Utilization and Bio-Efficacy of Carotenoids, Vitamin A and Its Vitaminoids in Nutricosmetics, Cosmeceuticals, and Cosmetics’ Applications with Skin-Health Promoting Properties
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
3. Vitamin A, Its Vitaminoids and Carotenoids’ General Profile and Health Benefits
3.1. Chemical Structures, Origin, and Isolation of Vitamin A and Its Vitaminoids
3.2. Vitamin A-Related Bioactive Compounds: Provitamins (Carotenoids)
3.3. Indicative Role and Functions of Vitamin A, Its Vitaminoids, and Carotenoids—Vitamin A Deficiency, Hypervitaminosis, and Toxicity
4. Vitamin A, Vitaminoids, and Carotenoids as Nutricosmetic and/or Cosmeceutical and Cosmetic Factors: Absorption, Conversion, Storage, Distribution, Metabolism, and Biochemical Mechanisms of Action
4.1. Mechanisms of Absorption, Conversion, Storage-Distribution, and Metabolic Pathways of Vitamin A, Its Vitaminoids, and Carotenoids
4.1.1. Oral Absorption and Metabolism of Vitamin A, Its Vitaminoids, and Carotenoids
4.1.2. Topical Absorption of Carotenoids, Vitamin A, and Its Vitaminoids
4.1.3. Other Absorption Pathways of Vitamin A, Its Vitaminoids, and Carotenoids
4.2. Biochemical Mechanisms of Action of Vitamin A, Its Vitaminoids, and Carotenoids: Gene Regulation and Mediation in Thromboinflammatory Pathways
5. Vitamin A, Vitaminoids, and Carotenoids’ General Role in Nutricosmetic, Cosmeceutical, and Cosmetic Applications
5.1. Anti-Aging and Photo-Protective Properties of Vitamin A, Retinoids, and Carotenoids
5.1.1. Anti-Aging Effect—Skin Regeneration
5.1.2. Photo-Protective Effect
5.2. Antioxidant and Anti-Inflammatory Profile of Vitamin A, Retinoids, and Carotenoids as Immunodefensive Mechanisms
5.3. Hyperpigmentation Improvement Activity of Vitamin A, Retinoids, and Carotenoids
5.4. Acne Treatment Activity of Vitamin A, Retinoids, and Carotenoids
5.5. Psoriasis Treatment with Vitamin A, Retinoids, and Carotenoids
5.6. Skin and Other Cancers’ Treatment Potential of Vitamin A, Retinoids, and Carotenoids
6. Vitamin A and Its Derivatives of Several Sources: Health-Promoting Skin Benefits for Nutricosmetic, Cosmeceutical, and Cosmetic Applications
6.1. Vitamin A, Vitaminoids, and Carotenoids of Animal Origin
| Animal Source | Type of Vitamin A Derivative | Hypothesis—Intervention (Type of Study) | Study Design—Parameters Examined | Results–Observed Benefits | Nutricosmetic, and/or Cosmeceutical, and/or Cosmetic Application | Year of Study | References | |
|---|---|---|---|---|---|---|---|---|
| Milk and Dairy | Goat cheese derived from goat milk | Isolated from goat milk samples, Rhodotorula glutinis P4M422, from which the carotenoid pigments β-carotene, torulene, and γ-carotene were extracted |
|
|
|
| 2020 | [52] |
| Cows with mastitis | Isolated from milk samples, Rhodotorula glutinis, from which the carotenoid pigment was extracted |
|
|
|
| 2022 | [155] | |
| Animal sources and fortified foods, including margarines and dairy products | Preformed vitamin A, retinol, and provitamin A carotenoids |
|
|
|
| 2007 | [156] | |
| Infraspinatus muscle, liver, and kidney of goats fed with a blend of palm and canola oil | α-carotene, β-carotene, and lycopene isomers |
|
|
|
| 2015 | [157] | |
| Sheep | β-carotene including lactoferrin, immunoglobulin (Ig)A, fat-soluble vitamins, and zinc |
|
|
|
| 2024 | [158] | |
| Cheese out of cow’s milk | Bacterioruberin |
|
|
|
| 2020 | [159] | |
| Eggs and Egg Yolk | (Chicken) Eggs | Lutein and zeaxanthin (1213 ± 1731 μg/day) |
|
|
|
| 2015 | [160] |
| (Chicken) egg and egg whites | Lutein and zeaxanthin |
|
|
|
| 2013 | [161] | |
| Modified hen eggs and egg-yolk-based beverage | Lutein and zeaxanthin |
|
|
|
| 2014 | [162] |
6.2. Vitamin A, Vitaminoids, and Carotenoids of Marine Origin
| Marine Source | Type of Marine | Type of Vitamin A Derivative | Hypothesis—Intervention (Type of Study) | Study Design—Parameters Examined | Results–Observed Benefits | Nutricosmetic, and/or Cosmeceutical, and/or Cosmetic Application | Year of Study | References |
|---|---|---|---|---|---|---|---|---|
| Shellfish | Scallop (Chlamys nobilis) | 2,2′-dihydroxy-astaxanthin from the isolated bacterium Brevundimonas scallop |
|
|
|
| 2020 | [50] |
| Algae | Dunaliella salina | α-carotene, lutein, zeaxanthin, all-trans-β-carotene, and 9-cis-β-carotene |
|
|
|
| 2017 | [166] |
| Dunaliella salina | β-carotene and retinol |
|
|
|
| 2023 | [167] | |
| Dunaliella salina | Phytoene and phytofluene |
|
|
|
| 2022 | [168] | |
| Dunaliella salina | All-trans-forms of α-carotene, β-carotene, lutein, and zeaxanthin, as well as 13-cis-β-carotene, 9-cis-α-carotene, and 9-cis-β-carotene |
|
|
|
| 2013 | [169] | |
| Haematococcus pluvialis | Astaxanthin capsules |
|
|
|
| 2018 | [170] | |
| Haematococcus pluvialis | Astaxanthin, astaxanthin monoester (AXME), and astaxanthin diester (AXDE) as well as retinol |
|
|
|
| 2013 | [171] | |
| Haematococcus pluvialis | Astaxanthin tablets and fish collagen hydrolysate tablets |
|
|
|
| 2014 | [172] | |
| Haematococcus pluvialis | Astaxanthin |
|
|
|
| 2020 | [173] | |
| Halimeda opuntia (Linnaeus) Lamoroux green seaweed | Total carotenoid content |
|
|
|
| 2022 | [174] | |
| Sargassum hemiphyllum (brown seaweed) | Fucoidan and Fucoxanthin |
|
|
|
| 2021 | [175] | |
| Marine algae-derived carotenoid | Fucoxanthin |
|
|
|
| 2020 | [176] | |
| Seaweed | Fucoxanthin |
|
|
|
| 2017 | [177] | |
| Sargassum glaucescens (brown seaweed algae extract) | Fucoxanthin |
|
|
|
| 2020 | [178] | |
| Chlorella sp. | β-carotene, lutein, and zeaxanthin |
|
|
|
| 2015 | [179] | |
| Chlorella vulgaris | β-carotene, lutein, and zeaxanthin |
|
|
|
| 2021 | [180] | |
| Bacteria | Gordonia Hongkongensis (EUFUS-Z928) (Isolated from the octocoral Eunicea fusca) | β-carotene |
|
|
|
| 2024 | [51] |
| Five cyanobacteria strains: Alkaliema aff. pantanalense LEGE15481, Cyanobium gracile LEGE12431, Nodosilinea (Leptolyngbya) antarctica LEGE13457, Cuspidothric issatschenkoi LEGE03282, and Leptolyngbya-like sp. LEGE13412 | All-trans- β-carotene, zeaxanthin, echinenone, and lutein |
|
|
|
| 2020 | [181] | |
| Cyanobium sp. (LEGE06113) | β-carotene, zeaxanthin, echinenone, and lutein |
|
|
|
| 2020 | [182] | |
| Three different cyanobacteria: Pseudanabaena sp., Spirulina sp., and Lyngbya sp. | β-carotene (less amounts of Myxoxanthophyll, zeaxanthin, canthaxanthin, and α-carotene |
|
|
|
| 2015 | [63] | |
| Phormidium sp. LEGE05292, Tychonema sp. LEGE07196, Synechocystis salina LEGE06155, and Cyanobium sp. LEGE07175 | β-carotene, zeaxanthin, echinenone, canthaxanthin, and lutein |
|
|
|
| 2020 | [183] | |
| Archaea | Haloterrigena turkmenica | Lycopene, phytoene, and lycopersene, as well as bacterioruberin carotenoids |
|
|
|
| 2017 | [184] |
| Natrialba sp. M6 (haloalkaliphilic archaeon) | C50 carotenoid Bacterioruberin |
|
|
|
| 2020 | [46] | |
| Halophilic Archaea | β-carotene and carotenoids in general |
|
|
|
| |||
| Halorubrum tebenquichense | Bacterioruberin |
|
|
|
| 2022 | [185] | |
| Halorubrum sp. BS2 | Bacterioruberin and Bisanhydro- bacterioruberin |
|
|
|
| 2020 | [186] | |
| Haloferax mediterranei |
|
|
|
| 2022 | [65] | ||
| Haloarcula sp. (several strains) | Bacterioruberin, Monoanhydro- bacterioruberin, Bisanhydro- bacterioruberin, 5-cis-26-cis- Bacterioruberin, 9-cis- Bacterioruberin, 13-cis- Bacterioruberin and 9-cis-26-cis- Bacterioruberin and 9 more in less significant amount |
|
|
|
| 2021 | [38,187] |
6.3. Vitamin A, Vitaminoids, and Carotenoids of Plant and Herb Origin
| Plant Source | Type of Plant/Herb | Type of Vitamin A Derivative | Hypothesis—Intervention (Type of Study) | Study Design—Parameters Examined | Results–Observed Benefits | Nutricosmetic, and/or Cosmeceutical, and/or Cosmetic Application | Year of Study | References |
|---|---|---|---|---|---|---|---|---|
| Fruits and Vegetables | Tomato powder | Phytoene and phytofluene |
|
|
|
| 2015 | [188] |
| Tomato-derived lycopene nanostructured lipid carriers (NLC) | Lycopene |
|
|
|
| 2015 | [189] | |
| Lycopene-rich tomato nutrient complex (TNC) | Lycopene |
|
|
|
| 2019 | [190] | |
| Lycopene capsule and tomato paste | Lycopene |
|
|
|
| 2015 | [191] | |
| Tomato extract, lutein, and lycopene | Lycopene and lutein |
|
|
|
| 2013 | [192] | |
| Tomato waste extracts | Lycopene and β-carotene |
|
|
|
| 2014 | [193] | |
| Tomato capsules | Lutein and lycopene |
|
|
|
| 2016 | [140] | |
| Tomato and marine algae-derived products | Phytoene and phytofluene |
|
|
|
| 2018 | [194] | |
| Carrots (Daucus carota L., cv. Flacoro and Nantejska) | β-carotene, phenolic acids, and flavonoids |
|
|
|
| 2022 | [41] | |
| Palmyra (Borassus flabellifer) and (soapberry (Sapindus mukorossi) and aloe vera and extraction by virgin coconut oil) | β-carotene and its isomers |
|
|
|
| 2023 | [47] | |
| Red guava (Psidium guajava L.) | All-trans-β-carotene, all-trans-lycopene, and their isomers |
|
|
|
| 2017 | [195] | |
| Pink guava (Psidium guajava L. Cv. ‘Criolla’) | All-trans-β-carotene, all-trans-lycopene, and 15-cis-lycopene |
|
|
|
| 2017 | [43] | |
| Sweet Orange | β-carotene |
|
|
|
| 2014 | [196] | |
| Red bell pepper extract (RBPE) (Capsicum annum L.) | β-carotene, β-cryptoxanthin, and capsanthin |
|
|
|
| 2022 | [197] | |
| Avocado (Persea americana) | Mostly the xanthophyll lutein and then zeaxanthin |
|
|
|
| 2020 | [198,199] | |
| Avocado (Persea americana) cv ‘Hass’ | β-carotene, all-trans-lutein, all-trans-lutein-5,6-Epoxide, all-trans- violaxanthin, all-trans-Neochrome, and Chrysanthemaxanthin |
|
|
|
| 2020 | [198,200] | |
| Avocado (Persea americana) | Lutein, α-carotene, β-carotene, and retinol |
|
|
|
| 2020 | [198,201] | |
| Field Pumpkin (Cucurbita pepo L. (CpL) | Lycopene, b-carotene, and lutein |
|
|
|
| 2024 | [42] | |
| Sweet Potato (Ipomoea batatas (L.) Lam) TNG66 | β-carotene, violaxanthin, lutein, zeaxanthin, α-carotene, and β-cryptoxanthin and their isomers |
|
|
|
| 2022 | [202] | |
| Spinach (Spinachia oleracea) | Lutein |
|
|
|
| 2020 | [203] | |
| Broccoli (Brassica oleracea) | β-carotene and other carotenoid phenolics |
|
|
|
| 2020 | [204] | |
| Waste apricot flesh (WAF) | Phytoene at the highest content of total carotenoids |
|
|
|
| 2023 | [44] | |
| Pineapple (Ananas comosus L. Merr.) | α-carotene, β-carotene, lutein, β-cryptoxanthin, lycopene, neoxanthin, violaxanthin, zeaxanthin, and retinol, as well as other constituents like tocopherol and vitamins C and E |
|
|
|
| 2015 | [205] | |
| Kernel oils from sour cherry (Prunus cerasus L.) | All-trans-β-carotene (mainly) and total carotenoids, along with fatty acids, total tocochromanols, tocopherols, sterols, and squalene |
|
|
|
| 2016 | [206] | |
| Mango (Ataulfo) | β-carotene |
|
|
|
| 2020 | [207] | |
| Cantaloupe melon (Cucumis melo L.) | β-carotene, α-carotene, lutein, and zeaxanthin |
|
|
|
| 2021 | [62] | |
| Cereal/ Grains | Gluten-free (GF) bread from pea flour by Chlorella sorokiniana | All-trans-lutein, all-trans-β-carotene, all-trans- zeaxanthin, and 9-cis-β-carotene along with other lutein, β-carotene, violaxanthin, and zeaxanthin |
|
|
|
| 2020 | [208] |
| Cereal product | High amounts of β-carotene (as well as γ-linolenic acid (GLA)) |
|
|
|
| 2018 | [209] | |
| Herbs and Spices | Paprika oleoresin (Capsicum annuum) | Mostly capsanthin and capsorubin |
|
|
|
| 2020 | [210] |
| Paprika oleoresin (Capsicum annuum) (zeaxanthin) and marigold flower oleoresin (lutein) | Zeaxanthin and lutein |
|
|
|
| 2017 | [211] | |
| Coriander (Coriandrum sativum L.) | Total carotenoids and β-carotene |
|
|
|
| 2012 | [212] | |
| Parsley (Petroselinum crispum) | Capsanthone (the only carotenoid) and other acids, furanocoumarins, and flavonoids |
|
|
|
| 2021 | [45] |
6.4. Vitamin A, Vitaminoids, and Carotenoids from Microorganism Sources
| Type of Microorganism | Microorganism | Type of Vitamin A Derivative | Hypothesis—Intervention (Type of Study) | Study Design—Parameters Examined | Results—Observed Benefits | Nutricosmetic, and/or Cosmeceutical, and/or Cosmetic Application | Year Study | References |
|---|---|---|---|---|---|---|---|---|
| Fungi | Blakeslea trispora and Mucor circinelloides | β-carotene |
|
|
|
| 2020 | [218] |
| Blakeslea trispora | Phytoene-rich β-carotene and lycopene |
|
|
|
| 2024 | [219] | |
| Mucor circinelloides | β-carotene |
|
|
|
| 2020 | [220] | |
| Neurospora crassa and Fusarium fujikuroi | Neurosporaxanthin |
|
|
|
| 2020 | [39] | |
| Yeast | Rhodotorula mucilaginosa (Pinaceae forest ecosystems) | β-carotene (mainly), torulene, and torularhodin |
|
|
|
| 2021 | [221,222] |
| Rhodotorula glutinis (P4M422) | Preformed Vitamin A |
|
|
|
| 2017 | [223] | |
| Sporidiobolus pararoseus | Torularhodin |
|
|
|
| 2019 | [224] | |
| Sporidiobolus pararoseus | Torulene and torularhodin |
|
|
|
| 2017 | [225] | |
| Phaffia Rhodozyma (NRRL-Y 17268) | β-carotene, astaxanthin, and lutein |
|
|
|
| 2015 | [226] | |
| Saccharomyces cerevisiae | β-carotene |
|
|
|
| 2022 | [227] | |
| Bacteria | Paracoccus aurantius (MBLB3053T) | Mostly astaxanthin |
|
|
|
| 2024 | [228] |
| Paracoccus carotinifaciens | Astaxanthin-rich extract from P. carotinifaciens (supplement) |
|
|
|
| 2018 | [229] | |
| Arthrobacter agilis DSM 20550T and Arthrobacter bussei DSM 109896T | Bacterioruberin |
|
|
|
| 2022 | [230] | |
| Citricoccus parietis AUCs | β-carotene |
|
|
|
| 2023 | [231] | |
| Exiquobacterium acetilicum S01 | Lycopene (Car-I), Diapolycopene-dioic-acid-diglucosyl-ester (Car-II), β-carotene (Car-III), zeaxanthin (Car-IV), astaxanthin (Car-V), and Keto-Myxocoxanthin (Car-VI) |
|
|
|
| 2020 | [232] | |
| Dietzia natronolimnaea (waste molasses and its hydrolysate) | Canthaxanthin |
|
|
|
| 2014 | [233] | |
| Escherichia coli | Carotenoid holoprotein and carotenoids in general (mainly β-carotene and violaxanthin) |
|
|
|
| 2015 | [234,235] | |
| Escherichia coli | Retinyl palmitate |
|
|
|
| 2020 | [236] | |
| Spirulinaplatensis | B-carotene |
|
|
|
| 2017 | [237] | |
| Spirulina platensis | β-carotene and zeaxanthin (blue–green) in this microalgae powder |
|
|
|
| 2015 | [238] | |
| Micrococcus Luteus (Q24) | Carotenoid pigments probiotics |
|
|
|
| 2024 | [64,239] | |
| Algae | Neochloris oleoabundans | Lutein, carotenoid monoesters, and violaxanthin |
|
|
|
| 2016 | [240] |
| Coelastrella oocystiformis. | β-carotene, astaxanthin, lutein, canthaxanthin and phytofluene |
|
|
|
| 2015 | [241] |
7. Recent Advances in the Delivery Systems of Vitamin A and Its Derivatives in Nutricosmetics, Cosmeceuticals, and Cosmetics Applications
8. Limitations and Future Perspectives of Vitamin A, Vitaminoids, and Carotenoids in Nutricosmetics, Cosmeceuticals, and Cosmetics Applications
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martínez-Cámara, S.; Ibañez, A.; Rubio, S.; Barreiro, C.; Barredo, J.-L. Main Carotenoids Produced by Microorganisms. Encyclopedia 2021, 1, 1223–1245. [Google Scholar] [CrossRef]
- Steenbock, H. White Corn vs. Yellow Corn and a Probable Relation Between the Fat-Soluble Vitamine and Yellow Plant Pigments. Science 1919, 50, 352–353. [Google Scholar] [CrossRef] [PubMed]
- Carazo, A.; Macáková, K.; Matoušová, K.; Krčmová, L.K.; Protti, M.; Mladěnka, P. Vitamin A Update: Forms, Sources, Kinetics, Detection, Function, Deficiency, Therapeutic Use and Toxicity. Nutrients 2021, 13, 1703. [Google Scholar] [CrossRef]
- Shastak, Y.; Pelletier, W. Pet Wellness and Vitamin A: A Narrative Overview. Animals 2024, 14, 1000. [Google Scholar] [CrossRef]
- Baswan, S.M.; Klosner, A.E.; Weir, C.; Salter-Venzon, D.; Gellenbeck, K.W.; Leverett, J.; Krutmann, J. Role of Ingestible Carotenoids in Skin Protection: A Review of Clinical Evidence. Photodermatol. Photoimmunol. Photomed. 2021, 37, 490–504. [Google Scholar] [CrossRef]
- Crupi, P.; Faienza, M.F.; Naeem, M.Y.; Corbo, F.; Clodoveo, M.L.; Muraglia, M. Overview of the Potential Beneficial Effects of Carotenoids on Consumer Health and Well-Being. Antioxidants 2023, 12, 1069. [Google Scholar] [CrossRef]
- Biskanaki, F.; Kalofiri, P.; Tertipi, N.; Sfyri, E.; Andreou, E.; Kefala, V.; Rallis, E. Carotenoids and Dermoaesthetic Benefits: Public Health Implications. Cosmetics 2023, 10, 120. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Stinco, C.M.; Mapelli-Brahm, P. Skin Carotenoids in Public Health and Nutricosmetics: The Emerging Roles and Applications of the UV Radiation-Absorbing Colourless Carotenoids Phytoene and Phytofluene. Nutrients 2019, 11, 1093. [Google Scholar] [CrossRef] [PubMed]
- Flieger, J.; Raszewska-Famielec, M.; Radzikowska-Büchner, E.; Flieger, W. Skin Protection by Carotenoid Pigments. Int. J. Mol. Sci. 2024, 25, 1431. [Google Scholar] [CrossRef]
- VanBuren, C.A.; Everts, H.B. Vitamin A in Skin and Hair: An Update. Nutrients 2022, 14, 2952. [Google Scholar] [CrossRef] [PubMed]
- Milani, M.; Colombo, F. Skin Anti-Aging Effect of Oral Vitamin A Supplementation in Combination with Topical Retinoic Acid Treatment in Comparison with Topical Treatment Alone: A Randomized, Prospective, Assessor-Blinded, Parallel Trial. Cosmetics 2023, 10, 144. [Google Scholar] [CrossRef]
- Cho, Y.S.; Kim, H.O.; Woo, S.M.; Lee, D.H. Use of Dexpanthenol for Atopic Dermatitis—Benefits and Recommendations Based on Current Evidence. J. Clin. Med. 2022, 11, 3943. [Google Scholar] [CrossRef] [PubMed]
- Stettler, H.; de Salvo, R.; Olsavszky, R.; Nanu, E.A.; Dumitru, V.; Trapp, S. Performance and Tolerability of a New Topical Dexpanthenol-Containing Emollient Line in Subjects with Dry Skin: Results from Three Randomized Studies. Cosmetics 2021, 8, 18. [Google Scholar] [CrossRef]
- Temova Rakuša, Ž.; Roškar, R. Quality Control of Vitamins A and E and Coenzyme Q10 in Commercial Anti-Ageing Cosmetic Products. Cosmetics 2021, 8, 61. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Zhang, H.; Ding, Z.; Han, J. The Application of Natural Carotenoids in Multiple Fields and Their Encapsulation Technology: A Review. Molecules 2024, 29, 967. [Google Scholar] [CrossRef]
- Kim, J.A.; Jang, J.-H.; Lee, S.-Y. An Updated Comprehensive Review on Vitamin A and Carotenoids in Breast Cancer: Mechanisms, Genetics, Assessment, Current Evidence, and Future Clinical Implications. Nutrients 2021, 13, 3162. [Google Scholar] [CrossRef]
- Villar, A.; Vázquez-González, I.; Vicente, F.; Salcedo, G.; González, L.; Botana, A.; Royo, L.J.; Eguinoa, P.; Busqué, J. Study of the Variability in Fatty Acids and Carotenoid Profiles: Laying the Ground for Tank Milk Authentication. Sustainability 2021, 13, 4506. [Google Scholar] [CrossRef]
- Sharma, C.; Kamle, M.; Kumar, P. Microbial-Derived Carotenoids and Their Health Benefits. Microbiol. Res. 2024, 15, 1670–1689. [Google Scholar] [CrossRef]
- Darvin, M.E.; Lademann, J.; von Hagen, J.; Lohan, S.B.; Kolmar, H.; Meinke, M.C.; Jung, S. Carotenoids in Human Skin In Vivo: Antioxidant and Photo-Protectant Role against External and Internal Stressors. Antioxidants 2022, 11, 1451. [Google Scholar] [CrossRef]
- Shastak, Y.; Pelletier, W.; Kuntz, A. Insights into Analytical Precision: Understanding the Factors Influencing Accurate Vitamin A Determination in Various Samples. Analytica 2024, 5, 54–73. [Google Scholar] [CrossRef]
- Shastak, Y.; Pelletier, W. Review of Liquid Vitamin A and E Formulations in Veterinary and Livestock Production: Applications and Perspectives. Vet. Sci. 2024, 11, 421. [Google Scholar] [CrossRef]
- Zhang, H.; Ren, X.; Yang, Z.; Lai, J. Vitamin A Concentration in Human Milk: A Meta-Analysis. Nutrients 2022, 14, 4844. [Google Scholar] [CrossRef] [PubMed]
- Górska-Warsewicz, H.; Rejman, K.; Laskowski, W.; Czeczotko, M. Milk and Dairy Products and Their Nutritional Contribution to the Average Polish Diet. Nutrients 2019, 11, 1771. [Google Scholar] [CrossRef] [PubMed]
- Stadnik, J. Nutritional Value of Meat and Meat Products and Their Role in Human Health. Nutrients 2024, 16, 1446. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.U.; Khan, A.; Naz, S.; Ullah, Q.; Puvača, N.; Laudadio, V.; Mazzei, D.; Seidavi, A.; Ayasan, T.; Tufarelli, V. Pros and Cons of Dietary Vitamin A and Its Precursors in Poultry Health and Production: A Comprehensive Review. Antioxidants 2023, 12, 1131. [Google Scholar] [CrossRef]
- Ionov, I.A.; Katerinich, O.O.; Kuchmistov, V.O.; Anisimova, O.V.; Griffin, D.K.; Romanov, M.N.; Zhukova, I.O. Vitamin E and A Availability in Goose Embryos and Goslings and Improvement of Reproduction Traits Depending on the Starting Temperature Regime of Egg Incubation. Poultry 2023, 2, 305–319. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, L.; Jiang, W.; Liu, Y.; Jiang, J.; Kuang, S.; Li, S.; Tang, L.; Tang, W.; Zhou, X.; et al. Dietary Vitamin A Improved the Flesh Quality of Grass Carp (Ctenopharyngodon Idella) in Relation to the Enhanced Antioxidant Capacity through Nrf2/Keap 1a Signaling Pathway. Antioxidants 2022, 11, 148. [Google Scholar] [CrossRef]
- Cabezuelo, M.T.; Zaragozá, R.; Barber, T.; Viña, J.R. Role of Vitamin A in Mammary Gland Development and Lactation. Nutrients 2020, 12, 80. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, R.; Meléndez-Martínez, A.J.; Vicario, I.M.; Alcalde, M.J. Carotenoid and Vitamin A Contents in Biological Fluids and Tissues of Animals as an Effect of the Diet: A Review. Food Rev. Int. 2015, 31, 319–340. [Google Scholar] [CrossRef]
- Darwish, W.S.; Ikenaka, Y.; Morshdy, A.E.; Eldesoky, K.I.; Nakayama, S.; Mizukawa, H.; Ishizuka, M. β-Carotene and Retinol Contents in the Meat of Herbivorous Ungulates with a Special Reference to Their Public Health Importance. J. Vet. Med. Sci. 2016, 78, 351–354. [Google Scholar] [CrossRef]
- Shastak, Y.; Pelletier, W. Vitamin A in Fish Well-Being: Integrating Immune Strength, Antioxidant Capacity and Growth. Fishes 2024, 9, 330. [Google Scholar] [CrossRef]
- Tarricone, S.; Ragni, M.; Carbonara, C.; Giannico, F.; Bozzo, F.; Petrontino, A.; Caputi Jambrenghi, A.; Colonna, M.A. Growth Performance and Flesh Quality of Sea Bass (Dicentrarchus Labrax) Fed with Diets Containing Olive Oil in Partial Replacement of Fish Oil—With or Without Supplementation with Rosmarinus Officinalis L. Essential Oil. Animals 2024, 14, 3237. [Google Scholar] [CrossRef] [PubMed]
- Dajnowska, A.; Tomaszewska, E.; Świątkiewicz, S.; Arczewska-Włosek, A.; Dobrowolski, P.; Domaradzki, P.; Rudyk, H.; Brezvyn, O.; Muzyka, V.; Kotsyumbas, I.; et al. Yolk Fatty Acid Profile and Amino Acid Composition in Eggs from Hens Supplemented with SS-Hydroxy-ß-Methylbutyrate. Foods 2023, 12, 3733. [Google Scholar] [CrossRef]
- Bjørklund, G.; Shanaida, M.; Lysiuk, R.; Butnariu, M.; Peana, M.; Sarac, I.; Strus, O.; Smetanina, K.; Chirumbolo, S. Natural Compounds and Products from an Anti-Aging Perspective. Molecules 2022, 27, 7084. [Google Scholar] [CrossRef]
- Kawczak, P.; Feszak, I.; Brzeziński, P.; Bączek, T. Structure–Activity Relationships and Therapeutic Applications of Retinoids in View of Potential Benefits from Drug Repurposing Process. Biomedicines 2024, 12, 1059. [Google Scholar] [CrossRef]
- Karrer, P.; Morf, B.; Schöpp, K. Isolation of Vitamin A. Nature 1932, 129, 88. [Google Scholar] [CrossRef]
- Holmes, H.N.; Corbet, R.E. The Isolation of Crystalline Vitamin A1. J. Am. Chem. Soc. 1937, 59, 2042–2047. [Google Scholar] [CrossRef]
- Lizama, C.; Romero-Parra, J.; Andrade, D.; Riveros, F.; Bórquez, J.; Ahmed, S.; Venegas-Salas, L.; Cabalín, C.; Simirgiotis, M.J. Analysis of Carotenoids in Haloarchaea Species from Atacama Saline Lakes by High Resolution UHPLC-Q-Orbitrap-Mass Spectrometry: Antioxidant Potential and Biological Effect on Cell Viability. Antioxidants 2021, 10, 1230. [Google Scholar] [CrossRef] [PubMed]
- Parra-Rivero, O.; Paes de Barros, M.; del Mar Prado, M.; Gil, J.-V.; Hornero-Méndez, D.; Zacarías, L.; Rodrigo, M.J.; Limón, M.C.; Avalos, J. Neurosporaxanthin Overproduction by Fusarium Fujikuroi and Evaluation of Its Antioxidant Properties. Antioxidants 2020, 9, 528. [Google Scholar] [CrossRef] [PubMed]
- Słowik-Borowiec, M.; Głąb, N.; Stach, S.; Szpyrka, E. A Miniaturized Sample Preparation Method for the Determination of Vitamins A and E in Food Products. Molecules 2023, 28, 3449. [Google Scholar] [CrossRef]
- Średnicka-Tober, D.; Kopczyńska, K.; Góralska-Walczak, R.; Hallmann, E.; Barański, M.; Marszałek, K.; Kazimierczak, R. Are Organic Certified Carrots Richer in Health-Promoting Phenolics and Carotenoids than the Conventionally Grown Ones? Molecules 2022, 27, 4184. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, R.; Castaldo, L.; Sessa, R.; Ricci, L.; Vardaro, E.; Izzo, L.; Grosso, M.; Ritieni, A.; Laneri, S. Chemical Profile and Promising Applications of Cucurbita Pepo L. Flowers. Antioxidants 2024, 13, 1476. [Google Scholar] [CrossRef]
- Rojas-Garbanzo, C.; Gleichenhagen, M.; Heller, A.; Esquivel, P.; Schulze-Kaysers, N.; Schieber, A. Carotenoid Profile, Antioxidant Capacity, and Chromoplasts of Pink Guava (Psidium guajava L. Cv. ‘Criolla’) during Fruit Ripening. J. Agric. Food Chem. 2017, 65, 3737–3747. [Google Scholar] [CrossRef]
- Suo, A.; Fan, G.; Wu, C.; Li, T.; Cong, K. Green Extraction of Carotenoids from Apricot Flesh by Ultrasound Assisted Corn Oil Extraction: Optimization, Identification, and Application. Food Chem. 2023, 420, 136096. [Google Scholar] [CrossRef] [PubMed]
- Staropoli, A.; Vassetti, A.; Salvatore, M.M.; Andolfi, A.; Prigigallo, M.I.; Bubici, G.; Scagliola, M.; Salerno, P.; Vinale, F. Improvement of Nutraceutical Value of Parsley Leaves (Petroselinum crispum) upon Field Applications of Beneficial Microorganisms. Horticulturae 2021, 7, 281. [Google Scholar] [CrossRef]
- Hegazy, G.E.; Abu-Serie, M.M.; Abo-Elela, G.M.; Ghozlan, H.; Sabry, S.A.; Soliman, N.A.; Abdel-Fattah, Y.R. In Vitro Dual (Anticancer and Antiviral) Activity of the Carotenoids Produced by Haloalkaliphilic Archaeon Natrialba sp. M6. Sci. Rep. 2020, 10, 5986. [Google Scholar] [CrossRef] [PubMed]
- Thevamirtha, C.; Balasubramaniyam, A.; Srithayalan, S.; Selvakumar, P.M. An Insight into the Antioxidant Activity of the Facial Cream, Solid Soap and Liquid Soap Made Using the Carotenoid Extract of Palmyrah (Borassus flabellifer) Fruit Pulp. Ind. Crops Prod. 2023, 195, 116413. [Google Scholar] [CrossRef]
- Dyaa, A.; Soliman, H.; Abdelrazak, A.; Samra, B.N.; Khojah, E.; Ahmed, A.F.; El-Esawi, M.A.; Elsayed, A. Optimization of Carotenoids Production from Rhodotorula sp. Strain ATL72 for Enhancing Its Biotechnological Applications. J. Fungi 2022, 8, 160. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Shen, M.; Yu, T.; Yang, X.; Li, Q.; Zhao, B.; Zou, J.; Zhang, M. Liquid Chromatography as Candidate Reference Method for the Determination of Vitamins A and E in Human Serum. J. Clin. Lab. Anal. 2020, 34, e23528. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, C.; Zhang, X.; Tan, K.; Zhang, H.; Cheng, D.; Ye, T.; Li, S.; Ma, H.; Zheng, H. A Novel Carotenoids-Producing Marine Bacterium from Noble Scallop Chlamys Nobilis and Antioxidant Activities of Its Carotenoid Compositions. Food Chem. 2020, 320, 126629. [Google Scholar] [CrossRef]
- Rhenals-Montoya, P.; Villamil, L.; Sánchez-Suárez, J.; Díaz, L.; Coy-Barrera, E. Optimized Carotenoid Production and Antioxidant Capacity of Gordonia Hongkongensis. Sci. Prog. 2024, 107, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Mata-Gómez, L.C.; Mapelli-Brahm, P.; Meléndez-Martínez, A.J.; Méndez-Zavala, A.; Morales-Oyervides, L.; Montañez, J. Microbial Carotenoid Synthesis Optimization in Goat Cheese Whey Using the Robust Taguchi Method: A Sustainable Approach to Help Tackle Vitamin A Deficiency. Foods 2023, 12, 658. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Amaya, D.B.; Esquivel, P.; Meléndez-Martínez, A.J. Comprehensive Update on Carotenoid Colorants from Plants and Microalgae: Challenges and Advances from Research Laboratories to Industry. Foods 2023, 12, 4080. [Google Scholar] [CrossRef]
- Rosales, A.; Crossa, J.; Cuevas, J.; Cabrera-Soto, L.; Dhliwayo, T.; Ndhlela, T.; Palacios-Rojas, N. Near-Infrared Spectroscopy to Predict Provitamin A Carotenoids Content in Maize. Agronomy 2022, 12, 1027. [Google Scholar] [CrossRef]
- Soulat, J.; Andueza, D.; Graulet, B.; Girard, C.L.; Labonne, C.; Aït-Kaddour, A.; Martin, B.; Ferlay, A. Comparison of the Potential Abilities of Three Spectroscopy Methods: Near-Infrared, Mid-Infrared, and Molecular Fluorescence, to Predict Carotenoid, Vitamin and Fatty Acid Contents in Cow Milk. Foods 2020, 9, 592. [Google Scholar] [CrossRef]
- Beshgetoor, D. Handbook of Vitamins. Am. J. Clin. Nutr. 2002, 75, 955. [Google Scholar] [CrossRef]
- Vállez-Gomis, V.; Carchano-Olcina, S.; Azorín, C.; Benedé, J.L.; Chisvert, A.; Salvador, A. Simultaneous Quantification of Vitamin A and Derivatives in Cosmetic Products by Liquid Chromatography with Ultraviolet Detection. Separations 2022, 9, 40. [Google Scholar] [CrossRef]
- Gradinaru, I.; Ciubotaru, B.I.; Butnaru, M.; Cojocaru, F.D.; Covașă, C.T.; Bibire, T.; Dascalu, M.; Bargan, A.; Cazacu, M.; Zaltariov, M.-F. The Impact of the Addition of Vitamins on a Silicone Lining Material to the Oral Mucosa Tissue—Evaluation of the Biocompatibility, Hydrolytic Stability and Histopathological Effect. Medicina 2023, 59, 1936. [Google Scholar] [CrossRef]
- Bas, T.G. Bioactivity and Bioavailability of Carotenoids Applied in Human Health: Technological Advances and Innovation. Int. J. Mol. Sci. 2024, 25, 7603. [Google Scholar] [CrossRef]
- Jiang, Y.; Ye, J.; Hu, Y.; Zhang, J.; Li, W.; Zhou, X.; Yu, M.; Yu, Y.; Yang, J.; Yang, W.; et al. Extraction and Synthesis of Typical Carotenoids: Lycopene, β-Carotene, and Astaxanthin. Molecules 2024, 29, 4549. [Google Scholar] [CrossRef] [PubMed]
- Freitas, J.V.; Junqueira, H.C.; Martins, W.K.; Baptista, M.S.; Gaspar, L.R. Antioxidant Role on the Protection of Melanocytes against Visible Light-Induced Photodamage. Free. Radic. Biol. Med. 2019, 131, 399–407. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, G.L.R.; Medeiros, I.; da Cruz Nascimento, S.S.; Viana, R.L.S.; Porto, D.L.; Rocha, H.A.O.; Aragão, C.F.S.; Maciel, B.L.L.; de Assis, C.F.; de Araújo Morais, A.H.; et al. Antioxidant Stability Enhancement of Carotenoid Rich-Extract from Cantaloupe Melon (Cucumis melo L.) Nanoencapsulated in Gelatin under Different Storage Conditions. Food Chem. 2021, 348, 129055. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, C.; Ghosh, T.; Bhayani, K.; Maurya, R.; Mishra, S. Antioxidant, Anti-Nephrolithe Activities and in Vitro Digestibility Studies of Three Different Cyanobacterial Pigment Extracts. Mar. Drugs 2015, 13, 5384–5401. [Google Scholar] [CrossRef] [PubMed]
- Mohana, D.C.; Thippeswamy, S.; Abhishek, R.U. Antioxidant, Antibacterial, and Ultraviolet-Protective Properties of Carotenoids Isolated from Micrococcus Spp. Radiat. Prot. Environ. 2013, 36, 168. [Google Scholar] [CrossRef]
- Giani, M.; Gervasi, L.; Loizzo, M.R.; Martínez-Espinosa, R.M. Carbon Source Influences Antioxidant, Antiglycemic, and Antilipidemic Activities of Haloferax Mediterranei Carotenoid Extracts. Mar. Drugs 2022, 20, 659. [Google Scholar] [CrossRef] [PubMed]
- MAN-000064; QMS Standard Operating Procedure (SOP) Template for Laboratory Operations. Food and Drug Administration Office of Regulatory Affairs, Office of Regulatory Science: Silver Spring, MD, USA, 2022; pp. 1–34.
- Rimkus, G.G.; Schubert, M.; Morgan, D.; Jungjohann, S. Rapid Direct Analysis of Retinyl Palmitate (Vitamin A) in Fortified Vegetable Oils by HPLC-FLD. Food Addit. Contam. Part A 2022, 39, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhang, S.; Qi, B.-K.; Li, H.; Xie, F.-Y.; Li, Y. Molecular Distillation-Induced Deacidification of Soybean Oil Isolated by Enzyme-Assisted Aqueous Extraction: Effect of Distillation Parameters. Appl. Sci. 2019, 9, 2123. [Google Scholar] [CrossRef]
- González-Peña, M.A.; Ortega-Regules, A.E.; Anaya de Parrodi, C.; Lozada-Ramírez, J.D. Chemistry, Occurrence, Properties, Applications, and Encapsulation of Carotenoids—A Review. Plants 2023, 12, 313. [Google Scholar] [CrossRef] [PubMed]
- Olmedilla-Alonso, B.; Rodríguez-Rodríguez, E.; Beltrán-de-Miguel, B.; Estévez-Santiago, R. Dietary β-Cryptoxanthin and α-Carotene Have Greater Apparent Bioavailability Than β-Carotene in Subjects from Countries with Different Dietary Patterns. Nutrients 2020, 12, 2639. [Google Scholar] [CrossRef]
- Sajovic, J.; Meglič, A.; Glavač, D.; Markelj, Š.; Hawlina, M.; Fakin, A. The Role of Vitamin A in Retinal Diseases. Int. J. Mol. Sci. 2022, 23, 1014. [Google Scholar] [CrossRef] [PubMed]
- Cardoso-Demartini, A.A.; Boguszewski, C.L.; Boguszewski, M.C.S. Potential Effects of Oral Isotretinoin on Growth Plate and Height. Endocrines 2023, 4, 281–292. [Google Scholar] [CrossRef]
- Amimo, J.O.; Michael, H.; Chepngeno, J.; Raev, S.A.; Saif, L.J.; Vlasova, A.N. Immune Impairment Associated with Vitamin A Deficiency: Insights from Clinical Studies and Animal Model Research. Nutrients 2022, 14, 5038. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, Y.; Qi, G.; Brand, D.; Zheng, S.G. Role of Vitamin A in the Immune System. J. Clin. Med. 2018, 7, 258. [Google Scholar] [CrossRef]
- Nagai, Y.; Ambinder, A.J. The Promise of Retinoids in the Treatment of Cancer: Neither Burnt Out Nor Fading Away. Cancers 2023, 15, 3535. [Google Scholar] [CrossRef] [PubMed]
- Bakac, E.R.; Percin, E.; Gunes-Bayir, A.; Dadak, A. A Narrative Review: The Effect and Importance of Carotenoids on Aging and Aging-Related Diseases. Int. J. Mol. Sci. 2023, 24, 15199. [Google Scholar] [CrossRef]
- O’Connor, C.; Varshosaz, P.; Moise, A.R. Mechanisms of Feedback Regulation of Vitamin A Metabolism. Nutrients 2022, 14, 1312. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, H.; Yokoi, N.; Sotozono, C. Immunohistochemistry in an Adult Case of Bitot’s Spots Caused by Vitamin A Deficiency. Diagnostics 2023, 13, 3676. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Deng, S.; Li, C.; Cao, J.; Wu, A.; Chen, M.; Ma, X.; Wu, S.; Lian, Z. The Role of Retinoic Acid in Spermatogenesis and Its Application in Male Reproduction. Cells 2024, 13, 1092. [Google Scholar] [CrossRef]
- Filipe Rosa, L.; Petersen, P.P.; Görtz, L.F.; Stolzer, I.; Kaden-Volynets, V.; Günther, C.; Bischoff, S.C. Vitamin A- and D-Deficient Diets Disrupt Intestinal Antimicrobial Peptide Defense Involving Wnt and STAT5 Signaling Pathways in Mice. Nutrients 2023, 15, 376. [Google Scholar] [CrossRef] [PubMed]
- El Soury, M.; Fornasari, B.E.; Carta, G.; Zen, F.; Haastert-Talini, K.; Ronchi, G. The Role of Dietary Nutrients in Peripheral Nerve Regeneration. Int. J. Mol. Sci. 2021, 22, 7417. [Google Scholar] [CrossRef] [PubMed]
- Gutowska, K.; Formanowicz, D.; Formanowicz, P. Interrelations between Iron and Vitamin A—Studied Using Systems Approach. Int. J. Mol. Sci. 2022, 23, 1189. [Google Scholar] [CrossRef] [PubMed]
- Mugware, A.; Motadi, S.A.; Bere, A.; Mushaphi, L.F. Iron and Vitamin A Status of Children Aged 0 to 36 Months in Thulamela Municipality, Vhembe District, South Africa. Children 2024, 11, 1018. [Google Scholar] [CrossRef]
- Luca, M.M.; Buzatu, R.; Bumbu, B.A. Evaluating the Protective Role of Vitamin A Supplementation in Periodontal Health: A Comprehensive Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 4775. [Google Scholar] [CrossRef] [PubMed]
- Tavares Antunes, P.; das Graças Vaz-Tostes, M.; Tomáz Sant’Ana, C.; Araújo de Faria, R.; Lopes Toledo, R.C.; Brunoro Costa, N.M. Bioavailability of Iron and the Influence of Vitamin a in Biofortified Foods. Agronomy 2019, 9, 777. [Google Scholar] [CrossRef]
- Yee, M.M.F.; Chin, K.-Y.; Ima-Nirwana, S.; Wong, S.K. Vitamin A and Bone Health: A Review on Current Evidence. Molecules 2021, 26, 1757. [Google Scholar] [CrossRef] [PubMed]
- Harrison, E.H. Mechanisms Involved in the Intestinal Absorption of Dietary Vitamin A and Provitamin A Carotenoids. Biochim. Et Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2012, 1821, 70–77. [Google Scholar] [CrossRef]
- Harrison, E.H. Mechanisms of Transport and Delivery of Vitamin A and Carotenoids to the Retinal Pigment Epithelium. Mol. Nutr. Food Res. 2019, 63, 1801046. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.; Saraiva, M.J. Transthyretin: A Multifaceted Protein. BioMolecular Concepts 2014, 5, 45–54. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosio, D.N.; Clugston, R.D.; Blaner, W.S. Vitamin A Metabolism: An Update. Nutrients 2011, 3, 63–103. [Google Scholar] [CrossRef]
- Urbach, J.; Rando, R.R. Isomerization of All-Trans-Retinoic Acid to 9-Cis-Retinoic Acid. Biochem. J. 1994, 299, 459–465. [Google Scholar] [CrossRef]
- Martin Ask, N.; Leung, M.; Radhakrishnan, R.; Lobo, G.P. Vitamin A Transporters in Visual Function: A Mini Review on Membrane Receptors for Dietary Vitamin A Uptake, Storage, and Transport to the Eye. Nutrients 2021, 13, 3987. [Google Scholar] [CrossRef] [PubMed]
- Reboul, E. Absorption of Vitamin A and Carotenoids by the Enterocyte: Focus on Transport Proteins. Nutrients 2013, 5, 3563–3581. [Google Scholar] [CrossRef] [PubMed]
- Shete, V.; Quadro, L. Mammalian Metabolism of β-Carotene: Gaps in Knowledge. Nutrients 2013, 5, 4849–4868. [Google Scholar] [CrossRef]
- Samara, I.; Moula, A.I.; Moulas, A.N.; Katsouras, C.S. The Effect of Retinoids in Vascular Smooth Muscle Cells: From Phenotyping Switching to Proliferation and Migration. Int. J. Mol. Sci. 2024, 25, 10303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, Y.; Li, R.; Chen, G. Transcriptional Factors Mediating Retinoic Acid Signals in the Control of Energy Metabolism. Int. J. Mol. Sci. 2015, 16, 14210–14244. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wang, Y.; Yu, D.; Zhang, S.; Zheng, F.; Ding, N.; Zhu, L.; Zhu, Q.; Sun, W.; Li, S.; et al. Association between Serum Vitamin A, Blood Lipid Level and Dyslipidemia among Chinese Children and Adolescents. Nutrients 2022, 14, 1444. [Google Scholar] [CrossRef]
- Shin, K.-C.; Seo, M.-J.; Kim, Y.-S.; Yeom, S.-J. Molecular Properties of β-Carotene Oxygenases and Their Potential in Industrial Production of Vitamin A and Its Derivatives. Antioxidants 2022, 11, 1180. [Google Scholar] [CrossRef]
- Harrison, E.H. Carotenoids, β-Apocarotenoids, and Retinoids: The Long and the Short of It. Nutrients 2022, 14, 1411. [Google Scholar] [CrossRef]
- Szymański, Ł.; Skopek, R.; Palusińska, M.; Schenk, T.; Stengel, S.; Lewicki, S.; Kraj, L.; Kamiński, P.; Zelent, A. Retinoic Acid and Its Derivatives in Skin. Cells 2020, 9, 2660. [Google Scholar] [CrossRef] [PubMed]
- Everts, H.B.; Akuailou, E.-N. Retinoids in Cutaneous Squamous Cell Carcinoma. Nutrients 2021, 13, 153. [Google Scholar] [CrossRef]
- Zsikó, S.; Csányi, E.; Kovács, A.; Budai-Szűcs, M.; Gácsi, A.; Berkó, S. Methods to Evaluate Skin Penetration In Vitro. Sci. Pharm. 2019, 87, 19. [Google Scholar] [CrossRef]
- Hasan, M.; Khatun, A.; Kogure, K. Iontophoresis of Biological Macromolecular Drugs. Pharmaceutics 2022, 14, 525. [Google Scholar] [CrossRef] [PubMed]
- Conserva, M.R.; Anelli, L.; Zagaria, A.; Specchia, G.; Albano, F. The Pleiotropic Role of Retinoic Acid/Retinoic Acid Receptors Signaling: From Vitamin A Metabolism to Gene Rearrangements in Acute Promyelocytic Leukemia. Int. J. Mol. Sci. 2019, 20, 2921. [Google Scholar] [CrossRef] [PubMed]
- Zasada, M.; Budzisz, E. Retinoids: Active Molecules Influencing Skin Structure Formation in Cosmetic and Dermatological Treatments. Postep. Dermatol. Alergol. 2019, 36, 392–397. [Google Scholar] [CrossRef]
- Saubenova, M.; Rapoport, A.; Venkatachalam, M.; Dufossé, L.; Yermekbay, Z.; Oleinikova, Y. Production of Carotenoids by Microorganisms. Fermentation 2024, 10, 502. [Google Scholar] [CrossRef]
- Lešić, S.; Ivanišević, Z.; Špiljak, B.; Tomas, M.; Šoštarić, M.; Včev, A. The Impact of Vitamin Deficiencies on Oral Manifestations in Children. Dent. J. 2024, 12, 109. [Google Scholar] [CrossRef]
- Rathi, R.; Sanshita; Kumar, A.; Vishvakarma, V.; Huanbutta, K.; Singh, I.; Sangnim, T. Advancements in Rectal Drug Delivery Systems: Clinical Trials, and Patents Perspective. Pharmaceutics 2022, 14, 2210. [Google Scholar] [CrossRef]
- Cholidis, P.; Kranas, D.; Chira, A.; Galouni, E.A.; Adamantidi, T.; Anastasiadou, C.; Tsoupras, A. Shrimp Lipid Bioactives with Anti-Inflammatory, Antithrombotic, and Antioxidant Health-Promoting Properties for Cardio-Protection. Mar. Drugs 2024, 22, 554. [Google Scholar] [CrossRef]
- Baeza-Morales, A.; Medina-García, M.; Martínez-Peinado, P.; Pascual-García, S.; Pujalte-Satorre, C.; López-Jaén, A.B.; Martínez-Espinosa, R.M.; Sempere-Ortells, J.M. The Antitumour Mechanisms of Carotenoids: A Comprehensive Review. Antioxidants 2024, 13, 1060. [Google Scholar] [CrossRef]
- Quan, T. Human Skin Aging and the Anti-Aging Properties of Retinol. Biomolecules 2023, 13, 1614. [Google Scholar] [CrossRef]
- Andreazzoli, M.; Longoni, B.; Angeloni, D.; Demontis, G.C. Retinoid Synthesis Regulation by Retinal Cells in Health and Disease. Cells 2024, 13, 871. [Google Scholar] [CrossRef] [PubMed]
- Kaulmann, A.; Bohn, T. Carotenoids, Inflammation, and Oxidative Stress--Implications of Cellular Signaling Pathways and Relation to Chronic Disease Prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation, Not Cholesterol, Is a Cause of Chronic Disease. Nutrients 2018, 10, 604. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R.; Redfern, S.; Tsoupras, A.; Zabetakis, I. Inflammation and Cardiovascular Disease: Are Marine Phospholipids the Answer? Food Funct. 2020, 11, 2861–2885. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.; Adamantidi, T.; Finos, M.A.; Philippopoulos, A.; Detopoulou, P.; Tsopoki, I.; Kynatidou, M.; Demopoulos, C.A. Re-Assessing the Role of Platelet Activating Factor and Its Inflammatory Signaling and Inhibitors in Cancer and Anti-Cancer Strategies. FBL 2024, 29, 345. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, L.; Yue, L.; Huang, Y.; Wang, B.; Liu, P. Uncovering Key Mechanisms and Intervention Therapies in Aging Skin. Cytokine Growth Factor Rev. 2024, 79, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Polcz, M.E.; Barbul, A. The Role of Vitamin A in Wound Healing. Nutr. Clin. Pract. 2019, 34, 695–700. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Mendonca, P.; Messeha, S.S.; Oriaku, E.T.; Soliman, K.F.A. The Anticancer Effects of Marine Carotenoid Fucoxanthin through Phosphatidylinositol 3-Kinase (PI3K)-AKT Signaling on Triple-Negative Breast Cancer Cells. Molecules 2024, 29, 61. [Google Scholar] [CrossRef]
- Park, H.-A.; Hayden, M.M.; Bannerman, S.; Jansen, J.; Crowe-White, K.M. Anti-Apoptotic Effects of Carotenoids in Neurodegeneration. Molecules 2020, 25, 3453. [Google Scholar] [CrossRef]
- Tozer, S.; O’Mahony, C.; Hannah, J.; O’Brien, J.; Kelly, S.; Kosemund-Meynen, K.; Alexander-White, C. Aggregate Exposure Modelling of Vitamin A from Cosmetic Products, Diet and Food Supplements. Food Chem. Toxicol. 2019, 131, 110549. [Google Scholar] [CrossRef]
- Tsimidou, M.Z.; Mantzouridou, F.T.; Nenadis, N. Chapter Two—Minor Bioactive Lipids. In Advances in Food and Nutrition Research; Gallegos, C., Ruiz-Méndez, M.-V., Eds.; Dietary Lipids: Nutritional and Technological Aspects; Academic Press: Cambridge, MA, USA, 2023; Volume 105, pp. 51–95. [Google Scholar]
- Islam, F.; Khan, J.; Zehravi, M.; Das, R.; Haque, M.A.; Banu, A.; Parwaiz, S.; Nainu, F.; Nafady, M.H.; Shahriar, S.M.S.; et al. Synergistic Effects of Carotenoids: Therapeutic Benefits on Human Health. Process Biochem. 2024, 136, 254–272. [Google Scholar] [CrossRef]
- Bufka, J.; Vaňková, L.; Sýkora, J.; Křížková, V. Exploring Carotenoids: Metabolism, Antioxidants, and Impacts on Human Health. J. Funct. Foods 2024, 118, 106284. [Google Scholar] [CrossRef]
- Puri, V.; Nagpal, M.; Singh, I.; Singh, M.; Dhingra, G.A.; Huanbutta, K.; Dheer, D.; Sharma, A.; Sangnim, T. A Comprehensive Review on Nutraceuticals: Therapy Support and Formulation Challenges. Nutrients 2022, 14, 4637. [Google Scholar] [CrossRef] [PubMed]
- Morganti, P.; Morganti, G.; Gagliardini, A.; Lohani, A. From Cosmetics to Innovative Cosmeceuticals—Non-Woven Tissues as New Biodegradable Carriers. Cosmetics 2021, 8, 65. [Google Scholar] [CrossRef]
- Hoang, H.T.; Moon, J.-Y.; Lee, Y.-C. Natural Antioxidants from Plant Extracts in Skincare Cosmetics: Recent Applications, Challenges and Perspectives. Cosmetics 2021, 8, 106. [Google Scholar] [CrossRef]
- Pérez-Sánchez, A.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Nutraceuticals for Skin Care: A Comprehensive Review of Human Clinical Studies. Nutrients 2018, 10, 403. [Google Scholar] [CrossRef]
- Ahmed, I.A.; Mikail, M.A. Diet and Skin Health: The Good and the Bad. Nutrition 2024, 119, 112350. [Google Scholar] [CrossRef]
- Balić, A.; Mokos, M. Do We Utilize Our Knowledge of the Skin Protective Effects of Carotenoids Enough? Antioxidants 2019, 8, 259. [Google Scholar] [CrossRef] [PubMed]
- Galasso, C.; Corinaldesi, C.; Sansone, C. Carotenoids from Marine Organisms: Biological Functions and Industrial Applications. Antioxidants 2017, 6, 96. [Google Scholar] [CrossRef]
- Peng, J.; Yuan, J.-P.; Wu, C.-F.; Wang, J.-H. Fucoxanthin, a Marine Carotenoid Present in Brown Seaweeds and Diatoms: Metabolism and Bioactivities Relevant to Human Health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef]
- Kontaxi, N.-I.; Panoutsopoulou, E.; Ofrydopolou, A.; Tsoupras, A. Anti-Inflammatory Benefits of Grape Pomace and Tomato Bioactives as Ingredients in Sun Oils against UV Radiation for Skin Protection. Appl. Sci. 2024, 14, 6236. [Google Scholar] [CrossRef]
- Michalak, M. Plant-Derived Antioxidants: Significance in Skin Health and the Ageing Process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef]
- Darvin, M.E.; Fluhr, J.W.; Meinke, M.C.; Zastrow, L.; Sterry, W.; Lademann, J. Topical Beta-Carotene Protects against Infra-Red-Light-Induced Free Radicals. Exp. Dermatol. 2011, 20, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Carotenoids: New Applications of “Old” Pigments. Phyton-Int. J. Exp. Bot. 2021, 90, 1041–1062. [CrossRef]
- Baran, M.T.; Miziak, P.; Bonio, K. Characteristics of Carotenoids and Their Use in the Cosmetics Industry. J. Educ. Health Sport 2020, 10, 192–196. [Google Scholar] [CrossRef]
- Meinke, M.C.; Friedrich, A.; Tscherch, K.; Haag, S.F.; Darvin, M.E.; Vollert, H.; Groth, N.; Lademann, J.; Rohn, S. Influence of Dietary Carotenoids on Radical Scavenging Capacity of the Skin and Skin Lipids. Eur. J. Pharm. Biopharm. 2013, 84, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Mayne, S.T.; Cartmel, B.; Scarmo, S.; Jahns, L.; Ermakov, I.V.; Gellermann, W. Resonance Raman Spectroscopic Evaluation of Skin Carotenoids as a Biomarker of Carotenoid Status for Human Studies. Arch. Biochem. Biophys. 2013, 539, 163–170. [Google Scholar] [CrossRef]
- Grether-Beck, S.; Marini, A.; Jaenicke, T.; Stahl, W.; Krutmann, J. Molecular Evidence That Oral Supplementation with Lycopene or Lutein Protects Human Skin against Ultraviolet Radiation: Results from a Double-blinded, Placebo-controlled, Crossover Study. Br. J. Dermatol. 2017, 176, 1231–1240. [Google Scholar] [CrossRef]
- Nishino, A.; Sugimoto, K.; Sambe, H.; Ichihara, T.; Takaha, T.; Kuriki, T. Effects of Dietary Paprika Xanthophylls on Ultraviolet Light-Induced Skin Damage: A Double-Blind Placebo-Controlled Study. J. Oleo Sci. 2018, 67, 863–869. [Google Scholar] [CrossRef]
- De Cássia Dias Mendes-Silva, T.; Fontenele da Silva Andrade, R.; Ootani, M.A.; Mendes, P.V.D.; de Queiroz Cavalcanti de Sá, R.A.; da Silva, M.R.F.; Souza, K.S.; dos Santos Correia, M.T.; de Oliveira, M.B.M. Biotechnological Potential of Carotenoids Produced by Extremophilic Microorganisms and Application Prospects for the Cosmetics Industry. Adv. Microbiol. 2020, 10, 397–410. [Google Scholar] [CrossRef]
- Motamedi, M.; Chehade, A.; Sanghera, R.; Grewal, P. A Clinician’s Guide to Topical Retinoids. J. Cutan. Med. Surg. 2022, 26, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Leyden, J.J.; Tanghetti, E.A.; Miller, B.; Ung, M.; Berson, D.; Lee, J. Once-Daily Tazarotene 0.1 % Gel versus Once-Daily Tretinoin 0.1 % Microsponge Gel for the Treatment of Facial Acne Vulgaris: A Double-Blind Randomized Trial. Cutis 2002, 69, 12–19. [Google Scholar]
- Bagatin, E.; de Sá Gonçalves, H.; Sato, M.; Almeida, L.M.C.; Miot, H.A. Comparable Efficacy of Adapalene 0.3% Gel and Tretinoin 0.05% Cream as Treatment for Cutaneous Photoaging. Eur. J. Dermatol. 2018, 28, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.; Kaur, J.; Masand, P.; Anurag; Parihar, V.K.; Sharma, A. Vitamins Strategies for Psoriasis: An Update on Current Scientific Evidence. J. Holist. Integr. Pharm. 2023, 4, 299–309. [Google Scholar] [CrossRef]
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in Skin Health, Repair, and Disease: A Comprehensive Review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef] [PubMed]
- Sauer, L.; Li, B.; Bernstein, P.S. Ocular Carotenoid Status in Health and Disease. Annu. Rev. Nutr. 2019, 39, 95–120. [Google Scholar] [CrossRef] [PubMed]
- Rotter, A.; Varamogianni-Mamatsi, D.; Zvonar Pobirk, A.; Gosenca Matjaž, M.; Cueto, M.; Díaz-Marrero, A.R.; Jónsdóttir, R.; Sveinsdóttir, K.; Catalá, T.S.; Romano, G.; et al. Marine Cosmetics and the Blue Bioeconomy: From Sourcing to Success Stories. iScience 2024, 27, 111339. [Google Scholar] [CrossRef]
- Papikinou, M.-A.; Pavlidis, K.; Cholidis, P.; Kranas, D.; Adamantidi, T.; Anastasiadou, C.; Tsoupras, A. Marine Fungi Bioactives with Anti-Inflammatory, Antithrombotic and Antioxidant Health-Promoting Properties Against Inflammation-Related Chronic Diseases. Mar. Drugs 2024, 22, 520. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, A.J.; Mandić, A.I.; Bantis, F.; Böhm, V.; Borge, G.I.A.; Brnčić, M.; Bysted, A.; Cano, M.P.; Dias, M.G.; Elgersma, A.; et al. A Comprehensive Review on Carotenoids in Foods and Feeds: Status Quo, Applications, Patents, and Research Needs. Crit. Rev. Food Sci. Nutr. 2022, 62, 1999–2049. [Google Scholar] [CrossRef]
- Chungchunlam, S.M.S.; Moughan, P.J. Comparative Bioavailability of Vitamins in Human Foods Sourced from Animals and Plants. Crit. Rev. Food Sci. Nutr. 2024, 64, 11590–11625. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as Natural Functional Pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef]
- Zerres, S.; Stahl, W. Carotenoids in Human Skin. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158588. [Google Scholar] [CrossRef]
- Naisi, S.; Bayat, M.; Zahraei Salehi, T.; Rahimian Zarif, B.; Yahyaraeyat, R. Antimicrobial and Anti-Biofilm Effects of Carotenoid Pigment Extracted from Rhodotorula Glutinis Strain on Food-Borne Bacteria. Iran J. Microbiol. 2023, 15, 79–88. [Google Scholar] [CrossRef]
- Larsson, S.C.; Bergkvist, L.; Näslund, I.; Rutegård, J.; Wolk, A. Vitamin A, Retinol, and Carotenoids and the Risk of Gastric Cancer: A Prospective Cohort Study2. Am. J. Clin. Nutr. 2007, 85, 497–503. [Google Scholar] [CrossRef]
- Adeyemi, K.D.; Sabow, A.B.; Ebrahimi, M.; Samsudin, A.A.; Sazili, A.Q. Fatty Acid Composition, Cholesterol and Antioxidant Status of Infraspinatus Muscle, Liver and Kidney of Goats Fed Blend of Palm Oil and Canola Oil. Ital. J. Anim. Sci. 2016, 15, 181–190. [Google Scholar] [CrossRef]
- Erkiert-Polguj, A.; Kazimierska, K.; Kalinowska-Lis, U. Assessment of the Impact of a Cosmetic Product with Sheep Colostrum on Acne Skin. Appl. Sci. 2024, 14, 2199. [Google Scholar] [CrossRef]
- Flegler, A.; Runzheimer, K.; Kombeitz, V.; Mänz, A.T.; Heidler von Heilborn, D.; Etzbach, L.; Schieber, A.; Hölzl, G.; Hüttel, B.; Woehle, C.; et al. Arthrobacter bussei sp. nov., a Pink-Coloured Organism Isolated from Cheese Made of Cow’s Milk. Int. J. Syst. Evol. Microbiol. 2020, 70, 3027–3036. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, M.N.; Valenzuela, F.; Robles, A.E.; Artalejo, E.; Aguilar, D.; Andersen, C.J.; Valdez, H.; Fernandez, M.L. One Egg per Day Improves Inflammation When Compared to an Oatmeal-Based Breakfast without Increasing Other Cardiometabolic Risk Factors in Diabetic Patients. Nutrients 2015, 7, 3449–3463. [Google Scholar] [CrossRef]
- Ballesteros, M.N.; Cabrera, R.M.; Saucedo, S.; Fernandez, M.L. Two Eggs per Day Increase Plasma Lutein and Zeaxanthin in a Pediatric Population Characterized by Low Intake of Fruits and Vegetables. J. Adv. Med. Med. Res. 2013, 3, 2203–2213. [Google Scholar] [CrossRef]
- Kelly, E.R.; Plat, J.; Haenen, G.R.M.M.; Kijlstra, A.; Berendschot, T.T.J.M. The Effect of Modified Eggs and an Egg-Yolk Based Beverage on Serum Lutein and Zeaxanthin Concentrations and Macular Pigment Optical Density: Results from a Randomized Trial. PLoS ONE 2014, 9, e92659. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo-Baños, M.; Garbayo, I.; Vílchez, C.; Bonete, M.J.; Martínez-Espinosa, R.M. Carotenoids from Haloarchaea and Their Potential in Biotechnology. Mar. Drugs 2015, 13, 5508–5532. [Google Scholar] [CrossRef] [PubMed]
- Gebregziabher, B.S.; Gebremeskel, H.; Debesa, B.; Ayalneh, D.; Mitiku, T.; Wendwessen, T.; Habtemariam, E.; Nur, S.; Getachew, T. Carotenoids: Dietary Sources, Health Functions, Biofortification, Marketing Trend and Affecting Factors—A Review. J. Agric. Food Res. 2023, 14, 100834. [Google Scholar] [CrossRef]
- Miao, Q.; Si, X.; Zhao, Q.; Zhang, H.; Qin, Y.; Tang, C.; Zhang, J. Deposition and Enrichment of Carotenoids in Livestock Products: An Overview. Food Chem. X 2024, 21, 101245. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.-F.; Liao, J.-Y.; Lu, Y.-Y.; Han, Y.-C.; Shen, Y.-C.; Venkatakrishnan, K.; Golovinskaia, O.; Wang, C.-K. Anti-Proliferative, Anti-Inflammatory and pro-Apoptotic Effects of Dunaliella Salina on Human KB Oral Carcinoma Cells. J. Food Biochem. 2017, 41, e12349. [Google Scholar] [CrossRef]
- Teixé-Roig, J.; Oms-Oliu, G.; Odriozola-Serrano, I.; Martín-Belloso, O. Enhancing in Vivo Retinol Bioavailability by Incorporating β-Carotene from Alga Dunaliella salina into Nanoemulsions Containing Natural-Based Emulsifiers. Food Res. Int. 2023, 164, 112359. [Google Scholar] [CrossRef]
- Havas, F.; Krispin, S.; Cohen, M.; Loing, E.; Farge, M.; Suere, T.; Attia-Vigneau, J. A Dunaliella Salina Extract Counteracts Skin Aging under Intense Solar Irradiation Thanks to Its Antiglycation and Anti-Inflammatory Properties. Mar. Drugs 2022, 20, 104. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.-J.; Lin, J.-T.; Chen, Y.-C.; Liu, S.-C.; Lu, F.-J.; Chang, T.-J.; Wang, M.; Lin, H.-W.; Chang, Y.-Y. Suppressive Effect of Carotenoid Extract of Dunaliella salina Alga on Production of LPS-Stimulated pro-Inflammatory Mediators in RAW264.7 Cells via NF-κB and JNK Inactivation. J. Funct. Foods 2013, 5, 607–615. [Google Scholar] [CrossRef]
- Ito, N.; Seki, S.; Ueda, F. The Protective Role of Astaxanthin for UV-Induced Skin Deterioration in Healthy People—A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2018, 10, 817. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.R.; Sindhuja, H.N.; Dharmesh, S.M.; Sankar, K.U.; Sarada, R.; Ravishankar, G.A. Effective Inhibition of Skin Cancer, Tyrosinase, and Antioxidative Properties by Astaxanthin and Astaxanthin Esters from the Green Alga Haematococcus Pluvialis. J. Agric. Food Chem. 2013, 61, 3842–3851. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.-S.; Cho, H.H.; Cho, S.; Lee, S.-R.; Shin, M.-H.; Chung, J.H. Supplementing with Dietary Astaxanthin Combined with Collagen Hydrolysate Improves Facial Elasticity and Decreases Matrix Metalloproteinase-1 and -12 Expression: A Comparative Study with Placebo. J. Med. Food 2014, 17, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z. Astaxanthin Improves Serum Cytokine Expression and Semen Quality of Diabetes Mellitus KKAy Mice. Chem. Biol. Interact. 2020, 332, 109303. [Google Scholar] [CrossRef] [PubMed]
- Nazarudin, M.F.; Yasin, I.S.M.; Mazli, N.A.I.N.; Saadi, A.R.; Azizee, M.H.S.; Nooraini, M.A.; Saad, N.; Ferdous, U.T.; Fakhrulddin, I.M. Preliminary Screening of Antioxidant and Cytotoxic Potential of Green Seaweed, Halimeda opuntia (Linnaeus) Lamouroux. Saudi J. Biol. Sci. 2022, 29, 2698–2705. [Google Scholar] [CrossRef] [PubMed]
- Shih, P.-H.; Shiue, S.-J.; Chen, C.-N.; Cheng, S.-W.; Lin, H.-Y.; Wu, L.-W.; Wu, M.-S. Fucoidan and Fucoxanthin Attenuate Hepatic Steatosis and Inflammation of NAFLD through Modulation of Leptin/Adiponectin Axis. Mar. Drugs 2021, 19, 148. [Google Scholar] [CrossRef]
- Sun, G.; Xin, T.; Zhang, R.; Liu, C.; Pang, Q. Fucoxanthin Attenuates Behavior Deficits and Neuroinflammatory Response in 1-Methyl-4-Phenyl-1,2,3,6 -Tetrahydropyridine-Induced Parkinson’s Disease in Mice. Pharmacogn. Mag. 2020, 16, 51–56. [Google Scholar] [CrossRef]
- Hitoe, S.; Shimoda, H. Seaweed Fucoxanthin Supplementation Improves Obesity Parameters in Mild Obese Japanese Subjects. Funct. Foods Health Dis. 2017, 7, 246–262. [Google Scholar] [CrossRef]
- Wang, P.-T.; Sudirman, S.; Hsieh, M.-C.; Hu, J.-Y.; Kong, Z.-L. Oral Supplementation of Fucoxanthin-Rich Brown Algae Extract Ameliorates Cisplatin-Induced Testicular Damage in Hamsters. Biomed. Pharmacother. 2020, 125, 109992. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Lee, Y.-J.; Chou, M.-C.; Chang, R.; Chiu, C.-H.; Liang, Y.-J.; Wu, L.-S. Astaxanthin Protects Steroidogenesis from Hydrogen Peroxide-Induced Oxidative Stress in Mouse Leydig Cells. Mar. Drugs 2015, 13, 1375–1388. [Google Scholar] [CrossRef]
- Serra, A.T.; Silva, S.D.; Pleno de Gouveia, L.; Alexandre, A.M.R.C.; Pereira, C.V.; Pereira, A.B.; Partidário, A.C.; Silva, N.E.; Bohn, T.; Gonçalves, V.S.S.; et al. A Single Dose of Marine Chlorella Vulgaris Increases Plasma Concentrations of Lutein, β-Carotene and Zeaxanthin in Healthy Male Volunteers. Antioxidants 2021, 10, 1164. [Google Scholar] [CrossRef]
- Lopes, G.; Clarinha, D.; Vasconcelos, V. Carotenoids from Cyanobacteria: A Biotechnological Approach for the Topical Treatment of Psoriasis. Microorganisms 2020, 8, 302. [Google Scholar] [CrossRef]
- Pagels, F.; Salvaterra, D.; Amaro, H.M.; Lopes, G.; Sousa-Pinto, I.; Vasconcelos, V.; Guedes, A.C. Bioactive Potential of Cyanobium sp. Pigment-Rich Extracts. J. Appl. Phycol. 2020, 32, 3031–3040. [Google Scholar] [CrossRef]
- Morone, J.; Lopes, G.; Preto, M.; Vasconcelos, V.; Martins, R. Exploitation of Filamentous and Picoplanktonic Cyanobacteria for Cosmetic Applications: Potential to Improve Skin Structure and Preserve Dermal Matrix Components. Mar. Drugs 2020, 18, 486. [Google Scholar] [CrossRef] [PubMed]
- Squillaci, G.; Parrella, R.; Carbone, V.; Minasi, P.; La Cara, F.; Morana, A. Carotenoids from the Extreme Halophilic Archaeon Haloterrigena Turkmenica: Identification and Antioxidant Activity. Extremophiles 2017, 21, 933–945. [Google Scholar] [CrossRef]
- Caimi, A.T.; Yasynska, O.; Rivas Rojas, P.C.; Romero, E.L.; Morilla, M.J. Improved Stability and Biological Activity of Bacterioruberin in Nanovesicles. J. Drug Deliv. Sci. Technol. 2022, 77, 103896. [Google Scholar] [CrossRef]
- Sahli, K.; Gomri, M.A.; Esclapez, J.; Gómez-Villegas, P.; Ghennai, O.; Bonete, M.-J.; León, R.; Kharroub, K. Bioprospecting and Characterization of Pigmented Halophilic Archaeal Strains from Algerian Hypersaline Environments with Analysis of Carotenoids Produced by Halorubrum sp. BS2. J. Basic Microbiol. 2020, 60, 624–638. [Google Scholar] [CrossRef]
- Safarpour, A.; Ebrahimi, M.; Shahzadeh Fazeli, S.A.; Amoozegar, M.A. Supernatant Metabolites from Halophilic Archaea to Reduce Tumorigenesis in Prostate Cancer In-Vitro and In-Vivo. Iran J. Pharm. Res. 2019, 18, 241–253. [Google Scholar]
- Von Oppen-Bezalel, L.; Fishbein, D.; Havas, F.; Ben-Chitrit, O.; Khaiat, A. The Photoprotective Effects of a Food Supplement Tomato Powder Rich in Phytoene and Phytofluene, the Colorless Carotenoids, a Preliminary Study. Glob. Dermatol. 2015, 2, 4. [Google Scholar] [CrossRef][Green Version]
- Okonogi, S.; Riangjanapatee, P. Physicochemical Characterization of Lycopene-Loaded Nanostructured Lipid Carrier Formulations for Topical Administration. Int. J. Pharm. 2015, 478, 726–735. [Google Scholar] [CrossRef]
- Groten, K.; Marini, A.; Grether-Beck, S.; Jaenicke, T.; Ibbotson, S.H.; Moseley, H.; Ferguson, J.; Krutmann, J. Tomato Phytonutrients Balance UV Response: Results from a Double-Blind, Randomized, Placebo-Controlled Study. Skin Pharmacol. Physiol. 2019, 32, 101–108. [Google Scholar] [CrossRef]
- Sokoloski, L.; Borges, M.; Bagatin, E. Lycopene Not in Pill, nor in Natura Has Photoprotective Systemic Effect. Arch. Dermatol. Res. 2015, 307, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Armoza, A.; Haim, Y.; Bashiri, A.; Wolak, T.; Paran, E. Tomato Extract and the Carotenoids Lycopene and Lutein Improve Endothelial Function and Attenuate Inflammatory NF-κB Signaling in Endothelial Cells. J. Hypertens. 2013, 31, 521–529, discussion 529. [Google Scholar] [CrossRef]
- Stajčić, S.; Ćetković, G.; Čanadanović-Brunet, J.; Djilas, S.; Mandić, A.; Četojević-Simin, D. Tomato Waste: Carotenoids Content, Antioxidant and Cell Growth Activities. Food Chem. 2015, 172, 225–232. [Google Scholar] [CrossRef]
- Havas, F.; Krispin, S.; Meléndez-Martínez, A.J.; von Oppen-Bezalel, L. Preliminary Data on the Safety of Phytoene- and Phytofluene-Rich Products for Human Use Including Topical Application. J. Toxicol. 2018, 2018, 5475784. [Google Scholar] [CrossRef]
- Vasconcelos, A.G.; Amorim, A.d.G.; dos Santos, R.C.; Souza, J.M.T.; de Souza, L.K.M.; Araújo, T.d.S.; Nicolau, L.A.D.; Carvalho, L.d.L.; de Aquino, P.E.A.; Martins, C.d.S.; et al. Lycopene Rich Extract from Red Guava (Psidium guajava L.) Displays Anti-Inflammatory and Antioxidant Profile by Reducing Suggestive Hallmarks of Acute Inflammatory Response in Mice. Food Res. Int. 2017, 99, 959–968. [Google Scholar] [CrossRef]
- Pons, E.; Alquézar, B.; Rodríguez, A.; Martorell, P.; Genovés, S.; Ramón, D.; Rodrigo, M.J.; Zacarías, L.; Peña, L. Metabolic Engineering of β-Carotene in Orange Fruit Increases Its In Vivo Antioxidant Properties. Plant Biotechnol. J. 2014, 12, 17–27. [Google Scholar] [CrossRef]
- Petito, N.d.L.; Devens, J.M.; Falcão, D.Q.; Dantas, F.M.L.; Passos, T.S.; Araujo, K.G.d.L. Nanoencapsulation of Red Bell Pepper Carotenoids: Comparison of Encapsulating Agents in an Emulsion Based System. Colorants 2022, 1, 132–148. [Google Scholar] [CrossRef]
- Marra, A.; Manousakis, V.; Zervas, G.P.; Koutis, N.; Finos, M.A.; Adamantidi, T.; Panoutsopoulou, E.; Ofrydopoulou, A.; Tsoupras, A. Avocado and Its By-Products as Natural Sources of Valuable Anti-Inflammatory and Antioxidant Bioactives for Functional Foods and Cosmetics with Health-Promoting Properties. Appl. Sci. 2024, 14, 5978. [Google Scholar] [CrossRef]
- Edwards, C.G.; Walk, A.M.; Thompson, S.V.; Reeser, G.E.; Erdman, J.W.; Burd, N.A.; Holscher, H.D.; Khan, N.A. Effects of 12-Week Avocado Consumption on Cognitive Function among Adults with Overweight and Obesity. Int. J. Psychophysiol. 2020, 148, 13–24. [Google Scholar] [CrossRef]
- Villa-Rodriguez, J.A.; Yahia, E.M.; González-León, A.; Ifie, I.; Robles-Zepeda, R.E.; Domínguez-Avila, J.A.; González-Aguilar, G.A. Ripening of ‘Hass’ Avocado Mesocarp Alters Its Phytochemical Profile and the in Vitro Cytotoxic Activity of Its Methanolic Extracts. South Afr. J. Bot. 2020, 128, 1–8. [Google Scholar] [CrossRef]
- Wang, L.; Tao, L.; Hao, L.; Stanley, T.H.; Huang, K.-H.; Lambert, J.D.; Kris-Etherton, P.M. A Moderate-Fat Diet with One Avocado per Day Increases Plasma Antioxidants and Decreases the Oxidation of Small, Dense LDL in Adults with Overweight and Obesity: A Randomized Controlled Trial. J. Nutr. 2020, 150, 276–284. [Google Scholar] [CrossRef]
- Hsu, H.-Y.; Chen, B.-H. A Comparative Study on Inhibition of Breast Cancer Cells and Tumors in Mice by Carotenoid Extract and Nanoemulsion Prepared from Sweet Potato (Ipomoea batatas L.) Peel. Pharmaceutics 2022, 14, 980. [Google Scholar] [CrossRef]
- Kavalappa, Y.P.; Gopal, S.S.; Ponesakki, G. Lutein Inhibits Breast Cancer Cell Growth by Suppressing Antioxidant and Cell Survival Signals and Induces Apoptosis. J. Cell. Physiol. 2021, 236, 1798–1809. [Google Scholar] [CrossRef] [PubMed]
- Borja-Martínez, M.; Lozano-Sánchez, J.; Borrás-Linares, I.; Pedreño, M.A.; Sabater-Jara, A.B. Revalorization of Broccoli By-Products for Cosmetic Uses Using Supercritical Fluid Extraction. Antioxidants 2020, 9, 1195. [Google Scholar] [CrossRef]
- Freitas, A.; Moldão-Martins, M.; Costa, H.S.; Albuquerque, T.G.; Valente, A.; Sanches-Silva, A. Effect of UV-C Radiation on Bioactive Compounds of Pineapple (Ananas comosus L. Merr.) by-Products. J. Sci. Food Agric. 2015, 95, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Górnaś, P.; Rudzińska, M.; Raczyk, M.; Mišina, I.; Soliven, A.; Segliņa, D. Composition of Bioactive Compounds in Kernel Oils Recovered from Sour Cherry (Prunus cerasus L.) by-Products: Impact of the Cultivar on Potential Applications. Ind. Crops Prod. 2016, 82, 44–50. [Google Scholar] [CrossRef]
- Fam, V.W.; Holt, R.R.; Keen, C.L.; Sivamani, R.K.; Hackman, R.M. Prospective Evaluation of Mango Fruit Intake on Facial Wrinkles and Erythema in Postmenopausal Women: A Randomized Clinical Pilot Study. Nutrients 2020, 12, 3381. [Google Scholar] [CrossRef]
- Diprat, A.B.; Silveira Thys, R.C.; Rodrigues, E.; Rech, R. Chlorella sorokiniana: A New Alternative Source of Carotenoids and Proteins for Gluten-Free Bread. LWT 2020, 134, 109974. [Google Scholar] [CrossRef]
- Mudroňová, D.; Karaffová, V.; Koščová, J.; Bartkovský, M.; Marcinčáková, D.; Popelka, P.; Klempová, T.; Čertík, M.; Mačanga, J.; Marcinčák, S. Effect of Fungal Gamma-Linolenic Acid and Beta-Carotene Containing Prefermented Feed on Immunity and Gut of Broiler Chicken. Poult. Sci. 2018, 97, 4211–4218. [Google Scholar] [CrossRef]
- Jimenez-Escobar, M.P.; Pascual-Mathey, L.I.; Beristain, C.I.; Flores-Andrade, E.; Jiménez, M.; Pascual-Pineda, L.A. In Vitro and In Vivo Antioxidant Properties of Paprika Carotenoids Nanoemulsions. LWT 2020, 118, 108694. [Google Scholar] [CrossRef]
- Morse, N.L.; Reid, A.-J.; St-Onge, M. An Open-Label Clinical Trial Assessing the Efficacy and Safety of Bend Skincare Anti-Aging Formula on Minimal Erythema Dose in Skin. Photodermatol. Photoimmunol. Photomed. 2018, 34, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Divya, P.; Puthusseri, B.; Neelwarne, B. Carotenoid Content, Its Stability during Drying and the Antioxidant Activity of Commercial Coriander (Coriandrum sativum L.) Varieties. Food Res. Int. 2012, 45, 342–350. [Google Scholar] [CrossRef]
- Azizi, M.; Zare, D.; Akhavan Sepahy, A.; Azin, M. Evaluating the Effect of Microbial Stimulation and Oxidative Stress on Increasing β-Carotene Production in Blakeslea Trispora. Microbiol. Metab. Biotechnol. 2021, 4, 1–11. [Google Scholar] [CrossRef]
- Naz, T.; Nosheen, S.; Li, S.; Nazir, Y.; Mustafa, K.; Liu, Q.; Garre, V.; Song, Y. Comparative Analysis of β-Carotene Production by Mucor Circinelloides Strains CBS 277.49 and WJ11 under Light and Dark Conditions. Metabolites 2020, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Viñals, N.; Alonso-Estrada, D.; Pacios-Michelena, S.; García-Cruz, A.; Ramos-González, R.; Faife-Pérez, E.; Michelena-Álvarez, L.G.; Martínez-Hernández, J.L.; Iliná, A. Current Advances in Carotenoid Production by Rhodotorula Sp. Fermentation 2024, 10, 190. [Google Scholar] [CrossRef]
- Li, J.; Bates, E.; Perera, D.S.; Palage, A.M.; Ward, V.C.A. Membrane Engineering for Carotenoid Production in Escherichia coli. SynBio 2024, 2, 349–362. [Google Scholar] [CrossRef]
- Promdonkoy, P.; Watcharawipas, A.; Bubphasawan, S.; Sansatchanon, K.; Suwanakitti, N.; Kocharin, K.; Runguphan, W. Metabolic Engineering of Saccharomyces Cerevisiae for Production of Canthaxanthin, Zeaxanthin, and Astaxanthin. J. Fungi 2024, 10, 433. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Xue, C.; Zhao, Y.; Zhang, H.; Rao, Z.; Yu, X. Blakeslea Trispora Photoreceptors: Identification and Functional Analysis. Appl. Environ. Microbiol. 2020, 86, e02962-19. [Google Scholar] [CrossRef] [PubMed]
- Mantzouridou, F.T.; Sferopoulou, E.; Thanou, P. Uncovering the Hidden Potential of Phytoene Production by the Fungus Blakeslea Trispora. Foods 2024, 13, 2882. [Google Scholar] [CrossRef] [PubMed]
- Naz, T.; Nazir, Y.; Nosheen, S.; Ullah, S.; Halim, H.; Fazili, A.B.A.; Li, S.; Mustafa, K.; Mohamed, H.; Yang, W.; et al. Redirecting Metabolic Flux towards the Mevalonate Pathway for Enhanced β-Carotene Production in M. Circinelloides CBS 277.49. BioMed Res. Int. 2020, 2020, 8890269. [Google Scholar] [CrossRef]
- Allahkarami, S.; Sepahi, A.A.; Hosseini, H.; Razavi, M.R. Isolation and Identification of Carotenoid-Producing Rhodotorula sp. from Pinaceae Forest Ecosystems and Optimization of in Vitro Carotenoid Production. Biotechnol. Rep. (Amst) 2021, 32, e00687. [Google Scholar] [CrossRef]
- Li, Z.; Li, C.; Cheng, P.; Yu, G. Rhodotorula mucilaginosa—Alternative Sources of Natural Carotenoids, Lipids, and Enzymes for Industrial Use. Heliyon 2022, 8, e11505. [Google Scholar] [CrossRef]
- Hernández-Almanza, A.; Navarro Macias V., E.; Aguilar, O.; Aguilar, M.; Aguilar, N.C. Carotenoids Extraction from Rhodotorula Glutinis Cells Using Various Techniques: A Comparative Study. Indian J. Exp. Biol. 2024, 55, 479–484. [Google Scholar]
- Liu, C.; Cui, Y.; Pi, F.; Guo, Y.; Cheng, Y.; Qian, H. Torularhodin Ameliorates Oxidative Activity in Vitro and D-Galactose-Induced Liver Injury via the Nrf2/HO-1 Signaling Pathway in Vivo. J. Agric. Food Chem. 2019, 67, 10059–10068. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Guo, Y.; Cheng, Y.; Han, M.; Zhang, W.; Qian, H. Torulene and Torularhodin, Protects Human Prostate Stromal Cells from Hydrogen Peroxide-Induced Oxidative Stress Damage through the Regulation of Bcl-2/Bax Mediated Apoptosis. Free Radic. Res. 2017, 51, 113–123. [Google Scholar] [CrossRef]
- Eliane, P.C.; Bruna, A.B.; Carolina, D.S.S.; Carlos, A.V.B.; Eliana, B.F.; Janaina, F.D.M.B. Carotenoids from Phaffia Rhodozyma: Antioxidant Activity and Stability of Extracts. Afr. J. Biotechnol. 2015, 14, 1982–1988. [Google Scholar] [CrossRef]
- Bu, X.; Lin, J.; Duan, C.; Koffas, M.A.G.; Yan, G. Dual Regulation of Lipid Droplet-Triacylglycerol Metabolism and ERG9 Expression for Improved β-Carotene Production in Saccharomyces Cerevisiae. Microb. Cell Factories 2022, 21, 3. [Google Scholar] [CrossRef]
- Hwang, C.Y.; Cho, E.-S.; Bae, E.H.; Jung, D.-H.; Seo, M.-J. Carotenoid-Producing Paracoccus aurantius sp. nov., Isolated from the West Coast of Dokdo Island, Republic of Korea. J. Microbiol. Biotechnol. 2024, 34, 2012–2022. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Ishibashi, T.; Maoka, T. Effect of Astaxanthin-Rich Extract Derived from Paracoccus Carotinifaciens on Cognitive Function in Middle-Aged and Older Individuals. J. Clin. Biochem. Nutr. 2018, 62, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Flegler, A.; Lipski, A. The C50 Carotenoid Bacterioruberin Regulates Membrane Fluidity in Pink-Pigmented Arthrobacter Species. Arch. Microbiol. 2021, 204, 70. [Google Scholar] [CrossRef]
- Hagaggi, N.S.A.; Abdul-Raouf, U.M. Production of Bioactive β-Carotene by the Endophytic Bacterium Citricoccus Parietis AUCs with Multiple in Vitro Biological Potentials. Microb. Cell Factories 2023, 22, 90. [Google Scholar] [CrossRef]
- Jinendiran, S.; Dahms, H.-U.; Dileep Kumar, B.S.; Kumar Ponnusamy, V.; Sivakumar, N. Diapolycopenedioic-Acid-Diglucosyl Ester and Keto-Myxocoxanthin Glucoside Ester: Novel Carotenoids Derived from Exiguobacterium acetylicum S01 and Evaluation of Their Anticancer and Anti-Inflammatory Activities. Bioorganic Chem. 2020, 103, 104149. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Razavi, S.H.; Mousavi, M. Carotenoid Production from Hydrolyzed Molasses by Dietzia Natronolimnaea HS-1 Using Batch, Fed-Batch and Continuous Culture. Ann. Microbiol. 2014, 64, 945–953. [Google Scholar] [CrossRef]
- De Carbon, C.B.; Thurotte, A.; Wilson, A.; Perreau, F.; Kirilovsky, D. Biosynthesis of Soluble Carotenoid Holoproteins in Escherichia Coli. Sci. Rep. 2015, 5, 9085. [Google Scholar] [CrossRef] [PubMed]
- Xinrui, D.; Bo, L.; Yihong, B.; Weifeng, L.; Yong, T. Metabolic Engineering of Escherichia Coli for High-Level Production of Violaxanthin. Microb. Cell Factories 2023, 22, 115. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.H.; Hwang, H.J.; Lee, J.E.; Oh, S.H.; Hwang, J.S.; Lee, B.Y.; Lee, P.C. Microbial Production of Retinyl Palmitate and Its Application as a Cosmeceutical. Antioxidants 2020, 9, 1130. [Google Scholar] [CrossRef]
- Gunes, S.; Tamburaci, S.; Dalay, M.C.; Deliloglu Gurhan, I. In Vitro Evaluation of Spirulina Platensis Extract Incorporated Skin Cream with Its Wound Healing and Antioxidant Activities. Pharm. Biol. 2017, 55, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Darvin, M.E.; Jung, S.; Schanzer, S.; Richter, H.; Kurth, E.; Thiede, G.; Meinke, M.C.; Lademann, J. Influence of the Systemic Application of Blue–Green Spirulina Platensis Algae on the Cutaneous Carotenoids and Elastic Fibers in Vivo. Cosmetics 2015, 2, 302–312. [Google Scholar] [CrossRef]
- McLoughlin, I.J.; Voss, A.L.; Hale, J.D.F.; Jain, R. Cosmetic Efficacy of the Topical Probiotic Micrococcus Luteus Q24 in Healthy Human Adults. Cosmetics 2024, 11, 122. [Google Scholar] [CrossRef]
- Castro-Puyana, M.; Pérez-Sánchez, A.; Valdés, A.; Ibrahim, O.H.M.; Suarez-Álvarez, S.; Ferragut, J.A.; Micol, V.; Cifuentes, A.; Ibáñez, E.; García-Cañas, V. Pressurized Liquid Extraction of Neochloris oleoabundans for the Recovery of Bioactive Carotenoids with Anti-Proliferative Activity against Human Colon Cancer Cells. Food Res. Int. 2017, 99, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Gupte, Y.; Iyer, G.; Desai, S.; Iyer, M.; Sawant, V.; Moramkar, N. Characterization of High Carotenoid Producing Coelastrella Oocystiformis and Its Anti-Cancer Potential. Int. J. Curr. Microbiol. Appl. Sceinces 2024, 4, 527–536. [Google Scholar]
- Milosheska, D.; Roškar, R. Use of Retinoids in Topical Antiaging Treatments: A Focused Review of Clinical Evidence for Conventional and Nanoformulations. Adv. Ther. 2022, 39, 5351. [Google Scholar] [CrossRef] [PubMed]
- De Souza Guedes, L.; Martinez, R.M.; Bou-Chacra, N.A.; Velasco, M.V.R.; Rosado, C.; Baby, A.R. An Overview on Topical Administration of Carotenoids and Coenzyme Q10 Loaded in Lipid Nanoparticles. Antioxidants 2021, 10, 1034. [Google Scholar] [CrossRef] [PubMed]
- Kulawik-Pióro, A.; Miastkowska, M. Polymeric Gels and Their Application in the Treatment of Psoriasis Vulgaris: A Review. Int. J. Mol. Sci. 2021, 22, 5124. [Google Scholar] [CrossRef] [PubMed]
- Elango, J.; Zamora-Ledezma, C.; Negrete-Bolagay, D.; Aza, P.N.D.; Gómez-López, V.M.; López-González, I.; Belén Hernández, A.; De Val, J.E.M.S.; Wu, W. Retinol-Loaded Poly(Vinyl Alcohol)-Based Hydrogels as Suitable Biomaterials with Antimicrobial Properties for the Proliferation of Mesenchymal Stem Cells. Int. J. Mol. Sci. 2022, 23, 15623. [Google Scholar] [CrossRef]
- Hamed, R.; Abu Nahia, B.J.; Alkilani, A.Z.; Al-Adhami, Y.; Obaidat, R. Recent Advances in Microneedling-Assisted Cosmetic Applications. Cosmetics 2024, 11, 51. [Google Scholar] [CrossRef]
- Sinopoli, A.; Caminada, S.; Isonne, C.; Santoro, M.M.; Baccolini, V. What Are the Effects of Vitamin A Oral Supplementation in the Prevention and Management of Viral Infections? A Systematic Review of Randomized Clinical Trials. Nutrients 2022, 14, 4081. [Google Scholar] [CrossRef] [PubMed]
- Al-Mekhlafi, H.M.; Al-Zabedi, E.M.; Al-Maktari, M.T.; Atroosh, W.M.; Al-Delaimy, A.K.; Moktar, N.; Sallam, A.A.; Abdullah, W.A.; Jani, R.; Surin, J. Effects of Vitamin A Supplementation on Iron Status Indices and Iron Deficiency Anaemia: A Randomized Controlled Trial. Nutrients 2014, 6, 190–206. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Lokesh, V.; Shang, X.; Shin, J.; Keum, Y.-S.; Lee, J.-H. Carotenoids: Dietary Sources, Extraction, Encapsulation, Bioavailability, and Health Benefits—A Review of Recent Advancements. Antioxidants 2022, 11, 795. [Google Scholar] [CrossRef] [PubMed]
- Conboy Stephenson, R.; Ross, R.P.; Stanton, C. Carotenoids in Milk and the Potential for Dairy Based Functional Foods. Foods 2021, 10, 1263. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Deshmukh, R.K.; Tripathi, S.; Gaikwad, K.K.; Das, S.S.; Sharma, D. Recent Advances in the Carotenoids Added to Food Packaging Films: A Review. Foods 2023, 12, 4011. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.d.C.; Passos, T.S.; Morais, A.H.A. Vitamin A Status Improvement in Obesity: Findings and Perspectives Using Encapsulation Techniques. Nutrients 2021, 13, 1921. [Google Scholar] [CrossRef] [PubMed]
- Mujica-Álvarez, J.; Gil-Castell, O.; Barra, P.A.; Ribes-Greus, A.; Bustos, R.; Faccini, M.; Matiacevich, S. Encapsulation of Vitamins A and E as Spray-Dried Additives for the Feed Industry. Molecules 2020, 25, 1357. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.; Estevinho, B.N.; Rocha, F. Microencapsulation of Vitamin A: A Review. Trends Food Sci. Technol. 2016, 51, 76–87. [Google Scholar] [CrossRef]
- Drosou, C.; Krokida, M. A Comparative Study of Encapsulation of β-Carotene via Spray-Drying and Freeze-Drying Techniques Using Pullulan and Whey Protein Isolate as Wall Material. Foods 2024, 13, 1933. [Google Scholar] [CrossRef]
- De Lima, P.M.; Dacanal, G.C.; Pinho, L.S.; de Sá, S.H.G.; Thomazini, M.; Favaro-Trindade, C.S. Combination of Spray-Chilling and Spray-Drying Techniques to Protect Carotenoid-Rich Extracts from Pumpkin (Cucurbita Moschata) Byproducts, Aiming at the Production of a Powdered Natural Food Dye. Molecules 2022, 27, 7530. [Google Scholar] [CrossRef]
- Pinho, L.S.; de Lima, P.M.; de Sá, S.H.G.; Chen, D.; Campanella, O.H.; da Costa Rodrigues, C.E.; Favaro-Trindade, C.S. Encapsulation of Rich-Carotenoids Extract from Guaraná (Paullinia Cupana) Byproduct by a Combination of Spray Drying and Spray Chilling. Foods 2022, 11, 2557. [Google Scholar] [CrossRef]
- Łupina, K.; Kowalczyk, D.; Kazimierczak, W. Gum Arabic/Gelatin and Water-Soluble Soy Polysaccharides/Gelatin Blend Films as Carriers of Astaxanthin—A Comparative Study of the Kinetics of Release and Antioxidant Properties. Polymers 2021, 13, 1062. [Google Scholar] [CrossRef] [PubMed]
- Pinna, N.; Blasi, F.; Schoubben, A. Optimization of Solid Lipid Nanoparticles for the Encapsulation of Carotenoids from Cucurbita Moschata Pulp. Eng. Proc. 2023, 37, 5. [Google Scholar] [CrossRef]
- Ortega-Regules, A.E.; Martínez-Thomas, J.A.; Schürenkämper-Carrillo, K.; de Parrodi, C.A.; López-Mena, E.R.; Mejía-Méndez, J.L.; Lozada-Ramírez, J.D. Recent Advances in the Therapeutic Potential of Carotenoids in Preventing and Managing Metabolic Disorders. Plants 2024, 13, 1584. [Google Scholar] [CrossRef]
- Torres-Montilla, S.; Rodriguez-Concepcion, M. Making Extra Room for Carotenoids in Plant Cells: New Opportunities for Biofortification. Prog. Lipid Res. 2021, 84, 101128. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Zhao, N.; Song, Q.; Du, Z.; Shu, P. Topical Retinoids: Novel Derivatives, Nano Lipid-Based Carriers, and Combinations to Improve Chemical Instability and Skin Irritation. J. Cosmet. Dermatol. 2024, 23, 3102–3115. [Google Scholar] [CrossRef] [PubMed]
- Spierings, N.M.K. Evidence for the Efficacy of Over-the-Counter Vitamin A Cosmetic Products in the Improvement of Facial Skin Aging: A Systematic Review. J. Clin. Aesthet. Dermatol. 2021, 14, 33–40. [Google Scholar] [PubMed]
- Martínez-Iglesias, O.; Naidoo, V.; Carrera, I.; Corzo, L.; Cacabelos, R. Natural Bioactive Products as Epigenetic Modulators for Treating Neurodegenerative Disorders. Pharmaceuticals 2023, 16, 216. [Google Scholar] [CrossRef]
- Acevedo, N.; Alashkar Alhamwe, B.; Caraballo, L.; Ding, M.; Ferrante, A.; Garn, H.; Garssen, J.; Hii, C.S.; Irvine, J.; Llinás-Caballero, K.; et al. Perinatal and Early-Life Nutrition, Epigenetics, and Allergy. Nutrients 2021, 13, 724. [Google Scholar] [CrossRef]
- Roche, F.C.; Harris-Tryon, T.A. Illuminating the Role of Vitamin A in Skin Innate Immunity and the Skin Microbiome: A Narrative Review. Nutrients 2021, 13, 302. [Google Scholar] [CrossRef] [PubMed]
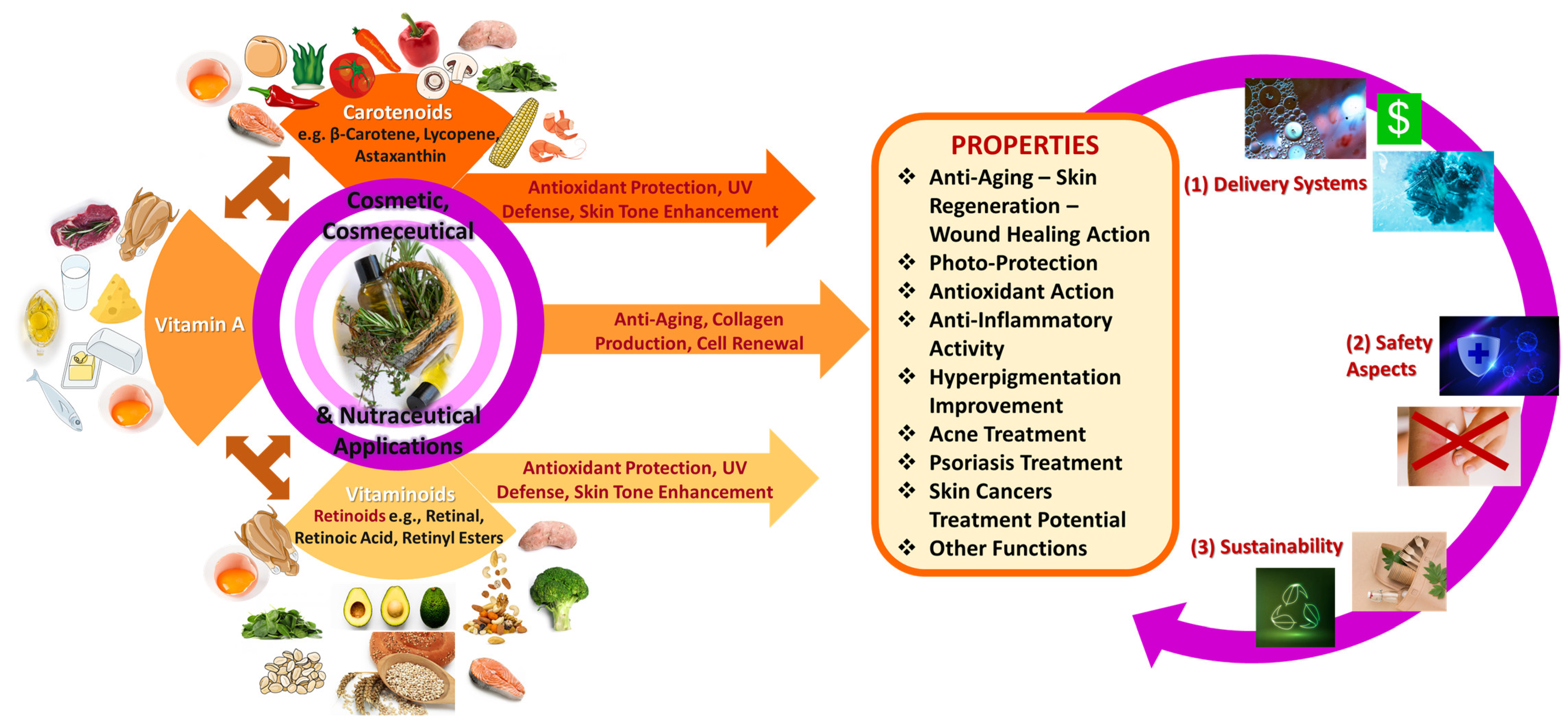
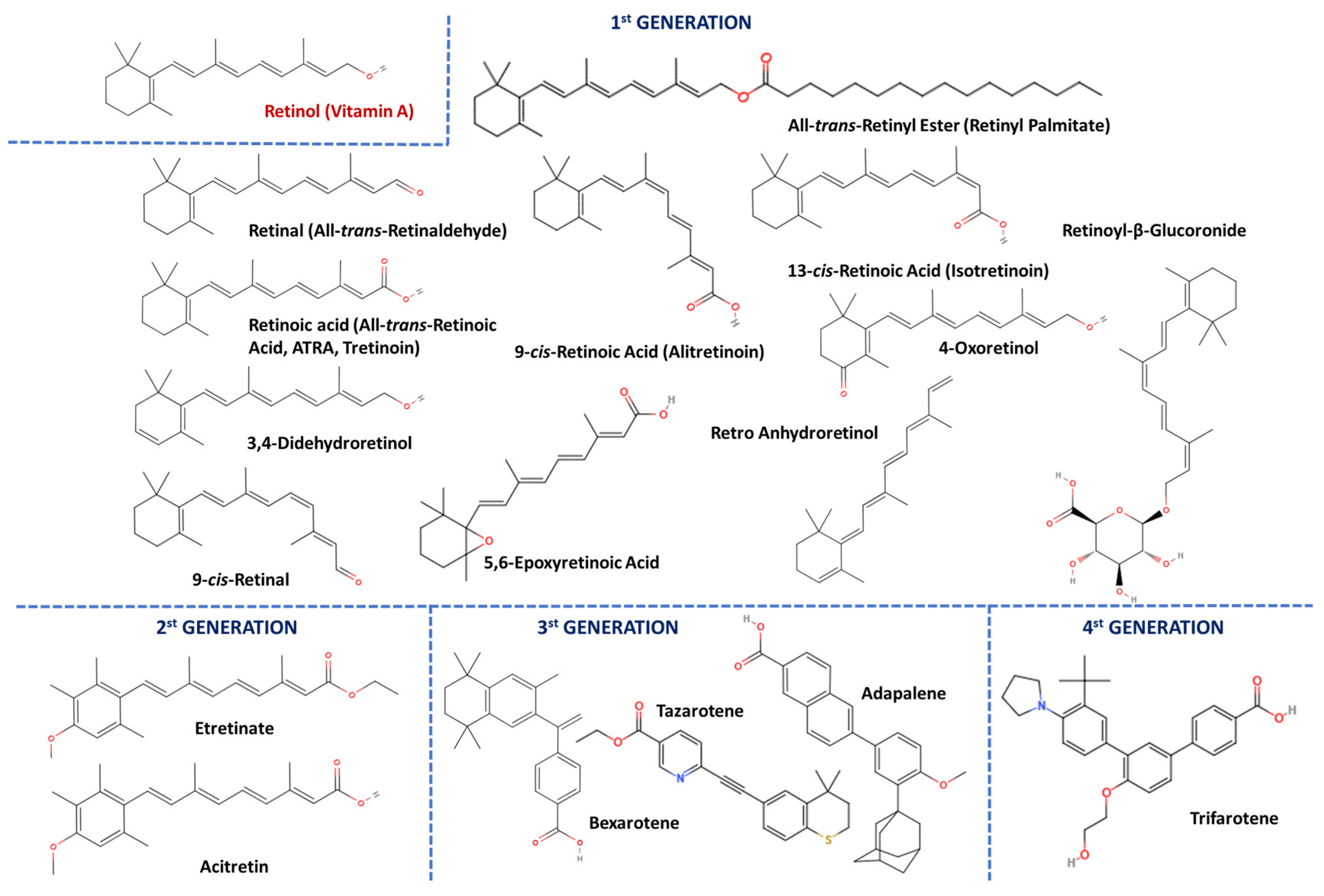
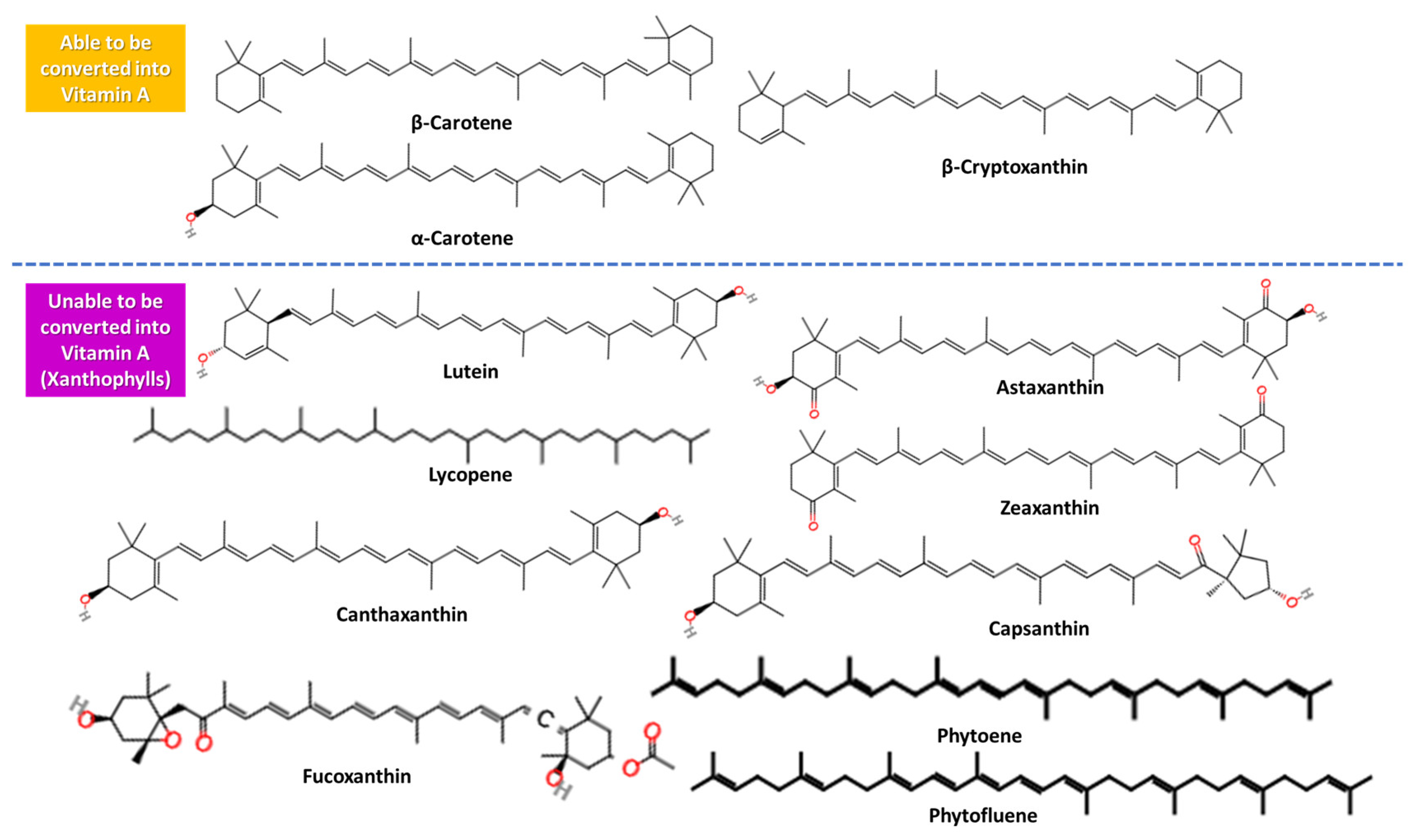

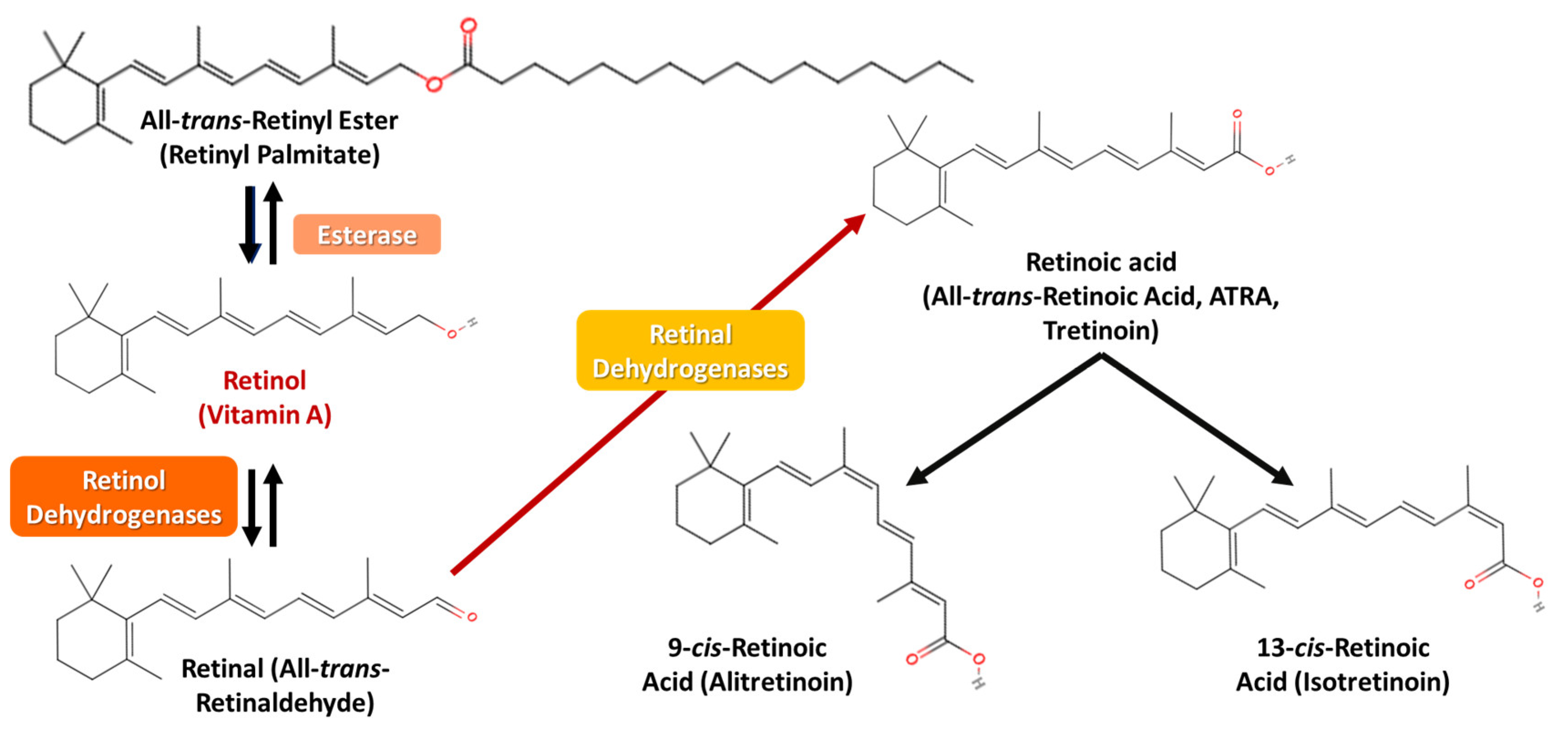
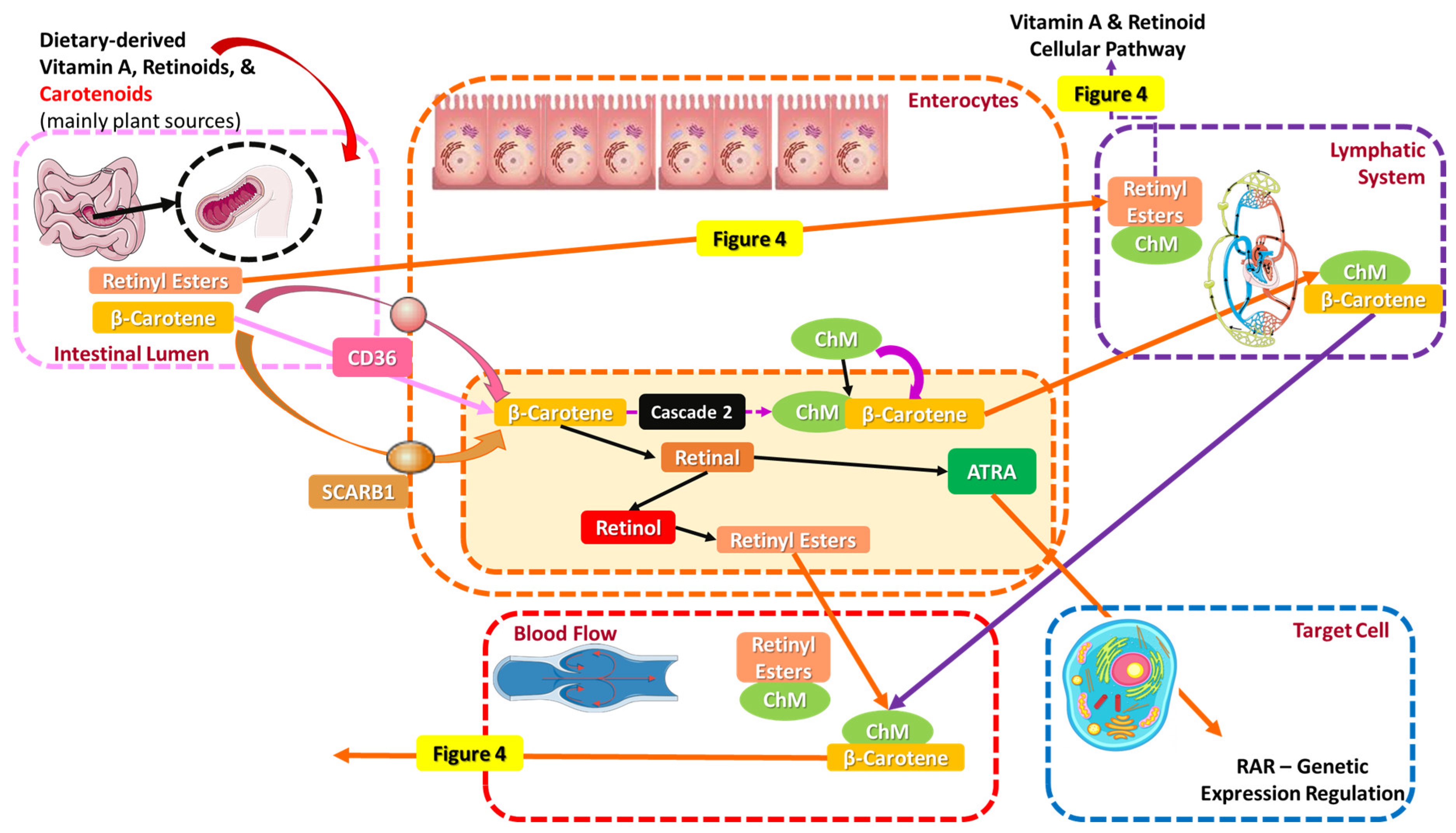

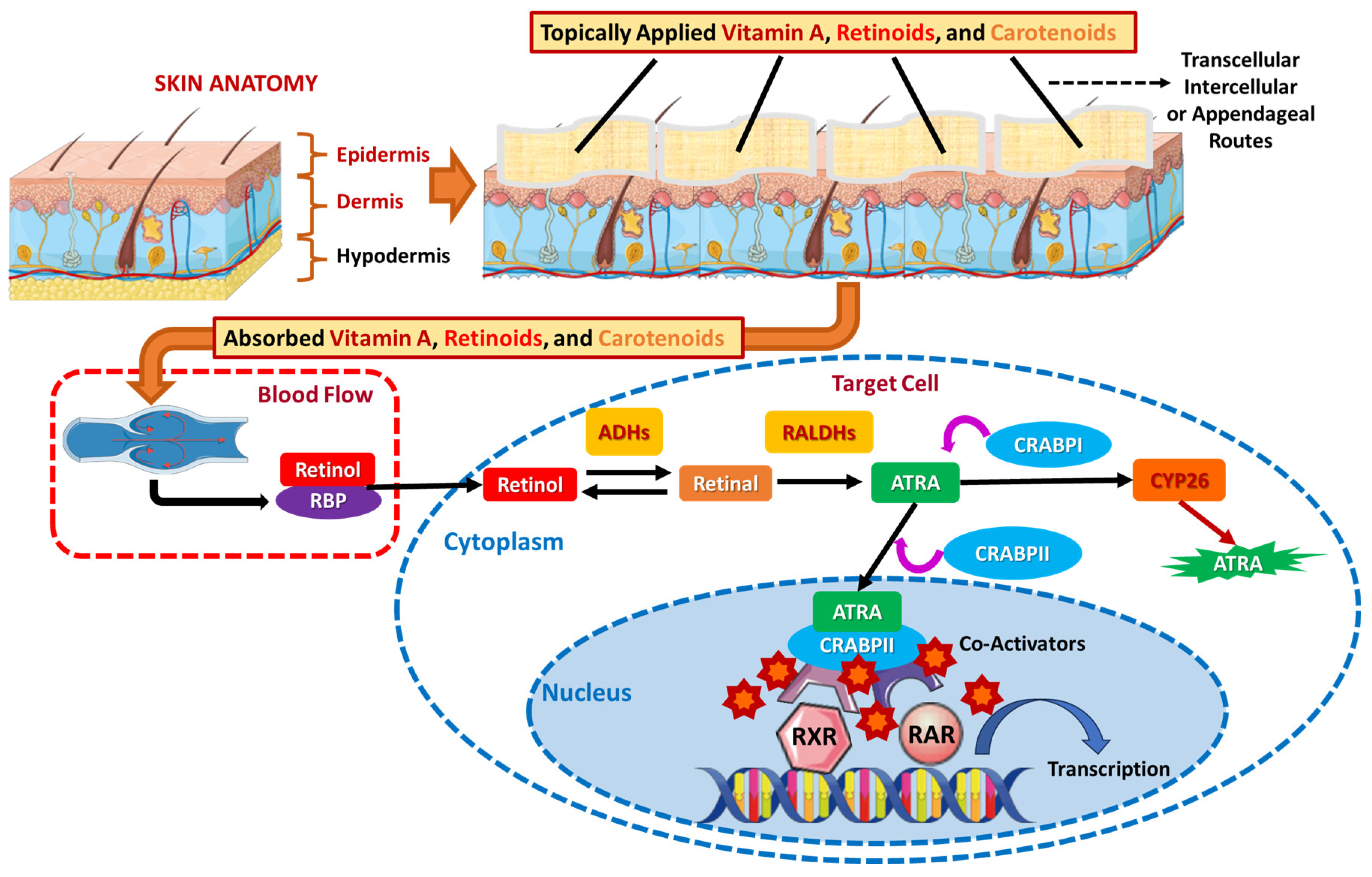
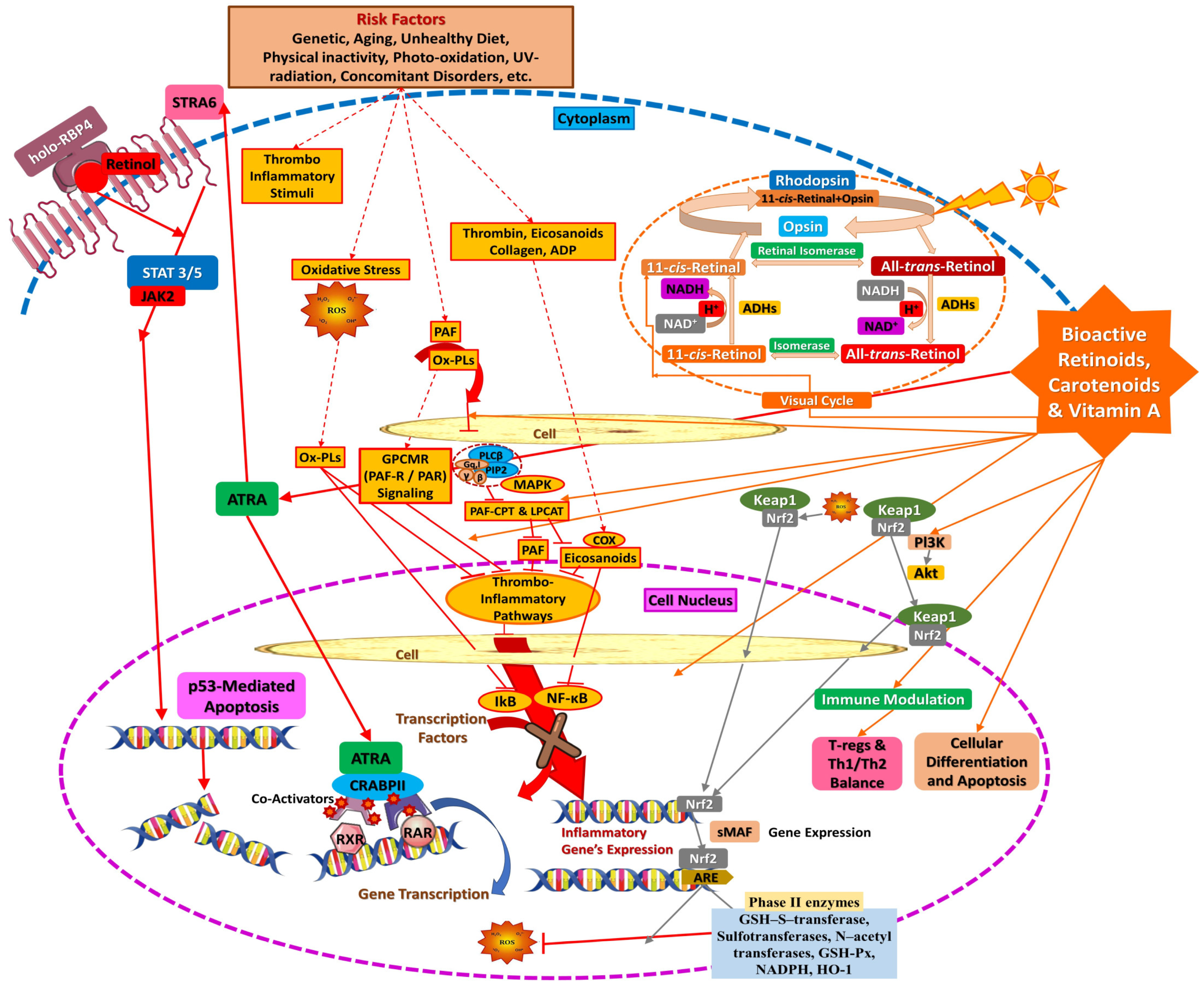

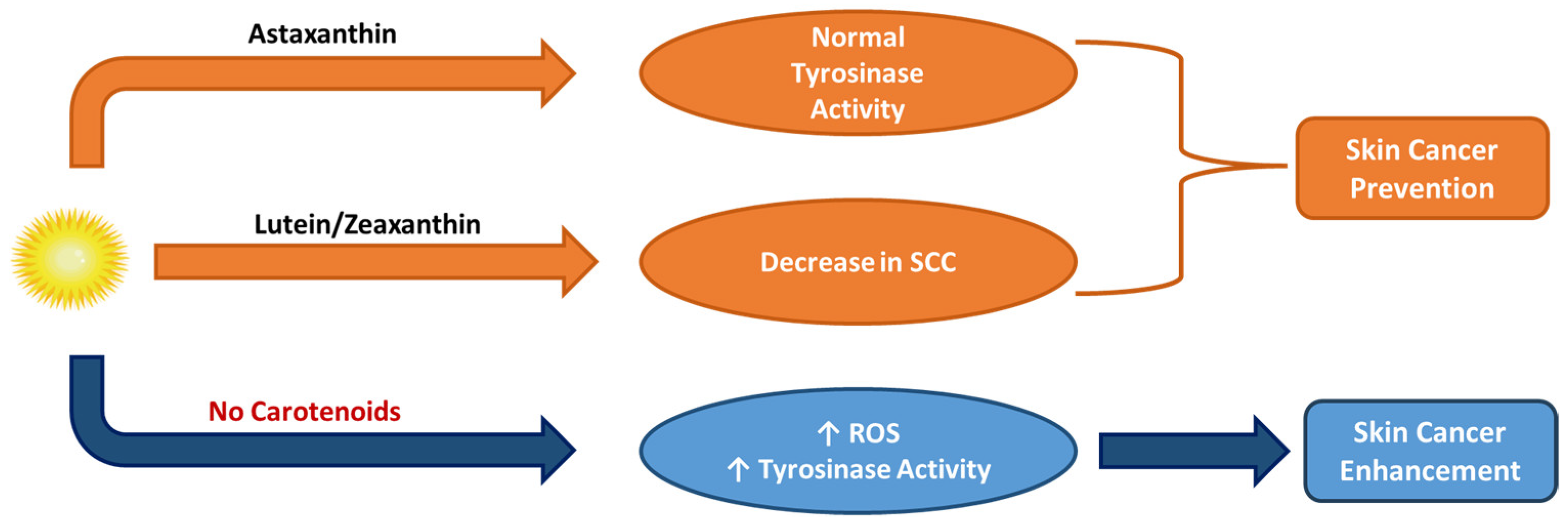
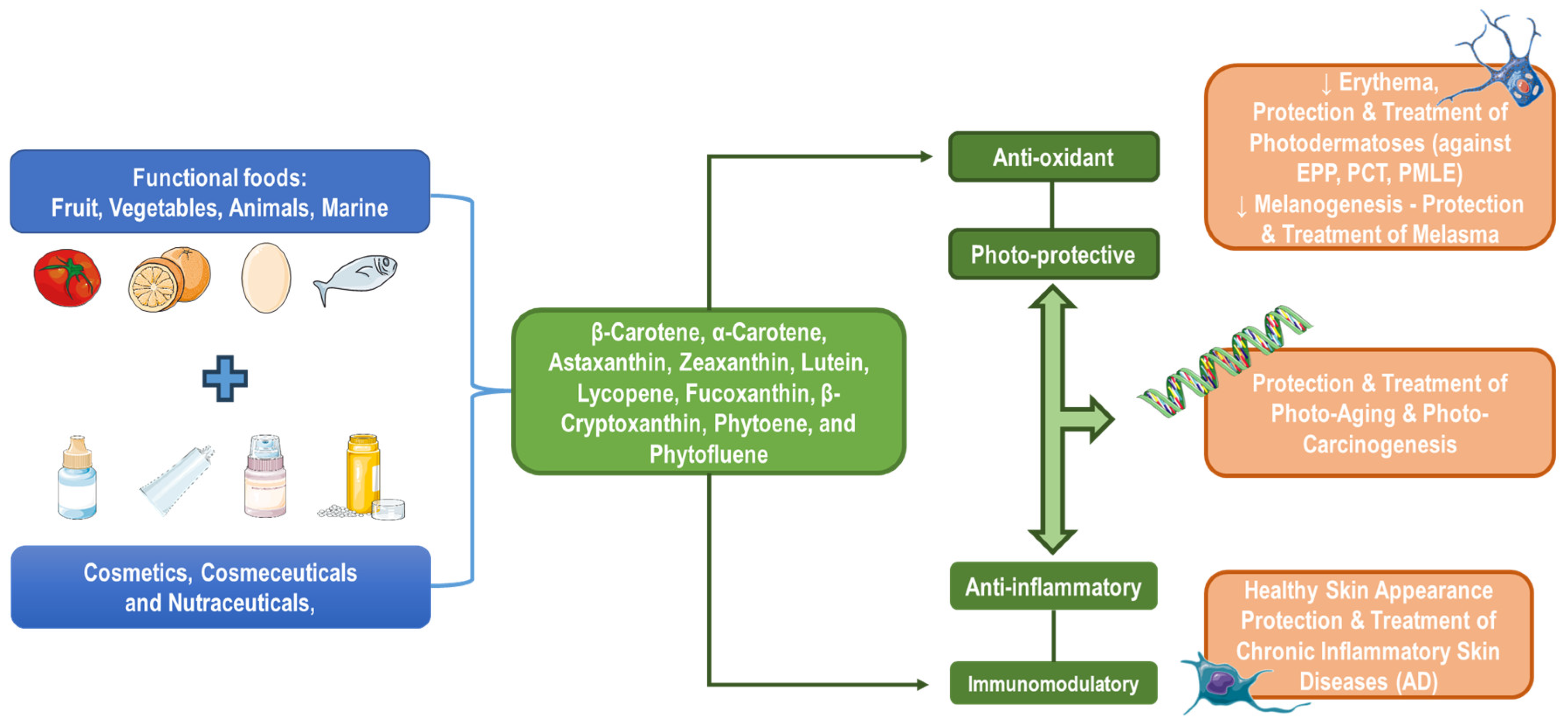

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamantidi, T.; Lafara, M.-P.; Venetikidou, M.; Likartsi, E.; Toganidou, I.; Tsoupras, A. Utilization and Bio-Efficacy of Carotenoids, Vitamin A and Its Vitaminoids in Nutricosmetics, Cosmeceuticals, and Cosmetics’ Applications with Skin-Health Promoting Properties. Appl. Sci. 2025, 15, 1657. https://doi.org/10.3390/app15031657
Adamantidi T, Lafara M-P, Venetikidou M, Likartsi E, Toganidou I, Tsoupras A. Utilization and Bio-Efficacy of Carotenoids, Vitamin A and Its Vitaminoids in Nutricosmetics, Cosmeceuticals, and Cosmetics’ Applications with Skin-Health Promoting Properties. Applied Sciences. 2025; 15(3):1657. https://doi.org/10.3390/app15031657
Chicago/Turabian StyleAdamantidi, Theodora, Maria-Parthena Lafara, Maria Venetikidou, Eleni Likartsi, Ioanna Toganidou, and Alexandros Tsoupras. 2025. "Utilization and Bio-Efficacy of Carotenoids, Vitamin A and Its Vitaminoids in Nutricosmetics, Cosmeceuticals, and Cosmetics’ Applications with Skin-Health Promoting Properties" Applied Sciences 15, no. 3: 1657. https://doi.org/10.3390/app15031657
APA StyleAdamantidi, T., Lafara, M.-P., Venetikidou, M., Likartsi, E., Toganidou, I., & Tsoupras, A. (2025). Utilization and Bio-Efficacy of Carotenoids, Vitamin A and Its Vitaminoids in Nutricosmetics, Cosmeceuticals, and Cosmetics’ Applications with Skin-Health Promoting Properties. Applied Sciences, 15(3), 1657. https://doi.org/10.3390/app15031657








