Silent Disruptors: The Multifaceted Impact of Phthalates Across Aquatic Invertebrate and Vertebrate Taxa
Abstract
1. Introduction
2. Effects of PAEs on Biota
2.1. Invertebrates
2.1.1. Cladocera
2.1.2. Echinoidea
2.1.3. Insecta (Chironomidae)
2.1.4. Gastropoda
2.1.5. Anellida
2.1.6. Bivalvia
2.1.7. Malacostraca
2.1.8. Copepoda
2.2. Anphibians
2.3. Reptiles
2.4. Mammals
2.5. Utilizing the Current Evidence in Ecological Risk Assessment
3. Conclusions
Ecological Risk Assessment and Policy Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Saleh, I.; Elkhatib, R. Screening of Phthalate Esters in 47 Branded Perfumes. Environ. Sci. Pollut. Res. 2016, 23, 455–468. [Google Scholar] [CrossRef]
- Giuliani, A.; Zuccarini, M.; Cichelli, A.; Khan, H.; Reale, M. Critical Review on the Presence of Phthalates in Food and Evidence of Their Biological Impact. Int. J. Environ. Res. Public Health 2020, 17, 5655. [Google Scholar] [CrossRef] [PubMed]
- Pham, C.L.T.; Hoang, N.B.; Nguyen, A.V.; Le, V.; Tran, N.M.T.; Pham, K.T.; Phung, H.D.; Chu, N.C.; Hoang, A.Q.; Minh, T.B.; et al. Distribution of Phthalic Acid Esters (PAEs) in Personal Care Products and Untreated Municipal Wastewater Samples: Implications for Source Apportionment and Ecological Risk Assessment. Water Air Soil. Pollut. 2025, 236, 33. [Google Scholar] [CrossRef]
- Singh, S.P.; Agarwal, A.K.; Gupta, T.; Maliyekkal, S.M. (Eds.) New Trends in Emerging Environmental Contaminants; Energy, Environment, and Sustainability; Springer: Singapore, 2022; ISBN 9789811683664. [Google Scholar]
- Tian, C.; Ni, J.; Chang, F.; Liu, S.; Xu, N.; Sun, W.; Xie, Y.; Guo, Y.; Ma, Y.; Yang, Z.; et al. Bio-Source of Di-n-Butyl Phthalate Production by Filamentous Fungi. Sci. Rep. 2016, 6, 19791. [Google Scholar] [CrossRef]
- Roy, R.N. Bioactive Natural Derivatives of Phthalate Ester. Crit. Rev. Biotechnol. 2020, 40, 913–929. [Google Scholar] [CrossRef] [PubMed]
- Pace, A.; Vaglica, A.; Maccotta, A.; Savoca, D. The Origin of Phthalates in Algae: Biosynthesis and Environmental Bioaccumulation. Environments 2024, 11, 78. [Google Scholar] [CrossRef]
- Semenov, A.A.; Enikeev, A.G.; Babenko, T.A.; Shafikova, T.N.; Gorshkov, A.G. Phthalates—A Strange Delusion of Ecologists. Theor. Appl. Ecol. 2021, 16–21. [Google Scholar] [CrossRef]
- Savoca, D.; Barreca, S.; Lo Coco, R.; Punginelli, D.; Orecchio, S.; Maccotta, A. Environmental Aspect Concerning Phthalates Contamination: Analytical Approaches and Assessment of Biomonitoring in the Aquatic Environment. Environments 2023, 10, 99. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiao, Y.; Li, Z.; Tao, Y.; Yang, Y. Hazards of Phthalates (PAEs) Exposure: A Review of Aquatic Animal Toxicology Studies. Sci. Total Environ. 2021, 771, 145418. [Google Scholar] [CrossRef]
- Lucaccioni, L.; Trevisani, V.; Passini, E.; Righi, B.; Plessi, C.; Predieri, B.; Iughetti, L. Perinatal Exposure to Phthalates: From Endocrine to Neurodevelopment Effects. Int. J. Mol. Sci. 2021, 22, 4063. [Google Scholar] [CrossRef]
- Warner, G.R.; Dettogni, R.S.; Bagchi, I.C.; Flaws, J.A.; Graceli, J.B. Placental Outcomes of Phthalate Exposure. Reprod. Toxicol. 2021, 103, 1–17. [Google Scholar] [CrossRef]
- Punhagui-Umbelino, A.P.F.; Frigoli, G.F.; de Aquino, A.M.; Jorge, B.C.; Alonso-Costa, L.G.; Erthal-Michelato, R.P.; Arena, A.C.; Scarano, W.R.; Fernandes, G.S.A. Phthalate Exposure During Pregnancy and Lactation Transgenerationally Impairs the Epididymis in the Offspring of Rats. J. Biochem. Mol. Toxicol. 2025, 39, e70379. [Google Scholar] [CrossRef]
- Sree, C.G.; Buddolla, V.; Lakshmi, B.A.; Kim, Y.-J. Phthalate Toxicity Mechanisms: An Update. Comp. Biochem. Physiol. Part. C Toxicol. Pharmacol. 2023, 263, 109498. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, L.; Chen, W.; Wang, Y.; Cui, K.; Chen, W.; Liu, J.; Jin, H.; Zhou, Z. Reproductive Toxicity and Multi/Transgenerational Effects of Emerging Pollutants on C. elegans. Toxics 2024, 12, 785. [Google Scholar] [CrossRef]
- Cheng, T.; Zhao, Y.; Li, X.; Lin, F.; Xu, Y.; Zhang, X.; Li, Y.; Wang, R.; Lai, L. Computation of Octanol−Water Partition Coefficients by Guiding an Additive Model with Knowledge. J. Chem. Inf. Model. 2007, 47, 2140–2148. [Google Scholar] [CrossRef]
- Van Der Spoel, D.; Manzetti, S.; Zhang, H.; Klamt, A. Prediction of Partition Coefficients of Environmental Toxins Using Computational Chemistry Methods. ACS Omega 2019, 4, 13772–13781. [Google Scholar] [CrossRef] [PubMed]
- Bergé, A.; Cladière, M.; Gasperi, J.; Coursimault, A.; Tassin, B.; Moilleron, R. Meta-Analysis of Environmental Contamination by Phthalates. Environ. Sci. Pollut. Res. 2013, 20, 8057–8076. [Google Scholar] [CrossRef]
- Net, S.; Sempéré, R.; Delmont, A.; Paluselli, A.; Ouddane, B. Occurrence, Fate, Behavior and Ecotoxicological State of Phthalates in Different Environmental Matrices. Environ. Sci. Technol. 2015, 49, 4019–4035. [Google Scholar] [CrossRef]
- Le, T.M.; Nguyen, H.M.N.; Nguyen, V.K.; Nguyen, A.V.; Vu, N.D.; Yen, N.T.H.; Hoang, A.Q.; Minh, T.B.; Kannan, K.; Tran, T.M. Profiles of Phthalic Acid Esters (PAEs) in Bottled Water, Tap Water, Lake Water, and Wastewater Samples Collected from Hanoi, Vietnam. Sci. Total Environ. 2021, 788, 147831. [Google Scholar] [CrossRef]
- Gao, D.; Li, Z.; Wang, H.; Liang, H. An Overview of Phthalate Acid Ester Pollution in China over the Last Decade: Environmental Occurrence and Human Exposure. Sci. Total Environ. 2018, 645, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Nehmeh, B.; Haydous, F.; Ali, H.; Hdaifi, A.; Abdlwahab, B.; Orm, M.B.; Abrahamian, Z.; Akoury, E. Emerging Contaminants in the Mediterranean Sea Endangering Lebanon’s Palm Islands Natural Reserve. RSC Adv. 2025, 15, 2034–2044. [Google Scholar] [CrossRef]
- Torres, N.I.; Yu, X.; Padilla, I.Y.; Macchiavelli, R.E.; Ghasemizadeh, R.; Kaeli, D.; Cordero, J.F.; Meeker, J.D.; Alshawabkeh, A.N. The Influence of Hydrogeological and Anthropogenic Variables on Phthalate Contamination in Eogenetic Karst Groundwater Systems. Environ. Pollut. 2018, 237, 298–307. [Google Scholar] [CrossRef]
- Elaiyaraja, A.; Mayilsamy, M.; Vimalkumar, K.; Nikhil, N.P.; Noorani, P.M.; Bommuraj, V.; Thajuddin, N.; Mkandawire, M.; Rajendran, R.B. Aquatic and Human Health Risk Assessment of Humanogenic Emerging Contaminants (HECs), Phthalate Esters from the Indian Rivers. Chemosphere 2022, 306, 135624. [Google Scholar] [CrossRef]
- Sharma, K.; Nayarisseri, A.; Singh, S.K. Biodegradation of Plasticizers by Novel Strains of Bacteria Isolated from Plastic Waste near Juhu Beach, Mumbai, India. Sci. Rep. 2024, 14, 30824. [Google Scholar] [CrossRef]
- Hu, X.; Gu, Y.; Huang, W.; Yin, D. Phthalate Monoesters as Markers of Phthalate Contamination in Wild Marine Organisms. Environ. Pollut. 2016, 218, 410–418. [Google Scholar] [CrossRef]
- Ye, T.; Kang, M.; Huang, Q.; Fang, C.; Chen, Y.; Shen, H.; Dong, S. Exposure to DEHP and MEHP from Hatching to Adulthood Causes Reproductive Dysfunction and Endocrine Disruption in Marine Medaka (Oryzias melastigma). Aquat. Toxicol. 2014, 146, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Haggerty, D.K.; Rappolee, D.A.; Ruden, D.M. Phthalate Exposure and Long-Term Epigenomic Consequences: A Review. Front. Genet. 2020, 11, 405. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Tao, Y.; Yang, Y.; Diogene, T.; Yu, H.; He, Z.; Han, W.; Chen, Z.; Wu, P.; Zhang, Y. Monobutyl Phthalate (MBP) Can Dysregulate the Antioxidant System and Induce Apoptosis of Zebrafish Liver. Environ. Pollut. 2020, 257, 113517. [Google Scholar] [CrossRef] [PubMed]
- Heudorf, U.; Mersch-Sundermann, V.; Angerer, J. Phthalates: Toxicology and Exposure. Int. J. Hyg. Environ. Health 2007, 210, 623–634. [Google Scholar] [CrossRef]
- Staples, C.A.; Adams, W.J.; Parkerton, T.F.; Gorsuch, J.W.; Biddinger, G.R.; Reinert, K.H. Aquatic Toxicity of Eighteen Phthalate Esters. Environ. Toxicol. Chem. 1997, 16, 875–891. [Google Scholar] [CrossRef]
- Mathieu-Denoncourt, J.; Wallace, S.J.; de Solla, S.R.; Langlois, V.S. Influence of Lipophilicity on the Toxicity of Bisphenol A and Phthalates to Aquatic Organisms. Bull. Environ. Contam. Toxicol. 2016, 97, 4–10. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, T.; Ban, Y.; Shen, C.; Shen, Q.; Chai, X.; Zhao, W.; Wei, J. Di-(2-Ethylhexyl) Phthalate Exposure Modulates Antioxidant Enzyme Activity and Gene Expression in Juvenile and Adult Daphnia Magna. Arch. Environ. Contam. Toxicol. 2018, 75, 145–156. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Bui, M.H.; Strady, E.; Kieu-Le, T.C.; Bui, B.T.; Dao, T.S. Single and Combined Effects of Di-2-Ethylhexyl Phthalate and Bisphenol A on Life Traits of the Tropical Micro-Crustacean Ceriodaphniacornuta. J. Sci. Technol. Eng. 2020, 62, 23–29. [Google Scholar] [CrossRef]
- Martino, C.; Savoca, D.; Mauro, M.; Byrne, M.; Hüffer, T.; Chiarelli, R.; Badalamenti, R.; Maccotta, A.; Arizza, V.; Vazzana, M. Heatwave Conditions Increase the Toxicity of Phthalates in Marine Organisms. Sci. Total Environ. 2025, 979, 179479. [Google Scholar] [CrossRef]
- Planelló, R.; Herrero, O.; Martínez-Guitarte, J.L.; Morcillo, G. Comparative Effects of Butyl Benzyl Phthalate (BBP) and Di(2-Ethylhexyl) Phthalate (DEHP) on the Aquatic Larvae of Chironomus Riparius Based on Gene Expression Assays Related to the Endocrine System, the Stress Response and Ribosomes. Aquat. Toxicol. 2011, 105, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Herrero, Ó.; Planelló, R.; Morcillo, G. The Plasticizer Benzyl Butyl Phthalate (BBP) Alters the Ecdysone Hormone Pathway, the Cellular Response to Stress, the Energy Metabolism, and Several Detoxication Mechanisms in Chironomus Riparius Larvae. Chemosphere 2015, 128, 266–277. [Google Scholar] [CrossRef]
- dos Santos Morais, G.; Vieira, T.B.; Santos, G.S.; Dolatto, R.G.; Cestari, M.M.; Grassi, M.T.; Antônio Navarro da Silva, M. Genotoxic, Metabolic, and Biological Responses of Chironomus Sancticaroli Strixino & Strixino, 1981 (Diptera: Chironomidae) after Exposure to BBP. Sci. Total Environ. 2020, 715, 136937. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Cao, W.; Sun, H.; Zhang, S.; Li, P.; Jiang, S.; Zhong, C. Dose-Dependent Effects of Di-(2-Ethylhexyl) Phthalate (DEHP) in Mussel Mytilus Galloprovincialis. Front. Mar. Sci. 2021, 8, 658361. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, H.; Liu, Y.; Zhan, J.; Li, W.; Yang, K.; Yi, X. Acute and Chronic Combined Effect of Polystyrene Microplastics and Dibutyl Phthalate on the Marine Copepod Tigriopus Japonicus. Chemosphere 2020, 261, 127711. [Google Scholar] [CrossRef]
- Prieto-Amador, M.; Caballero, P.; Martínez-Guitarte, J.-L. Analysis of the Impact of Three Phthalates on the Freshwater Gastropod Physella Acuta at the Transcriptional Level. Sci. Rep. 2021, 11, 11411. [Google Scholar] [CrossRef]
- Lee, S.K.; Veeramachaneni, D.N.R. Subchronic Exposure to Low Concentrations of Di-n-Butyl Phthalate Disrupts Spermatogenesis in Xenopus laevis Frogs. Toxicol. Sci. 2005, 84, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Owens, G.A.; Veeramachaneni, D.N.R. Exposure to Low Concentrations of Di-n-Butyl Phthalate During Embryogenesis Reduces Survivability and Impairs Development of Xenopus laevis Frogs. J. Toxicol. Environ. Health Part A 2005, 68, 763–772. [Google Scholar] [CrossRef]
- Xu, Y.; Gye, M.C. Developmental Toxicity of Dibutyl Phthalate and Citrate Ester Plasticizers in Xenopus laevis Embryos. Chemosphere 2018, 204, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Gao, J.; Wang, H. Influence of DEHP on Thyroid, Sex Steroid-related Genes and Gonadal Differentiation in Rana chensinensis Tadpoles. Environ. Toxicol. 2018, 33, 112–121. [Google Scholar] [CrossRef]
- Gardner, S.T.; Wood, A.T.; Lester, R.; Onkst, P.E.; Burnham, N.; Perygin, D.H.; Rayburn, J. Assessing Differences in Toxicity and Teratogenicity of Three Phthalates, Diethyl Phthalate, Di-n-Propyl Phthalate, and Di-n-Butyl Phthalate, Using Xenopus laevis Embryos. J. Toxicol. Environ. Health Part A 2016, 79, 71–82. [Google Scholar] [CrossRef]
- Cocci, P.; Capriotti, M.; Mosconi, G.; Palermo, F.A. Effects of Endocrine Disrupting Chemicals on Estrogen Receptor Alpha and Heat Shock Protein 60 Gene Expression in Primary Cultures of Loggerhead Sea Turtle (Caretta caretta) Erythrocytes. Environ. Res. 2017, 158, 616–624. [Google Scholar] [CrossRef]
- Bianchi, L.; Casini, S.; Vantaggiato, L.; Di Noi, A.; Carleo, A.; Shaba, E.; Armini, A.; Bellucci, F.; Furii, G.; Bini, L.; et al. A Novel Ex Vivo Approach Based on Proteomics and Biomarkers to Evaluate the Effects of Chrysene, MEHP, and PBDE-47 on Loggerhead Sea Turtles (Caretta caretta). Int. J. Environ. Res. Public Health 2022, 19, 4369. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-E.; Tang, B.; Liu, Y.; Luo, X.-J.; Mai, B.-X.; Covaci, A.; Poma, G. Occurrence, Biomagnification and Maternal Transfer of Legacy and Emerging Organophosphorus Flame Retardants and Plasticizers in Water Snake from an e-Waste Site. Environ. Int. 2019, 133, 105240. [Google Scholar] [CrossRef]
- Lemos, L.S.; Di Perna, A.C.; Steinman, K.J.; Robeck, T.R.; Quinete, N.S. Assessment of Phthalate Esters and Physiological Biomarkers in Bottlenose Dolphins (Tursiops truncatus) and Killer Whales (Orcinus orca). Animals 2024, 14, 1488. [Google Scholar] [CrossRef]
- Giovani, G.; Filippi, S.; Molino, C.; Peruffo, A.; Centelleghe, C.; Meschini, R.; Angeletti, D. Plastic Additive Di(2-Ethylhexyl)Phthalate (DEHP) Causes Cell Death and Micronucleus Induction on a Bottlenose Dolphin’s (Tursiops truncatus) in Vitro-Exposed Skin Cell Line. Front. Mar. Sci. 2022, 9, 958197. [Google Scholar] [CrossRef]
- Routti, H.; Harju, M.; Lühmann, K.; Aars, J.; Ask, A.; Goksøyr, A.; Kovacs, K.M.; Lydersen, C. Concentrations and Endocrine Disruptive Potential of Phthalates in Marine Mammals from the Norwegian Arctic. Environ. Int. 2021, 152, 106458. [Google Scholar] [CrossRef] [PubMed]
- Andvik, C.; Bories, P.; Harju, M.; Borgå, K.; Jourdain, E.; Karoliussen, R.; Rikardsen, A.; Routti, H.; Blévin, P. Phthalate Contamination in Marine Mammals off the Norwegian Coast. Mar. Pollut. Bull. 2024, 199, 115936. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Aceves, M.A.; Abo-Al-Ela, H.G.; Faggio, C. Impact of Phthalates and Bisphenols Plasticizers on Haemocyte Immune Function of Aquatic Invertebrates: A Review on Physiological, Biochemical, and Genomic Aspects. J. Hazard. Mater. 2021, 419, 126426. [Google Scholar] [CrossRef] [PubMed]
- Melillo, D.; Marino, R.; Italiani, P.; Boraschi, D. Innate Immune Memory in Invertebrate Metazoans: A Critical Appraisal. Front. Immunol. 2018, 9, 1915. [Google Scholar] [CrossRef]
- Faggio, C.; Tsarpali, V.; Dailianis, S. Mussel Digestive Gland as a Model Tissue for Assessing Xenobiotics: An Overview. Sci. Total Environ. 2018, 636, 220–229. [Google Scholar] [CrossRef]
- Galloway, T.S.; Depledge, M.H. Immunotoxicity in Invertebrates: Measurement and Ecotoxicological Relevance. Ecotoxicology 2001, 10, 5–23. [Google Scholar] [CrossRef]
- Seyoum, A.; Pradhan, A. Effect of Phthalates on Development, Reproduction, Fat Metabolism and Lifespan in Daphnia Magna. Sci. Total Environ. 2019, 654, 969–977. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Bui, N.H.; Dao, T.S. Transgenerational Effects of the Plasticizer Di-2-Ethylhexyl Phthalate on Survival, Growth, and Reproduction of Daphnia Magna. Vietnam. J. Sci. Technol. Eng. 2019, 61, 64–69. [Google Scholar] [CrossRef]
- Le, T.; Nguyen, V.; Bui, M.; Huynh, T.; Huynh, A.; Tran, V.; Vo, T.; Tran, T.; Dao, T. Single and Binary Effects of Di-2-ethylhexyl Phthalate and Trace Metals (Cd, Pb) on Life-history Traits of Daphnia Magna. Environ. Qual. Mgmt 2022, 32, 217–223. [Google Scholar] [CrossRef]
- Dao, T.-S.; Nguyen, V.-T.; Baduel, C.; Bui, M.-H.; Tran, V.T.; Pham, T.-L.; Bui, B.-T.; Dinh, K.V. Toxicity of Di-2-Ethylhexyl Phthalate and Tris (2-Butoxyethyl) Phosphate to a Tropical Micro-Crustacean (Ceriodaphnia cornuta) Is Higher in Mekong River Water than in Standard Laboratory Medium. Environ. Sci. Pollut. Res. 2022, 29, 39777–39789. [Google Scholar] [CrossRef]
- Cho, H.; Seol, Y.; Baik, S.; Sung, B.; Ryu, C.S.; Kim, Y.J. Mono(2-Ethylhexyl) Phthalate Modulates Lipid Accumulation and Reproductive Signaling in Daphnia Magna. Environ. Sci. Pollut. Res. 2022, 29, 55639–55650. [Google Scholar] [CrossRef] [PubMed]
- Shore, E.A.; Huber, K.E.; Garrett, A.D.; Pespeni, M.H. Four Plastic Additives Reduce Larval Growth and Survival in the Sea Urchin Strongylocentrotus Purpuratus. Mar. Pollut. Bull. 2022, 175, 113385. [Google Scholar] [CrossRef]
- Gambardella, C.; Miroglio, R.; Prieto Amador, M.; Castelli, F.; Castellano, L.; Piazza, V.; Faimali, M.; Garaventa, F. High Concentrations of Phthalates Affect the Early Development of the Sea Urchin Paracentrotus Lividus. Ecotoxicol. Environ. Saf. 2024, 279, 116473. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Kwak, I.-S. Expression of Chironomus Riparius Serine-Type Endopeptidase Gene under Di-(2-Ethylhexyl)-Phthalate (DEHP) Exposure. Comp. Biochem. Physiol. Part. B Biochem. Mol. Biol. 2008, 151, 349–354. [Google Scholar] [CrossRef]
- Zhou, J.; Cai, Z.-H.; Li, L.; Gao, Y.-F.; Hutchinson, T.H. A Proteomics Based Approach to Assessing the Toxicity of Bisphenol A and Diallyl Phthalate to the Abalone (Haliotis Diversicolor Supertexta). Chemosphere 2010, 79, 595–604. [Google Scholar] [CrossRef]
- Lu, Y.; Lin, M.; Aitken, R.J. Exposure of Spermatozoa to Dibutyl Phthalate Induces Abnormal Embryonic Development in a Marine Invertebrate Galeolaria Caespitosa (Polychaeta: Serpulidae). Aquat. Toxicol. 2017, 191, 189–200. [Google Scholar] [CrossRef]
- Yurdakok-Dikmen, B.; Turgut, Y.; Gunal, A.Ç.; Uyar, R.; Kuzukıran, O.; Filazi, A.; Erkoc, F. In Vitro Effects of Selected Endocrine Disruptors (DEHP, PCB118, BPA) on Narrow-Clawed Crayfish (Astacus leptodactylus) Primary Cells. In Vitro Cell. Dev. Biol.-Anim. 2020, 56, 783–791. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Lin, W.; Shi, C.; Li, R.; Mu, C.; Wang, C.; Ye, Y. Accumulation, Detoxification, and Toxicity of Dibutyl Phthalate in the Swimming Crab. Chemosphere 2022, 289, 133183. [Google Scholar] [CrossRef]
- Kloas, W.; Lutz, I. Amphibians as Model to Study Endocrine Disrupters. J. Chromatogr. A 2006, 1130, 16–27. [Google Scholar] [CrossRef]
- Orton, F.; Tyler, C.R. Do Hormone-modulating Chemicals Impact on Reproduction and Development of Wild Amphibians? Biol. Rev. 2015, 90, 1100–1117. [Google Scholar] [CrossRef]
- Mathieu-Denoncourt, J.; De Solla, S.R.; Langlois, V.S. Chronic Exposures to Monomethyl Phthalate in Western Clawed Frogs. Gen. Comp. Endocrinol. 2015, 219, 53–63. [Google Scholar] [CrossRef]
- Mathieu-Denoncourt, J.; Martyniuk, C.J.; Loughery, J.R.; Yargeau, V.; De Solla, S.R.; Langlois, V.S. Lethal and Sublethal Effects of Phthalate Diesters in Silurana tropicalis Larvae. Environ. Toxicol. Chem. 2016, 35, 2511–2522. [Google Scholar] [CrossRef]
- Ohtani, H.; Miura, I.; Ichikawa, Y. Effects of Dibutyl Phthalate as an Environmental Endocrine Disruptor on Gonadal Sex Differentiation of Genetic Males of the Frog Rana Rugosa. Environ. Health Perspect. 2000, 108, 1189. [Google Scholar] [CrossRef]
- Shen, O.; Wu, W.; Du, G.; Liu, R.; Yu, L.; Sun, H.; Han, X.; Jiang, Y.; Shi, W.; Hu, W.; et al. Thyroid Disruption by Di-n-Butyl Phthalate (DBP) and Mono-n-Butyl Phthalate (MBP) in Xenopus laevis. PLoS ONE 2011, 6, e19159. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jang, J.; Gye, M.C. The Xenopus laevis Teratogenesis Assay for Developmental Toxicity of Phthalate Plasticizers and Alternatives. Environ. Pollut. 2022, 300, 118985. [Google Scholar] [CrossRef] [PubMed]
- Arancio, A.L.; Cole, K.D.; Dominguez, A.R.; Cohenour, E.R.; Kadie, J.; Maloney, W.C.; Cilliers, C.; Schuh, S.M. Bisphenol A, Bisphenol AF, Di-n-Butyl Phthalate, and 17β-Estradiol Have Shared and Unique Dose-Dependent Effects on Early Embryo Cleavage Divisions and Development in Xenopus laevis. Reprod. Toxicol. 2019, 84, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Shen, Y.; Niu, Z.; Li, X. Effects of Cadmium and Diethylhexyl Phthalate on Skin Microbiota of Rana chinensis Tadpoles. Environ. Sci. Pollut. Res. 2023, 30, 64285–64299. [Google Scholar] [CrossRef]
- Shen, Y.; Jiang, Z.; Zhong, X.; Wang, H.; Liu, Y.; Li, X. Manipulation of Cadmium and Diethylhexyl Phthalate on Rana chensinensis Tadpoles Affects the Intestinal Microbiota and Fatty Acid Metabolism. Sci. Total Environ. 2022, 821, 153455. [Google Scholar] [CrossRef]
- Bissegger, S.; Pineda Castro, M.A.; Yargeau, V.; Langlois, V.S. Phthalates Modulate Steroid 5-Reductase Transcripts in the Western Clawed Frog Embryo. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2018, 213, 39–46. [Google Scholar] [CrossRef]
- Kamel, A.; Matten, S.R.; Lynn, S.G.; Wolf, J.C.; Fort, D.J. Amphibian Metamorphosis Assay: Investigation of the Potential Effects of Five Chemicals on the Hypothalamic-Pituitary Thyroid Axis of Xenopus laevis. Regul. Toxicol. Pharmacol. 2022, 134, 105241. [Google Scholar] [CrossRef]
- Lv, X.; Wu, Y.; Chen, G.; Yu, L.; Zhou, Y.; Yu, Y.; Lan, S.; Hu, J. The Strategy for Estrogen Receptor Mediated-Risk Assessment in Environmental Water: A Combination of Species Sensitivity Distributions and in Silico Approaches. Environ. Pollut. 2022, 309, 119763. [Google Scholar] [CrossRef]
- Muñoz, C.; Charles, S.; Vermeiren, P. Advancing Maternal Transfer of Organic Pollutants across Reptiles for Conservation and Risk Assessment Purposes. Environ. Sci. Technol. 2024, 58, 17567–17579. [Google Scholar] [CrossRef]
- Savoca, D.; Arculeo, M.; Vecchioni, L.; Cambera, I.; Visconti, G.; Melfi, R.; Arizza, V.; Palumbo Piccionello, A.; Buscemi, S.; Pace, A. Can Phthalates Move into the Eggs of the Loggerhead Sea Turtle Caretta caretta? The Case of the Nests on the Linosa Island in the Mediterranean Sea. Mar. Pollut. Bull. 2021, 168, 112395. [Google Scholar] [CrossRef]
- Savoca, D.; Arculeo, M.; Barreca, S.; Buscemi, S.; Caracappa, S.; Gentile, A.; Persichetti, M.F.; Pace, A. Chasing Phthalates in Tissues of Marine Turtles from the Mediterranean Sea. Mar. Pollut. Bull. 2018, 127, 165–169. [Google Scholar] [CrossRef]
- Di Renzo, L.; Mascilongo, G.; Berti, M.; Bogdanović, T.; Listeš, E.; Brkljača, M.; Notarstefano, V.; Gioacchini, G.; Giorgini, E.; Olivieri, V.; et al. Potential Impact of Microplastics and Additives on the Health Status of Loggerhead Turtles (Caretta caretta) Stranded Along the Central Adriatic Coast. Water Air Soil. Pollut. 2021, 232, 98. [Google Scholar] [CrossRef]
- van de Merve, J.; Hodge, M.; Whittier, J.; Ibrahim, K.; Lee, S. Persistent Organic Pollutants in the Green Sea Turtle Chelonia Mydas: Nesting Population Variation, Maternal Transfer, and Effects on Development. Mar. Ecol. Prog. Ser. 2010, 403, 269–278. [Google Scholar] [CrossRef]
- Stewart, K.R.; Keller, J.M.; Templeton, R.; Kucklick, J.R.; Johnson, C. Monitoring Persistent Organic Pollutants in Leatherback Turtles (Dermochelys coriacea) Confirms Maternal Transfer. Mar. Pollut. Bull. 2011, 62, 1396–1409. [Google Scholar] [CrossRef] [PubMed]
- Sinaei, M.; Bolouki, M. Metals in Blood and Eggs of Green Sea Turtles (Chelonia mydas) from Nesting Colonies of the Northern Coast of the Sea of Oman. Arch. Environ. Contam. Toxicol. 2017, 73, 552–561. [Google Scholar] [CrossRef]
- Gale, R.W.; Bergeron, J.M.; Willingam, E.J.; Crews, D. Turtle Sex Determination Assay: Mass Balance and Responses to 2,3,7,8-Tetrachlorodibenzo-p-Dioxin and 3,3′,4,4′,5-Pentachlorobiphenyl. Environ. Toxicol. Chem. 2002, 21, 2477–2482. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.K.; Gross, T.S.; Borgert, C.J. Topical Dose Delivery in the Reptilian Egg Treatment Model. Environ. Toxicol. Chem. 2007, 26, 914–919. [Google Scholar] [CrossRef]
- Keller, J.M. Forty-Seven Days of Decay Does Not Change Persistent Organic Pollutant Levels in Loggerhead Sea Turtle Eggs. Environ. Toxicol. Chem. 2013, 32, 747–756. [Google Scholar] [CrossRef]
- Sanjuan, O.N.; Sait, S.T.L.; Gonzalez, S.V.; Tomás, J.; Raga, J.A.; Asimakopoulos, A.G. Phthalate Metabolites in Loggerhead Marine Turtles (Caretta caretta) from the Mediterranean Sea (East Spain Region). Environ. Chem. Ecotoxicol. 2023, 5, 178–185. [Google Scholar] [CrossRef]
- Schaap, I.; Buedenbender, L.; Johann, S.; Hollert, H.; Dogruer, G. Impact of Chemical Pollution on Threatened Marine Mammals: A Systematic Review. J. Hazard. Mater. 2023, 459, 132203. [Google Scholar] [CrossRef]
- Brassea-Pérez, E.; Hernández-Camacho, C.J.; Labrada-Martagón, V.; Vázquez-Medina, J.P.; Gaxiola-Robles, R.; Zenteno-Savín, T. Oxidative Stress Induced by Phthalates in Mammals: State of the Art and Potential Biomarkers. Environ. Res. 2022, 206, 112636. [Google Scholar] [CrossRef]
- Gorini, F.; Tonacci, A.; Sanmartin, C.; Venturi, F. Phthalates and Non-Phthalate Plasticizers and Thyroid Dysfunction: Current Evidence and Novel Strategies to Reduce Their Spread in Food Industry and Environment. Toxics 2025, 13, 222. [Google Scholar] [CrossRef] [PubMed]
- Tranganida, A.; Hall, A.J.; Armstrong, H.C.; Moss, S.E.W.; Bennett, K.A. Consequences of in Vitro Benzyl Butyl Phthalate Exposure for Blubber Gene Expression and Insulin-Induced Akt Activation in Juvenile Grey Seals. Environ. Pollut. 2023, 316, 120688. [Google Scholar] [CrossRef] [PubMed]
- Dziobak, M.K.; Curtin, T.; Wells, R.S.; Takeshita, R.; Smith, C.R.; Zolman, E.; Toms, C.N.; Allen, R.F.; Hart, L.B. Comparing Phthalate Exposure between Bottlenose Dolphins (Tursiops truncatus) Residing in Urban and Rural Environments. Front. Mar. Sci. 2025, 12, 1554075. [Google Scholar] [CrossRef]
- Heindel, J.J.; Blumberg, B.; Cave, M.; Machtinger, R.; Mantovani, A.; Mendez, M.A.; Nadal, A.; Palanza, P.; Panzica, G.; Sargis, R.; et al. Metabolism Disrupting Chemicals and Metabolic Disorders. Reprod. Toxicol. 2017, 68, 3–33. [Google Scholar] [CrossRef]
- Nadal, A.; Quesada, I.; Tudurí, E.; Nogueiras, R.; Alonso-Magdalena, P. Endocrine-Disrupting Chemicals and the Regulation of Energy Balance. Nat. Rev. Endocrinol. 2017, 13, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Feige, J.N.; Gelman, L.; Rossi, D.; Zoete, V.; Métivier, R.; Tudor, C.; Anghel, S.I.; Grosdidier, A.; Lathion, C.; Engelborghs, Y.; et al. The Endocrine Disruptor Monoethyl-Hexyl-Phthalate Is a Selective Peroxisome Proliferator-Activated Receptor γ Modulator That Promotes Adipogenesis. J. Biol. Chem. 2007, 282, 19152–19166. [Google Scholar] [CrossRef]
- Klöting, N.; Hesselbarth, N.; Gericke, M.; Kunath, A.; Biemann, R.; Chakaroun, R.; Kosacka, J.; Kovacs, P.; Kern, M.; Stumvoll, M.; et al. Di-(2-Ethylhexyl)-Phthalate (DEHP) Causes Impaired Adipocyte Function and Alters Serum Metabolites. PLoS ONE 2015, 10, e0143190. [Google Scholar] [CrossRef]
- Völker, J.; Ashcroft, F.; Vedøy, Å.; Zimmermann, L.; Wagner, M. Adipogenic Activity of Chemicals Used in Plastic Consumer Products. Environ. Sci. Technol. 2022, 56, 2487–2496. [Google Scholar] [CrossRef]
- Bennett, K.A.; Robinson, K.J.; Moss, S.E.W.; Millward, S.; Hall, A.J. Using Blubber Explants to Investigate Adipose Function in Grey Seals: Glycolytic, Lipolytic and Gene Expression Responses to Glucose and Hydrocortisone. Sci. Rep. 2017, 7, 7731. [Google Scholar] [CrossRef]
- Khudyakov, J.I.; Champagne, C.D.; Meneghetti, L.M.; Crocker, D.E. Blubber Transcriptome Response to Acute Stress Axis Activation Involves Transient Changes in Adipogenesis and Lipolysis in a Fasting-Adapted Marine Mammal. Sci. Rep. 2017, 7, 42110. [Google Scholar] [CrossRef]
- Zanuttini, C. Les Contaminants Chez les Grands Dauphins (Tursiops truncatus) du Golfe Normand-Breton. Available online: https://www.gecc-normandie.org/wp-content/uploads/2018/07/Cyrielle-Zanuttini_Rapport-r%C3%A9sultats-janvier-2015.pdf (accessed on 28 November 2025).
- Dziobak, M.K.; Wells, R.S.; Pisarski, E.C.; Wirth, E.F.; Hart, L.B. Demographic Assessment of Mono(2-ethylhexyl) Phthalate (MEHP) and Monoethyl Phthalate (MEP) Concentrations in Common Bottlenose Dolphins (Tursiops truncatus) From Sarasota Bay, FL, USA. GeoHealth 2021, 5, e2020GH000348. [Google Scholar] [CrossRef] [PubMed]
- Vighi, M.; Borrell, A.; Sahyoun, W.; Net, S.; Aguilar, A.; Ouddane, B.; Garcia-Garin, O. Concentrations of Bisphenols and Phthalate Esters in the Muscle of Mediterranean Striped Dolphins (Stenella coeruleoalba). Chemosphere 2023, 339, 139686. [Google Scholar] [CrossRef] [PubMed]
- Hart, L.B.; Beckingham, B.; Wells, R.S.; Alten Flagg, M.; Wischusen, K.; Moors, A.; Kucklick, J.; Pisarski, E.; Wirth, E. Urinary Phthalate Metabolites in Common Bottlenose Dolphins (Tursiops truncatus) From Sarasota Bay, FL, USA. GeoHealth 2018, 2, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Zhang, X.; Xie, Y.; Wu, J.; Wu, Y. Occurrences and Potential Lipid-Disrupting Effects of Phthalate Metabolites in Humpback Dolphins from the South China Sea. J. Hazard. Mater. 2023, 441, 129939. [Google Scholar] [CrossRef]
- Rian, M.B.; Vike-Jonas, K.; Gonzalez, S.V.; Ciesielski, T.M.; Venkatraman, V.; Lindstrøm, U.; Jenssen, B.M.; Asimakopoulos, A.G. Phthalate Metabolites in Harbor Porpoises (Phocoena phocoena) from Norwegian Coastal Waters. Environ. Int. 2020, 137, 105525. [Google Scholar] [CrossRef]
- Xie, Z.; Zhang, X.; Wu, J.; Wu, Y. Risk Assessment of Phthalate Metabolites Accumulated in Fish to the Indo-Pacific Humpback Dolphins from Their Largest Habitat. Sci. Total Environ. 2023, 876, 163094. [Google Scholar] [CrossRef]
- Sambolino, A.; Alves, F.; Rodriguez, M.; Weyn, M.; Ferreira, R.; Correia, A.M.; Rosso, M.; Kaufmann, M.; Cordeiro, N.; Dinis, A. Phthalates and Fatty Acid Markers in Free-Ranging Cetaceans from an Insular Oceanic Region: Ecological Niches as Drivers of Contamination. Environ. Pollut. 2024, 360, 124693. [Google Scholar] [CrossRef]
- Martino, C. 2025. Available online: https://BioRender.com/froezxm (accessed on 28 November 2025).
| Phthalate | Structure | Molecular Weight g/mol | XLogP3 |
|---|---|---|---|
| Monoesters | |||
| Monomethyl phthalate (MMP) | 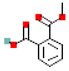 | 180.2 | 1.1 |
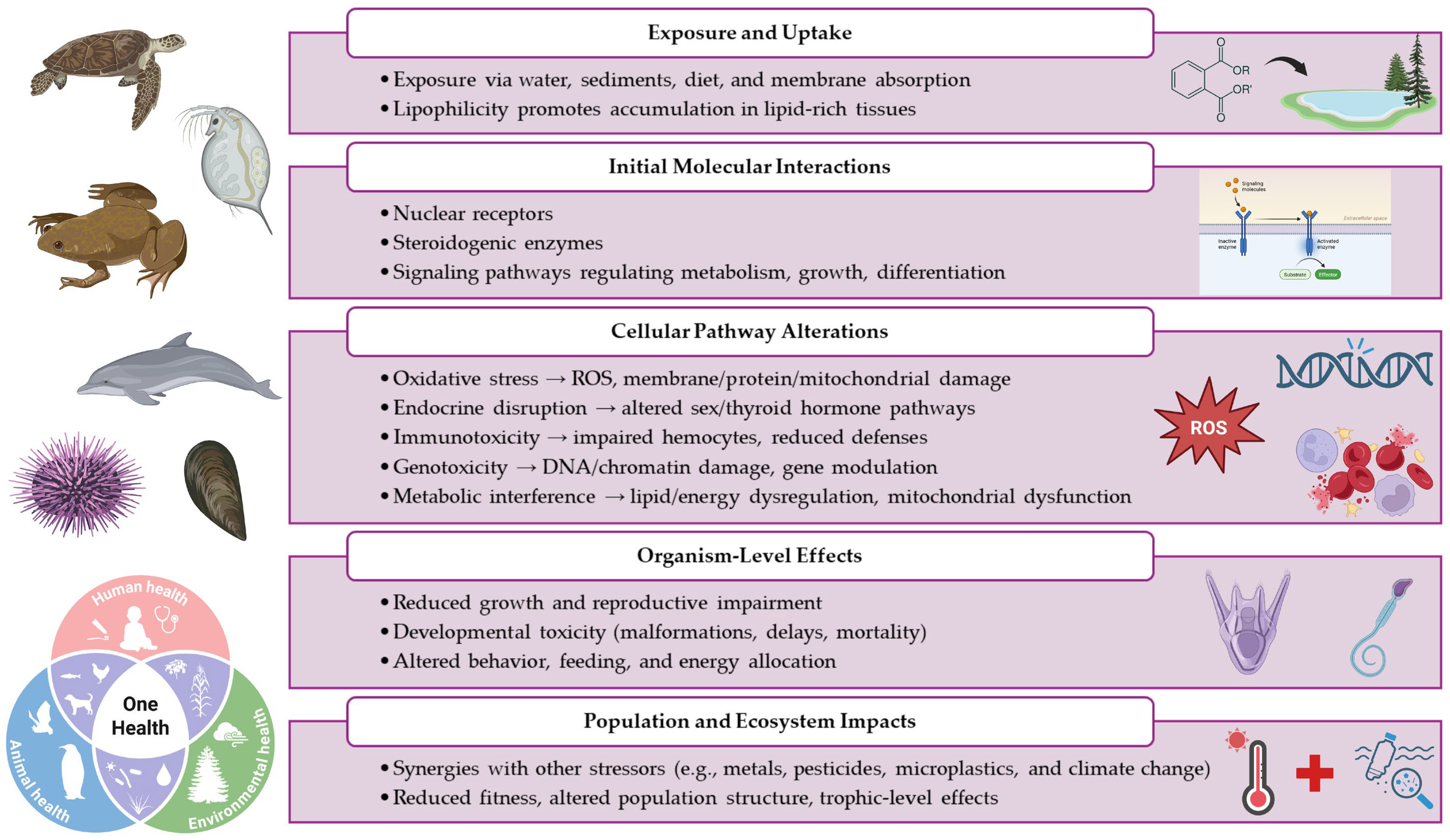
| Monoethyl phthalate (MEP) | 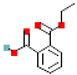 | 194.2 | 1.8 |
| Monobutyl phthalate (MbutP) |  | 222.2 | 3.1 |
| Mono(2-ethylhexyl) phthalate (MEHP) | 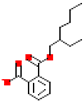 | 277.3 | 4.6 |
| Monodecyl phthalate (MdecP) | 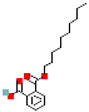 | 306.4 | 6.4 |
| Diesters | |||
| Dimethyl phthalate (DMP) | 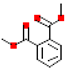 | 194.2 | 1.6 |
| Diethyl phthalate (DEP) | 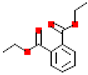 | 222.2 | 2.5 |
| Diisobutyl phthalate (DiBP) | 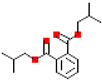 | 278.3 | 4.1 |
| Dibutyl phthalate (DnBP) | 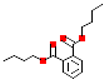 | 278.3 | 4.7 |
| Denzyl butyl phthalate (BBP) | 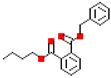 | 312.4 | 4.9 |
| Diheptyl phthalate (DHpP) | 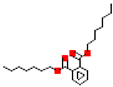 | 362.5 | 8.0 |
| Di(2-ethylhexyl) phthalate (DEHP) | 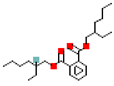 | 390.6 | 7.4 |
| Di-n-octyl phthalate (DnOP) | 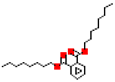 | 390.6 | 9.1 |
| Diisononyl phthalate (DiNP) | 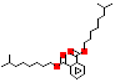 | 418.7 | 9.6 |
| Taxon/Species | Phthalate(s) | Concentration/LC50/EC50 | Main Effects | References |
|---|---|---|---|---|
| Invertebrates—Daphnia magna (juvenile) | DEHP | LC50: 0.83 → 0.56 mg L−1 (24–48 h) | Mortality, oxidative stress (↑MDA, SOD, GST) | [33] |
| Invertebrates—D. magna (adults) | LC50: 0.48 → 0.35 mg L−1 (24–48 h) | |||
| Invertebrates—Ceriodaphnia cornuta | DEHP | 50–500 µg L−1 | Reproduction ↑, growth alterations | [34] |
| Invertebrates—Arbacia lixula embryos | PAE mixture | EC50 reduced by 76% under heat stress | Developmental malformations, skeletal stunting, enzyme modulation | [35] |
| Invertebrates—Chironomus riparius | DEHP, BBP | 0.1–2000 µg L−1 | Gene expression changes (hsp70, EcR), GST ↓, survival ↓ | [36,37,38] |
| Invertebrates—Mytilus galloprovincialis | DEHP | 4–324 µg L−1 | Hormetic effects on antioxidant enzymes, metabolomic alterations | [39] |
| Invertebrates—Tigriopus japonicus | DBP ± microplastics | LC50: 1.23 mg L−1 | Antagonistic interaction with MPs, reproduction ↓ | [40] |
| Invertebrates—Physella acuta | DEP, BBP, DEHP | 0.1–1000 µg L−1 | Broad gene expression changes (DNA repair, detoxification, apoptosis) | [41] |
| Amphibians—Xenopus laevis | DBP | LC50: 13.3–14.5 mg L−1 | Mortality, malformations, spermatogenesis defects | [42,43,44] |
| Amphibians—Rana chensinensis | DEHP | 3.91 mg L−1 | Delayed metamorphosis, thyroid alterations, sex ratio skew | [45] |
| Amphibians—Xenopus laevis | DEP, DnPP, DBP | 1–100 mg L−1 | Teratogenic risk, growth inhibition, malformations | [46] |
| Reptiles—Caretta caretta (erythrocyte culture) | DiNP, DiDP, MEHP | 0.0278–44,667 µg L−1 | Cytotoxicity, ERα and HSP60 modulation, oxidative stress | [47,48] |
| Reptiles—Enhydris chinensis | DEHP, DnOP, DiBP | Field study | Bioaccumulation, maternal transfer to eggs | [49] |
| Mammals—Tursiops truncatus, Orcinus orca | DEP, DEHP | 5–88,675 ng mL−1 | Hormone correlations (aldosterone, cortisol), oxidative stress | [50] |
| Mammals—Tursiops truncatus (skin cells) | DEHP | 3.9–1953 mg L−1 | Micronucleus induction, aneugenic effects | [51] |
| Mammals—Arctic cetaceans, polar bears | DEHP, DiNP | <20–398 µg Kg w.w. | Receptor modulation (PPARγ, GR, THR) | [52] |
| Mammals—various cetaceans (Norway) | BBP, DEHP, DiNP, DiBP, DnOP | Blubber/levels variable | Bioaccumulation, interspecies differences | [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savoca, D.; Martino, C.; Maccotta, A.; Arizza, V.; Amorello, D.; Arrabito, G.; Orecchio, S. Silent Disruptors: The Multifaceted Impact of Phthalates Across Aquatic Invertebrate and Vertebrate Taxa. Appl. Sci. 2025, 15, 12937. https://doi.org/10.3390/app152412937
Savoca D, Martino C, Maccotta A, Arizza V, Amorello D, Arrabito G, Orecchio S. Silent Disruptors: The Multifaceted Impact of Phthalates Across Aquatic Invertebrate and Vertebrate Taxa. Applied Sciences. 2025; 15(24):12937. https://doi.org/10.3390/app152412937
Chicago/Turabian StyleSavoca, Dario, Chiara Martino, Antonella Maccotta, Vincenzo Arizza, Diana Amorello, Giuseppe Arrabito, and Silvia Orecchio. 2025. "Silent Disruptors: The Multifaceted Impact of Phthalates Across Aquatic Invertebrate and Vertebrate Taxa" Applied Sciences 15, no. 24: 12937. https://doi.org/10.3390/app152412937
APA StyleSavoca, D., Martino, C., Maccotta, A., Arizza, V., Amorello, D., Arrabito, G., & Orecchio, S. (2025). Silent Disruptors: The Multifaceted Impact of Phthalates Across Aquatic Invertebrate and Vertebrate Taxa. Applied Sciences, 15(24), 12937. https://doi.org/10.3390/app152412937









