Biotextronics System for the Prevention and Treatment of Lower Urinary Tract Infections
Abstract
1. Introduction
1.1. Lower Urinary Tract Inflammation
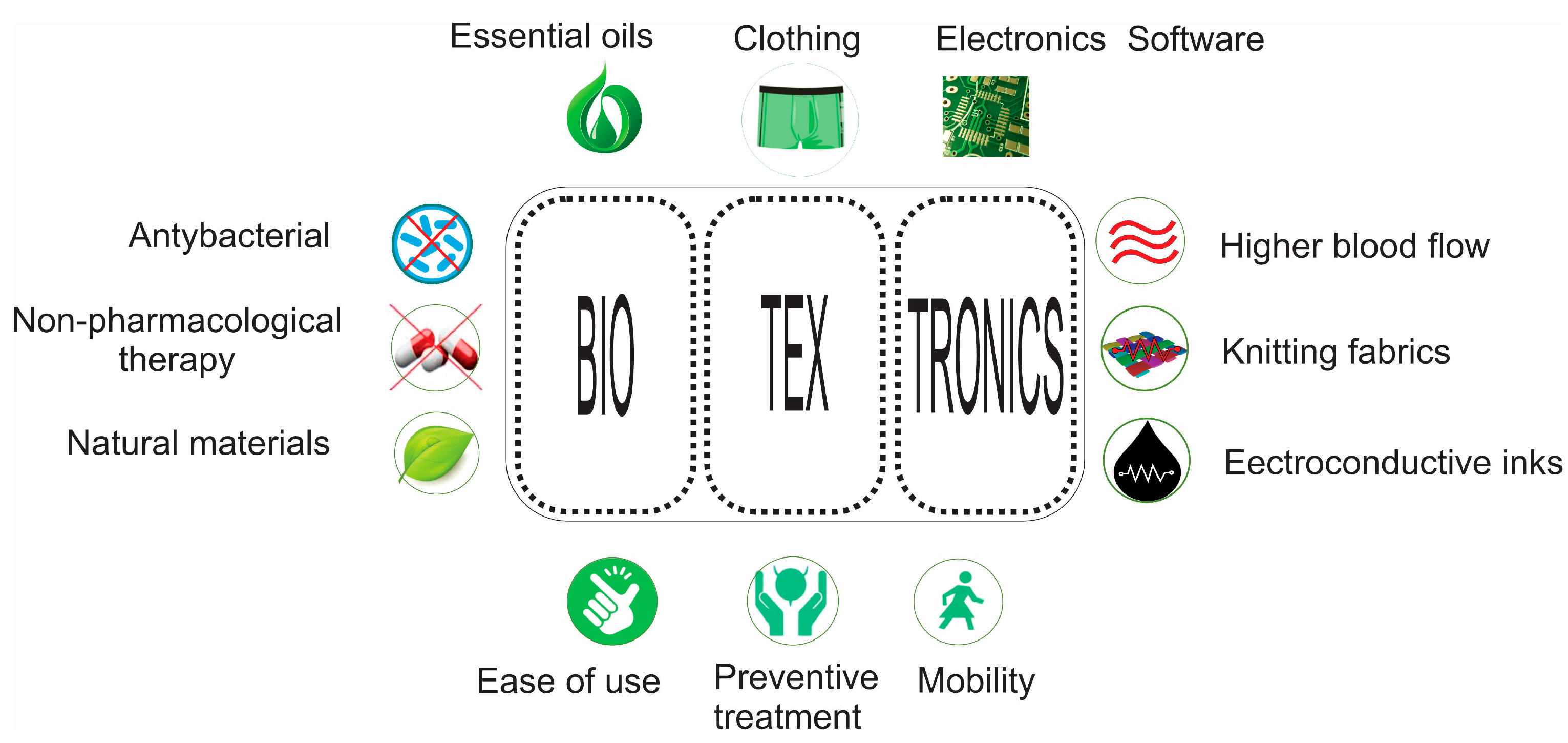
1.2. Textronics and Biotextronics in Healthcare
- High flexibility;
- Lightness;
- Resistance to mechanical and operational exposure;
- Resistance to moisture (sweat, washing);
- Resistance to weather conditions (variable temperature, rain, humidity).
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Gas Chromatography–Mass Spectrometry (GC-MS)
2.2.2. Model Insert Preparation
2.2.3. Solid Phase Microextraction (SPME)
- Temperature: 40 °C;
- Conditioning time (taq): 5 and 10 min;
- Extraction time (tex): 20 min;
- No stirring;
- Fiber: DVB/CAR/PDMS (ternary phase; Sigma-Aldrich, USA).
3. Results
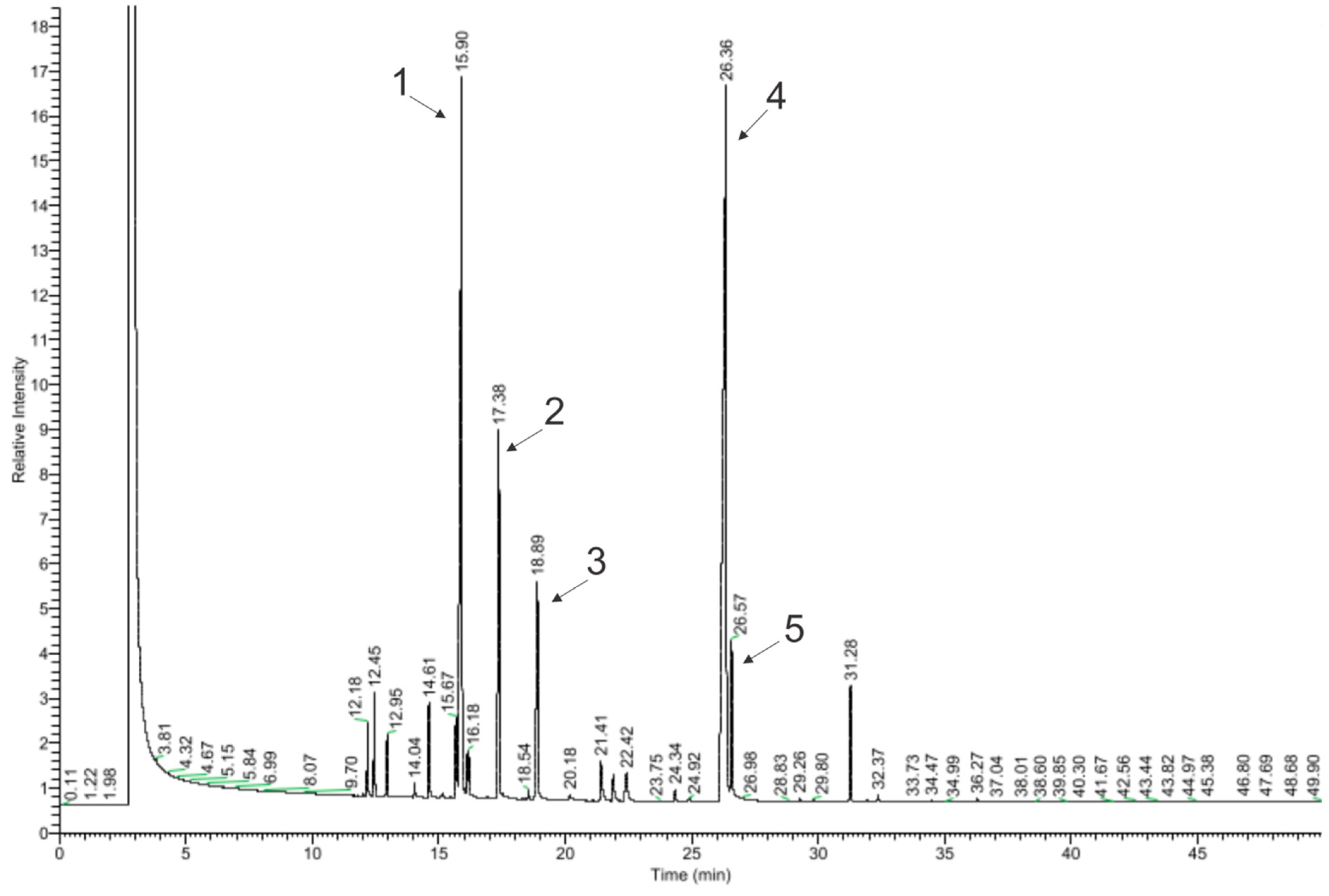
3.1. Chemical Compounds
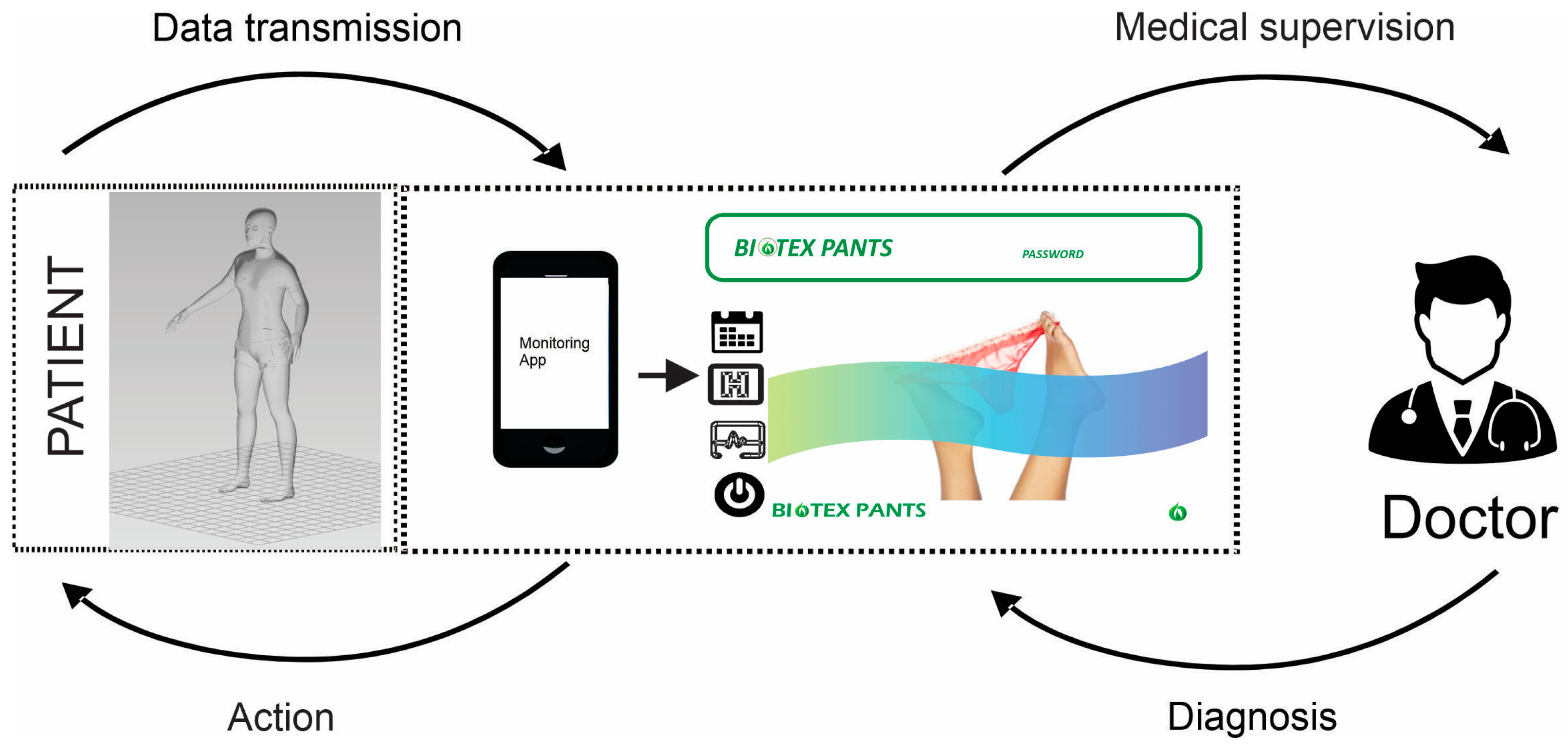
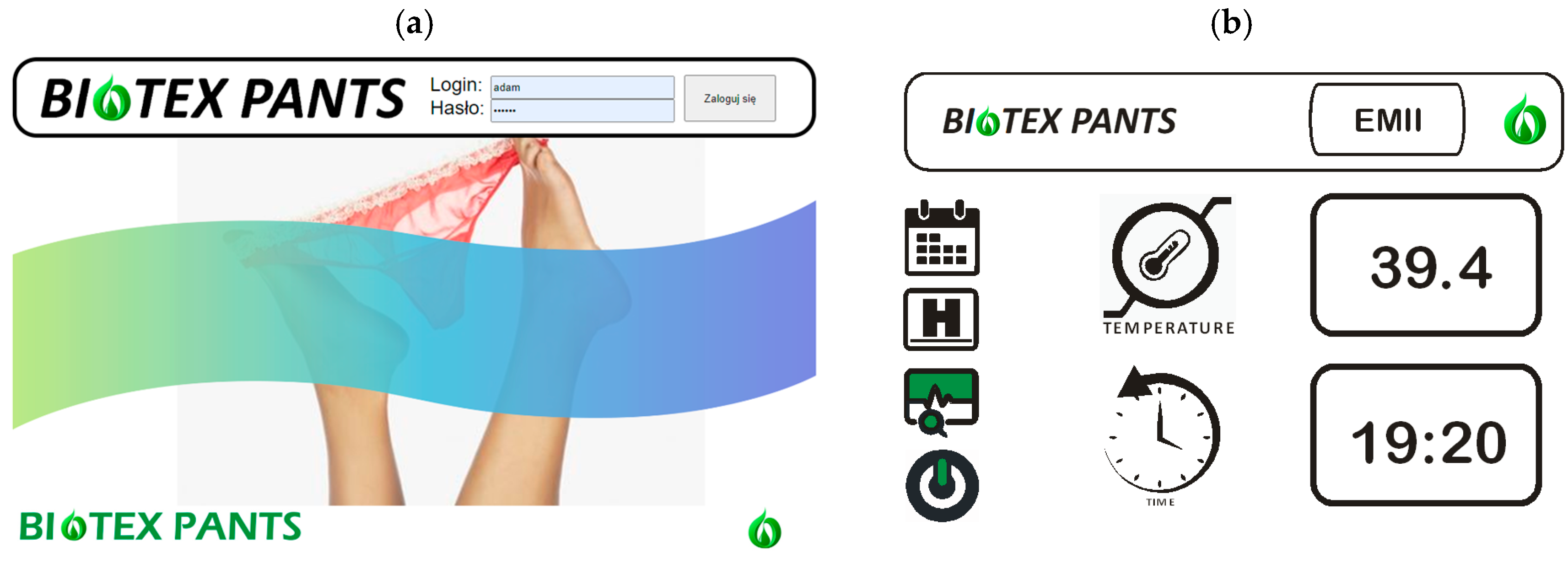
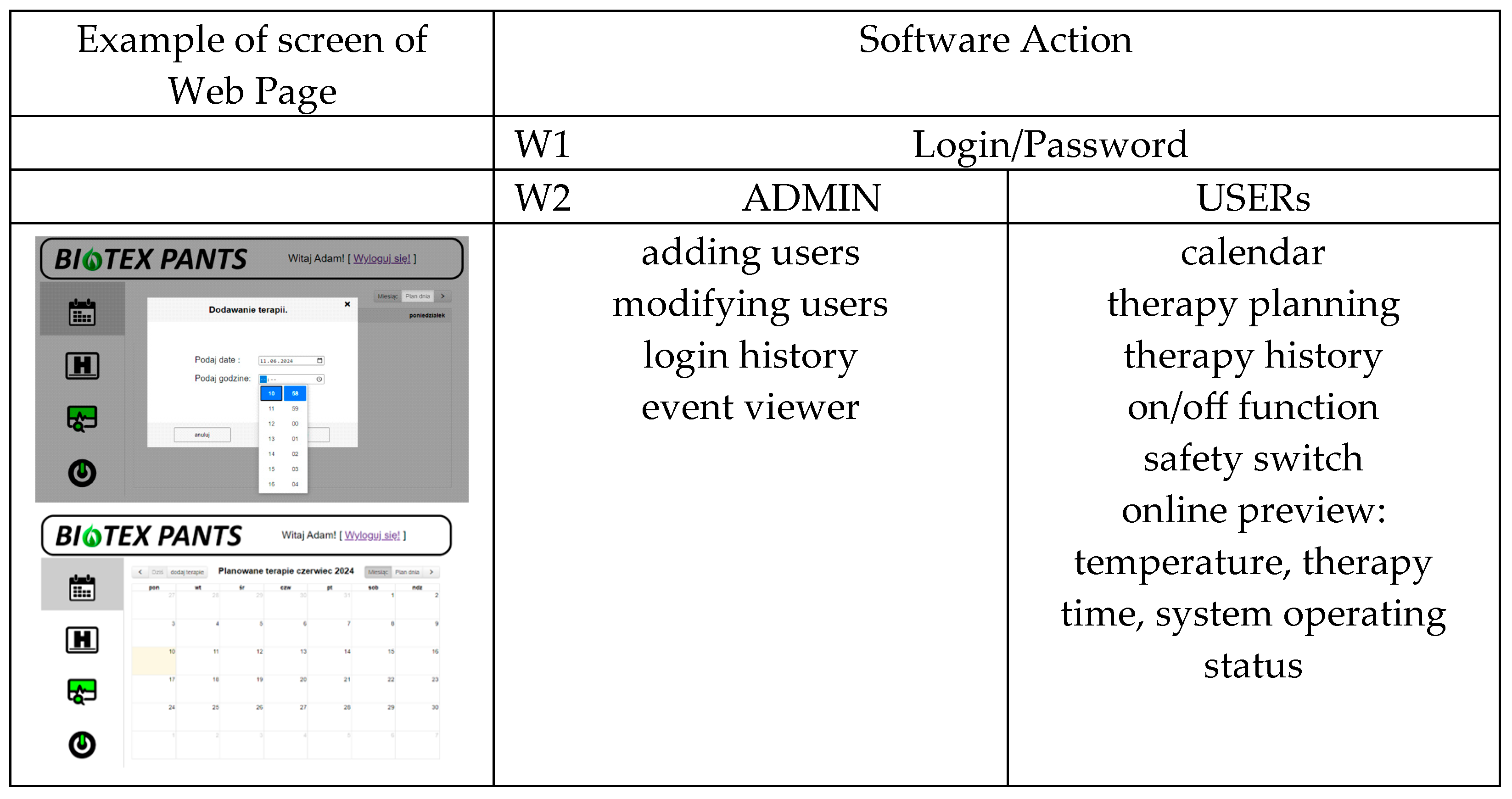
3.2. Software
4. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abelson, B.; Sun, D.; Que, L.; Nebel, R.A.; Baker, D.; Popiel, P.; Amundsen, C.L.; Chai, T.; Close, C.; DiSanto, M.; et al. Sex differences in lower urinary tract biology and physiology. Biol. Sex Differ. 2018, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Henning, P.H.; Jureidini, K.F. Urinary tract infection. Aust. Fam. Physician 1989, 18, 225–227. [Google Scholar]
- Dickson, K.; Zhou, J.; Lehmann, C. Lower Urinary Tract Inflammation and Infection: Key Microbiological and Immunological Aspects. J. Clin. Med. 2024, 13, 315. [Google Scholar] [CrossRef]
- Wasik-Olejnik, A. Recurrent Urinary Tract Infections; Prophylaxis and Treatment. Przewodnik Lekarza/Guide for GPs, pp. 18–23, 2009. Available online: https://www.termedia.pl/Recurrent-urinary-tract-infections-8211-prophylaxis-and-treatment,8,13276,1,1.html (accessed on 10 September 2025).
- Latham, R.H.; Running, K.; Stamm, W.E. Urinary tract infections in young adult women caused by Staphylococcus saprophyticus. JAMA 1983, 250, 3063–3066. [Google Scholar] [CrossRef]
- Miller, J.L.; Krieger, J.N. Urinary tract infections cranberry juice, underwear, and probiotics in the 21st century. Urol. Clin. North Am. 2002, 29, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Filipowska, B.; Rybicki, E.; Walawska, A.; Matyjas-Zgondek, E. New method for the antibacterial and antifungal modification of silver finished textiles. Fibres Text. East. Eur. 2011, 87, 124–128. [Google Scholar]
- Abu-Darwish, M.S.; Al-Ramamneh, E.A.-D.M.; Kyslychenko, V.S.; Karpiuk, U.V. The antimicrobial activity of essential oils and extracts of some medicinal plants grown in Ash-shoubak region—South of Jordan. Pak. J. Pharm. Sci. 2012, 25, 239–246. [Google Scholar]
- Song, G. Improving Comfort in Clothing. In Improving Comfort in Clothing; Elsevier: Amsterdam, The Netherlands, 2011; pp. 1–459. [Google Scholar] [CrossRef]
- Guennes, M.; Cunha, J.; Cabral, I. Smart Textile Design: A Systematic Review of Materials and Technologies for Textile Interaction and User Experience Evaluation Methods. Technologies 2025, 13, 251. [Google Scholar] [CrossRef]
- Frydrysiak, M.; Tesiorowski, L. Wearable textronic system for protecting elderly people. In Proceedings of the 2016 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Benevento, Italy, 15–18 May 2016; pp. 1–6. [Google Scholar] [CrossRef]
- Frydrysiak, M.; Tęsiorowski, Ł. Wearable Care System for Elderly People. Int. J. Pharma Med. Biol. Sci. 2016, 5, 171–177. [Google Scholar] [CrossRef]
- Łada-Tondyra, E.; Jakubas, A. Modern applications of textronic systems. Prz. Elektrotechniczny 2018, 94, 198–201. [Google Scholar] [CrossRef]
- Frydrysiak, E.; Kunicka-Styczyńska, A.; Śmigielski, K.; Frydrysiak, M. The impact of selected essential oils applied to non-woven viscose on bacteria that cause lower urinary tract infections—Preliminary studies. Molecules 2021, 26, 6854. [Google Scholar] [CrossRef]
- Łada-Tondyra, E.; Jakubas, A.; Jabłońska, B.; Stańczyk-Mazanek, E. The research and analysis of the bactericidal properties of the spacer knitted fabric with the UV-C system. Opto-Electron. Rev. 2021, 29, 192–200. [Google Scholar] [CrossRef]
- Park, J.-H.; Lee, J.-W.; Kim, Y.-C.; Prausnitz, M.R. The effect of heat on skin permeability. Int. J. Pharm. 2008, 359, 94–103. [Google Scholar] [CrossRef] [PubMed]
- NASA. Physiological Effects of Touch Temperature. 2023. Available online: https://www.nasa.gov/wp-content/uploads/2023/12/ochmo-tb-009-touch-temperature.pdf (accessed on 30 October 2025).
- Kędzia, A.; Dera-Tomaszewska, B.; Ziółkowska-Klinkosz, M.; Kędzia, A.W.; Kochańska, B.; Gębska, A. Aktywność olejku tymiankowego (Oleum Thymi) wobec bakterii tlenowych. Postępy Fitoter. 2012, 2, 67–71. [Google Scholar]
- Kędzia, A.; Ziółkowska-Klinkosz, M.; Lassmann, Ł.; Włodarkiewicz, A.; Kusiak, A.; Kochańska, B. Wrażliwość na olejek tymiankowy (Oleum Thymi) bakterii mikroaerofilnych wyizolowanych z zakażeń jamy ustnej. Postępy Fitoter. 2013, 3, 159–162. [Google Scholar]
- Lis, A.; Olejek z Tymianku Pospolitego. Najcenniejsze Olejki Eteryczne, Część I; Wydawnictwo Politechniki Łódzkiej: Łódź, Poland, 2013; pp. 344–354. [Google Scholar]
- Scholes, D.; Hooton, T.M.; Roberts, P.L.; Stapleton, A.E.; Gupta, K.; Stamm, W.E. Risk factors for recurrent urinary tract infection in young women. J. Infect. Dis. 2000, 182, 1177–1182. [Google Scholar] [CrossRef]
- Satyal, P.; Murray, B.L.; McFeeters, R.L.; Setzer, W.N. Essential oil characterization of thymus vulgaris from various geographical locations. Foods 2016, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential oils as antimicrobial agents—Myth or real alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef] [PubMed]
- Marshall, K. Interstitial cystitis: Understanding the syndrome. Altern. Med. Rev. 2003, 8, 426–437. [Google Scholar]
- Reyes-Jurado, F.; Navarro-Cruz, A.R.; Ochoa-Velasco, C.E.; Palou, E.; López-Malo, A.; Ávila-Sosa, R. Essential oils in vapor phase as alternative antimicrobials: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1641–1650. [Google Scholar] [CrossRef]
- Amiri, F.; Safiri, S.; Aletaha, R.; Sullman, M.J.M.; Hassanzadeh, K.; Kolahi, A.-A.; Arshi, S. Epidemiology of urinary tract infections in the Middle East and North Africa, 1990–2021. Trop. Med. Health 2025, 53, 16. [Google Scholar] [CrossRef] [PubMed]
- Allegranzi, B.; Tartari, E.; Pittet, D. “Seconds save lives e clean your hands”: The 5 May 2021 World Health Organization SAVE LIVES: Clean Your Hands campaign, Journal of Hospital Infection. J. Hosp. Infect. 2021, 111, 1–3. [Google Scholar] [CrossRef]
- Reda, E.M.; Mosaad, M.M. Essential Oils in Medical Textile Finishing. J. Text. Color. Polym. Sci. 2024, 22, 211–217. [Google Scholar] [CrossRef]
- Huges, S. The Best Essential Oil Diffusers. The Guardian. Available online: https://www.theguardian.com/fashion/2021/mar/13/the-best-essential-oil-diffusers (accessed on 13 September 2025).
- ScienceDirect Topics, Cosmetotextiles. ScienceDirect. Available online: https://www.sciencedirect.com/topics/engineering/cosmetotextiles (accessed on 30 October 2025).
- Necef, Ö.K.; Öndoğan, Z.; Birkocak, D.T.; Boz, S.; Kılıç, A.Ş.; Boyacı, B.; Sağduyu, İ.E. Evaluating the Efficacy of Cosmetic Textiles in Skin Hydration and Cellulite Management Through Wear Trials. Appl. Sci. 2024, 14, 11874. [Google Scholar] [CrossRef]
- Shokri, J.; Adibki, K. Application of Cellulose and Cellulose Derivatives in Pharmaceutical Industries. In Cellulose—Medical, Pharmaceutical and Electronic Applications; van de Ven, T., Godbout, L., Eds.; IntechOpen: London, UK, 2013; Chapter 3. [Google Scholar] [CrossRef]
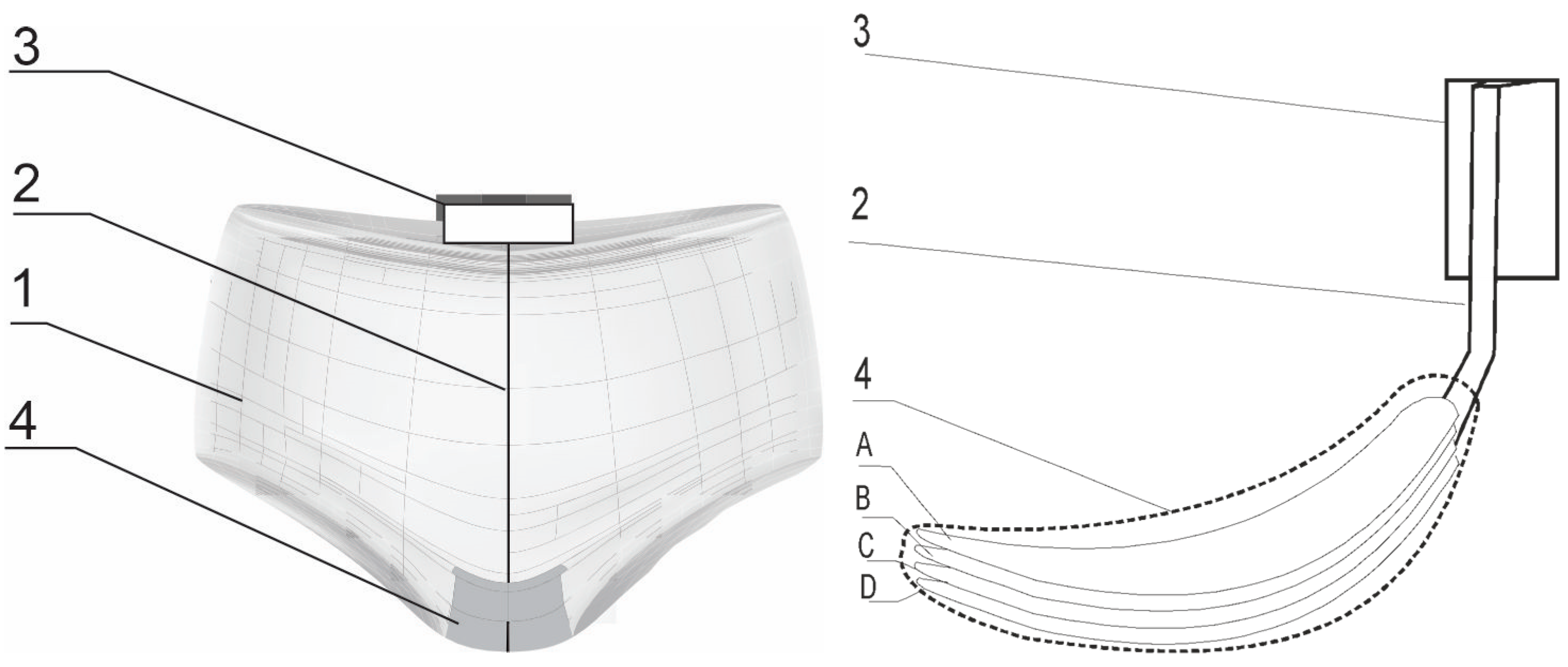
| Material Type | Mark on the Figure 2 | Surface Mass [g/m2] | Thickness [mm] | Manufacturer |
|---|---|---|---|---|
| Viscose nonwoven | A | 30 | 0.20 | Lentex S.A. (Pabianice, Poland) |
| Linen knitted fabric | B | 114 | 1.20 | PIEGATEX (Bytom, Poland) |
| Printed cotton knitted fabric | C | 150 | 0.74 | PIEGATEX (Bytom, poland) |
| Cotton fabric | D | 150 | 0.52 | Andropol (Lodz, Poland) |
| Compound | Producer | Target of Using |
|---|---|---|
| Nonwoven viscose | Lentex S.A. (Lubliniec, Poland) | Outer insert layer for EO immobilization |
| Thyme essential oil | Avicenna Oil® (Wrocław, Poland) | Antibacterial activity |
| Cellulose/microcrystalline cellulose | RETTENMAIER Polska Sp. z o.o. (Warszawa, Poland) | Carrier for EO |
| Agar-agar | Sigma-Aldrich® (St. Louis, MO, USA) | Film for EO immobilization |
| Organoleptic Description | Analytical Data | Chromatographic Profile |
|---|---|---|
| Clear liquid, yellow to dark reddish-brown in color, with a strong odor of thymol | Density (at 20 °C): 0.915–0.935 g/cm3 Refractive index (at 20 °C): 1.490–1.505 Optical rotation: −7° to +3° Flash point: 58 °C | α-thujene: 0.2–1.5% β-myrcene: 1.0–3.0% α-terpinene: 0.9–2.6% ρ-cymene: 14.0–28.0% γ-terpinene: 4.0–12.0% linalool: 1.5–6.5% terpinen-4-ol: 0.1–2.5% methyl carvacrol ether: 0.05–1.5% thymol: 37.0–55.0% carvacrol: 0.5–5.5% |
| No. | Chemical Compounds | Content in EO [%] | Content According to Manufacturer’s Specifications Avicenna Oil [%] | Content According to European Pharmacopoeia 7 [%] |
|---|---|---|---|---|
| 1 | Tricyclene | 0.04 ± 0.00 | NA | NA |
| 2 | α-Thujene | 1.28 ± 0.03 | 0.2–1.5 | NA |
| 3 | α-Pinene | 1.81 ± 0.03 | NA | NA |
| 4 | Camphene | 1.08 ± 0.02 | NA | NA |
| 5 | β-Pinene | 0.25 ± 0.00 1.81 ± 0.03 | NA | NA |
| 6 | Pseudolimonene | 0.05 ± 0.00 | NA | NA |
| 7 | α-Phellandrene | 0.09 ± 0.01 | NA | NA |
| 8 | Hydroxy-trans-sabinene | 0.05 ± 0.00 | NA | NA |
| 9 | α-Terpinene | 1.78 ± 0.04 | 0.9–2.6 | NA |
| 10 | p-Cymene | 23.84 ± 1.01 | 14.0–28.0 | 15.0–28.0 |
| 11 | β-Cymene | 0.05 ± 0.00 | NA | NA |
| 12 | 1.8-Cineole | 0.62 ± 0.02 | NA | NA |
| 13 | Limonene | 0.76 ± 0.02 | NA | NA |
| 14 | γ-Terpinene | 9.04 ± 0.27 | 4.0–12.0 | 5.0–10.0 |
| 15 | cis-Sabinene hydrate | 0.04 ± 0.00 | NA | NA |
| 16 | cis-Linalool oxide | 0.03 ± 0.00 | NA | NA |
| 17 | α-Terpinolene | 0.20 ± 0.00 | NA | NA |
| 18 | Linalool | 5.73 ± 0.65 | 1.5–6.5 | 4.0–6.5 |
| 19 | Camphor | 0.13 ± 0.01 | NA | NA |
| 20 | Camphor | 0.05 ± 0.00 | NA | NA |
| 21 | trans-Borneole | 1.04 ± 0.04 | NA | NA |
| 22 | Terpinen-4-ol | 0.72 ± 0.06 | 0.1–2.5 | 0.2–2.5 |
| 23 | α-Terpineol | 0.96 ± 0.05 | NA | NA |
| 24 | Methyl carvacrol ether | 0.26 ± 0.02 | 0.05–1.5 | NA |
| 25 | Linalyl anthranilate | 0.03 ± 0.00 | NA | NA |
| 26 | Linalyl anthranilate | 0.06 ± 0.00 | NA | NA |
| 27 | Thymol | 42.29 ± 2.25 | 37.0–55.0 | 36.0–55.0 |
| Time of Storage [Days] | Total Number of Identified Compounds | |||||
|---|---|---|---|---|---|---|
| EO:C | EO:MC | |||||
| 1:1 | 1:2 | 1:3 | 1:1 | 1:2 | 1:3 | |
| 0 | 16 | 19 | 19 | 20 | 21 | 27 |
| 7 | 15 | 5 | 9 | 10 | 10 | 8 |
| 14 | 6 | 4 | 5 | 5 | 7 | 4 |
| 28 | 4 | 4 | 5 | 3 | 2 | 2 |
| 56 | 5 | 3 | 6 | 5 | 5 | 3 |
| No. | Chemical Compounds | EO:C | EO:MC | EO | ||||
|---|---|---|---|---|---|---|---|---|
| 1:1 | 1:2 | 1:3 | 1:1 | 1:2 | 1:3 | |||
| On the day of preparation | ||||||||
| 1 | α-Thujene | 0.39 ± 0.01 | 0.46 ± 0.02 | 0.41 ± 0.03 | 0.36 ± 0.02 | 0.35 ± 0.02 | 0.37 ± 0.03 | 1.28 |
| 2 | α-Pinene | 0.73 ± 0.03 | 0.65 ± 0.04 | 0.60 ± 0.03 | 0.49 ± 0.03 | 0.62 ± 0.04 | 0.59 ± 0.01 | 1.81 |
| 3 | Camphene | 0.52 ± 0.02 | 0.50 ± 0.03 | 0.59 ± 0.05 | 0.43 ± 0.03 | 0.41 ± 0.02 | 0.47 ± 0.00 | 1.08 |
| 4 | Myrcene | 1.52 ± 0.05 | 1.46 ± 0.04 | 1.83 ± 0.05 | 1.84 ± 0.05 | 1.71 ± 0.05 | 1.72 ± 0.05 | ND |
| 5 | γ-Terpinene | 1.14 ± 0.03 | 1.03 ± 0.05 | 1.46 ± 0.04 | 1.54 ± 0.05 | 1.88 ± 0.06 | 2.10 ± 0.06 | 9.04 |
| 6 | p-Cymene | 51.10 ± 2.01 | 41.22 ± 1.52 | 47.61 ± 1.43 | 48.98 ± 1.50 | 36.24 ± 1.12 | 32.17 ± 0.98 | ND |
| 7 | Limonene | 2.24 ± 0.08 | 2.23 ± 0.08 | 2.37 ± 0.71 | 2.20 ± 0.06 | 1.94 ± 0.05 | 1.94 ± 0.06 | 0.76 |
| 8 | α-Phellandrene | ND | ND | ND | ND | 1.98 ± 0.05 | 0.15 ± 0.01 | ND |
| 9 | Sabinene | ND | ND | ND | ND | ND | 1.93 ± 0.05 | ND |
| 10 | 1.8-Cineol | 2.86 ± 0.08 | 2.21 ± 0.08 | 3.45 ± 0.09 | 3.27 ± 0.08 | 1.93 ± 0.05 | 1.93 ± 0.06 | 0.62 |
| 11 | β-Ocimene | ND | 19.49 ± 0.85 | ND | ND | 17.88 ± 0.85 | 18.30 ± 0.91 | ND |
| 12 | β-Ocimene | ND | ND | ND | ND | ND | 0.28 | ND |
| 13 | γ-Terpinene | 21.38 ± 0.64 | 19.49 ± 0.59 | 22.12 ± 0.68 | 20.54 ± 0.71 | 17.88 ± 0.59 | 16.37 ± 0.55 | ND |
| 14 | Terpinolene | ND | 0.23 ± 0.01 | 0.48 ± 0.02 | 0.27 ± 0.01 | 0.30 ± 0.01 | 0.40 ± 0.02 | ND |
| 15 | Linalool | 5.69 ± 0.17 | 3.55 ± 0.11 | 5.67 ± 0.11 | 7.61 ± 0.15 | 4.09 ± 0.98 | 4.18 ± 0.13 | 5.73 |
| 16 | Camphor | ND | ND | ND | 0.39± | ND | 0.17 ± 0.01 | ND |
| 17 | Borneole | 0.41 ± 0.02 | 0.18 ± 0.01 | 0.41 ± 0.04 | 0.50 ± 0.05 | 0.29 ± 0.02 | 0.24 ± 0.01 | ND |
| 18 | Terpinen-4-ol | 0.33 ± 0.01 | 0.26 ± 0.02 | 0.48 ± 0.04 | 0.59 ± 0.05 | 0.36 ± 0.01 | 0.29 ± 0.02 | 0.72 |
| 19 | α-Terpineol | ND | ND | 0.23 ± 0.01 | 0.42 ± 0.04 | 0.15 ± 0.02 | 0.15 ± 0.01 | 0.96 |
| 20 | Methyl carvacrol ether | 0.40 ± 0.01 | 0.24 ± 0.01 | 0.68 ± 0.02 | 0.44 ± 0.01 | 0.35 ± 0.02 | 0.56 ± 0.03 | ND |
| 21 | Thymol | 2.93 ± 0.05 | 2.52 ± 0.07 | 3.46 ± 0.10 | 4.17 ± 0.15 | 3.15 ± 0.98 | 3.75 ± 0.17 | 42.29 |
| 22 | Carvacrol | ND | 0.27 ± 0.01 | 0.23 ± 0.01 | 0.29 ± 0.02 | 0.22 ± 0.01 | 0.25 ± 0.01 | 2.87 |
| 23 | Kopaene | ND | ND | ND | ND | ND | 0.13 ± 0.00 | 0.04 |
| 24 | β-Caryophyllene | 7.99 ± 0.22 | 3.74 ± 0.11 | 7.41 ± 0.25 | 5.43 ± 0.18 | 7.86 ± 0.28 | 10.56 ± 0.34 | 2.42 |
| 25 | Aromadendrene | ND | ND | ND | ND | ND | 0.16 ± 0.01 | ND |
| 26 | α-Caryophyllene | 0.37 ± 0.02 | 0.24 ± 0.02 | 0.40 ± 0.02 | 0.25 ± 0.01 | 0.40 ± 0.03 | 0.63 ± 0.03 | 0.15 |
| 27 | δ-Cadinene | ND | ND | ND | ND | ND | 0.17 ± 0.01 | ND |
| After 7 days | ||||||||
| 1 | α-Thujene | 0.31 ± 0.01 | ND | ND | ND | ND | ND | NA |
| 2 | α-Pinene | 0.63 ± 0.02 | ND | ND | 1.76 ± 0.05 | ND | ND | NA |
| 3 | Camphene | 0.40 ± 0.02 | ND | ND | ND | ND | ND | NA |
| 4 | Myrcene | 1.55 ± 0.04 | ND | ND | 1.10 ± 0.03 | 1.69 ± 0,05 | ND | NA |
| 5 | p-Cymene | 58.19 ± 1.77 | 37.35 ± 1.12 | 46.70 ± 1.54 | 36.80 ± 1.32 | 48.46 ± 1.57 | 53.63 ± 1.63 | NA |
| 6 | β-Ocimene | ND | ND | ND | ND | ND | ND | NA |
| 7 | Limonene | 1.91 ± 0.56 | ND | ND | ND | ND | ND | NA |
| 8 | 1.8-Cineole | 3.59 ± 0.11 | 8.57 ± 0.22 | 7.31 ± 0.22 | 6.88 ± 0.21 | 6.56 ± 0.18 | 6.37 ± 0.15 | NA |
| 9 | γ-Terpinene | 14.64 ± 0.42 | 6.29 ± 0.17 | 5.34 ± 0.16 | 13.95 ± 0.42 | 9.24 ± 0.28 | 9.61 ± 0.30 | NA |
| 10 | Linalool | 8.07 ± 0.25 | 30.77 ± 0.85 | 18.80 ± 0.57 | 17.03 ± 0.47 | 16.17 ± 0.45 | 13.71 ± 0.38 | NA |
| 11 | Camphor | 0.83 ± 0.04 | ND | ND | ND | ND | ND | NA |
| 12 | Borneole | 0.47 ± 0.01 | ND | 0.92 ± 0.03 | 1.36 ± 0.04 | 1.26 ± 0.03 | 1.44 ± 0.05 | NA |
| 13 | Terpinen-4-ol | 0.61 ± 0.02 | ND | 2.79 ± 0.08 | 1.70 ± 0.04 | 1.22 ± 0.03 | 1.28 ± 0.04 | NA |
| 14 | Thymol methyl ether | 0.40 ± 0.01 | ND | 1.07 ± 0.03 | ND | 1.29 ± 0.04 | ND | NA |
| 15 | Thymol | 4.41 ± 0.14 | 17.01 ± 0.51 | 14.10 ± 0.42 | 17.34 ± 0.46 | 9.66 ± 0.30 | 10.17 ± 0.28 | NA |
| 16 | β-Caryophyllene | 4.00 ± 0.13 | ND | 2.97 ± 0.09 | 2.08 ± 0.06 | 4.45 ± 0.14 | 3.78 ± 0.11 | NA |
| After 14 days | ||||||||
| 1 | p-Cymene | 32.75 ± 1.02 | 9.88 ± 0.30 | 5.21 ± 0.16 | 20.63 ± 0.62 | 17.18 ± 0.48 | 7.11 ± 0.23 | NA |
| 2 | 1.8-Cineole | 3.72 ± 0.11 | 9.53 ± 0.27 | 14.42 ± 0.48 | 13.17 ± 0.41 | 9.90 ± 0.33 | 11.35 ± 0.34 | NA |
| 3 | γ-Terpinene | 5.04 ± 0.15 | ND | ND | ND | 4.09 ± 0.12 | ND | NA |
| 4 | Linalool | 30.16 ± 0.92 | 44.10 ± 1.28 | 38.23 ± 1.20 | 37.19 ± 1.18 | 33.09 ± 1.02 | 40.93 ± 1.11 | NA |
| 5 | Camphor | 5.58 ± 0.16 | ND | ND | ND | ND | ND | NA |
| 6 | Borneole | ND | ND | 3.30 ± 0.09 | 2.32 ± 0.07 | 4.16 ± 0.14 | ND | NA |
| 7 | Terpinen-4-ol | ND | ND | ND | ND | 5.11 ± 0.17 | ND | NA |
| 8 | Thymol | 22.74 ± 0.71 | 36.49 ± 1.15 | 38.84 ± 1.18 | 26.69 ± 0.79 | 26.48 ± 0.80 | 40.61 ± 1.21 | NA |
| After 28 days | ||||||||
| 1 | p-Cymene | 5.70 ± 0.18 | ND | 3.47 ± 0.11 | ND | ND | ND | NA |
| 2 | 1.8-Cineole | ND | 6.18 ± 0.19 | 10.35 ± 0.33 | 9.64 ± 0.27 | ND | 18.06 ± 0.55 | NA |
| 3 | Linalool | 44.87 ± 1.35 | 31.32 ± 0.95 | 43.78 ± 1.36 | 46.97 ± 1.48 | 43.47 ± 1.40 | ND | NA |
| 4 | Camphor | 9.38 ± 0.33 | 22.90 ± 0.82 | ND | ND | ND | ND | NA |
| 5 | Borneole | ND | ND | 3.33 ± 0.11 | ND | ND | ND | NA |
| 6 | Thymol | 40.05 ± 1.12 | 39.61 ± 1.07 | 39.08 ± 1.05 | 43.40 ± 1.21 | 56.53 ± 1.35 | 81.94 ± 2.45 | NA |
| After 56 days | ||||||||
| 1 | Linalool | 38.42 ± 1.24 | 30.08 ± 0.84 | 37.91 ± 1.18 | 37.73 ± 1.15 | 29.22 ± 0.83 | 31.06 ± 0.92 | NA |
| 2 | Camphor | 10.27 ± 0.33 | 17.24 ± 0.47 | ND | 12.60 ± 0.40 | 11.08 ± 0.38 | ND | NA |
| 3 | Borneole | ND | ND | 1.43 ± 0.04 | ND | ND | ND | NA |
| 4 | Terpinen-4-ol | 5.68 ± 0.18 | ND | 6.32 ± 0.20 | 6.02 ± 0.18 | 4.32 ± 0.15 | 12.77 ± 0.39 | NA |
| 5 | α-Terpineole | ND | ND | 4.25 ± 0.14 | ND | ND | ND | NA |
| 6 | Thymol | 42.41 ± 1.44 | 52.68 ± 1.62 | 47.98 ± 1.44 | 39.42 ± 1.21 | 50.58 ± 1.57 | 56.17 ± 1.75 | NA |
| 7 | Carvacrol | 3.22 ± 0.10 | ND | 2.11 ± 0.65 | 4.22 ± 0.15 | 4.80 ± 0.22 | ND | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frydrysiak, M.; Frydrysiak, E.; Śmigielski, K. Biotextronics System for the Prevention and Treatment of Lower Urinary Tract Infections. Appl. Sci. 2025, 15, 12448. https://doi.org/10.3390/app152312448
Frydrysiak M, Frydrysiak E, Śmigielski K. Biotextronics System for the Prevention and Treatment of Lower Urinary Tract Infections. Applied Sciences. 2025; 15(23):12448. https://doi.org/10.3390/app152312448
Chicago/Turabian StyleFrydrysiak, Michał, Emilia Frydrysiak, and Krzysztof Śmigielski. 2025. "Biotextronics System for the Prevention and Treatment of Lower Urinary Tract Infections" Applied Sciences 15, no. 23: 12448. https://doi.org/10.3390/app152312448
APA StyleFrydrysiak, M., Frydrysiak, E., & Śmigielski, K. (2025). Biotextronics System for the Prevention and Treatment of Lower Urinary Tract Infections. Applied Sciences, 15(23), 12448. https://doi.org/10.3390/app152312448








