The Influence of the Fruit Maceration Method on pH, Vitamin C and Total Phenolic Contents, and Antioxidant Activity of Japanese Quince and Quince Tinctures
Abstract
1. Introduction
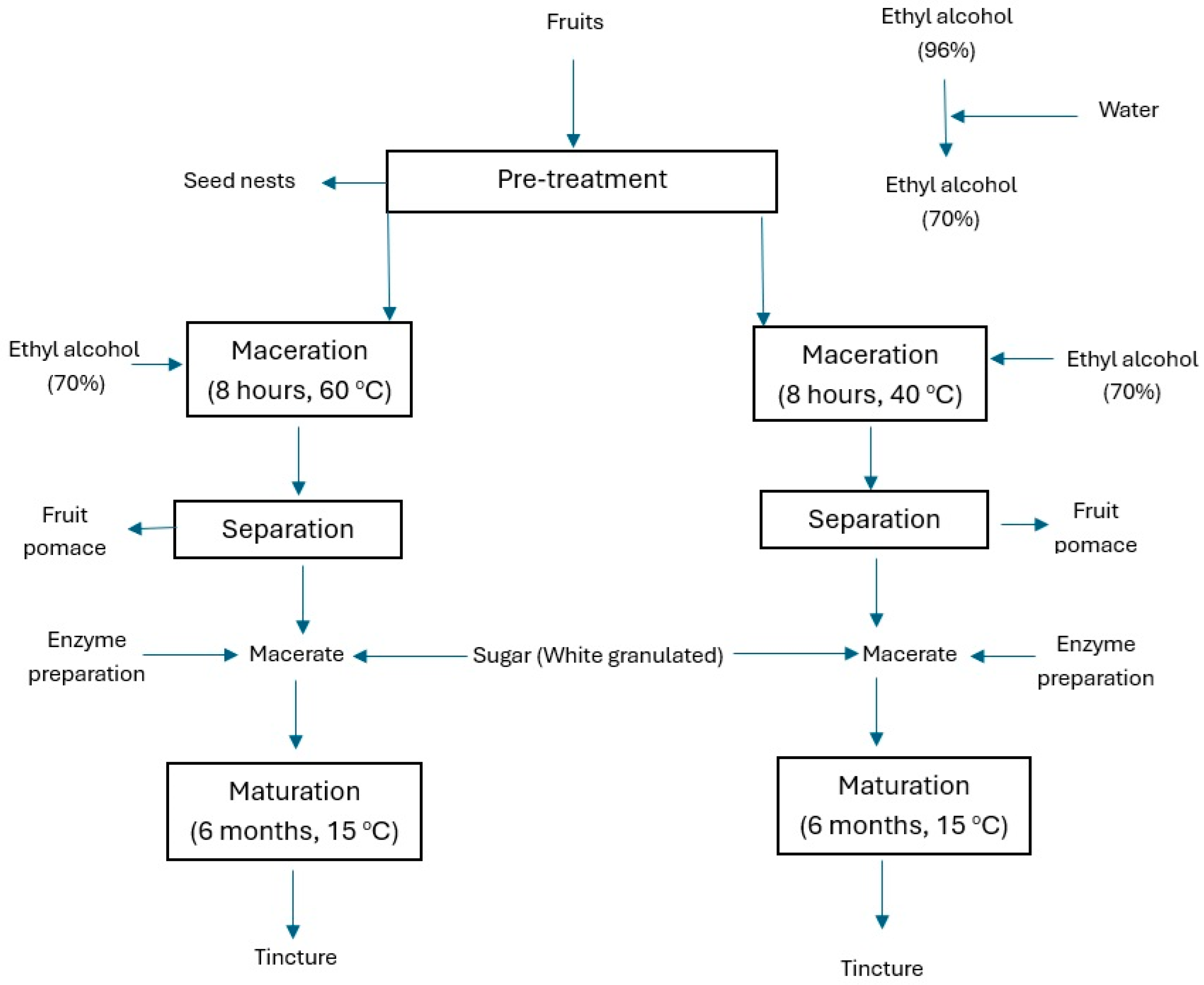
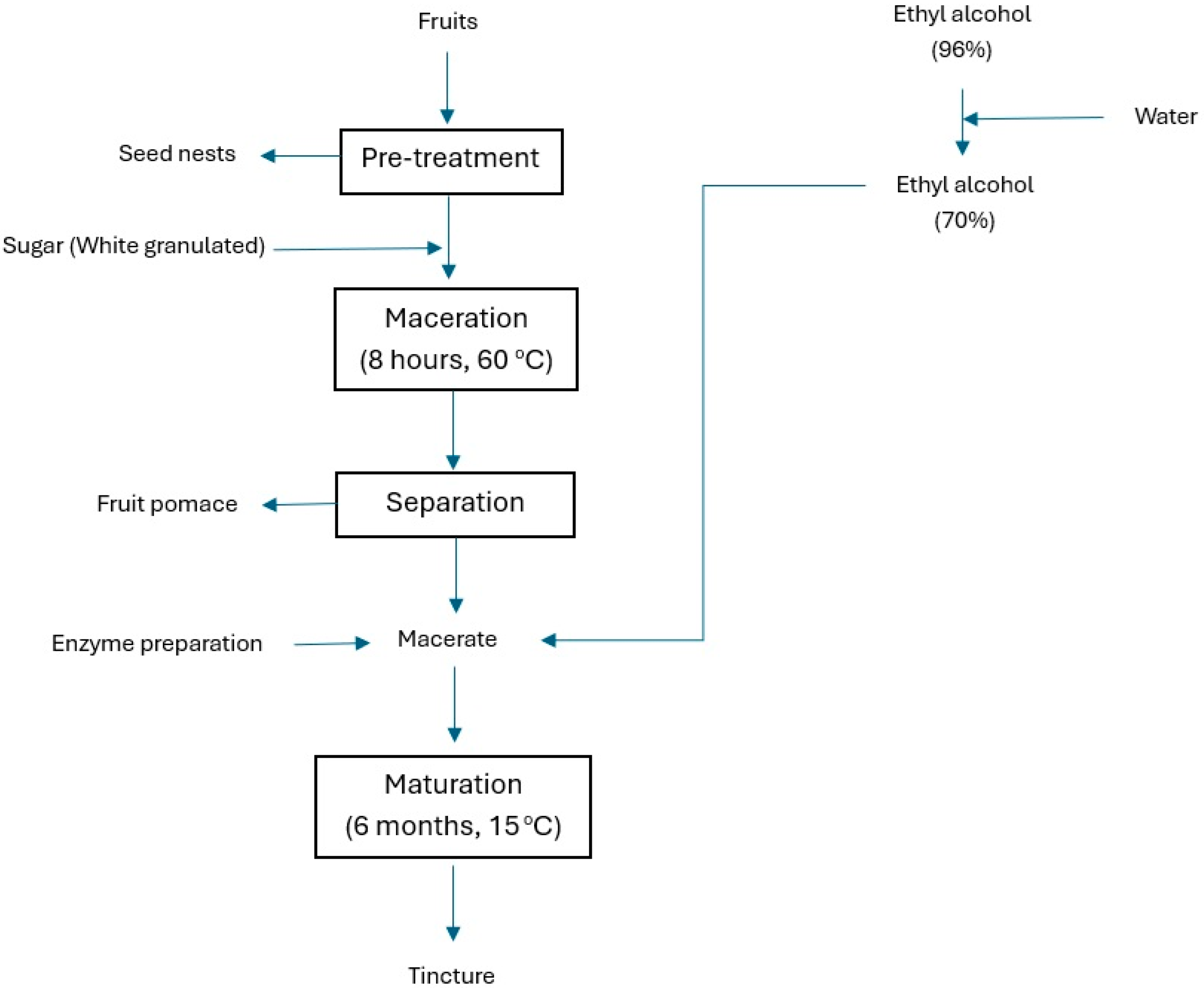
2. Materials and Methods
2.1. Chemicals
2.2. Material
2.3. Methods
2.3.1. pH Value
2.3.2. Vitamin C
2.3.3. Total Phenolic Compounds
2.3.4. DPPH Radical Scavenging Activity
2.3.5. Statistical Analysis
3. Results and Discussion
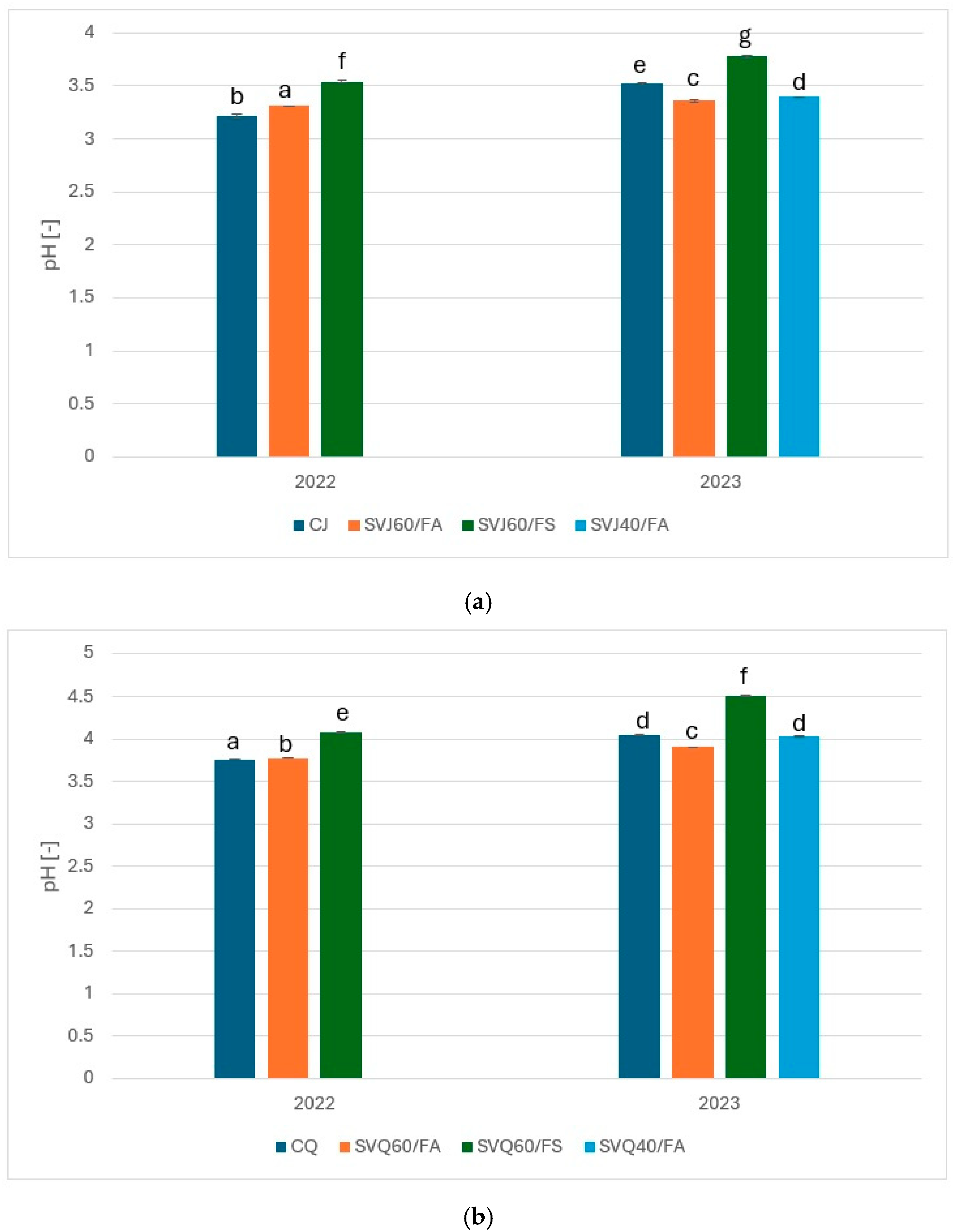
3.1. pH Value
3.2. Vitamin C Content
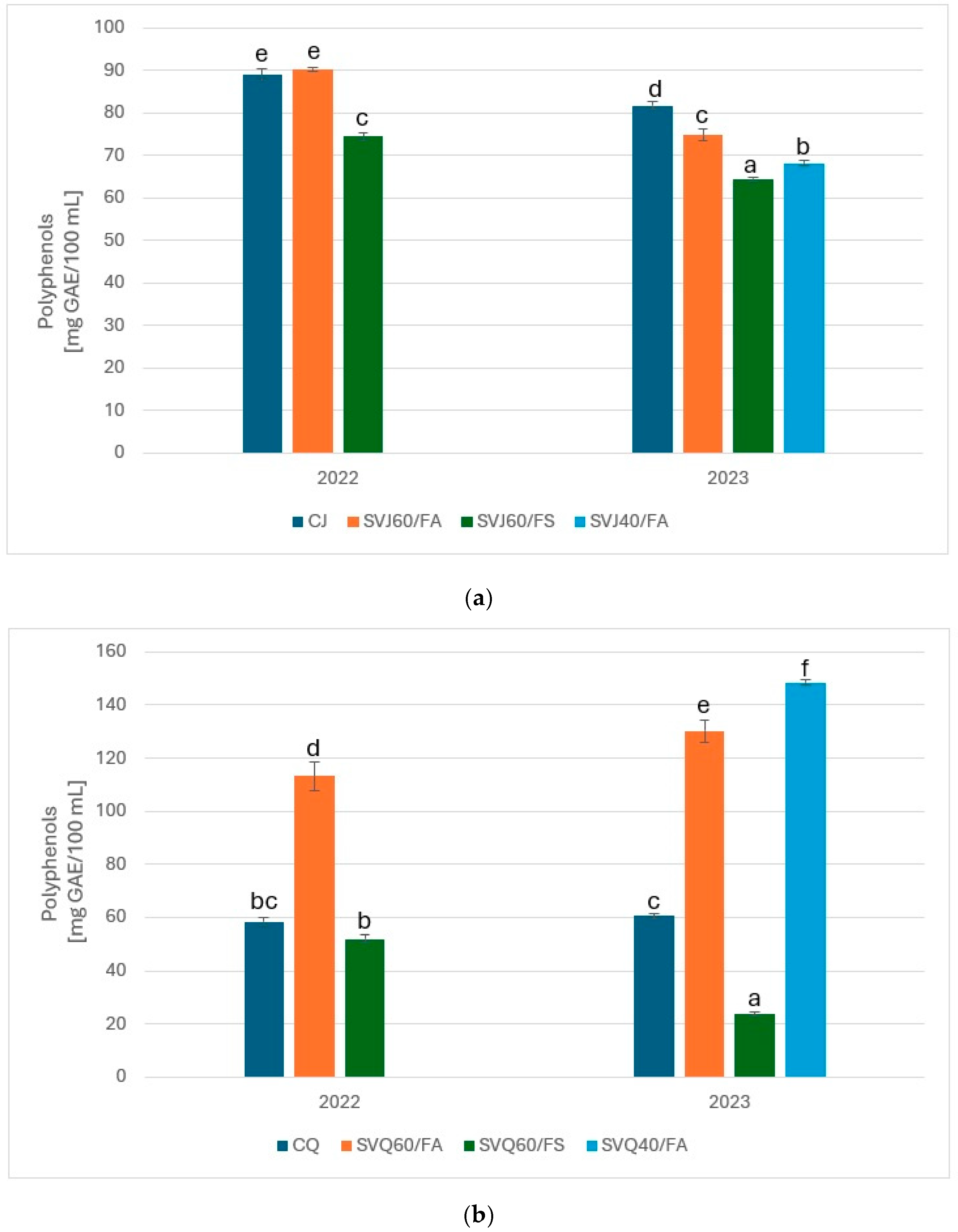
3.3. Total Polyphenol Content
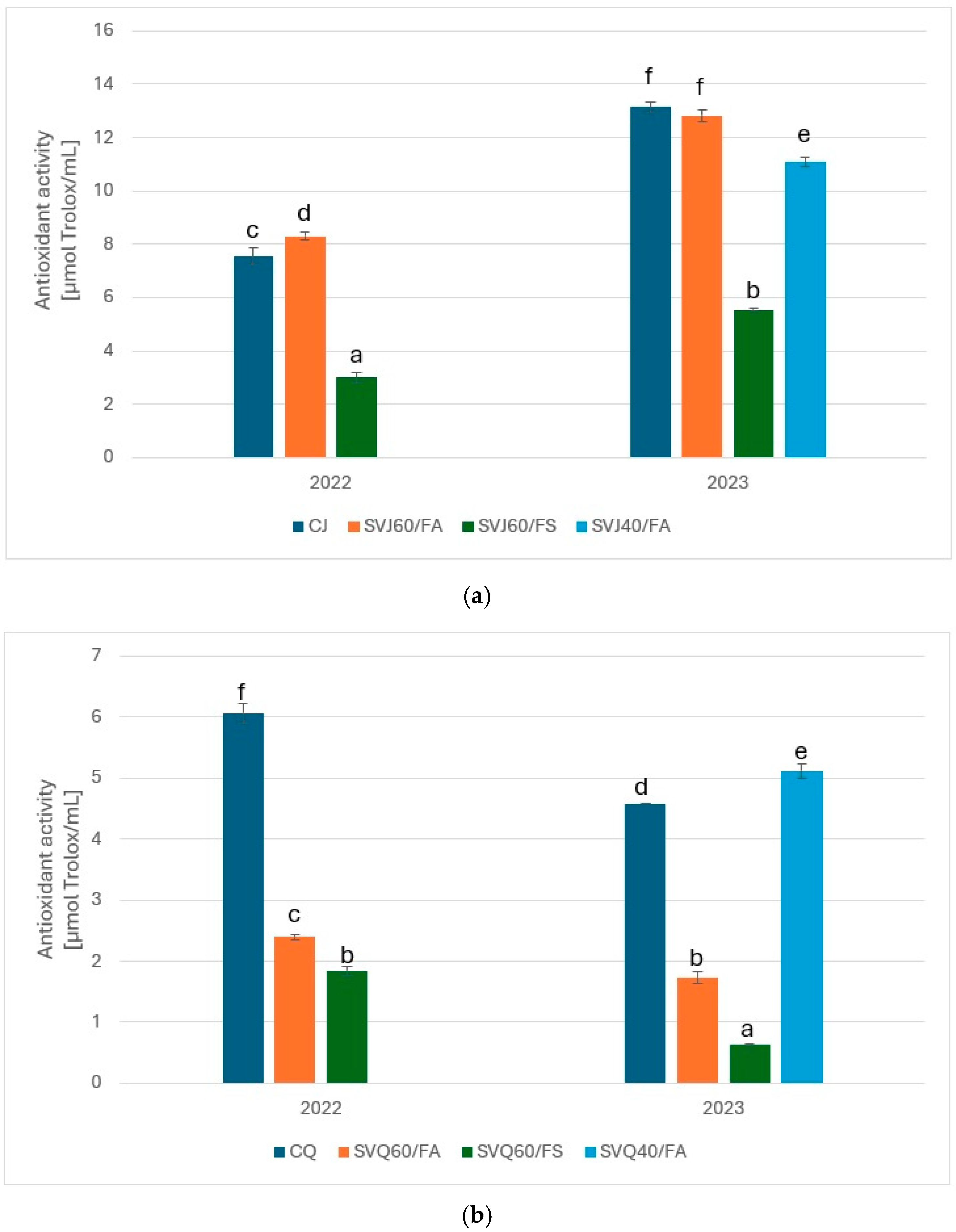
3.4. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Szydłowska, M.; Fiedoruk, A. Nalewki Domowe. Leksykon. Nalewki, Likiery, Miody; Wydawnictwo SBM Renata Gmitrzak: Warszawa, Poland, 2014; pp. 4–23. [Google Scholar]
- Śmiechowska, M.; Dmowski, P.; Skowierzak, L. Edible Flowers’ Antioxidant Properties and Polyphenols Content Reflect Their Applicability for Household and Craft Tincture Production. Appl. Sci. 2021, 11, 10095. [Google Scholar] [CrossRef]
- Regulation of the Minister of Agriculture and Rural Development of 14 February 2024 (Rozporządzenie Ministra Rolnictwa i Rozwoju Wsi z Dnia 14 Lutego 2024 r. Zmieniające Rozporządzenie w Sprawie Znakowania Poszczególnych Rodzajów Środków Spożywczych. Dziennik Ustaw Rzeczypospolitej Polskiej, Poz. 217, Warszawa, Dnia 19 Lutego 2024 r.). Available online: https://dziennikustaw.gov.pl/D2024000021701.pdf (accessed on 3 September 2025).
- Regulation (EU) 2019/787 of the European Parliament and of the Council of 17 April 2019 on the Definition, Description, Presentation and Labelling of Spirit Drinks, the Use of the Names of Spirit Drinks in the Presentation and Labelling of Other Foodstuffs, the Protection of Geographical Indications for Spirit Drinks, the Use of Ethyl Alcohol and Distillates of Agricultural Origin in Alcoholic Beverages, and Repealing Regulation (EC) No 110/2008. Available online: https://eur-lex.europa.eu/eli/reg/2019/787/oj/eng (accessed on 3 September 2025).
- Vacca, V.; Piga, A.; Del Caro, A.; Fenu, P.A.; Agabbio, M. Changes in phenolic compounds, colour and antioxidant activity in industrial red myrtle liqueurs during storage. Nahrung 2003, 47, 442–447. [Google Scholar] [CrossRef]
- Śliwińska, M.; Wiśniewska, P.; Dymerski, T.; Wardencki, W.; Namieśnik, J. The flavour of fruit spirits and fruit liqueurs: A review. Flavour Fragr. J. 2015, 30, 197–207. [Google Scholar] [CrossRef]
- Sokół-Łętowska, A.; Kucharska, A.Z.; Szumny, A.; Wińska, K.; Nawirska-Olszańska, A. Phenolic Composition Stability and Antioxidant Activity of Sour Cherry Liqueurs. Molecules 2018, 23, 2156. [Google Scholar] [CrossRef]
- Polak, J.; Bartoszek, M.; Bernat, R. Comprehensive comparison of antioxidant properties of tinctures. Sci. Rep. 2019, 9, 6148. [Google Scholar] [CrossRef]
- Polak, J.; Bartoszek, M. The study of antioxidant capacity of varieties of nalewka, a traditional Polish fruit liqueur, using EPR, NMR and UV-vis spectroscopy. J. Food Compos. Anal. 2015, 40, 114–119. [Google Scholar] [CrossRef]
- Śliwińska, M.; Wiśniewska, P.; Dymerski, T.; Wardencki, W.; Namieśnik, J. Application of Electronic Nose Based on Fast GC for Authenticity Assessment of Polish Homemade Liqueurs Called Nalewka. Food Anal. Methods 2016, 9, 2670–2681. [Google Scholar] [CrossRef]
- Jakopic, J.; Colaric, M.; Veberic, R.; Hudina, M.; Solar, A.; Stampar, F. How much do cultivar and preparation time influence on phenolics content in walnut liqueur? Food Chem. 2007, 104, 100–105. [Google Scholar] [CrossRef]
- Kucharska, A.Z.; Sokół-Łętowska, A.; Hudko, J.; Nawirska, A. Influence of the preparation procedure on the antioxidant activity and colour of liqueurs from cornelian cherry (Cornus mas L.). Pol. J. Food Nutr. Sci. 2007, 57, 343–347. [Google Scholar]
- Sokół-Łętowska, A.; Kucharska, A.Z.; Wińska, K.; Szumny, A.; Nawirska-Olszańska, A.; Mizgier, P.; Wyspiańska, D. Composition and antioxidant activity of red fruit liqueurs. Food Chem. 2014, 157, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Duchnik, W.; Muzykiewicz-Szymańska, A.; Kucharski, Ł.; Zielonka-Brzezicka, J.; Nowak, A.; Klimowicz, A. The Changes of Antioxidant Activity of Three Varieties of ‘Nalewka’, a Traditional Polish Fruit Alcoholic Beverage during Long-Term Storage. Appl. Sci. 2023, 13, 1114. [Google Scholar] [CrossRef]
- Marat, N.; Danowska-Oziewicz, M.; Narwojsz, A. Chaenomeles Species-Characteristics of Plant, Fruit and Processed Products: A Review. Plants 2022, 11, 3036. [Google Scholar] [CrossRef]
- Narwojsz, A.; Borowska, E.J.; Borowski, J.; Piłat, B. Ekstraktywność składników bioaktywnych w procesie otrzymywania nalewki z pigwy. Bromat. Chem. Toksykol. 2012, XLV, 567–572. [Google Scholar]
- Carbonell-Barrachina, Á.A.; Szychowski, P.J.; Vásquez, M.V.; Hernández, F.; Wojdyło, A. Technological aspects as the main impact on quality of quince liquors. Food Chem. 2015, 167, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Mudura, E.; Coldea, T.E.; Fărcaş, A. Quince peel extract addition to liqueur for improving antioxidant activity and phenolic content. Hop Med. Plants 2016, 24, 63–70. [Google Scholar] [CrossRef]
- Hellín, P.; Vila, R.; Jordán, M.J.; Laencina, J.; Rumpunen, K.; Ros, J.M. Characteristics and composition of Chaenomeles fruit juice. In Japanese Quince-Potential Fruit Crop for Northern Europe; Rumpunen, K., Ed.; Swedish University of Agricultural Sciences: Alnarp, Sweden, 2003; pp. 127–139. [Google Scholar]
- Jordán, M.J.; Vila, R.; Hellin, P.; Laencina, J.; Rumpunem, K.; Ros, J.M. Volatile compounds associated with the fragrance and flavour of Chaenomeles juice. In Japanese Quince-Potential Fruit Crop for Northern Europe; Rumpunen, K., Ed.; Swedish University of Agricultural Sciences: Alnarp, Sweden, 2003; pp. 149–157. [Google Scholar]
- Yang, L.; Ahmed, S.; Stepp, J.R.; Zhao, Y.; Zeng, M.J.; Pei, S.; Xue, D.; Xu, G. Cultural Uses, Ecosystem Services, and Nutrient Profile of Flowering Quince (Chaenomeles speciosa) in the Highlands of Western Yunnan, China. Econ. Bot. 2015, 69, 273–283. [Google Scholar] [CrossRef]
- Tarko, T.; Duda-Chodak, A.; Satora, P.; Sroka, P.; Pogoń, P.; Machalica, J. Chaenomeles japonica, Cornus mas, Morus nigra fruits characteristics and their processing potential. J. Food Sci. Technol. 2014, 51, 3934–3941. [Google Scholar] [CrossRef]
- Rumpunen, K. Chaenomeles: Potential new fruit crop for northern Europe. In Trends in New Crops and New Uses; Janick, J., Whipkey, A., Eds.; ASHS Press: Alexandria, Egypt, 2002; pp. 385–392. [Google Scholar]
- Zhang, S.Y.; Han, L.Y.; Zhang, H.; Xin, H.L. Chaenomeles speciosa: A review of chemistry and pharmacology. Biomed. Rep. 2014, 2, 12–18. [Google Scholar] [CrossRef]
- Strugała, P.; Cyboran-Mikołajczyk, S.; Dudra, A.; Mizgier, P.; Kucharska, A.Z.; Olejniczak, T.; Gabrielska, J. Biological Activity of Japanese Quince Extract and Its Interactions with Lipids, Erythrocyte Membrane, and Human Albumin. J. Membr. Biol. 2016, 249, 393–410. [Google Scholar] [CrossRef]
- Urbanavičiūtė, I.; Rubinskiene, M.; Viškelis, P. The Fatty Acid Composition and Quality of Oils from Post-Industrial Waste of Quince Chaenomeles japonica. Chem. Biodivers. 2019, 16, e1900352. [Google Scholar] [CrossRef]
- Seglina, D.; Krasnova, I.; Heidemane, G.; Ruisa, S. Influence of drying technology on the quality of dried candied Chaenomeles japonica during storage. Latvian J. Agron. 2009, 12, 113–118. [Google Scholar]
- Du, H.; Wu, J.; Li, H.; Zhong, P.X.; Xu, Y.J.; Li, C.H.; Ji, K.X.; Wang, L.S. Polyphenols and triterpenes from Chaenomeles fruits: Chemical analysis and antioxidant activities assessment. Food Chem. 2013, 141, 4260–4268. [Google Scholar] [CrossRef]
- Bieniasz, M.; Dziedzic, E.; Kaczmarczyk, E. The effect of storage and processing on vitamin C content in Japanese quince fruit. Folia Hort. 2017, 29, 83–93. [Google Scholar] [CrossRef]
- Thomas, M.; Guillemin, F.; Guillon, F.; Thibault, J.F. Pectins in the fruits of Japanese quince (Chaenomeles japonica). Carbohydr. Polym. 2003, 53, 361–372. [Google Scholar] [CrossRef]
- Khoubnasabjafari, M.; Jouyban, A. A review of phytochemistry and bioactivity of quince (Cydonia oblonga Mill.). J. Med. Plants Res. 2011, 5, 3577–3594. [Google Scholar]
- Sajid, S.M.; Zubair, M.; Waqas, M.; Nawaz, M.; Ahmad, Z. A Review on Quince (Cydonia oblonga): A Useful Medicinal Plant. Glob. Vet. 2015, 14, 517–524. [Google Scholar]
- Rasheed, M.; Hussain, I.; Rafiq, S.; Hayat, I.; Qayyum, A.; Ishaq, S.; Awan, M. Chemical composition and antioxidant activity of quince fruit pulp collected from different locations. Int. J. Food Prop. 2018, 21, 2320–2327. [Google Scholar] [CrossRef]
- Baroni, M.; Gastaminza, J.; Podio, N.S.; Lingua, M.S.; Wunderlin, D.A.; Rovasio, J.L.; Dotti, R.; Rosso, J.C.; Ghione, S.; Ribotta, P.D. Changes in the Antioxidant Properties of Quince Fruit (Cydonia oblonga Miller) during Jam Production At Industrial Scale. J. Food Qual. 2018, 2018, 1460758. [Google Scholar] [CrossRef]
- Majeed, T.; Wani, I.; Muzzaffar, S. Postharvest Biology and Technology of Quince. In Postharvest Biology and Technology of Temperate Fruits; Mir, S., Shah, M., Mir, M., Eds.; Springer: Cham, Switzerland, 2018; pp. 273–284. [Google Scholar]
- Papp, N.; Szabó, T.; Szabó, Z.; Nyéki, J.; Stefanovits-Bányai, É.; Hegedüs, A. Antioxidant capacity and total polyphenolic content in quince (Cydonia oblonga Mill.) fruit. Int. J. Hortic. Sci. 2013, 19, 33–35. [Google Scholar] [CrossRef]
- Moradi, S.; Saba, M.K.; Mozafari, A.A.; Abdollahi, H. Antioxidant Bioactive Compounds Changes in Fruit of Quince Genotypes Over Cold Storage. J. Food Sci. 2016, 81, H1833–H1839. [Google Scholar] [CrossRef]
- Hadi, S.T.; Fadhil, N.J.; Khalaf, A.S.; Alhadithi, H.J. Extraction of pectin from quince (Cydonia oblonga) fruit husk and using it in jam industry. Biochem. Cell. Arch. 2020, 20, 2163–2166. [Google Scholar]
- Baldwin, D.E. Sous vide cooking: A review. Int. J. Gastron. Food Sci. 2012, 1, 15–30. [Google Scholar] [CrossRef]
- Onyeaka, H.; Nwaizu, C.-C.; Ekaette, I. Mathematical modelling for thermally treated vacuum-packaged foods: A review on sous vide processing. Trends Food Sci. Tech. 2022, 126, 73–85. [Google Scholar] [CrossRef]
- Huarte, E.; Trius-Soler, M.; Dominguez-Fernández, M.; De Peña, M.-P.; Cid, C. (Poly)phenol characterization in white and red cardoon stalks: Could the sous-vide technique improve their bioaccessibility? Int. J. Food Sci. Nutr. 2022, 73, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Narwojsz, A.; Sawicki, T.; Piłat, B.; Tańska, M. Effect of heat treatment methods on Color, Bioactive Compound Content, and Antioxidant Capacity of Carrot Root. Appl. Sci. 2024, 15, 254. [Google Scholar] [CrossRef]
- Dordevic, D.; Kalcakova, L.; Lankovova, A.; Dordevic, S.; Pospiech, M.; Tremlova, B.; Kushkevych, I. Application of Sous-Vide Technology in the Processing of Different Apple Cultivars and Its Effect on Physico-chemical Properties. Eur. Food Res. Technol. 2025, 251, 15–19. [Google Scholar] [CrossRef]
- Kosewski, G.; Chanaj-Kaczmarek, J.; Dziedzic, K.; Jakubowski, K.; Lisiak, N.; Przysławski, J.; Drzymała-Czyż, S. Changes in Polyphenolic profile and Antioxidant Properties of Selected Raw and processed vegetables Under Different Cooking Methods. Appl. Sci. 2025, 15, 4677. [Google Scholar] [CrossRef]
- Alamprese, C.; Pompei, C.; Scaramuzzi, F. Characterization and antioxidant activity of nocino liqueur. Food Chem. 2005, 90, 495–502. [Google Scholar] [CrossRef]
- Szydłowska, M. Atlas Nalewek Polskich; Wydawnictwo SBM Renata Gmitrzak: Warszawa, Poland, 2022. [Google Scholar]
- Gökmen, V.; Kahraman, N.; Demir, N.; Acar, J. Enzymatically validated liquid chromatographic method for the determination of ascorbic and dehydroascorbic acids in fruit and vegetables. J. Chromatogr. A 2000, 881, 309–316. [Google Scholar] [CrossRef]
- Borowska, E.J.; Mazur, B.; Gadzała-Kopciuch, R.; Buszewski, B. Polyphenol, anthocyanin and resveratrol mass fractions and antioxidant properties of cranberry cultivars. Food Technol. Biotechnol. 2009, 47, 56–61. [Google Scholar]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Jarczyk, A.; Płocharski, W. Skład chemiczny owoców, warzyw i grzybów i ich znaczenie żywieniowe. In Technologia Produktów Owocowych i Warzywnych; Wydawnictwo Wyższej Szkoły Ekonomiczno-Humanistycznej im. Prof. Szczepana A. Pieniążka: Skierniewice, Poland, 2010; pp. 22–52. [Google Scholar]
- Moura, T.; Gaudy, D.; Jacob, M.; Cassanas, G. pH Influence on the Stability of Ascorbic Acid Spray-Drying Solutions. Pharm. Acta Helv. 1994, 69, 77–80. [Google Scholar] [CrossRef]
- Rojas, A.M.; Gerschenson, L.N. Ascorbic Acid Destruction in Aqueous Model Systems: An Additional Discussion. J. Sci. Food Agric. 2001, 81, 1433–1439. [Google Scholar] [CrossRef]
- Rahman, M.S.; Rahman, M.R.T. pH in Food Preservation. In Handbook of Food Preservation; Rahman, M.S., Ed.; CRC Press: Boca Raton, FL, USA, 2020; pp. 323–332. [Google Scholar]
- Kalemba-Drożdż, M.; Kwiecień, I.; Szewczyk, A.; Cierniak, A.; Grzywacz-Kisielewska, A. Fermented Vinegars from Apple Peels, Raspberries, Rosehips, Lavender, Mint, and Rose Petals: The Composition, Antioxidant Power, and Genoprotective Abilities in Comparison to Acetic Macerates, Decoctions, and Tinctures. Antioxidants 2020, 9, 1121. [Google Scholar] [CrossRef]
- Tabaszewska, M.; Najgebauer-Lejko, D. The content of selected phytochemicals and in vitro antioxidant properties of rose hip (Rosa canina L.) tinctures. NFS J. 2020, 21, 50–56. [Google Scholar] [CrossRef]
- Janda, K.; Kasprzak, M.; Wolska, J. Witamina C-budowa, właściwości, funkcje i występowanie. Pom. J. Life Sci. 2015, 61, 419–425. [Google Scholar] [CrossRef]
- Kazimierczak, R.; Kopczyńska, K.; Ponder, A.; Hallmann, E.; Zebrowska-Krasuska, M.; Srednicka-Tober, D. The Concentrations of Phenolic Compounds and Vitamin C in Japanese Quince (Chaenomeles japonica) Preserves. Foods 2025, 14, 1369. [Google Scholar] [CrossRef]
- Yin, X.; Chen, K.; Cheng, H.; Chen, X.; Feng, S.; Song, Y.; Liang, L. Chemical Stability of Ascorbic Acid Integrated into Commercial Products: A Review on Bioactivity and Delivery Technology. Antioxidants 2022, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Verbeyst, L.; Bogaerts, R.; Van der Plancken, I.; Hendrickx, M.; Van Loey, A. Modelling of Vitamin C Degradation during Thermal and High-Pressure Treatments of Red Fruit. Food Bioproc. Tech. 2013, 6, 1015–1023. [Google Scholar] [CrossRef]
- Jiang, T.; Zheng, X.; Li, J.; Jing, G.; Cai, L.; Ying, T. Integrated Application of Nitric Oxide and Modified Atmosphere Packaging to Improve Quality Retention of Button Mushroom (Agaricus bisporus). Food Chem. 2011, 126, 1693–1699. [Google Scholar] [CrossRef] [PubMed]
- Chetti, M.B.; Deepa, G.T.; Antony, R.T.; Khetagoudar, M.C.; Uppar, D.S.; Navalgatti, C.M. Influence of Vacuum Packaging and Long Term Storage on Quality of Whole Chilli (Capsicum annuum L.). J. Food Sci. Technol. 2014, 51, 2827–2832. [Google Scholar] [CrossRef]
- Lee, S.K.; Kader, A.A. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol. Technol. 2000, 20, 207–220. [Google Scholar] [CrossRef]
- Silva, B.M.; Andrade, P.B.; Martins, R.C.; Valentao, P.; Ferreres, F.; Seabra, R.M.; Ferreira, M.A. Quince (Cydonia oblonga Miller) fruit characterization using principal component analysis. J. Agric. Food Chem. 2004, 52, 8044–8051. [Google Scholar] [CrossRef]
- Serreli, G.; Jerković, I.; Gil, K.A.; Marijanović, Z.; Pacini, V.; Tuberoso, C.I.G. Phenolic Compounds, Volatiles and Antioxidant Capacity of White Myrtle Berry Liqueurs. Plant Foods Hum. Nutr. 2017, 72, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Sokół-Łętowska, A.; Kucharska, A.Z.; Nawirska-Olszańska, A. Wpływ dodatku cukru na jakość nalewek wiśniowych podczas przechowywania. Uniwersytet Ekonomiczny w Poznaniu. Zesz. Nauk. 2011, 205, 124–132. [Google Scholar]
- Wilk, M.; Seruga, P.; Nowicka, P. Effect of Processing Parameters on the Content of Bioactive Compounds of Prunus spinosa L. Fruit Tinctures. Foods 2025, 14, 3200. [Google Scholar] [CrossRef]
- Thoo, Y.Y.; Ho, S.K.H.; Liang, J.Y.; Ho, C.W.; Tan, C.P. Effects of binary solvent extraction system, extraction time and extraction temperature on phenolic antioxidants and antioxidant capacity from mengkudu (Morinda citrifolia). Food Chem. 2010, 120, 290–295. [Google Scholar] [CrossRef]
- Sokół-Łętowska, A. Związki Fenolowe w Nalewkach z Wybranych Owoców; Monografia; Wydawnictwo Uniwersytetu Przyrodniczego we Wrocławiu: Wrocław, Poland, 2013. [Google Scholar]
- Yu, L.; Wu, Y.; Liu, D.; Sheng, Z.; Liu, J.; Chen, H.; Feng, W. The kinetic behavior of antioxidant activity and the stability of aqueous and organic polyphenol extracts from navel orange peel. Food Sci. Technol. 2022, 42, e90621. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Wianowska, D.; Baraniak, B. The antioxidant properties of alcoholic extracts from Sambucus nigra L. (antioxidant properties of extracts). LWT-Food Sci. Technol. 2006, 39, 308–315. [Google Scholar] [CrossRef]
- Liyana-Pathirana, C.; Shahidi, F. Optimization of extraction of phenolic compounds from wheat using response surface methodology. Food Chem. 2005, 93, 47–56. [Google Scholar] [CrossRef]
- Sun, G.; Wang, J.; Wang, N.; Zhao, S.; Zhao, Y.; Yan, Y.; Tang, S.; Li, Z. Effects of Various Physical Field-Assisted Techniques and Combination methods on Extraction of Flavonoids and Polyphenols from Angelica keiskei. Sep. Sci. Plus 2015, 8, e70000. [Google Scholar] [CrossRef]
- Meregalli, M.M.; Puton, B.M.S.; Dal’Maso Camera, F.; Amaral, A.U.; Zeni, J.; Cansian, R.L.; Mignoni, M.L.; Backes, G.T. Conventional and ultrasound-assisted methods for extraction of bioactive compounds from red araçá peel (Psidium cattleianum Sabine). Arab. J. Chem. 2020, 13, 5800–5809. [Google Scholar] [CrossRef]
- Yunusova, H.M.; Turdieva, Z.V.; Ilkhamova, N.B. Development of Modern technology for Obtaining Tinctures with Sedative Effect. Cardiometry 2021, 21, 90–94. [Google Scholar] [CrossRef]
- Edo, G.I.; Nwachukwu, S.C.; Ali, A.B.M.; Yousif, E.; Jikah, A.N.; Zainulabdeen, K.; Ekokotu, H.A.; Isoje, E.F.; Igbuku, U.A.; Opiti, R.A.; et al. A Review on the Composition, Extraction and Applications of Phenolic Compounds. Ecol. Front. 2025, 45, 7–23. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marat, N.; Narwojsz, A.; Polak-Śliwińska, M.; Danowska-Oziewicz, M. The Influence of the Fruit Maceration Method on pH, Vitamin C and Total Phenolic Contents, and Antioxidant Activity of Japanese Quince and Quince Tinctures. Appl. Sci. 2025, 15, 12506. https://doi.org/10.3390/app152312506
Marat N, Narwojsz A, Polak-Śliwińska M, Danowska-Oziewicz M. The Influence of the Fruit Maceration Method on pH, Vitamin C and Total Phenolic Contents, and Antioxidant Activity of Japanese Quince and Quince Tinctures. Applied Sciences. 2025; 15(23):12506. https://doi.org/10.3390/app152312506
Chicago/Turabian StyleMarat, Natalia, Agnieszka Narwojsz, Magdalena Polak-Śliwińska, and Marzena Danowska-Oziewicz. 2025. "The Influence of the Fruit Maceration Method on pH, Vitamin C and Total Phenolic Contents, and Antioxidant Activity of Japanese Quince and Quince Tinctures" Applied Sciences 15, no. 23: 12506. https://doi.org/10.3390/app152312506
APA StyleMarat, N., Narwojsz, A., Polak-Śliwińska, M., & Danowska-Oziewicz, M. (2025). The Influence of the Fruit Maceration Method on pH, Vitamin C and Total Phenolic Contents, and Antioxidant Activity of Japanese Quince and Quince Tinctures. Applied Sciences, 15(23), 12506. https://doi.org/10.3390/app152312506




