Valorization of Agri-Food Waste into PHA and Bioplastics: From Waste Selection to Transformation
Abstract
1. Introduction
- −
- Food processing: about 39% of the waste.
- −
- Primary production (agriculture and livestock): about 33%.
- −
- Distribution and retail: about 5%.
- −
- Restaurants and catering: about 14%.
- LIFE Biogas. Focuses on the development of technologies for the production of biogas from organic waste. It also aims to improve the efficiency of converting waste into renewable energy and fertilizer [7].
- RES URBIS (Resources from Urban Bio-Waste). Focuses on the conversion of urban organic waste into bioplastics, in particular, polyhydroxyalkanoates or PHA’s. It explores different ways of integrating urban waste into biorefineries [8].
- AgriMax. Aims to valorize agricultural by-products and crop residues through biorefineries. In addition to converting them into bioproducts, the project focuses on reducing waste in the food supply chain and maximizing the use of agricultural by-products. The project yielded a number of products, including bioplastics, food additives (such as lycopene), and biocomposite fibers. The bioplastic obtained was polyhydroxyalkanoate (PHA), produced from agricultural and food waste. One of the fibers obtained was for biocomposites, using agricultural waste such as tomato bagasse and cereal husks. These fibers were integrated into bioplastics, creating biocomposite materials for applications such as sustainable packaging and other industrial products [9].
- WASTE2FUNC. The project yielded the production of biosurfactants, including sophorolipids, and lactic acid. The project aims to create a supply chain that collects organic waste and turns it into high-value-added products, helping to reduce the environmental impact of the agri-food sector [10].
- United States. In 2024, the United States Department of Agriculture (USDA) entered into 38 cooperative agreements with the objective of supporting innovative and scalable waste management schemes, with the ultimate goal of reducing and diverting food waste from landfills [11].
- China. One illustrative example is the AgriLoop project, an international collaboration since 2024 that involves 13 Chinese academic and industrial organizations and 22 European partners. The objective of this project is to develop sustainable processes for the conversion of agri-food waste into high-value-added products. A cascade biorefinery approach will be employed to develop sustainable integrated processes for the conversion of agri-food waste (including tomato, soybean, straw, potato, brewery, winery, livestock, and other materials) into high-value, environmentally friendly products. These products will include plant and microbial proteins, polyesters, and other bio-based chemicals for use in food, feed, health, and material applications, particularly within the agricultural sectors [12].
- Russia. The EU-funded SUST-RUS project developed until the end of 2011 a spatial, economic, and ecological model for the assessment of sustainability policies in Russia. The project team collated and verified a comprehensive database comprising social, economic, and environmental data, with the objective of reconciling disparate policy objectives and advancing sustainable development in the country [13].
- The United Arab Emirates (UAE). The management of sustainable agricultural waste has become a prominent issue in recent years. A notable case in point is the project that has been implemented in Hatta since 2024, where approximately 27 tons of agricultural waste is managed on a daily basis. The waste is transported in an appropriate manner to Warsan’s waste-to-energy plant, thereby contributing to a reduction in landfill waste and the generation of clean energy [14]. Therefore, the project bets for an energy valorization instead of a material valorization.
- Aerobic fermentation for bioplastics production:
- −
- Polyhydroxyalkanoates (PHAs). PHAs are biopolymers produced by microorganisms, such as bacteria, under specific microbial stress conditions: excess carbon and lack of nutrients (e.g., nitrogen or phosphorus). Their properties, similar to those of conventional plastics, together with their biodegradability, make them of particular interest in the search for sustainable alternatives to petroleum-based products [15]. These biopolymers can be produced from any agri-food waste that can be previously transformed into a VFA solution.
- −
- Polylactic acid (PLA). From whey, lactic acid can be produced by fermentation, which will be used as a raw material for the synthesis of PLA, a biodegradable bioplastic [16].
- −
- Polybutylene succinate (PBS). A biodegradable biopolymer derived from renewable raw materials or agricultural waste, such as agri-food by-products. It stands out for its biodegradability and is a flexible, resistant material used in applications such as packaging and single-use products, especially in the food and agricultural sector [17].
- Composting. Compost improves soil structure by increasing water-holding capacity and promoting biological activity. It is produced by the aerobic decomposition of organic matter in by-products such as fruit peels, vegetable waste, manure, etc. It is one of the most common forms of waste valorization [18].
- Vermicomposting. This is a variation in the previous process in which worms (e.g., Eisenia fetida) are used to speed up the process. The result is a biofertilizer with properties similar to or better than conventional compost because it contains a higher number of beneficial microorganisms and readily available nutrients [19].
- Biochar. Biochar is a solid by-product from the pyrolysis of agricultural and food waste that can be used as a soil amendment. It improves the soil’s ability to retain nutrients and water, while increasing the stability of organic matter in the soil [20].
- Anaerobic digestion. Agri-food waste is transformed in the absence of oxygen by microorganisms. The product is a gaseous mixture (biogas), and a stabilized residue or sludge (digestate) containing microorganisms responsible for the degradation of organic matter. The process is characterized by four distinct metabolic stages: hydrolysis, acidogenesis, acetogenesis, and methanogenesis. These stages are mediated by different groups of specialized microorganisms [21]. In terms of valorization, digestate is used as a biofertilizer. It may or may not undergo additional stabilization processes [22]. Furthermore, the gas phase is predominantly composed of CO2 and CH4, with the latter being a viable energy source [23].
- Dark fermentation. It is an anaerobic fermentation stopped at the acidogenesis step where agri-food waste produces a VFA solution. The VFA can be used to produce biofuels or high-value-added compounds such as bioplastics. The gas phase is rich in biohydrogen, which can be valorized [24].
- −
- Type of substrate. This influences the type and quantity of VFAs. Waste rich in carbohydrates generally favors the production of acetic acid, whereas waste rich in fat can increase the production of butyric acid.
- −
- Operating conditions. Factors such as pH, solids concentration, and temperature also influence the type and amount of VFA produced.
- −
- Microorganisms. The microorganisms present in the fermentation medium are also important factors in an anaerobic fermentation process. Genera such as Clostridium, Lactobacillus, and Bacteroides play an important role in VFA production [25].
- −
- Biofuel production. VFAs are used as precursors in biogas production, where they are converted to CH4 by methanogenesis during anaerobic digestion.
- −
- Food industry. VFAs are used as preservatives, acidity regulators, and food additives due to their antimicrobial properties.
- −
- Bioplastic production. They are used as feedstocks for aerobic fermentation in the production of polyhydroxyalkanoates (PHAs), biodegradable plastics used as alternatives to petroleum-based products.
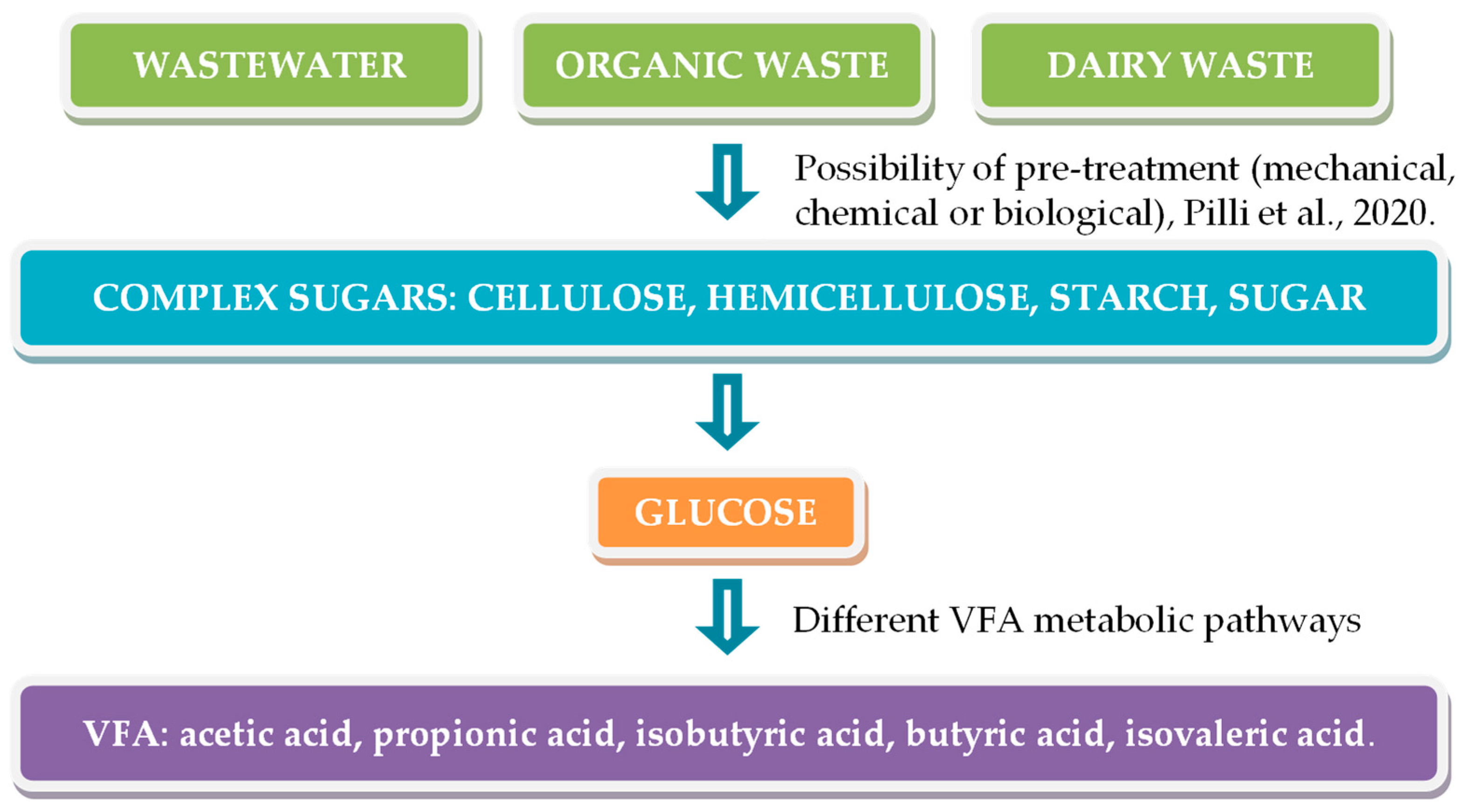
2. Production of Valeric- and Propionic-Rich VFA Streams from Agri-Food Wastes
2.1. Dark Fermentation: Method to Transform Agri-Food Waste into VFA
- (1)
- Hydrolysis. Complex organic polymers—carbohydrates, proteins, and lipids—are converted into simpler monomers: monosaccharides, amino acids, fatty acids, and glycerol. To do this, microorganisms secrete hydrolytic enzymes that break down the polymers.
- (2)
- Acidogenesis. The soluble monomers produced in the previous stage are fermented by acidogenic bacteria to produce VFAs, alcohols, CO2, and H2, among other compounds.
- (3)
- Acetogenesis. The VFAs and alcohols produced in the previous phase are converted to acetic acid, hydrogen, and CO2.
- (4)
- Methanogenesis. The compounds formed in acetogenesis are converted to biogas (mainly CH4 and CO2) by methanogenic archaea.
2.2. Agri-Food Waste Typically Used for VFA Production
- Vegetable waste. Agriculture, food processing, and surplus sales generate sugar-rich wastes that can be anaerobically fermented to produce VFAs. Their high carbohydrate content favors the formation of acetic, propionic, and valeric acids, depending on the fermentation conditions. Wainaina et al. reported in 2020 that these residues can produce a high yield of VFAs, especially when an appropriate carbon-to-nitrogen (C/N) ratio is achieved [37].
- Vegetable oil industry wastes. Residues from oil extraction, such as palm and soybean waste, are rich in lipids and carbohydrates. Almomani et al. found in 2020 that these wastes are highly valorized for VFA production, especially when co-fermented with other wastes containing higher amounts of carbohydrates [38].
- Whey. A by-product of the dairy industry that is an excellent source of carbohydrates and proteins. Jiang et al. already found in 2015 that bacteria such as Propionibacterium can enhance propionic acid production when the pH and retention time conditions are suitable [39].
- Fish industry waste. The valorization of fish waste, such as tuna, for the production of biochemical compounds has been studied. In 2017, Bermúdez-Penabad et al. published a study in which they improved the efficiency of the process by adjusting the operating conditions of the bioreactor. [40].
- Industrial wastewater from agri-food transformation sector. This type of waste is rich in organic compounds that are suitable for anaerobic fermentation to produce VFAs [41]. According to Agler et al. (2011), by adjusting the C/N ratio and maintaining a suitable pH between 5 and 6.5, it is possible to obtain a VFA profile with high levels of valeric and propionic acids. Recent studies have shown that the co-fermentation of agro-industrial residues can maximize the production of VFAs with these compounds [41]. This type of waste includes vinasses, a liquid by-product of alcohol distillation in beverage production. They contain carbohydrates, organic acids, and other fermentable compounds that can be converted into VFAs, such as acetic, propionic, and valeric acids, depending on the microorganisms and conditions used in the process. Subjecting vinasse to anaerobic digestion can result in the production of VFAs where the main component is acetic acid, with lower concentrations of propionic and valeric acids, as demonstrated by Rajes and Ganesan in their study published in 2011 [42].
- Low value-added starches. Derived from agricultural by-products, these residues can be pre-treated, for example, by enzymatic hydrolysis, to break them down into simple sugars that can be anaerobically fermented to produce VFAs. An example of low-value starch is brewer’s spent grain, which is rich in fiber and carbohydrates and can also be valorized by anaerobic fermentation to produce VFAs, including valeric and propionic acids, as shown by Sukphun et al. in their study (2021) [43]. Finally, Achinas et al. (2019) demonstrated that potato processing residues, such as peel or pulp, are also suitable for VFA production. Pretreatment and co-fermentation, as well as the adjustment of pH conditions and the inoculum/substrate ratio, can optimize valeric acid production [44].
2.2.1. Production of VFA, Rich in Valeric Acid and Propionic Acid, from Cheese Whey
- Substrate. A high Kjeldahl nitrogen content has been shown to increase the propionic acid yield to 78% of the total VFA production. Therefore, the characteristics and composition of the substrate are some of the most important factors influencing VFA production and composition [80].
- Retention times. In an anaerobic digestion process, a distinction must be made between the SRT—the average time the microorganisms are in the digester—and the HRT—the time the wastewater or sludge is in the digester. Both times coincide with suspended growth digesters where there is no recirculation. Calero et al. [77] studied three different HRTs in dark fermentation: four, six, and ten days at three different pH levels. They used a sequential bioreactor (SBR) type anaerobic reactor with a working volume of 2 L, with two daily cycles of 12 h, each with four stages: loading, reaction, sedimentation, and liquid extraction. It was operated at a temperature of 30 °C. Under these operating conditions, they concluded that the conditions that maximized the production of valeric and propionic acids were an increase in the SRT, up to 10 days, regardless of the pH used. When the SRT was increased from 4 to 10 at pH 5, propionic acid increased from 13% to 24% of the final VFA composition. Similarly, at pH 5.5 and 6, propionic acid increased from 17% to 24% and 24% to 36%, respectively. In the case of valeric acid, its concentration increased with increasing SRT independently of pH, from 12% to 25%, from 10% to 22%, and from 9% to 15% for pH 5, 5.5, and 6, respectively. Thus, increasing the SRT from 4 to 10 days tended to stimulate the formation of odd VFA (propionic and valeric) to the detriment of even VFA such as acetic and butyric.
- pH. A less acidic pH, a change from 5 to 6, stimulates the production of propionic acid and reduces that of valeric acid, regardless of the SRT used [77]. Other studies [81,82] use a different type of reactor, in this case, a continuous stirred tank reactor (CSTR) with an SRT of 48 h, also conclude that increasing the pH from 5.25 to 6 favors the production of propionic acid to the detriment of butyric acid. A distinct study was conducted by Yu and Fang [83], which showed that propionate is favored during the acidogenesis of dairy wastewater (simulated dairy wastewater, prepared from full-cream powdered milk) at pH 4.0–4.5.
- Organic loading rate (OLR). The organic loading rate indicates the amount of material used in the reactor feed, and can be expressed in terms of COD or volatile solids (VSs). Pérez Morales et al. [80], no clear trend was observed between changes in organic loading and VFA yield and production; Calero et al. [77] observed that an increase in organic loading had a negative effect on VFA production when whey was used as substrate. The increase in OLR from 3 to 12 g COD L−1 d−1 means a decrease in the acidification degree from 94% to 65%. Regarding the VFA profile, in the same study, it was concluded that a lower organic load improves the production of propionic and valeric acids; the highest ratio of both acids occurred at an OLR of 3 g COD L−1 d−1. The effect of OLR on the distribution of the different VFA is illustrated in the figure below (Figure 2).
2.2.2. Production of VFAs Rich in Valeric Acid and Propionic Acid from Low-Value-Added Starches
- (a)
- Potato industry waste
- Acetic acid was the main product treated at pH 7.0 and 11.0, representing 46–77% and 74–92% of the total VFAs, respectively, while butyric acid was the main product at pH 5.0 and uncontrolled pH, representing 11–75% and 38–82% of the VFA mixture, respectively.
- In the pH 7.0 treatment, the percentage of propionic acid increased as the fermentation progressed, while the acetic acid content decreased. The highest propionic acid production was obtained at pH 7.0. At this level, a favorable balance is achieved between the activity of acidogenic microorganisms and the production of propionates, while the production of other VFAs, which could predominate at lower or higher pH levels, is reduced.
- In particular, the percentage of butyric acid decreased with increasing pH. When treated at pH 5.0 and uncontrolled pH, the hexanoic acid content was about 20% of the total VFA at the end of fermentation.
- (b)
- Beer industry waste
2.2.3. Production of VFAs Rich in Valeric Acid and Propionic Acid from Low Value-Added Starches and Cheese Whey: Co-Fermentation
2.2.4. Production of VFAs Rich in Valeric Acid and Propionic Acid from Expired Juices
2.2.5. Production of VFAs Rich in Valeric Acid and Propionic Acid from Non-Agri-Food Waste
3. Production of Bioplastics from Volatile Fatty Acids
- In the glyoxysomes, the glyoxylate cycle converts acetyl-CoA to malate.
3.1. Microorganisms Responsible for the Transformation of VFA into PHA
3.1.1. Phosphorus-Accumulating Organisms
- PAO can utilize volatile fatty acids, such as acetic acid and propionic acid, found in sewage and other organic wastes. This allows the use of low-cost materials, creating a more economical and sustainable PHA production process, rather than relying on purified carbon sources such as glucose. This capability contributes to the circular economy concept by enabling the valorization of waste [133,134].
- PHA production in an anaerobic-aerobic cycle. Under anaerobic conditions, storage cells integrate PHAs using VFAs as a carbon source and store it in their cells as an energy reserve. In the aerobic phase, they consume this PHA for phosphorus accumulation, a process specific to PAOs. This anaerobic-aerobic cycle has the advantage that PHA production does not require continuous aeration, thus reducing energy consumption compared to other microorganisms that produce PHAs only under aerobic conditions [132].
- Flexibility in the production of different types of PHA. PAOs can produce different types of PHA, such as poly(3-hydroxybutyrate) (PHB) or poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) [135]. This flexibility in the type of PHA allows the mechanical and thermal properties of the bioplastic to be tailored to specific applications, such as biodegradable packaging or medical materials products [136].
- Compatibility with wastewater treatment. Already found in biological wastewater treatment systems due to their role in phosphorus removal, PHA production systems could be integrated into water treatment facilities, making use of existing infrastructure and eliminating the need for separating production facilities. This would allow simultaneous nutrient removal and bioplastic production, maximizing system efficiency [137].
3.1.2. Mechanism of PHA Accumulation. Accumulating Microorganisms
3.2. Accumulation of PHA Fermentation Conditions
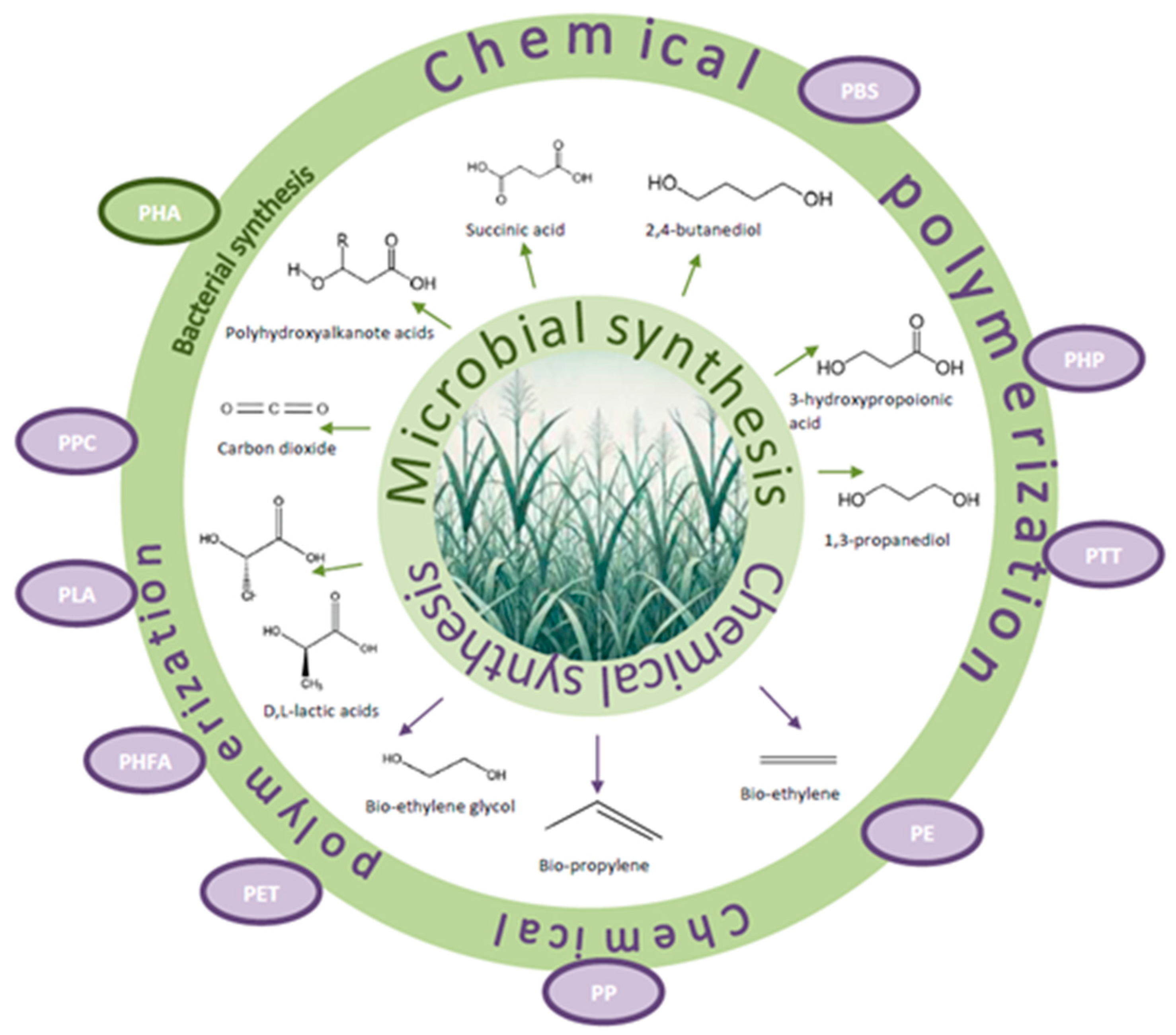
3.3. Physico-Chemical Properties of Bioplastics and Their Applications
3.3.1. Polyhydroxyalkanoates (PHAs)
3.3.2. Other Bioplastics
- (a)
- Poly(lactic acid) (PLA)
- (b)
- Poly(butylene succinate) (PBS).
- Disposable tableware and utensils, including tableware [231].
- (c)
- Poly(propylene carbonate) (PPC)
3.4. Economic Aspects and Technology Barriers
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- European Commission. Preparatory Study on Food Waste Across EU 27; Technical Report—2010-054; European Commission: Brussels, Belgium, 2010. [Google Scholar]
- European Parliament and Council of the European Union. Directive 2018/851 of the European Parliament and of the Council of 30 May 2018 amending Directive 2008/98/EC on waste. Off. J. Eur. Union 2018, L150, 109. [Google Scholar]
- MIT Climate. (n.d.). What Makes Methane a More Potent Greenhouse Gas Than Carbon Dioxide? Available online: https://climate.mit.edu/ask-mit/what-makes-methane-more-potent-greenhouse-gas-carbon-diox (accessed on 5 September 2024).
- MIT Climate. (n.d.). Why Do We Compare Methane to Carbon Dioxide over a 100-Year Timeframe? Are We Underrating Methane’s Impact? Available online: https://climate.mit.edu/ask-mit/why-do-we-compare-methane-carbon-dioxide-over-100-year-timeframe-are-we-underrating (accessed on 5 September 2024).
- European Commission. EU Methane Strategy; European Commission: Brussels, Belgium, 2020; Available online: https://ec.europa.eu/energy/sites/ener/files/eu_methane_strategy.pdf (accessed on 5 September 2024).
- European Research Executive Agency. 135 New Projects in the Areas of Food, Bioeconomy, Natural Resources, Agriculture, and Environment Are Starting Their Research; European Research Executive Agency: Brussels, Belgium, 2024. [Google Scholar]
- BiogasNet. (n.d.). LIFE Biogas. Available online: https://biogasnet.eu/es/ (accessed on 5 September 2024).
- CORDIS. (n.d.). RES URBIS (Resources from Urban Bio-Waste). Available online: https://cordis.europa.eu/project/id/730349/results/ (accessed on 5 September 2024).
- AgriMax Project. (n.d.). AgriMax: Valorising Agricultural Residues and By-Products Through Biorrefineries. Available online: https://www.cbe.europa.eu/projects/agrimax (accessed on 5 September 2024).
- Waste2Func Project. (n.d.). WASTE2FUNC: Converting Agrofood Residues into Valuable Products. Available online: https://www.waste2func.eu/en/ (accessed on 5 September 2024).
- U.S. Department of Agriculture (USDA). (n.d.). El USDA Invierte Unos 115 Millones de Dólares en Proyectos de Compostaje y Reducción de Residuos. Available online: https://www.nrcs.usda.gov/news/el-usda-invierte-unos-115-millones-de-dolares-en-proyectos-de-compostaje-y-reduccion-de (accessed on 5 September 2024).
- Agriloop Project. (n.d.). High-value Products from Agricultural Residues Through Sustainable Chains. Available online: https://www.agriloop-project.eu/about/ (accessed on 28 December 2024).
- CORDIS. A Sustainability Model for Russia. Available online: https://cordis.europa.eu/article/id/90572-a-sustainability-model-for-russia/en (accessed on 3 August 2017).
- Waste Recycling Magazine. Dubai Municipality Rolls out Comprehensive Waste Management, Recycling Project in Hatta. Available online: https://www.wasterecyclingmag.com/dubai-municipality-rolls-out-comprehensive-waste-management-recycling-project-in-hatta (accessed on 15 December 2024).
- Kourmentza, C.; Plácido, J.; Venetsaneas, N.; Burniol-Figols, A.; Varrone, C.; Gavala, H.N.; Reis, M.A.M. Recent Advances and Challenges towards Sustainable Polyhydroxyalkanoate (PHA) Production. Bioengineering 2017, 4, 55. [Google Scholar] [CrossRef] [PubMed]
- Dedenaro, G.; Costa, S.; Rugiero, I.; Pedrini, P.; Tamburini, E. Valorization of agri-food waste via fermentation: Production of L-lactic acid as a building block for the synthesis of biopolymers. Appl. Sci. 2016, 6, 379. [Google Scholar] [CrossRef]
- Scolaro, C.; Facchin, M.; Brahimi, S.; Belhamdi, H.; Gatto, V.; Beghetto, V. Agri-food wastes for bioplastics: European prospective on possible applications in their second life for a circular economy. Polymers 2022, 14, 2752. [Google Scholar] [CrossRef]
- Lim, S.L.; Lee, L.H.; Wu, T.Y. Sustainability of using composting and vermicomposting technologies for organic solid waste biotransformation: Recent overview, greenhouse gases emissions and economic analysis. J. Clean. Prod. 2016, 111, 262–278. [Google Scholar] [CrossRef]
- Aira, M.; Monroy, F.; Domínguez, J.; Mato, S. How earthworm density affects microbial biomass and activity in pig manure. Eur. J. Soil Biol. 2002, 38, 7–10. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. (Eds.) Biochar for Environmental Management: Science, Technology and Implementation, 2nd ed.; Routledge: London, UK, 2015; p. 976. [Google Scholar] [CrossRef]
- Lorenzo Acosta, Y.; Obaya Abreu, M.C. La digestión anaerobia. Aspectos teóricos. Parte I. ICIDCA. Sobre Deriv. Caña Azúcar 2005, 39, 35–48. [Google Scholar]
- Tambone, F.; Scaglia, B.; D’Imporzano, G.; Schievano, A.; Orzi, V.; Salati, S.; Adani, F. Assessing amendment and fertilizing properties of digestates from anaerobic digestion through a comparative study with digested sludge and compost. Chemosphere 2010, 81, 577–583. [Google Scholar] [CrossRef]
- Kapdan, I.K.; Kargi, F. Bio-hydrogen production from waste materials. Enzym. Microb. Technol. 2006, 38, 569–582. [Google Scholar] [CrossRef]
- Nasir, I.M.; Ghazi, T.I.M.; Omar, R. Anaerobic digestion technology in livestock manure treatment for biogas production: A review. Eng. Life Sci. 2012, 12, 258–269. [Google Scholar] [CrossRef]
- Lee, W.S.; Chua, A.S.M.; Yeoh, H.K.; Ngoh, G.C. A review of the production and applications of waste-derived volatile fatty acids. Chem. Eng. J. 2014, 235, 83–99. [Google Scholar] [CrossRef]
- NoAW Project. H2020 NoAW Project Overview. 2020. Available online: https://noaw2020.eu (accessed on 14 October 2024).
- Ward, A.J.; Hobbs, P.J.; Holliman, P.J.; Jones, D.L. Optimisation of the anaerobic digestion of agricultural resources. Bioresour. Technol. 2008, 99, 7928–7940. [Google Scholar] [CrossRef]
- PROMOFER Project. (n.d.). PROMOFER: Valorisation of Lignocellulosic Biomass and Food Industry Waste. Available online: https://promofer-project.eu/ (accessed on 15 October 2024).
- Jin, A.; del Valle, L.J.; Puiggalí, J. Copolymers and blends based on 3-hydroxybutyrate and 3-hydroxyvalerate units. Int. J. Mol. Sci. 2023, 24, 17250. [Google Scholar] [CrossRef]
- Philip, S.; Keshavarz, T.; Roy, I. Polyhydroxyalkanoates: Biodegradable polymers with a range of applications. J. Chem. Technol. Biotechnol. 2007, 82, 233–247. [Google Scholar] [CrossRef]
- Policastro, G.; Panico, A.; Fabbricino, M. Improving biological production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) co-polymer: A critical review. Rev. Environ. Sci. Bio/Technol. 2021, 20, 479–513. [Google Scholar] [CrossRef]
- Chalima, A.; de Castro, L.F.; Burgstaller, L.; Sampaio, P.; Carolas, A.L.; Gildemyn, S.; Velghe, F.; Ferreira, B.S.; Pais, C.; Neureiter, M.; et al. Waste-derived volatile fatty acids as carbon source for added-value fermentation approaches. FEMS Microbiol. Lett. 2021, 368, fnab054. [Google Scholar] [CrossRef]
- Łukajtis, R.; Hołowacz, I.; Kucharska, K.; Glinka, M.; Rybarczyk, P.; Przyjazny, A.; Kamiński, M. Hydrogen production from biomass using dark fermentation. Renew. Sustain. Energy Rev. 2018, 91, 665–694. [Google Scholar] [CrossRef]
- Bajpai, P. Basics of anaerobic digestion process. In Anaerobic Technology in Pulp and Paper Industry; Springer: Singapore, 2017; pp. 7–12. [Google Scholar]
- Pandey, A.K.; Pilli, S.; Bhunia, P.; Tyagi, R.; Surampalli, R.Y.; Zhang, T.C.; Kim, S.-H. Dark fermentation: Production and utilization of volatile fatty acid from different wastes—A review. Chemosphere 2022, 288, 132444. [Google Scholar] [CrossRef] [PubMed]
- Pilli, S.; Pandey, A.K.; Katiyar, A.; Pandey, K.; Tyagi, R.D. Pre-treatment technologies to enhance anaerobic digestion. In Sustainable Sewage Sludge Management and Resource Efficiency; IntechOpen: London, UK, 2020; p. 24. [Google Scholar]
- Wainaina, S.; Lukitawesa; Kumar, A.; Taherzadeh, M.J. Valorization of organic waste to biogas and volatile fatty acids: A review. Bioengineered 2020, 11, 628–648. [Google Scholar]
- Almomani, F.; Bhosale, R.R.; Khraisheh, M. Utilization of industrial oily food wastes for bioenergy recovery: A review. Renew. Energy 2020, 145, 2370–2390. [Google Scholar] [CrossRef]
- Jiang, L.; Cui, H.; Zhu, L.; Hu, Y.; Xu, X.; Li, S.; Huang, H. Enhanced propionic acid production from whey lactose with immobilized Propionibacterium acidipropionici and the role of trehalose synthesis in acid tolerance. Green Chem. 2014, 17, 250–259. [Google Scholar] [CrossRef]
- Bermúdez-Penabad, N.; Kennes, C.; Veiga, M.C. Anaerobic digestion of tuna waste for the production of volatile fatty acids. Waste Manag. 2017, 68, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Agler, M.T.; Wrenn, B.A.; Zinder, S.H.; Angenent, L.T. Waste to bioproduct conversion with undefined mixed cultures: The carboxylate platform. Trends Biotechnol. 2010, 29, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Rajesh Banu, J.; Ganesan, S. Anaerobic treatment of winery wastewater in fixed bed reactors. Bioresour. Technol. 2010, 101, 8557–8562. [Google Scholar] [CrossRef]
- Sukphun, P.; Sittijunda, S.; Reungsang, A. Volatile Fatty Acid Production from Organic Waste with the Emphasis on Membrane-Based Recovery. Fermentation 2021, 7, 159. [Google Scholar] [CrossRef]
- Achinas, S.; Li, Y.; Achinas, V.; Euverink, G.J.W. Biogas Potential from the Anaerobic Digestion of Potato Peels: Process Performance and Kinetics Evaluation. Energies 2019, 12, 2311. [Google Scholar] [CrossRef]
- Ryan, M.P.; Walsh, G. The biotechnological potential of whey. Rev. Environ. Sci. Bio/Technol. 2016, 15, 479–498. [Google Scholar] [CrossRef]
- Pintado, M.E.; Macedo, A.C.; Malcata, F.X. Technology, chemistry, and microbiology of whey cheeses: A review. Food Sci. Technol. Int. 2001, 7, 105–116. [Google Scholar] [CrossRef]
- Lavelli, V.; Beccalli, M.P. Cheese whey recycling in the perspective of the circular economy: Modeling processes and the supply chain to design the involvement of small and medium enterprises. Trends Food Sci. Technol. 2022, 126, 86–98. [Google Scholar] [CrossRef]
- Barba, F.J. An Integrated Approach for the Valorization of Cheese Whey. Foods 2021, 10, 564. [Google Scholar] [CrossRef] [PubMed]
- Jelen, P. Whey processing—Utilization and products. In Encyclopedia of Dairy Sciences, 2nd ed.; Fuquay, J.W., Fox, P.F., McSweeney, P.L.H., Eds.; Academic Press: Cambridge, MA, USA, 2011; pp. 731–737. [Google Scholar]
- Gregg, J.S.; Jürgens, J.; Happel, M.K.; Strøm-Andersen, N.; Tanner, A.N.; Bolwig, S.; Klitkou, A. Valorization of bio-residuals in the food and forestry sectors in support of a circular bioeconomy: A review. J. Clean. Prod. 2020, 267, 122093. [Google Scholar] [CrossRef]
- Moatsou, G.; Moschopoulou, E. Cheese and Whey: The Outcome of Milk Curdling. Foods 2021, 10, 1008. [Google Scholar] [CrossRef] [PubMed]
- Akpinar, A.; Yerlikaya, O.; Akan, E.; Karagozlu, C.; Kinik, O.; Uysal, H.R. The effect of packaging materials on physicochemical, microbiological, and sensorial properties of Turkish whey (Lor) cheese with some plants. J. Food Process. Preserv. 2022, 46, 17060. [Google Scholar] [CrossRef]
- Pires, A.F.; Marnotes, N.G.; Rubio, O.D.; Garcia, A.C.; Pereira, C.D. Dairy By-Products: A Review on the Valorization of Whey and Second Cheese Whey. Foods 2021, 10, 1067. [Google Scholar] [CrossRef]
- Zotta, T.; Solieri, L.; Iacumin, L.; Picozzi, C.; Gullo, M. Valorization of cheese whey using microbial fermentations. Appl. Microbiol. Biotechnol. 2020, 104, 2749–2764. [Google Scholar] [CrossRef] [PubMed]
- Bintsis, T.; Papademas, P. The evolution of fermented milks, from artisanal to industrial products: A critical review. Fermentation 2022, 8, 679. [Google Scholar] [CrossRef]
- Pala, C.; Scarano, C.; Venusti, M.; Sardo, D.; Casti, D.; Cossu, F.; Lamon, S.; Spanu, V.; Ibba, M.; Marras, M.; et al. Shelf life evaluation of ricotta fresca sheep cheese in modified atmosphere packaging. Ital. J. Food Saf. 2016, 5, 5502. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Macedo, A.; Bilau, J.; Cambóias, E.; Duarte, E. Integration of Membrane Processes for By-Product Valorization to Improve the Eco-Efficiency of Small/Medium Size Cheese Dairy Plants. Foods 2021, 10, 1740. [Google Scholar] [CrossRef]
- Macedo, A.; Azedo, D.; Duarte, E.; Pereira, C. Valorization of Goat Cheese Whey through an Integrated Process of Ultrafiltration and Nanofiltration. Membranes 2021, 11, 477. [Google Scholar] [CrossRef] [PubMed]
- Kaminarides, S.; Zagari, H.; Zoidou, E. Effect of whey fat content on the properties and yields of whey cheese and serum. J. Hell. Vet. Med. Soc. 2020, 71, 2149–2156. [Google Scholar] [CrossRef]
- Siso, M. The biotechnological utilization of cheese whey: A review. Bioresour. Technol. 1996, 57, 1–11. [Google Scholar] [CrossRef]
- Silanikove, N.; Leitner, G.; Merin, U.; Prosser, C.G. Recent advances in exploiting goat’s milk: Quality, safety, and production aspects. Small Rumin. Res. 2010, 89, 110–120. [Google Scholar]
- Pereira, C.D.; Henriques, M.; Gomes, D.; Gouveia, R.; Gomez-Zavaglia, A.; de Antoni, G. Fermented dairy products based on ovine cheese whey. J. Food Sci. Technol. 2015, 52, 7401–7408. [Google Scholar] [CrossRef]
- Henriques, M.H.F.; Gomes, D.M.G.S.; Pereira, C.J.D.; Gil, M.H.M. Effects of liquid whey protein concentrate on functional and sensorial properties of set yoghurts and fresh cheese. Food Bioprocess Technol. 2013, 6, 952–963. [Google Scholar] [CrossRef]
- Milani, F.; Wendorff, W. Goat and sheep milk products in the United States (USA). Small Rumin. Res. 2011, 101, 134–139. [Google Scholar] [CrossRef]
- Hejtmánková, A.; Pivec, V.; Trnková, E.; Dragounová, H. Differences in the composition of total and whey proteins in goat and ewe milk and their changes throughout the lactation period. Czech J. Anim. Sci. 2012, 57, 323–331. [Google Scholar]
- Moatsou, G.; Hatzinaki, A.; Samolada, M.; Anifantakis, E. Major whey proteins in ovine and caprine acid wheys from indigenous Greek breeds. Int. Dairy J. 2005, 15, 123–131. [Google Scholar] [CrossRef]
- Pintado, M.E.; Lopes da Silva, J.A.; Malcata, F.X. Comparative characterization of whey protein concentrates from ovine, caprine, and bovine breeds. LWT-Food Sci. Technol. 1999, 32, 231–237. [Google Scholar]
- Carlini, M.; Castellucci, S.; Moneti, M. Biogas production from poultry manure and cheese whey wastewater under mesophilic conditions in batch reactor. Energy Procedia 2015, 82, 811–818. [Google Scholar]
- Carvalho, F.; Prazeres, A.R.; Rivas, J. Cheese whey wastewater: Characterization and treatment. Sci. Total Environ. 2013, 445–446, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Tsermoula, P.; Khakimov, B.; Nielsen, J.H.; Engelsen, S.B. WHEY—The waste-stream that became more valuable than the food product. Trends Food Sci. Technol. 2021, 118 Pt A, 230–241. [Google Scholar] [CrossRef]
- Costa, C.; Azoia, N.G.; Coelho, L.; Freixo, R.; Batista, P.; Pintado, M. Proteins Derived from the Dairy Losses and By-Products as Raw Materials for Non-Food Applications. Foods 2021, 10, 135. [Google Scholar] [CrossRef] [PubMed]
- Augustin, M.; Udabage, P.; Juliano, P.; Clarke, P. Towards a more sustainable dairy industry: Integration across the farm–factory interface and the dairy factory of the future. Int. Dairy J. 2013, 31, 2–11. [Google Scholar] [CrossRef]
- Minj, S.; Anand, S. Whey Proteins and Its Derivatives: Bioactivity, Functionality, and Current Applications. Dairy 2020, 1, 233–258. [Google Scholar] [CrossRef]
- Zonfa, T.; Kamperidis, T.; Falzarano, M.; Lyberatos, G.; Polettini, A.; Pomi, R.; Rossi, A.; Tremouli, A. Two-Stage Process for Energy Valorization of Cheese Whey through Bio-Electrochemical Hydrogen Production Coupled with Microbial Fuel Cell. Fermentation 2023, 9, 306. [Google Scholar] [CrossRef]
- Čechmánková, J.; Skála, J.; Sedlařík, V.; Duřpeková, S.; Drbohlav, J.; Šalaková, A.; Vácha, R. The Synergic Effect of Whey-Based Hydrogel Amendment on Soil Water Holding Capacity and Availability of Nutrients for More Efficient Valorization of Dairy By-Products. Sustainability 2021, 13, 10701. [Google Scholar] [CrossRef]
- Ghaly, A.E.; Ramkumar, D.R. Controlling the pH of acid cheese whey in a two-stage anaerobic digester with sodium hydroxide. Energy Sources 1999, 21, 475–502. [Google Scholar]
- Ojemaye, M.O.; Adefisoye, M.A.; Okoh, A.I. Volatile fatty acids production from cheese whey: Influence of pH, solid retention time and organic loading rate. J. Environ. Manag. 2020, 275, 111234. [Google Scholar] [CrossRef]
- Molinuevo-Salces, B.; da Silva-Lacerda, V.; García-González, M.C.; Riaño, B. Production of Volatile Fatty Acids from Cheese Whey and Their Recovery Using Gas-Permeable Membranes. Recycling 2024, 9, 65. [Google Scholar] [CrossRef]
- Méndez-Acosta, H.O.; Hernandez-Martinez, L.; Sanchez-Garcia, J. Diagnosis and monitoring of volatile fatty acids production from raw cheese whey by multiscale time-series analysis. Appl. Sci. 2021, 11, 5803. [Google Scholar] [CrossRef]
- Pérez Morales, J.M. Estudio del Efecto de pH y Relación Sustrato/Inóculo Sobre la Producción de Ácidos Grasos Volátiles Durante la Fermentación de Lactosuero (Tesis de Maestría); Universidad Veracruzana, Xalapa de Enríquez: Veracruz, México, 2020. [Google Scholar]
- Domingos, J.M.B.; Martinez, G.A.; Scoma, A.; Fraraccio, S.; Kerckhof, F.-M.; Boon, N.; Reis, M.A.M.; Fava, F.; Bertin, L. Effect of operational parameters in the continuous anaerobic fermentation of cheese whey on titers, yields, productivities, and microbial community structures. ACS Sustain. Eng. 2018, 6, 14205–14216. [Google Scholar] [CrossRef]
- Mariz, D. Estudio de la Producción de Ácido Propiónico por Propionibacteriumacidipropionici en Cultivo Continuo. Ph.D. Thesis, Universidad Politécnica de Madrid, Madrid, Spain, 2011. Available online: https://oa.upm.es/7734/1/Djalma_Mariz.pdf (accessed on 23 November 2024).
- Yu, H.-Q.; Fang, H. Acidogenesis of dairy wastewater at various pH levels. Water Sci. Technol. 2002, 45, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Yan, B.; Wong, J.W.; Zhang, Y. Enhanced volatile fatty acids production from anaerobic fermentation of food waste: A mini-review focusing on acidogenic metabolic pathways. Bioresour. Technol. 2018, 248, 68–78. [Google Scholar] [CrossRef] [PubMed]
- FAO. Food and Agriculture Organization of the United Nations Statistics Division. 2020. Available online: https://www.fao.org/newsroom/detail/doubling-global-potato-production-in-10-years-is-possible/es (accessed on 23 November 2024).
- Liang, S.; McDonald, A.G.; Coats, E.R. Lactic acid production with undefined mixed culture fermentation of potato peel waste. Waste Manag. 2014, 34, 2022–2027. [Google Scholar] [CrossRef]
- Liang, S.; Gliniewicz, K.; Gerritsen, A.T.; McDonald, A.G. Analysis of microbial community variation during the mixed culture fermentation of agricultural peel wastes to produce lactic acid. Bioresour. Technol. 2016, 208, 7–12. [Google Scholar] [CrossRef]
- Sepelev, I.; Galoburda, R. Industrial potato peels waste application in food production: A review. Res. Rural. Dev. Int. Sci. Conf. 2015, 1, 130–136. [Google Scholar]
- Abdelraof, M.; Hasanin, M.S.; El -Saied, H. Ecofriendly green conversion of potato peel wastes to high productivity bacterial cellulose. Carbohydr. Polym. 2019, 211, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Chohan, N.A.; Aruwajoye, G.; Sewsynker-Sukai, Y.; Kana, E.G. Valorisation of potato peel wastes for bioethanol production using simultaneous saccharification and fermentation: Process optimization and kinetic assessment. Renew. Energy 2019, 146, 1031–1040. [Google Scholar] [CrossRef]
- Hijosa-Valsero, M.; Paniagua-García, A.I.; Díez-Antolínez, R. Industrial potato peel as a feedstock for biobutanol production. New Biotechnol. 2018, 46, 54–60. [Google Scholar] [CrossRef]
- Khawla, B.J.; Sameh, M.; Imen, G.; Donyes, F.; Dhouha, G.; Raoudha, E.G.; Oumèma, N.-E. Potato peel as feedstock for bioethanol production: A comparison of acidic and enzymatic hydrolysis. Ind. Crop. Prod. 2014, 52, 144–149. [Google Scholar] [CrossRef]
- Xie, Y.; Niu, X.; Yang, J.; Fan, R.; Shi, J.; Ullah, N.; Feng, X.; Chen, L. Active biodegradable films based on the whole potato peel incorporated with bacterial cellulose and curcumin. Int. J. Biol. Macromol. 2020, 150, 480–491. [Google Scholar] [CrossRef]
- Hossain, M.B.; Tiwari, B.K.; Gangopadhyay, N.; O’Donnell, C.P.; Brunton, N.P.; Rai, D.K. Ultrasonic extraction of steroidal alkaloids from potato peel waste. Ultrason. Sonochem. 2014, 21, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.D.; Fradinho, P.; Rodríguez-Seoane, P.; Falqué, E.; Santos, V.; Domínguez, H. Biorefinery concept for discarded potatoes: Recovery of starch and bioactive compounds. J. Food Eng. 2020, 275, 109886. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, G.; Zhang, L.; Sun, Z. Preparation of high performance H 2 S removal biochar by direct fluidized bed carbonization using potato peel waste. Process. Saf. Environ. Prot. 2017, 107, 281–288. [Google Scholar] [CrossRef]
- Yang, X.; Kwon, E.E.; Dou, X.; Zhang, M.; Kim, K.-H.; Tsang, D.C.; Ok, Y.S. Fabrication of spherical biochar by a two-step thermal process from waste potato peel. Sci. Total. Environ. 2018, 626, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; McDonald, A.G. Anaerobic digestion of pre-fermented potato peel wastes for methane production. Waste Manag. 2015, 46, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Kaparaju, P.; Rintala, J. Anaerobic co-digestion of potato tuber and its industrial by-products with pig manure. Resour. Conserv. Recycl. 2005, 43, 175–188. [Google Scholar] [CrossRef]
- Pistis, A.; Asquer, C.; Scano, E.A. Anaerobic digestion of potato industry by-products on a pilot-scale plant under thermophilic conditions. Environ. Eng. Manag. J. 2013, 12, 93–96. [Google Scholar]
- Dai, K.; Zhang, W.; Zeng, R.J.; Zhang, F. Production of chemicals in thermophilic mixed culture fermentation: Mechanism and strategy. Crit. Rev. Environ. Sci. Technol. 2019, 50, 1–30. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Q.; Wang, X.; Zhou, X.; Zhu, J. Effect of pH on volatile fatty acid production from anaerobic digestion of potato peel waste. Bioresour. Technol. 2020, 316, 123851. [Google Scholar] [CrossRef]
- Jankowska, E.; Chwiałkowska, J.; Stodolny, M.; Oleskowicz-Popiel, P. Effect of pH and retention time on volatile fatty acids production during mixed culture fermentation. Bioresour. Technol. 2015, 190, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, R.; Huang, L.; Wang, X.; Chou, S.; Zhu, J. Acidogenic fermentation of potato peel waste for volatile fatty acids production: Effect of initial organic load. J. Biotechnol. 2023, 374, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ullah, A.; Gao, X.; Shi, J. Synergistic Ball Milling–Enzymatic Pretreatment of Brewer’s Spent Grains to Improve Volatile Fatty Acid Production through Thermophilic Anaerobic Fermentation. Processes 2023, 11, 1648. [Google Scholar] [CrossRef]
- Guarda, E.C.; Oliveira, A.C.; Antunes, S.; Freitas, F.; Castro, P.M.L.; Duque, A.F.; Reis, M.A.M. A Two-Stage Process for Conversion of Brewer’s Spent Grain into Volatile Fatty Acids through Acidogenic Fermentation. Appl. Sci. 2021, 11, 3222. [Google Scholar] [CrossRef]
- Sarkar, O.; Rova, U.; Christakopoulos, P.; Matsakas, L. Green hydrogen and platform chemicals production from acidogenic conversion of brewery spent grains co-fermented with cheese whey wastewater: Adding value to acidogenic CO2. Sustain. Energy Fuels 2021, 6, 778–790. [Google Scholar] [CrossRef]
- del Campo, A.G.; Fernández, F.J.; Cañizares, P.; Rodrigo, M.A.; Pinar, F.J.; Lobato, J. Energy recovery of biogas from juice wastewater through a short high temperature PEMFC stack. Int. J. Hydrogen Energy 2014, 39, 6937–6943. [Google Scholar] [CrossRef]
- Shi, B.; Huang, J.; Yin, Z.; Han, W.; Qiu, S.; Tang, J.; Hou, P. Riboflavin boosts fermentative valeric acid generation from waste activated sludge. BioResources 2020, 15, 3962–3969. Available online: https://bioresources.cnr.ncsu.edu/wp-content/uploads/2020/04/BioRes_15_2_3962_Shi_HYHQTH_Riboflavin_Boosts_Fermentative_Valeric_Acid_Activated_Sludge_17183.pdf (accessed on 12 December 2024). [CrossRef]
- Zhang, A.; Yang, S.-T. Propionic acid production from glycerol by metabolically engineered Propionibacterium acidipropionici. Process. Biochem. 2009, 44, 1346–1351. [Google Scholar] [CrossRef]
- Bodie, E.A.; Goodman, N.; Schwartz, R.D. Production of propionic acid by mixed cultures ofPropionibacterium shermanii andLactobacillus casei in autoclave-sterilized whey. J. Ind. Microbiol. Biotechnol. 1987, 1, 349–353. [Google Scholar] [CrossRef]
- Lewis, V.P.; Yang, S.-T. Propionic acid fermentation by Propionibacterium acidipropionici: Effect of growth substrate. Appl. Microbiol. Biotechnol. 1992, 37, 437–442. [Google Scholar] [CrossRef]
- Morales, J.; Choi, J.-S.; Kim, D.-S. Production rate of propionic acid in fermentation of cheese whey with enzyme inhibitors. Environ. Prog. 2006, 25, 228–234. [Google Scholar] [CrossRef]
- Suwannakham, S.; Yang, S.-T. Enhanced propionic acid fermentation by Propionibacterium acidipropionici mutant obtained by adaptation in a fibrous-bed bioreactor. Biotechnol. Bioeng. 2005, 91, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Feng, L.; Chen, Y.; Sun, H.; Shen, Q.; Li, X.; Chen, H. Alkyl polyglucose enhancing propionic acid enriched short-chain fatty acids production during anaerobic treatment of waste activated sludge and mechanisms. Water Res. 2015, 73, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Chalima, A.; Hatzidaki, A.; Karnaouri, A.; Topakas, E. Integration of a dark fermentation effluent in a microalgal-based biorefinery for the production of high-added value omega-3 fatty acids. Appl. Energy 2019, 241, 130–138. [Google Scholar] [CrossRef]
- Reddy, M.V.; Kumar, G.; Mohanakrishna, G.; Shobana, S.; Al-Raoush, R.I. Review on the Production of Medium and Small Chain Fatty Acids through Waste Valorization and CO2 Fixation. Bioresour. Technol. 2020, 309, 123400. [Google Scholar] [CrossRef]
- Llamas, M.; Dourou, M.; González-Fernández, C.; Aggelis, G.; Tomás-Pejó, E. Screening of oleaginous yeasts for lipid production using volatile fatty acids as substrate. Biomass Bioenergy 2020, 138, 105553. [Google Scholar] [CrossRef]
- Morales-Sánchez, D.; Martinez-Rodriguez, O.A.; Kyndt, J.; Martinez, A. Heterotrophic growth of microalgae: Metabolic aspects. World J. Microbiol. Biotechnol. 2015, 31, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chalima, A.; Oliver, L.; de Castro, L.F.; Karnaouri, A.; Dietrich, T.; Topakas, E. Utilization of Volatile Fatty Acids from Microalgae for the Production of High Added Value Compounds. Fermentation 2017, 3, 54. [Google Scholar] [CrossRef]
- Turon, V.; Baroukh, C.; Trably, E.; Latrille, E.; Fouilland, E.; Steyer, J.P. Use of fermentative metabolites for heterotrophic microalgae growth: Yields and kinetics. Bioresour. Technol. 2015, 175, 342–349. [Google Scholar] [CrossRef] [PubMed]
- VenkataMohan, S.; PrathimaDevi, M. Fatty acid rich effluent from acidogenic biohydrogen reactor as substrate for lipid accumulation in heterotrophic microalgae with simultaneous treatment. Bioresour. Technol. 2012, 123, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Turon, V.; Trably, E.; Fouilland, E.; Steyer, J.P. Potentialities of dark fermentation effluents as substrates for mi-croalgae growth: A review. Process Biochem. 2016, 51, 1843–1854. [Google Scholar] [CrossRef]
- Turon, V.; Trably, E.; Fouilland, E.; Steyer, J.-P. Growth of Chlorella sorokiniana on a mixture of volatile fatty acids: The effects of light and temperature. Bioresour. Technol. 2015, 198, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Ryu, B.G.; Kim, W.; Heo, S.W.; Kim, D.; Choi, G.G.; Yang, J.W. Advanced treatment of residual nitrogen from biologically treated coke effluent by a microalga-mediated process using volatile fatty acids (VFA) under stepwise mixotrophic conditions. Bioresour. Technol. 2015, 191, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Chen, Z.; Li, P.; Duan, R.; Ren, N. Lipid production for biofuels from hydrolyzate of waste activated sludge by heterotrophic Chlorella protothecoides. Bioresour. Technol. 2013, 143, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-N.; Yuan, M.; Shen, Z.-H.; Peng, K.-M.; Lu, L.-J.; Huang, X.-F. Bioconversion of volatile fatty acids derived from waste activated sludge into lipids by Cryptococcus curvatus. Bioresour. Technol. 2016, 211, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Du, W.; Zhao, X.; Zhang, G.; Liu, D. Microbial oil production from various carbon sources and its use for biodiesel preparation. Biofuels Bioprod. Biorefining 2012, 7, 65–77. [Google Scholar] [CrossRef]
- Ageitos, J.M.; Vallejo, J.A.; Veiga-Crespo, P.; Villa, T.G. Oily yeasts as oleaginous cell factories. Appl. Microbiol. Biotechnol. 2011, 90, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Hesselmann, R.P.; Werlen, C.; Hahn, D.; van der Meer, J.R.; Zehnder, A.J. Enrichment, Phylogenetic Analysis and Detection of a Bacterium That Performs Enhanced Biological Phosphate Removal in Activated Sludge. Syst. Appl. Microbiol. 1999, 22, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Zilles, J.L.; Peccia, J.; Kim, M.-W.; Hung, C.-H.; Noguera, D.R. Involvement of Rhodocyclus -Related Organisms in Phosphorus Removal in Full-Scale Wastewater Treatment Plants. Appl. Environ. Microbiol. 2002, 68, 2763–2769. [Google Scholar] [CrossRef] [PubMed]
- Mino, T.; Arun, V.; Tsuzuki, Y.; Matsuo, T. Microbial selection of polyphosphate-accumulating bacteria in activated sludge wastewater treatment. Water Sci. Technol. 1998, 37, 27–44. [Google Scholar]
- Nielsen, P.H.; McIlroy, S.J.; Albertsen, M.; Nierychlo, M. Re-evaluating the microbiology of the enhanced biological phosphorus removal process. Curr. Opin. Biotechnol. 2019, 57, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Seviour, R.J.; Mino, T.; Onuki, M. The microbiology of biological phosphorus removal in activated sludge systems. FEMS Microbiol. Rev. 2003, 27, 99–127. [Google Scholar] [CrossRef] [PubMed]
- Madison, L.L.; Huisman, G.W. Metabolic Engineering of Poly(3-Hydroxyalkanoates): From DNA to Plastic. Microbiol. Mol. Biol. Rev. 1999, 63, 21–53. [Google Scholar] [CrossRef]
- Rai, R.; Keshavarz, T.; Roether, J.A.; Boccaccini, A.R.; Roy, I. Medium chain length polyhydroxyalkanoates, promising new biomedical materials for the future. Mater. Sci. Eng. R Rep. 2011, 72, 29–47. [Google Scholar] [CrossRef]
- Rittmann, B.E.; McCarty, P.L. Environmental Biotechnology: Principles and Applications; McGraw-Hill: New York, NY, USA, 2011. [Google Scholar]
- Lu, J.; Tappel, R.C.; Nomura, C.T. Mini-review: Biosynthesis of poly(hydroxyalkanoates). Polym. Rev. 2009, 49, 226–248. [Google Scholar] [CrossRef]
- Jiang, G.; Hill, D.J.; Kowalczuk, M.; Johnston, B.; Adamus, G.; Irorere, V.; Radecka, I. Carbon Sources for Polyhydroxyalkanoates and an Integrated Biorefinery. Int. J. Mol. Sci. 2016, 17, 1157. [Google Scholar] [CrossRef] [PubMed]
- Grage, K.; McDermott, P.; Rehm, B.H.A. Engineering Bacillus megaterium for production of functional intracellular materials. Microb. Cell Factories 2017, 16, 211. [Google Scholar] [CrossRef] [PubMed]
- Lovely, S.; Kumar, S.; Srivastava, A.K.; Shivakumar, S. Optimized batch cultivation and scale-up of Bacillus thuringiensis for high-yield production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Bioresour. Technol. 2024, 409, 131220. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.; Lin, M.; Jin, W.; Chen, C.; Liu, G. Biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) from volatile fatty acids by Cupriavidusnecator. J. Basic Microbiol. 2023, 63, 128–139. [Google Scholar] [CrossRef]
- Morlino, M.S.; Serna García, R.; Savio, F.; Zampieri, G.; Morosinotto, T.; Treu, L.; Campanaro, S. Cupriavidus necator as a Platform for Polyhydroxyalkanoate Production: An Overview of Strains, Metabolism, and Modeling Approaches. Biotechnol. Adv. 2023, 69, 108264. [Google Scholar]
- Jawed, K.; Irorere, V.U.; Bommareddy, R.R.; Minton, N.P.; Kovács, K. Establishing Mixotrophic Growth of Cupriavidus necator H16 on CO2 and Volatile Fatty Acids. Fermentation 2022, 8, 125. [Google Scholar] [CrossRef]
- Schlegel, H.G.; Gottschalk, G.; VON Bartha, R. Formation and Utilization of Poly-β-Hydroxybutyric Acid by Knallgas Bacteria (Hydrogenomonas). Nature 1961, 191, 463–465. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Salerno, A.; Braunegg, G. Polyhydroxyalkanoates: Basics, production and applications of microbial biopolyesters. In Bio-Based Plastics; Wiley: Hoboken, NJ, USA, 2013; pp. 137–170. [Google Scholar]
- Vu, D.H.; Mahboubi, A.; Root, A.; Heinmaa, I.; Taherzadeh, M.J.; Åkesson, D. Thorough investigation of the effects of cultivation factors on polyhydroalkanoates (PHAs) production by Cupriavidusnecator from food waste-derived volatile fatty acids. Fermentation 2022, 8, 605. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Gurav, R.; Choi, T.-R.; Jung, H.-R.; Yang, S.-Y.; Moon, Y.-M.; Song, H.-S.; Jeon, J.-M.; Choi, K.-Y.; Yang, Y.-H. Bioconversion of plant biomass hydrolysate into bioplastic (polyhydroxyalkanoates) using Ralstonia eutropha 5119. Bioresour. Technol. 2018, 271, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Saratale, G.D.; Oh, M.-K. Characterization of poly-3-hydroxybutyrate (PHB) produced from Ralstonia eutropha using an alkali-pretreated biomass feedstock. Int. J. Biol. Macromol. 2015, 80, 627–635. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Franca, R.D.G.; Dionísio, M.; Sevrin, C.; Grandfils, C.; Reis, M.A.M.; Lourenço, N.D. Polyhydroxyalkanoates from a Mixed Microbial Culture: Extraction Optimization and Polymer Characterization. Polymers 2022, 14, 2155. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, J.M. Producción de Polihidroxibutirato a Partir de Residuos Agroindustriales (Tesis de Maestría); Universidad Nacional de Colombia: Manizales, Colombia, 2010. [Google Scholar]
- Cal, A.J.; Kibblewhite, R.E.; Sikkema, W.D.; Torres, L.F.; Hart-Cooper, W.M.; Orts, W.J.; Lee, C.C. Production of polyhydroxyalkanoate copolymers containing 4-hydroxybutyrate in engineered Bacillus megaterium. Int. J. Biol. Macromol. 2021, 168, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.L.M.; Yepes-Pérez, M.; Contreras, K.A.C.; Moreno, P.E.Z. Production of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) by Bacillus megaterium LVN01 Using Biogas Digestate. Appl. Microbiol. 2024, 4, 1057–1078. [Google Scholar] [CrossRef]
- Alkotaini, B.; Sathiyamoorthi, E.; Kim, B.S. Potential of Bacillus megaterium for production of polyhydroxyalkanoates using the red algae Gelidiumamansii. Biotechnol. Bioprocess Eng. 2015, 20, 856–860. [Google Scholar] [CrossRef]
- Vu, D.H.; Wainaina, S.; Taherzadeh, M.J.; Åkesson, D.; Ferreira, J.A. Production of polyhydroxyalkanoates (PHAs) by Bacillus megaterium using food waste acidogenic fermentation-derived volatile fatty acids. Bioengineered 2021, 12, 2480–2498. [Google Scholar] [CrossRef] [PubMed]
- Melanie, S.; Winterburn, J.B.; Devianto, H. Production of Biopolymer Polyhydroxyalkanoates (PHA) by Extreme Halophilic Marine Archaea Haloferax mediterranei in Medium with Varying Phosphorus Concentration. J. Eng. Technol. Sci. 2018, 50, 255–271. [Google Scholar] [CrossRef]
- Chmelová, D.; Legerská, B.; Ondrejovič, M.; Miertuš, S. Optimization of Propagation Medium for Enhanced Polyhydroxyalkanoate Production by Pseudomonas oleovorans. Fermentation 2021, 8, 16. [Google Scholar] [CrossRef]
- Możejko-Ciesielska, J.; Marciniak, P.; Szacherska, K. Polyhydroxyalkanoates Synthesized by Aeromonas Species: Trends and Challenges. Polymers 2019, 11, 1328. [Google Scholar] [CrossRef]
- Lee, S.H.; Oh, D.H.; Ahn, W.S.; Lee, Y.; Choi, J.-I.; Lee, S.Y. Production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) by high-cell-density cultivation of Aeromonas hydrophila. Biotechnol. Bioeng. 2000, 67, 240–244. [Google Scholar] [CrossRef]
- Chen, G.Q.; Zhang, G.; Park, S.J.; Lee, S.Y. Industrial scale production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Appl. Microbiol. Biotechnol. 2001, 57, 50–55. [Google Scholar] [PubMed]
- Shen, X.-W.; Yang, Y.; Jian, J.; Wu, Q.; Chen, G.-Q. Production and characterization of homopolymer poly(3-hydroxyvalerate) (PHV) accumulated by wild type and recombinant Aeromonas hydrophila strain 4AK4. Bioresour. Technol. 2009, 100, 4296–4299. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-Q.; Patel, M.K. Plastics Derived from Biological Sources: Present and Future: A Technical and Environmental Review. Chem. Rev. 2012, 112, 2082–2099. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Feng, H.; Liu, N.; Zhao, X. Biomass-Derived 2,3-Butanediol and Its Application in Biofuels Production. Energies 2023, 16, 5802. [Google Scholar] [CrossRef]
- Chen, G.Q. Plastics from Bacteria, Natural Functions and Applications; Springer: Heidelberg, Germany, 2010; p. 116. [Google Scholar]
- Qin, Y.; Wang, X.H. Carbon dioxide-based copolymers: Environmental benefits of PPC, an industrially viable catalyst. Biotechnol. J. 2010, 5, 1164. [Google Scholar] [CrossRef]
- Deng, Y.; Lin, X.-S.; Zheng, Z.; Deng, J.-G.; Chen, J.-C.; Ma, H.; Chen, G.-Q. Poly(hydroxybutyrate-co-hydroxyhexanoate) promoted production of extracellular matrix of articular cartilage chondrocytes in vitro. Biomaterials 2003, 24, 4273–4281. [Google Scholar] [CrossRef] [PubMed]
- Steinbüchel, A.; Valentin, H.E. Diversity of bacterial polyhydroxyalkanoic acids. FEMS Microbiol. Lett. 1995, 128, 219–228. [Google Scholar] [CrossRef]
- Wang, S.; Chen, W.; Xiang, H.; Yang, J.; Zhou, Z.; Zhu, M. Modification and Potential Application of Short-Chain-Length Polyhydroxyalkanoate (SCL-PHA). Polymers 2016, 8, 273. [Google Scholar] [CrossRef] [PubMed]
- Doi, Y. Microbial synthesis, physical properties, and biodegradability of polyhydroxyalkanoates. Macromol. Symp. 1995, 98, 585–599. [Google Scholar] [CrossRef]
- Marschessault, R.; Monasterios, C.; Morin, F.; Sundararajan, P. Chiral poly(β-hydroxyalkanoates): An adaptable helix influenced by the alkane side-chain. Int. J. Biol. Macromol. 1990, 12, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-Q.; Wu, Q. The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials 2005, 26, 6565–6578. [Google Scholar] [CrossRef] [PubMed]
- de Lucas, A.; Rodríguez, L.; Villaseñor, J.; Fernández, F. Biodegradation kinetics of stored wastewater substrates by a mixed microbial culture. Biochem. Eng. J. 2005, 26, 191–197. [Google Scholar] [CrossRef]
- Rivera Briso, A.L.; Serrano Aroca, Á. Métodos de refuerzo mecánico del poli(3-hidroxibutirato-co-3-hidroxivalerato) para aplicaciones industriales avanzadas. Nereis Rev. Iberoam. Interdiscip. De Métodos Model. Y Simulación 2018, 10, 79–94. [Google Scholar]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef] [PubMed]
- Li, A.R.; Ding, W.; Rai, Y.; Roether, J.A.; Schubert, B.; Boccaccini, D.W. Preparation and characterization of PHBV microsphere/45S5 bioactive glass composite scaffolds with vancomycin releasing function. Mater. Sci. Eng. C 2014, 41, 320–328. [Google Scholar] [CrossRef]
- Sultana, M.; Wang, N. PHBV/PLLA-based composite scaffolds fabricated using an emulsion freezing/freeze-drying technique for bone tissue engineering: Surface modification and in vitro biological evaluation. Biofabrication 2012, 4, 5003. [Google Scholar] [CrossRef]
- Gheibi, A.A.; Khoshnevisan, A.; Ketabchi, K.; Derakhshan, N.; Babadi, M.A. Application of electrospun nanofibrous PHBV scaffold in neural graft and regeneration: A mini-review. Nanomed. Res. J. 2016, 1, 107–111. [Google Scholar]
- Landa-Salgado, V.; Cruz-Monterrosa, P.; Hernández-Guzmán, R.G.; Reséndiz-Cruz, F.J. Nanotecnología en la industria alimentaria: Bionanocompuestos en empaques de alimentos. Agroproductividad 2017, 10, 34–40. [Google Scholar]
- Guerrero, I.N.; Carvalho, A.B.; Madrona, C.B.; Cestari, G.S.; Scapin, L.A.; Prado, M.R.S. Envases alternativos biodegradables y activos con aceites esenciales para productos cárnicos. Eurocarne 2015, 238. [Google Scholar]
- Wu, K.; Xue, J.; Li, K.; Sun, H.; Liu, J. Improvement of PHBV scaffolds with bioglass for cartilage tissue engineering. PLoS ONE 2013, 8, e71563. [Google Scholar] [CrossRef]
- Asrar, J.; Valentin, H.E.; Berger, P.A.; Tran, M.; Padgette, S.R.; Garbow, J.R. Biosynthesis and Properties of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) Polymers. Biomacromolecules 2002, 3, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- KANEKA The Dreamology Company. Kaneka. 2022. Available online: https://www.kaneka.be/ (accessed on 12 December 2024).
- Qiu, Y.; Fu, J.; Sun, B.; Ma, X. Sustainable nanocomposite films based on SiO2 and biodegradable poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBH) for food packaging. e-Polymers 2021, 21, 072–081. [Google Scholar] [CrossRef]
- Hu, Y.-J.; Wei, X.; Zhao, W.; Liu, Y.-S.; Chen, G.-Q. Biocompatibility of poly(3-hydroxybutyrate-co-3-hydroxyvalerate-co-3-hydroxyhexanoate) with bone marrow mesenchymal stem cells. Acta Biomater. 2009, 5, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.W.; Wu, Q.; Chen, G.Q. Reduced mouse cell growth by increased hydrophilicity of microbial polyhy-droxyalkanoates via hyaluronan coating. Biomaterials 2003, 24, 4621–4629. [Google Scholar] [CrossRef] [PubMed]
- Tebaldi, M.L.; Chaves Maia, A.L.; Poletto, F.; Vieira de Andrade, F.; Ferreira Soares, D.C. Poly(-3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV): Current advances in synthesis methodologies, antitumor applications and biocompatibility. J. Drug Deliv. Sci. Technol. 2019, 51, 115–126. [Google Scholar]
- Wang, Y.-W.; Wu, Q.; Chen, G.-Q. Attachment, proliferation and differentiation of osteoblasts on random biopolyester poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) scaffolds. Biomaterials 2004, 25, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Andreeßen, B.; Taylor, N.; Steinbüchel, A. Poly(3-Hydroxypropionate): A Promising Alternative to Fossil Fuel-Based Materials. Appl. Environ. Microbiol. 2014, 80, 6574–6582. [Google Scholar] [CrossRef]
- Zinn, M.; Witholt, B.; Egli, T. Occurrence, synthesis and medical application of bacterial polyhydroxyalkanoate. Adv. Drug Deliv. Rev. 2001, 53, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Pouton, C.W.; Akhtar, S. Biosynthetic polyhydroxyalkanoates and their potential in drug delivery. Adv. Drug Deliv. Rev. 1996, 18, 133–162. [Google Scholar] [CrossRef]
- Martin, D.P.; Williams, S.F. Biodegradable polymers: Biochemical engineering for sustainable development. Bio-Chem. Eng. J. 2003, 16, 97–105. [Google Scholar]
- Anderson, A.J.; Dawes, E.A. Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhy-droxyalkanoates. Microbiol. Rev. 1990, 54, 450–472. [Google Scholar] [CrossRef] [PubMed]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, L.; Wang, Z.; Chen, G. Biosynthesis and characterization of 3-hydroxyalkanoate terpolyesters with adjustable properties by Aeromonas hydrophila. Biotechnol. Bioeng. 2009, 104, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.P.; Chen, G.Q. Production and characterization of terpolyester poly(3-hydroxybutyrate-co-4-hydroxybutyrate-co-3-hydroxyhexanoate) by recombinant Aeromonas hydrophila 4AK4 harboring genes phapcj. Biochem. Eng. J. 2008, 38, 384–389. [Google Scholar] [CrossRef]
- Akaraonye, E.; Keshavarz, T.; Roy, I. Production of polyhydroxyalkanoates: The future green materials of choice. J. Chem. Technol. Biotechnol. 2010, 85, 732–743. [Google Scholar] [CrossRef]
- Tan, G.-Y.A.; Chen, C.-L.; Li, L.; Ge, L.; Wang, L.; Razaad, I.M.N.; Li, Y.; Zhao, L.; Mo, Y.; Wang, J.-Y. Start a Research on Biopolymer Polyhydroxyalkanoate (PHA): A Review. Polymers 2014, 6, 706–754. [Google Scholar] [CrossRef]
- Markl, E.; Grünbichler, H.; Lackner, M. PHB—Bio Based and BiodegradableReplacement for PP: A Review. Nov. Tech. Nutr. Food Sci. 2018, 2, 206–209. [Google Scholar]
- Hankermeyer, C.R.; Tjeerdema, R.S. Polyhydroxybutyrate: Plastic made and degraded by microorganisms. In Reviews of Environmental Contamination and Toxicology; Ware, G.W., Ed.; Springer: New York, NY, USA, 1999; pp. 1–24. [Google Scholar]
- Chanprateep, S. Current trends in biodegradable polyhydroxyalkanoates. J. Biosci. Bioeng. 2010, 110, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Brandl, H.; Gross, R.A.; Lenz, R.W.; Fuller, R.C. Pseudomonas Oleovorans as a Source of Poly(Beta-Hydroxyalkanoates) for Potential Applications as Biodegradable Polyesters. Appl. Environ. Microbiol. 1988, 54, 1977–1982. [Google Scholar] [CrossRef]
- Gogotov, I.N.; Gerasin, V.A.; Knyazev, Y.V.; Antipov, E.M.; Barazov, S.K. Composite biodegradable materials based on polyhydroxyalkanoate. Appl. Biochem. Microbiol. 2010, 46, 607–613. [Google Scholar] [CrossRef]
- Raza, Z.A.; Abid, S.; Banat, I.M. Polyhydroxyalkanoates: Characteristics, production, recent developments and applications. Int. Biodeterior. Biodegrad. 2018, 126, 45–56. [Google Scholar] [CrossRef]
- Mondal, S.; Subramaniam, C. Xenobiotic Contamination of Water by Plastics and Pesticides Revealed through Real-Time, Ultrasensitive, and Reliable Surface-Enhanced Raman Scattering. ACS Sustain. Chem. Eng. 2020, 8, 7639–7648. [Google Scholar] [CrossRef]
- Zan, L.; Tian, L.; Liu, Z.; Peng, Z. A new polystyrene–TiO2 nanocomposite film and its photocatalytic degradation. Appl. Catal. A Gen. 2004, 264, 237–242. [Google Scholar] [CrossRef]
- Lagaron, J.M.; Lopez-Rubio, A. Nanotechnology for bioplastics: Opportunities, challenges and strategies. Trends Food Sci. Technol. 2011, 22, 611–617. [Google Scholar] [CrossRef]
- Garrison, T.F.; Murawski, A.; Quirino, R.L. Bio-Based Polymers with Potential for Biodegradability. Polymers 2016, 8, 262. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Lactic acid production from lignocellulosic biomass: Overview and limits of process technology. BioMed Res. Int. 2013, 2013, 1–10. [Google Scholar]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Fermentative production of lactic acid from brewer’s spent grain. J. Sci. Food Agric. 2006, 86, 1237–1243. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338. [Google Scholar] [CrossRef]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Emerging technologies for the pretreatment of lignocellulosic biomass. Bioresour. Technol. 2018, 262, 310–318. [Google Scholar] [CrossRef]
- Yu, L.; Dean, K.; Li, L. Polymer blends and composites from renewable resources. Prog. Polym. Sci. 2006, 31, 576–602. [Google Scholar] [CrossRef]
- Rojas, A.M.; Montaño, L.P.; Bastidas, M.J. Producción de ácido láctico a partir del lactosuero utilizando Lactobacillus delbrueckii subsp. bulgaricus y Streptococcusthermophilus. Rev. Colomb. Química 2015, 44, 5–10. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Crystallization from the melt of poly(lactide)s with different optical purities and their blends. Macromol. Chem. Phys. 1996, 197, 3483–3499. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, V. New emerging trends in synthetic biodegradable polymers—Polylactide: A critique. Eur. Polym. J. 2007, 43, 4053–4074. [Google Scholar] [CrossRef]
- AIMPLAS. Envase Alimentario: Nuevo PLA Flexible y con Mayor Resistencia a Impactos, Potencial Sustituto del Polipropileno. 2022. Available online: https://www.aimplas.es/blog/envase-alimentario-nuevo-pla-flexible-y-con-mayor-resistencia-a-impactos-potencial-sustituto-del-polipropileno/ (accessed on 12 December 2024).
- Datta, R.; Tsai, S.; Patrick, B.; Moon, S.; Frank, J. Technological and economic potential of poly lactic acid and lactic acid derivatives. In Proceedings of the International Congress on Chemicals from Biotechnology, Hannover, Germany, 18–20 October 1993; pp. 1–18. [Google Scholar]
- Litchfield, J. Microbial production of lactic acid. Appl. Microbiol. 1996, 42, 45–95. [Google Scholar]
- Domínguez, J.; Vásquez, M. Effect of the operational conditions on the L-lactic acid production by Rhizopus oryzae. Cienc. Y Tecnol. Aliment. 1999, 2, 113–118. [Google Scholar] [CrossRef][Green Version]
- Aversa, C.; Barletta, M.; Puopolo, M.; Vesco, S. Cast extrusion of low gas permeability bioplastic sheets in PLA/PBS and PLA/PHB binary blends. Polym. Sci. 2020, 59, 231–240. [Google Scholar] [CrossRef]
- Su, S.; Kopitzky, R.; Tolga, S.; Kabasci, S. Polylactide (PLA) and Its Blends with Poly(butylene succinate) (PBS): A Brief Review. Polymers 2019, 11, 1193. [Google Scholar] [CrossRef]
- Ihn, K.J.; Yoo, E.S.; Im, S.S. Structure and morphology of poly(tetramethylene succinate) crystals. Macromolecules 1995, 28, 2460–2464. [Google Scholar] [CrossRef]
- Ichikawa, Y.; Suzuki, J.; Washiyama, J.; Moteki, Y.; Noguchi, K.; Okuyama, K. Strain-induced crystal modification in poly(tetramethylene succinate). Polymer 1994, 35, 3338–3339. [Google Scholar] [CrossRef]
- Horii, F.; Hirai, A.; Murayama, K.; Kitamaru, R.; Suzuki, T. Molecular mobilities of individual constituent carbons of solid polyesters above Tg as studied by carbon-13 nuclear magnetic resonance spectroscopy. Macromolecules 1983, 16, 273–278. [Google Scholar] [CrossRef]
- Kuwabara, K.; Gan, Z.; Nakamura, T.; Abe, H.; Doi, Y. Temperature dependence of the molecular motion in the crystalline region of biodegradable poly(butylene adipate), poly(ethylene succinate), and poly(butylene succinate). Polym. Stab. 2004, 84, 105–114. [Google Scholar] [CrossRef]
- Ichikawa, Y.; Kondo, H.; Igarashi, Y.; Noguchi, K.; Okuyama, K.; Washiyama, J. Crystal structures of α and β forms of poly(tetramethylene succinate). Polymer 2000, 41, 4719–4727. [Google Scholar] [CrossRef]
- Aziman, N.; Kian, L.K.; Jawaid, M.; Sanny, M.; Alamery, S. Morphological, Structural, Thermal, Permeability, and Antimicrobial Activity of PBS and PBS/TPS Films Incorporated with Biomaster-Silver for Food Packaging Application. Polymers 2021, 13, 391. [Google Scholar] [CrossRef] [PubMed]
- Koitabashi, M.; Noguchi, M.T.; Sameshima-Yamashita, Y.; Hiradate, S.; Suzuki, K.; Yoshida, S.; Watanabe, T.; Shinozaki, Y.; Tsushima, S.; Kitamoto, H.K. Degradation of biodegradable plastic mulch films in soil environment by phylloplane fungi isolated from gramineous plants. AMB Express 2012, 2, 40. [Google Scholar] [CrossRef] [PubMed]
- Ayu, R.S.; Khalina, A.; Harmaen, A.S.; Zaman, K.; MohdNurrazi, N.; Isma, T.; Lee, C.H. Effect of empty fruit bunch reinforcement in PolyButylene-Succinate/Modified Tapioca Starch blend for agricultural mulch films. Sci. Rep. 2020, 10, 1166. [Google Scholar] [CrossRef] [PubMed]
- Guidotti, G.; Soccio, M.; Siracusa, V.; Gazzano, M.; Salatelli, E.; Munari, A.; Lotti, N. Novel Random PBS-Based Copolymers Containing Aliphatic Side Chains for Sustainable Flexible Food Packaging. Polymers 2017, 9, 724. [Google Scholar] [CrossRef] [PubMed]
- Pivsa-Art, W.; Chaiyasat, A.; Pivsa-Art, S.; Yamane, H.; Ohara, H. Preparation of polymer blends between poly(lactic acid) and poly(butylene adipate-co-terephthalate) and biodegradable polymers as compatibilizers. Energy Procedia 2013, 34, 549–554. [Google Scholar] [CrossRef]
- Brunner, C.T.; Baran, E.T.; Pinho, E.D.; Reis, R.L.; Neves, N.M. Performance of biodegradable microcapsules of poly(butylene succinate), poly(butylene succinate-co-adipate) and poly(butylene terephthalate-co-adipate) as drug encapsulation systems. Colloids Surf. B Biointerfaces 2011, 84, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Babu, R.P.; Oconnor, K.; Seeram, R. Current progress on bio-based polymers and their future trends. Prog. Biomater. 2013, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Aliotta, L.; Vannozzi, A.; Panariello, L.; Gigante, V.; Coltelli, M.-B.; Lazzeri, A. Sustainable Micro and Nano Additives for Controlling the Migration of a Biobased Plasticizer from PLA-Based Flexible Films. Polymers 2020, 12, 1366. [Google Scholar] [CrossRef] [PubMed]
- Coltelli, M.-B.; Aliotta, L.; Vannozzi, A.; Morganti, P.; Panariello, L.; Danti, S.; Neri, S.; Fernandez-Avila, C.; Fusco, A.; Donnarumma, G.; et al. Properties and Skin Compatibility of Films Based on Poly(Lactic Acid) (PLA) Bionanocomposites Incorporating Chitin Nanofibrils (CN). J. Funct. Biomater. 2020, 11, 21. [Google Scholar] [CrossRef]
- Gowman, A.; Wang, T.; Rodriguez-Uribe, A.; Mohanty, A.K.; Misra, M. Bio-poly(butylene succinate) and its com-posites with grape pomace: Mechanical performance and thermal properties. ACS Omega 2018, 3, 15205–15216. [Google Scholar] [CrossRef]
- Cui, S.; Li, L.; Wang, Q. Fabrication of (PPC/NCC)/PVA composites with inner-outer double constrained structure and improved glass transition temperature. Carbohydr. Polym. 2018, 191, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.P.; Li, Z.; Liu, B.H.; Song, L.N. Degradation and stability of PPC. China Synthetic Resin and Plastics 2019, 36, 80–85. [Google Scholar]
- Udipi, K.; Gillham, J.K. Poly(ethylene carbonate) and poly(propylene carbonate): Transitions and thermomechanical spectra. J. Appl. Polym. Sci. 1974, 18, 1575–1580. [Google Scholar] [CrossRef]
- Li, X.; Meng, L.; Zhang, Y.; Qin, Z.; Meng, L.; Li, C.; Liu, M. Research and Application of Polypropylene Carbonate Composite Materials: A Review. Polymers 2022, 14, 2159. [Google Scholar] [CrossRef]
- Wang, X.; Pan, H.; Jia, S.; Lu, Z.; Han, L.; Zhang, H. Mechanical properties, thermal behavior, miscibility, and light stability of the poly(butylene adipate-co-terephthalate)/poly(propylene carbonate)/polylactide mulch films. Polym. Bull. 2022, 79, 1–17. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, J.; Yao, Z.; Luo, S.; Tian, L.; Tian, C.; Sun, Y. Preliminary Findings of Polypropylene Carbonate (PPC) Plastic Film Mulching Effects on the Soil Microbial Community. Agriculture 2022, 12, 406. [Google Scholar] [CrossRef]
- Jha, S.; Akula, B.; Enyioma, H.; Novak, M.; Amin, V.; Liang, H. Biodegradable Biobased Polymers: A Review of the State of the Art, Challenges, and Future Directions. Polymers 2024, 16, 2262. [Google Scholar] [CrossRef] [PubMed]
- Getino, L.; Martín, J.L.; Chamizo-Ampudia, A. A Review of Polyhydroxyalkanoates: Characterization, Production, and Application from Waste. Microorganisms 2024, 12, 2028. [Google Scholar] [CrossRef] [PubMed]
- UvaDoc. Polyhydroxyalkanoates (PHA) Production from Biogas in Waste Treatment Plants: A Realistic Alternative to Conventional Biogas Valorization. 2020. Available online: https://uvadoc.uva.es/handle/10324/46543 (accessed on 12 December 2024).
- Gironi, F.; Piemonte, V. Bioplastics and Petroleum-based Plastics: Strengths and Weaknesses. Energy Sources Part A Recover. Util. Environ. Eff. 2011, 33, 1949–1959. [Google Scholar] [CrossRef]
- Prosperi, M.; Sisto, R.; Lombardi, M.; Zhu, X. Production of bioplastics for agricultural purposes: A supply chain study. Riv. DI Studi Sulla Sostenibilita 2018, 119–136. [Google Scholar] [CrossRef]
- Endres, H.J. Bioplastics. In Biorefineries. Advances in Biochemical Engineering/Biotechnology; Wagemann, K., Tippkötter, N., Eds.; Springer International Publishing: Cham, Switzerland, 2017; Volume 166, pp. 427–468. ISBN 978-3-319-97119-3. [Google Scholar]
- Song, J.H.; Murphy, R.J.; Narayan, R.; Davies, G.B.H. Biodegradable and compostable alternatives to conventional plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2127–2139. [Google Scholar] [CrossRef] [PubMed]
- Leong, Y.K.; Show, P.L.; Lan, J.C.-W.; Loh, H.-S.; Lam, H.L.; Ling, T.C. Economic and environmental analysis of PHAs production process. Clean Technol. Environ. Policy 2017, 19, 1941–1953. [Google Scholar] [CrossRef]
- Choi, J.-I.; Lee, S.Y. Efficient and economical recovery of poly(3-hydroxybutyrate) from recombinantEscherichia coli by simple digestion with chemicals. Biotechnol. Bioeng. 1999, 62, 546–553. [Google Scholar] [CrossRef]
- Dalton, B.; Bhagabati, P.; De Micco, J.; Padamati, R.B.; O’connor, K. A Review on Biological Synthesis of the Biodegradable Polymers Polyhydroxyalkanoates and the Development of Multiple Applications. Catalysts 2022, 12, 319. [Google Scholar] [CrossRef]
- Gerassimidou, S.; Martin, O.V.; Diaz, G.Y.F.; Wan, C.; Komilis, D.; Iacovidou, E. Systematic Evidence Mapping to Assess the Sustainability of Bioplastics Derived from Food Waste: Do We Know Enough? Sustainability 2022, 15, 611. [Google Scholar] [CrossRef]
- DIRECTIVE (EU) 2019/904 of the European Parliament and of the Council of 5 June 2019 on the Reduction of the Impact of Certain Plastic Products on the Environment. Official Journal of the European Union L 155/1-19. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32019L0904 (accessed on 12 December 2024).
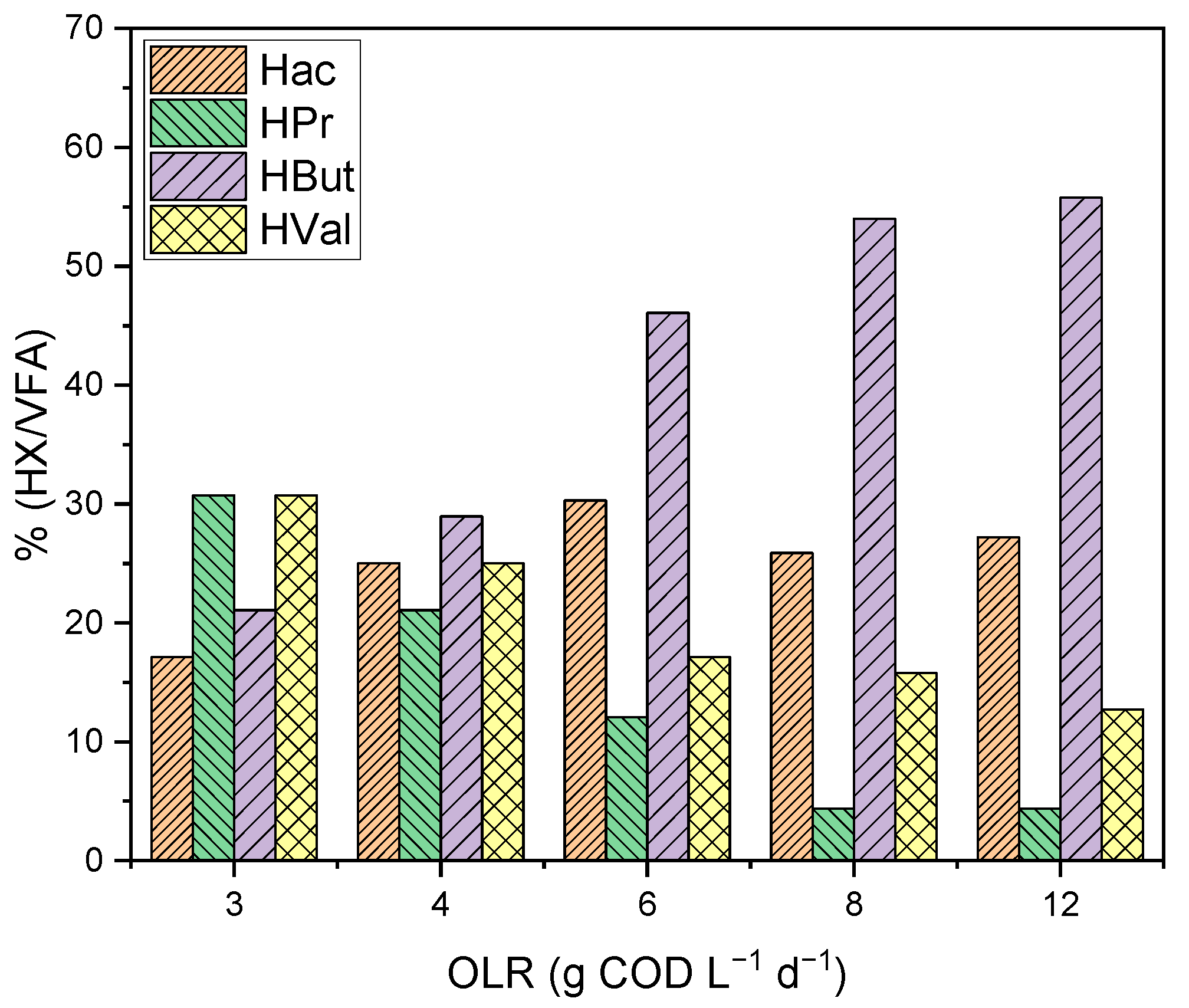


| Waste | pH | T (°C) | OLR | SRT (days = d) | Additives and Other Considerations | VFA Concentration | Valeric and Propionic Acid Yield (%) |
|---|---|---|---|---|---|---|---|
| Whey [77] | 6 (just for propionic acid) | 35 | Low: 3 g COD/(L·d) | STR = 10 | - | - | -Propionic: 36 (pH = 6, STR = 10 d) -Valeric: 25 (pH = 5, STR = 10 d) |
| Potato peel [102] | 7 for propionic acid | 37 | - | 5 d (propionic acid) | - | -Propionic: 180 mg COD/gVSfed -Acetic: 289.5 mg COD/gVSfed. -Butyric: 157.2 mg COD/gVSfed Hexanoic: 89.4 mg COD/gVSfed | - |
| Bagasse [106] | 6 | 30 | 10 g COD/(L·d) | 7 d | Pretreatment of hydrolysis to BSG | - | -Propionic: 1,2% COD basis -Valeric: 2% COD basis |
| Whey and bagasse [107] | 6–6.5 | 35 | 40 g COD/(L·d) | 48 d (propionic acid) 56 d (valeric acid) | Recirculation of CO2 and H2 generated that increased propionic concentration | -Propionic: 3.85 g COD/L (d 48). -Acetic: 10.56 g COD/L (d 48). -Butyric: 20.42 g COD/L (d 56). -Valeric: 3.77 g COD/L (d 56). -Caproic: 13.02 g COD/L (d 56). -with CO2 recirculation. | - |
| Activated Sludge [109] | Nearly 9 | 35 | 13.4 g/(L·d) of MLVSS | 21 d | Rivoflavin | - | Valeric acid: 62.8% with a riboflavin dose of 5 mM |
| Activated Sludge [115] | No pH control. Initial pH was 6.8 ± 2 | 25 | - | 8 d | Alkylpolyglucose | Propionic: 1,3 g COD/L | Propionic acid:43,9% at AOG of 0.3 g/g TSS |
| Substrate | Bacteria Involved | Type of PHA |
|---|---|---|
| Simple sugar | Alcaligenes latus, Ralstonia eutropha, Haloferax mediterranei, Azotobacter vinelandii | -P3HB -Adding organic acids: P(3HB-3HV), P(3HB-4HB) |
| Pseudomonas putida, Pseudomonas citronellolis | -P3HHx, P3HO, and other medium chain PHA (mcl-PHA) | |
| Aeromonas hydrophila, Aeromona scaviae, Rhodospirillum rubrum | -Adding organic acids or alcohols: P(3HB-co-3HHx), P(3HB-co-3HO) and other scl-mcl copolymers. | |
| Bacillus megatherium [140] | -PHB | |
| Bacillus thuringiensis [141] | -PHBV | |
| Tryacylglycerols | Aeromonas hydrophila | -P3HB-3HHx |
| Pseudomonas oleovroans, Pseudomonas stutzeri, Pseudomonas citronellolis | -P3HHx, P3HO and other mcl-PHA. | |
| Cupravidus necator [142] | -P3HB, P(3HB-3HV) | |
| Hydrocarbons | Pseudomonas oleovorans, Pseudomonas citronellolis | -P3HHx, P3HO, P3HD and other mcl-PHA. |
| Strain | Fermentation Conditions | Yield | Type |
|---|---|---|---|
| Cupravidus necator DSM 545 [147] | -Mix of volatile fatty acids. -pH 7 -C/N 6 | 1.5 g/L of biomass and 56% of PHA accumulation | PHBV with 91–96% of hidroxybutyrate content. |
| Bacillus megaterium LVN01 [153] | -Biogas digestate. -pH 7 -30.8 °C | 0.360 g/L of PHA and 0.133 g/L of DCW. | PHBV |
| Haloferaxmediterranei [156] | -Phosphate limitation (KH2PO4). -37 °C | 0.95 g/Lwith 15.6% dry biomass | PHBV |
| Pseudomonas oleovorans DSM 1045 [157] | -Synthetic medium (8.4 g/L glucose + 5.7 g/L sodium ammonium phosphate +35.44 mM phosphate buffer) -30 °C | 1.7 g/L of biomass | mcl-PHA |
| Aeromonas hydrophila 4AK4 [158,159,160,161] | -Sodium gluconate -P limitation | 4.63 g/L of biomass with 35.1% of PHA | P(3HB) |
| -Lauric acid + glucose -P limitation | 16.2 g/L of biomass with 51.5% of PHA | P(3HB-co-HHx) | |
| -Undecanoic acid | 5.01 g/L of biomass with 45.2% of PHA | P(3HV) |
| PHA Type | Description | Physio-Chemical Properties | Applications | Other Considerations |
|---|---|---|---|---|
| scl-PHA (Short-chain-length PHA) | PHA with monomers of 3 to 5 carbon atoms [168]. | The monomer, P(3HB) is highly crystalline [169] brittle and high melting temperature [135]. | Packaging, fiber, biomedical [168]. | High biodegradability [168]. |
| mcl-PHA (Medium-chain length PHA) | PHA with monomers of 6 to 14 carbon atoms [168]. | In general, are flexible [136] Mechanical strength due to their crystalline parts [170]. | Due to their flexibility, they are suitable for food packaging and tissue engineering [136]. | Biodegradable and biocompatible [136]. |
| PHB (Polyhydroxibutirate) | PHA homopolymer [162]. | Hight crystallinity. Stiffness. Fragile at low temperatures. Sensitive to thermal degradation [171]. | Biomedical applications such as matrix for in vitro cell growth, implant patches or sutures [171]. | Biodegradable and biocompatible [171,172]. |
| PHBV (Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)) | Copolymer. It results from the incorporation of 3 units of 3-hydroxyvalerate (HV) into PHB. [173]. | Compared to PHB, it has a lower melting temperature and higher viscosity and flexibility [173]. | Tissue engineering [173,174,175,176,177] Disposable items: bags, packaging. Food packaging [178,179]. | Biodegradable and biocompatible. Reduced cytotoxicity [180]. |
| PHBH (Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) | Copolymer resulting from the incorporation of mcl-PHA monomer (poly(3-hydroxyhexnoate)) into poly(3-hydroxybutyrate) [181]. | High elasticity, low crystallinity, high elongation at break [181]. | Packaging materials [182], coffee capsules, straws, cutlery, shopping bags, fishery items [183]. | Biodegradable and non-toxic [183]. |
| PHBVH (Poly(3-hydroxybutyrate-co-3-hydroxyvalerate-co-3-hydroxyhexanoate) | Copolymer that can be produced by recombinant Aeromonas hydrophila [184]. | Rougher surface and higher hydrophobicity than poly (L-lactic acid) and PHBH. High thermal degradation temperature [184]. | Tissue engineering [184]. | Biocompatible for several cells: fibroblast [185], chondrocytes [186], osteoblasts [187]. |
| PHP (Poly (3-hydroxypropionate) | scl-PHA [188]. | High flexibility, low rigidity [188]. | Drug capsules [189,190]. | Biodegradable and biocompatible [189,190]. |
| Polymer | Melting Point (°C) | Tg (°C) | ME (GPa) | EF (MPa) | E (%) | Biodegradation |
|---|---|---|---|---|---|---|
| P(3HB) | 180 | 4 | 3.5 | 40 | 5 | High rate |
| P(3HB-co-20 mol% 3HV) | 145 | −1 | 0.8 | 20 | 50 | High rate |
| P(3HB-co-6 mol% 3HV) | 133 | −8 | 0.2 | 17 | 680 | High rate |
| P(3HV) | 103 | −15.8 | - | - | - | High rate |
| P(75.3% 3HB-co-13.1% 3HV-co-11.7% 3HHx) | 102.3 | −1.8 | - | - | - | High rate |
| P(73.8% 3HB-co-7.6% 4HB-co-18.6% 3HHx) | - | −11.7 | 0.003 | - | 143 | High rate |
| Polypropylene | 176 | −10 | 1.7 | 38 | 400 | Slow rate |
| LDPE | 130 | −30 | 0.2 | 10 | 620 | Slow rate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arriaga, M.; Pinar, F.J.; Izarra, I.; Amo, J.d.; Vicente, J.; Fernández-Morales, F.J.; Mena, J. Valorization of Agri-Food Waste into PHA and Bioplastics: From Waste Selection to Transformation. Appl. Sci. 2025, 15, 1008. https://doi.org/10.3390/app15031008
Arriaga M, Pinar FJ, Izarra I, Amo Jd, Vicente J, Fernández-Morales FJ, Mena J. Valorization of Agri-Food Waste into PHA and Bioplastics: From Waste Selection to Transformation. Applied Sciences. 2025; 15(3):1008. https://doi.org/10.3390/app15031008
Chicago/Turabian StyleArriaga, Marta, Francisco Javier Pinar, Irene Izarra, Jesús del Amo, Javier Vicente, Francisco Jesús Fernández-Morales, and Javier Mena. 2025. "Valorization of Agri-Food Waste into PHA and Bioplastics: From Waste Selection to Transformation" Applied Sciences 15, no. 3: 1008. https://doi.org/10.3390/app15031008
APA StyleArriaga, M., Pinar, F. J., Izarra, I., Amo, J. d., Vicente, J., Fernández-Morales, F. J., & Mena, J. (2025). Valorization of Agri-Food Waste into PHA and Bioplastics: From Waste Selection to Transformation. Applied Sciences, 15(3), 1008. https://doi.org/10.3390/app15031008







