Sequential Extraction Transformation of Brown Onion Skin into Cellulose-Based Enzyme Immobilization Carrier
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
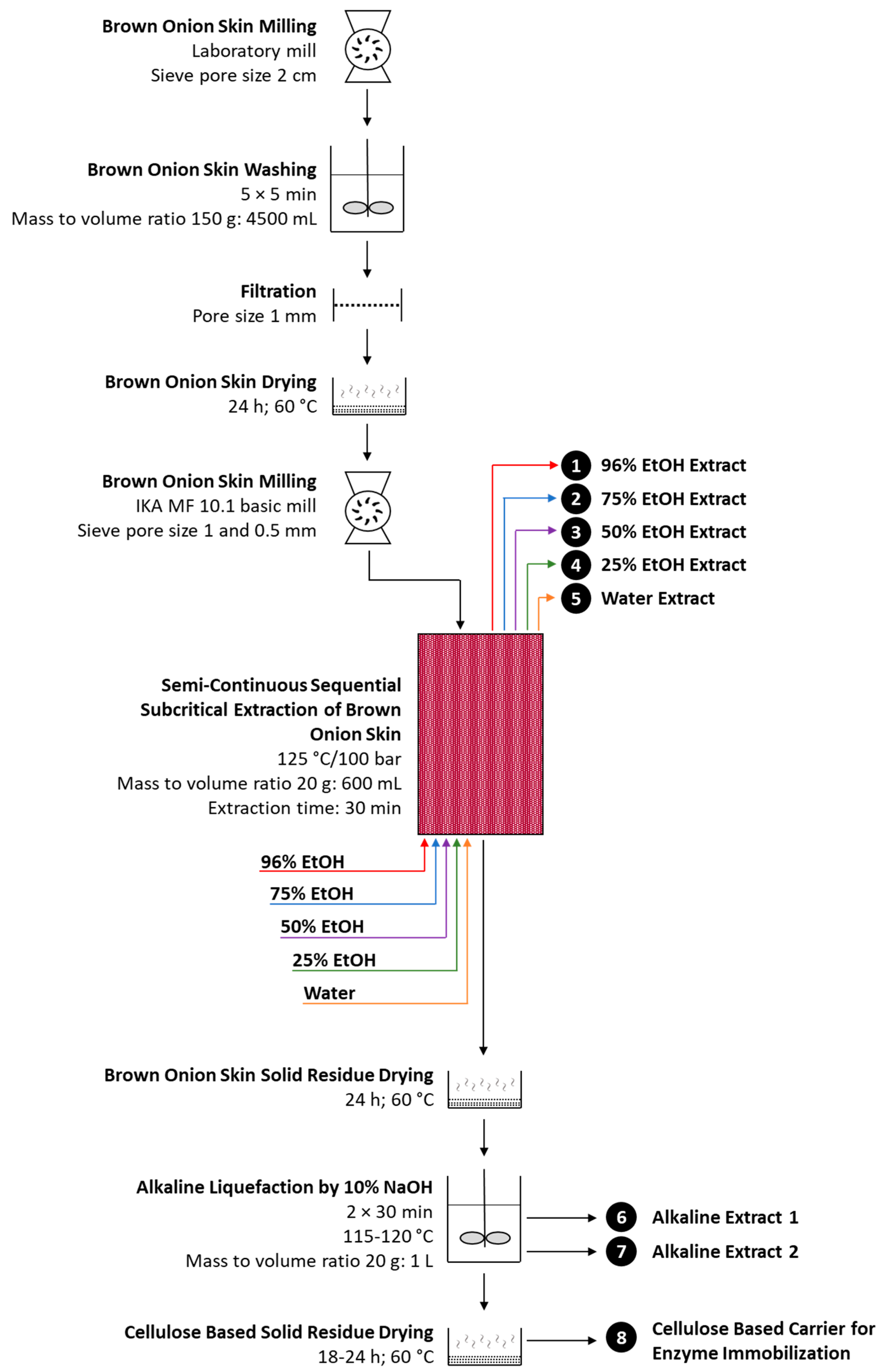
2.2. Semi-Continuous Sequential Subcritical Extraction of Brown Onion Skin
2.3. Alkaline Liquefaction of Sequentially Extracted Brown Onion Skin
2.4. Physical-Chemical Analysis of Brown Onion Skin and Solid Residues Obtained After Subcritical Extraction and Alkaline Liquefaction
2.5. Chemical Analysis of Extracts
2.6. Immobilization of Burkholderia cepacia Lipase on BOS-Derived Cellulose-Based Immobilization Carrier
2.7. Statistical Analysis
3. Results and Discussion
3.1. Transformation of Brown Onion Skin to a Cellulose-Based Enzyme Immobilization Carrier: A Process Design Overview
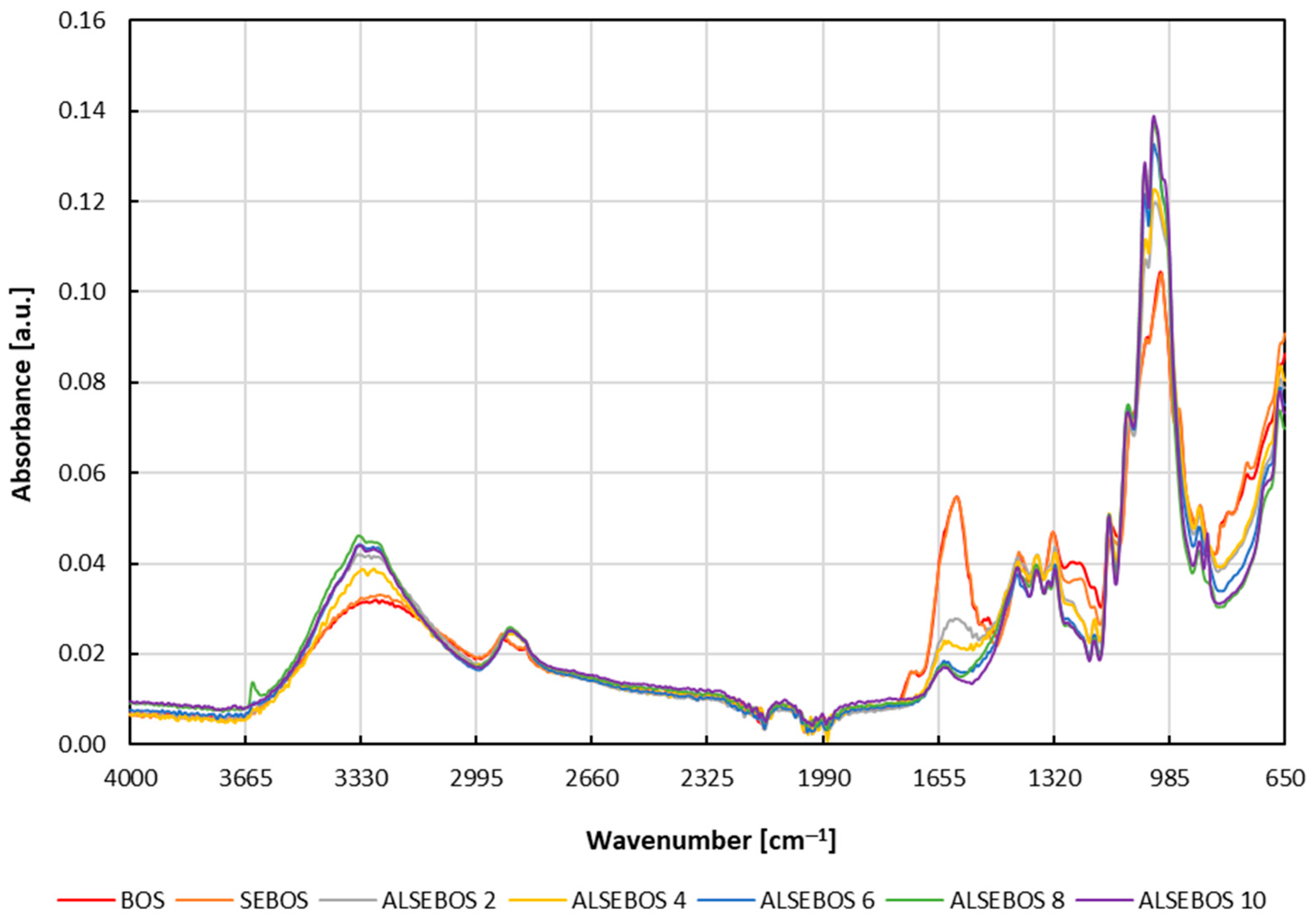
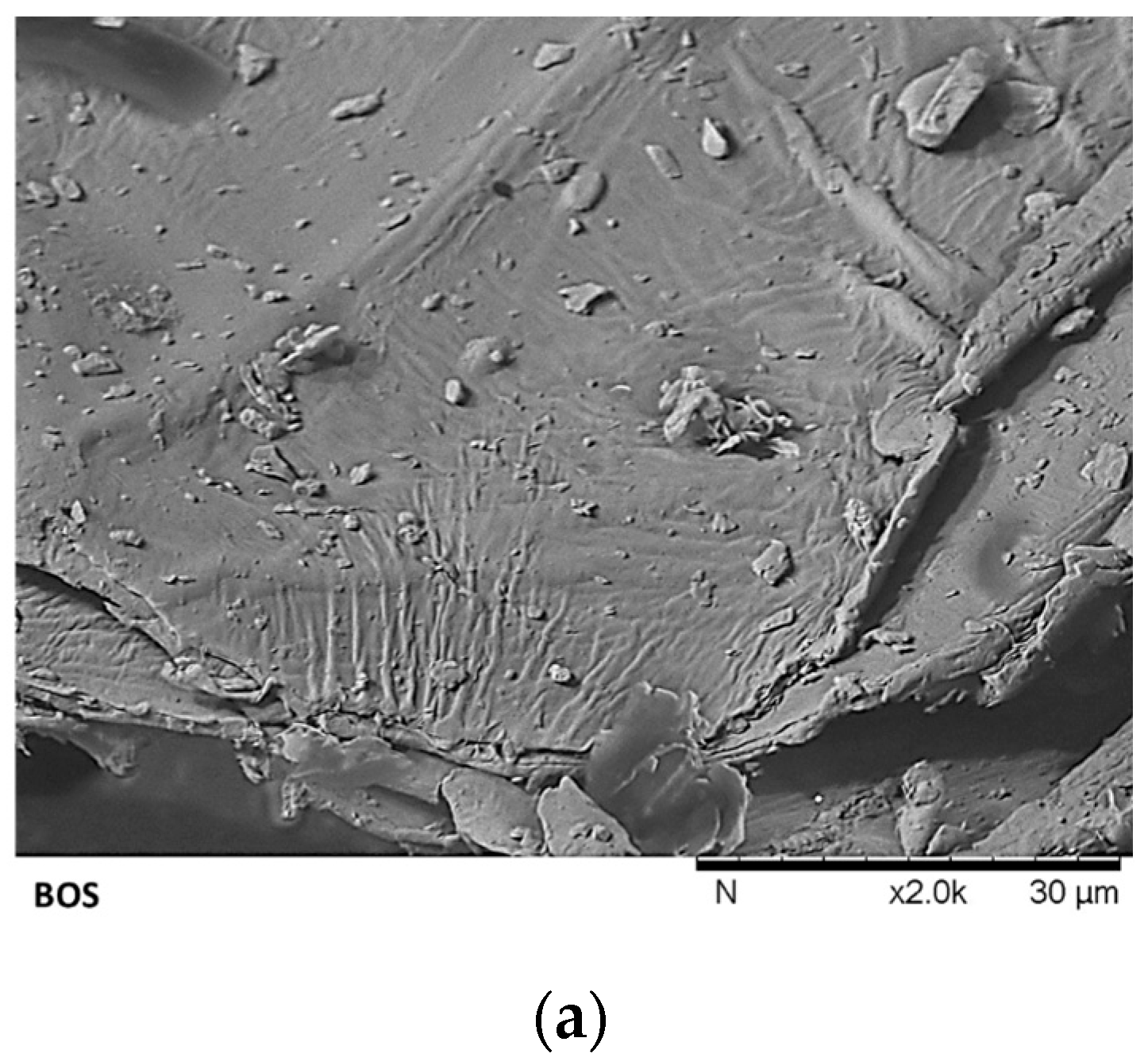
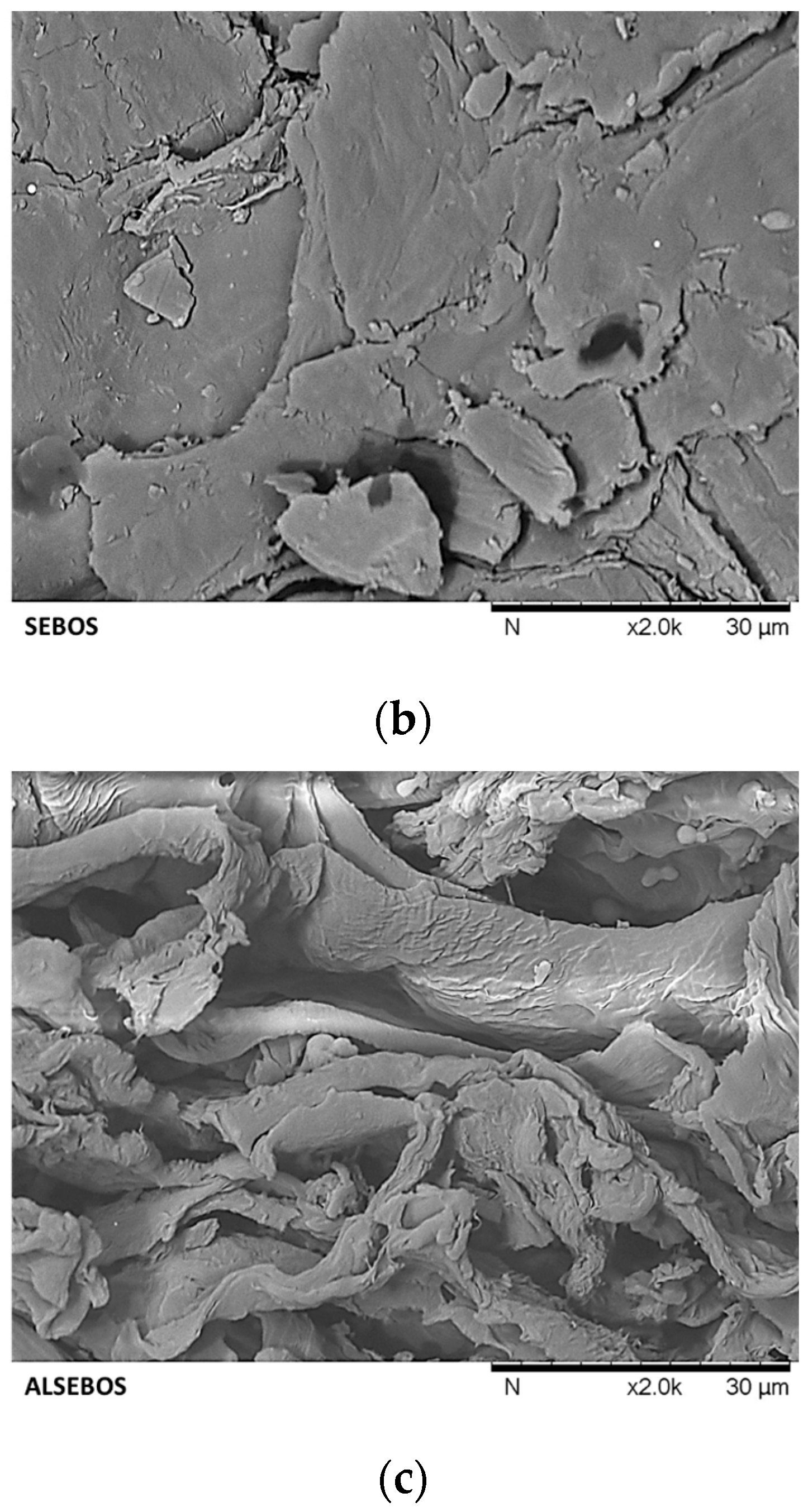
3.2. Physical-Chemical Characterization of Brown Onion Skin and Solid Residues Obtained During Transformation Process
3.3. Physical-Chemical Characterization of Extracts Obtained During Brown Onion Skin Transformation
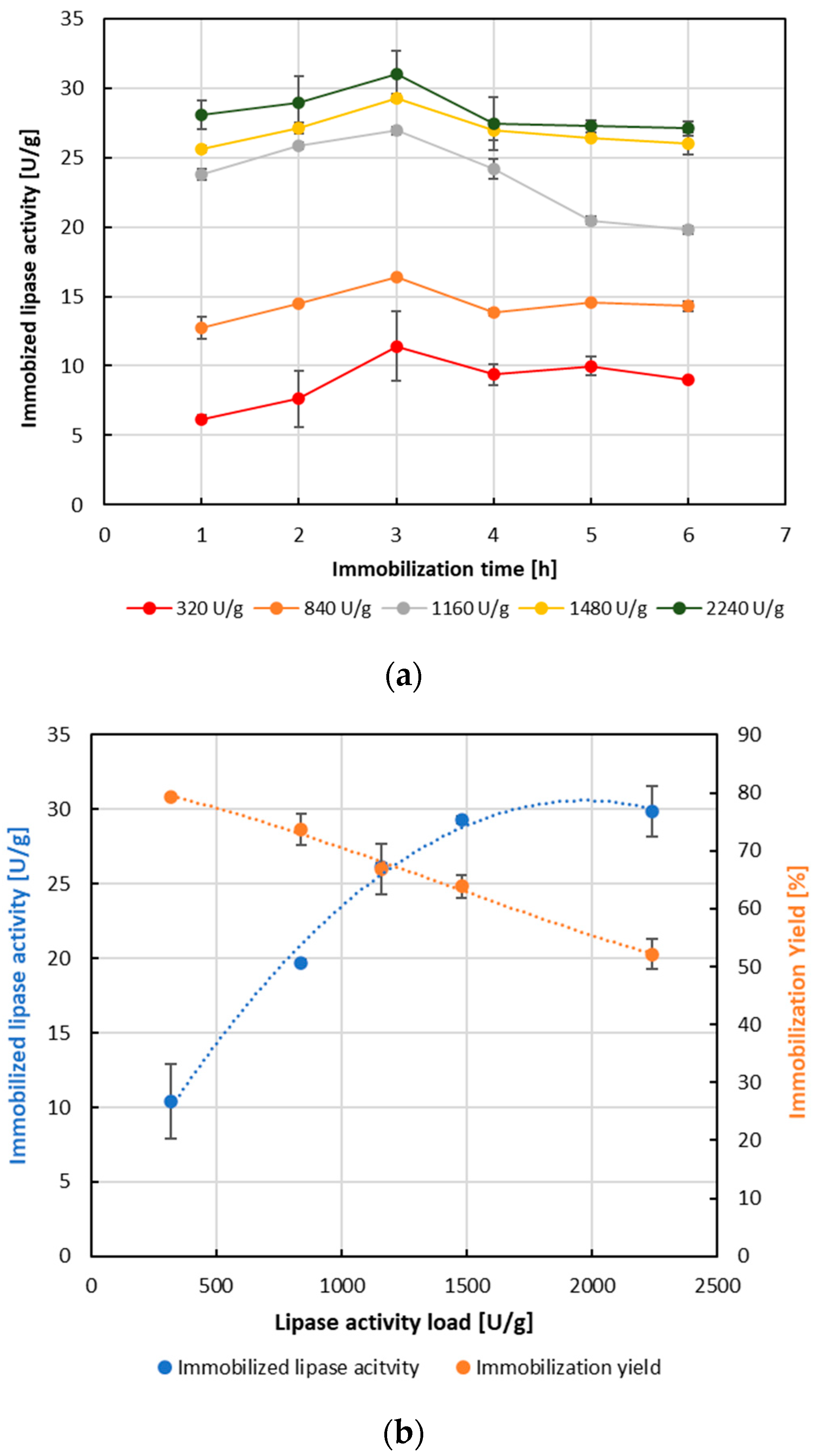
3.4. Immobilization of Burkholderia cepacia Lipase onto BOS-Derived Cellulose-Based Immobilization Carrier by Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BOS | Brown onion skin |
| SEBOS | Sequentially extracted brown onion skin |
| ALSEBOS | Alkaline-liquefied sequentially extracted brown onion skin |
| HPLC | High-performance liquid chromatography |
| BCL | Burkholderia cepacia lipase |
| SubWE | Subcritical water extraction |
| FTIR-ATR | Fourier transform infrared-attenuated total reflectance |
| ScSeqSubE | Semi-continuous sequential subcritical extraction |
References
- Jaganmohanrao, L. Valorization of Onion Wastes and By-Products Using Deep Eutectic Solvents as Alternate Green Technology Solvents for Isolation of Bioactive Phytochemicals. Food Res. Int. 2025, 206, 115980. [Google Scholar] [CrossRef]
- Kyei, S.K.; Frimpong, A.J.; Agorku, E.S.; Eke, W.I.; Akaranta, O. Onion (Allium cepa L.) Skin Waste for Industrial Applications: A Sustainable Strategy for Value Addition and Circular Economy. Bioresour. Technol. Rep. 2025, 29, 102094. [Google Scholar] [CrossRef]
- Kumar, M.; Barbhai, M.D.; Hasan, M.; Punia, S.; Dhumal, S.; Radha; Rais, N.; Chandran, D.; Pandiselvam, R.; Kothakota, A.; et al. Onion (Allium cepa L.) Peels: A Review on Bioactive Compounds and Biomedical Activities. Biomed. Pharmacother. 2022, 146, 112498. [Google Scholar] [CrossRef]
- Osojnik Črnivec, I.G.; Skrt, M.; Šeremet, D.; Sterniša, M.; Farčnik, D.; Štrumbelj, E.; Poljanšek, A.; Cebin, N.; Pogačnik, L.; Smole Možina, S.; et al. Waste Streams in Onion Production: Bioactive Compounds, Quercetin and Use of Antimicrobial and Antioxidative Properties. Waste Manag. 2021, 126, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Benítez, V.; Mollá, E.; Martín-Cabrejas, M.A.; Aguilera, Y.; López-Andréu, F.J.; Cools, K.; Terry, L.A.; Esteban, R.M. Characterization of Industrial Onion Wastes (Allium cepa L.): Dietary Fibre and Bioactive Compounds. Plant Foods Hum. Nutr. 2011, 66, 48–57. [Google Scholar] [CrossRef]
- Coventry, E.; Noble, R.; Mead, A.; Whipps, J.M. Control of Allium White Rot (Sclerotium cepivorum) with Composted Onion Waste. Soil Biol. Biochem. 2002, 34, 1037–1045. [Google Scholar] [CrossRef]
- Jaime, L.; Mollá, E.; Fernández, A.; Martín-Cabrejas, M.A.; López-Andréu, F.J.; Esteban, R.M. Structural Carbohydrate Differences and Potential Source of Dietary Fiber of Onion (Allium cepa L.) Tissues. J. Agric. Food Chem. 2002, 50, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Reddy, J.P.; Rhim, J.-W. Extraction and Characterization of Cellulose Microfibers from Agricultural Wastes of Onion and Garlic. J. Nat. Fibers 2018, 15, 465–473. [Google Scholar] [CrossRef]
- Báez, J.; Marra, G.; Olt, V.; Fernández-Fernández, A.M.; Medrano, A. Potential of Onion Byproducts as a Sustainable Source of Dietary Fiber and Antioxidant Compounds for Its Application as a Functional Ingredient. In Proceedings of the International Electronic Conference on Foods, Virtual, 14 October 2023; MDPI: Basel, Switzerland, 2023; p. 67. [Google Scholar]
- Ko, M.-J.; Cheigh, C.-I.; Cho, S.-W.; Chung, M.-S. Subcritical Water Extraction of Flavonol Quercetin from Onion Skin. J. Food Eng. 2011, 102, 327–333. [Google Scholar] [CrossRef]
- Lizcano-Delgado, Y.Y.; Martínez-Vázquez, O.T.; Cristiani-Urbina, E.; Morales-Barrera, L. Onion Peel: A Promising, Economical, and Eco-Friendly Alternative for the Removal of Divalent Cobalt from Aqueous Solutions. Processes 2024, 12, 1263. [Google Scholar] [CrossRef]
- Sagar, N.A.; Khar, A.; Vikas; Tarafdar, A.; Pareek, S. Physicochemical and Thermal Characteristics of Onion Skin from Fifteen Indian Cultivars for Possible Food Applications. J. Food Qual. 2021, 2021, 7178618. [Google Scholar] [CrossRef]
- Benito-Román, Ó.; Alonso-Riaño, P.; Díaz De Cerio, E.; Sanz, M.T.; Beltrán, S. Semi-Continuous Hydrolysis of Onion Skin Wastes with Subcritical Water: Pectin Recovery and Oligomers Identification. J. Environ. Chem. Eng. 2022, 10, 107439. [Google Scholar] [CrossRef]
- Benito-Román, Ó.; Melgosa, R.; Illera, A.E.; Sanz, M.T.; Beltrán, S. Kinetics of Extraction and Degradation of Pectin Derived Compounds from Onion Skin Wastes in Subcritical Water. Food Hydrocoll. 2024, 153, 109957. [Google Scholar] [CrossRef]
- Benito-Román, Ó.; Blanco, B.; Sanz, M.T.; Beltrán, S. Subcritical Water Extraction of Phenolic Compounds from Onion Skin Wastes (Allium cepa Cv. Horcal): Effect of Temperature and Solvent Properties. Antioxidants 2020, 9, 1233. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.S.; Cho, E.J.; Moon, J.-H.; Bae, H.-J. Onion Skin Waste as a Valorization Resource for the By-Products Quercetin and Biosugar. Food Chem. 2015, 188, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Jin, E.Y.; Lim, S.; Kim, S.O.; Park, Y.-S.; Jang, J.K.; Chung, M.-S.; Park, H.; Shim, K.-S.; Choi, Y.J. Optimization of Various Extraction Methods for Quercetin from Onion Skin Using Response Surface Methodology. Food Sci. Biotechnol. 2011, 20, 1727–1733. [Google Scholar] [CrossRef]
- Katsampa, P.; Valsamedou, E.; Grigorakis, S.; Makris, D.P. A Green Ultrasound-Assisted Extraction Process for the Recovery of Antioxidant Polyphenols and Pigments from Onion Solid Wastes Using Box–Behnken Experimental Design and Kinetics. Ind. Crops Prod. 2015, 77, 535–543. [Google Scholar] [CrossRef]
- Kim, S.-W.; Ko, M.-J.; Chung, M.-S. Extraction of the Flavonol Quercetin from Onion Waste by Combined Treatment with Intense Pulsed Light and Subcritical Water Extraction. J. Clean. Prod. 2019, 231, 1192–1199. [Google Scholar] [CrossRef]
- Munir, M.T.; Kheirkhah, H.; Baroutian, S.; Quek, S.Y.; Young, B.R. Subcritical Water Extraction of Bioactive Compounds from Waste Onion Skin. J. Clean. Prod. 2018, 183, 487–494. [Google Scholar] [CrossRef]
- Salak, F.; Daneshvar, S.; Abedi, J.; Furukawa, K. Adding Value to Onion (Allium cepa L.) Waste by Subcritical Water Treatment. Fuel Process. Technol. 2013, 112, 86–92. [Google Scholar] [CrossRef]
- Trigueros, E.; Benito-Román, Ó.; Oliveira, A.P.; Videira, R.A.; Andrade, P.B.; Sanz, M.T.; Beltrán, S. Onion (Allium cepa L.) Skin Waste Valorization: Unveiling the Phenolic Profile and Biological Potential for the Creation of Bioactive Agents through Subcritical Water Extraction. Antioxidants 2024, 13, 205. [Google Scholar] [CrossRef]
- Velisdeh, Z.J.; Najafpour Darzi, G.; Poureini, F.; Mohammadi, M.; Sedighi, A.; Bappy, M.J.P.; Ebrahimifar, M.; Mills, D.K. Turning Waste into Wealth: Optimization of Microwave/Ultrasound-Assisted Extraction for Maximum Recovery of Quercetin and Total Flavonoids from Red Onion (Allium cepa L.) Skin Waste. Appl. Sci. 2024, 14, 9225. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.Y. Enzyme Immobilization on Cellulose Matrixes. J. Bioact. Compat. Polym. 2016, 31, 553–567. [Google Scholar] [CrossRef]
- Corici, L.; Ferrario, V.; Pellis, A.; Ebert, C.; Lotteria, S.; Cantone, S.; Voinovich, D.; Gardossi, L. Large Scale Applications of Immobilized Enzymes Call for Sustainable and Inexpensive Solutions: Rice Husks as Renewable Alternatives to Fossil-Based Organic Resins. RSC Adv. 2016, 6, 63256–63270. [Google Scholar] [CrossRef]
- Cespugli, M.; Lotteria, S.; Navarini, L.; Lonzarich, V.; Del Terra, L.; Vita, F.; Zweyer, M.; Baldini, G.; Ferrario, V.; Ebert, C.; et al. Rice Husk as an Inexpensive Renewable Immobilization Carrier for Biocatalysts Employed in the Food, Cosmetic and Polymer Sectors. Catalysts 2018, 8, 471. [Google Scholar] [CrossRef]
- Ittrat, P.; Chacho, T.; Pholprayoon, J.; Suttiwarayanon, N.; Charoenpanich, J. Application of Agriculture Waste as a Support for Lipase Immobilization. Biocatal. Agric. Biotechnol. 2014, 3, 77–82. [Google Scholar] [CrossRef]
- Brígida, A.I.S.; Pinheiro, Á.D.T.; Ferreira, A.L.O.; Gonçalves, L.R.B. Immobilization of Candida Antarctica Lipase B by Adsorption to Green Coconut Fiber. Appl. Biochem. Biotechnol. 2008, 146, 173–187. [Google Scholar] [CrossRef]
- Jasińska, K.; Zieniuk, B.; Jankiewicz, U.; Fabiszewska, A. Bio-Based Materials versus Synthetic Polymers as a Support in Lipase Immobilization: Impact on Versatile Enzyme Activity. Catalysts 2023, 13, 395. [Google Scholar] [CrossRef]
- Ostojčić, M.; Brekalo, M.; Stjepanović, M.; Bilić Rajs, B.; Velić, N.; Šarić, S.; Djerdj, I.; Budžaki, S.; Strelec, I. Cellulose Carriers from Spent Coffee Grounds for Lipase Immobilization and Evaluation of Biocatalyst Performance. Sustainability 2025, 17, 9633. [Google Scholar] [CrossRef]
- Gong, R.; Zhang, J.; Zhu, J.; Wang, J.; Lai, Q.; Jiang, B. Loofah Sponge Activated by Periodate Oxidation as a Carrier for Covalent Immobilization of Lipase. Korean J. Chem. Eng. 2013, 30, 1620–1625. [Google Scholar] [CrossRef]
- Ondul, E.; Dizge, N.; Albayrak, N. Immobilization of Candida Antarctica A and Thermomyces Lanuginosus Lipases on Cotton Terry Cloth Fibrils Using Polyethyleneimine. Colloids Surf. B Biointerfaces 2012, 95, 109–114. [Google Scholar] [CrossRef]
- Nikolic, T.; Kostic, M.; Praskalo, J.; Pejic, B.; Petronijevic, Z.; Skundric, P. Sodium Periodate Oxidized Cotton Yarn as Carrier for Immobilization of Trypsin. Carbohydr. Polym. 2010, 82, 976–981. [Google Scholar] [CrossRef]
- Girelli, A.M.; Salvagni, L.; Tarola, A.M. Use of Lipase Immobilized on Celluse Support for Cleaning Aged Oil Layers. J. Braz. Chem. Soc. 2012, 23, 585–592. [Google Scholar] [CrossRef]
- Vikas, V.; Kumar, R.; Pundir, C.S. Covalent Immobilization of Lipase onto Onion Membrane Affixed on Plastic Surface: Kinetic Properties and Application in Milk Fat Hydrolysis. Indian J. Biotechnol. 2007, 6, 479–484. [Google Scholar]
- Kumar, J.; D’Souza, S.F. Inner Epidermis of Onion Bulb Scale: As Natural Support for Immobilization of Glucose Oxidase and Its Application in Dissolved Oxygen Based Biosensor. Biosens. Bioelectron. 2009, 24, 1792–1795. [Google Scholar] [CrossRef]
- Wang, F.; Yao, J.; Russel, M.; Chen, H.; Chen, K.; Zhou, Y.; Ceccanti, B.; Zaray, G.; Choi, M.M.F. Development and Analytical Application of a Glucose Biosensor Based on Glucose Oxidase/O-(2-Hydroxyl)Propyl-3-Trimethylammonium Chitosan Chloride Nanoparticle-Immobilized Onion Inner Epidermis. Biosens. Bioelectron. 2010, 25, 2238–2243. [Google Scholar] [CrossRef]
- Brekalo, M.; Rajs, B.B.; Aladić, K.; Jakobek, L.; Šereš, Z.; Krstović, S.; Jokić, S.; Budžaki, S.; Strelec, I. Multistep Extraction Transformation of Spent Coffee Grounds to the Cellulose-Based Enzyme Immobilization Carrier. Sustainability 2023, 15, 13142. [Google Scholar] [CrossRef]
- ISO 6865:2000; Animal Feeding Stuffs—Determination of Crude Fibre Content—Method with Intermediate Filtration. International Standard Organization (ISO): Geneva, Switzerland, 2000.
- Matić, P.; Sabljić, M.; Jakobek, L. Validation of Spectrophotometric Methods for the Determination of Total Polyphenol and Total Flavonoid Content. J. AOAC Int. 2017, 100, 1795–1803. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Yue, F.; Zhang, J.; Xu, J.; Niu, T.; Lü, X.; Liu, M. Effects of Monosaccharide Composition on Quantitative Analysis of Total Sugar Content by Phenol-Sulfuric Acid Method. Front. Nutr. 2022, 9, 963318. [Google Scholar] [CrossRef]
- Bitter, T.; Muir, H.M. A Modified Uronic Acid Carbazole Reaction. Anal. Biochem. 1962, 4, 330–334. [Google Scholar] [CrossRef]
- Bonet-Ragel, K.; López-Pou, L.; Tutusaus, G.; Benaiges, M.D.; Valero, F. Rice Husk Ash as a Potential Carrier for the Immobilization of Lipases Applied in the Enzymatic Production of Biodiesel. Biocatal. Biotransform. 2018, 36, 151–158. [Google Scholar] [CrossRef]
- Cui, C.; Tao, Y.; Li, L.; Chen, B.; Tan, T. Improving the Activity and Stability of Yarrowia Lipolytica Lipase Lip2 by Immobilization on Polyethyleneimine-Coated Polyurethane Foam. J. Mol. Catal. B Enzym. 2013, 91, 59–66. [Google Scholar] [CrossRef]
- Mustranta, A.; Forssell, P.; Poutanen, K. Applications of Immobilized Lipases to Transesterification and Esterification Reactions in Nonaqueous Systems. Enzym. Microb. Technol. 1993, 15, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Trbojević Ivić, J.; Veličković, D.; Dimitrijević, A.; Bezbradica, D.; Dragačević, V.; Gavrović Jankulović, M.; Milosavić, N. Design of Biocompatible Immobilized Candida rugosa Lipase with Potential Application in Food Industry. J. Sci. Food Agric. 2016, 96, 4281–4287. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; Ur Rehman, D.I. Fourier Transform Infrared (FTIR) Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Thomas, S. Spectroscopic Tools. Available online: https://www.science-and-fun.de/tools/ (accessed on 25 October 2025).
- Kostryukov, S.G.; Matyakubov, H.B.; Masterova, Y.Y.; Kozlov, A.S.; Pryanichnikova, M.K.; Pynenkov, A.A.; Khluchina, N.A. Determination of Lignin, Cellulose, and Hemicellulose in Plant Materials by FTIR Spectroscopy. J. Anal. Chem. 2023, 78, 718–727. [Google Scholar] [CrossRef]
- Javier-Astete, R.; Jimenez-Davalos, J.; Zolla, G. Determination of Hemicellulose, Cellulose, Holocellulose and Lignin Content Using FTIR in Calycophyllum Spruceanum (Benth.) K. Schum. and Guazuma Crinita Lam. PLoS ONE 2021, 16, e0256559. [Google Scholar] [CrossRef]
- Lu, X.; Ross, C.F.; Powers, J.R.; Rasco, B.A. Determination of Quercetins in Onion (Allium cepa) Using Infrared Spectroscopy. J. Agric. Food Chem. 2011, 59, 6376–6382. [Google Scholar] [CrossRef]
- Lu, X.; Wang, J.; Al-Qadiri, H.M.; Ross, C.F.; Powers, J.R.; Tang, J.; Rasco, B.A. Determination of Total Phenolic Content and Antioxidant Capacity of Onion (Allium cepa) and Shallot (Allium oschaninii) Using Infrared Spectroscopy. Food Chem. 2011, 129, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A Review on Alkaline Pretreatment Technology for Bioconversion of Lignocellulosic Biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef]
- Samota, M.K.; Sharma, M.; Kaur, K.; Sarita; Yadav, D.K.; Pandey, A.K.; Tak, Y.; Rawat, M.; Thakur, J.; Rani, H. Onion Anthocyanins: Extraction, Stability, Bioavailability, Dietary Effect, and Health Implications. Front. Nutr. 2022, 9, 917617. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Pandey, V.K.; Dash, K.K.; Zanwar, S.; Singh, R. Natural Bio-Colorant and Pigments: Sources and Applications in Food Processing. J. Agric. Food Res. 2023, 12, 100628. [Google Scholar] [CrossRef]
- Deveoglu, O. A Review on Onion Skin, a Natural Dye Source. J. Text. Color. Polym. Sci. 2022, 19, 307–319. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.; Jesionowski, T.; Pinelo, M. A General Overview of Support Materials for Enzyme Immobilization: Characteristics, Properties, Practical Utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An Overview of Technologies for Immobilization of Enzymes and Surface Analysis Techniques for Immobilized Enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Restrepo, Y.A.; Orrego, C.E. Immobilization of Enzymes and Cells on Lignocellulosic Materials. Environ. Chem. Lett. 2020, 18, 787–806. [Google Scholar] [CrossRef]
- Khan, M.R. Immobilized Enzymes: A Comprehensive Review. Bull. Natl. Res. Cent. 2021, 45, 207. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, Y.; Simpson, B. Food Enzymes Immobilization: Novel Carriers, Techniques and Applications. Curr. Opin. Food Sci. 2022, 43, 27–35. [Google Scholar] [CrossRef]
- Kamdem Tamo, A.; Doench, I.; Deffo, G.; Zambou Jiokeng, S.L.; Doungmo, G.; Fotsop, C.G.; Tonleu Temgoua, R.C.; Montembault, A.; Serghei, A.; Njanja, E.; et al. Lignocellulosic Biomass and Its Main Structural Polymers as Sustainable Materials for (Bio)Sensing Applications. J. Mater. Chem. A 2025, 13, 24185–24253. [Google Scholar] [CrossRef]
- Jensen, C.U.; Rodriguez Guerrero, J.K.; Karatzos, S.; Olofsson, G.; Iversen, S.B. Fundamentals of HydrofactionTM: Renewable Crude Oil from Woody Biomass. Biomass Conv. Bioref. 2017, 7, 495–509. [Google Scholar] [CrossRef]
- Khodayari, A.; Thielemans, W.; Hirn, U.; Van Vuure, A.W.; Seveno, D. Cellulose-Hemicellulose Interactions—A Nanoscale View. Carbohydr. Polym. 2021, 270, 118364. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, W.; Blasiak, W. Modeling Study of Woody Biomass: Interactions of Cellulose, Hemicellulose, and Lignin. Energy Fuels 2011, 25, 4786–4795. [Google Scholar] [CrossRef]
- Rodrigues, P.D.O.; Corrêa, A.G.; Baffi, M.A.; Pasquini, D. Potential Applications of Hemicellulose. In Handbook of Biomass; Thomas, S., Hosur, M., Pasquini, D., Jose Chirayil, C., Eds.; Springer Nature: Singapore, 2023; pp. 1–31. ISBN 978-981-19-6772-6. [Google Scholar]
- Sun, Y.; Cheng, J. Hydrolysis of Lignocellulosic Materials for Ethanol Production: A Review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Gao, S.; Wang, Y.; Diao, X.; Luo, G.; Dai, Y. Effect of Pore Diameter and Cross-Linking Method on the Immobilization Efficiency of Candida Rugosa Lipase in SBA-15. Bioresour. Technol. 2010, 101, 3830–3837. [Google Scholar] [CrossRef]
- Cantone, S.; Ferrario, V.; Corici, L.; Ebert, C.; Fattor, D.; Spizzo, P.; Gardossi, L. Efficient Immobilisation of Industrial Biocatalysts: Criteria and Constraints for the Selection of Organic Polymeric Carriers and Immobilisation Methods. Chem. Soc. Rev. 2013, 42, 6262. [Google Scholar] [CrossRef] [PubMed]
- Dal Magro, L.; de Moura, K.S.; Backes, B.E.; de Menezes, E.W.; Benvenutti, E.V.; Nicolodi, S.; Klein, M.P.; Fernandez-Lafuente, R.; Rodrigues, R.C. Immobilization of Pectinase on Chitosan-Magnetic Particles: Influence of Particle Preparation Protocol on Enzyme Properties for Fruit Juice Clarification. Biotechnol. Rep. 2019, 24, e00373. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshi, N.; Singhal, N.K. Methods, Applications, and Challenges of Enzyme Immobilization on Nanomaterials. In Enzyme Immobilization with Nanomaterials: Applications and Challenges; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2025; Volume 1508, pp. 1–28. [Google Scholar]
- Zhao, L.; Zhang, Y.; Yang, Y.; Yu, C. Silica-Based Nanoparticles for Enzyme Immobilization and Delivery. Chem.–Asian J. 2022, 17, e202200573. [Google Scholar] [CrossRef] [PubMed]
- Zdravkov, B.; Čermák, J.; Šefara, M.; Janků, J. Pore Classification in the Characterization of Porous Materials: A Perspective. Open Chem. 2007, 5, 385–395. [Google Scholar] [CrossRef]
- Lira, R.K.dS.; Zardini, R.T.; de Carvalho, M.C.C.; Wojcieszak, R.; Leite, S.G.F.; Itabaiana, I., Jr. Agroindustrial Wastes as a Support for the Immobilization of Lipase from Thermomyces Lanuginosus: Synthesis of Hexyl Laurate. Biomolecules 2021, 11, 445. [Google Scholar] [CrossRef]
- Feng, W.; Hao, Z.; Li, M. Isolation and Structure Identification of Flavonoids. In Flavonoids—From Biosynthesis to Human Health; Justino, G.C., Ed.; InTech: Rijeka, Croatia, 2017; ISBN 978-953-51-3423-7. [Google Scholar]
- Bijlsma, J.; De Bruijn, W.J.C.; Velikov, K.P.; Vincken, J.-P. Unravelling Discolouration Caused by Iron-Flavonoid Interactions: Complexation, Oxidation, and Formation of Networks. Food Chem. 2022, 370, 131292. [Google Scholar] [CrossRef] [PubMed]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors Affecting Their Stability and Degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef] [PubMed]
- Urréjola, S.; Sánchez, A.; Hervello, M.F. Solubilities of Sodium, Potassium, and Copper(II) Sulfates in Ethanol−Water Solutions. J. Chem. Eng. Data 2011, 56, 2687–2691. [Google Scholar] [CrossRef]

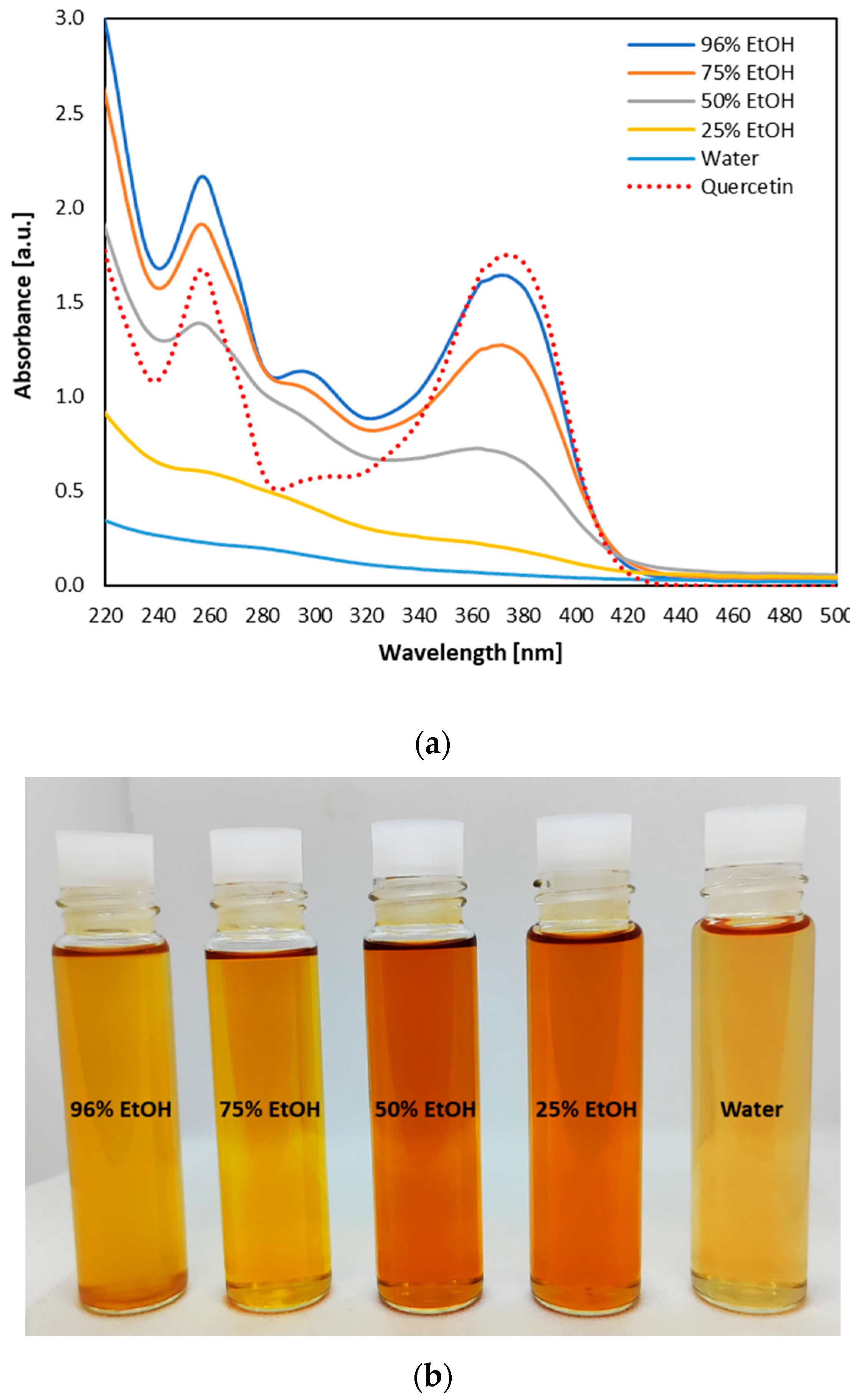
| Parameter | BOS | SEBOS | ALSEBOS |
|---|---|---|---|
| Dry matter [g/100 g] | 92.27 ± 0.12 | 91.01 ± 0.09 | 97.54 ± 0.37 |
| Proteins [g/100 gd.w.b.] | 3.06 ± 0.04 | 3.11 ± 0.05 | 0.62 ± 0.02 |
| Fats [g/100 gd.w.b.] | 0.75 ± 0.28 | n.d. 1 | n.d. |
| Crude fiber [g/100 gd.w.b.] | 37.29 ± 1.62 | 48.55 ± 0.91 | 70.96 ± 3.01 |
| Total polyphenols [g/100 gd.w.b.] | 4.23 ± 0.20 | 0.30 ± 0.07 | n.d. |
| Total flavonoids [g/100 gd.w.b.] | 4.34 ± 0.35 | 0.24 ± 0.08 | n.d. |
| Ash [g/100 gd.w.b.] | 10.81 ± 0.56 | 10.98 ± 0.24 | 6.44 ± 0.42 |
| Physical Property | BOS | SEBOS | ALSEBOS |
|---|---|---|---|
| Average particle size diameter [μm] | 364 | 377 | 247 |
| Specific surface area [m2/g] | 4.038 | 3.318 | 3.821 |
| Total pore volume [cm3/g] | 8.441 × 10−3 | 4.525 × 10−3 | 7.193 × 10−3 |
| Pore diameter [nm] | 4.876 | 4.318 | 5.573 |
| Process | Product | Mass Yield [%] |
|---|---|---|
| Delivery from industry | - | 100 |
| Milling (size 2–5 cm), washing, drying (60 °C) and milling (size 0.5 mm) of brown onion skin | Brown Onion Skin (BOS) | 70.9 ± 0.89 |
| Semi-continuous sequential subcritical extraction | Sequentially Extracted Brown Onion Skin (SEBOS) | 56.10 ± 3.86 |
| Alkaline liquefaction by 10% NaOH | Alkaline Liquefied Sequentially Extracted Brown Onion Skin (ALSEBOS) | 16.62 ± 1.55 |
| Parameter | 96% EtOH | 75% EtOH | 50% EtOH | 25% EtOH | Water | Total |
|---|---|---|---|---|---|---|
| Dry matter [mg/g] | 31.71 ± 3.43 | 26.29 ± 1.91 | 46.23 ± 2.07 | 34.73 ± 2.67 | 30.25 ± 2.45 | 169.20 ± 2.51 |
| Proteins [mg/g] | 15.37 ± 1.22 | 12.04 ± 1.53 | 7.09 ± 1.41 | 2.48 ± 0.89 | 0.23 ± 0.09 | 37.22 ± 1.03 |
| Total sugars [mg/g] 1 | 3.83 ± 0.67 | 5.11 ± 0.85 | 8.28 ± 0.54 | 13.27 ± 1.18 | 9.36 ± 1.28 | 39.86 ± 0.90 |
| Arabinose [mg/g] 2 | 0.12 ± 0.01 | n.d. 3 | 2.66 ± 0.16 | 5.30 ± 0.34 | 5.01 ± 0.01 | 13.10 ± 0.21 |
| Glucose [mg/g] 2 | 0.09 ± 0.01 | 1.07 ± 0.13 | 1.83 ± 0.24 | 2.59 ± 0.24 | 3.08 ± 0.09 | 8.66 ± 0.22 |
| Rhamnose [mg/g] 2 | 2.01 ± 0.14 | n.d. | 1.85 ± 0.09 | 3.92 ± 0.01 | 4.13 ± 0.27 | 11.91 ± 0.25 |
| Xylose [mg/g] 2 | 0.13 ± 0.04 | n.d. | 2.01 ± 0.17 | 3.11 ± 0.33 | 3.98 ± 0.19 | 9.22 ± 0.29 |
| Uronic acid [mg/g] 4 | 0.05 ± 0.03 | 0.27 ± 0.05 | 2.28 ± 0.16 | 7.16 ± 0.53 | 7.74 ± 0.27 | 17.50 ± 0.21 |
| Total polyphenols [mg/g] 5 | 11.87 ± 1.59 | 7.63 ± 0.91 | 8.34 ± 1.14 | 0.42 ± 0.26 | n.d. | 28.26 ± 0.89 |
| Total flavonoids [mg/g] 6 | 18.53 ± 2.24 | 11.69 ± 1.60 | 4.84 ± 1.06 | 1.15 ± 0.96 | n.d. | 36.21 ± 1.36 |
| Quercetin [mg/g] 7 | 6.35 ± 0.89 | 2.64 ± 0.52 | 0.61 ± 0.28 | 0.14 ± 0.03 | 0.08 ± 0.00 | 9.81 ± 0.39 |
| Quercetin-4-glucoside [mg/g] 7 | 0.51 ± 0.07 | 0.58 ± 0.08 | 0.47 ± 0.12 | 0.08 ± 0.01 | 0.03 ± 0.00 | 1.67 ± 0.12 |
| Total flavanols [mg/g] 8 | 6.79 ± 0.91 | 3.20 ± 0.54 | 0.87 ± 0.39 | 0.17 ± 0.04 | 0.08 ± 0.00 | 11.11 ± 0.40 |
| Component | 1st Alkaline Liquefaction Extract | 2nd Alkaline Liquefaction Extract |
|---|---|---|
| Proteins [mg/g] | 19.05 ± 1.74 | 4.74 ± 0.80 |
| Total sugars [mg/g] | 58.03 ± 5.17 | 4.60 ± 0.89 |
| Uronic acids [mg/g] | 10.18 ± 0.30 | 0.70 ± 0.09 |
| Total polyphenols [mg/g] | 6.59 ± 1.00 | n.d. 1 |
| Total flavonoids [mg/g] | 0.50 ± 0.25 | n.d. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brekalo, M.; Ostojčić, M.; Stjepanović, M.; Rajs, B.B.; Matić, P.; Šarić, S.; Stanojev, J.; Aladić, K.; Barron, L.J.; Jokić, S.; et al. Sequential Extraction Transformation of Brown Onion Skin into Cellulose-Based Enzyme Immobilization Carrier. Appl. Sci. 2025, 15, 12970. https://doi.org/10.3390/app152412970
Brekalo M, Ostojčić M, Stjepanović M, Rajs BB, Matić P, Šarić S, Stanojev J, Aladić K, Barron LJ, Jokić S, et al. Sequential Extraction Transformation of Brown Onion Skin into Cellulose-Based Enzyme Immobilization Carrier. Applied Sciences. 2025; 15(24):12970. https://doi.org/10.3390/app152412970
Chicago/Turabian StyleBrekalo, Mirna, Marta Ostojčić, Marija Stjepanović, Blanka Bilić Rajs, Petra Matić, Stjepan Šarić, Jovana Stanojev, Krunoslav Aladić, Lidija Jakobek Barron, Stela Jokić, and et al. 2025. "Sequential Extraction Transformation of Brown Onion Skin into Cellulose-Based Enzyme Immobilization Carrier" Applied Sciences 15, no. 24: 12970. https://doi.org/10.3390/app152412970
APA StyleBrekalo, M., Ostojčić, M., Stjepanović, M., Rajs, B. B., Matić, P., Šarić, S., Stanojev, J., Aladić, K., Barron, L. J., Jokić, S., Djerdj, I., Strelec, I., & Budžaki, S. (2025). Sequential Extraction Transformation of Brown Onion Skin into Cellulose-Based Enzyme Immobilization Carrier. Applied Sciences, 15(24), 12970. https://doi.org/10.3390/app152412970











