Effect of Different Biostimulant Application Forms on Some Geometrical and Mechanical Properties of Soybean Seeds
Abstract
1. Introduction
2. Materials and Methods
- -
- Ultimate force (maximum force)—Fmax (N);
- -
- Deformation at the ultimate force—Lmax (mm);
- -
- Compression work up to the maximum force—W (mJ).
- -
- Conventional compression resistance factor as the value of the ultimate force to deformation at the ultimate force—RF = Fmax/Lmax (N∙mm−1) [42];
- -
- Compression work related to the seed thickness—W/dt (mJ∙mm−1);
- -
- Compression work related to the seed mass—W/m (mJ∙g−1).
Statistical Analysis
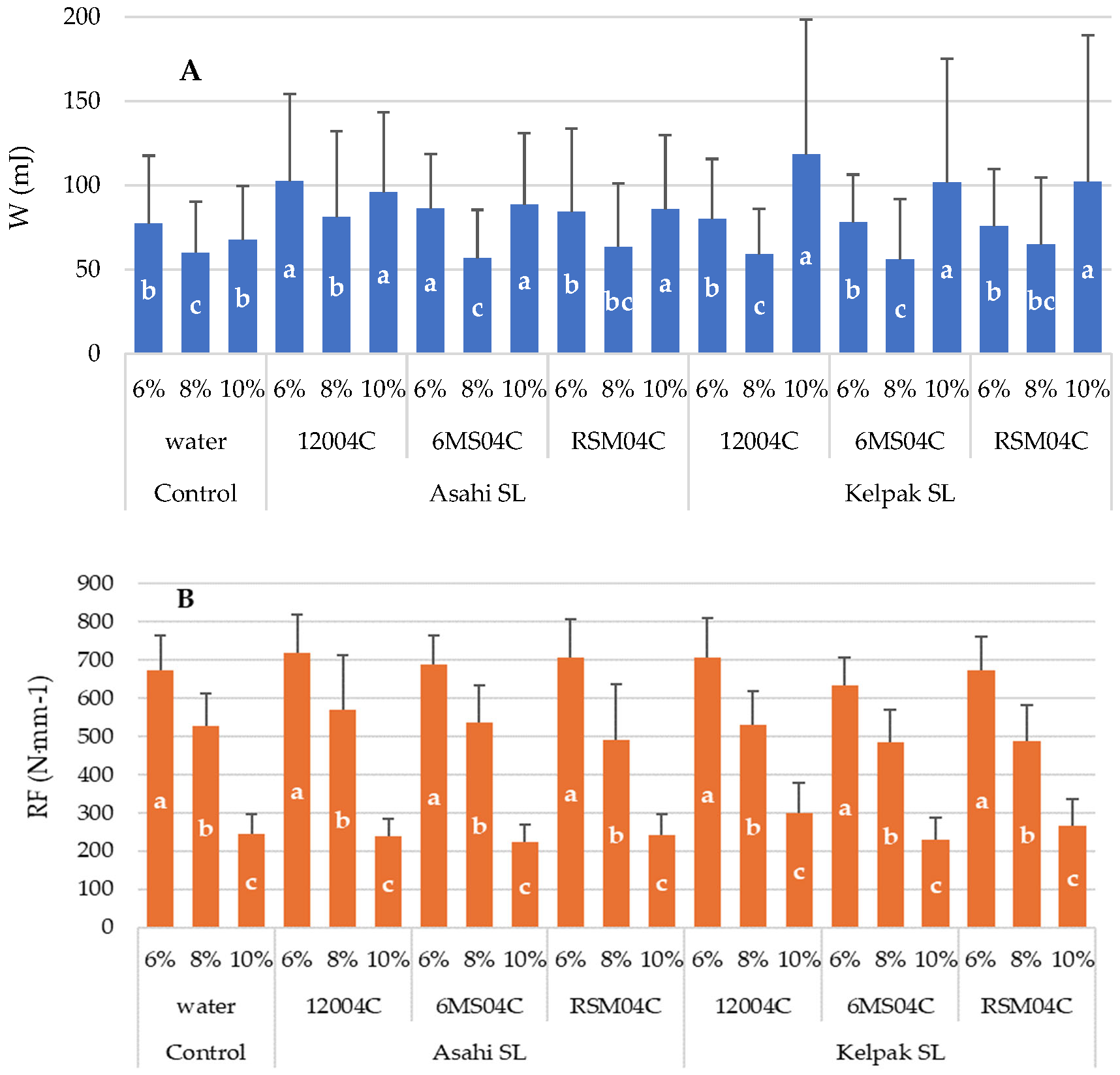
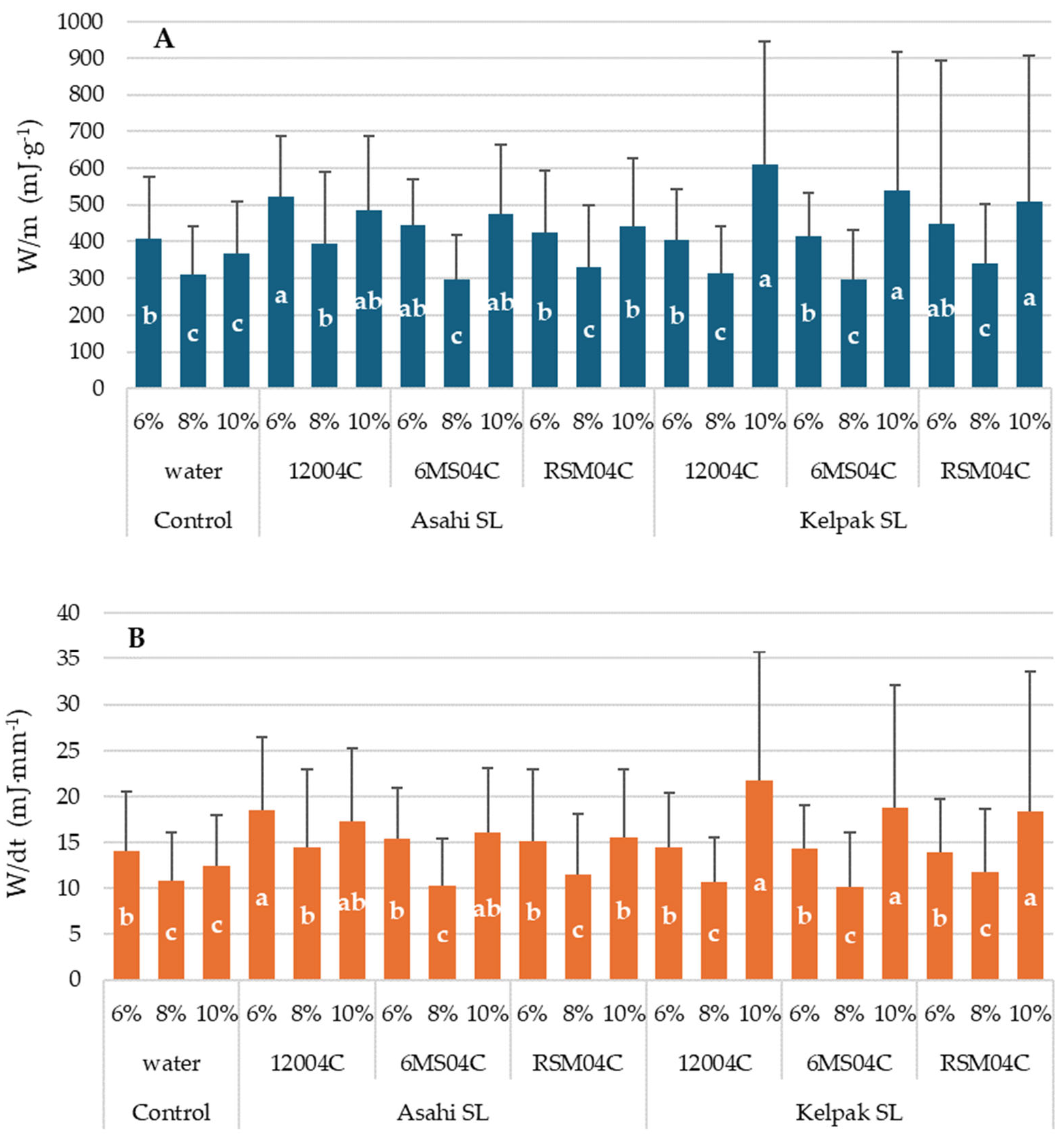
3. Results
4. Discussion
5. Conclusions
- (1)
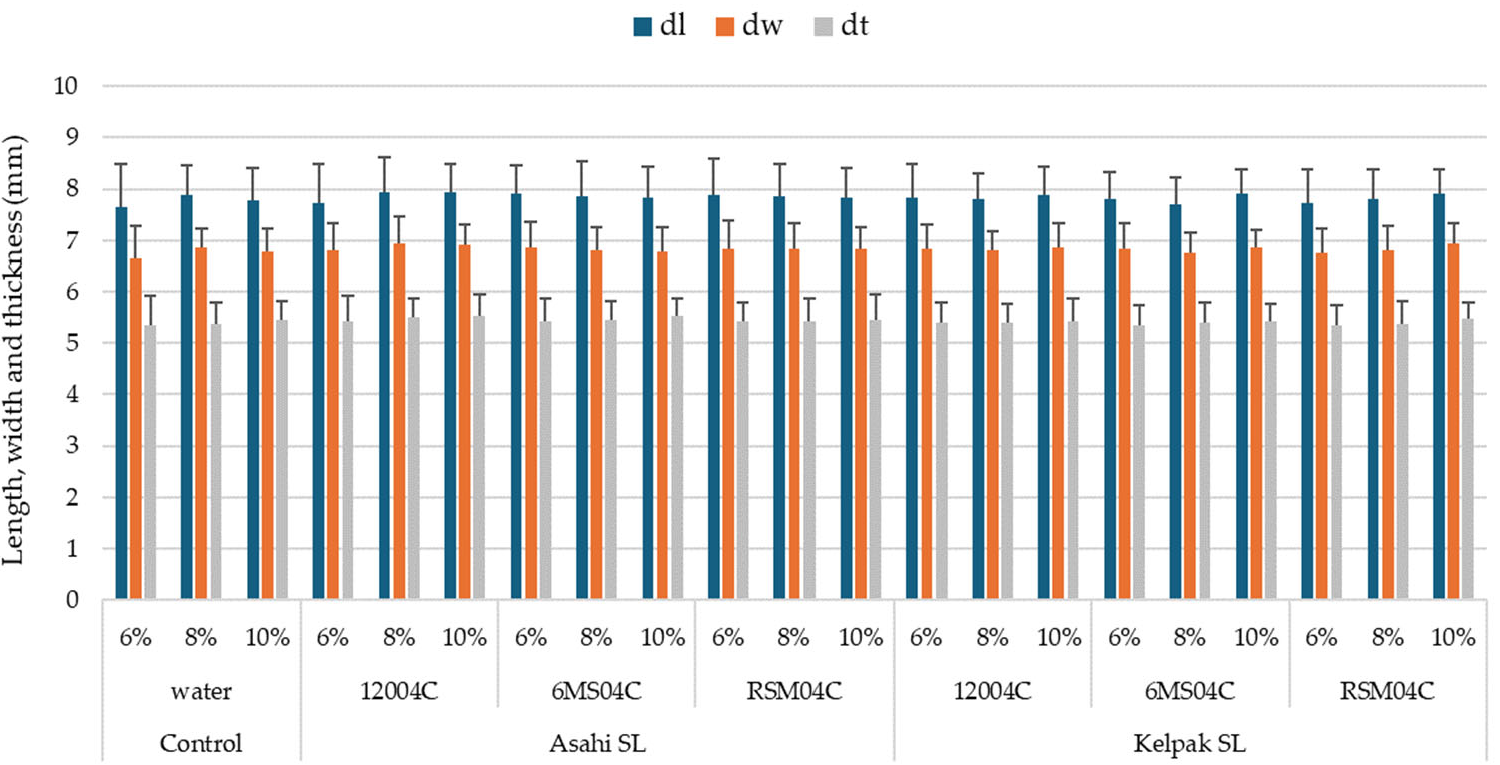
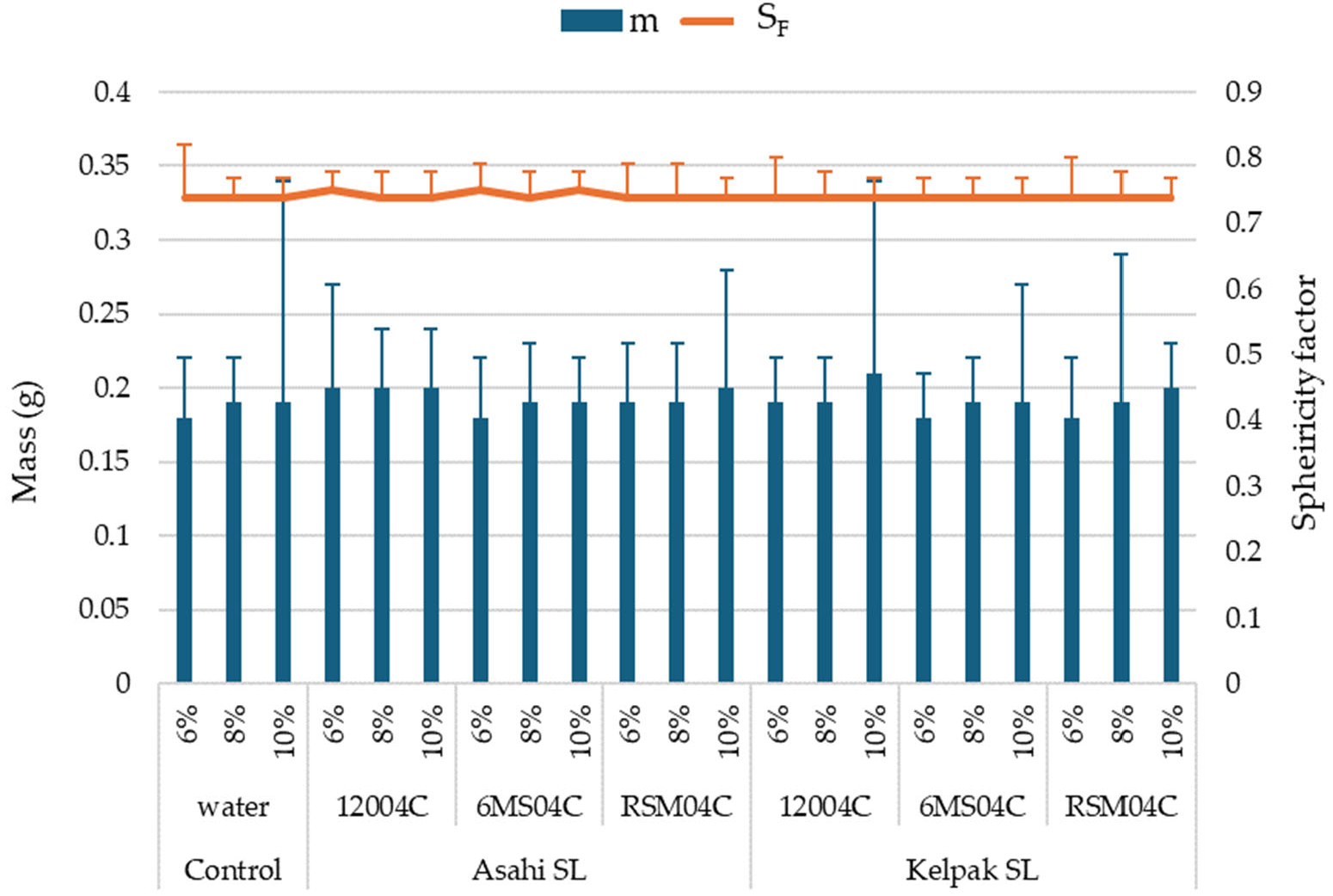
- No significant differences were determined in terms of the seed size (thickness, width, length) and seed mass between the variants with the use of a biostimulant and the control. The use of the biostimulants Asahi SL and Kelpak SL, irrespective of the form of application or moisture content in seeds, did not significantly affect the sphericity of seeds of the soybean.
- (2)
- The use of a 12004C universal spray nozzle resulted in the attainment of the highest values of ultimate force in all the experimental variants. This could be a consequence of the form of sprayed liquid, supplied as finer droplets, which helps the preparation to penetrate deeper into the plant’s structure. An exception was the use of Asahi SL applied with a 12004C universal sprayer nozzle combined with seed moisture of 10%.
- (3)
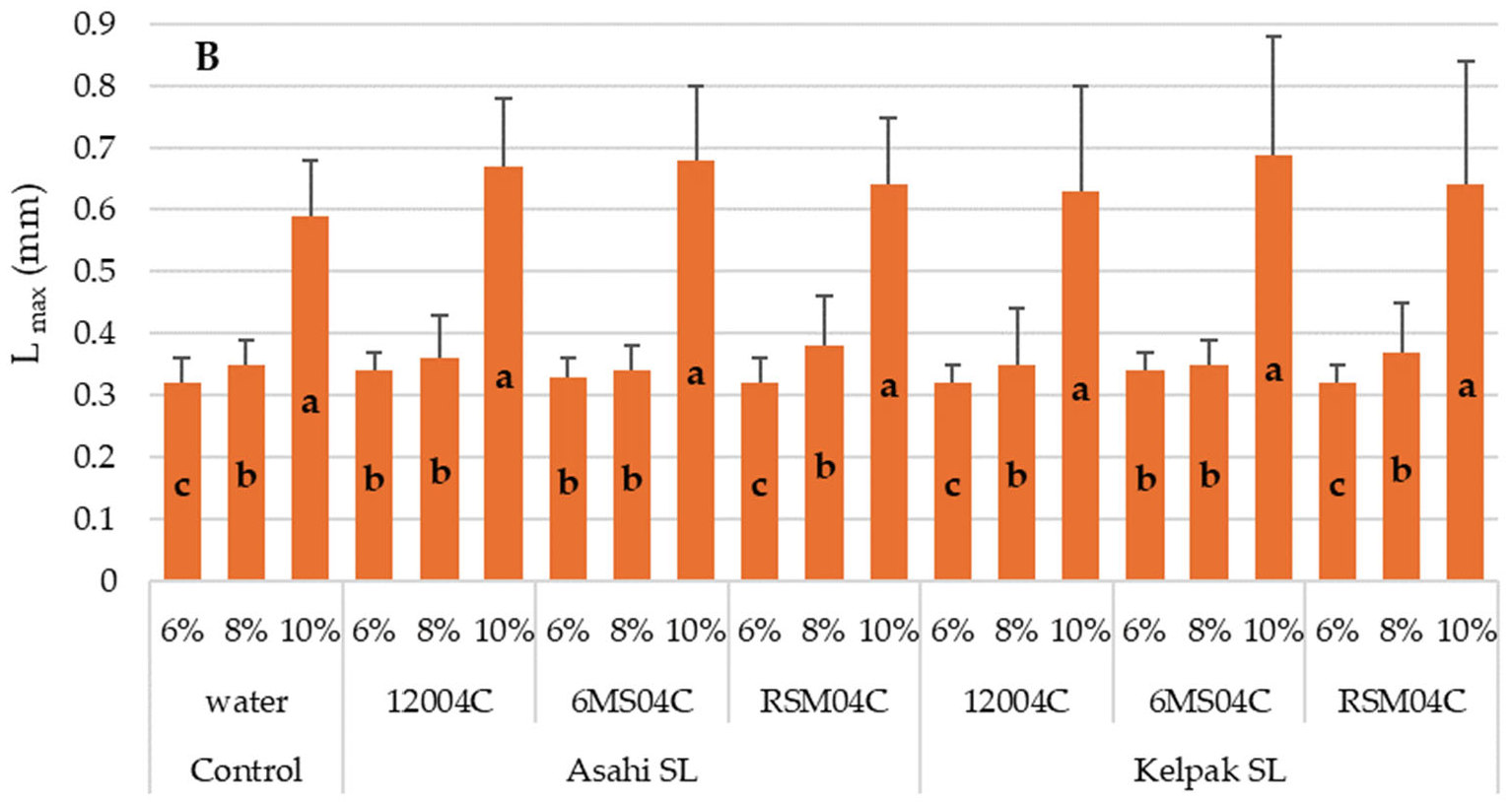
- Lower seed moisture (6%) led to a higher resistance of seeds to mechanical damage and a higher RF.
- (4)
- Kelpak SL, for all forms of application, improved the mechanical resistance of seeds compared to the control, which could be attributed to the natural composition of this biostimulant.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of the World’s Land and Water Resources for Food and Agriculture—Systems at Breaking Point; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture Development, Pesticide Application and its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- du Jardin, P. Plant biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural Uses of Plant Biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Wally, O.S.; Critchley, A.T.; Hiltz, D.; Craigie, J.S.; Han, X.; Zaharia, L.I.; Abrams, S.R.; Prithiviraj, B. Regulation of Phytohormone Biosynthesis and Accumulation in Arabidopsis Following Treatment with Commercial Extract from the Marine Macroalga Ascophyllum nodosum. J. Plant Growth Regul. 2013, 32, 324–339. [Google Scholar] [CrossRef]
- Kocira, S.; Sujak, A.; Kocira, A.; Wójtowicz, A.; Oniszczuk, A. Effect of Fylloton Application on Photosynthetic Activity of Moldavian Dragonhead (Dracocephalum moldavica L.). Agric. Agric. Sci. Procedia 2015, 7, 108–112. [Google Scholar] [CrossRef]
- Shukla, P.S.; Borza, T.; Critchley, A.T.; Prithiviraj, B. Seaweed—Based Compounds and Products for Sustainable Protection Against Plant Pathogens. Mar. Drugs 2021, 19, 59. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sharma, K.; Chahal, H.S.; Kaur, H.; Hasanain, M. Seaweed-Derived Plant Bootsters: Revolutionizing Sustainable Farming and Soil Health. Front. Soil Sci. 2025, 5, 1504045. [Google Scholar] [CrossRef]
- Ertani, A.; Nardi, S.; Francioso, O.; Pizzeghello, D.; Tinti, A.; Schiavon, M. Metabolite-Targeted Analysis and Physiological Traits of Zea mays L. in Response to Application of a Leonardite-Humate and Lignosulfonate-Based Products for Their Evaluation as Potential Biostimulants. Agron. J. 2019, 9, 445. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Biostimulants in Agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef]
- Shukla, P.S.; Prithiviraj, B. Ascophyllum Nodosum Biostimulant Improves the Growth of Zea Mays Grown Under Phosphorus Impoverished Conditions. Front. Plant Sci. 2021, 11, 601843. [Google Scholar] [CrossRef]
- Rouphael, Y.; Carillo, P.; Garcia-Perez, P.; Cardarelli, M.; Senizza, B.; Miras-Moreno, B.; Lucini, L. Plant Biostimulants from Seaweeds or Vegetal Proteins Enhance the Salinity Tolerance in Greenhouse Lettuce by Modulating Plant Metabolism in a Distinctive Manner. Sci. Hortic. 2022, 305, 111368. [Google Scholar] [CrossRef]
- Michalek, W.; Kocira, A.; Findura, P.; Szparaga, A.; Kocira, S. The Influence of Biostimulant Asahi SL on the Photosynthetic Activity of Selected Cultivars of Phaseolus vulgaris L. ASPE 2018, 20, 1286–1301. [Google Scholar]
- Findura, P.; Hara, P.; Szparaga, A.; Kocira, S.; Czerwińska, E.; Bartoš, P.; Treder, K. Evaluation of the Effects of Allelopathic Aqueous Plant Extracts, as Potential Preparations for Seed Dressing, on the Modulation of Cauliflower Seed Germination. Agriculture 2020, 10, 122. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The Role of Biostimulants and Bioeffectors as Alleviators of Abiotic Stress in Crop Plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Kocira, S. Effect of Amino Acid Biostimulant on the Yield and Nutraceutical Potential of Soybean. Chil. J. Agric. Res. 2019, 79, 17–25. [Google Scholar] [CrossRef]
- Sozoniuk, M.; Świeca, M.; Bohatá, A.; Bartoš, P.; Bedrníček, J.; Lorenc, F.; Jarošová, M.; Perná, K.; Stupková, A.; Lencová, J.; et al. Selection of Reference Genes for Expression Profiling in Biostimulation Research of Soybean. Chem. Biol. Technol. Agric. 2024, 11, 130. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohardi, M.Z.; Rathar, P.; Prithiviraj, B. Seaweed Extracts as Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants Application in Horticultural Crops Under Abiotic Stress Conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulant Properties of Seaweed Extracts in Plants: Implications towards Sustainable Crop Production. Plants 2021, 10, 531. [Google Scholar] [CrossRef]
- Repke, R.A.; Silva, D.M.R.; dos Santos, J.C.C.; de Almeida Sliva, M. Alleviation of Drought Stress in Soybean by Applying a Biostimulant Based on Amino Acids and Macro- and Micronutrients. Agron. J. 2022, 12, 2244. [Google Scholar] [CrossRef]
- Rymuza, K.; Radzka, E.; Cała, J. The Effect of Applied Biostimulants on the Yielding of Three Non-genetic Modified Soybean Cultivars. Agriculture 2023, 13, 900. [Google Scholar] [CrossRef]
- Karpiński, P.; Kocira, S. Possibilities of Using a Multispectral Camera to Assess the Effects of Biostimulant Application in Soybean Cultivation. Sensors 2025, 25, 3464. [Google Scholar] [CrossRef] [PubMed]
- Krawczuk, A.; Parafiniuk, S.; Przywara, A.; Huyghebeart, B.; Rabier, F.; Limbourg, Q.; Mostade, O.; Kocira, S. Technical Parameters of Sprying with a Biostimulant as a Determinant of Biometrical Properties and Yield of a Soybean Seeds. J. Agric. Eng. 2021, 25, 171–179. [Google Scholar] [CrossRef]
- Krawczuk, A.; Ogrodniczek, J.; Bohata, A.; Bartos, P.; Olšan, P.; Findura, P.; Kocira, S. Physical Properties of Plant Extracts with Biostimulant Potential Produced Using Cold Plasma and Low-Pressure Microwave Discharge. J. Agric. Eng. 2024, 28, 277–285. [Google Scholar] [CrossRef]
- Forster, W.A.; Kimberlay, M.O.; Zabkiewicz, J.A. A Universal Spray Droplet Adhesion Model. ASABE 2005, 48, 1321–1330. [Google Scholar] [CrossRef]
- Nuyttens, D.; Beatens, K.; De Schampheliere, M.; Sonck, B. Effect of Nozzle Type, Size and Pressure on Spray Droplet Characteristics. Biosyst. Eng. 2007, 97, 333–345. [Google Scholar] [CrossRef]
- Khan, F.A.; Khorsandi, F.; Ali, M.; Ghafoor, A.; Khan, R.A.Z.; Umair, M.; Shahzaib; Rehman, A.; Hussain, Z. Spray Drift Reduction Management in Agriculture: A review. Prog. Agric. Eng. Sci. 2024, 20, 1–36. [Google Scholar] [CrossRef]
- Ozkan, H.E. Effect of Major Variables on Drift Distances of Spray Droplets; Ohio State University Extension Service, Publication AEX: Columbus, OH, USA, 2016; pp. 816–900. [Google Scholar]
- Butler Ellis, M.C.; Lane, A.G.; O’Sullivan, C.M.; Jones, S. Wind Tunel Investigation of the Ability of Drift-Reducing Nozzles to Provide Mitigation Measures for Bystander Exposure to Pesticides. Biosyst. Eng. 2021, 202, 152–164. [Google Scholar] [CrossRef]
- Butler Ellis, M.C.; Alanis, R.; Lane, A.G.; Tuck, C.R.; Nuyttens, D.; van de Zande, J.C. Wind Tunnel Measurements and Model Predictions for Estimating Spray Drift Reduction Under Field Conditions. Biosyst. Eng. 2017, 154, 25–34. [Google Scholar] [CrossRef]
- Moorea, D.R.J.; McCarroll-Butler, C.A.; Avanasic, R.; Chen, W.; White, M.; Brain, R.A. How Protective to the Environment is the Pesticide Risk Assessment and Registration Process in the United States? J. Regul. Sci. 2021, 9, 1–20. [Google Scholar] [CrossRef]
- Szewczyk, A. Analiza Ustawienia, Parametrów i Warunków Pracy Rozpylacza w Aspekcie Jakości Opryskiwania Upraw Polowych; Wydawnictwo Uniwersytetu Przyrodniczego we Wrocławiu: Wrocław, Poland, 2010; Volume 97, pp. 1–131. [Google Scholar]
- de Oliveira, R.B.; Precipito, L.M.B.; Gandalfo, M.A.; de Oliveira, J.V.; Lucio, F.R. Effect of Droplet Size and Leaf Surface on Retention of 2,4-D Formulations. Crop Prot. 2019, 119, 97–101. [Google Scholar] [CrossRef]
- Lodwik, D.; Pietrzyk, J.; Malesa, W. Analysis of Volume Distribution and Evaluation of the Spraying Spectrum in Terms of Spraying Quality. Appl. Sci. 2020, 10, 2395. [Google Scholar] [CrossRef]
- Subr, A.; Parafiniuk, S.; Milanowski, M.; Krawczuk, A.; Kachel, M. Study of Deposited Spray Quality of Spraying Agents with Different Physical Properties. Plant Arch. 2020, 20, 6109–6114. [Google Scholar]
- Koszel, M. Ocena Jakości Oprysku w Sytuacji Różnego Stopnia Zużycia i Różnych Eksploatacyjnych Parametrów Rozpylaczy Płaskostrumieniowych. J. Agric. Eng. 2009, 8, 55–60. [Google Scholar]
- Ozkan, H.E. Strategies to Minimize Spray Drift; Ohio State University Extension Service, Publication AEX: Columbus, OH, USA, 2022. [Google Scholar]
- ASAE Standard S352.2; Moisture Measurement—Ungrounded Grains and Seeds. American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2006.
- Kibar, H.; Öztürk, T. Physical and Mechanical Properties of Soybean. Int. Agrophys. 2008, 30, 239–244. [Google Scholar]
- Żabiński, A.; Mudryk, K. Wybrane Właściwości Fizyczne Nasion Krajowych i Zagranicznych Odmian Soczewicy Jadalnej. J. Agric. Eng. 2009, 9, 319–329. [Google Scholar]
- Mora, C.F.; Kwan, A.K.H. Sphericity, Shape Factor and Convexity Measurement of Coarse Aggregate for Concreto Using Digital Image Processing. Cem. Concr. Res. 2000, 30, 351–385. [Google Scholar] [CrossRef]
- Sayinci, B.; Demir, B.; Açik, N. Comparision of spray nozzles in terms of spray coverage and drop distribution uniformity at low volume. Turk. J. Agric. For. 2020, 44, 262–270. [Google Scholar] [CrossRef]
- Yu, Y.; Zhu, H.; Ozkan, H.E.; Derksen, R.C.; Krause, C.R. Evaporation and Deposition Coverage Area of Droplets Containing Insecticides and Spray Additives on Hydrophilic, Hydrophobic, and Crabapple Leaf Surfaces. Trans. ASAE 2009, 52, 39–49. [Google Scholar] [CrossRef]
- Krawczuk, A.; Huyghebaert, B.; Rabier, F.; Parafiniuk, S.; Przywara, A.; Koszel, M.; Lorencowicz, E.; Kocira, S. The Technical Parameters of Seaweed Biostimulant Spray Application as a Factor in the Economic Viability of Soybean Production. Appl. Sci. 2023, 13, 1051. [Google Scholar] [CrossRef]
- Kuźniar, P.; Jarecki, W.; Bobrecka-Jamro, D. Właściwości Mechaniczne Nasion Wybranych Roślin Strączkowych a ich Masa i Grubość. J. Agric. Eng. 2013, 4, 171–177. [Google Scholar]
- Sosnowski, S.; Kuźniar, P. Effect of Dynamic Loading on the Quality of Soybean. Int. Agrophys. 1999, 13, 125–132. [Google Scholar]
- Hebda, T.; Frączek, J. Wpływ Wybranych Czynników na Wartość Wskaźnika Odkształcenia Nasienia. J. Agric. Eng. 2005, 11, 171–180. [Google Scholar]
- Deshpande, S.D.; Bal, S.; Ojha, T.P. Physical properties of soybean. J Agric. Eng. Res. 1993, 56, 89–98. [Google Scholar] [CrossRef]
- Hauth, M.R.; Bothelho, F.M.; Hoscher, R.H.; de C.C. Bothello, S.; de Oliveira, G.H.H. Physical Properties of Different Soybean Cultivars During Drying. Eng. Agric. 2018, 38, 590–598. [Google Scholar] [CrossRef]
- Polat, R.; Atay, U.; Saglam, C. Some Physical and Aerodynamic Properties of Soybean. J. Agron. 2006, 5, 74–78. [Google Scholar] [CrossRef]
- Ashalou, M.O.; Noibi, A.O. Effect of Moisture Content on Some Mechanical Properties of Soybean (Glycine max) Varieties. Afr. J. Food Sci. Technol. 2013, 4, 211–220. [Google Scholar]
- Bako, T.; Mamai, E.A.; Bature, B.J. Physical and Mechanical Properties of Soya Bean Seeds in Relations to the Design of oil Extractors. J. Postharvest Technol. 2019, 7, 50–61. [Google Scholar]
- Alonge, A.F. The Effect of Moisture Content on Mechanical Properties of Soybean (Glycine max (L) Merr.). J. Agric. Res. Dev. 2003, 2, 60–69. [Google Scholar] [CrossRef]
- Tavakoli, H.; Rajabipour, A.; Mohtasebi, S.S. Moisture-Dependent Some Engineering Properties of Soybean Grains. Agric. Eng. Int. 2009, 11, 1110. [Google Scholar]
- Kruszelnicka, W.; Chen, Z.; Ambrose, K. Moisture-Dependent Physical-Mechanical Properties of Maize, Rice, and Soybeans as Related to Handling and Processing. Materials 2022, 15, 8729. [Google Scholar] [CrossRef]
- Lamidi, W.A.; Ogunlade, C.A.; Olaniyan, A.R.; Shittu, K.A.; Murtadha, M.A.; Ajibade, A.F.; Fadeyibi, A. Moisture Dependent: Physical Properties of Baobab Seeds (Adansonia digitata L.). J. Agric. Eng. 2023, 27, 33–46. [Google Scholar] [CrossRef]
- Kuźniar, P.; Szpunar-Krok, E.; Findura, P.; Buczek, J.; Bobrecka-Jamro, D. Physical and Chemical Properties of Soybean Seeds Determine their Susceptibility to Mechanical Damage. Zemdirb. Agric. 2016, 103, 183–192. [Google Scholar] [CrossRef]
- Kocira, S.; Szparaga, A.; Kocira, A.; Czerwińska, E.; Wójtowicz, A.; Borowiecka-Mielniczuk, U.; Koszel, M.; Findura, P. Modeling Biometric Traits, Yield and Nutritional and Antioxidiant Properties of Seeds of Three Soybean Cultivars Through the Application of Biostimulant Containing Seaweed and Amino Acids. Front. Plant Sci. 2018, 9, 388. [Google Scholar] [CrossRef]
- Iwanicka, N. Agronomic, Qualitative and Economic Effects of Using Selected Biostimulants in the Cultivation of Common Bean (Phaseolus vulgaris L.), Orzeł Variety. Ph.D. Thesis, Instytut Nauk o Żywności Człowieka i Rolnictwie—Państwowa Akademia Nauk Stosowanych w Chełmie, Chełm, Poland, 2022. Available online: https://up.lublin.pl/wp-content/uploads/2022/12/Rozprawa-doktorska-Natalia-Iwanicka.pdf (accessed on 13 October 2025).

| Biostimulant | Characteristics | Number of Sprays | Concentration |
|---|---|---|---|
| Kelpak SL | Contains Ecklonia maxima extract obtained by cold cellular-burst technology. Composition: auxins (11 mg·L−1) and cytokinins (0.031 mg·L−1), alginates, brassinosteroids, gibberellins, phlorotannins (Eckol), polyamines. In crop cultivation, it is recommended to apply the biostimulant 1 to 3 times in a dose of 2–4 L·ha−1. | Single spray (BBCH 12–13) | 0.7% 1.0% |
| Double spray (BBCH 12–13 and BBCH 61) | 0.7% 1.0% | ||
| Asahi SL | Contains active substances from the group of nitrophenols, present naturally in plant cells. Composition: 0.3% sodium para–nitrophenolate, 0.2% sodium ortho-nitrophenolate, and 0.1% of sodium nitroguaiacolate. In crop cultivation, it is recommended to apply the biostimulant 1 to 3 times, in a dose of 0.5–0.6 L·ha−1, at 7–30 day intervals, carrying out the first spraying at the stage when the second true leaf unfolds. | Single spray (BBCH 12–13) | 0.1% 1.0% |
| Double spray (BBCH 12–13 and BBCH 61) | 0.1% 0.2% |
| Factor | dt (mm) | dw (mm) | dl (mm) | Mass (g) | SF | Fmax (N) | Lmax (mm) | W (mJ) | W/m (mJ∙g−1) | W/dt (mJ∙mm−1) | RF (N∙mm−1) | Results of Statistical Test |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biostimulant | 7.83 | 4.17 | 5.82 | 5.86 | 9.41 | 11.21 | 5.15 | 20.99 | 30.12 | 25.30 | 2.95 | H |
| 0.0199 * | 0.1241 | 0.0545 | 0.0535 | 0.0091 * | 0.0037 * | 0.0762 | <0.0001 * | <0.0001 * | <0.0001 * | 0.2294 | p-value | |
| Type of spraying | 3.07 | 6.16 | 2.92 | 7.74 | 6.58 | 40.85 | 4.67 | 37.80 | 44.22 | 36.85 | 17.41 | H |
| 0.3814 | 0.1041 | 0.4043 | 0.0524 | 0.0864 | <0.0001 * | 0.1971 | <0.0001 * | <0.0001 * | <0.0001 * | 0.0006 * | p-value | |
| Moisture | 0.32 | 1.57 | 2.56 | 1.11 | 4.23 | 763.73 | 1404.96 | 176.44 | 249.47 | 203.32 | 1637.23 | H |
| 0.8517 | 0.4550 | 0.2785 | 0.5754 | 0.1204 | <0.0001 * | <0.0001 * | <0.0001 * | <0.0001 * | <0.0001 * | <0.0001 * | p-value |
| Characteristics | Control | Asahi SL | Kelpak |
|---|---|---|---|
| SF | 0.743 ± 0.032 a | 0.744 ± 0.036 a | 0.739 ± 0.032 a |
| Fmax (N) | 180.31 ± 49.04 a | 190.63 ± 54.04 b | 186.90 ± 43.97 b |
| m (g) | 0.185 ± 0.039 a | 0.191 ± 0.040 a | 0.187 ± 0.034 a |
| dt (mm) | 5.420 ± 0.388 a | 5.473 ± 0.383 b | 5.423 ± 0.344 a |
| Lmax (mm) | 0.417 ± 0.136 a | 0.451 ± 0.170 a | 0.446 ± 0.188 a |
| W (mJ) | 67.361 ± 33.519 a | 75.549 ± 33.457 b | 70.894 ± 32.084 a |
| W/dt (mJ∙mm−1) | 12.249 ± 5.521 a | 13.892 ± 5.854 b | 12.913 ± 5.473 a |
| W/m (mJ∙g−1) | 361.479 ± 152.340 a | 414.181 ± 165.899 b | 383.191 ± 156.572 a |
| RF (N∙mm−1) | 482.154 ± 193.836 a | 490.199 ± 218.188 a | 477.297 ± 186.625 a |
| Characteristics | Control | 12004C | 6MS04C | RSM04C |
|---|---|---|---|---|
| SF | 0.743 ± 0.032 a | 0.742 ± 0.033 a | 0.742 ± 0.032 a | 0.740 ± 0.036 a |
| Fmax (N) | 180.31 ± 49.04 a | 198.22 ± 50.13 b | 183.00 ± 45.00 a | 185.08 ± 49.59 a |
| m (g) | 0.185 ± 0.039 a | 0.192 ± 0.039 a | 0.186 ± 0.035 a | 0.188 ± 0.038 a |
| dt (mm) | 5.420 ± 0.388 a | 5.464 ± 0.380 a | 5.441 ± 0.345 a | 5.439 ± 0.369 a |
| Lmax (mm) | 0.417 ± 0.136 a | 0.445 ± 0.173 a | 0.457 ± 0.191 a | 0.444 ± 0.172 a |
| W (mJ) | 67.361 ± 33.519 a | 80.607 ± 36.152 b | 72.041 ± 32.982 a | 71.675 ± 34.895 a |
| W/dt (mJ∙mm−1) | 12.249 ± 5.521a | 14.328 ± 5.791 b | 13.009 ± 5.483 a | 12.773 ± 5.515 a |
| W/m (mJ∙g−1) | 361.479 ± 152.340 a | 429.639 ± 169.519 b | 386.401 ± 154.725 a | 380.858 ± 157.490 a |
| RF (N∙mm−1) | 482.154 ± 193.836 ab | 508.779 ± 207.765 a | 465.266 ± 196.070 b | 477.252 ± 203.075 b |
| Characteristics | 6% | 8% | 10% |
|---|---|---|---|
| SF | 0.744 ± 0.035 a | 0.741 ± 0.034 a | 0.740 ± 0.031 a |
| Fmax (N) | 224.00 ± 16.00 a | 182.90 ± 43.73 b | 155.71 ± 33.53 c |
| m (g) | 0.187 ± 0.039 a | 0.188 ± 0.038 a | 0.189 ± 0.036 a |
| dt (mm) | 5.442 ± 0.393 a | 5.445 ± 0.367 a | 5.445 ± 0.345 a |
| Lmax (mm) | 0.327 ± 0.036 a | 0.355 ± 0.052 a | 0.643 ± 0.140 b |
| W (mJ) | 79.445 ± 32.177 a | 60.226 ± 31.006 b | 81.446 ± 36.636 a |
| W/dt (mJ∙mm−1) | 14.6783 ± 5.537 a | 11.069 ± 5.507 b | 15.073 ± 6.590 a |
| W/m (mJ∙g−1) | 430.435 ± 145.633 a | 323.282 ± 146.079 b | 440.305 ± 182.414 a |
| RF (N∙mm−1) | 684.203 ± 93.560 a | 520.495 ± 105.119 b | 247.814 ± 61.974 c |
| rs | SF | Fmax (N) | Lmax (mm) | W (mJ) | W/dt (mJ∙mm−1) | W/m (mJ∙mg−1) | RF (N·mm−1) |
|---|---|---|---|---|---|---|---|
| m (g) | 0.06 | 0.45 * | 0.18 | 0.51 * | 0.42 * | 0.17 | 0.21 |
| dt (mm) | 0.43 * | 0.39 * | 0.20 | 0.45 * | 0.35 * | 0.16 | 0.17 |
| dw (mm) | −0.06 | 0.42 * | 0.18 | 0.48 * | 0.40 * | 0.17 | 0.18 |
| dl (mm) | −0.22 | 0.43 * | 0.19 | 0.49 * | 0.42 * | 0.20 | 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przywara, A.; Różańska-Boczula, M.; Parafiniuk, S.; Kocira, S.; Żelazna, A.; Łysiak, G. Effect of Different Biostimulant Application Forms on Some Geometrical and Mechanical Properties of Soybean Seeds. Appl. Sci. 2025, 15, 12593. https://doi.org/10.3390/app152312593
Przywara A, Różańska-Boczula M, Parafiniuk S, Kocira S, Żelazna A, Łysiak G. Effect of Different Biostimulant Application Forms on Some Geometrical and Mechanical Properties of Soybean Seeds. Applied Sciences. 2025; 15(23):12593. https://doi.org/10.3390/app152312593
Chicago/Turabian StylePrzywara, Artur, Monika Różańska-Boczula, Stanisław Parafiniuk, Sławomir Kocira, Agnieszka Żelazna, and Grzegorz Łysiak. 2025. "Effect of Different Biostimulant Application Forms on Some Geometrical and Mechanical Properties of Soybean Seeds" Applied Sciences 15, no. 23: 12593. https://doi.org/10.3390/app152312593
APA StylePrzywara, A., Różańska-Boczula, M., Parafiniuk, S., Kocira, S., Żelazna, A., & Łysiak, G. (2025). Effect of Different Biostimulant Application Forms on Some Geometrical and Mechanical Properties of Soybean Seeds. Applied Sciences, 15(23), 12593. https://doi.org/10.3390/app152312593








