Figures S4, S5 and S8). Increases in plant length and shoot fresh weight were also monitored by Metwail et al. [93] using Bacillus sp. in faba bean varieties (Vicia faba L.) under mitigation of salinity stress. Our results are in agreement with studies conducted on white clover and wheat where inoculation with Bacillus subtilis increased shoot and root length and biomass under conditions of increased salinity [94,95]. The results showed that the local variety, without treatment (no-priming application) at both salinity levels, showed a significant reduction in all growth parameters compared to the other treatments. Regarding the effect of the remaining treatments on the variety, at the low salinity level, the variety utilized the biopriming/hydropriming application (T4) and dramatically improved, with statistically significant differences, all shoot and root growth parameters, compared to the no-priming treatment. Increased stress resistance at a low level of 50 mM NaCl was also documented by Sahana Basu et al. [96] in a pot experiment, where inoculation with Bacillus subtilis increased chlorophyll content, plant height and other biochemical parameters of chickpea plants. A study by BiBi et al. [97] showed that hydropriming improved germination and growth parameters in potted rapeseed (canola) plants watered with 0.5% NaCl compared to the control. At the high salinity level, the results showed that the local variety benefited from the biopriming application (T3), mainly increasing vegetative characteristics such as fresh and dry shoot weight, shoot length and biomass, and the biopriming/hydropriming application (T4) increasing root characteristics such as fresh root weight, dry root weight and root/shoot ratio (Figure 8 and Figures S6, S7 and S9). A study by Allah et al. [98] documented salinity tolerance of chickpea plants inoculated with Bacillus subtilis and increased growth compared to control plants under severe salt stress (200 mM NaCl). Also, Nawaz et al. [99], using a salt-sensitive and a salt-resistant wheat variety, found that the damage caused by salt stress can be mitigated using PGPR and results in improved growth and yield, such as fresh and dry weight of roots and shoots, root length (RL), and biomass of the sensitive variety. An important finding at high salinity levels, as well as at the advanced growth stage, was that the control of the local variety showed relatively satisfactory performance only for the characteristics of shoot length and root length. When evaluating these results alongside established knowledge about the adaptability of local populations to challenging conditions, we hypothesize that the local variety may have struggled to fully utilize its potential in pots and controlled environments, or it may not possess inherent resistance to salt stress. A plethora of studies conducted for evaluating salt tolerance of diverse genetic material including landraces of rice, grass pea and alfalfa, respectively, demonstrated that there is a salinity–genotype interaction, indicating that there are diverse responses of the different genotypes (including many local varieties) to salinity stress [100,101,102]. In general, the results showed that the commercial variety had a better response to salinity stress compared to the local variety under control conditions. Priming treatments that increased shoot growth parameters, biopriming (T3) or root growth parameters, biopriming/hydropriming (T4), helped the varieties to cope better with stress. Similar conclusions were deduced in a study by Güleç Şen et al. [22] in a greenhouse pot experiment, with three local populations and two commercial varieties of Lathyrus sativus, where they also found that the commercial variety had a better response to salinity stress, and they set a critical salinity level threshold for grass pea at 120 mM, without any seed priming treatment.
5. Conclusions
The aim of the study was to evaluate the effect of seed priming techniques on the growth parameters of a commercial and local variety under salt stress. Under NaCl treatments, the local variety significantly mitigated the effects of stress, utilizing the biopriming/hydropriming (T4) and biopriming (T3) treatments. The biopriming application (T3) improved the growth parameters of both varieties, but it was better utilized by the local variety. Regarding the controls, the commercial variety responded better than the local variety to controlled growth conditions, confirming its reputation as a very good commercial variety with stable performance and tolerance to abiotic stresses. A future field experiment may provide additional information and a more representative picture of the potential of the two varieties and the effect of priming techniques on their growth parameters.
The purpose of the study was to evaluate the impact of priming techniques on the growth parameters of a commercial and local variety with and without NaCl application. Biopriming (T3) improved root and shoot traits in both varieties under normal conditions but was utilized more efficiently by the local variety. Under NaCl treatments, the local variety significantly mitigated the effects of stress, mainly using the biopriming/hydropriming treatment (T4), while the commercial variety primarily benefited from biopriming (T3). Regarding the controls, the commercial variety performed better than the local variety under controlled growth conditions, confirming its reputation as an excellent commercial variety with consistent yield and resistance to abiotic stresses. A future field experiment will provide additional information and a more representative picture of the potential of both varieties and the effect of priming techniques on their growth parameters.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app15041692/s1, Figure S1: Biplot of objects (characteristics) and component loadings (treatments) for the two varieties (Maleme-107 and Sofades). Figure S2: Mean values of eight agronomic characteristics. Figure S3: Mean values of the 8 agronomic traits at an advanced stage of development, without salinity, only for the controls. Figure S4: One-way ANOVA was applied to the collected data and the statistical significance of 12 treatment mean values was determined using the LSD value of p < 0.05. Figure S5: Mean values of 12 treatments for all agronomical characteristics for Maleme-107. Figure S6: One-way ANOVA was applied to the collected data and the statistical significance of 12 treatment mean values was determined using the LSD value of p < 0.05. Figure S7: Mean values of 12 treatments for all agronomical characteristics for Sofades. Figure S8: Biplot of objects (characteristics) and component loadings (treatments). Figure S9: Biplot of objects (characteristics) and component loadings (treatments).
Author Contributions
Conceptualization, E.T., A.B. and D.C.; methodology, M.G., A.P., C.K., C.C. and N.K.; software, M.G.; validation, E.T. and M.G.; formal analysis, E.T. and M.G.; investigation, M.G.; resources, E.T., A.B., D.C. and P.J.B.; data curation, E.T. and M.G.; writing—original draft preparation, M.G., E.T. and A.P.; writing—review and editing, E.T., D.C., P.J.B., A.B., M.G., A.P., C.K., C.C. and N.K.; visualization, M.G.; supervision, E.T. and D.C.; project administration, M.G.; funding acquisition, A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by PRIMA (Partnership for Research and Innovation in the Mediterranean Area) Programme-H2020, Call Multi-Topic 2021, Research and Innovation Activities—RIA, Project “Boosting technologies of orphan legumes towards resilient farming systems in the Greater Mediterranean Region: from bench to open field (BENEFIT-Med)” ID: 1726 (2022–2025).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available due to privacy.
Acknowledgments
The authors would like to thank Dimitrios Vlachostergios for providing Maleme-107 seeds and Agroland for providing Sofades seeds.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, M.W.; Grusak, M.A.; Pinto, E.; Gomes, A.; Ferreira, H.; Balázs, B.; Centofanti, T.; Ntatsi, G.; Savvas, D.; Karkanis, A. The biology of legumes and their agronomic, economic, and social impact. In The Plant Family Fabaceae: Biology and Physiological Responses to Environmental Stresses; Springer Nature: Singapore, 2020; pp. 3–25. [Google Scholar] [CrossRef]
- Pickson, R.B.; Gui, P.; Chen, A.; Boateng, E. Climate change and food security nexus in Asia: A regional comparison. Ecol. Inform. 2023, 76, 102038. [Google Scholar] [CrossRef]
- Mirón, I.J.; Linares, C.; Díaz, J. The influence of climate change on food production and food safety. Environ. Res. 2023, 216, 114674. [Google Scholar] [CrossRef]
- Lee, C.-C.; Zeng, M.; Luo, K. How does climate change affect food security? Evidence from China. Environ. Impact Assess. Rev. 2024, 104, 107324. [Google Scholar] [CrossRef]
- Aguilera, E.; Díaz-Gaona, C.; García-Laureano, R.; Reyes-Palomo, C.; Guzmán, G.I.; Ortolani, L.; Sánchez-Rodríguez, M.; Rodríguez-Estévez, V. Agroecology for adaptation to climate change and resource depletion in the Mediterranean region. A review. Agric. Syst. 2020, 181, 102809. [Google Scholar] [CrossRef]
- Araújo, S.S.; Beebe, S.; Crespi, M.; Delbreil, B.; González, E.M.; Gruber, V.; Lejeune-Henaut, I.; Link, W.; Monteros, M.J.; Prats, E. Abiotic stress responses in legumes: Strategies used to cope with environmental challenges. Crit. Rev. Plant Sci. 2015, 34, 237–280. [Google Scholar] [CrossRef]
- Terzopoulos, P.; Bebeli, P. Phenotypic diversity in Greek tomato (Solanum lycopersicum L.) landraces. Sci. Hortic. 2010, 126, 138–144. [Google Scholar] [CrossRef]
- Cooper, H.D.; Spillane, C.; Hodgkin, T. Broadening the Genetic Base of Crops: An Overview; CABI Publishing, CAB International: Oxon, UK, 2001; pp. 1–23. [Google Scholar] [CrossRef]
- Lazaridi, E.; Kapazoglou, A.; Gerakari, M.; Kleftogianni, K.; Passa, K.; Sarri, E.; Papasotiropoulos, V.; Tani, E.; Bebeli, P.J. Crop landraces and indigenous varieties: A valuable source of genes for plant breeding. Plants 2024, 13, 758. [Google Scholar] [CrossRef] [PubMed]
- Antolín, M.C.; Toledo, M.; Pascual, I.; Irigoyen, J.J.; Goicoechea, N. The exploitation of local Vitis vinifera L. biodiversity as a valuable tool to cope with climate change maintaining berry quality. Plants 2020, 10, 71. [Google Scholar] [CrossRef]
- Ribaut, J.-M.; Ragot, M. Modernising breeding for orphan crops: Tools, methodologies, and beyond. Planta 2019, 250, 971–977. [Google Scholar] [CrossRef]
- Lambein, F.; Travella, S.; Kuo, Y.-H.; Van Montagu, M.; Heijde, M. Grass pea (Lathyrus sativus L.): Orphan crop, nutraceutical or just plain food? Planta 2019, 250, 821–838. [Google Scholar] [CrossRef] [PubMed]
- Rubiales, D.; Emeran, A.A.; Flores, F. Adaptation of grass pea (Lathyrus sativus) to Mediterranean environments. Agronomy 2020, 10, 1295. [Google Scholar] [CrossRef]
- Patto, M.V.; Skiba, B.; Pang, E.; Ochatt, S.; Lambein, F.; Rubiales, D. Lathyrus improvement for resistance against biotic and abiotic stresses: From classical breeding to marker assisted selection. Euphytica 2006, 147, 133–147. [Google Scholar] [CrossRef]
- Goufa, M.; Makeroufas, E.; Gerakari, M.; Sarri, E.; Ragkos, A.; Bebeli, P.J.; Balestrazzi, A.; Tani, E. Understanding the Potential to Increase Adoption of Orphan Crops: The Case of Lathyrus spp. Cultivation in Greece. Agronomy 2024, 14, 108. [Google Scholar] [CrossRef]
- Gonçalves, L.; Rubiales, D.; Bronze, M.R.; Vaz Patto, M.C. Grass pea (Lathyrus sativus L.)—A sustainable and resilient answer to climate challenges. Agronomy 2022, 12, 1324. [Google Scholar] [CrossRef]
- Chettri, P.; Atta, K.; Pal, A. Comparative Physiology of Drought and Salinity Stress in Grass Pea (Lathyrus sativus L.) Seedlings. Int. J. Environ. Clim. Chang. 2021, 11, 111–119. [Google Scholar] [CrossRef]
- Tokarz, B.; Wójtowicz, T.; Makowski, W.; Jędrzejczyk, R.J.; Tokarz, K.M. What is the difference between the response of grass pea (Lathyrus sativus L.) to salinity and drought stress?—A physiological study. Agronomy 2020, 10, 833. [Google Scholar] [CrossRef]
- Khosravi, Z.; Pourmohammad, A.; ALILOO, A.A.; Shahabivand, S.; Hassanpouraghdam, M.B.; Topcu, H. In vitro salinity stress mediates grass pea genotypes’ (Lathyrus sativus L.) responses. Turk. J. Agric. For. 2022, 46, 340–351. [Google Scholar] [CrossRef]
- Arslan, M.; Aydınoğlu, B. Effect of salinity (NaCl) stress on germination and seedling growth characteristics in grass pea (Lathyrus sativus L.). Germination Seedl. 2018, 7, 49–54. [Google Scholar]
- Güleç Şen, K.; Başaran, U.; Çopur Doğrusöz, M.; Gülümser, E.; Mut, H. Growth and Biochemical Responses of Grass Pea (Lathyrus sativus L.) Genotypes Under Salt (NaCl) Stress Generated by Irrigation Water, and Changes in Soil pH and EC. Gesunde Pflanz. 2023, 75, 667–675. [Google Scholar] [CrossRef]
- Verma, S.R.; Solanki, H.A. Alleviating Salinity Stress During Seed Germinating by Priming Techniques: A Review. Int. J. Sci. Res. Sci. Technol. 2021, 8, 198–202. [Google Scholar] [CrossRef]
- Stavropoulou, A. About the action of metabolites of plant growth-promoting rhizobacteria Bacillus subtilis on plant salt tolerance (I). Arch. Phytopathol. Plant Prot. 2011, 44, 1867–1882. [Google Scholar] [CrossRef]
- Siddika, A.; Rashid, A.A.; Khan, S.N.; Khatun, A.; Karim, M.M.; Prasad, P.V.; Hasanuzzaman, M. Harnessing plant growth-promoting rhizobacteria, Bacillus subtilis and B. aryabhattai to combat salt stress in rice: A study on the regulation of antioxidant defense, ion homeostasis, and photosynthetic parameters. Front. Plant Sci. 2024, 15, 1419764. [Google Scholar] [CrossRef] [PubMed]
- Omafuvbe, B. Effect of salt on the fermentation of soybean (Glycine max) into padawan using Bacillus subtilis as starter culture. Afr. J. Biotechnol. 2006, 5, 1001–1005. [Google Scholar]
- Nadeem, M.; Li, J.; Yahya, M.; Wang, M.; Ali, A.; Cheng, A.; Wang, X.; Ma, C. Grain legumes and fear of salt stress: Focus on mechanisms and management strategies. Int. J. Mol. Sci. 2019, 20, 799. [Google Scholar] [CrossRef]
- Lastochkina, O. Bacillus subtilis-mediated abiotic stress tolerance in plants. In Bacilli and Agrobiotechnology: Phytostimulation and Biocontrol; Springer: Berlin/Heidelberg, Germany, 2019; Volume 2, pp. 97–133. [Google Scholar] [CrossRef]
- Kaschuk, G.; Auler, A.C.; Vieira, C.E.; Dakora, F.D.; Jaiswal, S.K.; da Cruz, S.P. Coinoculation impact on plant growth promotion: A review and meta-analysis on coinoculation of rhizobia and plant growth-promoting bacilli in grain legumes. Braz. J. Microbiol. 2022, 53, 2027–2037. [Google Scholar] [CrossRef]
- Ibarra-Villarreal, A.L.; Gándara-Ledezma, A.; Godoy-Flores, A.D.; Herrera-Sepúlveda, A.; Díaz-Rodríguez, A.M.; Parra-Cota, F.I.; de los Santos-Villalobos, S. Salt-tolerant Bacillus species as a promising strategy to mitigate the salinity stress in wheat (Triticum turgidum subsp. durum). J. Arid. Environ. 2021, 186, 104399. [Google Scholar] [CrossRef]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef]
- Ferreira, N.C.; Mazzuchelli, R.d.C.L.; Pacheco, A.C.; Araujo, F.F.d.; Antunes, J.E.L.; Araujo, A.S.F.d. Bacillus subtilis improves maize tolerance to salinity. Ciência Rural. 2018, 48, e20170910. [Google Scholar] [CrossRef]
- Damalas, C.A.; Koutroubas, S.D.; Fotiadis, S. Hydro-priming effects on seed germination and field performance of faba bean in spring sowing. Agriculture 2019, 9, 201. [Google Scholar] [CrossRef]
- Abdelhamid, M.T.; El-Masry, R.R.; Darwish, D.S.; Abdalla, M.M.; Oba, S.; Ragab, R.; EL Sabagh, A.; El Kholy, M.H.; Omer, E. Mechanisms of seed priming involved in salt stress amelioratio. In Priming and Pretreatment of Seeds and Seedlings: Implication in Plant Stress Tolerance and Enhancing Productivity in Crop Plants; Springer: Berlin/Heidelberg, Germany, 2019; pp. 219–251. [Google Scholar] [CrossRef]
- Paparella, S.; Araújo, S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed priming: State of the art and new perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Komala, N.; Sumalatha, G.; Gurumurthy, R.; Surendra, P. Seed quality enhancement techniques. J. Pharmacogn. Phytochem. 2018, 7, 3124–3128. [Google Scholar]
- Forni, C.; Borromeo, I. The utilization of seed priming as a tool to overcome salt and drought stresses: Is still a long way to go? Seeds 2023, 2, 406–420. [Google Scholar] [CrossRef]
- Dutta, P. Seed priming: New vistas and contemporary perspectives. In Advances in Seed Priming; Springer: Berlin/Heidelberg, Germany, 2018; pp. 3–22. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Gaurav, A.K.; Srivastava, S.; Verma, J.P. Plant growth-promoting bacteria: Biological tools for the mitigation of salinity stress in plants. Front. Microbiol. 2020, 11, 1216. [Google Scholar] [CrossRef]
- Pagano, A.; Macovei, A.; Balestrazzi, A. Molecular dynamics of seed priming at the crossroads between basic and applied research. Plant Cell Rep. 2023, 42, 657–688. [Google Scholar] [CrossRef]
- Devika, O.S.; Singh, S.; Sarkar, D.; Barnwal, P.; Suman, J.; Rakshit, A. Seed priming: A potential supplement in integrated resource management under fragile intensive ecosystems. Front. Sustain. Food Syst. 2021, 5, 654001. [Google Scholar] [CrossRef]
- Upadhyay, S.; Singh, D. Effect of salt-tolerant plant growth-promoting rhizobacteria on wheat plants and soil health in a saline environment. Plant Biol. 2015, 17, 288–293. [Google Scholar] [CrossRef]
- Swetha, L.N.; Dayal, A. Effect of Seed Biopriming on Germination & Seedling Attributes in Chickpea (Cicer arietinum L.). Int. J. Environ. Clim. Chang. 2024, 14, 431–440. [Google Scholar] [CrossRef]
- Neshat, M.; Abbasi, A.; Hosseinzadeh, A.; Sarikhani, M.R.; Dadashi Chavan, D.; Rasoulnia, A. Plant growth promoting bacteria (PGPR) induce antioxidant tolerance against salinity stress through biochemical and physiological mechanisms. Physiol. Mol. Biol. Plants 2022, 28, 347–361. [Google Scholar] [CrossRef]
- Lastochkina, O.; Aliniaeifard, S.; Garshina, D.; Garipova, S.; Pusenkova, L.; Allagulova, C.; Fedorova, K.; Baymiev, A.; Koryakov, I.; Sobhani, M. Seed priming with endophytic Bacillus subtilis strain-specifically improves growth of Phaseolus vulgaris plants under normal and salinity conditions and exerts anti-stress effect through induced lignin deposition in roots and decreased oxidative and osmotic damages. J. Plant Physiol. 2021, 263, 153462. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Zhao, X.Q.; Javed, M.T.; Khan, K.S.; Bano, A.; Shen, R.F.; Masood, S. Bacillus pumilus enhances tolerance in rice (Oryza sativa L.) to combined stresses of NaCl and high boron due to limited uptake of Na+. Environ. Exp. Bot. 2016, 124, 120–129. [Google Scholar] [CrossRef]
- Haroon, U.; Munis, M.F.H.; Liaquat, F.; Khizar, M.; Elahi, M.; Chaudhary, H.J. Biofilm formation and flocculation potential analysis of halotolerant Bacillus tequilensis and its inoculation in soil to mitigate salinity stress of chickpea. Physiol. Mol. Biol. Plants 2023, 29, 277–288. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Alaraidh, I.A.; Alsahli, A.A.; Alamri, S.A.; Ali, H.M.; Alayafi, A.A. Bacillus firmus (SW5) augments salt tolerance in soybean (Glycine max L.) by moduLating root system architecture, antioxidant defense systems and stress-responsive genes expression. Plant Physiol. Biochem. 2018, 132, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Desoky, E.-S.M.; Saad, A.M.; El-Saadony, M.T.; Merwad, A.-R.M.; Rady, M.M. Plant growth-promoting rhizobacteria: Potential improvement in antioxidant defense system and suppression of oxidative stress for alleviating salinity stress in Triticum aestivum (L.) plants. Biocatal. Agric. Biotechnol. 2020, 30, 101878. [Google Scholar] [CrossRef]
- Biswas, S.; Seal, P.; Majumder, B.; Biswas, A.K. Efficacy of seed priming strategies for enhancing salinity tolerance in plants: An overview of the progress and achievements. Plant Stress 2023, 9, 100186. [Google Scholar] [CrossRef]
- Nautiyal, C.S.; Srivastava, S.; Chauhan, P.S.; Seem, K.; Mishra, A.; Sopory, S.K. Plant growth-promoting bacteria Bacillus amyloliquefaciens NBRISN13 modulates gene expression profile of leaf and rhizosphere community in rice during salt stress. Plant Physiol. Biochem. 2013, 66, 1–9. [Google Scholar] [CrossRef]
- Martínez-Álvarez, J.C.; Castro-Martínez, C.; Sánchez-Peña, P.; Gutiérrez-Dorado, R.; Maldonado-Mendoza, I.E. Development of a powder formulation based on Bacillus cereus sensu lato strain B25 spores for biological control of Fusarium verticillioides in maize plants. World J. Microbiol. Biotechnol. 2016, 32, 1–10. [Google Scholar] [CrossRef]
- Lobo, C.B.; Tomás, M.S.J.; Viruel, E.; Ferrero, M.A.; Lucca, M.E. Development of low-cost formulations of plant growth-promoting bacteria to be used as inoculants in beneficial agricultural technologies. Microbiol. Res. 2019, 219, 12–25. [Google Scholar] [CrossRef]
- Hashmi, I.; Paul, C.; Al-Dourobi, A.; Sandoz, F.; Deschamps, P.; Junier, T.; Junier, P.; Bindschedler, S. Comparison of the plant growth-promotion performance of a consortium of Bacilli inoculated as endospores or as vegetative cells. FEMS Microbiol. Ecol. 2019, 95, fiz147. [Google Scholar] [CrossRef]
- Hatala, J.A. Spatiotemporal Dynamics of Carbon Dioxide and Methane Fluxes from Agricultural and Restored Wetlands in the California Delta. Ph.D. Thesis, University of California, Berkeley, CA, USA, 2013. [Google Scholar]
- Tarchoun, N.; Saadaoui, W.; Hamdi, K.; Falleh, H.; Pavli, O.; Ksouri, R.; Petropoulos, S.A. Seed Priming and Biopriming in Two Squash Landraces (Cucurbita maxima Duchesne) from Tunisia: A Sustainable Strategy to Promote Germination and Alleviate Salt Stress. Plants 2024, 13, 2464. [Google Scholar] [CrossRef] [PubMed]
- Blake, C.; Christensen, M.N.; Kovács, Á.T. Molecular aspects of plant growth promotion and protection by Bacillus subtilis. Mol. Plant-Microbe Interact. 2021, 34, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, M.K. Seed priming: A low-cost technology for resource-poor farmers in improving pulse productivity. In Advances in Seed Priming; Springer: Berlin/Heidelberg, Germany, 2018; pp. 187–208. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Imran, M.; Ahmad, I.; Atif, M.; Alghamdi, S.S. Improving the productivity and profitability of late sown chickpea by seed priming. Int. J. Plant Prod. 2019, 13, 129–139. [Google Scholar] [CrossRef]
- Catiempo, R.L.; Photchanachai, S.; Bayogan, E.R.V.; Wongs-Aree, C. Impact of hydropriming on germination and seedling establishment of sunflower seeds at elevated temperature. Plant Soil Environ. 2021, 67, 491–498. [Google Scholar] [CrossRef]
- Patel, S.; Bhattacharya, C.; Pandhi, N. Screening of Bacillus sp. (OQ654027) Mediated Seed Bio-Priming Enhance Plant-Growth-Promotion for Sustainable Crop Production of Groundnut and Chickpea. Curr. Agric. Res. J. 2023, 11, 865–880. [Google Scholar] [CrossRef]
- Kumari, P.; Meena, M.; Gupta, P.; Dubey, M.K.; Nath, G.; Upadhyay, R. Plant growth promoting rhizobacteria and their biopriming for growth promotion in mung bean (Vigna radiata (L.) R. Wilczek). Biocatal. Agric. Biotechnol. 2018, 16, 163–171. [Google Scholar] [CrossRef]
- Jabborova, D.; Enakiev, Y.; Sulaymanov, K.; Kadirova, D.; Ali, A.; Annapurna, K. Plant growth promoting bacteria Bacillus subtilis promote growth and physiological parameters of Zingiber officinale Roscoe. Plant Sci. Today 2021, 8, 66–71. [Google Scholar] [CrossRef]
- Miljaković, D.; Marinković, J.; Tamindžić, G.; Đorđević, V.; Tintor, B.; Milošević, D.; Ignjatov, M.; Nikolić, Z. Bio-priming of soybean with Bradyrhizobium japonicum and Bacillus megaterium: Strategy to improve seed germination and the initial seedling growth. Plants 2022, 11, 1927. [Google Scholar] [CrossRef]
- Solouki, H.; Kafi, M.; Nabati, J.; Ahmadi, M.J.; Nezami, A.; Ahmady, R.S. Seed biopriming and plant growth-promoting bacteria improve nutrient absorption and dry matter production of fenugreek (Trigonella foenum-graecum) plants. S. Afr. J. Bot. 2023, 162, 296–303. [Google Scholar] [CrossRef]
- Pedrini, S.; Balestrazzi, A.; Madsen, M.D.; Bhalsing, K.; Hardegree, S.P.; Dixon, K.W.; Kildisheva, O.A. Seed enhancement: Getting seeds restoratio-ready. Restoratio Ecol. 2020, 28, S266–S275. [Google Scholar] [CrossRef]
- Nevill, P.G.; Cross, A.T.; Dixon, K.W. Ethical seed sourcing is a key issue in meeting global restoratio targets. Curr. Biol. 2018, 28, R1378–R1379. [Google Scholar] [CrossRef] [PubMed]
- Meissen, J.C.; Galatowitsch, S.M.; Cornett, M.W. Risks of overharvesting seed from native tallgrass prairies. Restoratio Ecol. 2015, 23, 882–891. [Google Scholar] [CrossRef]
- Commission, E. Key Policy Objectives of the CAP 2023-27. Available online: https://agriculture.ec.europa.eu/common-agricultural-policy/cap-overview/cap-2023-27/key-policy-objectives-cap-2023-27_en (accessed on 10 March 2023).
- Roujean, J.-L.; Breon, F.-M. Estimating PAR absorbed by vegetation from bidirectional reflectance measurements. Remote Sens. Environ. 1995, 51, 375–384. [Google Scholar] [CrossRef]
- Price, J.C. Estimating vegetation amount from visible and near infrared reflectances. Remote Sens. Environ. 1992, 41, 29–34. [Google Scholar] [CrossRef]
- Starks, P.; Zhao, D.; Phillips, W.; Coleman, S. Herbage mass, nutritive value and canopy spectral reflectance of bermudagrass pastures. Grass Forage Sci. 2006, 61, 101–111. [Google Scholar] [CrossRef]
- Sadras, V.; Lake, L.; Leonforte, A.; McMurray, L.; Paull, J. Screening field pea for adaptation to water and heat stress: Associations between yield, crop growth rate and seed abortion. Field Crops Res. 2013, 150, 63–73. [Google Scholar] [CrossRef]
- Maphosa, L.; Richards, M.F.; Norton, S.L.; Nguyen, G.N. Breeding for abiotic stress adaptation in chickpea (Cicer arietinum L.): A comprehensive review. Crop Breed. Genet. Genom. 2020, 4, e200015. [Google Scholar] [CrossRef]
- Lake, L.; Sadras, V.O. Screening chickpea for adaptation to water stress: Associations between yield and crop growth rate. Eur. J. Agron. 2016, 81, 86–91. [Google Scholar] [CrossRef]
- Inman, D.; Khosla, R.; Mayfield, T. On-the-go active remote sensing for efficient crop nitrogen management. Sens. Rev. 2005, 25, 209–214. [Google Scholar] [CrossRef]
- Patwa, N.; Pandey, V.; Gupta, O.P.; Yadav, A.; Meena, M.R.; Ram, S.; Singh, G. Unravelling wheat genotypic responses: Insights into salinity stress tolerance in relation to oxidative stress, antioxidant mechanisms, osmolyte accumulation and grain quality parameters. BMC Plant Biol. 2024, 24, 875. [Google Scholar] [CrossRef]
- Goto, K.; Goto, T.; Nmor, J.C.; Minematsu, K.; Gotoh, K. Evaluating salinity damage to crops through satellite data analysis: Application to typhoon affected areas of southern Japan. Nat. Hazards 2015, 75, 2815–2828. [Google Scholar] [CrossRef]
- Duan, Z.; Wang, X.; Sun, L.; Zhou, M.; Luo, Y. An insight into effect of soil salinity on vegetation dynamics in the exposed seafloor of the Aral Sea. Sci. Total Environ. 2024, 951, 175615. [Google Scholar] [CrossRef]
- Brunner, P.; Li, H.; Kinzelbach, W.; Li, W.; Dong, X. Extracting phreatic evaporatio from remotely sensed maps of evapotranspiratio. Water Resour. Res. 2008, 44, W08428. [Google Scholar] [CrossRef]
- Ivushkin, K.; Bartholomeus, H.; Bregt, A.K.; Pulatov, A.; Franceschini, M.H.; Kramer, H.; van Loo, E.N.; Roman, V.J.; Finkers, R. UAV based soil salinity assessment of cropland. Geoderma 2019, 338, 502–512. [Google Scholar] [CrossRef]
- Klimek-Kopyra, A.; Zajac, T.; Oleksy, A.; Kulig, B.; Slizowska, A. The value of different vegetative indices (NDVI, GAI) for the assessment of yield potential of pea (Pisum sativum L.) at different growth stages and under varying management practices. Acta Agrobot. 2018, 71, 1733. [Google Scholar] [CrossRef]
- Kakabouki, I.; Stavropoulos, P.; Roussis, I.; Mavroeidis, A.; Bilalis, D. Contribution of arbuscular mycorrhizal fungi (AMF) in improving the growth and yield performances of flax (Linum usitatissimum L.) to salinity stress. Agronomy 2023, 13, 2416. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004, 166, 3–16. [Google Scholar] [CrossRef]
- Wei, T.-J.; Li, G.; Cui, Y.-R.; Xie, J.; Gao, X.-A.; Teng, X.; Zhao, X.-Y.; Guan, F.-C.; Liang, Z.-W. Variation Characteristics of Root Traits of Different Alfalfa Cultivars under Saline-Alkaline Stress and their Relationship with Soil Environmental Factors. Phyton (0031-9457) 2024, 93, 29–43. [Google Scholar] [CrossRef]
- Wang, Q.H.; Han, W.; Hou, Y.Y.; Feng, L.; Ye, Z.; Gu, H.; Chen, B. Responses of main characters of root system to salt stress among cotton varieties with different salt tolerance. J. Appl. Ecol. 2018, 29, 865–873. [Google Scholar] [CrossRef]
- Thakur, M.; Sharma, P.; Anand, A. Seed priming-induced early vigor in crops: An alternate strategy for abiotic stress tolerance. In Priming and Pretreatment of Seeds and Seedlings: Implication in Plant Stress Tolerance and Enhancing Productivity in Crop Plants; Springer: Berlin/Heidelberg, Germany, 2019; pp. 163–180. [Google Scholar] [CrossRef]
- Seo, D.H.; Seomun, S.; Choi, Y.D.; Jang, G. Root development and stress tolerance in rice: The key to improving stress tolerance without yield penalties. Int. J. Mol. Sci. 2020, 21, 1807. [Google Scholar] [CrossRef]
- El-Hendawy, S.E.; Hu, Y.; Yakout, G.M.; Awad, A.M.; Hafiz, S.E.; Schmidhalter, U. Evaluating salt tolerance of wheat genotypes using multiple parameters. Eur. J. Agron. 2005, 22, 243–253. [Google Scholar] [CrossRef]
- Diya, A.; Beena, R.; Jayalekshmy, V. Physiological, Biochemical and Molecular Mechanisms of Seed Priming: A Review. Legume Res. Int. J. 2024, 47, 159–166. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Ge, J.; Li, R.; Zhang, R.; Zhang, Y.; Huo, Z.; Xu, K.; Wei, H.; Dai, Q. Improved physiological and morphological traits of root synergistically enhanced salinity tolerance in rice under appropriate nitrogen application rate. Front. Plant Sci. 2022, 13, 982637. [Google Scholar] [CrossRef]
- Metwali, E.M.; Abdelmoneim, T.S.; Bakheit, M.A.; Kadasa, N.M. Alleviation of salinity stress in faba bean (Vicia faba L.) plants by inoculation with plant growth promoting rhizobacteria (PGPR). Plant Omics 2015, 8, 449–460. [Google Scholar]
- Han, Q.-Q.; Lü, X.-P.; Bai, J.-P.; Qiao, Y.; Paré, P.W.; Wang, S.-M.; Zhang, J.-L.; Wu, Y.-N.; Pang, X.-P.; Xu, W.-B. Beneficial soil bacterium Bacillus subtilis (GB03) augments salt tolerance of white clover. Front. Plant Sci. 2014, 5, 525. [Google Scholar] [CrossRef] [PubMed]
- Gul, S.; Javed, S.; Azeem, M.; Aftab, A.; Anwaar, N.; Mehmood, T.; Zeshan, B. Application of Bacillus subtilis for the alleviation of salinity stress in different cultivars of Wheat (Tritium aestivum L.). Agronomy 2023, 13, 437. [Google Scholar] [CrossRef]
- Basu, S.; Kumari, S.; Subhadarshini, P.; Rishu, A.K.; Shekhar, S.; Kumar, G. Plant growth promoting rhizobacterium Bacillus sp. BSE01 alleviates salt toxicity in chickpea (Cicer arietinum L.) by conserving ionic, osmotic, redox and hormonal homeostasis. Physiol. Plant. 2023, 175, e14076. [Google Scholar] [CrossRef]
- BiBi, R.; Elahi, N.N.; Danish, S.; Alahmadi, T.A.; Ansari, M.J. Enhancing germination and growth of canola (Brassica napus L.) through hydropriming and NaCl priming. Sci. Rep. 2024, 14, 14026. [Google Scholar] [CrossRef]
- Abd_Allah, E.F.; Alqarawi, A.A.; Hashem, A.; Radhakrishnan, R.; Al-Huqail, A.A.; Al-Otibi, F.O.N.; Malik, J.A.; Alharbi, R.I.; Egamberdieva, D. Endophytic bacterium Bacillus subtilis (BERA 71) improves salt tolerance in chickpea plants by reguLating the plant defense mechanisms. J. Plant Interact. 2018, 13, 37–44. [Google Scholar] [CrossRef]
- Nawaz, A.; Shahbaz, M.; Asadullah; Imran, A.; Marghoob, M.U.; Imtiaz, M.; Mubeen, F. Potential of salt tolerant PGPR in growth and yield augmentation of wheat (Triticum aestivum L.) under saline conditions. Front. Microbiol. 2020, 11, 2019. [Google Scholar] [CrossRef]
- Soofinia, S.; Pourmohammad, A.; Aliloo, A.; Alizadeh, K. The Response of Early-Maturing Grass Pea (Lathyrus sativus) Genotypes to Different Levels of Salinity Stress. J. Crop Breed. 2024, 16, 61–73. [Google Scholar] [CrossRef]
- Ali, M.N.; Yeasmin, L.; Gantait, S.; Goswami, R.; Chakraborty, S. Screening of rice landraces for salinity tolerance at seedling stage through morphological and molecular markers. Physiol. Mol. Biol. Plants 2014, 20, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Al-Farsi, S.M.; Al-Sadi, A.M.; Ullah, A.; Farooq, M. Salt tolerance in alfalfa landraces of omani origin: Morpho-biochemical, mineral, and genetic diversity assessment. J. Soil Sci. Plant Nutr. 2021, 21, 1484–1499. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).