Use of Wickerhamomyces anomalus Strains from Biologically Aged Wines to Improve the Sensorial Profile of Young White Wines
Abstract
1. Introduction
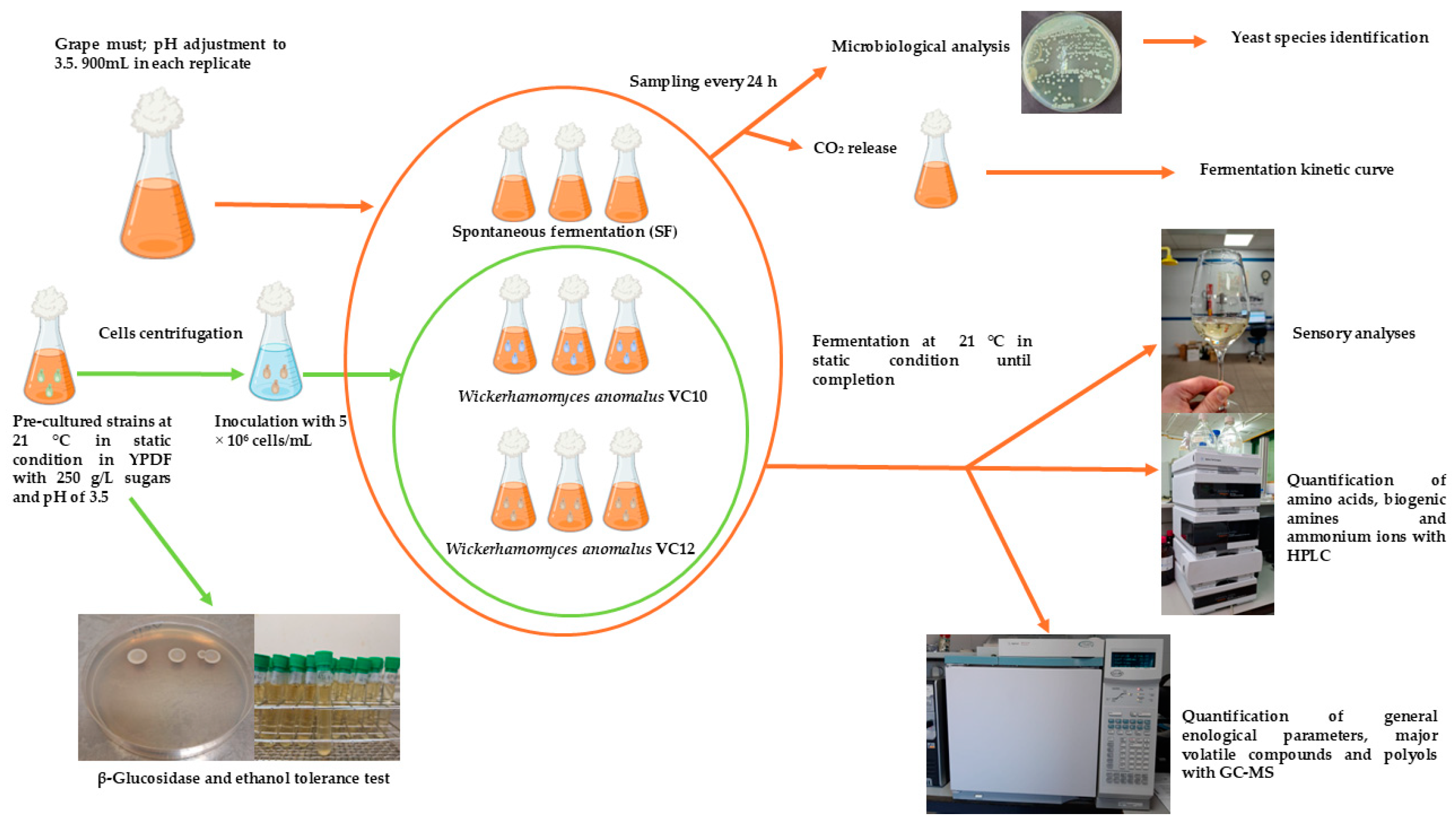
2. Materials and Methods
2.1. Microorganisms, Media, and Growth Conditions
2.2. β-Glucosidase and Ethanol Tolerance Test
2.3. Fermentation Conditions
2.4. Measurement of Enological Parameters
2.5. Quantification of Major Aroma Compounds and Polyols
2.6. Microbiological Analysis
2.7. Quantification of Amino Acids, Biogenic Amines and Ammonium Ions
2.8. Sensory Analyses
2.9. Statistical Analyses
3. Results and Discussion
3.1. β-Glucosidase and Ethanol Tolerance Test
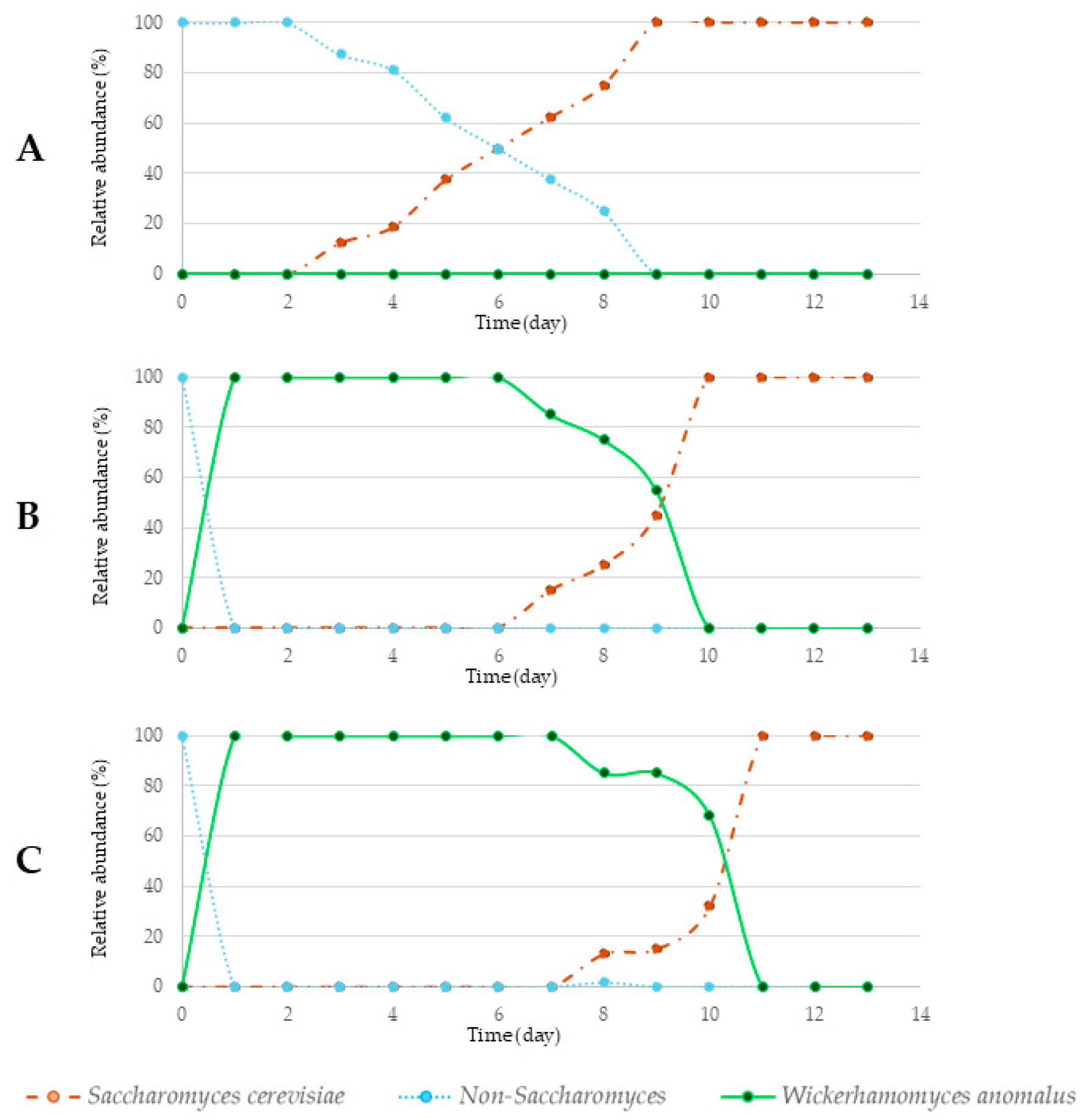
3.2. Alcoholic Fermentation Rates
3.3. Microbiological Analysis
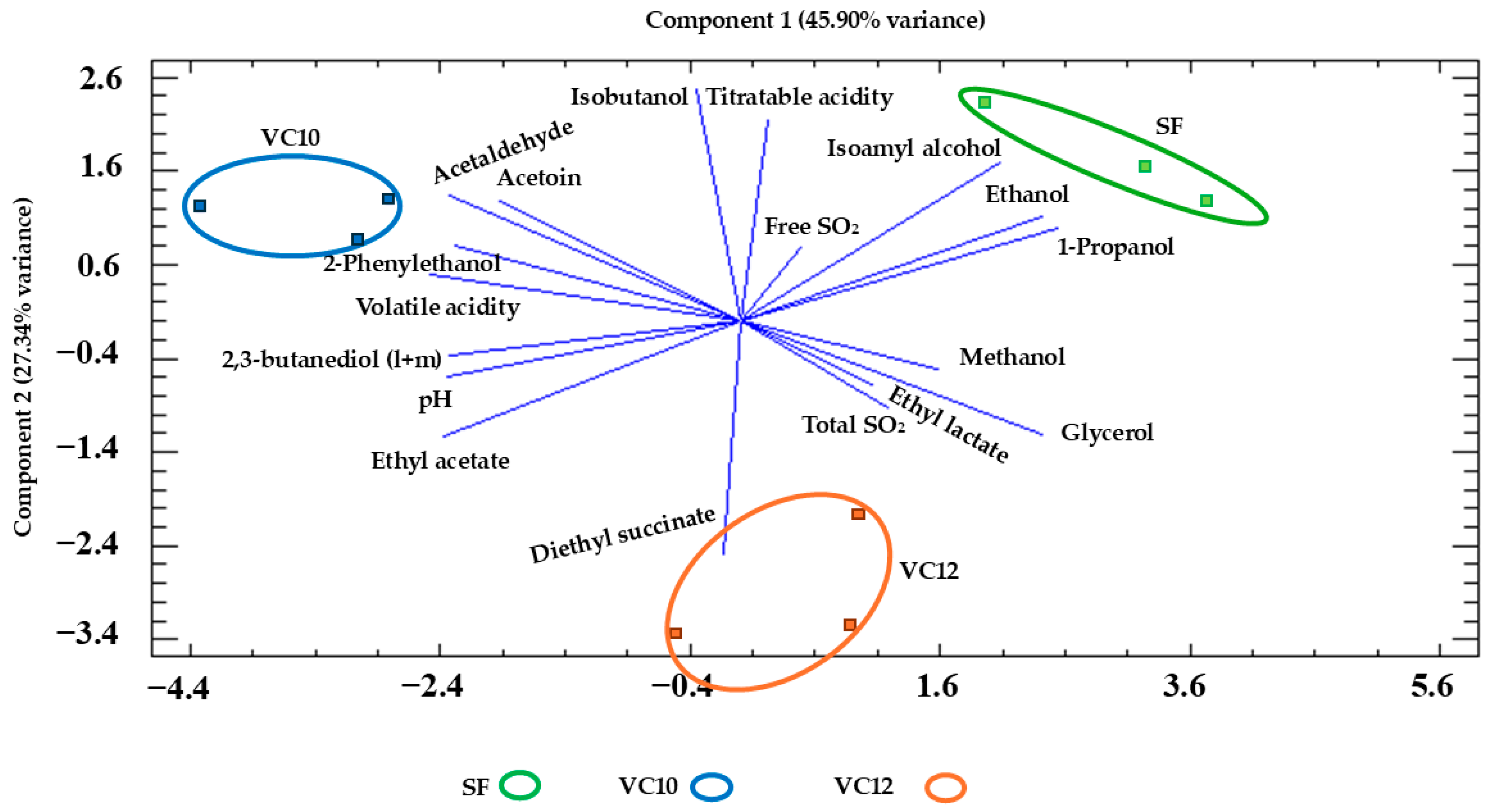
3.4. Enological Parameters and Major Aroma Compounds and Polyols
3.5. Nitrogen Compounds
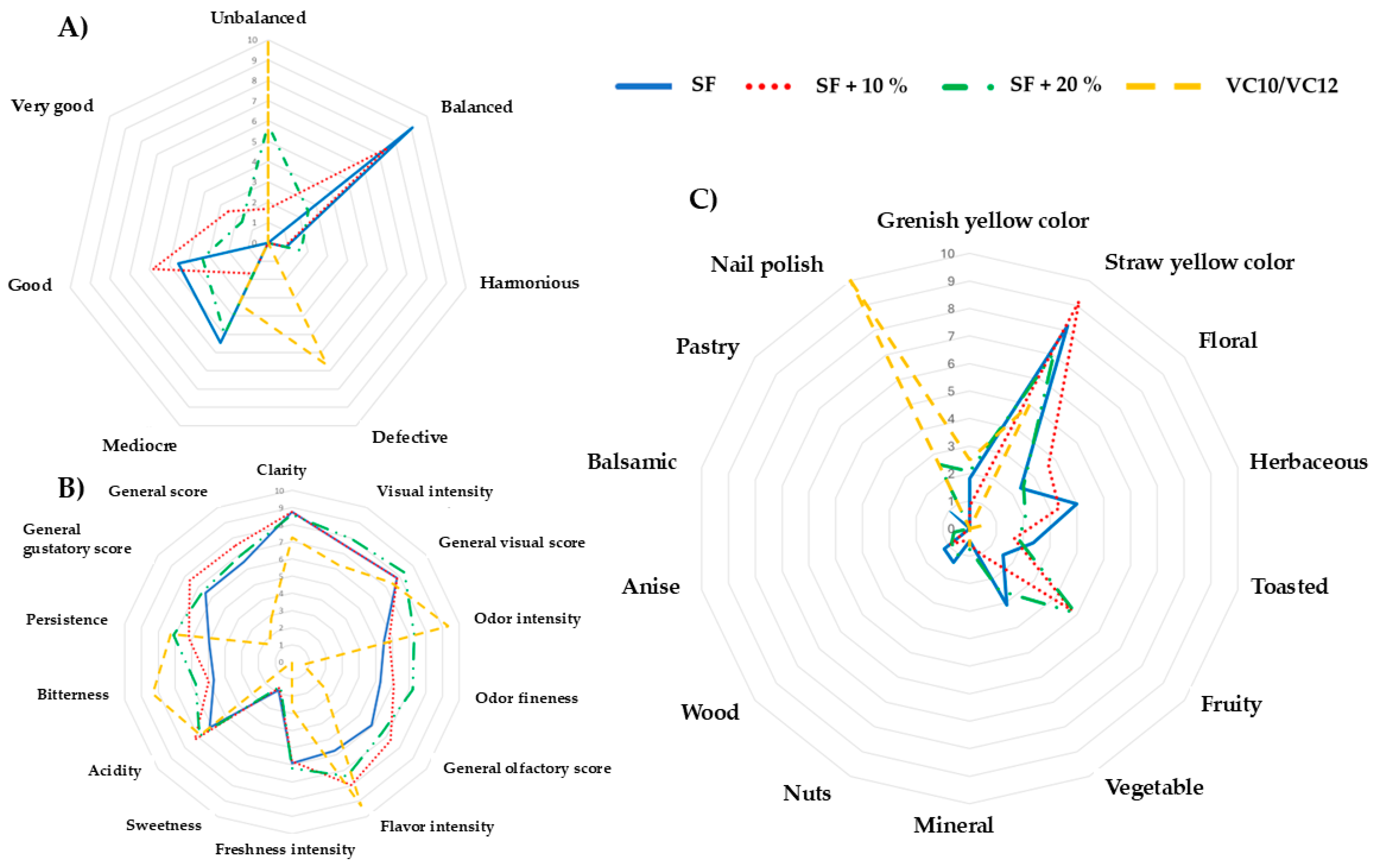
3.6. Sensory Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Padilla, B.; Gil, J.V.; Manzanares, P. Challenges of the Non-Conventional Yeast Wickerhamomyces anomalus in Winemaking. Fermentation 2018, 4, 68. [Google Scholar] [CrossRef]
- Wang, J.; Yan, J.; Gao, H.; Li, X.; Dong, Z.; Yan, S.; Shi, F. Comparative Metabolic Profiling of Cabernet Sauvignon Wines Reveals the Potential of Different Wickerhamomyces anomalus Co-Fermented with Commercial Saccharomyces cerevisiae. LWT 2023, 186, 115229. [Google Scholar] [CrossRef]
- Martín-García, F.J.; Palacios-Fernández, S.; López de Lerma, N.; García-Martínez, T.; Mauricio, J.C.; Peinado, R.A. The Effect of Yeast, Sugar and Sulfur Dioxide on the Volatile Compounds in Wine. Fermentation 2023, 9, 541. [Google Scholar] [CrossRef]
- Roudil, L.; Russo, P.; Berbegal, C.; Albertin, W.; Spano, G.; Capozzi, V. Non-Saccharomyces Commercial Starter Cultures: Scientific Trends, Recent Patents and Innovation in the Wine Sector. Recent. Pat. Food Nutr. Agric. 2020, 11, 27–39. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; Tesfaye, W.; Bañuelos, M.A.; González, C.; Suárez Lepe, J.A. Lachancea thermotolerans Applications in Wine Technology. Fermentation 2018, 4, 53. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; Escott, C.; del Fresno, J.M.; Bañuelos, M.A.; Suárez-Lepe, J.A. Applications of Metschnikowia pulcherrima in Wine Biotechnology. Fermentation 2019, 5, 63. [Google Scholar] [CrossRef]
- Vicente, J.; Calderón, F.; Santos, A.; Marquina, D.; Benito, S. High Potential of Pichia kluyveri and Other Pichia Species in Wine Technology. Int. J. Mol. Sci. 2021, 22, 1196. [Google Scholar] [CrossRef] [PubMed]
- Sola, I.M.; Evers, L.D.; Wojeicchowski, J.P.; Assis, T.M.d.; Marinho, M.T.; Demiate, I.M.; Alberti, A.; Nogueira, A. Impact of Pure, Co-, and Sequential Fermentations with Hanseniaspora Sp. and Saccharomyces cerevisiae on the Volatile Compounds of Ciders. Fermentation 2024, 10, 177. [Google Scholar] [CrossRef]
- Comitini, F.; Agarbati, A.; Canonico, L.; Galli, E.; Ciani, M. Purification and Characterization of WA18, a New Mycocin Produced by Active in Wine Against Spoilage Yeasts. Microorganisms 2020, 9, 56. [Google Scholar] [CrossRef]
- Fernández de Ullivarri, M.; Bulacios, G.A.; Navarro, S.A.; Lanza, L.; Mendoza, L.M.; Chalón, M.C. The Killer Yeast Wickerhamomyces anomalus Cf20 Exerts a Broad Anti-Candida Activity through the Production of Killer Toxins and Volatile Compounds. Med. Mycol. 2020, 58, 1102–1113. [Google Scholar] [CrossRef]
- López-Enríquez, L.; Vila-Crespo, J.; Rodríguez-Nogales, J.M.; Fernández-Fernández, E.; Ruipérez, V. Modulation of the Aromatic Profile of Verdejo Wine through Sequential Inoculation of Wickerhamomyces anomalus and Saccharomyces cerevisiae. Fermentation 2023, 9, 977. [Google Scholar] [CrossRef]
- Ruiz-Muñoz, M.; Cordero-Bueso, G.; Benítez-Trujillo, F.; Martínez, S.; Pérez, F.; Cantoral, J.M. Rethinking about Flor Yeast Diversity and Its Dynamic in the “Criaderas and Soleras” Biological Aging System. Food Microbiol. 2020, 92, 103553. [Google Scholar] [CrossRef]
- Carbonero-Pacheco, J.; Moreno-García, J.; Moreno, J.; García-Martínez, T.; Mauricio, J.C. Revealing the Yeast Diversity of the Flor Biofilm Microbiota in Sherry Wines Through Internal Transcribed Spacer-Metabarcoding and Matrix-Assisted Laser Desorption/Ionization Time of Flight Mass Spectrometry. Front. Microbiol. 2021, 12, 825756. [Google Scholar] [CrossRef]
- Ruiz-Muñoz, M.; Hernández-Fernández, M.; Cordero-Bueso, G.; Martínez-Verdugo, S.; Pérez, F.; Cantoral, J.M. Non-Saccharomyces Are Also Forming the Veil of Flor in Sherry Wines. Fermentation 2022, 8, 456. [Google Scholar] [CrossRef]
- Martínez-García, R.; Mauricio, J.C.; García-Martínez, T.; Peinado, R.A.; Moreno, J. Towards a Better Understanding of the Evolution of Odour-Active Compounds and the Aroma Perception of Sparkling Wines during Ageing. Food Chem. 2021, 357, 129784. [Google Scholar] [CrossRef]
- Muñoz-Castells, R.; Moreno, J.; García-Martínez, T.; Mauricio, J.C.; Moreno-García, J. Chemometric Differentiation of White Wines from a Low-Aromatic Grape Obtained by Spontaneous Fermentation, Enriched with Non-Saccharomyces, or with a High-Glutathione-Producing Saccharomyces Yeast. Fermentation 2023, 9, 1023. [Google Scholar] [CrossRef]
- Valcárcel-Muñoz, M.J.; Guerrero-Chanivet, M.; Rodríguez-Dodero, M.d.C.; García-Moreno, M.d.V.; Guillén-Sánchez, D.A. Analytical and Chemometric Characterization of Fino and Amontillado Sherries during Aging in Criaderas Y Solera System. Molecules 2022, 27, 365. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.; Belda, I.; Beisert, B.; Navascués, E.; Marquina, D.; Calderón, F.; Rauhut, D.; Santos, A.; Benito, S. Analytical Impact of Metschnikowia pulcherrima in the Volatile Profile of Verdejo White Wines. Appl. Microbiol. Biotech. 2018, 102, 8501–8509. [Google Scholar] [CrossRef]
- Lepe, J.A.S.; Leal, B.Í. Microbiología Enológica: Fundamentos de Vinificación; Mundi-Prensa Libros: Madrid, Spain, 2004; ISBN 9788484761846. [Google Scholar]
- OIV. 2024. Available online: https://www.oiv.int/en (accessed on 1 November 2024).
- Peinado, R.A.; Moreno, J.A.; Muñoz, D.; Medina, M.; Moreno, J. Gas Chromatographic Quantification of Major Volatile Compounds and Polyols in Wine by Direct Injection. J. Agric. Food Chem. 2004, 52, 6389–6393. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Alonso, S.; Hermosín-Gutiérrez, I.; García-Romero, E. Simultaneous HPLC Analysis of Biogenic Amines, Amino Acids, and Ammonium Ion as Aminoenone Derivatives in Wine and Beer Samples. J. Agric. Food Chem. 2007, 55, 608–613. [Google Scholar] [CrossRef]
- Li, Y.; Long, H.; Jiang, G.; Gong, X.; Yu, Z.; Huang, M.; Guan, T.; Guan, Y.; Liu, X. Analysis of the Ethanol Stress Response Mechanism in Wickerhamomyces anomalus Based on Transcriptomics and Metabolomics Approaches. BMC Microbiol. 2022, 22, 275. [Google Scholar] [CrossRef] [PubMed]
- López-Enríquez, L.; Vila-Crespo, J.; Rodríguez-Nogales, J.M.; Fernández-Fernández, E.; Ruipérez, V. Non-Saccharomyces Yeasts from Organic Vineyards as Spontaneous Fermentation Agents. Foods 2023, 12, 3644. [Google Scholar] [CrossRef]
- Satora, P.; Tarko, T.; Sroka, P.; Blaszczyk, U. The Influence of Wickerhamomyces anomalus Killer Yeast on the Fermentation and Chemical Composition of Apple Wines. FEMS Yeast Res. 2014, 14, 729–740. [Google Scholar] [CrossRef]
- Oro, L.; Feliziani, E.; Ciani, M.; Romanazzi, G.; Comitini, F. Volatile Organic Compounds from Wickerhamomyces anomalus, Metschnikowia pulcherrima and Saccharomyces cerevisiae Inhibit Growth of Decay Causing Fungi and Control Postharvest Diseases of Strawberries. Int. J. Food Microbiol. 2018, 265, 18–22. [Google Scholar] [CrossRef]
- Liu, Z.; Tian, J.; Yan, H.; Li, D.; Wang, X.; Liang, W.; Wang, G. Ethyl Acetate Produced by Is a Potential Biocontrol Agent against Tomato Fruit Rot Caused by Phytophtora nicotianae. Front. Microbiol. 2022, 13, 978920. [Google Scholar]
- Kruis, A.J.; Levisson, M.; Mars, A.E.; van der Ploeg, M.; Garcés Daza, F.; Ellena, V.; Kengen, S.W.M.; van der Oost, J.; Weusthuis, R.A. Ethyl Acetate Production by the Elusive Alcohol Acetyltransferase from Yeast. Metab. Eng. 2017, 41, 92–101. [Google Scholar] [CrossRef]
- Domizio, P.; Romani, C.; Lencioni, L.; Comitini, F.; Gobbi, M.; Mannazzu, I.; Ciani, C. Outlining a Future for Non-Saccharomyces Yeasts: Selection of Putative Spoilage Wine Strains to Be Used in Association with Saccharomyces cerevisiae for Grape Juice Fermentation. Int. J. Food Microbiol. 2011, 147, 170–180. [Google Scholar] [CrossRef]
- Fu, Z.; Sun, B.; Li, X.; Fan, G.; Teng, C.; Alaa, A.; Jia, Y. Isolation and Characterization of a High Ethyl Acetate-Producing Yeast from Laobaigan Daqu and Its Fermentation Conditions for Producing High-Quality Baijiu. Biotechnol. Biotechnol. Equip. 2018, 32, 1218–1227. [Google Scholar] [CrossRef]
- Fredlund, E.; Druvefors, U.A.; Olstorpe, M.N.; Passoth, V.; Schnürer, J. Influence of Ethyl Acetate Production and Ploidy on the Anti-Mould Activity of Pichia anomala. FEMS Microbiol. Lett. 2004, 238, 133–137. [Google Scholar]
- Carbonero-Pacheco, J.; Constanta-Mustafa, F.; Muñoz-Castells, R.; Mauricio, J.C.; Moreno, J.; García-Martínez, T.; Moreno-García, J. Microbial Biocapsules as Generally Recognized-As-Safe Fungal-Based Immobilized Cell Technology for Precision Sequential Fermentations of Grape Must. Fermentation 2024, 10, 498. [Google Scholar] [CrossRef]
- Izquierdo Cañas, P.M.; García-Romero, E.; Heras Manso, J.M.; Fernández-González, M. Influence of Sequential Inoculation of Wickerhamomyces anomalus and Saccharomyces cerevisiae in the Quality of Red Wines. Eur. Food Res. Tech. 2014, 239, 279–286. [Google Scholar] [CrossRef]
- Aplin, J.J.; White, K.P.; Edwards, C.G. Growth and Metabolism of Non-Saccharomyces Yeasts Isolated from Washington State Vineyards in Media and High Sugar Grape Musts. Food Microbiol. 2019, 77, 158–165. [Google Scholar] [CrossRef]
- Kruis, A.J.; Mars, A.E.; Kengen, S.W.M.; Borst, J.W.; van der Oost, J.; Weusthuis, R.A. Alcohol Acetyltransferase Eat1 Is Located in Yeast Mitochondria. Appl. Environ. Microbiol. 2018, 84, e01640-18. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Teng, C.; Xu, D.; Fu, Z.; Liu, P.; Wu, Q.; Yang, R.; Li, X. Improving Ethyl Acetate Production in Baijiu Manufacture by Wickerhamomyces anomalus and Saccharomyces cerevisiae Mixed Culture Fermentations. BioMed Res. Int. 2019, 2019, 1470543. [Google Scholar] [CrossRef] [PubMed]
- Zha, M.; Sun, B.; Wu, Y.; Yin, S.; Wang, C. Improving Flavor Metabolism of Saccharomyces cerevisiae by Mixed Culture with Wickerhamomyces anomalus for Chinese Baijiu Making. J. Biosci. Bioeng. 2018, 126, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Mitri, S.; Koubaa, M.; Maroun, R.G.; Rossignol, T.; Nicaud, J.-M.; Louka, N. Bioproduction of 2-Phenylethanol through Yeast Fermentation on Synthetic Media and on Agro-Industrial Waste and By-Products: A Review. Foods 2022, 11, 109. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Du, Z.; Zhu, H.; Guo, X.; He, X. Protective Effects of Arginine on Saccharomyces cerevisiae Against Ethanol Stress. Sci. Rep. 2016, 6, 31311. [Google Scholar] [CrossRef]
- Qin, J.; Zhou, Y.J.; Krivoruchko, A.; Huang, M.; Liu, L.; Khoomrung, S.; Siewers, V.; Jiang, B.; Nielsen, J. Modular Pathway Rewiring of Saccharomyces cerevisiae Enables High-Level Production of L-Ornithine. Nat. Commun. 2015, 6, 8224. [Google Scholar] [CrossRef] [PubMed]
- Bach, B.; Sauvage, F.-X.; Dequin, S.; Camarasa, C. Role of γ-Aminobutyric Acid as a Source of Nitrogen and Succinate in Wine. Am. J. Enol. Vitic. 2009, 60, 508–516. [Google Scholar] [CrossRef]
- Cao, J.; Barbosa, J.M.; Singh, N.K.; Locy, R.D. GABA Shunt Mediates Thermotolerance in Saccharomyces cerevisiae by Reducing Reactive Oxygen Production. Yeast 2013, 30, 129–144. [Google Scholar] [CrossRef]
- Nishimura, A.; Ichikawa, K.; Nakazawa, H.; Tanahashi, R.; Morita, F.; Sitepu, I.; Boundy-Mills, K.; Fox, G.; Takagi, H. The Cdc25/Ras/cAMP-Dependent Protein Kinase A Signaling Pathway Regulates Proline Utilization in Wine Yeast Saccharomyces cerevisiae under a Wine Fermentation Model. Biosci. Biotechnol. Biochem. 2022, 86, 1318–1326. [Google Scholar] [CrossRef]
- Jiang, X.; Lu, Y.; Liu, S.Q. Effects of Different Yeasts on Physicochemical and Oenological Properties of Red Dragon Fruit Wine Fermented with Saccharomyces cerevisiae, Torulaspora delbrueckii and Lachancea thermotolerans. Microorganisms 2020, 8, 315. [Google Scholar] [CrossRef]
- Canonico, L.; Comitini, F.; Oro, L.; Ciani, M. Sequential Fermentation with Selected Immobilized Non-Saccharomyces Yeast for Reduction of Ethanol Content in Wine. Front. Microbiol. 2016, 7, 278. [Google Scholar] [CrossRef] [PubMed]

| Compound | Method of Detection | CAS | OT (mg/L) | Odor/Flavor Description | SF | VC10 | VC12 |
|---|---|---|---|---|---|---|---|
| Acetaldehyde (mg/L) | GC–FID | 75-07-0 | 10 | Over-ripe apple | 59.46 ± 1.26 b | 72.48 ± 1.44 c | 56.93 ± 0.58 a |
| Ethyl acetate (mg/L) | 141-78-6 | 7.5 | Pineapple, varnish, balsamic | 45.34 ± 0.16 a | 1159.35 ± 1.37 c | 998.73 ± 7.51 b | |
| 1,1-Diethoxyethane (mg/L) | 105-57-7 | 1 | Refreshing, pleasant, fruity-green | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| Methanol (mg/L) | 67-56-1 | 668 | Chemical, medicinal | 58.63 ± 6.55 a | 50.71 ± 7.07 a | 59.15 ± 1.16 a | |
| 1-Propanol (mg/L) | 71-23-8 | 830 | Ripe fruit, alcohol | 66.56 ± 0.14 c | 43.04 ± 0.19 a | 49.34 ± 3.05 b | |
| Isobutanol (mg/L) | 78-83-1 | 40 | Alcohol, wine, nail polish | 37.73 ± 0.09 b | 37.95 ± 0.02 b | 33.77 ± 0.99 a | |
| Isoamyl alcohol (mg/L) | 123-51-3 | 30 | Alcohol, nail polish | 211.23 ± 0.07 b | 185.8 ± 0.14 a | 183.52 ± 5.61 a | |
| Acetoin (mg/L) | 53584-56-8 | 30 | Buttery, creamy | 24.97 ± 4.03 ab | 32.32 ± 2.28 b | 21.85 ± 4.46 a | |
| Ethyl lactate (mg/L) | 97-64-3 | 7.5 | Strawberry, raspberry, buttery | 15.05 ± 0.20 a | 14.62 ± 0.20 a | 14.99 ± 0.66 a | |
| 2,3-butanediol (l + m) (mg/L) | 24347-58-8 | 668 | Buttery, creamy | 193.82 ± 18.47 a | 288.10 ± 3.51 b | 239.05 ± 70.87 ab | |
| Diethyl succinate (mg/L) | 123-25-1 | 100 | Over-ripe, lavender | 5.84 ± 0.23 a | 6.52 ± 0.12 b | 10.31 ± 0.32 c | |
| 2-Phenylethanol (mg/L) | 60-12-8 | 10 | Floral | 17.58 ± 0.89 a | 19.65 ± 0.94 b | 17.80 ± 0.65 a | |
| Glycerol (mg/L) | 56-81-5 | - | Confers body and smoothness and a sweet taste | 4260 ± 90.00 b | 3980 ± 50.00 a | 4300 ± 10.00 b | |
| Ethanol (% v/v) | According to OIV | 64-17-5 | 10 | Alcoholic | 10.77 ± 0.03 c | 10.32 ± 0.04 a | 10.40 ± 0.04 b |
| pH | - | - | - | 3.06 ± 0.01 a | 3.11 ± 0.02 c | 3.09 ± 0.01 b | |
| Volatile acidity (g/L) | 64-19-7 | 200 | Vinegar | 0.09 ± 0.02 a | 0.14 ± 0.01 b | 0.10 ± 0.02 a | |
| Titratable acidity (g/L) | - | - | - | 6.61 ± 0.04 b | 6.54 ± 0.14 ab | 6.31 ± 0.13 a | |
| Free SO2 (mg/L) | - | - | - | 8.66 ± 1.15 a | 8.66 ± 1.52 a | 8.00 ± 1.00 a | |
| Total SO2 (mg/L) | - | - | - | 37.66 ± 2.51 a | 37.00 ± 1.73 a | 38.66 ± 1.52 a |
| Compound (mg/L) | CAS | Initial Must | SF-4 | VC10-4 | VC12-4 | SF-8 | VC10-8 | VC12-8 | SF-F | VC10-F | VC12-F |
|---|---|---|---|---|---|---|---|---|---|---|---|
| L-aspartic acid | 56-84-8 | N.D. | 16.53 ± 2.31 b | 18.14 ± 3.74 b | 15.95 ± 3.30 b | N.D. | N.D. | 2.06 ± 0.2 a | N.D. | N.D. | N.D. |
| L-glutamic acid | 56-86-0 | N.D. | 42.81 ± 9.90 b | 43.47 ± 13.12 b | 37.02 ± 9.77 b | 10.07 ± 0.22 a | N.D. | N.D. | N.D. | N.D. | N.D. |
| L-glutamine | 56-85-9 | N.D. | 3.24 ± 0.65 a | 3.24 ± 0.83 a | 3.15 ± 0.20 a | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| L-histidine | 71-00-1 | 118.42 ± 31.55 b | 47.39 ± 4.38 a | 43.73 ± 8.22 a | 40.01 ± 6.37 a | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| glycine | 56-40-6 | 8.65 ± 2.48 a | 15.78 ± 2.52 b | 14.50 ± 1.92 b | 14.58 ± 2.16 b | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| L-threonine | 72-19-5 | 28.14 ± 12.54 a | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| L-citrulline | 372-75-8 | 11.82 ± 0.43 a | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| L-arginine | 74-79-3 | 1847 ± 112.44 e | 468.30 ± 22.79 d | 443.61 ± 79.91 d | 412.77 ± 63.00 d | 6.79 ± 0.58 a | 216.33 ± 35.77 b | 305.22 ± 9.01 c | N.D. | N.D. | N.D. |
| L-alanine | 56-41-7 | 49.09 ± 0.98 c | 35.11 ± 7.02 b | 41.10 ± 9.82 bc | 39.04 ± 8.73 bc | 2.03 ± 0.34 a | 2.97 ± 0.40 a | 2.26 ± 0.34 a | 2.94 ± 2.32 a | N.D. | N.D. |
| γ-Aminobutyric acid | 56-12-2 | 411.00 ± 7.78 d | 20.19 ± 5.37 b | 18.56 ± 5.08 b | 18.81 ± 7.09 b | N.D. | 13.07 ± 0.87 ab | 16.08 ± 4.49 ab | 9.27 ± 1.03 a | 31.54 ± 1.95 c | 32.71 ± 2.10 c |
| L-α-amino-n-Butyric acid | 2835-81-6 | 35.15 ± 7.32 c | 42.35 ± 9.50 c | 40.10 ± 8.29 c | 39.25 ± 10.97 c | N.D. | 2.95 ± 5.12 ab | 10.5 ± 0.16 b | N.D. | N.D. | N.D. |
| L-proline | 147-85-3 | 70.05 ± 3.39 d | 31.76 ± 3.55 c | 31.80 ± 3.63 c | 31.77 ± 4.74 c | 15.33 ± 0.39 b | 12.44 ± 3.64 ab | 12.59 ± 1.50 a | 15.54 ± 0.60 b | 13.16 ± 0.06 ab | 13.31 ± 0.69 ab |
| Ammonium chloride | 12125-02-9 | N.D. | 19.51 ± 5.47 a | 19.68 ± 3.64 a | 20.64 ± 5.43 a | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| L-valine | 72-18-4 | 6.15 ± 0.17 a | 30.76 ± 4.31 b | 28.88 ± 4.08 b | 28.24 ± 4.74 b | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| L-isoleucine | 73-32-5 | N.D. | 8.17 ± 1.48 b | 6.85 ± 0.72 a | 6.95 ± 1.18 a | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| L-tryptophan | 73-22-3 | N.D. | 12.25 ± 1.52 a | 11.73 ± 2.06 a | 11.05 ± 1.76 a | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| L-leucine | 61-90-5 | N.D. | 9.34 ± 1.82 b | 7.40 ± 1.09 a | 7.44 ± 1.17 a | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| L-phenylalanine | 63-91-2 | 6.44 ± 0.99 a | 12.06 ± 1.70 c | 8.7 ± 1.62 b | 8.62 ± 2.36 b | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| L-ornithine | 70-26-8 | 223.75 ± 6.74 f | 63.53 ± 3.69 bcd | 62.27 ± 12.78 bc | 56.48 ± 9.21 b | 6.67 ± 1.51 a | 72.67 ± 1.81 e | 67.33 ± 4.22 de | 4.04 ± 1.02 a | N.D. | N.D. |
| L-lysine | 56-87-1 | 121.33 ± 2.48 d | 11.35 ± 7.01 c | 11.35 ± 2.53 c | 13.20 ± 1.29 c | N.D. | 4.67 ± 1.01 b | 4.33 ± 2.13 ab | N.D. | N.D. | N.D. |
| putrescine | 110-60-1 | 75.36 ± 2.82 c | 17.59 ± 0.82 b | 17.06 ± 3.00 b | 15.84 ± 2.53 ab | 16.29 ± 0.56 ab | 14.90 ± 0.40 ab | 15.48 ± 0.64 ab | 15.33 ± 0.55 ab | 14.04 ± 0.28 a | 14.16 ± 0.61 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carbonero-Pacheco, J.; García-Jiménez, Á.; García-García, J.C.; Santos-Dueñas, I.M.; García-Martínez, T.; Moreno, J.; Moreno-García, J.; Mauricio, J.C. Use of Wickerhamomyces anomalus Strains from Biologically Aged Wines to Improve the Sensorial Profile of Young White Wines. Appl. Sci. 2025, 15, 1546. https://doi.org/10.3390/app15031546
Carbonero-Pacheco J, García-Jiménez Á, García-García JC, Santos-Dueñas IM, García-Martínez T, Moreno J, Moreno-García J, Mauricio JC. Use of Wickerhamomyces anomalus Strains from Biologically Aged Wines to Improve the Sensorial Profile of Young White Wines. Applied Sciences. 2025; 15(3):1546. https://doi.org/10.3390/app15031546
Chicago/Turabian StyleCarbonero-Pacheco, Juan, Álvaro García-Jiménez, Juan Carlos García-García, Inés M. Santos-Dueñas, Teresa García-Martínez, Juan Moreno, Jaime Moreno-García, and Juan Carlos Mauricio. 2025. "Use of Wickerhamomyces anomalus Strains from Biologically Aged Wines to Improve the Sensorial Profile of Young White Wines" Applied Sciences 15, no. 3: 1546. https://doi.org/10.3390/app15031546
APA StyleCarbonero-Pacheco, J., García-Jiménez, Á., García-García, J. C., Santos-Dueñas, I. M., García-Martínez, T., Moreno, J., Moreno-García, J., & Mauricio, J. C. (2025). Use of Wickerhamomyces anomalus Strains from Biologically Aged Wines to Improve the Sensorial Profile of Young White Wines. Applied Sciences, 15(3), 1546. https://doi.org/10.3390/app15031546










