Superconductivity and Cryogenics in Medical Diagnostics and Treatment: An Overview of Selected Applications
Abstract
1. Introduction
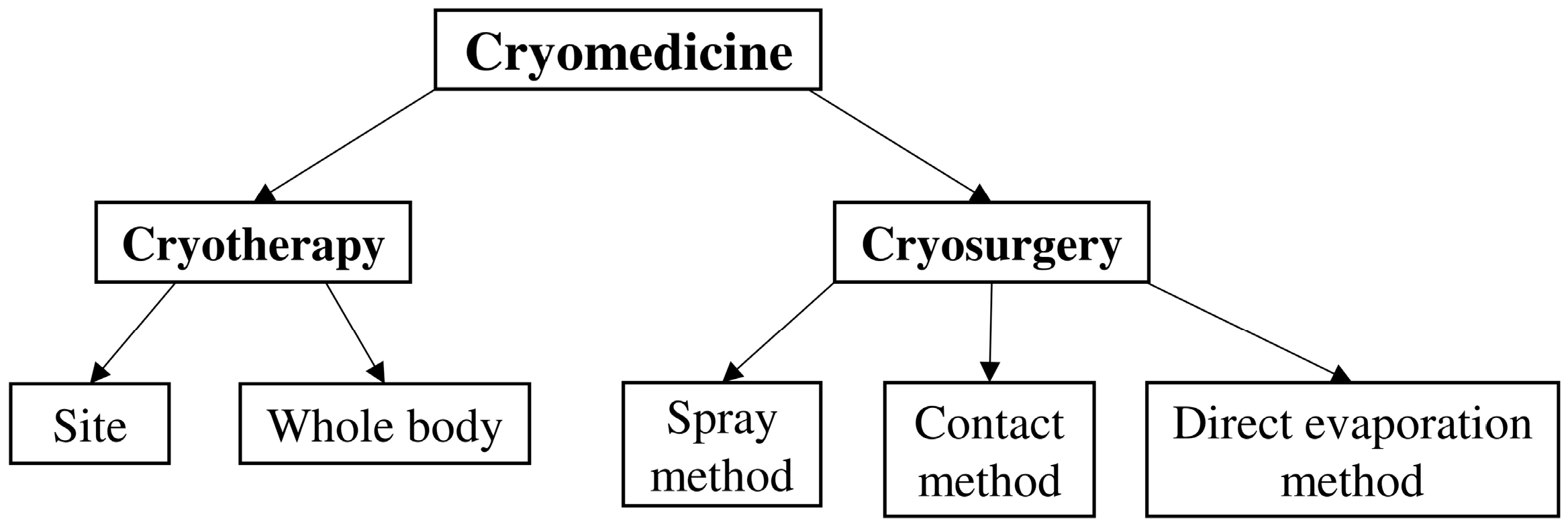
2. Cryogenics Applications in Medicine
2.1. Site Cryotherapy
2.2. Cryotherapy and Total Body Cryostimulation
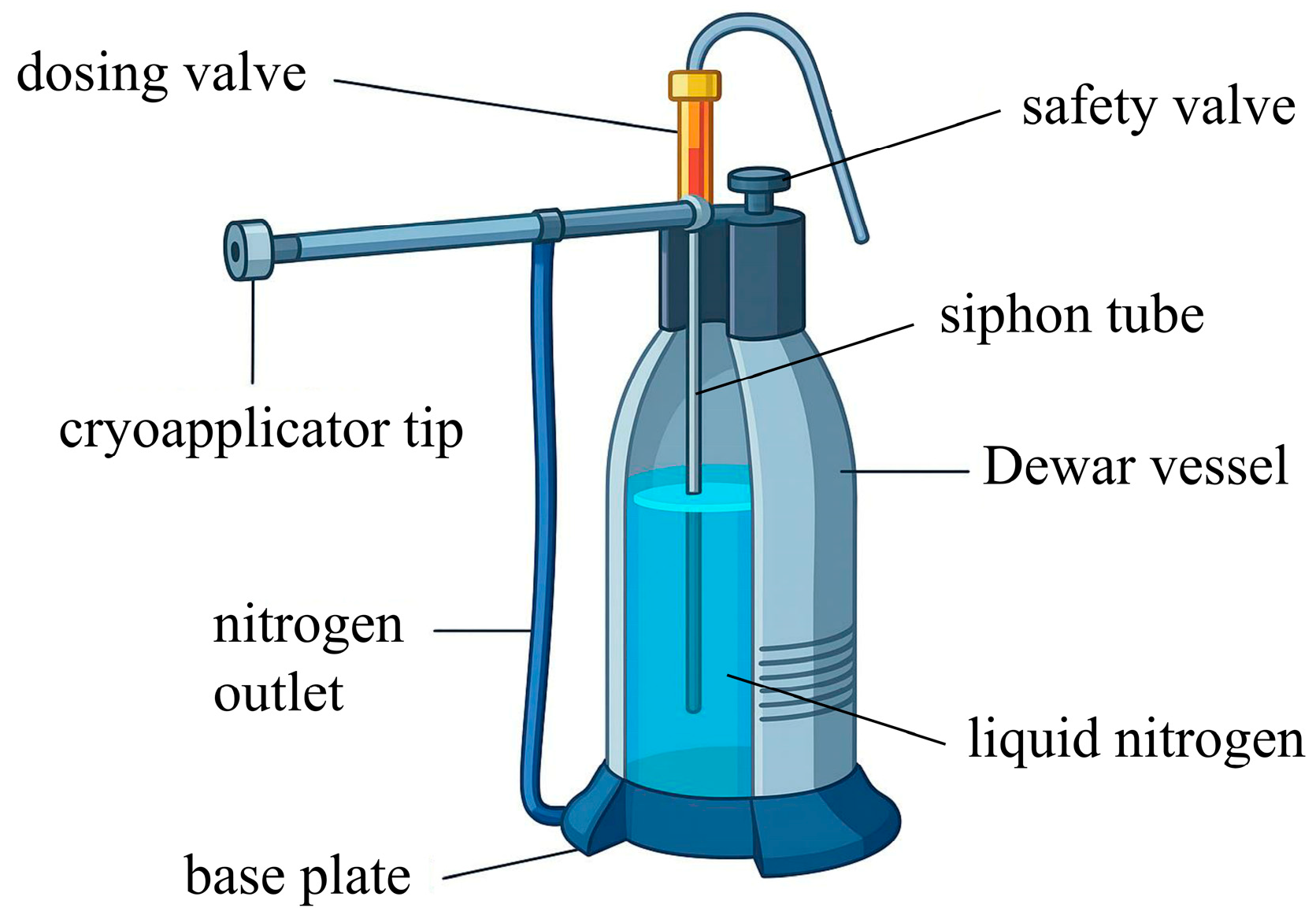
2.3. Equipment Used in Cryosurgery
3. Superconductivity Applications in Medicine
3.1. Cyclotrons for Radiotherapy
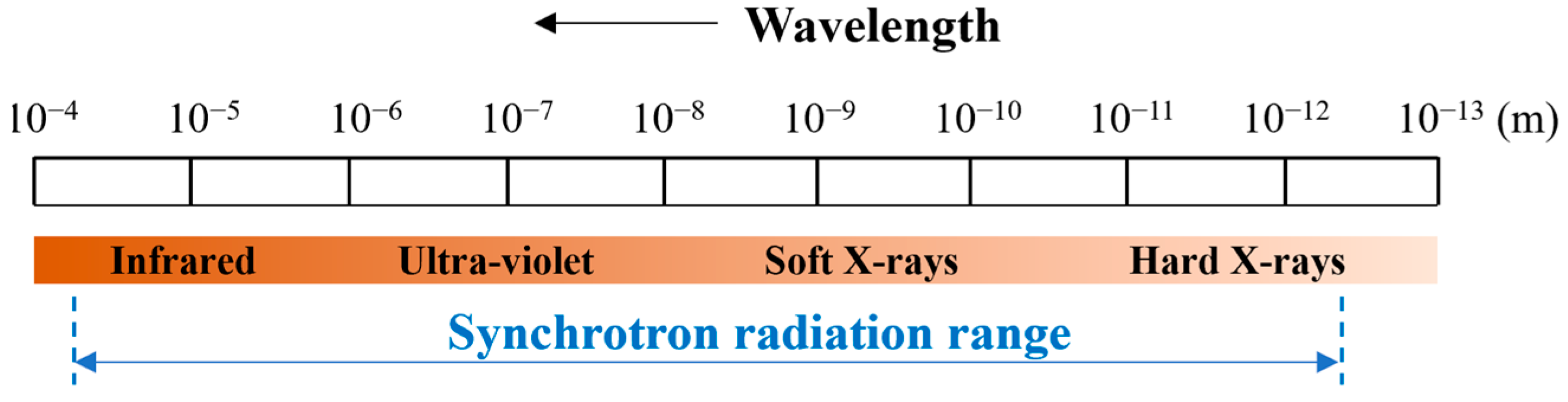
3.2. Radiation Oncology
- X-rays have low LET and limited DL;
- Protons also exhibit low LET, but achieve good DL;
- Neutrons offer higher LET, but poor DL;
- Carbon ions combine both high LET and high DL.
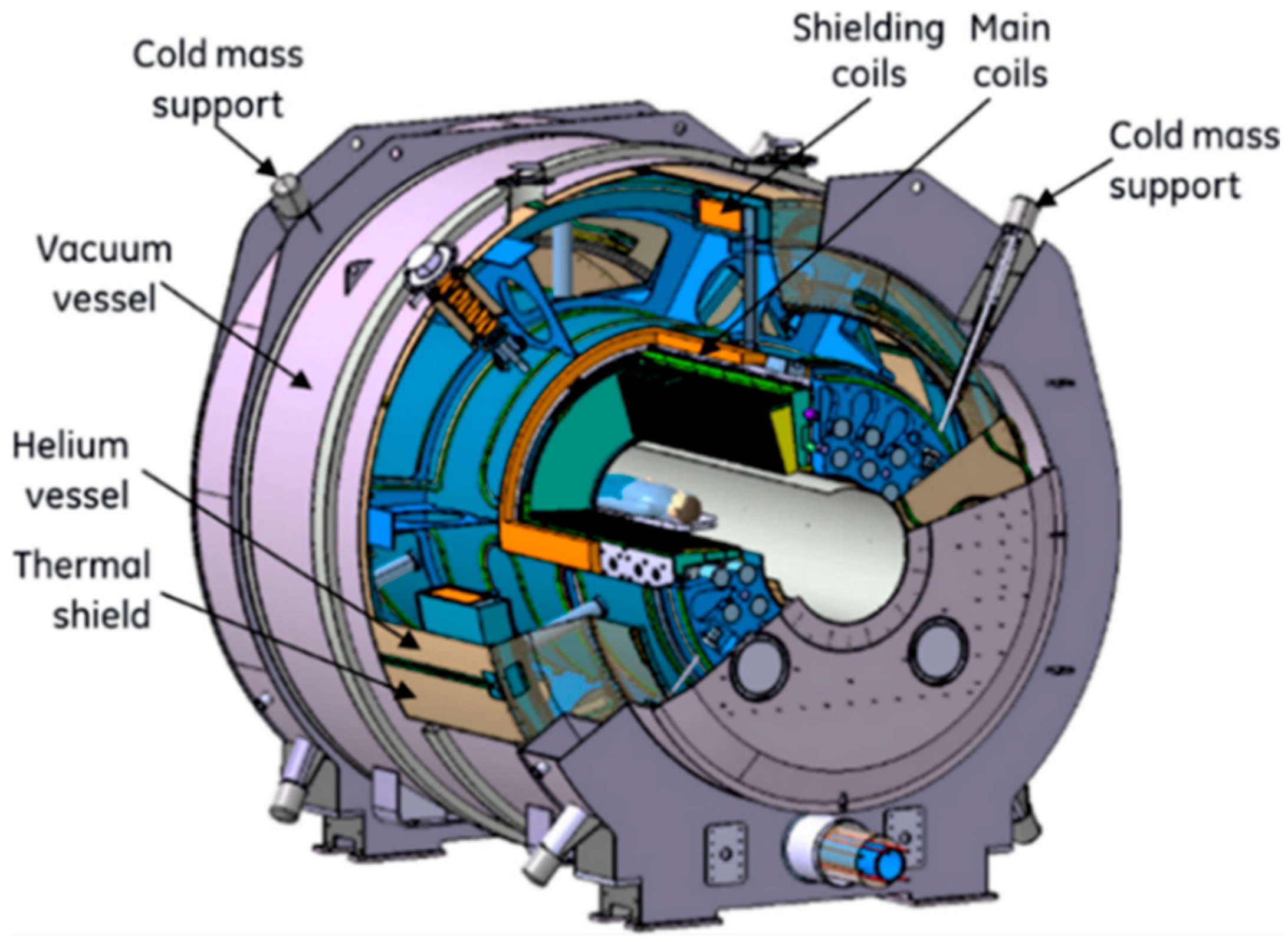
3.3. Magnetic Resonance Imaging
3.4. Ultra-High-Field MRI
3.5. Low- and Ultra-Low-Field MRI
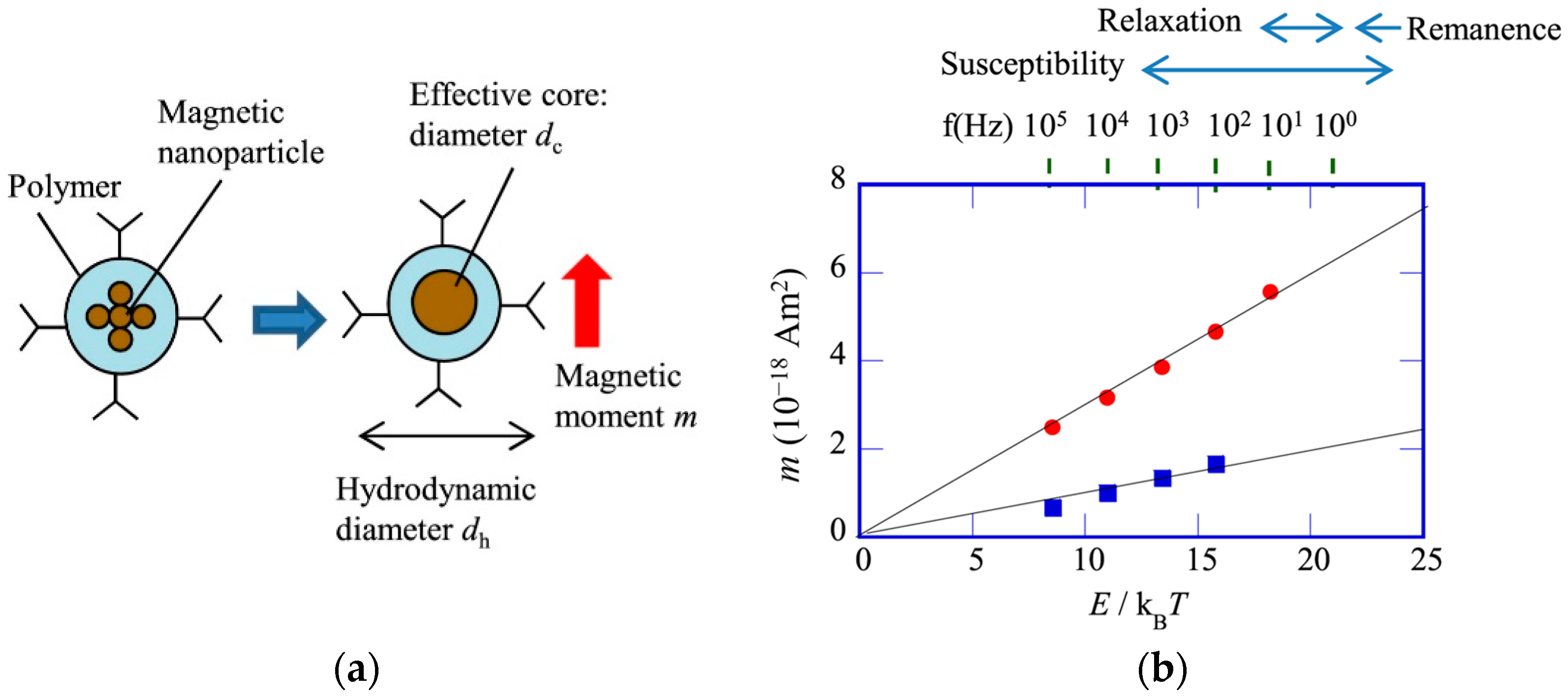
3.6. Biofunctionalized Magnetic Nanoparticles for Immunoassay
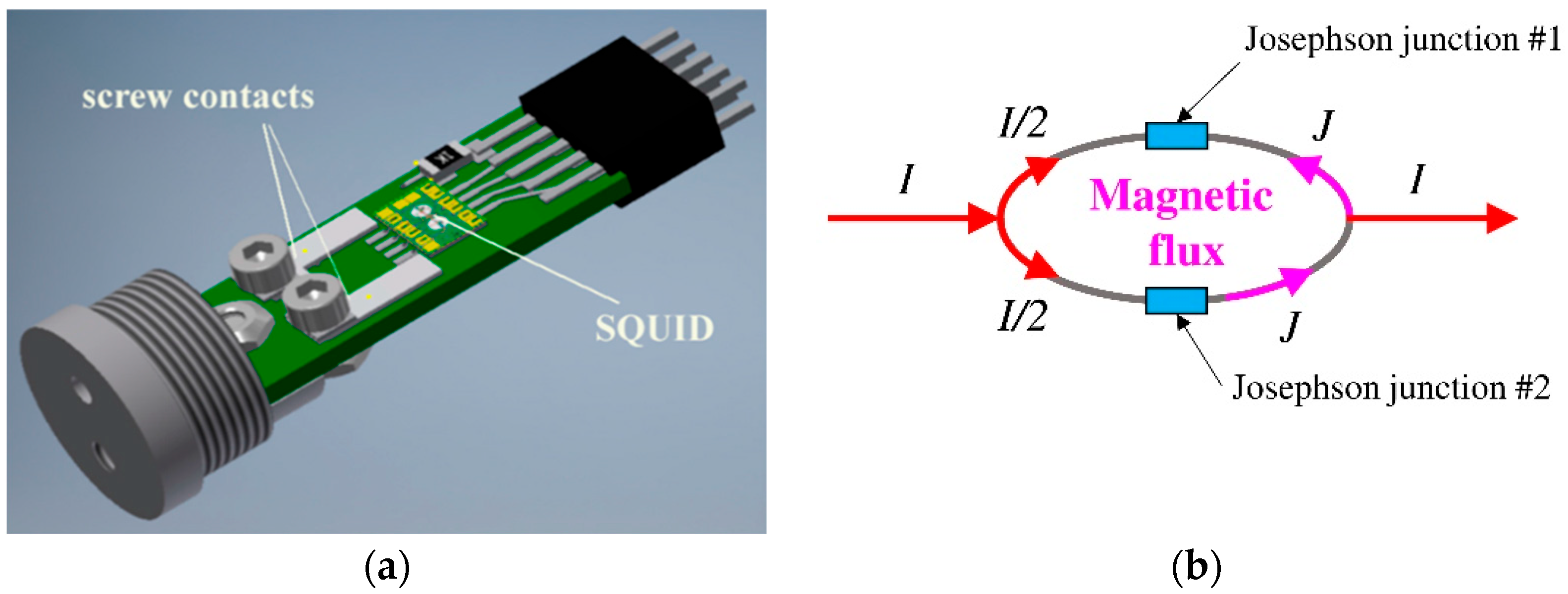
3.7. High-Sensitivity Magnetic Relaxometry Using SQUIDs
- (a).
- It works by using the quantum mechanical properties of superconductors to detect tiny changes in magnetic fields;
- (b).
- It measures the magnetic flux through a superconducting loop, which is converted into a measurable voltage or current, allowing for extremely sensitive measurements of magnetic fields.
4. Prospects for Future Applications of Cryogenics and Superconductivity in Medicine
- (a).
- Targeted drug delivery: MNPs can be functionalized with drugs to be guided to specific sites, like tumors, using external magnetic fields, which increases drug accumulation in the target tissue and reduces side effects.
- (b).
- Controlled release: Releasing the therapeutic agent from the MNPs can be triggered remotely by a magnetic field at the precise time and location.
- (c).
- Hyperthermial properties: MNPs can be used to locally heat and ablate diseased tissue, such as cancer cells, when an alternating magnetic field is applied.
- (d).
- Gene therapy (magnetofection): MNPs can act as carriers to deliver therapeutic genes to target cells under the external magnetic field.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stryczewska, H.D.; Stępień, M.; Boiko, O. Plasma and Superconductivity for the Sustainable Development of Energy and the Environment. Energies 2022, 15, 4092. [Google Scholar] [CrossRef]
- Assunçao, R.; Eckl, F.; Ramos, C.P.; Correia, C.B.; Neto, R.C. Oxygen liquefaction economical value in the development of the hydrogen economy. Int. J. Hydrogen Energy 2024, 62, 109–118. [Google Scholar] [CrossRef]
- Weisend, J.G., II. Superfluid. In How a Quantum Fluid Revolutionized Modern Science; Springer Nature: London, UK, 2023; pp. 3–10. [Google Scholar]
- Banaszkiewicz, T.; Chorowski, M.; Gizicki, W.; Jedrusyna, A.; Kielar, J.; Malecha, Z.; Piotrowska, A.; Polinski, J.; Rogala, Z.; Sierpowski, K.; et al. Liquefied Natural Gas in Mobile Applications—Opportunities and Challenges. Energies 2020, 13, 5673. [Google Scholar] [CrossRef]
- Chen, B.S.; Zeng, Y.P.; Luo, E.R.; Peng, N.; Zou, A.H. An innovative method for helium refrigeration liquefaction utilizing transonic nozzle. Energy 2025, 314, 134283. [Google Scholar] [CrossRef]
- Sharafi, M.; Borghei-Rad, S.M.; Hezavehei, M.; Shahverdi, A.; Benson, J.D. Cryopreservation of Semen in Domestic Animals: A Review of Current Challenges, Applications, and Prospective Strategies. Animals 2022, 12, 3271. [Google Scholar] [CrossRef]
- Rezaie, H.; Ziabasharhagh, M.; Mafi, M. A review of hydrogen liquefaction, current situation and its future. In Proceedings of the International Conference on Engineering & Applied Sciences, Dubai, United Arab Emirates, 10 March 2016. [Google Scholar]
- Omori, K.M.; Mazon-Cartagena, R.; Fernández-Torres, M.J.; Caballero, J.A.; Ravagnani, M.A.S.S.; Pavão, L.V.; Costa, C.B.B. Sensitivity Analysis in Simple Cycles for Hydrogen Liquefaction. Processes 2025, 13, 3076. [Google Scholar] [CrossRef]
- Li, Z.A.; Xu, M.J.; Wang, J.H.; Zhang, F. Recent Advances in Cryogenic 3D Printing Technologies. Adv. Eng. Mater. 2022, 24, 2200245. [Google Scholar] [CrossRef]
- Tuoriniemi, J.T.; Knuuttila, T.A. Nuclear cooling and spin properties of rhodium down to picokelvin temperatures. Phys. B Condens. Matter 2000, 280, 474–478. [Google Scholar] [CrossRef]
- Mizunami, T.; Tatehata, H.; Kawashima, H. High-sensitivity cryogenic fibre-Bragg-grating temperature sensors using Teflon substrates. Meas. Sci. Technol. 2001, 12, 914–917. [Google Scholar]
- Wu, M.C.; DeHaven, S.L. High-sensitivity cryogenic temperature sensors using pressurized fiber Bragg gratings. In Proceedings of the SPIE Photonics Europe, Strasbourg, France, 3–7 April 2006; Volume 6189, p. 61890O. [Google Scholar]
- Deppner, C.; Herr, W.; Cornelius, M.; Stromberger, P.; Sternke, T.; Grzeschik, C.; Grote, A.; Rudolph, R.; Krutzik, M.; Wenzlawski, A.; et al. Collective-Mode Enhanced Matter-Wave Optics. Phys. Rev. Lett. 2021, 127, 100401. [Google Scholar] [CrossRef]
- Rjabinin, J.N.; Shubnikow, L.W. Magnetic Properties and Critical Currents of Supra-conducting Alloys. Nature 1935, 135, 581–582. [Google Scholar] [CrossRef]
- Ginzburg, V.L.; Landau, L.D. On the Theory of Superconductivity. J. Exptl. Theoret. Phys. 1950, 20, 138–167. [Google Scholar]
- Bardeen, J.; Cooper, L.N.; Schrieffer, J.R. Theory of Superconductivity. Phys. Rev. 1957, 108, 1175–1204. [Google Scholar] [CrossRef]
- Braginski, A.I. Consequences of Brian Josephson’s Theoretical Discovery. J. Supercond. Nov. Magn. 2021, 34, 1597–1600. [Google Scholar] [CrossRef]
- Bednorz, J.G.; Muller, K.A. The new approach to high-Tc superconductivity. Rev. Mod. Phys. 1988, 60, 585–600. [Google Scholar] [CrossRef]
- Nagamatsu, J.; Nakagawa, N.; Muranaka, T.; Zenitani, Y.; Akimitsu, J. Superconductivity at 39 K in magnesium diboride. Nature 2001, 410, 63–64. [Google Scholar] [CrossRef]
- Takahashi, H.; Igawa, K.; Arii, K.; Kamihara, Y.; Hirano, M.; Hosono, H. Superconductivity at 43 K in an iron-based layered compound LaO1-xFxFeAs. Nature 2008, 53, 376–378. [Google Scholar] [CrossRef] [PubMed]
- Physics of Iron-Based High Temperature Superconductors. Available online: https://boulderschool.yale.edu/sites/default/files/files/MatsudaNote.pdf (accessed on 17 November 2025).
- Cao, Y.; Fatemi, V.; Fang, S.; Watanabe, K.; Taniguchi, T.; Kaxiras, E.; Jarillo-Herrero, P. Unconventional superconductivity in magic-angle graphene superlattices. Nature 2018, 556, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Eremets, M.I.; Minkov, V.S.; Drozdov, A.P.; Kong, P.P.; Ksenofontov, V.; Shylin, S.I.; Bud’ko, S.L.; Prozorov, R.; Balakirev, F.F.; Sun, D.; et al. High-Temperature Superconductivity in Hydrides: Experimental Evidence and Details. J. Supercond. Nov. Magn. 2022, 35, 965–977. [Google Scholar] [CrossRef]
- Podbielska, A.; Skrzek, A. Application of Low Temperatures in Biomedicine/Zastosowanie Niskich Temperatur w Biomedycynie, 1st ed.; Wrocław University of Technology Publishing House: Wrocław, Poland, 2012. [Google Scholar]
- Alonso, J.R.; Antaya, T.A. Superconductivity in Medicine. Rev. Accel. Sci. Technol. 2012, 5, 227–263. [Google Scholar] [CrossRef]
- Flukiger, R. Overview of Superconductivity and Challenges in Applications. Rev. Accel. Sci. Technol. 2012, 5, 1–23. [Google Scholar] [CrossRef]
- Cipria, K.; Lopez, E.; Naso, V. Investigation of the use of Pulse Tube in cell cryopreservation systems. Cryobiology 2010, 61, 225–230. [Google Scholar] [CrossRef]
- Janowski, T.; Stryczewska, H.D.; Wac-Włodarczyk, A. Superconducting and Plasma Technologies in Energy/Technologie Nadprzewodnikowe i Plazmowe w Energetyce, 1st ed.; Lublin Scientific Society Publishing House: Lublin, Poland, 2009. (In Polish) [Google Scholar]
- Kriogenika w Zastosowaniach Przemysłowych, Medycznych i Badawczych. Cryogenics in Industrial, Medical and Research Applications. Available online: https://www.klimatyzacja.pl/chlodnictwo/artykuly/kriogenika/kriogenika-w-zastosowaniach-przemyslowych-medycznych-i-badawczych (accessed on 16 November 2025). (In Polish).
- Chorowski, M. Cryogenics, Basics and Applications/Kriogenika, Podstawy i Zastosowania; IPPU Masta: Gdańsk, Poland, 2007. (In Polish) [Google Scholar]
- Tomaru, T.; Suzuki, T.; Haruyama, T.; Shintomi, T.; Yamamoto, A.; Koyama, T.; Li, R. Vibration analysis of cryocoolers. Cryogenics 2004, 44, 309–317. [Google Scholar] [CrossRef]
- Szkliniarz, K. Cyclotron Production and Study of Radioisotopes Used in Medical Diagnostics and Therapy/Cyklotronowa Produkcja i Badanie Radioizotopów Stosowanych w Diagnostyce i Terapii Medycznej. Ph.D. Thesis, Silesian University, Katowice, Poland, 2016. [Google Scholar]
- Körber, R.; Storm, J.H.; Seton, H.; Mäkelä, J.P.; Paetau, R.; Parkkonen, L.; Pfeiffer, C.; Riaz, B.; Schneiderman, J.F.; Dong, H.; et al. SQUIDs in biomagnetism: A roadmap towards improved healthcare. Supercond. Sci. Technol. 2016, 29, 113001. [Google Scholar] [CrossRef]
- Oladapo, B.I.; Zahedi, S.A.; Chaluvadi, S.C.; Bollapalli, S.S.; Ismail, M. Model design of a superconducting quantum interference device of magnetic field sensors for magnetocardiography. Biomed. Signal Process. Control. 2018, 46, 116–120. [Google Scholar] [CrossRef]
- Chu, W.T. Accelerators and Medicine. J. Korean Phys. Soc. 2007, 50, 1385–1389. [Google Scholar] [CrossRef]
- Levy, R.P.; Blakely, E.A.; Chu, W.T.; Coutrakon, G.B.; Hug, E.B.; Kraft, G.; Tsujii, H. The Current Status and Future Directions of Heavy Charged Particle Therapy in Medicine. AIP Conf. Proc. 2009, 1099, 410–425. [Google Scholar]
- Iwata, Y.; Noda, K. 8.10—Ion Linac and Synchrotron. Compr. Biomed. Phys. 2014, 8, 153–168. [Google Scholar]
- Hathaway, H.J.; Butler, K.S.; Adolphi, N.L.; Lovato, D.M.; Belfon, R.; Fegan, D.; Monson, T.C.; Trujillo, J.E.; Tessier, T.E.; Bryant, H.C.; et al. Detection of breast cancer cells using targeted magnetic nanoparticles and ultra-sensitive magnetic field sensors. Breast Cancer Res. 2011, 13, R108. [Google Scholar] [CrossRef] [PubMed]
- Nawrocki, W. Introduction to Quantum Metrology: Quantum Standards and Instrumentation, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Iwata, Y.; Noda, K.; Shirai, T.; Murakami, T.; Furukawa, T.; Mori, S.; Fujita, T.; Itano, A.; Shouda, K.; Mizushima, K.; et al. Design of a superconducting rotating gantry for heavy-ion therapy. Phys. Rev. ST Accel. Beams 2012, 15, 044701. [Google Scholar] [CrossRef]
- Iwata, Y.; Fujimoto, T.; Matsuba, S.; Fujita, T.; Sato, S.; Furukawa, T.; Hara, Y.; Mizushima, K.; Saraya, Y.; Tansho, R.; et al. Beam commissioning of a superconducting rotating-gantry for carbon-ion radiotherapy. Nucl. Instrum. Methods Phys. Res. A 2016, 834, 71–80. [Google Scholar] [CrossRef]
- Pełka, B. Synchrotron radiation in biology and medicine. Synchrotron Radiat. Nat. Sci. 2007, 6, 99–107. [Google Scholar]
- Kisiel, A. Synchrotron as a tool: Applications of synchrotron radiation in solid-state spectroscopy. Synchrotron Radiat. Nat. Sci. 2006, 5, 145–167. [Google Scholar]
- Sun, X.J.; Chen, F.S.; Yang, X.C.; Chen, W.; Bian, X.J.; Li, M.X.; Ge, R.; Xu, M.F.; Gao, Y.; Wang, J.C.; et al. Superconducting multipole wiggler with large magnetic gap for HEPS-TF. Nucl. Sci. Tech. 2022, 33, 16. [Google Scholar] [CrossRef]
- Wilkinson, B.; Hill, M.A.; Parsons, J.L. The Cellular Response to Complex DNA Damage Induced by Ionising Radiation. Int. J. Mol. Sci. 2023, 24, 4920. [Google Scholar] [CrossRef]
- Catterall, M. The assessment of the results of neutron therapy. Int. J. Radiat. Oncol. Biol. Phys. 1982, 8, 1573–1580. [Google Scholar] [CrossRef]
- Laramore, G.E.; Krall, J.M.; Griffin, T.W.; Duncan, W.; Richter, M.P.; Saroja, K.R.; Maor, M.H.; Davis, L.W. Neutron versus photon irradiation for unresectable salivary gland tumors: Final report of an RTOG-MRC randomized clinical trial. Int. J. Radiat. Oncol. Biol. Phys. 1993, 27, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Iwata, Y.; Fujita, T.; Furukawa, T.; Hara, Y.; Matsuba, S.; Mizushima, K.; Murakami, T.; Noda, K.; Saotome, N.; Saraya, Y.; et al. Superconducting gantry for carbon-ion radiotherapy. In Proceedings of the 9th International Particle Accelerator Conference IPAC2018, Vancouver, BC, Canada, 29 April–4 May 2018. [Google Scholar]
- Huang, G.; Albers, P.; Mookerji, N.; Pfanner, T.; Hui, A.; Mittal, R.; Broomfield, S.; Dean, L.; Martin, B.S.; Jacobsen, N.-E.; et al. Three-dimensional spatial localization and volume estimation of prostate tumors using 18F-PSMA-1007 PET/CT versus multiparametric MRI. Eur. J. Nucl. Med. Mol. Imaging 2025, 52, 1642–1648. [Google Scholar] [CrossRef] [PubMed]
- Kimberly, W.T.; Sorby-Adams, A.J.; Webb, A.G.; Beekman, R.; Bowry, R.; Schiff, S.J.; de Havenon, A.; Shen, F.X.; Sze, G.; Schaefer, P.; et al. Brain imaging with portable low-field MRI. Nat. Rev. Bioeng. 2023, 1, 617–630. [Google Scholar] [CrossRef]
- Sorby-Adams, A.J.; Guo, J.N.F.; Laso, P.; Kirsch, J.E.; Zabinska, J.; Guarniz, A.L.G.; Schaefer, P.W.; Payabvash, S.; de Havenon, A.; Rosen, M.S.; et al. Portable, low-field magnetic resonance imaging for evaluation of Alzheimer’s disease. Nat. Commun. 2024, 15, 10488. [Google Scholar] [CrossRef]
- Glowacki, B.A. Hydrogen Cryomagnetic a Common Solution for Metallic and Oxide Superconductors. Materials 2025, 18, 3665. [Google Scholar] [CrossRef]
- Zabinska, J.; de Havenon, A.; Sheth, K.N. Recent advances in portable, low-field magnetic resonance imaging in cerebrovascular disease. Curr. Opin. Neurol. 2025, 38, 35–39. [Google Scholar] [CrossRef]
- Shoghli, A.; Chow, D.; Kuoy, E.; Yaghmai, V. Current role of portable MRI in diagnosis of acute neurological conditions. Front. Neurol. 2023, 14, 1255858. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Yang, J.; Nittala, M.R.; Velazquez, A.E.; Huddleston, B.L.; Rugnath, N.A.; Adari, N.; Yajurvedi, A.K.; Komanduri, A.; Yang, C.C.; et al. Changing Role of PET/CT in Cancer Care with a Focus on Radiotherapy. Cureus 2022, 14, e32840. [Google Scholar] [CrossRef]
- Özütemiz, C.; White, M.; Elvendahl, W.; Eryaman, Y.; Marjanska, M.; Metzger, G.J.; Patriat, R.; Kulesa, J.; Harel, N.; Watanabe, Y.; et al. Use of a Commercial 7-T MRI Scanner for Clinical Brain Imaging: Indications, Protocols, Challenges, and Solutions-A Single-Center Experience. Am. J. Roentgenol. 2023, 221, 788–805. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, D.A.; Beckett, A.J.S.; Vu, A.T.; Stockmann, J.; Huber, L.; Ma, S.M.T.; Ahn, S.; Setsompop, K.; Cao, X.Z.; Park, S.; et al. Next-generation MRI scanner designed for ultra-high-resolution human brain imaging at 7 Tesla. Nat. Methods 2023, 20, 1833. [Google Scholar] [CrossRef] [PubMed]
- Platt, T.; Ladd, M.E.; Paech, D. 7 Tesla and Beyond: Advanced Methods and Clinical Applications in Magnetic Resonance Imaging. Investig. Radiol. 2021, 56, 705–725. [Google Scholar] [CrossRef] [PubMed]
- Boulant, N.; Quettier, L.; The Iseult Consortium. Commissioning of the Iseult CEA 11.7 T whole-body MRI: Current status, gradient–magnet interaction tests and first imaging experience. Magn. Reson. Mater. Phy. 2023, 36, 175–189. [Google Scholar] [CrossRef]
- TSAR—Topological Solitons in AntifeRroics. Available online: https://www.tsar-fetopen.eu/#consortium (accessed on 8 February 2025).
- U Scientists Scan World’s First 10.5-Tesla Human MRI Image|RIO. Available online: https://research.umn.edu/news/u-scientists-scan-worlds-first-105-tesla-human-mri-image (accessed on 8 February 2025).
- Clarke, J.; Hatridge, M.; Mößle, M. SQUID-Detected Magnetic Resonance Imaging in Microtesla Fields. Annu. Rev. Biomed. Eng. 2007, 9, 383–413. [Google Scholar] [CrossRef]
- Bandettini, P.A.; Petridou, N.; Bodurka, J. Direct Detection of Neuronal Activity with MRI: Fantasy, Possibility, or Reality? Appl. Magn. Reson. 2005, 29, 65–88. [Google Scholar] [CrossRef]
- Wald, L.L.; McDaniel, P.C.; Witzel, T.; Stockmann, J.P.; Zimmerman-Cooley, C. Low-Cost and Portable MRI. Magn. Reason. Imaging 2020, 52, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Nakamura, T.; Higashi, O.; Enpuku, K. Magnetic fractionation and characterization of magnetic nanoparticles for magnetic particle imaging. Jpn. J. Appl. Phys. 2018, 57, 080302. [Google Scholar] [CrossRef]
- Enpuku, K.; Yang, S.Y.; Chieh, J.J. Magnetic nanoparticles for immunoassay. Supercond. Sci. Technol. 2016, 29, 113001. [Google Scholar]
- Rogalla, H.; Kes, P.H. 100 Years of Superconductivity, 1st ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Faley, M.I.; Kostyurina, E.A.; Kalashnikov, K.V.; Maslennikov, Y.V.; Koshelets, V.P.; Dunin-Borkowski, R.E. Superconducting Quantum Interferometers for Non-destructive Evaluation. Sensors 2017, 17, 2798. [Google Scholar] [CrossRef] [PubMed]
- Ohta, H.; Matsui, T.; Uchikawa, Y. Studies on response of human hippocampus to random somatosensory stimuli by a SQUID system in a superconducting magnetic shield. IEEE Trans. Appl. Supercond. 2011, 21, 469–472. [Google Scholar] [CrossRef]
- Escalona-Vargas, D.; Siegel, E.R.; Oliphant, S.; Eswaran, H. Evaluation of pelvic floor muscles in pregnancy and postpartum with non-invasive magnetomyography. IEEE J. Transl. Eng. Health Med. 2022, 10, 1800106. [Google Scholar] [CrossRef]
- Zhang, Z.; He, A.; Xu, Z.; Yang, K.; Kong, X. Neuromuscular Magnetic Field Measurement Based on Superconducting Bio-Sensors. Micromachines 2023, 14, 1768. [Google Scholar] [CrossRef]
- Krause, H.J.; Dong, H. Biomagnetic Sensing. In Label-Free Biosensing; Schöning, M., Poghossian, A., Eds.; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Malmivuo, J.; Plonsey, R. Principles and Applications of Bioelectric and Biomagnetic Fields, 1st ed.; Oxford University Press: Oxford, UK, 1995. [Google Scholar]
- Ruffieux, S. High-Temperature Superconducting Magnetometers for on-Scalp MEG. Ph.D. Thesis, Chalmers University of Technology, Göteborg, Sweden, 2020. [Google Scholar]
- Zhu, K.; Kiourti, A. A Review of Magnetic Field Emissions From the Human Body: Sources, Sensors, and Uses. IEEE Open J. Antennas Propag. 2022, 3, 732–744. [Google Scholar] [CrossRef]
- Voitovych, I.D.; Primin, M.A.; Sosnytskyy, V.N. Application of SQUIDs for registration of biomagnetic signals. Low Temp. Phys. 2012, 38, 311–320. [Google Scholar] [CrossRef]
- Ohta, H.; Matsui, T.; Uchikawa, Y. A Whole-Head SQUID System in a Superconducting Magnetic Shield. IEEE Trans. Appl. Supercon. 2007, 17, 730–733. [Google Scholar] [CrossRef]
- Iqbal, S.; Saleem, S. A Perspective on Medical Applications of High Temperature Superconductors. J. Bioeng. Biomed. Sci. 2014, 4, e119. [Google Scholar]
- Maciejczyk, A.; Sztuder, A. Proton therapy should be developed in Poland. Nowotw. J. Oncol. 2017, 2, 65–70. [Google Scholar] [CrossRef]



| P * | Researchers | Discoveries |
|---|---|---|
| Cryogenics | Louis-Paul Cailletet, French physicist and industrialist, and Raoul-Pierre Pictet, Swiss physicist. | 1877—first attempts to condense oxygen to 90 K [2]. |
| Karol Olszewski, Polish chemist, mathematician, and physicist, together with Zygmunt Wróblewski, chemist and physicist, Cracow Laboratory. | 1883—the world’s first successful liquefaction of oxygen, nitrogen, and carbon dioxide from atmospheric air in a stable state carried out at the Cracow Laboratory [3]. 1884—Olszewski was the pioneer in liquefying hydrogen under dynamic conditions, reaching a then-unprecedented low temperature of −225 °C (48 K). In 1895, he also succeeded in liquefying argon [4]. | |
| H. Kamerlingh Onnes, Dutch physicist, University of Leiden | 1908—Helium liquefaction [5]. | |
| Christopher Polge, British biologist, is considered the father of modern cryopreservation. | 1949—C. Polge discovered the cryoprotective properties of glycerol, which enabled the first effective freezing of semen [6]. | |
| Carl von Linde, German engineer and inventor and Georges Claude, French engineer and inventor. | 1985—C. von Linde was the first to apply the methods of air condensation on an industrial scale, followed by air rectification in 1902. In the process of air liquefaction, Linde used the Joule-Thomson effect for pre-cooled air in a recuperative heat exchanger [7]. 1902—G. Claude used an expander cycle to condense the air [8]. | |
| Ming C. Leu et al. from Missouri University of Science and Technology, Rolla, MI, USA. | 1999—the investigators employed an inkjet-based dispensing system to deposit water in sequential layers, each of which was subjected to rapid cryogenic solidification. This approach was subsequently designated as the RFP technique [9]. | |
| Scientists of Helsinki University of Technology’s Low-Temperature Lab in Espoo, Finland. | 2000—nuclear spin temperatures in picokelvin range (below 100 pK) were reported for an experiment [10]. | |
| Mizunami K. et al. and Wu M.C. et al., one of the first developed high-sensitivity cryogenic fiber Bragg grating temperature sensors. | 2001—Mizunami K. investigated low-temperature sensing using a fiber Bragg grating fixed on Teflon substrate [11]. 2006—Wu M.C. studied cryogenic temperature sensing using a pressurized fiber Bragg grating [12]. | |
| Markus K. et al., German and French teams from Hannover, Bremen, Mainz, Berlin, Orsay and Toulouse. | 2021—setting the current world record for effective temperatures at 38 pK through matter-wave lensing of rubidium [13]. | |
| Superc. | H. Kamerlingh Onnes, Dutch physicist, University of Leiden. | 1911—phenomena of current flow without resistance discovery. Mercury cooled by liquid helium to 4.2 K becomes a superconductor [5]. |
| JN Rjabinin i Lev Shubnikov, Ukrainian researchers from the USSR. | 1935—experimentally discovered type II superconductors, which they published in [14]. | |
| Superconductivity | Lev Landau and Vitaly Ginzburg, Soviet physicists. | 1950—discovered and developed the theory of two types of superconductors I and II, which they published in their paper [15]. |
| J. Bardeen (IL, USA), C. Cooper (OH, USA) and J.R. Schiffer (Birmingham, England). | 1957—BCS Theory—the basis of the theory is the Cooper pair: two electrons with opposite spin and momentum, bound together in a system with resultant spin and momentum equal to zero [16]. | |
| Brian D. Josephson, University of Cambridge, UK. | 1962—Josephson stationary phenomenon. System consisting of two superconductors separated by a layer of insulator that plays the role of a barrier to the flowing current—macroscopic quantum phenomenon [17]. | |
| J. Georg Bednorz and K. Alex Müllerz, IBM Zurich. | 1986—HTS Superconducting properties at temperatures higher than helium—ceramic composites [18]. | |
| J. Nagamatsu et al., Japanese Scientists | 2001—discovery of MgB2 (Tc = 39 K) [19]. | |
| Takahashi H, Igawa K, Arii K, Kamihara Y, Hirano M, Hosono H., Japanese Scientists. | 2008—first superconducting Fe-based pnictides, with Tc values up to 56 K [20,21]. | |
| Cao Y. et al., scientists from universities and institutes of MIT, Harvard, National Institute for Materials Science of Japan. | 2018—reported the emergence of intrinsic, unconventional superconductivity—phenomena not accounted for by weak electron–phonon coupling—in a two-dimensional superlattice formed by stacking two graphene layers rotated with respect to one another by a small twist angle [22]. | |
| Eremets M.I. et al. together with Swedish and American scientists. | 2022—described in detail the experiments revealing high-temperature superconductivity in hydrides under high pressures [23]. |
| Parameter | Symbol | Value |
|---|---|---|
| critical temperature | Tc | Temperature below which a superconductor exhibits superconductivity at zero critical field strength and zero critical current. |
| lower critical magnetic field strength | Hc1 | Magnetic field strength at which the first fluxion enters the volume of a type II superconductor, causing a departure from ideal diamagnetism. |
| upper critical magnetic strength | Hc2 | Maximum magnetic field strength below which a type II superconductor is in the mixed state. |
| critical current | Ic | Maximum direct current that can be considered to flow in a superconductor without resistance. |
| critical current density | Jc | Electric current density at the critical current, determined for the whole superconducting cross-section or the unstable part thereof, when a stabilizer is present. |
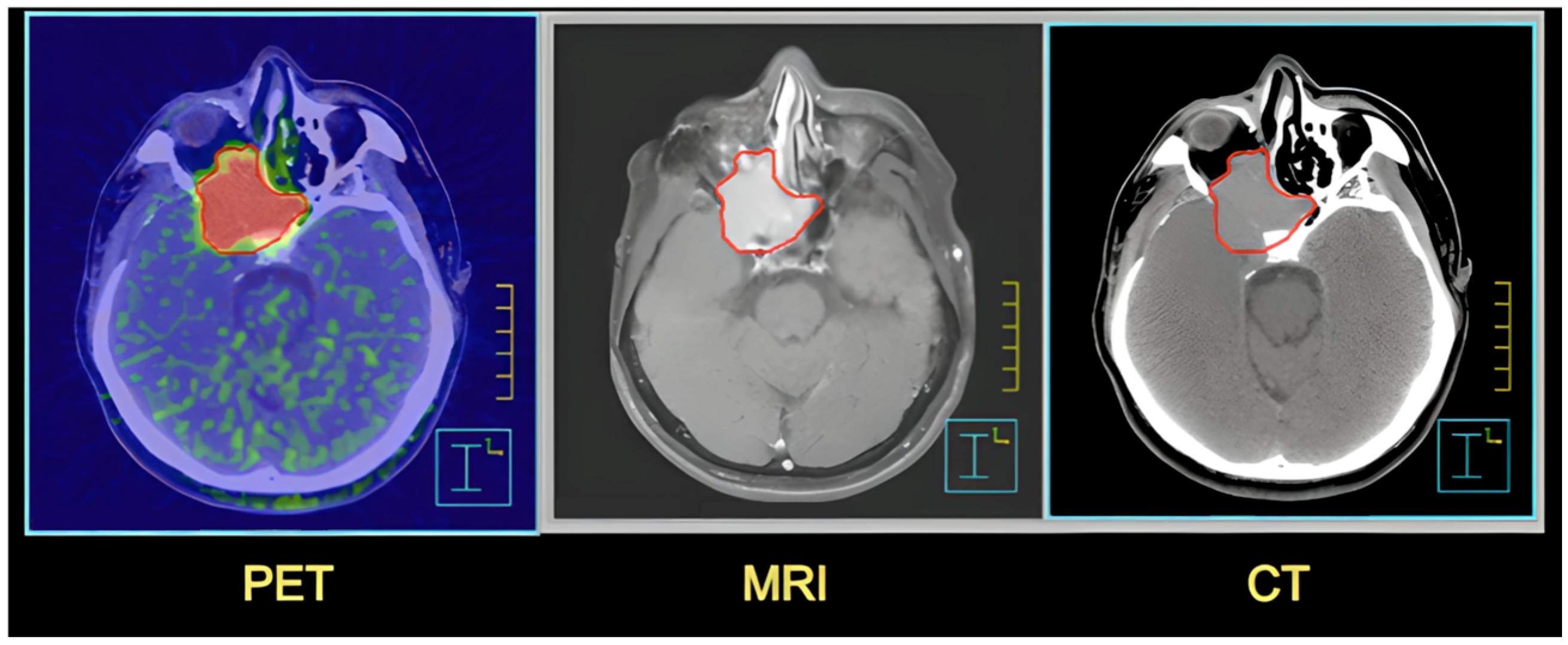
| Feature | CT | MRI and NMR | PET |
|---|---|---|---|
| Operation principle | X-rays | Magnetic field and radio waves | Radioactive tracer and detection of emitted positrons |
| Applications | Imaging of bone structures, injuries, in emergency situations | Imaging of soft tissues, organs, nervous system, joints (MRI); lab chemical analysis techniques (NMR) | Detection and assessment of metabolic activity in cells, e.g., tumors |
| Safety | Exposure to ionizing radiation | Safer-does not use ionizing radiation | Depends on the tracer used, but generally safe |
| Contraindications | Pregnancy, frequent exposure to radiation bodies | Medical implants, metallic foreign, pacemakers | Specific to the tracer used |
| Examination time | Short (a few to several minutes) | Longer (several to several dozen minutes) | Variable (depends on the examination) |
| Additional comments | Contrast agents: Iodine-based contrast agents are used in CT scans | Gadolinium-based compounds are used in MRI scans | Particularly useful in the diagnosis of oncological diseases, examinations are combined, PET/CT or PET/MRI, to obtain a more complete clinical picture |
| Source | Range | Frequency | Bandwidth |
|---|---|---|---|
| Brain (MEG) | 100 fT–1 pT | 0.5-500 Hz (clinically revelant < 70 Hz) | ~500 Hz/70 Hz |
| Heart (MCG) | 500–100 pT | <75 Hz | 75 Hz |
| Nerve (MNG) | 5 fT–8 pT | 6–500 Hz | 494 Hz |
| Spine (MSG) | 1–100 fT | 100–5000 Hz | 4900 Hz |
| Hand/Leg/Head/Muscle (MMG) | 1 fT–1 pT | 1–300 Hz | 300 Hz |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boiko, O.; Stryczewska, H.D. Superconductivity and Cryogenics in Medical Diagnostics and Treatment: An Overview of Selected Applications. Appl. Sci. 2025, 15, 12579. https://doi.org/10.3390/app152312579
Boiko O, Stryczewska HD. Superconductivity and Cryogenics in Medical Diagnostics and Treatment: An Overview of Selected Applications. Applied Sciences. 2025; 15(23):12579. https://doi.org/10.3390/app152312579
Chicago/Turabian StyleBoiko, Oleksandr, and Henryka Danuta Stryczewska. 2025. "Superconductivity and Cryogenics in Medical Diagnostics and Treatment: An Overview of Selected Applications" Applied Sciences 15, no. 23: 12579. https://doi.org/10.3390/app152312579
APA StyleBoiko, O., & Stryczewska, H. D. (2025). Superconductivity and Cryogenics in Medical Diagnostics and Treatment: An Overview of Selected Applications. Applied Sciences, 15(23), 12579. https://doi.org/10.3390/app152312579






