Abstract
Arsenic (As), a well-known ‘traditional’ environmental contaminant, and carbamazepine (CBZ), an emerging contaminant of a pharmaceutical category, are both frequently detected in the environment and have been shown to exhibit toxicity at exposure concentrations present in the environment. This study aimed to assess the single and combined exposure effects of these pollutants on the adult common vole (Microtus arvalis L.), a model mammalian organism. This study assessed As and CBZ accumulation, their biotransformation processes, and antioxidant enzyme defence responses after sole and combined exposure. The animals were fed a diet containing either a low (1.25 mg/kg) or high level (166 mg/kg) of As. Moreover, half of the animals were exposed to CBZ via drinking water infused with 10 ng/L of CBZ, and the second half had the use of drinking water devoid of CBZ. The results showed enhanced total As and As species contents in the organs of the As + CBZ exposure group compared to the As exposure group. High As exposure in the As + CBZ group did not cause an enhanced uptake of CBZ in the liver compared to the CBZ exposure group. There was a potential accumulation of CBZ in the liver of the CBZ exposed groups (CBZ and As + CBZ), raising concerns about potential toxic effects in mammals from long-term exposure. Glutathione peroxidase (GPx) activity, reflecting the antioxidant enzyme defence responses against single and co-exposure of the two pollutants, showed that the CBZ group exhibited comparable activity to the control group, while the As group had down-regulation and the As + CBZ group had up-regulation. These findings suggest that the CBZ group experienced minimal oxidative stress conditions, similar to the control group. The As group showed a rapid adaptation response to curtail or offset potential oxidative stress tissue damage conditions, compared to the slow adaptation/response in the As + CBZ group. The findings of this experiment indicate that the possible interactions of various environmental pollutants could alter the potential effects of the individual pollutants after a sole exposure. These findings indicate the necessity of investigating these interactions for better understanding of the potential risk of these pollutants in real environmental conditions.
1. Introduction
Emerging contaminants (ECs) or trace pollutants are natural or artificial substances that are present in the environment at trace concentrations (at nanogram and microgram per dm3 levels in the matrix). At such low concentrations, certain ECs have been scientifically proven to induce adverse impacts on the environment and cause health damage to humans and animals. However, the potential negative effects resulting from the presence of numerous ECs on receptors remain unknown or subject to speculation [1,2]. The presence of these trace pollutants in the environment has generated growing public attention and concerns globally, especially among the scientific community in recent decades. This is because before this period, ECs have been released into the environment unregulated and unmonitored. Among them, pharmaceuticals such as carbamazepine (CBZ) have been extensively investigated regarding their potential impact on biological systems.
Carbamazepine is an emerging contaminant of the pharmaceutical category that has gained notoriety in the scientific literature due to its frequent detection in the environment globally [3,4]. It is mainly used as an antiepileptic medicine, with many non-epileptic uses, including the treatment of depression, neuropathic pain, mental disorders, alcohol and drug withdrawal syndrome, diabetes, and restless leg syndrome [5,6,7]. The wide-ranging therapeutic usage of CBZ has culminated in its high worldwide consumption of over 1000 tonnes per year [8], and its massive consumption pattern leads to its continuous release into the environment. Moreover, this substance is highly refractory to all forms of degradation (physical, biological, and chemical) in the environment, and its removal from wastewater and sewage sludge treatment processes is limited, representing <45% for water, and <30% for sewage sludge [9,10,11,12].
From a global perspective, CBZ has been quantified (ng/L to µg/L levels) in various water resources, including freshwater bodies [4,13,14], marine environments [11,15], and groundwater [10,16,17,18]. It has also been detected in drinking water globally [19,20,21]. In the Czech Republic, CBZ has been detected in drinking and surface waters, where the highest CBZ levels in drinking water reached up to 18.5 ng/L [22,23].
At environmentally relevant concentrations, the toxic effects of this drug on non-target terrestrial animals have been speculated. However, few studies have been conducted to demonstrate its toxicity in model laboratory animals. For example, CBZ contained in drinking water at environmentally relevant concentrations is reported to have crossed the biological barriers of pregnant mice to the foetus’s brain, a situation the authors speculated can induce functional disabilities in the neurological system of the foetus [24]. There is also empirical scientific evidence that shows impairment of early-stage development in embryos of chicks exposed to CBZ at concentrations reported in treated wastewater [25]. However, based on a comprehensive literature review, studies to assess bioaccumulation, metabolic pathways, and potential health risks from exposure to CBZ at subtherapeutic concentrations in non-target small terrestrial mammals are limited.
Furthermore, thus far, although there have been just a few, these toxicity or risk assessment studies have focused on terrestrial small mammals’ exposure to CBZ alone at environmental concentrations under laboratory conditions [26]. On the contrary, in their natural terrestrial environment, these animals are often exposed to mixtures of different pollutants via food, accidental soil ingestion, and drinking water. Often, soils are contaminated with a mixture of many different classes/groups of potentially hazardous substances, including risk elements (REs), polycyclic aromatic hydrocarbons, persistent organic pollutants (POPs) such as polychlorinated biphenyls, chlorinated pesticides, etc., and their potential bioavailability/bioaccessibility for various organisms has already been widely investigated [27,28,29,30]. The negative health effects on animal and human organisms resulting from exposure to each class of pollutant are generally known as well [31,32,33]. However, data concerning the mutual effect on the environment of a cocktail of the above-mentioned compounds are scarce.
Among the REs group, arsenic (As) deserves special attention. Arsenic is well-known as a ‘traditional’ environmental contaminant and pollutant of global public health concern. Studies carried out worldwide have shown that the uptake of As from environmental sources and As-contaminated food by both humans and experimental animals can result in various disease conditions [34,35,36,37]. Moreover, inorganic As is present in biological systems as either arsenite or arsenate and can be metabolized via enzymatic methylation into various organometallic compounds such as methylarsonic (MMA) and dimethylarsinic acid (DMA). In this context, the methylated As compounds were proven as less toxic in biological systems compared to the inorganic ones. Therefore, the potential risk of As and its compounds in environmental pollution cannot be underestimated. Arsenic and CBZ at concentrations detected in the environment are known to exert their toxicities in biological systems by causing the generation of excessive reactive oxygen species (ROS), which can overwhelm the organism’s antioxidant defence system, leading to oxidative stress [37,38].
Small terrestrial mammals can be exposed simultaneously to both ‘traditional’ pollutants (e.g., REs) and ECs such as pharmaceuticals, where the potential mutual effects due to the interaction between both groups of contaminants on these organisms remain currently unknown. In a rare case, exposure of common beet plants to a mixture of REs and ECs resulted in an antagonistic relationship between the two groups of pollutants [39]. The present study prioritised the combined exposure to CBZ and As due to their frequent occurrences in the environment, with the common vole (Microtus arvalis L.) as the selected mammalian model organism for laboratory experiments. The choice of a small terrestrial mammal such as M. arvalis for this research was based on their proven effectiveness as bioindicators for monitoring the terrestrial environment [40,41]. In addition, they have been effectively utilised as surrogates in previous studies for the assessment of potential health risks to humans and terrestrial animals that were exposed to inorganic and organic contaminants [42,43]. Arsenic is among the most hazardous pollutants in the Czech Republic due to elevated As content in soils in various areas [44,45,46]. The choice of CBZ for this study was based on our previous experiences with animal exposure to this pollutant [47]. We hypothesized potential worsening of the pollutant effects in the case of combined compared to sole exposure. This experiment can provide novel information and add to the general knowledge concerning the potential impact of multiple sources of environmental pollution on wildlife.
Therefore, the present study had two objectives:
- (a)
- Assess the impact of the sole and co-exposure effects of CBZ and As oral uptake by M. arvalis by evaluating their accumulation and biotransformation (methylation) within the investigated animal organism as compared to the control group and in relation to each other. In this context, CBZ was present in the drinking water, whereas As-bearing soil was incorporated into the diet of voles to simulate unintentional consumption of soil under natural conditions.
- (b)
- Determine the activity of glutathione peroxidase (GPx) in blood plasma, and use it as the main indicator to assess the antioxidant enzyme defence response to the As and CBZ individual and combined uptake by the animals.
Based on these findings, the impact of the co-exposure of small terrestrial mammals to two different pollutants can be understood, and these findings can stimulate interest in the deeper investigation of the mutual adverse effects of various substances occurring in the environment on wildlife.
2. Materials and Methods
2.1. Ethical Approval
The use of experimental animals for this study complied with the present laws of the Czech Republic Act No. 246/1992 coll. on Protection against Animal Cruelty and EU Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. The experimental procedure was approved by a committee from the Ministry of Education, Youth and Sports, Czech Republic, with approval code MSMT-1082/2017-2. The provision of animal care was supervised and authorised by Zuzana Čadková, (Ph.D.), holder of Certificate No. CZ 02201 issued by the Central Commission for Animal Welfare of the Czech Republic. The in vivo experiment was chosen because the production of antioxidant enzymes is complex and species-dependent, and, therefore, it is not easy to simulate these processes in vitro. The official database of the alternative standardized methods to replace experimental animals with in vitro methods [48] do not offer any alternative in the case of CBZ, and in the case of GPx, the information is limited to laboratory animals only, not including any wild species.
2.2. Animal Experiment
2.2.1. Experimental Design
Fifty-two adult (50% males, 50% females) common voles (M. arvalis) endured the 6 weeks of the in vivo feeding study. The animals originated from North Bohemian areas of the Czech Republic, where the parental generation was caught directly in their natural conditions, and the 2nd generation was used for this experiment. The animals were kept at the Demonstrational and Experimental Centre, Czech University of Life Sciences, Prague, where they were housed in plastic boxes E3, which are standard for laboratory rodent husbandry. The common voles were allowed to acclimatise for 14 days. Within these 14 days, the animals consumed commercially produced pellet feeds and tap water ad libitum under the natural 12 h light/12 h dark cycle at a controlled temperature of 22 ± 2 °C.
After the acclimatisation period, the animals were divided randomly into four groups: Control, CBZ, As, and As + CBZ (Table 1). These groupings were based on (i) whether the experimental diet fed to the animals was below or above the indicated value of As and (ii) whether the common voles’ drinking water (tap water) contained 10 ng/L of CBZ or not. Thus, Control and CBZ groups received the diet containing very low As content soil, whereas the As and As + CBZ groups received the diet amended with very high As content soil. The groups marked “CBZ” received carbamazepine in their drinking water. M. arvalis given the same treatment were accommodated in two plastic boxes (males and females separately). The diet and water consumption were weekly recorded during the study (for details, see Table 1). The experiment was terminated after 6 weeks and all of the voles were euthanised. The animals were anaesthetised via inhalation followed by dislocation of the C1 vertebra.

Table 1.
Experimental design.
2.2.2. Characteristics of Experimental Soils
To assess the animal’s oral exposure to As through feed, two surface arable soils (0–20 cm) from two locations in the Czech Republic were selected for the experiment. These soils were chosen based on their markedly dissimilar pseudototal contents of As. One of these soils was named Cítov soil because it was sampled from the surroundings of the town of Cítov, Czech Republic (pHCaCl2 = 7.0; oxidisable carbon, Cox = 1.03%; cation exchange capacity, CEC = 110 mmol+/kg; for more details see Mercl et al. [49]). Table 2 documents the pseudototal (Aqua regia soluble) element contents of this soil, showing low contents of all the elements compared to the so-called preventive values given by the Public Notice 153/2016 [50], which contains the threshold levels of various pollutants in arable soils in the Czech Republic. The other soil was named Mokrsko soil (Cox = 2.20%, pHCaCl2 = 5.5, CEC = 105 mmol+/kg; see Tremlová et al. [51] for more details) because this soil was sampled from the surroundings of a gold deposit close to the village of Mokrsko, Czech Republic. Table 2 shows that the Mokrsko soil is extremely polluted with As, whereas the other risk element contents fall under the preventive values indicated in the Public Notice 153/2016 [50]. The two soils were air-dried, ground in a mortar, and passed through a 2 mm plastic sieve before being used to enrich the animal feed.

Table 2.
Pseudo total contents of As and other selected risk elements (mean ± standard deviation in mg/kg) in Citov and Morkrsko soils and their respective preventive values.
2.2.3. Preparations of Experimental Diet and CBZ Solution
Commercially supplied total-pathogen-free pellet feeds (for breeding mice and rats) from Velaz s.r.o, Prague, Czech Republic, were used to feed the untreated experimental animals. The main nutritional values of the commercially supplied pellet feed, as declared by the manufacturer, are moisture = 11.1%, crude ash = 6.1%, crude fibre = 4.5%, crude protein = 22.5%, Ca = 7.06 g/kg, K = 10.14 g/kg, Mg = 2.06 g/kg, Fe = 191 mg/kg, Se = 0.26 mg/kg, Zn = 85.0 mg /kg, vitamin A = 26,250 IU/kg, and vitamin E = 133 mg/ kg. For the preparation of the diet to feed the treatment animals, defined portions of individual soils were mixed with the commercially supplied pellet feeds to obtain a final percentage of 10% soil (w/w) of the final weight of the diet. The commercially supplied pellet feeds were milled to a fine powder and thoroughly mixed with the experimental soils. Then, the feed–soil homogeneous mixture was re-pelleted to serve as an experimental diet for the investigated animals. The 10% soil rate was chosen according to previous experiments [52]. The final content of As in the feeding mixtures was 1.25 mg/kg for the low As diet and 166 mg/kg for the high As diet.
Regarding the preparation of CBZ (>99% purity, purchased from Sigma–Aldrich, St. Louis, MO, USA), 10 ng of CBZ per litre of tap water was prepared freshly every week and divided among individual 500 mL water bottles to serve as the drinking water for the CBZ and As + CBZ groups. The choice of 10 ng/L CBZ was based on its realistic concentration in freshwater bodies [4].
2.2.4. Tissue Sample Collection
Immediately after vertebral dislocation, the whole cardiac blood sample of each specimen was withdrawn into vacutainer® tubes coated with spray-dried K3EDTA (FLmedical, Torreglia, Italy). After centrifugation, blood plasma was drawn off and stored in 2 mL Eppendorf tubes at −80 °C for later analyses of glutathione peroxidase activities. Subsequently, the common voles in all four groups were dissected to remove the liver, kidney, lung, and spleen samples. All the organs mentioned above were washed with deionised water, weighed, freeze-dried, then stored in Petri dishes and kept at a freezer temperature of −20 °C until laboratory analyses. Total As content was determined in the livers, kidneys, lungs, and spleens, while As species, total CBZ, and its metabolites were determined only in the liver.
2.3. Analytical Procedures
2.3.1. Determination of Total As Content in Animal Tissues and Experimental Diets
A 200 mg aliquot of homogenised freeze-dried organ tissue samples were decomposed in a mixture of HNO3 (Analpure®, Analytika, Prague, Czech Republic) and H2O2 (Rotipuran®, Carl Roth, Karlsruhe, Germany) in a microwave oven (Discover SP-D, CEM Corp., Charlotte, NC, USA). The concentration of total As was measured by inductively coupled plasma mass spectrometry (ICP-MS; Agilent 7700x, Agilent Technologies Inc., Santa Clara, CA, USA) operated in He mode. For quality assurance of the analytical results, certified reference material, specifically bovine liver (BCR-185R), and procedural blanks were analysed in parallel. In the reference material containing 0.033 ± 0.003 mg/kg of As, the value 0.037 ± 0.006 mg/kg (n = 3) was determined.
2.3.2. Extraction of As Species and As Speciation Using High-Performance Liquid Chromatography in the Liver
Arsenic species were released into an aqueous solution by applying a slightly modified microwave-assisted extraction procedure developed by Lin et al. [53]. The individual extracts were centrifuged (2680× g) for 10 min, passed through a syringe filter (0.2 µm, NYLON), combined and homogenised. The extract was subjected to As speciation analysis by employing a high-performance liquid chromatography system (HPLC, Agilent 1260, Agilent Technologies Inc., USA) hyphenated to ICP-MS as an As detector. The HPLC system was equipped with an anion exchange column PRP-X100 (150 × 4.6 mm, 10 µm; Hamilton, Reno, NV, USA), which was eluted with a gradient with 4 mmol L−1 NH4NO3 (A) and 60 mmol L−1 NH4NO3 (B) at a flow rate of 0.95 mL min−1 at pH = 8.7, similar to Button et al. [54]. Standard solutions at 0.1, 0.5, 2.5, 10 and 50 µg L−1 of each As species to be analysed were prepared using dissolution and dilution of NaAsO2 (AsIII), Na2HAsO4 × 7H2O (AsV), and dimethylarsinic acid (DMA), all purchased from Fluka (Buchs, Switzerland), while monomethylarsonate (MMA) and arsenobetaine were synthesised in house. All individual samples were tested in duplicate.
2.3.3. CBZ and Its Metabolites Determination
The QuEChERS—Quick, Easy, Cheap, Effective, Rugged, Safe—method was used to analyse CBZ in the liver tissues. The extraction followed the procedure previously described by Zita et al. [47]. Chromatographic separation of CBZ was performed using an ultrahigh-performance liquid chromatography system (Agilent 1290 Infinity II, Agilent Technologies, Santa Clara, CA, USA) with 0.005% HCOOH and MeOH as mobile phases A and B, respectively. A triple quadrupole mass spectrometer (MS; 6495 B, Agilent Technologies, Santa Clara, CA, USA) equipped with electrospray ionisation was used and operated in dMRM mode. Matrix-matched calibration prepared from control tissues was used for quantification.
2.3.4. Glutathione Peroxidase (GPx) Activity in Blood Plasma
The procedure employed to determine glutathione peroxidase activity was based on Lawrence and Burk [55], Lawrence and Burk [56], and Sigma–Aldrich [57]. The blood plasma was diluted appropriately with a potassium phosphate buffer with EDTA and sodium azide according to standard operating procedures before the analyses. For the determination of the GPx activity, a spectrophotometer (UV-1800, Shimadzu (Kyoto, Japan) with electronic stirrer model 300 (Rank Brothers Limited) and a CPS100-6 cell thermoelectrically temperature-controlled cell positioner (Shimadzu) was used, and the absorbance was recorded at 340 nm. Samples were measured in triplicate.
2.4. Calculation of Inorganic As Methylation Capacities of the Experimental Animal Groups
To assess the differential inorganic As methylation capacities of the experimental animal groups, the following formulae were applied:
where total As is the sum of As(III), As(V), MMA and DMA [58,59].
2.5. Data Processing and Statistical Analyses
Data processing and plotting of graphs were conducted using Microsoft Excel 365. The graphs represent the median values with median absolute deviation as the error bars. Statistica 12.0 software (DataBon s.r.o., Prague, Czech Republic) was utilised for all statistical analyses. The Shapiro–Wilk test was used to check the normality of all of the studied parameters. The individual datasets deviated from normal distribution parameters. Therefore, the non-parametric Kruskal–Wallis test, followed by a multiple comparison test, was employed to evaluate significant differences between the measured parameters. A value of p < 0.05 was deemed to be statistically significant. The raw data as well as the detailed results of the statistical analyses are summarized in the Supplementary Materials.
3. Results
3.1. Body Weights of the Experimental Animal Groups
No significant differences (p > 0.05) were found between the final median body weights of the control group and those of all the other experimental groups. This suggests that the As and CBZ exposure levels used in this study had negligible harmful effects on the overall body weight conditions of the animals (Figure 1). However, the final median body weight of the As group was significantly greater than that of the As + CBZ group. This can be explained by the notably greater initial median body weight of the As group than the As +CBZ group before the beginning of the experiment.
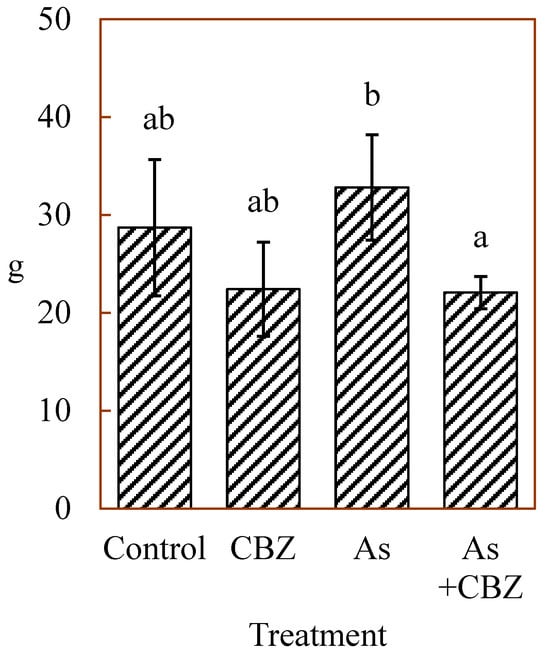
Figure 1.
Body weights of experimental animals (medians ± median absolute deviations) in grams (g); the columns marked by the same letter did not significantly differ at p < 0.05 within individual groups.
3.2. Total As Concentrations in the Various Organs of the Experimental Animals
Figure 2 represents the median total As concentrations expressed as mg/kg DW in livers (Figure 2a), kidneys (Figure 2b), spleens (Figure 2c), and lungs (Figure 2d) of the studied animals. The groups As and As + CBZ had significantly higher tissue total As concentrations compared to the control and the CBZ groups in all tissues analysed. Despite higher numerical total As concentrations in the As + CBZ group, no significant difference from the As group was detected. Likewise, the total As concentration in the control and CBZ were similar in all tissues analysed. Regarding the median total As concentrations in the liver (Figure 2a), statistically significant differences (p < 0.05) were determined between the very high As treatment groups (the As group and the As + CBZ group) and the very low As treatment groups (the control group and the CBZ group). In the kidney (Figure 2b), the control and CBZ groups appeared to show comparable and low total As concentrations. In contrast, the groups exposed to very high As (the As group and the As + CBZ group) showed increased total As concentrations (p < 0.05) in comparison to both the control and CBZ groups. Also, from the data, the As + CBZ group tended to accumulate the greatest amount of total As, but with no statistical difference (p > 0.05) when compared to the As group.
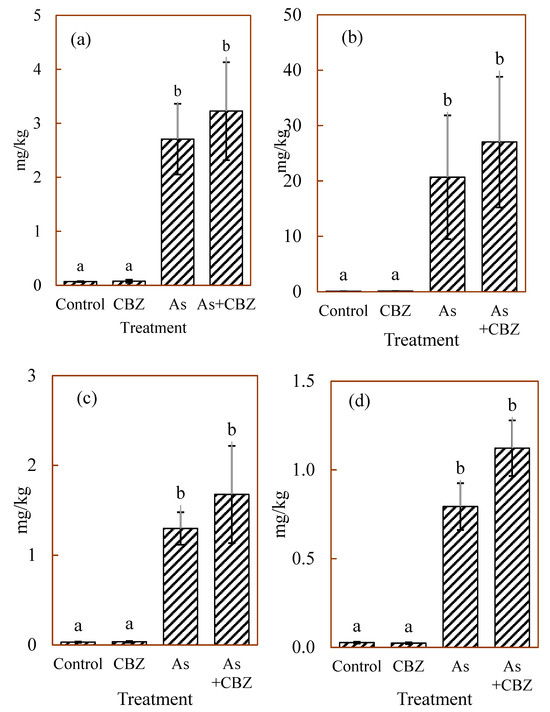
Figure 2.
The median arsenic concentrations in mg/kg DW of (a) liver, (b) kidney, (c) spleen, (d) lungs of experimental animal groups; the columns marked by the same letter did not significantly differ at p < 0.05 within individual groups.
The total As concentrations in the spleens of the studied animals are shown in Figure 2c. The control and CBZ groups appeared to demonstrate comparable and low levels of total As concentrations. On the other hand, the As and As + CBZ groups had an enhanced total As amount (p < 0.05) than the control and CBZ groups. Furthermore, the As + CBZ group appeared to have accumulated the highest quantity of total As than the As group, but the difference was not statistically significant (p > 0.05). Figure 2d shows the total As concentrations in the lungs among the treatment animal groups after the termination of the experiment. The data show that the control and CBZ groups had low variations and similar and low concentrations of total As. However, the As and As + CBZ groups showed high variations and accumulated more (p < 0.05) total As than the control and the CBZ groups. Even though the As + CBZ group seemed to have accumulated the most total As in the lungs, it was not statistically significant (p > 0.05) compared to the As group.
In summary, the sequence of total As concentrations across all studied organs was as follows: As + CBZ group > As group >> CBZ group ≡ control group. Furthermore, when comparing the studied organs, kidney > liver > spleen > lungs was the order of total As concentrations across the individual treatment animal groups.
3.3. Individual As Species Concentrations and Methylation Capacities in the Liver of Experimental Animals
Figure 3 shows inorganic As(III) (Figure 3a), inorganic As(V) (Figure 3b), dimethylarsinic acid (DMA; Figure 3c), and monomethylarsonic acid (MMA; Figure 3d) concentrations in the dried liver of the experimental animal groups, expressed in mg/kg. The As(III) concentrations in the control and CBZ groups tended to show low variations with similarly low concentrations, while the As and As + CBZ groups accumulated higher levels (p < 0.05) and were characterised by wider variations than the control and the CBZ groups.
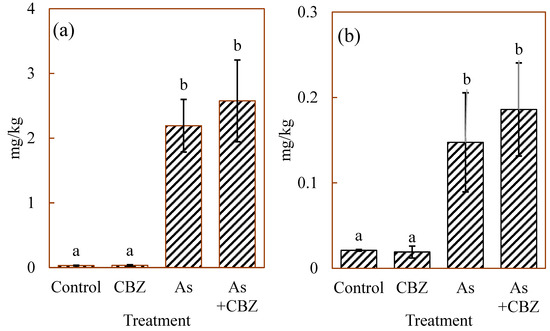
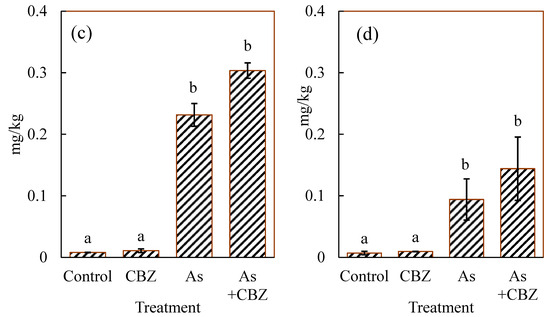
Figure 3.
(a) As (III), (b) As (V), (c) DMA, and (d) MMA median concentrations in the dried liver of the experimental animal groups; the columns marked by the same letter did not significantly differ at p < 0.05 within individual groups.
From the data, the As + CBZ group seems to have accumulated the highest level of As(III) species. However, there were no statistically significance differences between the As + CBZ group and the As group. Similar patterns were observed for all the remaining As species as shown in Figure 3. The As methylation capacities expressed in percentages of inorganic As (% iAs), % MMA, and % DMA are shown in Figure 4. From the findings, by comparing the % iAs from the rest of the experimental animal groups with that of the control group, the CBZ group documented markedly low percentages, and the As and As + CBZ groups recorded high percentages, where the As + CBZ group showed inconsiderably low % iAs compared to the As group.
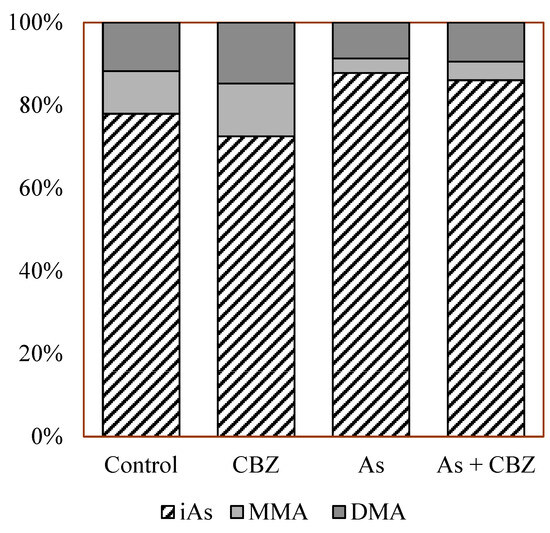
Figure 4.
The percentage of inorganic arsenic (iAs), DMA, and MMA in the liver of the experimental animal groups.
The sequence of % iAs among the groups was as follows: As group~As + CBZ group > Co group > CBZ group. Regarding % MMA, the CBZ group had the highest, whereas the As and As + CBZ groups had the lowest percentages compared to the control, with the As + CBZ group having inconsiderably more % MMA than the As group. The order of % MMA among the experimental animal groups was CBZ group > Co group > As + CBZ group ~ As group. For % DMA, a pattern similar to the %MMA was observed, that is, CBZ group > Co group > As + CBZ group ~ As group.
3.4. CBZ and Its Metabolites in the Liver of the Experimental Animals
Figure 5 shows the median concentrations of CBZ in the liver of the experimental animal groups, measured in picogram (pg) of CBZ per 1 g of dried liver. From the data, the control and the As groups showed CBZ levels below the limit of detection (10 pg/g). However, the CBZ group and As +CBZ group documented CBZ levels above the limit of detection with high variations among individual members of these groups, particularly within the As + CBZ group. The As + CBZ group seemed to show a lower CBZ level compared to the CBZ group. Nevertheless, no statistically significant difference (p > 0.05) was recorded between the CBZ and As + CBZ groups. Among the CBZ metabolites determined in the liver of the various animal groups, only minor proportions of CBZ 10, 11-epoxide were detected in the CBZ and As + CBZ groups. However, since the CBZ 10, 11-epoxide levels were approximately equal to the detection limits, these values were not considered.
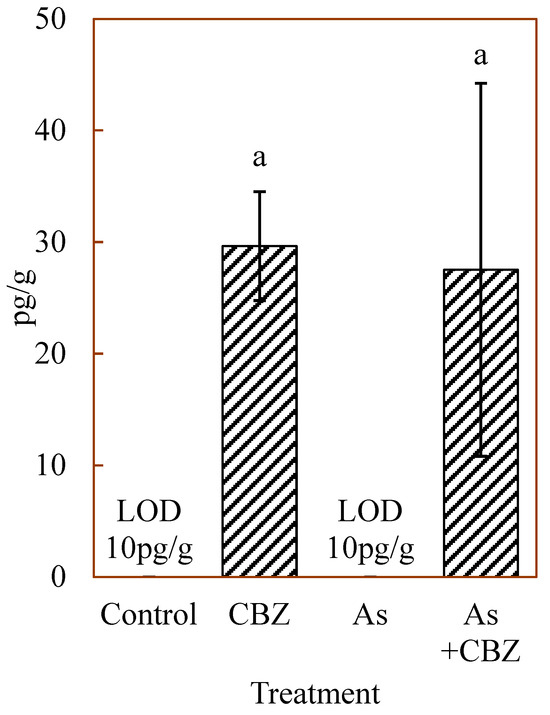
Figure 5.
Median concentrations of carbamazepine in the dried liver of the experimental animal groups; the columns marked by the same letter did not significantly differ at p < 0.05 within individual groups.
3.5. Glutathione Peroxidase (GPx) Activity in the Blood Plasma of the Experimental Animals
Figure 6 demonstrates glutathione peroxidase activity in the blood plasma of the experimental animals. All the treatment animal groups showed high variations, especially the As + CBZ group. The GPx activities in the blood plasma of the control and the CBZ groups appeared comparable. The As group showed insignificantly lower activity of this enzyme compared to the control and the CBZ groups. The highest GPx activity was recorded in the As + CBZ group with a significant difference (p < 0.05) compared to the As group.
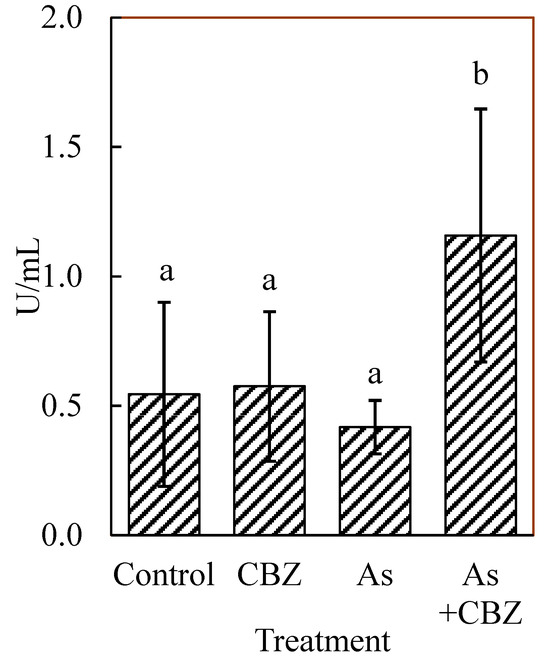
Figure 6.
Glutathione peroxidase (GPx) activity in the blood plasma of the experimental animal groups; the columns marked by the same letter did not significantly differ at p < 0.05 within individual groups.
4. Discussion
This in vivo study has some identified limitations that may have adversely impacted the statistical significance of the parameters determined among the investigated animal groups. These limitations include the short duration of the experiment and high variability within the groups. The high variability of the obtained data in this study is most likely caused by using wild specimens instead of the more commonly used laboratory inbred strains of rodents. The laboratory inbred strains are genetically virtually identical and possess characteristics that promote better stability and reproducibility of results [60]. However, since the purpose of this study was to mimic natural environmental conditions as closely as possible, we considered the use of the wild specimens (common voles) to be appropriate, justified, and suitable for this purpose. Moreover, the mutual interactions of the two studied environmental pollutants presents a pioneering study, where the interpretation of the data is hampered by a lack of comparable experiments. The statistical significance is relevant for evaluating the effects of pollutant exposure on health risks in both animal and human studies. However, biologically relevant adverse effects that emerge from the data, with the potential to cause significant consequences for the health of both animals and humans, are of greater importance. Therefore, apparent biological patterns should be considered of primary importance even when pair-wise comparison analyses fail to show statistical significance [61,62]. Hence, despite these limitations and the lack of statistical significance in the determined parameters among the animal groups in some cases, the current study’s findings revealed noticeable biological trends that merit attention.
4.1. Total As Concentrations in Different Organs and As Species Concentrations in the Liver of the Experimental Animals
The findings of total As concentrations in the liver, the kidney, the spleen, and the lungs showed a similar pattern, though with varying concentrations in each organ. These patterns indicate that the control and the CBZ groups appeared to have a similarly low intake of total As, leading to comparable low concentrations in all the studied organs. This implies that even though the CBZ group was fed a diet containing As because the As exposure dose in the diet was very low, the interaction between the two contaminants within the animals did not enhance As intake compared to the control group. That is why total As concentrations across all the studied organs in the control and CBZ groups were comparable.
According to Hughes et al. [63], Souza et al. [64], Li et al. [65], and Shao et al. [66], the amount of total As intake and its concentrations in tissues and organs of experimental mice increased in a dose-dependent manner and, at high As exposure dose, it was retained longer within the body. These observations of previous studies are consistent with the findings of this work. In this study, the As exposure dose was a significant factor that determined the intake and concentrations of total As in the studied organs across all the experimental animal groups. In this regard, the CBZ group was exposed to a very low dose of As in their diet, and even the presence of CBZ in their drinking water did not cause an enhanced intake of As, which manifested in low concentrations comparable to that of the control group in all the studied organs. However, the As and As + CBZ groups who were exposed to very high doses of As showed high intake and increased As concentrations in all the studied organs. Notably, the As + CBZ group had the highest total As concentrations, indicating this animal group accumulated the most total As across all the studied organs. This indicates that apart from the very high As exposure dose that facilitated total As intake in this group, CBZ-modulated effects at very high As doses are anticipated to have led to enhanced uptake of total As, leading to its greater accumulation than in the As group. These statements are speculative only, because the CBZ effect was not statistically proven, but it opens a space for further investigation to elucidate these potential interactions.
Previous studies on high As exposure dose effects have linked As accumulation to disease conditions such as metabolic disorders, nervous and cardiovascular system damage, and malignant transformation in the skin, lungs, liver, and bladder [67,68]. Shao et al. [66] observed through histopathological examinations that in the organs of mice exposed to a very high arsenic dose, the degree of damage correlated with the increase in As concentration in these organs. Therefore, in this research work, even though histopathological examinations were not conducted on the organs of mice exposed to very high As doses, we anticipate that any potential organ damage due to such exposure will be exacerbated in those of the As + CBZ group compared to the As group.
Arsenic, as a redox-sensitive element, is present primarily in soils as As(V) in aerobic conditions, while it is predominantly present as As(III) under anaerobic conditions. It is also known that the presence of As in organic forms is mainly DMA(V) and MMA(V) in the soils [69,70]. In the scientific literature, the cellular intake of different As species is known to be facilitated by phosphate transporters, aquaglyceroporins, facilitative glucose transporters, and organic anion-transporting polypeptides [71]. From the data of the current research, CBZ’s potential influence on enhanced intake of As only occurred at very high As doses. However, the mechanisms of this pharmaceutical on any of these cellular As transporters to enhance As intake were not investigated in this study and are subject to future research.
In the present study, in all the experimental animal groups, the order of total As concentrations in the organs was as follows: kidney > liver > spleen > lungs. Identifying specific As target organs is crucial for understanding the mode of action of As-influenced toxicity and carcinogenicity [63]. The findings of this study indicated that the kidney, the liver, and the spleen were the major target organs for As distribution and concentration after absorption, while minor distributions and concentrations were observed in the lungs. In that regard, Hughes et al. [63] and Kenyon et al. [72] observed the kidney as the predominant target organ after As absorption and distribution in mice. This is due to arsenate (which possesses similar physicochemical features as phosphate) reabsorption by phosphate transporters in the renal proximal tubules [63]. The liver is a target organ since it is the major site where the As methylation process occurs [73,74]. Cui and Okayasu [75] observed that the spleen was the organ that accumulated the most As in rats. According to Cui and Okayasu [75] and Soria et al. [76], the presence of ‘white lymphocyte-enriched pulp’ and ‘red erythrocyte-enriched pulp with macrophages’ in the spleen are potential As accumulation target sites. Contrary to the findings of this study, which documented minor concentrations of As in the lungs, previous research works have indicated the human lungs as a major target for As concentration [77,78]. According to Kenyon et al. [72], Khairul et al. [73], Li et al. [65], and Yi et al. [79], the pattern of As distribution and accumulation in organs in mammals may vary significantly due to exposure duration, the applied protocol of dose administration, species of the animal, and the presence of GSH and other thiol (-SH)-containing groups.
In this study, we assessed As species’ methylation (metabolism) and their concentrations in the experimental animal groups solely in the liver. The liver was chosen because it is known to be the major site where inorganic As methylation occurs when As is administered orally [59,74]. Four identifiable quantities of As species were measured in the liver of all the experimental animal groups, comprising As(III), As(V), DMA, and MMA. These findings suggested the As exposure dose, even in the control group, was able to trigger methylation reactions. From the results, the As species concentration pattern mirrored the trend observed in the total As concentrations in the liver and in the other investigated organs in this study. The control and the CBZ groups seemed to show low and comparable concentrations of all the studied As species, while the As and As + CBZ groups appeared to show elevated concentrations of all the As species, with the As + CBZ group appearing to have accumulated the highest amount of each As species.
These findings imply that the concentration of the As species in the liver was dependent on the dose of As exposure. This pattern mirrored the total As concentration in the liver and other organs in this research. This is consistent with the findings of Shao et al. [66], who also observed dose-dependent concentrations of As species in various investigated organs in mice exposed to As. These results also reinforce the already made point that CBZ may have enhanced the intake of As in the As + CBZ group. Even though we anticipated the predominant form of As exposure in this study was As(V), it is noteworthy that the concentration of As(III) was the highest when As species were compared within individual groups. Even the control group was not an exception. The As + CBZ group had the highest concentration based on intergroup comparisons of As species.
It has already been established that the pathway of inorganic As metabolism or biotransformation starts with the reduction of As(V) to As(III) in vivo where anaerobic conditions exist, after which As(III), because of its high affinity for thiol compounds, binds to GSH and other thiol compounds inside the cells [80,81]. The liver has high levels of GSH, especially in mice. Thus, this leads to high quantities of As(III)–(GS)3 complexes, which serve as the substrate for As3MT [80,82,83]. This probably explains the reason why the concentration of As(III) was the highest among the As species within the individual groups, even in the control group. The type of As species that accumulates in a target organ is presumed to be the dominant cause of its toxicity [79]. Therefore, in this current research, As(III) may be the As species predominantly associated with the highest As toxicity among the four As species determined in the liver of animals exposed to very high As doses (As and As + CBZ groups).
4.2. Inorganic As Methylation in the Liver of the Experimental Animals
The methylation of inorganic As species in mammals occurs through a specific enzymatic process. The pathway involves, first, the reduction of inorganic As(V) to inorganic As(III), which is then followed by oxidative methylation processes to produce monomethylarsonic acid—As MMA(V) and dimethylarsinic acid—As DMA(V) metabolites [80]. The methylation of inorganic As species is widely known as a detoxification process since the methylated As species MMA(V) and DMA(V) are less toxic than the inorganic forms [84,85]. However, this assertion has been questioned since the intermediate MMA(III) and DMA(III) metabolites are known to be more toxic As species [85,86]. In the literature, for easy understanding, the assessment of inorganic As methylation capacity in mammals has been categorised into primary methylation (first phase of methylation) and secondary methylation (second phase of methylation) [87]. The interpretation is that the primary methylation represents the proportion of MMA transformed from the total amount of As species present, and the proportion of DMA derived from the total As species indicates secondary methylation [88].
In this study, we calculated the % iAs (the sum of AsIII and AsV), the % MMA, and the % DMA to represent their relative proportions per the total sum of detectable As species (AsIII + AsV + MMA + DMA) in percentages. The % iAs represents the relative proportion of the inorganic As that is not methylated/metabolised in the liver. % MMA shows the efficiency of the first phase of the methylation process, and % DMA represents the efficiency of the second phase of the methylation process [59].
From the results, % iAs among the animal groups was in the order As group~As + CBZ group > CO group > CBZ group. For the % MMA and % DMA, the sequence was CBZ group > CO group > As + CBZ group~As group. The CBZ group had the lowest % iAs, suggesting that it has the least unmetabolised inorganic As species in the liver after the termination of the experiment. In other words, this means the CBZ group was the most efficient or the fastest in transforming the inorganic As species to MMA and DMA metabolites. This is corroborated by the highest % MMA and % DMA in the CBZ group compared to the other animal groups, indicating the highest primary and secondary methylation capacities. The As and As + CBZ groups had high and comparable % iAs, suggesting these groups had comparably high amounts of unmetabolised/unmethylated inorganic As. This also implied the inefficient capacities of these animal groups to metabolise or methylate inorganic As species to MMA and DMA. This is confirmed by the lowest % MMA and % DMA recorded for these groups.
Recent findings have shown that the predominant pathway for inorganic metabolism is the enzymatic process catalysed by arsenite methyltransferase enzyme (As3MT) leading to methylated As species of MMA(V) and DMA(V) [59,80,83]. Styblo et al. [59], observed that As3MT expression correlated negatively with % iAs and positively with % DMA in the liver. These previous findings suggest that an efficient As3MT catalysed methylation reaction leads to lower % iAs and higher % DMA. In other words, lower unmetabolised inorganic As species and higher secondary methylation capacity of inorganic As. According to Twaddle et al. [80] and Doerge et al. [83], the liver has high levels of GSH, especially in mice. Thus, this leads to high quantities of As(III)-(GS)3 complexes, which serve as the substrate for As3MT. However, at high % iAs, As3MT becomes saturated from the accumulated As(III) and this may cause the inhibition of the enzyme, leading to a decrease in the capacity to convert inorganic As to the methylated metabolites [59,89].
In this study, the As and As + CBZ groups recorded comparably enhanced % iAs and similarly low and comparable % MMA and %DMA, implying an accumulation of unmetabolised inorganic As species, especially As(III), and exhibited low primary and secondary methylation capacities, which we speculate could be partly due to the saturation of As3MT and inhibition by the high concentration of As(III). However, the CBZ group exhibited less % iAs and higher % MMA and % DMA than even the control group. The modulating role of CBZ on As3MT activity cannot be ascertained since it was not part of the investigation in this study.
4.3. Concentrations of CBZ and Its Metabolites in the Liver of Experimental Animals
This study assessed the potential concentrations of CBZ and its metabolites in the liver of experimental rodents (common voles) exposed to CBZ concentrations detected in surface water. The exposure was conducted through the CBZ spiked drinking water of the CBZ and the As + CBZ groups. Furthermore, we sought to establish if the concentration level of this drug in the liver could initiate metabolic processes. These evaluations have become imperative because, at the concentrations quantified in surface waters, data regarding potential CBZ concentrations and metabolic pathways in non-target terrestrial mammals are scarce in the literature. However, such investigations have been extensively conducted on aquatic organisms [90,91,92]. Based on the findings from this study, the control and the As groups showed CBZ levels below the limit of detection (10 pg/g). These results were expected considering that these two experimental animal groups were not administered drinking water containing CBZ.
On the other hand, significantly, the CBZ and As + CBZ groups documented CBZ concentrations above the detection limit. This indicates that CBZ exposure at environmentally relevant concentrations has the potential to accumulate within the liver of the model mammals, even though the observed concentration levels were very low. From the literature, CBZ accumulation in the liver induces the expression of CYP3A4 enzymes, with the predominant metabolic pathway being the formation of CBZ-10-11-epoxide as the primary metabolite, which is then followed by hydroxylation to CBZ-10,11-trans-dihydroxy [91,93]. There were minor quantities of the primary metabolite, specifically CBZ 10, 11-epoxide detected, but very close to the limit of detection; thus, they were not considered in subsequent evaluations. Since the quantities of the primary metabolite of CBZ were close to the detection limit, we speculate that the concentration levels may not be enough to have triggered the increased expression of CYP3A4 enzymes. Therefore, the metabolisation of CBZ in this study cannot be ascertained. However, long-term exposure beyond that of this study may be conducted in the future to test if the bioavailable levels can induce the enzymatic process leading to CBZ metabolisation. These results also indicate that the very high exposure dose of As in the As + CBZ group did not interact with CBZ to enhance the concentrations of the pharmaceutical.
4.4. The Activity of the Antioxidant Enzyme Glutathione Activity (GPx) in the Blood Plasma of the Experimental Animal Groups
Glutathione peroxidase (GPx) activities in the experimental animal groups were measured in enzyme unit (U) per millilitre (ml) of blood plasma. In mammals, GPx, together with catalase (CAT) and superoxide dismutase (SOD), constitute an enzymatic antioxidant defence system that curtails or offsets the toxic effects posed by ROS. Glutathione peroxidase is an important selenoenzyme responsible for catalysing the reduction of H2O2 and organic hydroperoxides to water or alcohol, respectively, as a way of lessening their toxicity [94]. Oxidative stress occurs when there is an excessive generation of ROS compared to the antioxidant defence system of the organism. This results in the development of aging and several diseases or pathological conditions [64,95].
From the results, the GPx activities in the blood plasma of the control and the CBZ groups appeared to show proximate figures, suggesting the GPx responses of the control and the CBZ were similar. The results also appeared to show that the As group had the lowest activity, while the As + CBZ group appeared to have the highest activity. In this study, time-dependent GPx defence responses/activities were not conducted, but their measurement was conducted at the end of the experiment. The lowest GPx activity of the As group, we speculate, could be due to a faster GPx response to counteract the potential released of hydroperoxides and hydrogen peroxides following exposure to the very high dose of As, leading to its depletion at the time of terminating the experiment. Conversely, in the As + CBZ group with the highest GPx activity, we anticipate showing a slower GPx response due to the potential overwhelmingly released H2O2 and hydroperoxides. This is substantiated by the observation that the bioavailability of As in the organs of the As + CBZ group was higher than those of the As group, suggesting a potentially higher release of H2O2 and hydroperoxides in the As + CBZ group compared to that of the As group. According to Souza et al. [64], a higher concentration (bioavailability) of As correlates with an increased H2O2 concentration. A high release of ROS elicits an intrinsic antioxidant response, which will then be suppressed with time [64,96]. Based on this observation, we anticipate a reduction in GPx activity in the As + CBZ group over time.
In assessing time-dependent activities of catalase (CAT) and GPx in the liver of mice exposed to high As doses for 3, 6, 9 and 12 months, Das and Santra [97] observed a dramatic increase in CAT and GPx activities at 3 months. However, at 9 and 12 months, the activities of these antioxidant enzymes were significantly reduced. Furthermore, Santra et al. [98] observed a similar trend in GPx activity with time following exposure to high doses of As. These observations corroborated our speculation that the GPx activities trend observed in the As and As + CBZ groups were time-dependent.
A reduction in GPx activity, as observed in the As group, could be caused by rapid adaptation leading to a reduction in the concentration of the enzyme substrate (reduced glutathione). Under such circumstances, there are two probable situations occurring: (a) an increase in the level of peroxides may ensue and cause oxidative stress to the animals in this group, or (b) the decrease in the enzyme substrate can lead to a corresponding reduction in H2O2 levels to curtail or offset oxidative stress conditions [99,100]. These findings remain questionable and need to be elucidated in future research. Regarding the As + CBZ group, which exhibited the highest GPx activity at the termination of the experiment, we anticipate that this may be due to ongoing adaptation to curtail or offset oxidative stress-induced damage, suggesting that the animals in this group were experiencing oxidative stress at the time the experiment ended.
5. Conclusions
This study indicated that there is a potential CBZ modulating effect, which led to an enhanced intake of As, where the total As and As species accumulation in the organs of the As + CBZ exposure group tended to increase compared to the As exposure group. However, the capacity to methylate the more toxic inorganic As species to its less toxic methylated forms was comparable in the As and As +CBZ groups, suggesting As toxicity could potentially be more pronounced in the As + CBZ group than the As group. This study also found that high As exposure interacted with CBZ in the As + CBZ group but did not cause an enhanced uptake of CBZ in the liver compared to the CBZ alone exposure group. It was observed that exposure to CBZ at concentrations detected normally in surface water caused its accumulation in the liver of the CBZ exposed groups (CBZ and As + CBZ), raising concerns about potential toxic effects in mammals from long-term exposure. However, the concentration levels detected were too low to assess the metabolic pathway of the drug at subtherapeutic exposure concentrations in the investigated mammalian organisms. Glutathione peroxidase (GPx) activity, used to assess the antioxidant enzyme defence responses against single and co-exposure of the two pollutants, showed the CBZ group exhibited comparable activity to that of the control group, while the As group showed down-regulation and the As + CBZ group showed up-regulation. These results suggest that the CBZ group experienced minimal oxidative stress conditions similar to the control group. The As group showed a rapid adaptation/response to curtail or offset potential oxidative stress tissue damage conditions compared to the slow adaptation/response in the As + CBZ group. These aspects are novel and deserve further elucidation. Moreover, the animals were exposed to a whole cocktail of pollutants, and their potential impact should be investigated as a whole in further research. Therefore, the potential mutual effects of emerging pollutants and risk elements should be targeted in future research because the co-exposure of wildlife to both groups of environmental pollutants cannot be avoided.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app15031662/s1. Figure S1: The results of statistical evaluation of the data; Table S1: The total arsenic contents in the individual organs (mg/kg) and initial and final body weights of the animals (g); Table S2: Total As and As species contents (mg/kg) in the liver samples; Table S3: GPx activity in blood plasma of the animals (U/mL).
Author Contributions
Conceptualization, J.S. and Z.Č.; methodology and analyses, F.B., J.Š., C.S., M.G., L.P. and F.M.; data processing, F.B. and J.Š.; writing—original draft preparation, F.B.; writing—review and editing, J.S., Z.Č. and P.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the grant project of GAČR 23-07811S.
Institutional Review Board Statement
The experimental procedure was approved by a committee from the Ministry of Education, Youth and Sports, Czech Republic; with approval code MSMT-1082/2017-2. The provision of animal care was supervised and authorized by Zuzana Čadková, (Ph.D.), holder of Certificate No. CZ 02201 issued by Central Commission for Animal Welfare of the Czech Republic.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Geissen, V.; Mol, H.; Klumpp, E.; Umlauf, G.; Nadal, M.; van der Ploeg, M.; van de Zee, S.E.; Ritsema, C.J. Emerging pollutants in the environment: A challenge for water resource management. Int. Soil. Water Conserv. Res. 2015, 3, 57–65. [Google Scholar] [CrossRef]
- Naidu, R.; Arias Espana, V.A.; Liu, Y.; Jit, J. Emerging contaminants in the environment: Risk-based analysis for better management. Chemosphere 2016, 154, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Gu, X.; Zeng, Q.; Mao, Z.; Liang, X.; Martyniuk, C.J. Carbamazepine disrupts molting hormone signaling and inhibits molting and growth of Eriocheir sinensis at environmentally relevant concentrations. Aquat. Toxicol. 2019, 208, 138–145. [Google Scholar] [CrossRef]
- Wilkinson, J.L.; Boxall, A.B.A.; Kolpin, D.W.; Leung, K.M.Y.; Lai, R.W.S.; Wong, D.; Ntchantcho, R.; Pizarro, J.; Mart, J.; Echeverr, S.; et al. Pharmaceutical pollution of the world’s rivers. Proc. Natl. Acad. Sci. USA 2022, 119, e2113947119. [Google Scholar] [CrossRef]
- Ambrosio, A.F.; Soares-da-Silva, P.; Carvalho, C.M.; Carvalho, A.P. Mechanisms of action of carbamazepine and its derivatives, oxcarbazepine, BIA 2-093, and BIA 2-024. Neurochem. Res. 2002, 27, 121–130. [Google Scholar] [CrossRef]
- Deng, Y.; Ok, Y.S.; Mohan, D.; Pittman, C.U.; Dou, X. Carbamazepine removal from water by carbon dot-modified magnetic carbon nanotubes. Environ. Res. 2019, 169, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Frickle-Galindo, I.; LLerena, A.; Jung-Cook, H.; Lopez-Lopez, M. Carbamazepine adverse drug reactions. Expert. Rev. Clin. Pharmacol. 2018, 11, 705–718. [Google Scholar] [CrossRef]
- Zhang, Y.; Geißen, S.U.; Gal, C. Carbamazepine and diclofenac: Removal in wastewater treatment plants and occurrence in water bodies. Chemosphere 2008, 73, 1151–1161. [Google Scholar] [CrossRef]
- Brezina, E.; Prasse, C.; Meyer, J.; Mückter, H.; Ternes, T.A. Investigation and risk evaluation of the occurrence of carbamazepine, oxcarbazepine, their human metabolites and transformation products in the urban water cycle. Environ. Pollut. 2017, 225, 261–269. [Google Scholar] [CrossRef]
- Ebrahimzadeh, S.; Castiglioni, S.; Riva, F.; Zuccato, E.; Azzellino, A. Carbamazepine levels related to the demographic indicators in groundwater of densely populated area. Water 2021, 13, 2539. [Google Scholar] [CrossRef]
- Mezzelani, M.; Gorbi, S.; Regoli, F. Pharmaceuticals in the aquatic environments: Evidence of emerged threat and future challenges for marine organisms. Mar. Envl Res. 2018, 140, 41–60. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Xiang, J.; Que, C.; Chen, F.; Xu, G. Occurrence and fate of psychiatric pharmaceuticals in the urban water system of Shanghai, China. Chemosphere 2015, 138, 486–493. [Google Scholar] [CrossRef]
- Lindim, C.; de Zwart, D.; Cousins, I.T.; Kutsarova, S.; Kühne, R.; Schüürmann, G. Exposure and ecotoxicological risk assessment of mixtures of top prescribed pharmaceuticals in Swedish freshwaters. Chemosphere 2019, 220, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Meyer, W.; Reich, M.; Beier, S.; Behrendt, J.; Gulyas, H.; Otterpohl, R. Measured and predicted environmental concentrations of carbamazepine, diclofenac, and metoprolol in small and medium rivers in northern Germany. Environ. Monit. Assess. 2016, 188, 487. [Google Scholar] [CrossRef] [PubMed]
- Almeida, Â.; Soares, A.M.V.M.; Esteves, V.I.; Freitas, R. Occurrence of the antiepileptic carbamazepine in water and bivalves from marine environments: A review. Environ. Toxicol. Pharmacol. 2021, 86, 103661. [Google Scholar] [CrossRef]
- Musolff, A.; Leschik, S.; Möder, M.; Strauch, G.; Reinstorf, F.; Schirmer, M. Temporal and spatial patterns of micropollutants in urban receiving waters. Environ. Pollut. 2009, 157, 3069–3077. [Google Scholar] [CrossRef]
- Osenbrück, K.; Gläser, H.R.; Knöller, K.; Weise, S.M.; Möder, M.; Wennrich, R.; Schirmer, M.; Reinstorf, F.; Busch, W.; Strauch, G. Sources and transport of selected organic micropollutants in urban groundwater underlying the city of Halle (Saale), Germany. Water Res. 2007, 41, 3259–3270. [Google Scholar] [CrossRef] [PubMed]
- Vulliet, E.; Cren-Olivé, C. Screening of pharmaceuticals and hormones at the regional scale, in surface and groundwaters intended to human consumption. Environ. Pollut. 2011, 159, 2929–2934. [Google Scholar] [CrossRef]
- Riva, F.; Castiglioni, S.; Fattore, E.; Manenti, A.; Davoli, E.; Zuccato, E. Monitoring emerging contaminants in the drinking water of Milan and assessment of the human risk. Int. J. Hyg. Environ. Health 2018, 221, 451–457. [Google Scholar] [CrossRef]
- Simazaki, D.; Kubota, R.; Suzuki, T.; Akiba, M.; Nishimura, T.; Kunikane, S. Occurrence of selected pharmaceuticals at drinking water purification plants in Japan and implications for human health. Water Res. 2015, 76, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ye, D.; Li, X.; Jia, Y.; Zhao, L.; Liu, S.; Xu, J.; Du, J.; Tian, L.; Li, J.; et al. Occurrence of pharmaceuticals and personal care products in bottled water and assessment of the associated risks. Environ. Int. 2021, 155, 106651. [Google Scholar] [CrossRef] [PubMed]
- Datel, J.V.; Hrabankova, A. Pharmaceuticals load in the Svihov water reservoir (Czech Republic) and impacts on quality of treated drinking water. Water 2020, 12, 1387. [Google Scholar] [CrossRef]
- Kozisek, F.; Pomykacova, I.; Jeligova, H.; Cadek, V.; Svobodova, V. Survey of human pharmaceuticals in drinking water in the Czech Republic. J. Water Health 2013, 11, 84–97. [Google Scholar] [CrossRef]
- Kaushik, G.; Huber, D.P.; Aho, K.; Finney, B.; Bearden, S.; Zarbalis, K.S.; Thomas, M.A. Maternal exposure to carbamazepine at environmental concentrations can cross intestinal and placental barriers. Biochem. Biophysl Res. Commun. 2016, 474, 291–295. [Google Scholar] [CrossRef]
- Kohl, A.; Golan, N.; Cinnamon, Y.; Genin, O.; Chefetz, B.; Sela-Donenfeld, D. A proof of concept study demonstrating that environmental levels of carbamazepine impair early stages of chick embryonic development. Environ. Int. 2019, 129, 583–594. [Google Scholar] [CrossRef]
- Santariová, M.; Zadinová, K.; Vostrá-Vydrová, H.; Kolářová, M.F.; Kurhan, S.; Chaloupková, H. Effect of environmental concentration of carbamazepine on the behaviour and gene expression of laboratory rats. Animals 2023, 13, 2097. [Google Scholar] [CrossRef]
- Dean, J.R.; Ma, R. Approaches to assess the oral bioaccessibility of persistent organic pollutants: A critical review. Chemosphere 2007, 68, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Petruzzelli, G.; Pedron, F.; Rosellini, I. Bioavailability and bioaccessibility in soil: A short review and a case study. AIMS Environ. Sci. 2020, 7, 208–224. [Google Scholar] [CrossRef]
- Tang, X.; Tang, L.; Zhu, Y.; Xing, B.; Duan, J.; Zheng, M. Assessment of the bioaccessibility of polycyclic aromatic hydrocarbons in soils from Beijing using an intro test. Environ. Pollut. 2006, 140, 279–285. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Gu, C.; Bian, Y.; Liu, Z.; Jia, M.; Wang, F.; Wang, D.; Jiang, X. Prediction of polycyclic aromatic hydrocarbon bioaccessibility to earthworms in spiked soils by composite extraction with hydroxypropyl-ß-cyclodextrin and organic acids. Pedosphere 2017, 27, 502–510. [Google Scholar] [CrossRef]
- Chen, F.; Muhammad, F.G.; Khan, Z.I.; Ahmad, K.; Malik, I.S.; Ashfaq, A.; Naeem, M.; Nadeem, M.; Ma, J.; Awan, M.U.M.; et al. Bioaccumulation and transfer of Zinc in soil plant and animal system: A health risk assessment for grazing animals. Environ. Sci. Pollut. Res. 2022, 29, 2718–2727. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.L.; Lambert, G.H.; Hsu, C.C.; Hsu, M.M.L. Yucheng: Health effects of prenatal exposure to polychlorinated biphenyls and dibenzofurans. Int. Arch. Occup. Environ. Health 2004, 77, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Misaki, K.; Takamura–Enya, T.; Ogawa, H.; Takamori, K.; Yanagida, M. Tumour-promoting activity of polycyclic aromatic hydrcarbons and their oxygenated or nitrated derivative. Mutagenesis 2016, 31, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Alka, S.; Shahir, S.; Ibrahim, N.; Ndejiko, M.J.; Vo, D.V.N.; Manan, F.A. Arsenic removal technologies and future trends: A mini review. J. Clean. Prod. 2021, 278, 123805. [Google Scholar] [CrossRef]
- Podgorski, J.; Berg, M. Global threat of arsenic in groundwater. Science 2020, 368, 845–850. [Google Scholar] [CrossRef]
- Tseng, C.H.; Huang, Y.K.; Huang, Y.L.; Chung, C.J.; Yang, M.H.; Chen, C.J.; Hsueh, Y.M. Arsenic exposure, urinary arsenic speciation, and peripheral vascular disease in blackfoot disease-hyperendemic villages in Taiwan. Toxicol. Appl. Pharmacol. 2005, 206, 299–308. [Google Scholar] [CrossRef]
- Xu, M.; Rui, D.; Yan, Y.; Xu, S.; Niu, Q.; Feng, G.; Wang, Y.; Li, S.; Jing, M. Oxidative Damage Induced by Arsenic in Mice or Rats: A Systematic Review and Meta-Analysis. Biol. Trace Elem. Res. 2017, 176, 154–175. [Google Scholar] [CrossRef]
- Gasca-Pérez, E.; Galar-Martínez, M.; García-Medina, S.; Pérez-Coyotl, I.A.; Ruiz-Lara, K.; Cano-Viveros, S.; Pérez-Pastén, B.R.; Gómez-Oliván, L.M. Short-term exposure to carbamazepine causes oxidative stress on common carp (Cyprinus carpio). Environ. Toxicol. Pharmacol. 2019, 66, 96–103. [Google Scholar] [CrossRef]
- Papaioannou, D.; Koukoulakis, P.H.; Lambropoulou, D.; Papageorgiou, M.; Kalavrouziotis, I.K. The dynamics of the pharmaceutical and personal care product interactive capacity under the effect of artificial enrichment of soil with heavy metals and of wastewater reuse. Sci. Total Environ. 2019, 662, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Gall, J.E.; Boyd, R.S.; Rajakaruna, N. Transfer of heavy metals through terrestrial food webs: A review. Environ. Monit. Assess. 2015, 187, 201. [Google Scholar] [CrossRef] [PubMed]
- Skácel, F.; Pekárek, J. Monitoring of lead, cadmium and mercury in environmental samples at the regional station of the integrated background monitoring network of GEMS in Czechoslovakia. Sci. Total Environ. 1992, 115, 261–276. [Google Scholar] [CrossRef]
- Hamers, T.; van den Berg, J.H.J.; van Gestel, C.A.M.; van Schooten, F.J.; Murk, A.J. Risk assessment of metals and organic pollutants for herbivorous and carnivorous small mammal food chains in a polluted floodplain (Biesbosch, The Netherlands). Environ. Pollut. 2006, 144, 581–595. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Estival, J.; Smits, J.E.G. Small mammals as sentinels of oil sands related contaminants and health effects in northeastern Alberta, Canada. Ecotoxicol. Environ. Safe 2016, 124, 285–295. [Google Scholar] [CrossRef]
- Yudovich, E.Y.; Ketris, M.P. Arsenic in coal: A review. Int. J. Coal Geol. 2005, 61, 141–196. [Google Scholar] [CrossRef]
- Skála, J.; Boahen, F.; Száková, J.; Vácha, R.; Tlustoš, P. Arsenic and lead in soil: Impacts on element mobility and bioaccessibility. Environ. Geochem. Health 2022, 44, 943–959. [Google Scholar] [CrossRef] [PubMed]
- Zadrapova, D.; Titera, A.; Szakova, J.; Cadkova, Z.; Cudlin, O.; Najmanova, J.; Tlustos, P. Mobility and bioaccessibility of risk elements in the area affected by the long-term opencast coal mining. J. Environ. Sci. Health A Tox Hazard. Subst. Environ. Eng. 2019, 54, 1159–1169. [Google Scholar] [CrossRef]
- Zita, L.; Kurhan, S.; Krunt, O.; Chmelíková, E.; Kraus, A.; Čítek, J.; Klouček, P.; Stupka, R. The effect of carbamazepine on performance, carcass value, hematological and biochemical blood parameters, and detection of carbamazepine and its metabolites in tissues, internal organs, and body fluids in growing rabbits. Animals 2023, 13, 2041. [Google Scholar] [CrossRef]
- European Commission. Joint Research Centre (JRC) (2019): EURL ECVAM Dataset on Alternative Methods to Animal Experimentation (DB-ALM). European Commission, Joint Research Centre (JRC) [Dataset] PID. Available online: http://data.europa.eu/89h/b7597ada-148d-4560-9079-ab0a5539cad3 (accessed on 8 January 2025).
- Mercl, F.; Košnář, Z.; Maršík, P.; Vojtíšek, M.; Dušek, J.; Száková, J.; Tlustoš, P. Pyrolysis of biosolids as an effective tool to reduce the uptake of pharmaceuticals by plants. J. Hazard. Mat. 2021, 405, 124278. [Google Scholar] [CrossRef]
- Public Notice No.153/2016 About the Conditions for the Protection of the Agricultural Soil Quality; Legal code of The Czech Republic: Brno, Czech Republic, 2016; pp. 2692–2699.
- Tremlová, J.; Száková, J.; Tlustoš, P. An assessment of possible effect of risk elements contained in soil on human organism. Chem. Listy 2010, 104, 349–352. (In Czech) [Google Scholar]
- Vlčková, V.; Malinová, M.; Koubková, B.; Száková, J.; Zídek, V.; Fučíková, A.; Zídková, J.; Kolihová, D.; Tlustoš, P. The long-term effect of diet amended by risk elements contaminated soils on risk element penetration and physiological parameters of rats. Czech J. Anim. Sci. 2014, 59, 416–427. [Google Scholar] [CrossRef]
- Lin, Y.; Sun, Y.; Wang, X.; Chen, S.; Wu, Y.; Fu, F. Universal method for the speciation analysis in various seafood based on microwave-assisted extraction and ion chromatography-inductively coupled plasma mass spectrometry. Microchem. J. 2020, 159, 105592. [Google Scholar] [CrossRef]
- Button, M.; Moriarty, M.M.; Watts, M.J.; Zhang, J.; Koch, I.; Reimer, K.J. Arsenic speciation in field-collected and laboratory-exposed earthworms lumbricus terrestris. Chemosphere 2011, 85, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R.A.; Burk, R.F. Glutathione peroxidase activity in selenium—Deficient rat liver. Biochem. Biophys. Res. Commun. 1974, 71, 952–958. [Google Scholar] [CrossRef]
- Lawrence, R.A.; Burk, R.F. Species, tissue and subcellular distribution of non-Se-dependent glutathione peroxidase activity. J. Nutr. 1978, 108, 211–215. [Google Scholar] [CrossRef] [PubMed]
- EC 1.11.19; Enzymatic Assay of Gluthathione Peroxidase. Sigma-Aldrich: Burlington, MA, USA, 2000.
- Hsieh, R.; Huang, Y.; Shiue, H.; Huang, S.; Lin, M.; Mu, S.; Chung, C.; Hsueh, Y. Arsenic methylation capacity and developmental delay in preschool children in Taiwan. Int. J. Hyg. Environ. Health 2014, 217, 678–686. [Google Scholar] [CrossRef]
- Stýblo, M.; Douillet, C.; Bangma, J.; Eaves, L.A.; de Villena, F.P.; Fry, R. Differential metabolism of inorganic arsenic in mice from genetically diverse Collaborative Cross strains. Arch. Toxicol. 2019, 93, 2811–2822. [Google Scholar] [CrossRef] [PubMed]
- Katoh, H. International Harmonization of Laboratory Animals. In Microbial Status and Genetic Evaluation of Mice and Rats: Proceedings of the 1999 US/Japan Conference; National Academies Press (US): Washington, DC, USA, 2000. [Google Scholar]
- EPA. Guidelines for Developmental Toxicity Risk Assessment. Fed. Regist. 1991, 56, 63798–63826. [Google Scholar]
- EFSA. Statistical Significance and Biological Relevance. EFSA J. 2011, 9, 2372. [Google Scholar]
- Hughes, M.F.; Kenyon, E.M.; Edwards, B.C.; Mitchell, C.T.; Razo, L.M.D.; Thomas, D.J. Accumulation and metabolism of arsenic in mice after repeated oral administration of arsenate. Toxicol. Appl. Pharmacol. 2003, 191, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.M.O.; Grotto, D.; Batista, B.L.; Júnior, F.B. Distribution of arsenic and oxidative stress in mice after rice ingestion. J. Trace Elem. Med. Biol. 2017, 44, 192–200. [Google Scholar] [CrossRef]
- Li, J.; Guo, Y.; Duan, X.; Li, B. Tissue- and region-specific accumulation of arsenic species, especially in the brain of mice, after long-term arsenite exposure in drinking water. Biol. Trace Elem. Res. 2020, 198, 168–176. [Google Scholar] [CrossRef]
- Shao, J.; Li, X.; Luo, Y.; Fang, H.; Lin, F.; Zheng, G.; Lu, F.; Guo, L.; Sun, Y. Distribution of arsenic species and pathological characteristics of tissues of the mice fed with arsenic–supplemented food simulating rice. J. Toxicol. Sci. 2021, 46, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Ratnaike, R.N. Acute and chronic arsenic toxicity. Postgrad. Med. J. 2003, 79, 391–396. [Google Scholar] [CrossRef]
- Zhou, Q.; Xi, S. A review on arsenic carcinogenesis: Epidemiology, metabolism, genotoxicity and epigenetic changes. Regul. Toxicol. Pharmacol. 2018, 99, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Jiménez, E.; Esteban, E.; Peñalosa, J.M. The fate of arsenic in soil-plant systems. Rev. Environ. Contam. Toxicol. 2012, 215, 1–37. [Google Scholar] [PubMed]
- Zhao, F.J.; McGrath, S.P.; Meharg, A.A. Arsenic as a food-chain contaminant: Mechanisms of plant uptake and metabolism and mitigation strategies. Ann. Rev. Plant Biol. 2010, 61, 535–559. [Google Scholar] [CrossRef]
- Maciaszczyk-Dziubinska, E.; Wawrzycka, D.; Wysocki, R. Arsenic and antimony transporters in eukaryotes. Int. J. Mol. Sci. 2012, 13, 3527–3548. [Google Scholar] [CrossRef]
- Kenyon, E.M.; Hughes, M.F.; Adair, B.M.; Highfill, J.H.; Crecelius, E.A.; Clewell, H.J.; Yager, J.W. Tissue distribution and urinary excretion of inorganic arsenic and its methylated metabolites in C57BL6 mice following subchronic exposure to arsenate in drinking water. Toxicol. Appl. Pharmacol. 2008, 232, 448–455. [Google Scholar] [CrossRef]
- Khairul, I.; Wang, Q.Q.; Jiang, Y.H.; Wang, C.; Naranmandura, H. Metabolism, toxicity and anticancer activities of arsenic compounds. Oncotarget 2017, 8, 23905–23926. [Google Scholar] [CrossRef]
- Thomas, D.J.; Li, J.; Waters, S.B.; Xing, W.; Adair, B.M.; Drobna, Z.; Devesa, V.; Styblo, M. Arsenic (+3 oxidation state) methyltransferase and the methylation of arsenicals. Exp. Biol. Med. 2007, 232, 3–13. [Google Scholar]
- Cui, X.; Okayasu, R. Arsenic accumulation, elimination, and interaction with copper, zinc and manganese in liver and kidney of rats. Food Chem. Toxicol. 2008, 46, 3646–3650. [Google Scholar] [CrossRef]
- Soria, E.A.; Pérez, R.D.; Queralt, I.; Pérezd, C.A.; Bongiovannie, G.A. Immunotoxicological effects of arsenic bioaccumulation on spatial metallomics and cellular enzyme response in the spleen of male Wistar rats after oral intake. Toxicol. Lett. 2017, 266, 65–73. [Google Scholar] [CrossRef]
- Putila, J.J.; Guo, N.L. Association of arsenic exposure with lung cancer incidence rates in the United States. PLoS ONE 2011, 6, e25886. [Google Scholar] [CrossRef]
- Ren, C.; Zhou, Y.; Liu, W.; Wang, Q. Paraxodical effects of arsenic in the lungs. Environ. Health Prevent Med. 2021, 26, 80. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Gao, S.; Xia, J.; Li, C.; Zhao, Y.; Zhang, Y.; Liang, A.; Ji, S. Study of the accumulation and distribution of arsenic species and association with arsenic toxicity in rats after 30 days of oral realgar administration. J. Ethnopharmacol. 2020, 247, 111576. [Google Scholar] [CrossRef] [PubMed]
- Twaddle, N.C.; Vanlandingham, M.; Churchwell, M.I.; Doerge, D.R. Metabolism and disposition of arsenic species from controlled oral dosing with sodium arsenite in adult female CD-1 mice. I. Pilot study to determine dosing, analytical measurements, and sampling strategies. Food Chem. Toxicol. 2018, 111, 482–493. [Google Scholar] [CrossRef]
- Watanabe, T.; Hirano, S. Metabolism of arsenic and its toxicological relevance. Arch. Toxicol. 2013, 87, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, G.M.; Sampayo-Reyest, A.; Aposhian, H.V. Arsenic binding protins of mammalian systems: I. Isolation of three arsnite-binding proteins of rabbit liver. Toxicology 1994, 93, 175–193. [Google Scholar] [CrossRef] [PubMed]
- Doerge, D.R.; Twaddle, N.C.; Churchwell, M.I.; Beland, F.A. Reduction by, ligand exchange among, and covalent binding to glutathione and cellular thiols link metabolism and disposition of dietary arsenic species with toxicity. Environ. Int. 2020, 144, 106086. [Google Scholar] [CrossRef]
- Drobna, Z.; Naranmandura, H.; Kubachka, K.M.; Edwards, B.C.; Herbin-Davis, K.; Styblo, M.; Le, X.C.; Creed, J.T.; Maeda, N.; Hughes, M.F.; et al. Disruption of the arsenic (+3 oxidation state) methyltransferase gene in the mouse alters the phenotype for methylation of arsenic and affects distribution and retention of orally administered arsenate. Chem. Res. Toxicol. 2009, 22, 1713–1720. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.J. Arsenic methylation—Lessons from three decades of research. Toxicology 2021, 457, 152800. [Google Scholar] [CrossRef]
- Styblo, M.; Del Razo, L.M.; Vega, L.; Germolec, D.R.; LeCluyse, E.L.; Hamilton, G.A.; Reed, W.; Wang, C.; Cullen, W.R.; Thomas, D.J. Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Arch. Toxicol. 2000, 74, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Xu, Y.; Li, X.; Jin, Y.; Li, B.; Sun, X. Urinary Arsenic Metabolites in Children and Adults Exposed to Arsenic in Drinking Water in Inner Mongolia, China. Environ. Health Perspect. 2007, 115, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Peña, L.C.S.; Hernández, A.B.; Razo, L.M.D. Decreased arsenic disposition and alteration of its metabolic profile in mice coexposed to fluoride. Biol. Trace Elem. Res. 2024, 202, 1594–1602. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, G.; Lin, Z.; Luo, Y.; Fang, H.; Yang, L.; Xie, J.; Guo, L. Determination of arsenicals in mouse tissues after simulated exposure to arsenic from rice for sixteen weeks and the effects on histopathological features. Ecotoxicol. Environ. Safe 2020, 200, 110742. [Google Scholar] [CrossRef]
- Chen, F.; Gong, Z.; Kelly, B.C. Rapid analysis of pharmaceuticals and personal care products in fish plasma micro-aliquots using liquid chromatography tandem mass spectrometry. J. Chromatogr. 2015, 1383, 104–111. [Google Scholar] [CrossRef]
- Daniele, G.; Fieu, M.; Joachim, S.; Bado-Nilles, A.; Beaudouin, R.; Baudoin, P.; James-Casas, A.; Andres, S.; Bonnard, M.; Bonnard, I.; et al. Determination of carbamazepine and 12 degradation products in various compartments of an outdoor aquatic mesocosm by reliable analytical methods based on liquid chromatography-tandem mass spectrometry. Environ. Sci. Pollut. Res. 2017, 24, 16893–16904. [Google Scholar] [CrossRef] [PubMed]
- Valdés, M.E.; Amé, M.V.; Bistoni, M.A.; Wunderlin, D.A. Occurrence and bioaccumulation of pharmaceutical in a fish species inhabiting the Suquía River basin (Córdoba, Argentina). Sci. Total Environ. 2014, 472, 389–396. [Google Scholar] [CrossRef]
- Higuchi, S.; Yano, A.; Takai, S.; Tsuneyama, K.; Fukami, T.; Nakajima, M.; Yokoi, T. Metabolic activation and inflammation reactions involved in carbamazepine-induced liver injury. Toxicol. Sci. 2012, 130, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Pan, X.; Wei, G.; Hua, Y. Research progress of glutathione peroxidase family (GPX) in redoxidation. Front. Pharmacol. 2023, 14, 1147414. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, Y.; Liu, Y.; Xing, Y.; Miao, C.; Zhao, Y.; Chang, X.; Zhang, Q. The role of oxidative stress and natural antioxidants in ovarian aging. Front. Pharmacol. 2021, 14, 617843. [Google Scholar] [CrossRef]
- Nandi, D.; Patra, R.; Swarup, D. Oxidative stress indices and plasma biochemical parameters during oral exposure to arsenic in rats. Food Chem. Toxicol. 2006, 44, 1579–1584. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Santra, A. Implications of oxidative stress and hepatic cytokine (TNF-α and IL-6) response in the pathogenesis of hepatic collagenesis in chronic arsenic toxicity. Toxicol. Appl. Pharmacol. 2005, 204, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Santra, A.; Maiti, A.; Das, S.; Lahiri, S.; Charkaborty, S.K.; Mazumder, D.N. Hepatic damage caused by chronic arsenic toxicity in experimental animals. J. Clin. Toxicol. 2000, 38, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Cavar, S.; Bošnjak, Z.; Klapec, T.; Barišić, K.; Cepelak, I.; Jurasović, J.; Milić, M. Blood selenium, glutathione peroxidase activity and antioxidant supplementation of subjects exposed to arsenic via drinking water. Environ. Toxicol. Pharmacol. 2010, 29, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Shila, S.; Subathra, M.; Devi, M.A.; Panneerselvam, C. Arsenic intoxication-induced reduction of glutathione level and of the activity of related enzymes in rat brain regions: Reversal by DL-a-lipoic acid. Arch. Toxicol. 2005, 79, 140–146. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).