2. Materials and Methods
General Procedure. Reactions were performed in a well-dried flask under an Argon atmosphere. Solvents for extraction and chromatography were of reagent grade and used as received. Solvents for the reaction were stored in molecular sieves, which were prepared by heating in a microwave for 1 min and then drying with a vacuum pump (this drying procedure was repeated three times). The column chromatography was performed with silica gel 60 (70–230 mesh) using a mixture of EtOAc/hexane as eluent. The 1H- and 13C-NMR spectra were, respectively, recorded on a 400 MHz and 100 MHz NMR spectrometer in deuterated chloroform (CDCl3) with tetramethylsilane (TMS) as an internal reference unless noted otherwise.
Experimental Procedures for the Synthesis of Grifolin 1
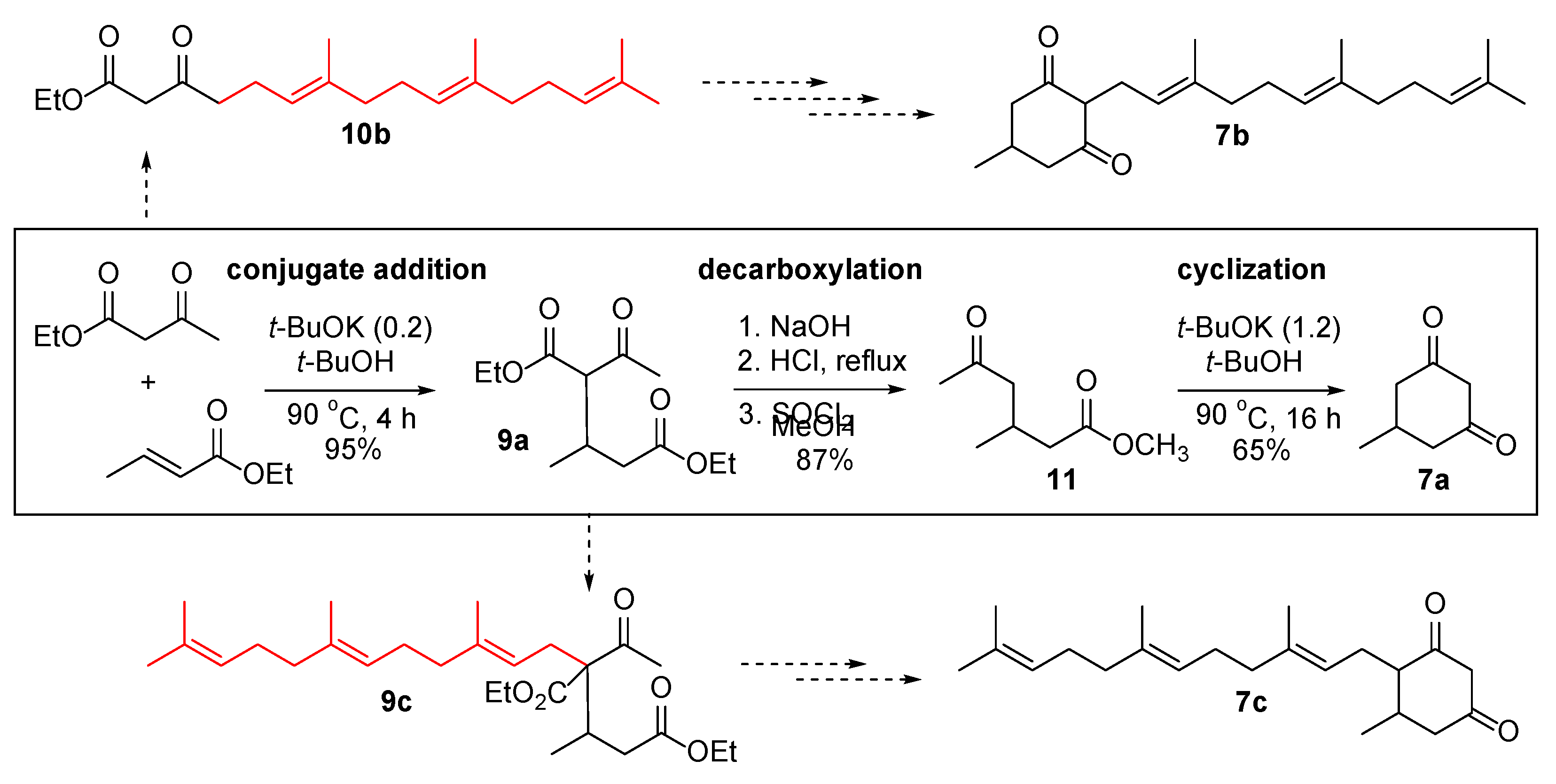
Ethyl (6E,10E)-7,11,15-trimethyl-3-oxohexadeca-6,10,14-trienoate (10b). To a stirred solution of ethyl acetoacetate (10.41 g, 80.00 mmol) in THF (40 mL) at 0 °C under an argon atmosphere, NaH (60% in mineral oil, 3.84 g, 96.00 mmol) was carefully added in several portions. The mixture was stirred at 0 °C for 1 h; then, 1.6 M hexane solution of n-BuLi (55.0 mL, 88.00 mmol) was added. Stirring at 0 °C for 30 min, the mixture was treated with a THF solution (10 mL) of farnesyl bromide (21.95 g, 76.95 mmol). The reaction mixture was stirred at 0 °C for 4.5 h, and quenched with 1 M HCl. The mixture was extracted with EtOAc, dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure to give the crude product (31.45 g), which was purified by SiO2 flash column chromatography (3–20% EtOAc/hexane eluent) to produce the coupling product 10b (19.20 g, 57.41 mmol) in 75% yield as yellow oil (a mixture of keto and enol forms).
Data for 10b: Rf = 0.65 (4:1 hexane/EtOAc); 1H NMR (CDCl3) δ = 1.28 (t, J = 7.2 Hz, 3H), 1.59 (s, 3H), 1.60 (s, 3H), 1.61 (s, 3H), 1.68 (s, 3H), 1.93–2.02 (m, 4H), 2.02–2.11 (m, 4H), 2.29 (dt, Jd = 6.8, Jt = 7.2 Hz, 2H), 2.57 (t, J = 7.2 Hz, 2H), 3.43 (s, 2H), 4.20 (q, J = 7.2 Hz, 2H), 5.04–5.14 (m, 3H) ppm; 13C NMR (CDCl3, keto form) δ = 14.2, 16.0, 16.1, 17.7, 22.2, 25.7, 26.6, 26.8, 39.7, 39.8, 43.1, 49.4, 61.4, 122.2, 124.1, 124.4, 131.3, 135.1, 136.8, 167.2, 202.6 ppm; IR ν = 3469 (w), 2967, 2929, 1740, 1716, 1652, 1447, 1375, 1315, 1279, 1236, 1176, 1153, 1096, 1026, 843, 804, 750 cm−1; HRMS (ESI) calcd for C21H34O3+Na 357.2406, found 357.2401.
Ethyl 4-hydroxy-6-methyl-2-oxo-3-((2E,6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-yl)cyclohex-3-ene-1-carboxylate (12b). To a stirred solution of the coupling product 10b (3.00 g, 8.97 mmol) and ethyl crotonate (1.02 g, 8.97 mmol) in t-BuOH (30 mL), t-BuOK (201 mg, 1.79 mmol) was added. The mixture was heated at 90 °C for 2 h, and the solution became cloudy. A stoichiometric amount of t-BuOK (1.01 g, 8.97 mmol) was added and the mixture was heated at 90 °C for 16 h. Upon cooling to room temperature, the mixture was concentrated under reduced pressure. The crude mixture was diluted with EtOAc, washed with 1 M HCl, dried over anhydrous Na2SO4, filtered, and concentrated to give an orange oily product (3.76 g). The crude product was purified by SiO2 flash column chromatography (20–40% EtOAc/hexane eluent) to produce the cyclized product 12b (3.12 g, 7.76 mmol) in 86% yield as a light-yellow liquid (a mixture of diastereomers).
Data for 12b: Rf = 0.56 (2:3 EtOAc/hexane); 1H NMR (CDCl3) δ = 1.07 (d, J = 6.4 Hz, 3H), 1.30 (t, J = 7.2 Hz, 3H), 1.60 (s, 6H), 1.68 (s, 3H), 1.75 (s, 3H), 2.10–2.26 (m, 9H), 2.44–2.60 (m, 2H), 3.00–3.16 (m, 2H), 3.02 (d, J = 11.6 Hz, 1H), 4.17–4.32 (m, 2H), 5.05 (t, J = 6.8 Hz, 1H), 5.09 (t, J = 6.8 Hz, 1H), 5.23 (t, J = 7.2 Hz, 1H), 7.05 (br s, 1H) ppm; 13C NMR (CDCl3) δ = 14.3, 16.1, 16.2, 17.7, 19.7, 21.5, 25.7, 26.1, 26.7, 30.8, 35.8, 39.7, 39.7, 60.5, 61.0, 112.5, 121.4, 123.3, 124.3, 131.4, 136.0, 140.8, 170.6, 171.5, 193.0 ppm; IR ν = 3435, 2974, 2937, 2876, 1724, 1623, 1456, 1405, 1376, 1302, 1242, 1222, 1154, 1095, 1025, 925, 852, 794, 735 cm−1; HRMS (ESI) calcd. for C25H38O4+H 403.2848, found 403.2844.
3-Hydroxy-5-methyl-2-((2E,6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-yl)cyclohex-2-en-1-one (7b′). The above cyclized product 12b (1.37 g, 3.40 mmol) was dissolved in 1 M NaOH (20 mL) and heated at 100 °C for 2 h. The mixture was cooled to room temperature and acidified with 1 M HCl (30 mL). The mixture was extracted with EtOAc, dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure to give orange sticky oil (1.34 g). The above crude product was then dissolved in toluene (20 mL) and heated to 110 °C for 17 h. Upon cooling to room temperature, the mixture was concentrated under reduced pressure. The crude product was purified by SiO2 flash column chromatography (20–40% EtOAc/hexane eluent) to give a keto 7b/enol 7b′ mixture of 1,3-cyclohexanedione (750 mg, 2.27 mmol) in 67% yield as an off-white solid.
Data for 7b′: Rf = 0.45 and 0.09 (2:3 EtOAc/hexane); 1H NMR (CDCl3) δ = 1.06 (d, J = 6.0 Hz, 3H), 1.59 (s, 3H), 1.60 (s, 3H), 1.68 (s, 3H), 1.75 (s, 3H), 1.90–2.24 (m, 11H), 2.30–2.56 (m, 2H), 3.05 (d of A of ABq, JAB = 16.4, Jd = 7.6 Hz, 1H), 3.13 (d of B of ABq, JAB = 16.4, Jd = 7.2 Hz, 1H), 5.02–5.14 (m, 2H), 5.22 (dt, Jd = 1.2, Jt = 7.2 Hz, 1H), 6.85 (br s, 1H) ppm; 13C NMR (CDCl3) δ = 16.0, 16.1, 16.2, 17.8, 21.0, 21.3, 25.8, 25.8, 26.3, 26.7, 28.3, 39.7, 39.7, 113.5, 121.9, 123.6, 124.4, 131.4, 135.8, 139.4, 172.2, 198.4 ppm; IR ν = 3403 (w), 2962, 2918, 2849, 1732, 1614, 1456, 1380, 1249, 1215, 1059, 911, 787, 731 cm−1; HRMS (ESI) calcd for C22H34O2+H 331.2637, found 331.2639.
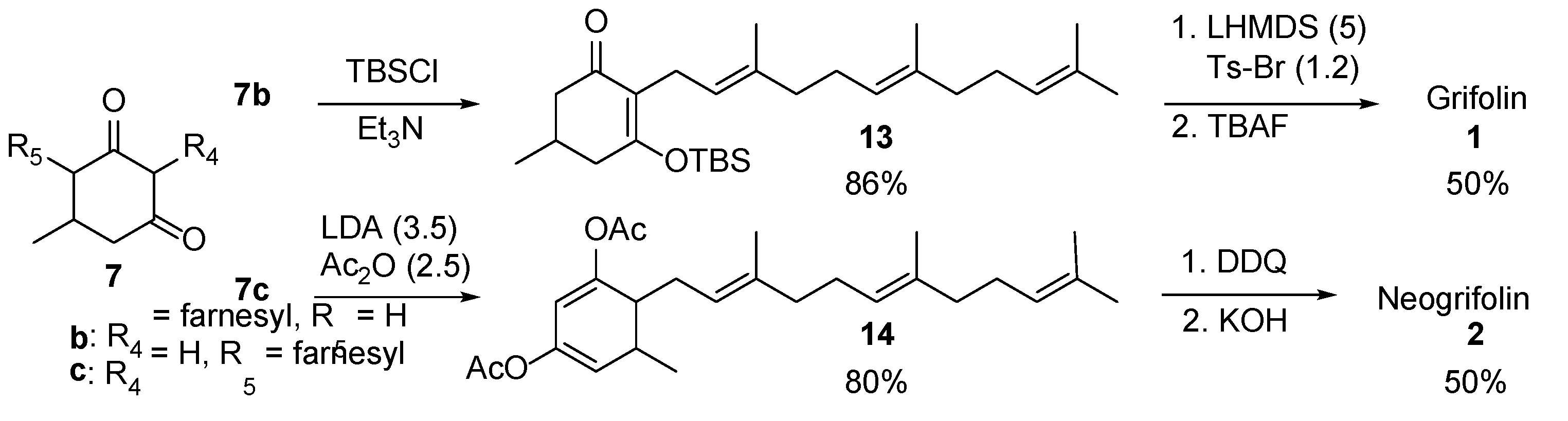
3-((tert-Butyldimethylsilyl)oxy)-5-methyl-2-((2E,6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-yl)cyclohex-2-en-1-one (13). To a stirred solution of the enol form of cyclohexane-1,3-dione 7b′ (430 mg, 1.30 mmol) in CH2Cl2 (10 mL) at 0 °C, tert-butyldimethylsilyl chloride (0.20 g, 1.37 mmol) and Et3N (0.19 mL, 1.37 mmol) were added. The mixture was stirred at room temperature for 15 min and concentrated under reduced pressure. The crude product was diluted with hexane, and the insoluble white precipitate was filtered and rinsed with hexane (5 × 10 mL). The combined hexane solutions were concentrated under reduced pressure and purified by SiO2 flash column chromatography (5–10% EtOAc/hexane) to afford TBS-ether 13 (495 mg, 1.11 mmol) in 86% yield as a colorless oil.
Data for 13: Rf = 0.56 (4:1 hexane/EtOAc); 1H NMR (CDCl3) δ = 0.24 (s, 3H), 0.25 (s, 3H), 0.97 (s, 9H), 1.05 (d, J = 6.0 Hz, 3H), 1.57 (s, 3H), 1.60 (s, 3H), 1.68 (s, 6H), 1.90–2.22 (m, 11H), 2.36–2.46 (m, 2H), 2.95 (d, J = 6.4 Hz, 2H), 5.00 (t, J = 6.4 Hz, 1H), 5.09 (t, J = 6.8 Hz, 2H) ppm; 13C NMR (CDCl3) δ = −3.4, −3.2, 16.0, 16.3, 17.7, 18.3, 21.1, 21.6, 25.6, 25.7, 26.7, 26.8, 28.6, 39.6, 39.7, 39.7, 45.2, 121.8, 122.6, 124.4, 124.4, 131.2, 134.7, 134.9, 168.1, 199.0 ppm; IR ν = 2957, 2929, 2858, 1718, 1657, 1617, 1472, 1463, 1370, 1318, 1254, 1232, 1113, 1053, 1005, 937, 899, 826, 813, 784, 741 cm−1; HRMS (FAB) calcd for C28H49O2Si 445.3502, found 445.3509.
Grifolin: 5-methyl-2-((2E,6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-yl)benzene-1,3-diol (1). To a stirred solution of TBS-ether 13 (98 mg, 0.22 mmol) in THF (5 mL)/hexane (10 mL) at −78 °C under an argon atmosphere, 1 M THF solution of LHMDS (1.1 mL, 1.10 mmol) was added. The mixture was stirred at −78 °C for 1 h and a solution of Ts-Br (60 mg, 0.25 mol) in THF (2 mL) was added dropwise to the reaction mixture. The reaction was quenched with saturated NH4Cl solution (2 mL) in 15 min at −78 °C. The mixture was extracted with EtOAc, washed with H2O, dried over anhydrous Na2SO4, filtered, and concentrated. The crude product was then treated with 1 M THF solution of TBAF (0.44 mL, 0.44 mol). The reaction mixture was stirred at room temperature for 15 min and quenched with 1 M HCl. The mixture was extracted with EtOAc, washed with H2O, dried over anhydrous Na2SO4, filtered, and concentrated. The crude product was purified by SiO2 flash column chromatography (7–15% EtOAc/hexane) to give grifolin 1 (37 mg, 0.11 mmol) in 50% yield as an orange-yellow oil.
Data for 1: 1H NMR (CDCl3) δ = 1.59 (s, 3H), 1.60 (s, 3H), 1.68 (s, 3H), 1.81 (s, 3H), 1.92–2.01 (m, 2H), 2.01–2.16 (m, 6H), 2.21 (s, 3H), 3.39 (d, J = 7.2 Hz, 2H), 5.02–5.16 (m, 4H), 5.27 (t, J = 7.2 Hz, 1H), 6.24 (s, 2H) ppm; 13C NMR (CDCl3) δ = 16.0, 16.2, 17.7, 21.1, 22.2, 25.7, 26.4, 26.7, 39.7, 39.7, 109.0, 110.4, 121.7, 123.6, 124.4, 131.3, 135.6, 137.5, 138.9, 154.8 ppm; IR ν = 3608, 3430 (br), 3015, 2922, 2852, 1760, 1699, 1628, 1589, 1449, 1336, 1211, 1041, 988, 909, 822, 756 cm−1; HRMS (ESI) calcd. for C22H32O2+H 329.2481, found 329.2476.
Experimental Procedures for the Synthesis of Neogrifolin 2
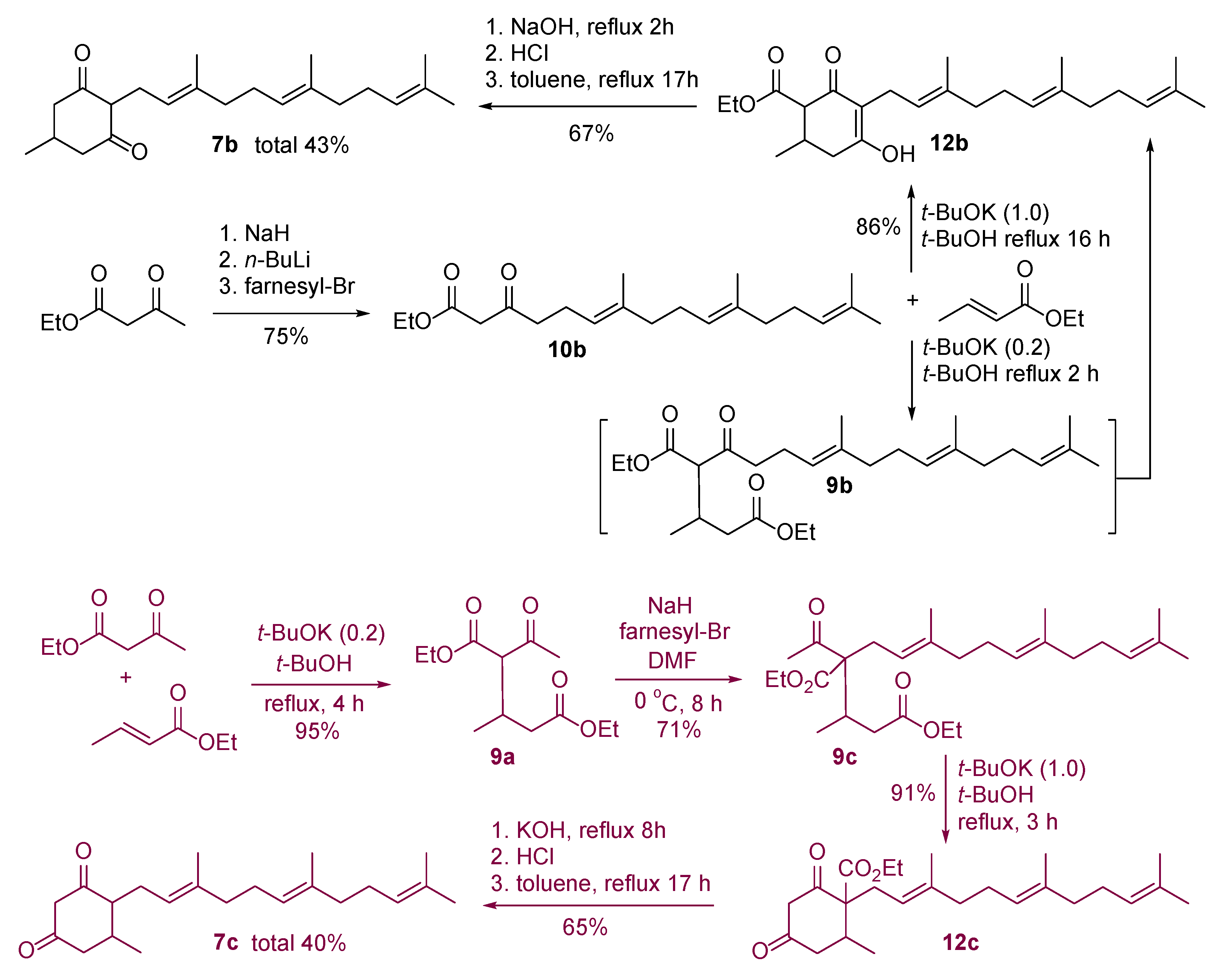
Diethyl 2-acetyl-3-methylpentanedioate (9a). To a stirred solution of ethyl acetoacetate (7.00 g, 53.76 mmol) in t-BuOH (50 mL) at 25 °C under an argon atmosphere, ethyl crotonate (6.13 g, 53.76 mmol) and t-BuOK (1.21 g, 10.75 mmol) were added. The reaction mixture was heated at 90 °C for 4 h and the mixture was quenched with 1 M HCl. The mixture was extracted with EtOAc, dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure to give the crude product (12.94 g), which was purified by SiO2 flash column chromatography (7–20% EtOAc/hexane eluent) to produce the addition product 9a (11.45 g, 51.07 mmol) in 95% yield as a light-yellow liquid (~1.1:1 mixture of diastereomers).
Data for 9a: Rf = 0.36 (4:1 hexane/EtOAc); 1H NMR (CDCl3) δ = 1.03 (d, J = 7.2 Hz, 3H) 1.01* (d, J = 6.8 Hz, 3H), 1.23–1.30 (m, 6H; OCH2CH3), 2.24 (s, 3H), 2.22–2.30 (m, 1H), 2.40 (dd, J = 15.6, 6.4 Hz, 1H), 2.48* (dd, J = 15.6, 4.0 Hz, 1H), 2.70–2.82 (m, 1H), 3.47* (d, J = 8.4 Hz, 1H), 3.55 (d, J = 8.0 Hz, 1H), 3.99–4.24 (m, 4H; OCH2CH3) ppm; 13C NMR (CDCl3, major peaks) 14.2, 14.3, 17.4, 29.8, 30.1, 38.9, 60.6, 61.5, 64.1, 168.9, 172.2, 202.7 ppm; IR ν = 3370 (w), 2975, 2929, 1730, 1603, 1446, 1383, 1367, 1298, 1223, 1186, 1095, 1025, 915, 848, 732 cm−1; HRMS (ESI) calcd. for C12H20O5+Na 267.1208, found 267.1203.
*: peaks from the minor stereoisomer.
Diethyl 2-acetyl-3-methyl-2-((2E,6E)-3,7,11-trimethyldodeca-2,6,10-trien-1yl)pentanedioate (9c). To a stirred solution of the coupling product 9a (1.50 g, 6.14 mmol) in DMF (20 mL) at 0 °C under an argon atmosphere, NaH (60% in mineral oil, 0.18 g, 7.37 mmol) was carefully added in several portions. Stirring at 0 °C for 1.5 h, the mixture was treated with a DMF solution (10 mL) of farnesyl bromide (1.40 g, 4.91 mmol). The reaction mixture was stirred at 0 °C for 8 h and quenched with 1 M HCl. The mixture was extracted with EtOAc, dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure to give the crude product (2.87 g), which was purified by SiO2 flash column chromatography (3–10% EtOAc/hexane eluent) to produce the coupling product 9c (1.54 g, 3.44 mmol) in 71% yield as a yellow oil (~2:1 mixture of diastereomers).
Data for 9c: Rf = 0.66 (1:4 EtOAc/hexane); 1H NMR (CDCl3) δ = 0.94 (d, J = 7.2 Hz, 3H) 0.96* (d, J = 6.8 Hz, 3H), 1.23–1.32 (m, 6H; OCH2CH3), 1.58 (s, 3H), 1.60 (s, 3H), 1.62 (s, 3H), 1.68 (s, 3H), 1.93–2.09 (m, 10H), 2.13 (s, 3H), 2.15* (s, 3H), 2.52–2.69 (m, 2H), 2.72–2.82 (m, 1H), 4.08–4.27 (m, 4H; OCH2CH3), 4.97 (t, J = 8.0 Hz, 1H), 5.07 (t, J = 6.8 Hz, 1H), 5.09 (t, J = 6.8 Hz, 1H) ppm; 13C NMR (CDCl3, major peaks) δ = 14.2, 14.3, 15.4, 16.0, 16.3, 17.7, 25.7, 26.6, 26.8, 28.3, 30.7, 32.5, 38.4, 39.7, 39.9, 60.4, 61.1, 66.3, 117.8, 123.9, 124.4, 131.3, 135.2, 138.8, 171.5, 172.9, 205.0 ppm; IR ν = 2979, 2929, 1733, 1708, 1446, 1374, 1354, 1301, 1266, 1221, 1178, 1095, 1029, 860, 736 cm−1; HRMS (ESI) calcd. for C27H44O5+Na 471.3086, found 471.3082.
*: peaks from the minor stereoisomer.
Ethyl 2-methyl-4,6-dioxo-1-((2E,6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-yl)cyclohexane-1-carboxylate (12c). To a stirred solution of coupling product 9c (1.80 g, 4.01 mmol) in t-BuOH (20 mL), t-BuOK (0.45 g, 4.01 mmol) was added. The mixture was heated at 80 °C for 3 h. The mixture was cooled to room temperature, quenched with 1 M HCl, extracted with EtOAc, dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The crude product was purified by SiO2 flash column chromatography (15–50% EtOAc/hexane eluent) to produce the cyclized product 12c (1.45 g, 3.63 mmol) in 91% yield as a light-yellow liquid (it contains a diastereomer).
Data for 12c: Rf = 0.37 (2:3 EtOAc/hexane); 1H NMR (CDCl3) δ = 1.09 (d, J = 6.0 Hz, 3H), 1.23 (t, J = 7.2 Hz, 3H), 1.58 (s, 3H), 1.60 (s, 3H), 1.66 (s, 3H), 1.68 (s, 3H), 1.91–2.11 (m, 8H), 2.46–2.62 (m, 3H), 2.70 (dd, J = 14.4, 8.8 Hz, 1H), 2.88 (dd, J = 14.4, 6.0 Hz, 1H), 3.40 (d, J = 14.4 Hz, 1H), 3.54 (d, J = 14.4 Hz, 1H), 4.10–4.24 (m, 2H; OCH2CH3), 4.93 (t, J = 7.4 Hz, 1H), 5.04 (t, J = 7.2 Hz, 1H), 5.07 (t, J = 6.8 Hz, 1H) ppm; 13C NMR (CDCl3) δ = 14.1, 15.8, 16.1, 16.4, 17.7, 25.7, 26.4, 26.7, 30.2, 30.5, 39.7, 40.0, 44.7, 57.7, 61.7, 62.9, 117.1, 123.8, 124.2, 131.4, 135.5, 140.6, 169.9, 202.9, 203.2 ppm; IR ν = 2967, 2915, 2855, 1733, 1596, 1445, 1410, 1384, 1366, 1297, 1217, 1188, 1160, 1095, 1019, 944, 850, 754 cm−1; HRMS (ESI) calcd. for C25H38O4+H 403.2848, found 403.2847.
5-methyl-4-((2E,6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-yl)cyclohexane-1,3-dione (7c). The above cyclized product 12c (300 mg, 0.74 mmol) in THF was treated with 50% KOH (15 mL), and heated at reflux for 8 h. The mixture was cooled to room temperature and acidified with 50% HCl (25 mL). The reaction mixture was extracted with EtOAc, dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure to give orange sticky oil (0.27 g). The crude product was then dissolved in toluene (20 mL) and heated at 110 °C for 17 h. Upon cooling to room temperature, the mixture was concentrated under reduced pressure. The crude product was purified by SiO2 flash column chromatography (15–40% EtOAc/hexane eluent) to give an 1,3-cyclohexanedione 7c (160 mg, 0.48 mmol) in 65% yield as a light-yellow liquid.
Data for 7c: Rf = 0.20 (EtOAc/hexane); 1H NMR (CDCl3) δ = 1.08 (d, J = 6.8 Hz, 3H), 1.59 (s, 3H), 1.60 (s, 3H), 1.65 (s, 3H), 1.68 (s, 3H), 1.93–2.13 (m, 9H), 2.18–2.55 (m, 4H), 2.73 (ddd, J = 12.0, 4.8, 1.2 Hz, 1H), 3.34 (d, J = 17.2 Hz, 1H), 3.43 (dd, J = 17.2, 1.2 Hz, 1H), 5.07 (t, J = 6.8 Hz, 1H), 5.08 (t, J = 6.8 Hz, 2H) ppm; 13C NMR (CDCl3) δ = 16.0, 16.2, 17.7, 20.1, 25.7, 26.4, 26.7, 26.8, 29.1, 39.7, 39.7, 46.7, 56.1, 57.5, 119.9, 123.9, 124.3, 131.4, 135.2, 138.0, 203.7, 205.6 ppm; IR ν = 2962, 2915, 1732, 1592, 1448, 1381, 1297, 1265, 1220, 1185, 1161, 1106, 1021, 910, 843, 734 cm−1; HRMS (ESI) calcd. for C22H34O2+H 331.2636, found 331.2637.
5-Methyl-6-((2E,6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-yl)cyclohexa-1,3-diene-1,3-diyl diacetate (14). To a stirred solution of cyclohexane-1,3-dione 7c (520 mg, 1.57 mmol) in THF (20 mL) at −78 °C under an argon atmosphere, 2 M THF solution of LDA (2.75 mL, 5.50 mmol) was added. The resulting mixture was stirred at −78 °C for 1 h before acetic anhydride (0.38 mL, 4.00 mmol) and DMAP (0.19 g, 1.57 mmol) were added. The reaction mixture was stirred at −78 °C for 30 min and quenched with 1 M HCl. The mixture was extracted with EtOAc, dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The crude product was purified by SiO2 flash column chromatography (2.5–7% EtOAc/hexane eluent) to produce diacetate 14 (521 mg, 1.25 mmol) in 80% yield (a ~1:1 mixture of diastereomers) as a light-yellow oil.
Data for 14: Rf = 0.66 (1:4 EtOAc/hexane); 1H NMR (CDCl3) δ = 1.08 (d, J = 7.2 Hz, 3H), 1.59 (s, 3H), 1.60 (s, 6H), 1.68 (s, 3H), 1.94–2.12 (m, 10H), 2.13 (s, 3H), 2,14 (s, 3H), 2.18–2.24 (m, 1H), 2.32–2.41 (m, 1H), 5.04–5.13 (m, 3H), 5.22 (dd, J = 6.0, 1.6 Hz, 1H), 5.61 (d, J = 1.6 Hz, 1H) ppm; 13C NMR (CDCl3)* δ = 16.0 (16.0), 17.7, 19.1, 20.4 (20.9), 21.0 (21.3), 21.5, 25.7, 26.5 (26.6), 26.8, 29.3 (31.6), 31.3 (31.9), 39.7, 39.8 (39.9), 43.9 (46.0), 107.0 (108.9), 112.8 (113.4), 120.9 (121.1), 123.8 (124.3), 124.1 (124.4), 131.3 (131.4), 135.1 (135.3), 138.0 (138.5), 142.9 (153.1), 166.7 (167.8), 168.8 (169.0), 183.7 (185.1) ppm; IR ν = 2962, 2922, 2855, 1765, 1614, 1448, 1367, 1275, 1190, 1119, 1096, 1006, 896, 846, 773, 734 cm−1; HRMS (ESI) calcd. C26H38O4+H+ for. 415.2848, found 415.2845.
*: diastereomeric peaks are notified in parenthesis.
5-Methyl-4-((2E,6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-yl)-1,3-phenylene diacetate. To a stirred solution of diacetate 14 (130 mg, 0.31 mmol) in CH2Cl2, DDQ (0.10 g, 0.44 mmol) was added. The mixture was stirred at 25 °C for 5 h and quenched with H2O. The mixture was then extracted with CH2Cl2, dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The crude product was purified by SiO2 flash column chromatography (5–10% EtOAc/hexane eluent) to produce the aromatized diacetate (66 mg, 0.16 mmol) in 52% yield as a colorless oil.
Data: Rf = 0.54 (EtOAc/hexane); 1H NMR (CDCl3) δ = 1.58 (s, 3H), 1.59 (s, 3H), 1.67 (s, 3H), 1.73 (s, 3H), 1.92–2.10 (m, 8H), 2.26 (s, 3H), 2.28 (s, 3H), 2.29 (s, 3H), 3.22 (d, J = 6.4 Hz, 2H), 4.98 (t, J = 6.4 Hz, 1H), 5.04–5.13 (m, 2H), 6.70 (d, J = 2.4 Hz, 1H), 6.80 (d, J = 2.4 Hz, 1H) ppm; 13C NMR (CDCl3) δ = 16.0, 16.2, 17.7, 19.8, 20.9, 21.1, 25.7, 25.8, 26.6, 26.7, 39.6, 39.7, 113.5, 120.9, 121.2, 124.0, 124.4, 129.7, 131.3, 135.1, 135.8, 139.2, 148.4, 149.1, 169.3, 169.3 ppm; IR ν = 2919, 1769, 1617, 1588, 1480, 1441, 1367, 1289, 1195, 1119, 1046, 1017, 977, 902, 833, 772 cm−1; HRMS (FAB) calcd. for. C26H36O4+H+ 413.2692, found 413.2701.
Neogrifolin: 5-methyl-4-((2E,6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-yl)benzene-1,3-diol (2). To a stirred solution of the above aromatized diacetate (40 mg, 0.097 mmol) in MeOH (5 mL) at 0 °C a 50% aqueous solution of KOH (28 mg, 0.5 mmol) was added. The mixture was stirred at 25 °C for 10 min and quenched with 1 M HCl. The mixture was then extracted with EtOAc, dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The crude product was purified by SiO2 flash column chromatography (10–15% EtOAc/hexane) to produce neogrifolin 2 (31 mg, 0.094 mmol) in 97% yield as a colorless oil.
Data for 2: 1H NMR (CDCl3) δ = 1.58 (s, 3H), 1.59 (s, 3H), 1.67 (s, 3H), 1.79 (s, 3H), 1.93–2.14 (m, 8H), 2.23 (s, 3H), 3.29 (d, J = 6.8 Hz, 2H), 4.44 (s, 1H), 5.07 (dt, Jt = 6.8, Jd = 1.2 Hz, 2H), 5.08 (s, 1H), 5.15 (dt, Jt = 6.8, Jd = 1.2 Hz, 1H), 6.21 (d, J = 2.0 Hz, 1H), 6.26 (d, J = 2.0 Hz, 1H) ppm; 13C NMR (CDCl3) δ = 16.0, 16.2, 17.7, 20.1, 20.1, 25.1, 25.7, 26.4, 26.7, 39.7, 101.0, 109.6, 117.9, 122.0, 123.7, 124.4, 131.3, 135.5, 137.8, 138.5, 154.2, 155.5 ppm; IR ν = 3396 (br s), 2965, 2919, 2854, 1596, 1508, 1465, 1449, 1377, 1329, 1279, 1201, 1167, 1138, 1048, 980, 831, 791, 756 cm−1; HRMS (FAB)calcd. for C22H32O2 328.2402, found 328.2401.