Artificial Intelligence in Honey Pollen Analysis: Accuracy and Limitations of Pollen Classification Compared with Palynological Expert Assessment
Featured Application
Abstract
1. Introduction
- To perform a comparative assessment of pollen grain classification in unifloral honeys by the AI model and by the palynology expert, using a set of morphological features including shape, size, type and number of apertures, surface ornamentation and pollen wall thickness.
- To evaluate the accuracy of the AI multi-class classification model in relation to expert classification under conditions of standard sample preparation for honey analysis.
- To identify which morphological features have a significant impact on the agreement between the AI model and the expert, and to determine the influence of image quality (e.g., depth of field) on classification outcomes.
- To formulate recommendations for implementing the AI model in honey quality control and food fraud prevention systems (Vulnerability Assessment and Critical Control Points, VACCP, and vulnerability assessment plans), as well as to indicate limitations that require further improvement.
2. Material and Methods
2.1. Characteristics of the Research Material
2.2. Artificial Intelligence Model and Training Pipeline
2.3. Expert Classification and Labeling
2.4. Validation Procedure and Evaluation Metrics
2.5. Statistical Analysis
3. Results
3.1. Sample Characteristics and Morphological Features
3.2. Classification Performance of the AI Model Relative to the Expert
3.2.1. Overall Performance
3.2.2. Class-Wise Classification Performance
3.2.3. Structure of Classification Errors and Prediction Confidence
3.3. Analysis of the Impact of Morphological Features and Image Quality
3.3.1. Morphological Features and Classification Agreement
3.3.2. Image Quality—Model Confidence and Classification Errors
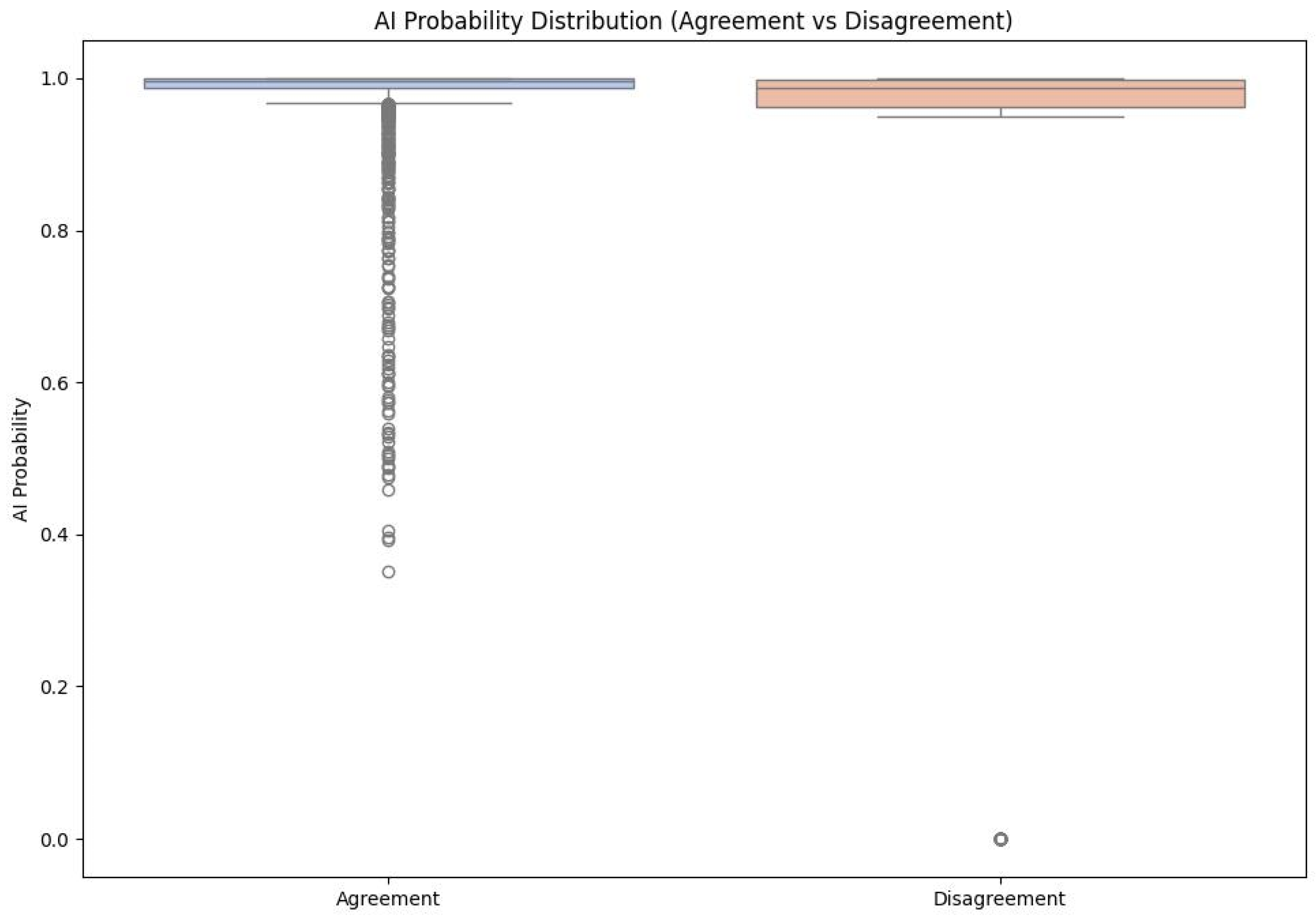
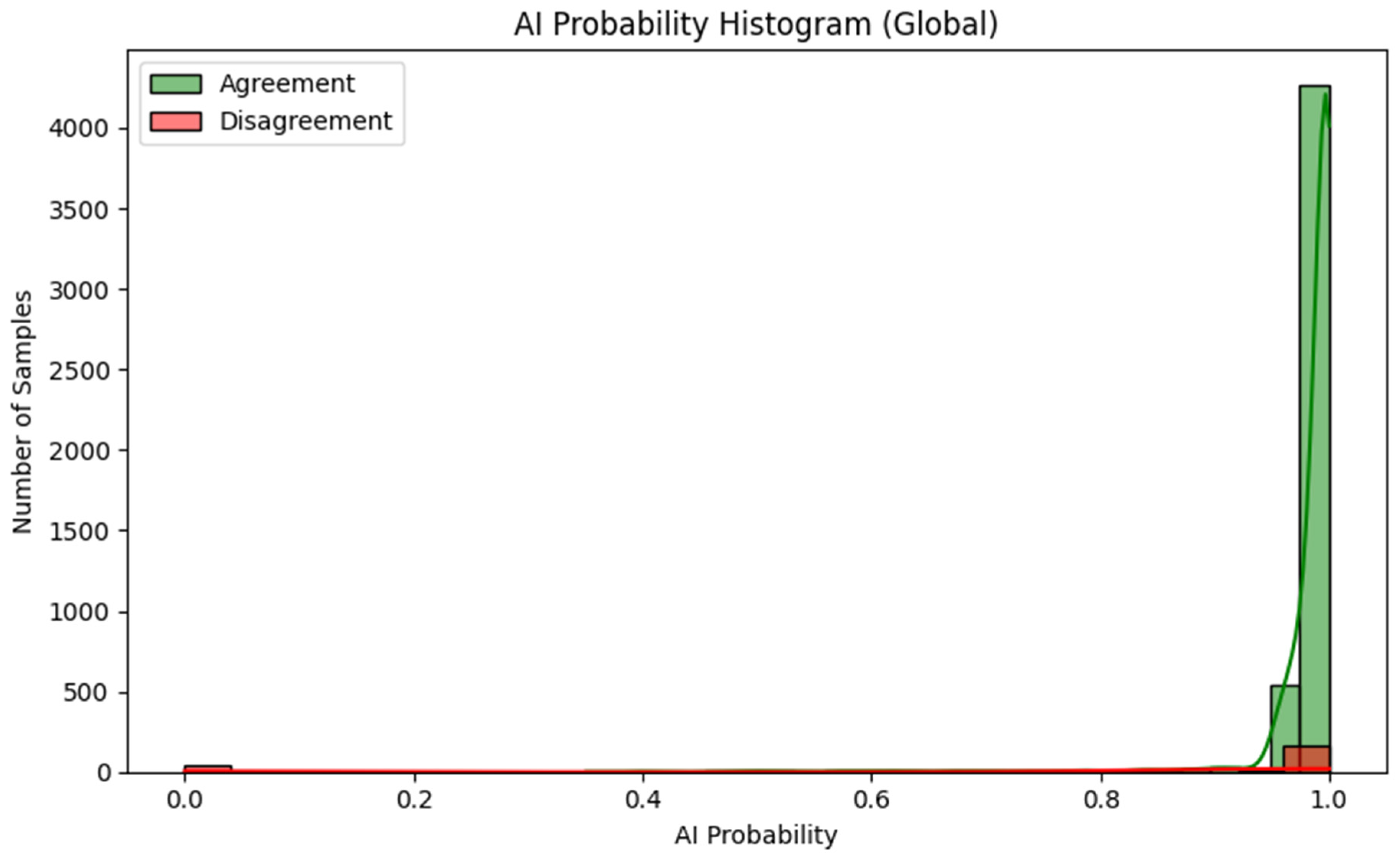
- For observations classified correctly by the AI model, in agreement with the expert, the distribution is dominated by a very narrow range of high prediction probability values, the vast majority of decisions have a probability close to 1.0.
- For misclassified observations, the distribution of prediction probability is clearly wider: the average confidence level is lower, intermediate values (approx. 0.80–0.95) occur more frequently and there are individual cases with very low probability, although some incorrect classifications are still associated with high declared confidence.
- This shape of the distributions indicates that information on prediction probability (AI probability) can serve as a useful warning indicator: low or intermediate values signal an increased risk of error, even though they do not completely eliminate the possibility of “confident but wrong” model decisions.
- With suboptimal depth of field (part of the grain remains outside the focal plane or strong contamination of the slide is visible in the frame);
- With reduced contrast between the grain and the background;
- With the presence of background artefacts (air bubbles, crystals, wax fragments) that hinder automatic segmentation.
4. Discussion
4.1. System Limitations and Directions for Further Research
4.2. Implementation Potential and Interoperability of the AI System
5. Summary and Conclusions
- The AI system achieves classification performance comparable to that of a palynology expert, particularly for dominant pollen classes, which confirms its usefulness as a decision-support tool in melissopalynological analysis (H1).
- Morphological traits of pollen grains (especially exine ornamentation and exine thickness), together with image quality parameters (depth of field, contrast, level of background noise), are key determinants of classification performance. The prediction probability (confidence score) assigned by the model can be used as a practical indicator of classification uncertainty and as a criterion for flagging samples for repeated expert review (H2–H3).
- Extending and diversifying the training dataset, in particular by adding rare classes, morphologically similar plant taxa and material from other geographic regions, is essential for further improving the model’s generalisation capability and its usefulness in international applications.
- The system has substantial implementation potential in honey laboratories and in food authenticity control systems, provided that standardised imaging procedures are maintained, minimum image quality criteria are defined and integration with existing IT infrastructure (LIMS, quality systems) is ensured. This justifies further work on operational testing, integration with other analytical tools and the development of explainable AI modules, including embedding the system within VACCP plans and honey fraud prevention procedures (H4).
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tkacz, E.; Rujna, P.; Więcławek, W.; Lewandowski, B.; Mika, B.; Sieciński, S. Application of 2D extension of Hjorth’s descriptors to distinguish defined groups of bee pollen images. Foods 2024, 13, 3193. [Google Scholar] [CrossRef] [PubMed]
- Wilczyńska, A.; Banach, J.K.; Żak, N.; Grzywińska-Rąpca, M. Preliminary studies on the use of an electrical method to assess the quality of honey and distinguish its botanical origin. Appl. Sci. 2024, 14, 12060. [Google Scholar] [CrossRef]
- Zhang, X.-H.; Gu, H.-W.; Liu, R.-J.; Qing, X.-D.; Nie, J.-F. A comprehensive review of the current trends and recent advancements on the authenticity of honey. Food Chem. X 2023, 19, 100850. [Google Scholar] [CrossRef]
- Fakhlaei, R.; Selamat, J.; Khatib, A.; Razis, A.F.A.; Sukor, R.; Ahmad, S.; Babadi, A.A. The toxic impact of honey adulteration: A review. Foods 2020, 9, 1538. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Amaral, J.S.; Beatriz, M.; Oliveira, P.P.; Mafra, I. A comprehensive review on the main honey authentication issues: Production and origin. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1072–1100. [Google Scholar] [CrossRef]
- Zhang, G.; Abdulla, W. Explainable AI-driven wavelength selection for hyperspectral imaging of honey products. Food Chem. Adv. 2023, 3, 100491. [Google Scholar] [CrossRef]
- Islam, M.K.; Barbour, E.; Locher, C. Authentication of Jarrah (Eucalyptus marginata) honey through its nectar signature and assessment of its typical physicochemical characteristics. Peer. J. Anal. Chem. 2024, 6, e33. [Google Scholar] [CrossRef]
- Tsagkaris, A.; Koulis, G.A.; Danezis, G.P.; Martakos, I.; Dasenaki, M.; Georgiou, C.A.; Thomaidis, N.S. Honey authenticity: Analytical techniques, state of the art and challenges. RSC Adv. 2021, 11, 11273–11294. [Google Scholar] [CrossRef]
- Jurica, K.; Brčić Karačonji, I.; Lasić, D.; Bursać Kovačević, D.; Putnik, P. Unauthorized Food Manipulation as a Criminal Offense: Food Authenticity, Legal Frameworks, Analytical Tools and Cases. Foods 2021, 10, 2570. [Google Scholar] [CrossRef]
- Moore, J.C.; Spink, J.; Lipp, M. Development and application of a database of food ingredient fraud and economically motivated adulteration from 1980 to 2010. J. Food Sci. 2012, 77, R118–R126. [Google Scholar] [CrossRef]
- Everstine, K.D.; Chin, H.B.; Lopes, F.A.; Moore, J.C. Database of Food Fraud Records: Summary of Data from 1980 to 2022. J. Food Prot. 2024, 87, 100227. [Google Scholar] [CrossRef]
- European Commission. EU Coordinated Action “From the Hives” (Honey 2021–2022). Available online: https://food.ec.europa.eu/food-safety/eu-agri-food-fraud-network/eu-coordinated-actions/honey-2021-2022_en (accessed on 3 November 2025).
- European Commission. The EU Agri-Food Fraud Network. 2022. Available online: https://food.ec.europa.eu/food-safety/eu-agri-food-fraud-network_en (accessed on 3 November 2025).
- FEEDM. Statement on the Need of Harmonisation of Analytical Methods for Honey Authenticity. 2023. Available online: https://www.feedm.com/publications/f-e-e-d-m-statements/ (accessed on 3 November 2025).
- Banach, J.K.; Rujna, P.; Lewandowski, B. Integrated Process Oriented Approach for Digital Authentication of Honey in Food Quality and Safety Systems A Case Study from a Research and Development Project. Appl. Sci. 2025, 15, 7850. [Google Scholar] [CrossRef]
- Escriche, I.; Juan-Borrás, M.; Visquert, M.; Valiente, J.M. An overview of the challenges when analysing pollen for monofloral honey classification. Food Control 2023, 143, 109305. [Google Scholar] [CrossRef]
- PN-88/A-77626; Miod Pszczeli. Polski Komitet Normalizacyjny: Warsaw, Poland, 1988. (In Polish)
- The Council of the European Union. Council Directive 2001/110/EC of 20 December 2001 Relating to Honey; Council of the European Union: Brussels, Belgium, 2001.
- Adamchuk, L.; Sukhenko, V.; Akulonok, O.; Bilotserkivets, T.; Vyshniak, V.; Lisohurska, D.; Lisohurska, O.; Slobodyanyuk, N.; Shanina, O.; Galyasnyj, I. Methods for determining the botanical origin of honey. Slovak J. Food Sci. 2020, 14, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Sotiropoulou, N.S.; Xagoraris, M.; Revelou, P.-K.; Kaparakou, E.H.; Kanakis, C.; Pappas, C.; Tarantilis, P.A. The use of SPME-GC-MS, IR and Raman techniques for botanical and geographical authentication and detection of adulteration of honey. Foods 2021, 10, 1671. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, P.M. Fourier Transform Infrared Spectroscopy Use in Honey Characterization and Authentication: A systematic review. ACS Food Sci. Technol. 2024, 4, 1817–1828. [Google Scholar] [CrossRef]
- Cozzolino, D. Advances in spectrometric techniques in food analysis and authentication. Foods 2023, 12, 438. [Google Scholar] [CrossRef]
- Trifković, J.; Andrić, F.; Ristivojević, P.; Guzelmeric, E.; Yesilada, E. Analytical methods in tracing honey authenticity. J. AOAC Int. 2023, 100, 827–839. [Google Scholar] [CrossRef]
- Soares, S.; Rodrigues, F.; Delerue-Matos, C. Towards DNA-Based Methods Analysis for Honey: An Update. Molecules 2023, 28, 2106. [Google Scholar] [CrossRef]
- Chenchouni, H.; Laallam, H. Revolutionizing food quality assessment: Unleashing the potential of artificial intelligence for enhancing honey physicochemical, biochemical, and melissopalynological insights. J. Saudi Soc. Agric. Sci. 2024, 23, 312–325. [Google Scholar] [CrossRef]
- Teye, E.; Amuah, C.L.; Lamptey, F.P.; Obeng, F.; Nyorkeh, R. Artificial intelligence for honey integrity in Ghana: A feasibility study on the use of smartphone images coupled with multivariate algorithms. Smart Agric. Technol. 2024, 8, 100453. [Google Scholar] [CrossRef]
- Chippa, P.; Hu, S.; Pound, M.; Yawar, S.A.; Baniulis, D. Honey authentication using AI-based pollen analysis: A UK review. Br. Food J. 2025, 127, 4512–4529. [Google Scholar] [CrossRef]
- Mahmood, T.; Choi, J.; Park, K.R. Artificial intelligence-based classification of pollen grains using attention-guided pollen features aggregation network. J. King Saud Univ.-Comput. Inf. Sci. 2023, 35, 740–756. [Google Scholar] [CrossRef]
- Le, T.N.; Nguyen, D.M.; Giang, A.C.; Pham, H.T.; Le, T.L.; Vu, H. Identification of Botanical Origin from Pollen Grains in Honey Using Computer Vision-Based Techniques. AgriEngineering 2025, 7, 282. [Google Scholar] [CrossRef]
- Valiente González, J.M.; Martín-Osuna, J.J.; Peral, A.M.; Escriche, I. Assisting monofloral honey classification by automated pollen identification based on convolutional neural networks. Ecol. Inform. 2025, 90, 103340. [Google Scholar] [CrossRef]
- Shamrat, F.M.J.M.; Idris, M.Y.I.; Zhou, X.; Khalid, M.; Sharmin, S.; Sharmin, Z.; Ahmed, K.; Moni, M.A. PollenNet: A novel architecture for high precision pollen grain classification through deep learning and explainable AI. Heliyon 2024, 10, e38596. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhao, J.; Xu, Z.; Wei, J.; Wang, Q.; Shen, F.; Yang, X.; Guo, Z. AIpollen: An Analytic Website for Pollen Identification Through Convolutional Neural Networks. Plants 2024, 13, 3118. [Google Scholar] [CrossRef]
- Viertel, P.; König, M. Pattern recognition methodologies for pollen grain image classification: A survey. Mach. Vis. Appl. 2022, 33, 18. [Google Scholar] [CrossRef]
- Gonçalves, A.B.; Souza, J.S.; Silva, G.G.D.; Cereda, M.P.; Pott, A.; Naka, M.H.; Pistori, H. Feature extraction and machine learning for the classification of brazilian savannah pollen grains. PLoS ONE 2016, 11, e0157044. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, S.; Zhang, Y.; Feng, Y.; Liu, J.; Zhu, H. Artificial intelligence in food safety: A decade review and bibliometric analysis. Foods 2023, 12, 1242. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, H.-W.; Yin, X.-L.; Geng, T. Deep learning in food safety and authenticity detection: An integrative review and future prospects. Trends Food Sci. Technol. 2024, 146, 104396. [Google Scholar] [CrossRef]
- Deng, Z.; Wang, T.; Zheng, Y.; Zhang, W.; Yun, Y.-H. Deep learning in food authenticity: Recent advances and future trends. Trends Food Sci. Technol. 2024, 144, 104344. [Google Scholar] [CrossRef]
- Ropodi, A.I.; Panagou, E.Z.; Nychas, G.J.E. Data Mining Derived from Food Analyses Using Non-Invasive/Non-Destructive Analytical Techniques; Determination of Food Authenticity, Quality & Safety in Tandem with Computer Science Disciplines. Trends Food Sci. Technol. 2016, 50, 11–25. [Google Scholar] [CrossRef]
- Bidyalakshmi, T.; Jyoti, B.; Mansuri, S.M.; Srivastava, A.; Mohapatra, D.; Kalnar, Y.B.; Narsaiah, K.; Indore, N. Application of artificial intelligence in food processing: Current status and future prospects. Food Eng. Rev. 2025, 17, 27–54. [Google Scholar] [CrossRef]
- Balakrishnan, P.; Leema, A.A.; Jothiaruna, N.; Assudani, P.J.; Sankar, K.; Kulkarni, M.B.; Bhaiyya, M. Artificial Intelligence for Food Safety: From Predictive Models to Real-World Safeguards. Trends Food Sci. Technol. 2025, 163, 105153. [Google Scholar] [CrossRef]
- Lukacs, M.; Toth, F.; Horvath, R.; Solymos, G.; Alpár, B.; Varga, P.; Kertesz, I.; Gillay, Z.; Baranyai, L.; Felfoldi, J.; et al. Advanced Digital Solutions for Food Traceability: Enhancing Origin, Quality, and Safety Through NIRS, RFID, Blockchain, and IoT. J. Sens. Actuator Netw. 2025, 14, 21. [Google Scholar] [CrossRef]
- Dabbene, F.; Gay, P.; Tortia, C. Traceability issues in food supply chain management: A review. Biosyst. Eng. 2014, 120, 65–80. [Google Scholar] [CrossRef]
- Olsen, P.; Borit, M. How to define traceability. Trends Food Sci. Technol. 2013, 29, 142–150. [Google Scholar] [CrossRef]
- ISO 22000:2018; Food Safety Management Systems—Requirements for Any Organization in the Food Chain. International Organization for Standardization: Geneva, Switzerland, 2018.
- ISO/IEC 17025:2017; General Requirements for the Competence of Testing and Calibration Laboratories. International Organization for Standardization: Geneva, Switzerland, 2017.
- Jia, W.; Georgouli, K.; Martinez-Del Rincon, J.; Koidis, A. Challenges in the Use of AI-Driven Non-Destructive Spectroscopic Tools for Rapid Food Analysis. Foods 2024, 13, 846. [Google Scholar] [CrossRef]
- Gallardo, R.; García-Orellana, C.J.; González-Velasco, H.M.; Garcia-Manso, A.; Tormo-Molina, R.; Macías-Macías, M.; Abengózar, E. Automated multifocus pollen detection using deep learning. Multimed. Tools Appl. 2024, 83, 72097–72112. [Google Scholar] [CrossRef]
- Rodopoulou, M.A.; Tananaki, C.; Dimou, M.; Liolios, V.; Kanelis, D.; Goras, G.; Thrasyvoulou, A. The determination of the botanical origin in honeys with over-represented pollen: Combination of melissopalynological, sensory and physicochemical analysis. J. Sci. Food Agric. 2018, 98, 2705–2712. [Google Scholar] [CrossRef] [PubMed]
- Shakoori, Z.; Mehrabian, A.; Minai, D.; Salmanpour, F.; Khajoei Nasab, F. Assessing the quality of bee honey on the basis of melissopalynology as well as chemical analysis. PLoS ONE 2023, 18, e0289702. [Google Scholar] [CrossRef]
- Wäldchen, J.; Mäder, P. Plant Species Identification Using Computer Vision Techniques: A Systematic Literature Review. Arch. Comput. Methods Eng. 2018, 25, 507–543. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, Y.; Wang, G.; Zhang, H. Deep learning for plant identification in natural environment. Comput. Intell. Neurosci. 2017, 1, 7361042. [Google Scholar] [CrossRef] [PubMed]
- Brar, D.S.; Aggarwal, A.K.; Nanda, V.; Saxenac, S.; Gautamc, S. AI and CV based 2D-CNN algorithm: Botanical authentication of Indian honey. Sustain. Food Technol. 2024, 2, 373–385. [Google Scholar] [CrossRef]
- Manning, L.; Soon, J.M. Food fraud vulnerability assessment: Reliable data sources and effective assessment approaches. Trends Food Sci. Technol. 2019, 91, 159–168. [Google Scholar] [CrossRef]
- Popping, B.; Buck, N.; Bánáti, D.; Brereton, P.; Gendel, S.; Hristozova, N.; Chaves, S.M.; Saner, S.; Spink, J.; Wunderlin, D. Food inauthenticity: Authority activities, guidance for food operators, and mitigation tools. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4776–4811. [Google Scholar] [CrossRef]
- Manning, L.; MacLeod, A.; James Ch Thompson, M.; Oyeyinka, S.; Cowen, N.; Skoczylis, J.; Onarinde, B.A. Food fraud prevention strategies: Building an effective verification ecosystem. Compr. Rev. Food Sci. Food Saf. 2024, 23, e70036. [Google Scholar] [CrossRef]
- Banach, J.K.; Rujna, P.; Pietrzak-Fiećko, R. Quality management of honey in the bani world: Consumer perceptions and market responses. Scientific Papers of Silesian University of Technology. Organization and Management. 2026; in press. [Google Scholar]
- Sawyer, R. Pollen Identification for Beekeepers; University College Cardiff Press: Cardiff, UK, 1981. [Google Scholar]
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of melissopalynology. Bee World 1978, 59, 139–157. [Google Scholar] [CrossRef]
- Athanasiou, G.; Arcos, J.L.; Cerquides, J. Enhancing medical image segmentation: Ground truth optimization through evaluating uncertainty in expert annotations. Mathematics 2023, 11, 3771. [Google Scholar] [CrossRef]
- Sylolypavan, A.; Sleeman, D.; Wu, H.; Sim, M. The impact of inconsistent human annotations on AI driven clinical decision making. NPJ Digit. Med. 2023, 6, 26. [Google Scholar] [CrossRef]
- Nabi, I.R.; Cardoen, B.; Khater, I.M.; Gao, G.; Wong, T.H.; Hamarneh, G. AI analysis of super-resolution microscopy: Biological discovery in the absence of ground truth. J. Cell Biol. 2024, 223, e202311073. [Google Scholar] [CrossRef]
- Cofre, S.; Sanchez, C.; Quezada-Figueroa, G.; López-Cortés, X.A. Validity and accuracy of artificial intelligence-based dietary intake assessment methods: A systematic review. Br. J. Nutr. 2025, 133, 1241–1253. [Google Scholar] [CrossRef]
- Bourel, B.; Marchant, R.; de Garidel-Thoron, T.; Tetard, M.; Barboni, D.; Gally, Y.; Beaufort, L. Automated recognition by multiple convolutional neural networks of modern, fossil, intact and damaged pollen grains. Comput. Geosci. 2020, 140, 104498. [Google Scholar] [CrossRef]
- Zhou, J.; Brereton, P.; Campbell, K. Progress towards achieving intelligent food assurance systems. Food Control 2024, 164, 110548. [Google Scholar] [CrossRef]
- Punyasena, S.W.; Haselhorst, D.S.; Kong, S.; Fowlkes, C.C.; Moreno, J.E. Automated identification of diverse Neotropical pollen samples using convolutional neural networks. Methods Ecol. Evol. 2022, 13, 2049–2064. [Google Scholar] [CrossRef]
- Haider, A.; Iqbal, S.Z.; Bhatti, I.A.; Alim, M.B.; Waseem, M.; Iqbal, M.; Khaneghah, A.M. Food authentication, current issues, analytical techniques, and future challenges: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13360. [Google Scholar] [CrossRef]
- Treiblmaier, H.; Garaus, M. Using blockchain to signal quality in the food supply chain: The impact on consumer purchase intentions and the moderating effect of brand familiarity. Int. J. Inf. Manag. 2023, 68, 102514. [Google Scholar] [CrossRef]
- FAO/WHO. International and National Regulatory Strategies to Counter Food Fraud; FAO: Rome, Italy, 2019; Available online: https://openknowledge.fao.org/handle/20.500.14283/ca5299en (accessed on 2 November 2025).
- European Food Safety Authority (EFSA). Consolidated Annual Activity Report 2021; EFSA: Parma, Italy, 2022; Available online: https://www.efsa.europa.eu/sites/default/files/2022-03/ar2021.pdf (accessed on 2 November 2025).
- FoodDrinkEurope. Guidance on Food Fraud Vulnerability Assessment and Mitigation. 2020. Available online: https://www.fooddrinkeurope.eu/policy-area/food-fraud/ (accessed on 2 November 2025).
- Hall, D.C. Managing Fraud in Food Supply Chains: The Case of Honey Laundering. Sustainability 2023, 15, 14374. [Google Scholar] [CrossRef]
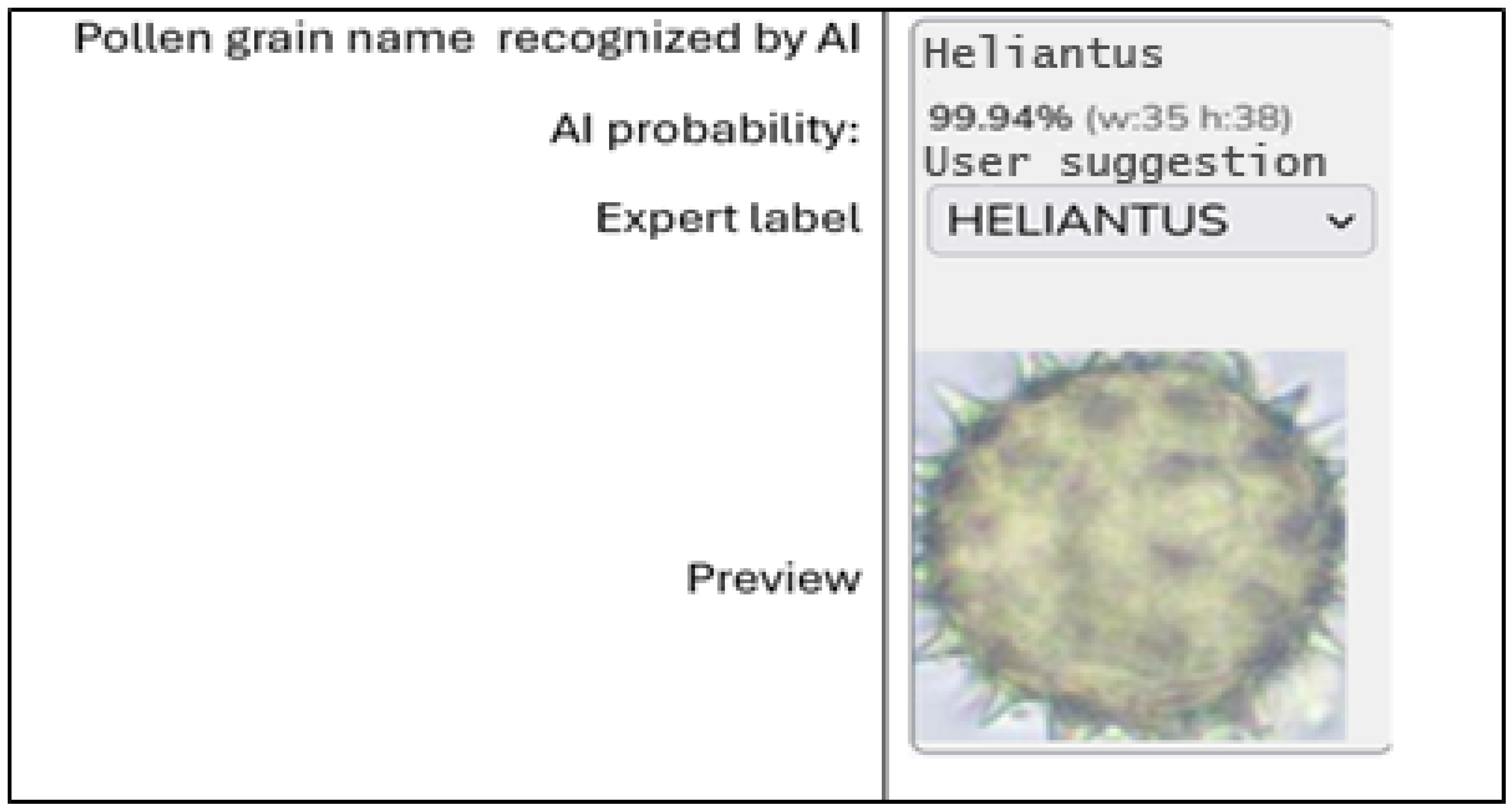
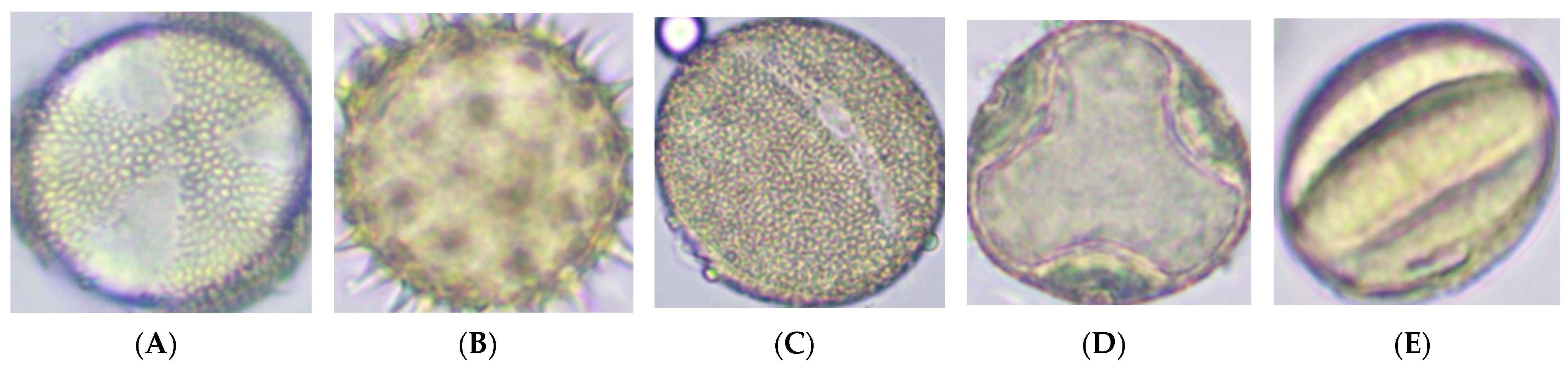
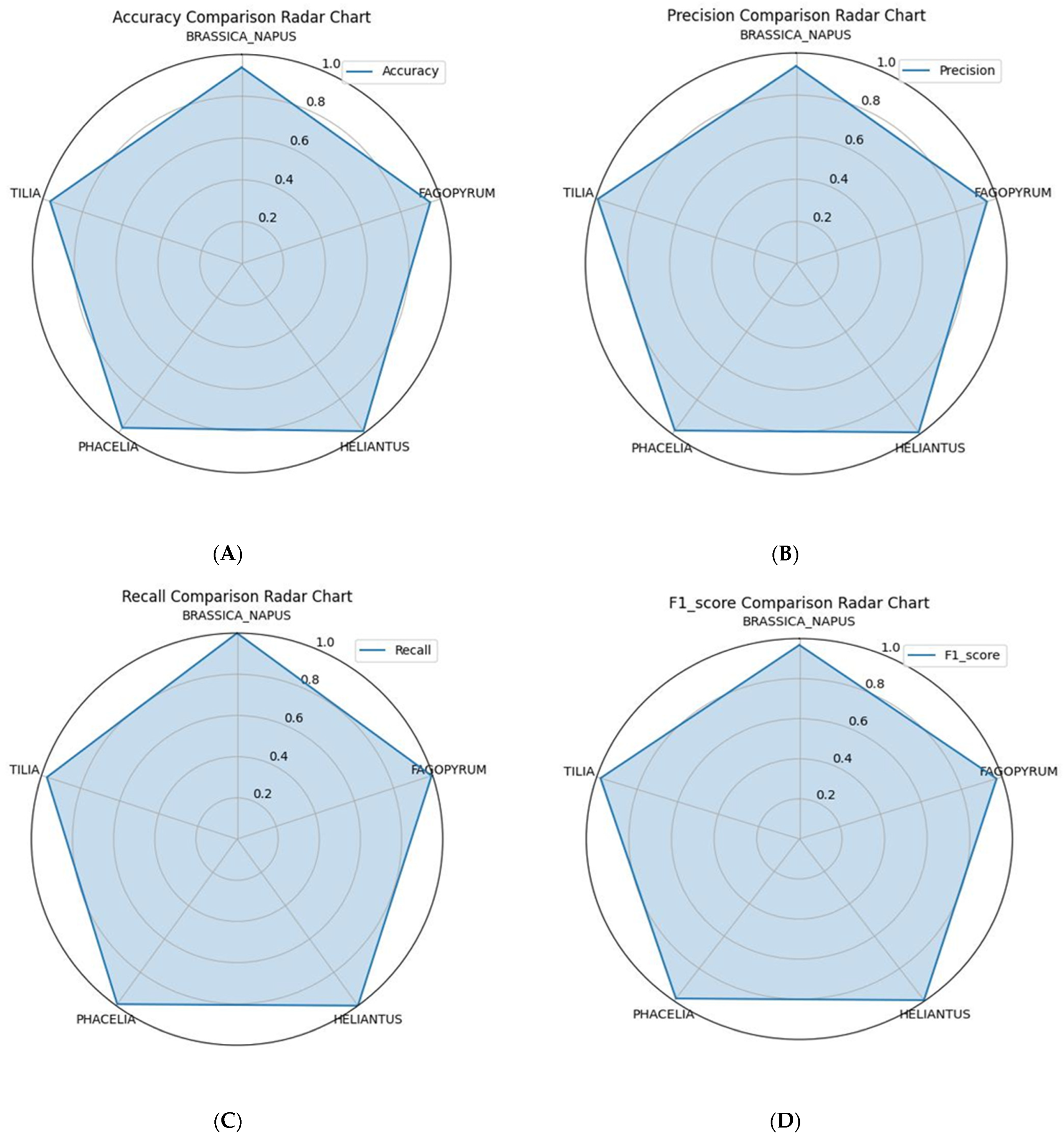
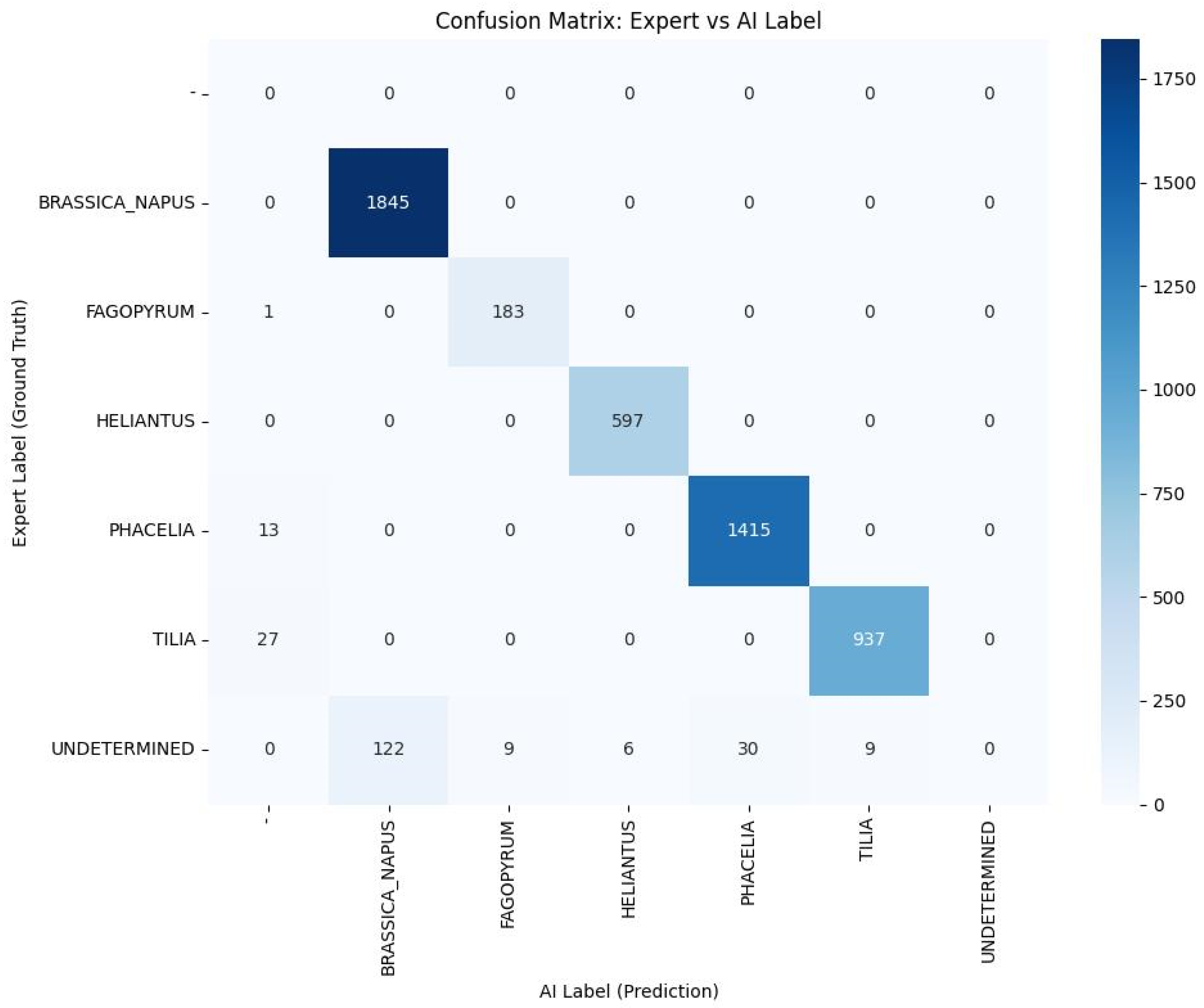
| Morphological Features | Operational Description (Validation Standard) |
|---|---|
| Shape | spherical, ellipsoidal, triangular, irregular |
| Size (μm) | diameter in micrometers |
| Apertures | number and type of openings (e.g., tricolpate, trizonate) |
| Exine ornamentation | surface texture, which may be smooth, spiny, or reticulate |
| Wall thickness (exine) | Visually assessed as thin, intermediate or thick; in the dataset this feature was encoded in three ordinal categories (light/medium/bold) corresponding to thin, medium and thick pollen walls. |
| Color and transparency | auxiliary criterion—omitted due to differences in sample preparation methods and photography parameters (depth of field) |
| Metric | Description | Significance in the Study Context |
|---|---|---|
| Accuracy | Percentage of correct classifications out of all cases | Assessment of the overall effectiveness of the AI model |
| Accuracy confidence interval | A range of values that is likely to contain the true population parameter | Helps to understand the reliability of model’s performance estimate |
| Accuracy standard error | The standard deviation of a sampling distribution, measuring how much a sample statistic (like the mean) would vary across repeated samples from the same population | Helps to understand the generalizability and precision of model’s metrics |
| Precision (per class) | Proportion of correct predictions among all assignments to a given class | Determines the model’s ability to avoid false positives for each pollen class |
| Recall (per class) | Proportion of correct predictions relative to the actual number of samples in a given class | Evaluation of the model’s sensitivity, its ability to capture true cases |
| F1-score | Harmonic mean of precision and recall | Balanced measure of classification effectiveness—particularly useful for imbalanced data |
| Confusion matrix | Matrix of actual and predicted labels | Identification of classification error patterns between pollen classes |
| Kappa coefficient (κ) | Measure of agreement between AI and expert, corrected for chance agreement | Verification of the classifier’s independent agreement with the expert reference assessment |
| Percent agreement | Percentage indicator of consistent classifications between AI and expert | Measurement of agreement without correction for randomness |
| Morphological Feature | Category | n/Proportion (%) * | Notes/Plant Taxa |
|---|---|---|---|
| Shape | spherical/ sphero-ellipsoidal | 5001/96.3 | Brassica napus, Helianthus annuus, Phacelia tanacetifolia, Tilia cordata |
| triangular | 193/3.7 | Fagopyrum esculentum | |
| Size (Ø) | <20 µm | 0/0 | no very small grains |
| 20–40 µm | 4591/88.4 | majority of taxa | |
| >40 µm | 603/11.6 | Mainly Helianthus annuus | |
| Aperture type and number | tricolpate | 3133/60.3 | Brassica napus, Fagopyrum esculentum, Tilia cordata |
| tricolporate | 603/11.6 | Helianthus annuus | |
| triporate | 1458/28.1 | Phacelia tanacetifolia | |
| Exine ornamentation | echinate | 2061/39.7 | Including Helianthus, Phacelia |
| reticulate | 3133/60.3 | Including Brassica, Fagopyrum, Tilia | |
| Pollen wall thickness | Light * | 1458/28.1 | mainly Phacelia tanacetifolia |
| Medium * | 2150/41.5 | intermediate category | |
| Bold * | 1576/30.4 | Including Tilia cordata | |
| Missing data * | 10/0.2 | single cases |
| Parameter | Value |
|---|---|
| Number of analysed pollen grain images | 5194 |
| Number of samples with identical AI and expert labels | 4977 |
| AI–expert agreement (%) | 95.8 |
| Mean accuracy (per class) | 0.96 |
| Mean precision (per class) | 0.97 |
| Mean recall (per class) | 0.99 |
| Mean F1-score (per class) | 0.98 |
| Cohen’s kappa | 0.9436 |
| Standard error (kappa) | 0.0037 |
| 95% CI for kappa | 0.9362–0.9509 |
| Pollen Type | Number of Samples | Samples with Identical AI and Expert Label | AI–Expert Agreement (%) | Accuracy | Precision | Recall | F1-Score | SD |
|---|---|---|---|---|---|---|---|---|
| Brassica napus | 1967 | 1845 | 93.8 | 0.94 | 0.94 | 1.00 | 0.97 | 0.03 |
| Helianthus annuus | 603 | 597 | 99.0 | 0.99 | 0.99 | 1.00 | 0.99 | 0.07 |
| Fagopyrum esculentum | 193 | 183 | 94.8 | 0.95 | 0.95 | 0.99 | 0.97 | 0.00 |
| Phacelia tanacetifolia | 1458 | 1415 | 97.1 | 0.97 | 0.98 | 0.99 | 0.99 | 0.01 |
| Tilia cordata | 973 | 937 | 96.3 | 0.96 | 0.99 | 0.97 | 0.98 | 0.08 |
| Exine Ornamentation | Number of Grains | AI–Expert Agreement (%) (Accuracy) | Mean Prediction Probability AI | Accuracy Standard Error | Accuracy Confidence Interval Low—High |
|---|---|---|---|---|---|
| Echinate | 2061 | 97.6 | 0.981 | 0.003 | 0.969–0.983 |
| Reticulate | 3133 | 94.6 | 0.974 | 0.004 | 0.938–0.954 |
| Exine Thickness | Number of Grains | AI–Expert Agreement (%) (Accuracy) | Mean Prediction Probability AI | Accuracy Standard Error | Accuracy Confidence Interval Low–High |
|---|---|---|---|---|---|
| Bold | 1576 | 97.3 | 0.957 | 0.004 | 0.965–0.981 |
| Light | 1458 | 97.0 | 0.984 | 0.004 | 0.962–0.979 |
| Medium | 2150 | 93.8 | 0.986 | 0.005 | 0.928–0.945 |
| Aperture Type | Number of Grains | AI–Expert Agreement (%) (Accuracy) | Mean Prediction Probability AI | Accuracy Standard Error | Accuracy Confidence Interval Low–High |
|---|---|---|---|---|---|
| Tricolporate | 603 | 99.0 | 0.973 | 0.004 | 0.982–0.997 |
| Triporate | 1458 | 97.1 | 0.984 | 0.004 | 0.961–0.980 |
| Tricolpate | 3133 | 94.6 | 0.974 | 0.004 | 0.938–0.954 |
| Criterion | Traditional Methods (Expert) | AI-Based Pollen Classification System |
|---|---|---|
| Sample preparation | Needs to be prepared according to the standard, approx. 20–40 min. per sample | Needs to be prepared according to the standard, approx. 20–40 min. per sample |
| Analysis time per sample | Approx. 1–2 h per sample (microscopic counting and classification) | Less than 2 min per prepared sample (for already prepared and imaged slides) |
| Required expertise | Specialist in melissopalynology | trained system operator |
| Reproducibility of results | Limited, subject to operator bias | high, algorithmically reproducible |
| Operational costs | High for large sample volumes due to labour-intensive manual classification of each pollen grain | Relatively lower for large sample volumes after system implementation, as classification is performed automatically on acquired images (main costs relate to IT infrastructure and software licensing) |
| Scalability | Limited by human resources | High, parallel processing possible |
| Integration with IT systems | Usually limited, manual reporting | Full integration (API, data export) |
| Validation and auditability | Mainly paper or PDF documentation | Automatic logs, XAI support possible |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banach, J.K.; Lewandowski, B.; Rujna, P. Artificial Intelligence in Honey Pollen Analysis: Accuracy and Limitations of Pollen Classification Compared with Palynological Expert Assessment. Appl. Sci. 2025, 15, 13009. https://doi.org/10.3390/app152413009
Banach JK, Lewandowski B, Rujna P. Artificial Intelligence in Honey Pollen Analysis: Accuracy and Limitations of Pollen Classification Compared with Palynological Expert Assessment. Applied Sciences. 2025; 15(24):13009. https://doi.org/10.3390/app152413009
Chicago/Turabian StyleBanach, Joanna Katarzyna, Bartosz Lewandowski, and Przemysław Rujna. 2025. "Artificial Intelligence in Honey Pollen Analysis: Accuracy and Limitations of Pollen Classification Compared with Palynological Expert Assessment" Applied Sciences 15, no. 24: 13009. https://doi.org/10.3390/app152413009
APA StyleBanach, J. K., Lewandowski, B., & Rujna, P. (2025). Artificial Intelligence in Honey Pollen Analysis: Accuracy and Limitations of Pollen Classification Compared with Palynological Expert Assessment. Applied Sciences, 15(24), 13009. https://doi.org/10.3390/app152413009






