Antidiabetic and Anti-Inflammatory Potential of Sorbus aucuparia Fruits (Rowanberries) from Romania
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. The Obtaining of the Extracts
2.2.1. Ultrasound-Assisted Extraction
2.2.2. Accelerated Solvent Extraction
2.3. Analytical Methods
2.3.1. Total Polyphenols Determination (TPF) and Flavonoids Content (TFC)
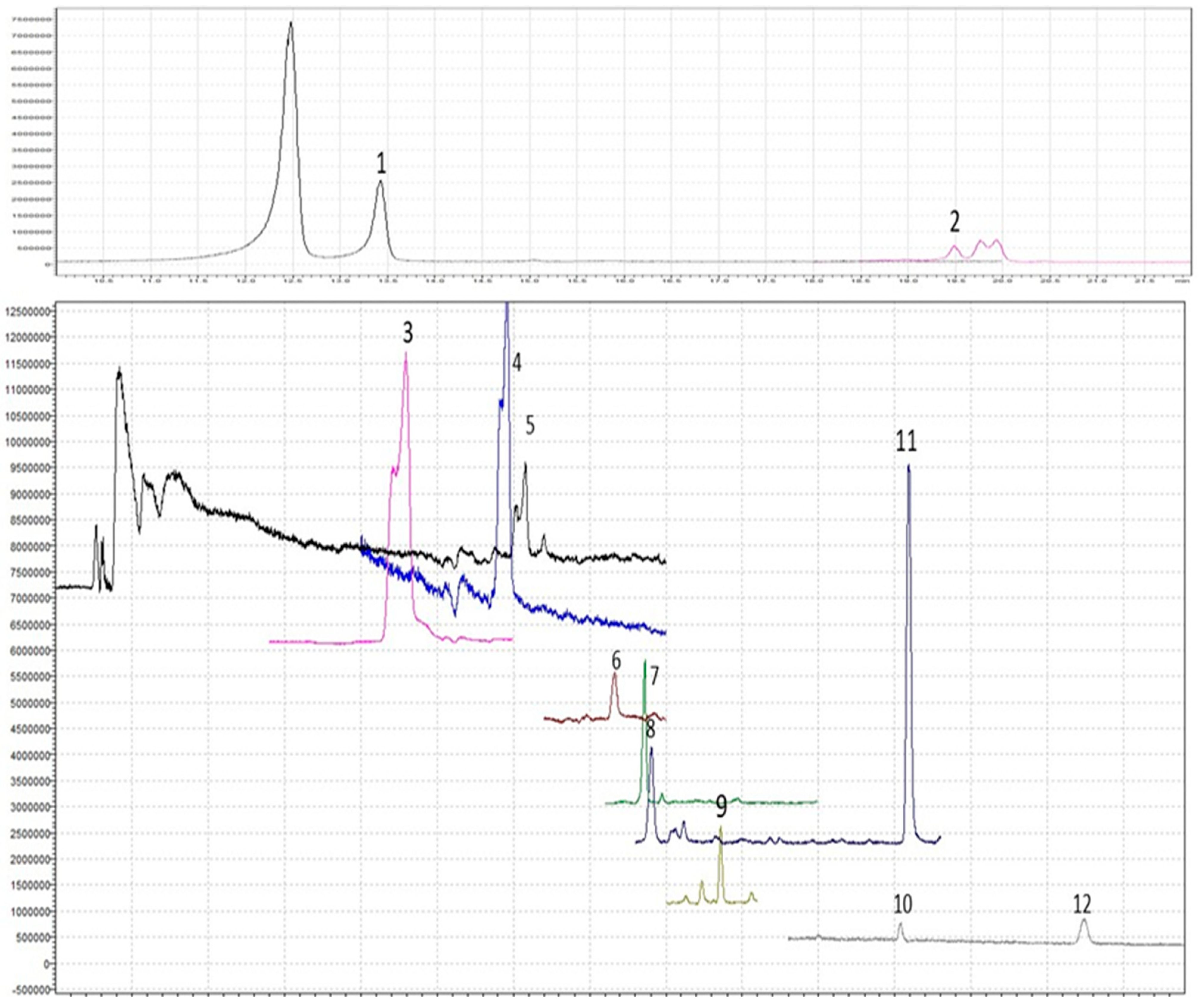
2.3.2. HPLC Analysis of Individual Phenolic Compounds
2.4. Antioxidant Assays
2.4.1. DPPH Radical Scavenging Activity
2.4.2. Determination of Antioxidant Activity by Reducing Power
2.4.3. ABTS Assay
2.5. Enzymes Inhibition Activity
2.5.1. Testing the Antidiabetic Potential of the Extracts
- Testing the α-amylase inhibition capacity
- α-Glucosidase Inhibition Assay
2.5.2. Testing the Anti-Inflammatory Potential of the Extracts
- Hyaluronidase inhibition assay
- Lipoxygenase inhibition assay
2.6. In Vitro Cytotoxicity Tests
2.6.1. Cell Culture
2.6.2. MTT Cell Viability Assay
2.7. Statistical Analysis
3. Results and Discussions
3.1. Phytochemical Analysis and Antioxidant Capacity
3.2. Testing Antidiabetic Potential—α-Amylase and α-Glucosidase Inhibition
3.3. Testing Anti-Inflammatory Potential—Lipoxygenase (LOX) and Hyaluronidase (HYA) Inhibition Activity
3.4. Cytocompatibility of S. aucuparia Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| UAE | Ultrasound-assisted extraction |
| ASE | Accelerated solvent extraction |
| HPLC-MS | High performance liquid chromatography—mass spectrometry |
| HYA | Hyaluronidase |
| LOX | Lipoxygenase |
| TEAC | Trolox Equivalent Antioxidant Capacity |
| SD | Standard deviation |
References
- Adhikari, B.; Marasini, B.P.; Rayamajhee, B.; Bhattarai, B.R.; Lamichhane, G.; Khadayat, K.; Adhikari, A.; Khanal, S.; Parajuli, N. Potential roles of medicinal plants for the treatment of viral diseases focusing on COVID-19: A review. Phyther. Res. 2021, 35, 1298–1312. [Google Scholar] [CrossRef]
- Pereira, C.; Barros, L.; Santos-Bruega, C.; Prieto, A.M. Natural bioactives used as additives in food applications. Front. Nutr. 2022, 9, 1063942. [Google Scholar] [CrossRef]
- Anunciato, T.P.; da Rocha Filho, P.A. Carotenoids and polyphenols in nutricosmetics, nutraceuticals, and cosmeceuticals. J. Cosmet. Dermatol. 2012, 11, 51–54. [Google Scholar] [CrossRef]
- Zhang, R.; McClements, D.J. Enhancing nutraceutical bioavailability by controlling the composition and structure of gastrointestinal contents: Emulsionbased delivery and excipient systems. Food Struct. 2016, 10, 21–36. [Google Scholar] [CrossRef]
- Al Ali, M.; Alqubaisy, M.; Aljaafari, M.N.; Al Ali, A.O.; Baqais, L.; Molouki, A.; Abushelaibi, A.; Lai, K.S.; Lim, S.E. Nutraceuticals: Transformation of conventional foods into health promoters/disease preventers and safety considerations. Molecules 2021, 26, 2540. [Google Scholar] [CrossRef] [PubMed]
- Nasri, H.; Baradaran, A.; Shirzad, H.; Rafieian-Kopaei, M. New concepts in nutraceuticals as alternative for pharmaceuticals. Int. J. Prev. Med. 2014, 5, 1487–1499. [Google Scholar] [PubMed]
- Pitchaiah, G.; Akula, A.; Chandi, V. Anticancer Potential of Nutraceutical Formulations in MNU-induced Mammary Cancer in Sprague Dawley Rats. Pharmacogn. Mag. 2017, 13, 46–50. [Google Scholar]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Affuso, F.; Ruvolo, A.; Micillo, F.; Sacc, L.; Fazio, S. Effects of a nutraceutical combination (berberine, red yeast rice and policosanols) on lipid levels and endothelial function randomized, double-blind, placebo-controlled study. Nutr. Metab. Cardiovasc. Dis. NMCD 2010, 20, 656–661. [Google Scholar] [CrossRef]
- Ruchi, S.; Amanjot, K.; Sourav, T.; Keerti, B.; Sujit, B. Role of nutraceuticals in health care: A review. Int. J. Green Pharm. 2017, 11, 386–394. [Google Scholar]
- Sołtys, A.; Galanty, A.; Podolak, I. Ethnopharmacologically important but underestimated genus Sorbus: A comprehensive review. Phytochem. Rev. 2020, 19, 491–526. [Google Scholar] [CrossRef]
- Olszewska, M.A.; Presler, A.; Michel, P. Profiling of phenolic compounds and antioxidant activity of dry extracts from the selected Sorbus species. Molecules 2012, 17, 3093–3113. [Google Scholar] [CrossRef]
- Polat, R.; Satıl, F. An ethnobotanical survey of medicinal plants in Edremit Gulf (Balikesir–Turkey). J. Ethnopharmacol. 2012, 139, 626–641. [Google Scholar] [CrossRef] [PubMed]
- Vogl, S.; Picker, P.; Mihaly-Bison, J.; Fakhrudin, N.; Atanasov, A.G.; Heiss, E.H.; Wawrosch, C.; Reznicek, G.; Dirsch, V.M.; Saukel, J.; et al. Ethnopharmacological in vitro studies on Austria’s folk medicine—An unexplored lore in vitro anti-inflammatory activities of 71 Austrian traditional herbal drugs. J. Ethnopharmacol. 2013, 149, 750–771. [Google Scholar] [CrossRef] [PubMed]
- Shikov, A.N.; Pozharitskaya, O.N.; Makarov, V.G.; Wagner, H.; Verpoorte, R.; Heinrich, M. Medicinal plants of the Russian Pharmacopoeia; their history and applications. J. Ethnopharmacol. 2014, 154, 481–536. [Google Scholar] [CrossRef]
- Pranskuniene, Z.; Ratkeviciute, K.; Simaitiene, Z.; Pranskunas, A.; Bernatoniene, J. Ethnobotanical study of cultivated plants in Kaišiadorys District, Lithuania: Possible trends for new herbal based medicines. Evid. Based. Complement. Altern. Med. 2019, 3940397. [Google Scholar] [CrossRef]
- Sak, K.; Jürisoo, K.; Raal, A. Estonian folk traditional experiences on natural anticancer remedies: From past to the future. Pharm. Biol. 2014, 52, 855–866. [Google Scholar] [CrossRef]
- Neves, J.M.; Matos, C.; Moutinho, C.; Queiroz, G.; Gomes, L.R. Ethnopharmacological notes about ancient uses of medicinal plants in Trás-os-Montes (northern of Portugal). J. Ethnopharmacol. 2009, 124, 270–283. [Google Scholar] [CrossRef]
- Olszewska, M.A. Variation in the phenolic content and in vitro antioxidant activity of Sorbus aucuparia leaf extracts during vegetation. Acta Pol. Pharm. Drug Res. 2011, 68, 937–944. [Google Scholar]
- Gaivelyte, K.; Jakstas, V.; Razukas, A.; Janulis, V. Variation in the contents of neochlorogenic acid, chlorogenic acid and three quercetin glycosides in leaves and fruits of Rowan (Sorbus) species and varieties from collections in Lithuania. Nat. Prod. Commun. 2013, 8, 1105–1110. [Google Scholar] [CrossRef]
- Kylli, P.; Nohynek, L.; Puupponen-Pimiä, R.; Westerlund-Wikstrom, B.; McDougall, G.; Stewart, D.; Heinonen, M. Rowanberry phenolics: Compositional analysis and bioactivities. J. Agric. Food Chem. 2010, 58, 11985–11992. [Google Scholar] [CrossRef]
- Zymone, K.; Raudone, L.; Raudonis, R.; Marksa, M.; Ivanauskas, L.; Janulis, V. Phytochemical profiling of fruit powders of twenty Sorbus L. cultivars. Molecules 2018, 23, 2593. [Google Scholar] [CrossRef]
- Bobinaitė, R.; Grootaert, C.; Van Camp, J.; Šarkinas, A.; Liaudanskas, M.; Žvikas, V.; Viškelis, P.; Venskutonis, P.R. Chemical composition, antioxidant, antimicrobial and antiproliferative activities of the extracts isolated from the pomace of rowanberry (Sorbus aucuparia L.). Food Res. Intern. 2020, 136, 109310. [Google Scholar] [CrossRef]
- Yang, B.; Kortesniemi, M. Clinical evidence on potential health benefits of berries. Curr. Opin. Food Sci. 2015, 2, 36–42. [Google Scholar] [CrossRef]
- Baby, B.; Antony, P.; Vijayan, R. Antioxidant and anticancer properties of berries. Cri. Rev. Food Sci. Nutr. 2018, 58, 2491–2507. [Google Scholar] [CrossRef] [PubMed]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Moi. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef]
- Matczak, M.; Michel Marchelak, A.P.; Owczarek, A.; Piszczan, A.; Kolodziejczyk-Czepas, J.; Nowak, P.; Olszewska, M.A. Sorbus domestica L. leaf extracts as functional products: Phytochemical profiling, cellular safety, pro-inflammatory enzymes inhibition and protective effects against oxidative stress in vitro. J. Funct. Foods. 2018, 40, 207–218. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxidative Med. Cell. Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef] [PubMed]
- Broholm, S.L.; Gramsbergen, S.M.; Nyberg, N.T.; Jager, A.K.; Staerk, D. Potential of Sorbus berry extracts for management of type 2 diabetes: Metabolomics investigation of 1H NMR spectra, α-amylase and α-glucosidase inhibitory activities, and in vivo anti-hyperglycaemic activity of S. norvegica. J. Ethnopharmacol. 2019, 242, 112061. [Google Scholar] [CrossRef]
- Xiao, J.; Högger, P. Dietary polyphenols and type 2 diabetes: Current insights and future perspectives. Curr. Med. Chem. 2015, 22, 23–38. [Google Scholar] [CrossRef]
- Edirisinghe, I.; Burton-Freeman, B. Anti-diabetic actions of Berry polyphenols—Review on proposed mechanisms of action. J. Berry Res. 2016, 6, 237–250. [Google Scholar] [CrossRef]
- Kato-Schwartz, C.G.; Corrêa, R.C.G.; Lima, D.S.; Sá-Nakanishi, A.B.; Gonçalves, G.A.; Seixas, F.A.V.; Haminiuk, C.W.I.; Barros, L.; Ferreira, I.C.F.R.; Bracht, A.; et al. Potential anti-diabetic properties of Merlot grape pomace extract: An in vitro, in silico and in vivo study of α-amylase and α-glucosidase inhibition. Food Res. Intern. 2020, 137, 109462. [Google Scholar] [CrossRef] [PubMed]
- Sajid, A.; Afzal, M.; Sajid, A.; Manzoor, Q.; Ahmed, E.; Sharif, A.; Younas, S. NMR structure elucidation and molecular modeling of lipoxygenase and cholinesterase inhibiting steroids from Hypericum oblongifolium. Curr. Org. Chem. 2022, 26, 1798–1806. [Google Scholar] [CrossRef]
- Loncaric, M.; Strelec, I.; Moslavac, T.; Subaric, D.; Pavic, V.; Molnar, M. Lipoxygenase inhibition by plant extracts. Biomolecules 2021, 11, 152. [Google Scholar] [CrossRef]
- González-Peña, D.; Colina-Coca, C.; Char, C.D.; Cano, M.P.; Ancos, B.; Sánchez-Moreno, C. Hyaluronidase inhibiting activity and radical scavenging potential of flavonols in processed onion. J. Agric. Food Chem. 2013, 61, 4862–4872. [Google Scholar] [CrossRef]
- Sajid, A.; Sajid, A.; Ahmed, E.; Sharif, A.; Manzoor, Q.; Al-Mijalli, S.H.; Iqbal, M. In-vitro and molecular docking studies of plant secondary metabolites isolated from Hypericum oblongifolium as antibacterial agents and lipoxygenase (5-LOX) inhibitors. J. Mol. Struct. 2024, 1312, 138549. [Google Scholar] [CrossRef]
- Hu, C.; Ma, S. Recent development of lipoxygenase inhibitors as anti-inflammatory agents. Med. Chem. Commun. 2018, 9, 212–225. [Google Scholar] [CrossRef]
- Perera, H.D.S.M.; Samarasekeraa, J.K.R.R.; Handunnettib, S.M.; Weerasenaba, O.V.D.S.J. In vitro anti-inflammatory and anti-oxidant activities of Sri Lankan medicinal plants. Ind. Crops Prod. 2016, 94, 610–620. [Google Scholar] [CrossRef]
- Steinhilber, D.; Hofmann, B. Recent advances in the search for novel5-lipoxygenase inhibitors. Basic Clin. Pharmacol. Toxicol. 2014, 114, 70–77. [Google Scholar] [CrossRef]
- Liyanaarachchia, G.D.; Samarasekeraa, J.K.R.R.; Mahanamab, K.R.R.; Hemalal, K.D.P. Tyrosinase, elastase, hyaluronidase, inhibitory and antioxidant activity of Sri Lankan medicinal plants for novel cosmeceuticals. Ind. Crops Prod. 2018, 111, 597–605. [Google Scholar] [CrossRef]
- Girish, K.S.; Kemparaju, K.; Nagaraju, S.; Vishwanath, B.S. Hyaluronidase inhibitors: A biological and therapeutic perspective. Curr. Med. Chem. 2009, 16, 2261–2288. [Google Scholar] [CrossRef]
- Sudha, P.N.; Rose, M.H. Beneficial effects of hyaluronic acid. Adv. Food Nutr. Res. 2014, 72, 137–176. [Google Scholar]
- Ölgen, S.; Kaessler, A.; Zühal Kiliç-Kurt, Z.; Jose, J. Investigation of aminomethyl indole derivatives as hyaluronidase inhibitors. Z. Naturforschung C 2014, 65, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Michalea, R.; Stathopoulou, K.; Polychronopoulos, P.; Benaki, D.; Mikros, E.; Aligiannis, N. Efficient identification of Acetylcholinesterase and Hyaluronidase inhibitors from Paeonia parnassica extracts through a Hetero Covariance Approach. J. Ethnopharmacol. 2020, 257, 111547. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef] [PubMed]
- Cano-Lamadrid, M.; Mozafari, L.; Martínez-Zamora, L.; Lorca, F.; García-Gomez, P.; Artes-Hernandez, F. Obtaining carotenoid encapsulates with polysaccharides carriers after pilot scale accelerated solvent extraction and ultrasound-assisted extraction from, industrial tomato by-product. Food Res. Int. 2025, 203, 115908. [Google Scholar] [CrossRef]
- Bazinet, L.; Doyen, A. Antioxidants, mechanisms, and recovery by membrane processes. Crit. Rev. Food Sci. Nutr. 2017, 57, 677–700. [Google Scholar] [CrossRef]
- Conidi, C.; Drioli, E.; Cassano, A. Membrane-based agro-food production processes for polyphenol separation, purification and concentration. Curr. Opin. Food Sci. 2018, 17, 149–164. [Google Scholar] [CrossRef]
- Paun, G.; Neagu, E.; Alecu, A.; Albu, C.; Seciu-Grama, A.-M.; Radu, G.L. Evaluating the Antioxidant and Antidiabetic Properties of Medicago sativa and Solidago virgaurea Polyphenolic-Rich Extracts. Molecules 2024, 29, 326. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods Enzymology; Academic Press: San Diego, CA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Lin, J.-Y.; Tang, C.-Y. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007, 101, 140–147. [Google Scholar] [CrossRef]
- Neagu, E.; Paun, G.; Albu, C.; Eremia, S.A.-M.V.; Radu, G.L. Artemisia abrotanum and Symphytum officinale Polyphenolic Compounds-Rich Extracts with Potential Application in Diabetes Management. Metabolites 2023, 13, 354. [Google Scholar] [CrossRef] [PubMed]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and mechanism of antioxidant activity using the DPPH free radical method. LWT Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Epure, A.; Pârvu, A.E.; Vlase, L.; Benedec, D.; Hanganu, D.; Gheldiu, A.M.; Toma, V.A.l.; Oniga, I. Phytochemical profile, anti-738 oxidant, cardioprotective and nephroprotective activity of romanian chicory extract. Plants 2021, 10, 64. [Google Scholar] [CrossRef]
- Berker, K.; Guclu, K.; Tor, I.; Apak, R. Comparative evaluation of Fe (III) reducing power-based antioxidant capacity assays in the presence of phenanthroline, batho-phenanthroline, tripyridyltriazine (FRAP) and ferricyanide reagents. Talanta 2007, 72, 1157–1165. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure–antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Queiroz, D.P.K.; Ferreira, A.G.; Lima, A.S.; Lima, E.S.; Lima, M.P. Isolation and identification of α-glucosidase, α-amylase and lipase inhibitors from Hortia longifolia. Int. J. Pharm. Pharm. Sci. 2013, 5, 336–339. [Google Scholar]
- Ranilla, L.G.; Kwon, Y.I.; Apostolidis, E.; Shetty, K. Phenolic compounds, antioxidant activity and in vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin America. Bioresour. Technol. 2010, 101, 4676–4689. [Google Scholar] [CrossRef]
- Sahasrabudhe, A.; Deodhar, M. Anti-hyaluronidase, antielastase activity of Garcinia indica. Int. J. Bot. 2010, 6, 299–303. [Google Scholar] [CrossRef]
- Hamberg, M.; Samuelsson, B. On the specificity of the oxygenation of unsaturated fatty acids catalyzed by soybean lipoxidase. J. Biol. Chem. 1967, 242, 5329–5335. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices Part 5: Tests for In Vitro Cytotoxicity. ISO: Genewa, Switzerland, 2009.
- Atkinson, I.; Seciu-Grama, A.M.; Serafim, A.; Petrescu, S.; Voicescu, M.; Anghel, E.M.; Marinescu, C.; Mitran, R.A.; Mocioiu, O.C.; Cusu, J.P.; et al. Bioinspired 3D scaffolds with antimicrobial, drug delivery, and osteogenic functions for bone regeneration. Drug Deliv. Transl. Res. 2024, 14, 1028–1047. [Google Scholar] [CrossRef]
- Moreira, S.A.; Pinto, C.A.; da Cruz Alexandre, E.M.; Pintado, M.E.; Saraiva, J.M. Assisted Extraction of Phenolic Compounds by Pressure-Based Technologies. In Technologies to Recover Polyphenols from AgroFood By-Products and Wastes; Academic Press: Cambridge, MA, USA, 2022; pp. 113–135. [Google Scholar]
- Becerra-Herrera, M.; Lazzoi, M.R.; Sayago, A.; Beltrán, R.; Del Sole, R.; Vasapollo, G. Extraction and determination of phenolic compounds in the berries of Sorbus americana Marsh and Lonicera oblongifolia (Goldie) Hook. Food Anal. Methods 2015, 8, 2554–2559. [Google Scholar] [CrossRef]
- Šavikin, K.P.; Zduni’c, G.M.; Krsti’c-Miloševi’c, D.B.; Šircelj, H.J.; Steševi’c, D.D. Sorbus aucuparia and Sorbus aria as a source of antioxidant phenolics, tocopherols, and pigments. Chem. Biodivers. 2017, 14, e1700329. [Google Scholar] [CrossRef]
- Arvinte, O.M.; Senila, L.; Becze, A.; Amariei, S. Rowanberry—A Source of Bioactive Compounds and Their Biopharmaceutical Properties. Plants 2023, 12, 3225. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jiang, Z.; Lu, H.; Xu, Z.; Tong, R.; Shi, J.; Jia, G. Recent advances of natural polyphenols activators for Keap1-Nrf2 signaling pathway. Chem. Biodivers. 2019, 16, e1900400. [Google Scholar] [CrossRef] [PubMed]
- Milenkovic, D.; Jude, B.; Morand, C. miRNA as molecular target of polyphenols underlying their biological effects. Free Radic. Biol. Med. 2013, 64, 40–51. [Google Scholar] [CrossRef]
- Perrone, P.; D’Angelo, S. Hormesis and health: Molecular mechanisms and the key role of polyphenols. Food Chem. Adv. 2025, 7, 101030. [Google Scholar] [CrossRef]
- Sarv, V.; Venskutonis, P.R.; Bhat, R. The Sorbus spp.—Underutilised Plants for Foods and Nutraceuticals: Review on Polyphenolic Phytochemicals and Antioxidant Potential. Antioxidants 2020, 9, 813. [Google Scholar] [CrossRef]
- Tian, Y.; Liimatainen, J.; Alanne, A.L.; Lindstedt, A.; Liu, P.; Sinkkonen, J.; Kallio, H.; Yang, B. Phenolic compounds extracted by acidic aqueous ethanol from berries and leaves of different berry plants. Food Chem. 2017, 220, 266–281. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Slatnar, A.; Stampar, F.; Veberic, R. HPLC–MSn identification and quantification of flavonol glycosides in 28 wild and cultivated berry species. Food Chem. 2012, 135, 2138. [Google Scholar] [CrossRef] [PubMed]
- Isaikina, N.V.; Kalinkina, G.I.; Razina, T.G.; Zueva, E.P.; Rybalkina, O.Y.; Ulirich, A.V.; Fedorova, E.P.; Shilova, A.B. Sorbus aucuparia L. Fruit Is a Source of the Drug for Increasing the Efficiency of Tumor Chemotherapy. Russ. J. Bioorg. Chem. 2018, 44, 899–905. [Google Scholar] [CrossRef]
- Hasbal, G.; Yilmaz-Ozden, T.; Can, A. Antioxidant and antiacetylcholinesterase activities of Sorbus torminalis (L.) Crantz (wild service tree) fruits. J. Food Drug Anal. 2015, 23, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Fomenko, S.E.; Kushnerova, N.F.; Sprygin, V.G.; Drugova, E.S.; Momot, T.V. Chemical Composition and Biological Action of Rowanberry Extract. Russ. J. Bioorg. Chem. 2016, 42, 764–769. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Hopia, A.I.; Heinonen, M. Berry phenolics and their antioxidant activity. J. Agric. Food Chem. 2001, 49, 4076–4082. [Google Scholar] [CrossRef]
- Turumtay, H.; Midilli, A.; Turumtay, E.A.; Demir, A.; Selvi, E.K.; Budak, E.E.; Er, H.; Kocaimamoglu, F.; Baykal, H.; Belduz, A.O.; et al. Gram (-) microorganisms DNA polymerase inhibition, antibacterial and chemical properties of fruit and leaf extracts of Sorbus acuparia and Sorbus caucasica var. yaltirikii. Biomed. Chromatogr. 2017, 31, e3901. [Google Scholar] [CrossRef]
- Mlcek, J.; Rop, O.; Jurikova, T.; Sochor, J.; Fisera, M.; Balla, S.; Baron, M.; Hrabe, J. Bioactive compounds in sweet rowanberry fruits of interspecific rowan crosses. Cent. Eur. J. Biol. 2014, 9, 1078–1086. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazic, V.; Abbasa, M.; Kambohd, A.A.; Khane, G.J.; Shumzaidf, M.; Ahmadg, F.; Babazadehh, D.; Fangi, F.X.; Modarresi-Ghazanij, F. Chlorogenic acid (CGA): A pharmacological review and call for further. BioMed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Forino, M.; Tenore, G.C.; Tartaglione, L.; Carmela, D.; Novellino, E.; Ciminiello, P. (1S,3R,4S,5R)5-O-Caffeoylquinic acid: Isolation, stereo-structure characterization and biological activity. Food Chem. 2015, 178, 306–310. [Google Scholar] [CrossRef]
- David, A.V.A.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Li, X.; Wang, Z.; Xiao, W.; He, Z.; Xiong, Z.; Zhao, L. Extraction optimization of accelerated solvent extraction for eight active compounds from Yaobitong capsule using response surface methodology: Comparison with ultrasonic and reflux extraction. J. Chromatogr. A 2020, 1620, 460984. [Google Scholar] [CrossRef]
- Culas, M.S.; Kaur, L.; Popovich, D.G.; Rashidinejad, A. Comparative efficiency of extraction techniques for bioactive compounds in Cinnamomum zeylanicum. Food Chem. 2025, 493, 145891. [Google Scholar] [CrossRef] [PubMed]
- Boath, A.S.; Stewart, D.; McDougall, G.J. Berry components inhibit α-glucosidase in vitro: Synergies between acarbose and polyphenols from black currant and rowanberry. Food Chem. 2012, 135, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhang, G.; Zhang, X.; Gao, J.; Zhou, Z.; Fan, J. Polyphenols from Sorbus aucuparia ameliorate insulin resistance and metabolic disorders in diabetic mice. Curr. Top. Nutraceutical Res. 2016, 14, 227–233. [Google Scholar]
- Rutkowska, M.; Kolodziejczyk-Czepas, J.; Owczarek, A.; Zakrzewska, A.; Magiera, A.; Olszewska, M.A. Novel insight into biological activity and phytochemical composition of Sorbus aucuparia L. fruits: Fractionated extracts as inhibitors of protein glycation and oxidative/nitrative damage of human plasma components. Food Res. Int. 2021, 147, 110526. [Google Scholar] [CrossRef]
- Chandran, A.K.; Stach, M.; Kucharska, A.Z.; Sokół-Łętowska, A.; Szumny, A.; Moreira, H.; Szyjka, A.; Barg, E.; Kolniak-Ostek, J. Comparison of polyphenol and volatile compounds and in vitro antioxidant, anti-inflammatory, antidiabetic, anti-ageing, and anticancer activities of dry tea leaves. LWT 2025, 222, 117632. [Google Scholar] [CrossRef]
- Abid, A.; Wafa, Z.; Belguidoum, M.; Touahria, T.; Mekhadmi, N.E.; Dekmouche, M.; Bechki, L.; Bireche, K.; Boussebaa, W.; Al-Farga, A. Exploring the anti-inflammatory, sedative, antidiabetic, and antioxidant potential in in-vitro and in-vivo models and phenolic profiling of Atractylis aristata Batt. J. Ethnopharmacol. 2024, 330, 118252. [Google Scholar] [CrossRef]
- Owczarek, K.; Lewandowska, U. The impact of dietary polyphenols on COX-2 expression in colorectal cancer. Nutr. Cancer 2017, 69, 1105–1118. [Google Scholar] [CrossRef]
- Huang, J.; Xie, M.; He, L.; Song, X.; Cao, T. Chlorogenic acid: A review on its mechanisms of anti-inflammation, disease treatment, and related delivery systems. Front. Pharmacol. 2023, 14, 1218015. [Google Scholar] [CrossRef]
- Rutkowska, M.; Owczarek, A.; Kolodziejczyk-Czepas, J.; Michel, P.; Piotrowska, D.G.; Kapusta, P.; Nowak, P.; Olszewska, M.A. Identification of bioactivity markers of Sorbus domestica leaves in chromatographic, spectroscopic and biological capacity tests: Application for the quality control. Phytochem. Lett. 2019, 30, 278–287. [Google Scholar] [CrossRef]
- Olszewska, M.A.; Kolodziejczyk-Czepas, J.; Rutkowska, M.; Magiera, A.; Michel, P.; Rejman, M.W.; Nowak, P.; Owczarek, A. The effect of standardised flower extracts of Sorbus aucuparia L. on proinflammatory enzymes, multiple oxidants, and oxidative/nitrative damage of human plasma components in vitro. Oxidative Med. Cell. Longev. 2019, 2019, 9746358. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Lee, Y.J.; Jang, H.J.; Kim, A.R.; Hong, S.; Kim, T.W.; Kim, M.Y.; Lee, J.; Lee, Y.G.; Cho, J.Y. Anti-inflammatory activity of Sorbus commixta water extract and its molecular inhibitory mechanism. J. Ethnopharmacol. 2011, 134, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; An, H.-J. β-sitosteryl-3-O-β-glucopyranoside isolated from the bark of Sorbus commixta ameliorates proinflammatory mediators in RAW 264.7 macrophages. Immunopharmacol. Immunotoxicol. 2014, 36, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, M.H.; Ahmed, E.; Hameed, S.; Malik, A.; Fatima, I.; Ashraf, M. (Cashmirols A and B, new Lipoxygenase Inhibiting Triterpenes from Sorbus cashmiriana. Chem. Biodivers. 2009, 6, 1471–1476. [Google Scholar] [CrossRef]
- Raspé, O.; Findlay, C.; Jacquemart, A.L. Sorbus aucuparia L. J. Ecol. 2000, 88, 910–930. [Google Scholar] [CrossRef]

| Samples | Extraction Method | Total Polyphenols Concentration ± SD (CA μg/mL) | Flavonoids Concentration ± SD (RU μg/mL) | Yield (%) | |
|---|---|---|---|---|---|
| S. aucuparia fruit extracts | ASE | Microfiltrate | 2265.62 ± 52.63 | 377.88 ± 17.32 | 14.09 |
| Concentrate | 2692.53 ± 37.89 | 433.88 ± 21.12 | 29.06 | ||
| UAE | Microfiltrate | 1246.85 ± 28.39 | 191.12 ± 5.23 | 13.13 | |
| Concentrate | 1576.24 ± 32.78 | 230.88 ± 10.32 | 22.12 | ||
| Compound | Extract ASE | Extract UAE | ||
|---|---|---|---|---|
| Microfiltrate Conc ± SD μg/mL | Concentrate Conc ± SD μg/mL | Microfiltrate Conc ± SD μg/mL | Concentrate Conc ± SD μg/mL | |
| Coumaric acid | 0.39 ± 0.01 | 0.45 ± 0.21 | 0.22 ± 0.01 | 0.26 ± 0.01 |
| Gallic acid | - | - | - | - |
| Caffeic acid | 32.67 ± 0.12 | 34.41 ± 1.21 | 16.40 ± 0.73 | 16.27 ± 1.12 |
| Luteolin | 0.04 ± 0.01 | 0.04 ± 0.001 | 0.02 ± 0.001 | 0.02 ± 0.001 |
| Kaempferol | 0.13 ± 0.01 | 0.14 ± 0.001 | 0.09 ± 0.004 | 0.10 ± 0.001 |
| Ellagic acid | 1.59 ± 0.02 | 3.81 ± 0.02 | 0.92 ± 0.04 | 2.20 ± 0.12 |
| Quercetin | 1.45 ± 0.01 | 1.66 ± 0.01 | 1.33 ± 0.01 | 1.28 ± 0.01 |
| p-hydroxybenzoic acid | 14.71 ± 0.89 | 15.09 ± 0.89 | 6.93 ± 0.03 | 8.81 ± 0.28 |
| Myricetin | - | - | - | - |
| Chlorogenic acid | 473.54 ± 20.12 | 526.08 ± 23.35 | 273.98 ± 10.81 | 296.80 ± 12.35 |
| Quercitrin | 2.52 ± 0.13 | 3.81 ± 0.14 | 1.34 ± 0.01 | 1.46 ± 0.01 |
| Quercetin 3-β-D-glucoside | 15.82 ± 0.89 | 17.27 ± 0.56 | 21.28 ± 1.29 | 23.37 ± 1.12 |
| Rutin | 13.68 ± 0.78 | 36.07 ± 1.23 | 4.85 ± 0.14 | 6.31 ± 0. 23 |
| Epicatechin | 4.65 ± 0.23 | 4.75 ± 0.12 | 1.15 ± 0.01 | 1.92 ± 0.02 |
| Samples | DPPH Radical Scavenging Activity IC50 (µg/mL) | Reducing Power Activity IC50 (µg/mL) | TEACABTS (μmolTrolox/g) | ||||
|---|---|---|---|---|---|---|---|
| ASE | UAE | ASE | UAE | ASE | UAE | ||
| S. aucuparia fruit extracts | Microfiltrate | 43.57 ± 1.62 * | 54.98 ± 2.15 * | 52.14 ± 2.62 * | 75.74 ± 3.25 * | 41.90 ± 2.97 * | 22.61 ± 0.90 |
| Concentrate | 39.07 ± 0.32 * | 50.98 ± 1.1 * | 47.36 ± 1.65 * | 49.61 ± 1.2 * | 50.81 ± 2.87 * | 32.33 ± 1.10 * | |
| Vitamin C | 46.47 ± 1.38 | 36.32 ± 1.25 | 68.61 ± 2.32 | ||||
| Samples | α-Amylase Inhibition IC50 (µg/mL) | α-Glucosidase Inhibition IC50 (µg/mL) | |||
|---|---|---|---|---|---|
| ASE | UAE | ASE | UAE | ||
| S. aucuparia extracts | Microfiltrate | 32.87 ± 2.15 * | 48.38 ± 3.56 * | 15.26 ± 0.98 * | 25.21 ± 1.36 * |
| Concentrate | 23.74 ± 1.32 * | 38.23 ± 2.53 * | 13.50 ± 0.96 * | 21.53 ± 1.25 * | |
| Acarbose | 22.65 ± 1.27 | 20.19 ± 1.67 | |||
| Samples | Lipoxygenase Inhibition IC50 (µg/mL) | Hyaluronidase Inhibition IC50 (µg/mL) | |||
|---|---|---|---|---|---|
| ASE | UAE | ASE | UAE | ||
| S. aucuparia extracts | Microfiltrate | 40.11 ± 2.21 * | 73.63 ± 4.35 * | 70.67 ± 2.53 * | 65.82 ± 3.16 * |
| Concentrate | 24.30 ± 1.54 * | 31.01 ± 2.36 * | 43.04 ± 2.19 * | 48.49 ± 3.15 * | |
| Ibuprofen | 26.91 ± 1.27 | 51.54 ± 3.67 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neagu, E.; Paun, G.; Albu, C.; Badea, G.; Seciu-Grama, A.M.; Radu, G.L. Antidiabetic and Anti-Inflammatory Potential of Sorbus aucuparia Fruits (Rowanberries) from Romania. Appl. Sci. 2025, 15, 12585. https://doi.org/10.3390/app152312585
Neagu E, Paun G, Albu C, Badea G, Seciu-Grama AM, Radu GL. Antidiabetic and Anti-Inflammatory Potential of Sorbus aucuparia Fruits (Rowanberries) from Romania. Applied Sciences. 2025; 15(23):12585. https://doi.org/10.3390/app152312585
Chicago/Turabian StyleNeagu, Elena, Gabriela Paun, Camelia Albu, Georgiana Badea, Ana Maria Seciu-Grama, and Gabriel Lucian Radu. 2025. "Antidiabetic and Anti-Inflammatory Potential of Sorbus aucuparia Fruits (Rowanberries) from Romania" Applied Sciences 15, no. 23: 12585. https://doi.org/10.3390/app152312585
APA StyleNeagu, E., Paun, G., Albu, C., Badea, G., Seciu-Grama, A. M., & Radu, G. L. (2025). Antidiabetic and Anti-Inflammatory Potential of Sorbus aucuparia Fruits (Rowanberries) from Romania. Applied Sciences, 15(23), 12585. https://doi.org/10.3390/app152312585








