Green Valorization of Alfalfa into Sustainable Lignocellulosic Films for Packaging Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Lignocellulosic Residue, Film Preparation, Film Optimization, and Characterization
2.3. Statistical Analysis
3. Results and Discussion
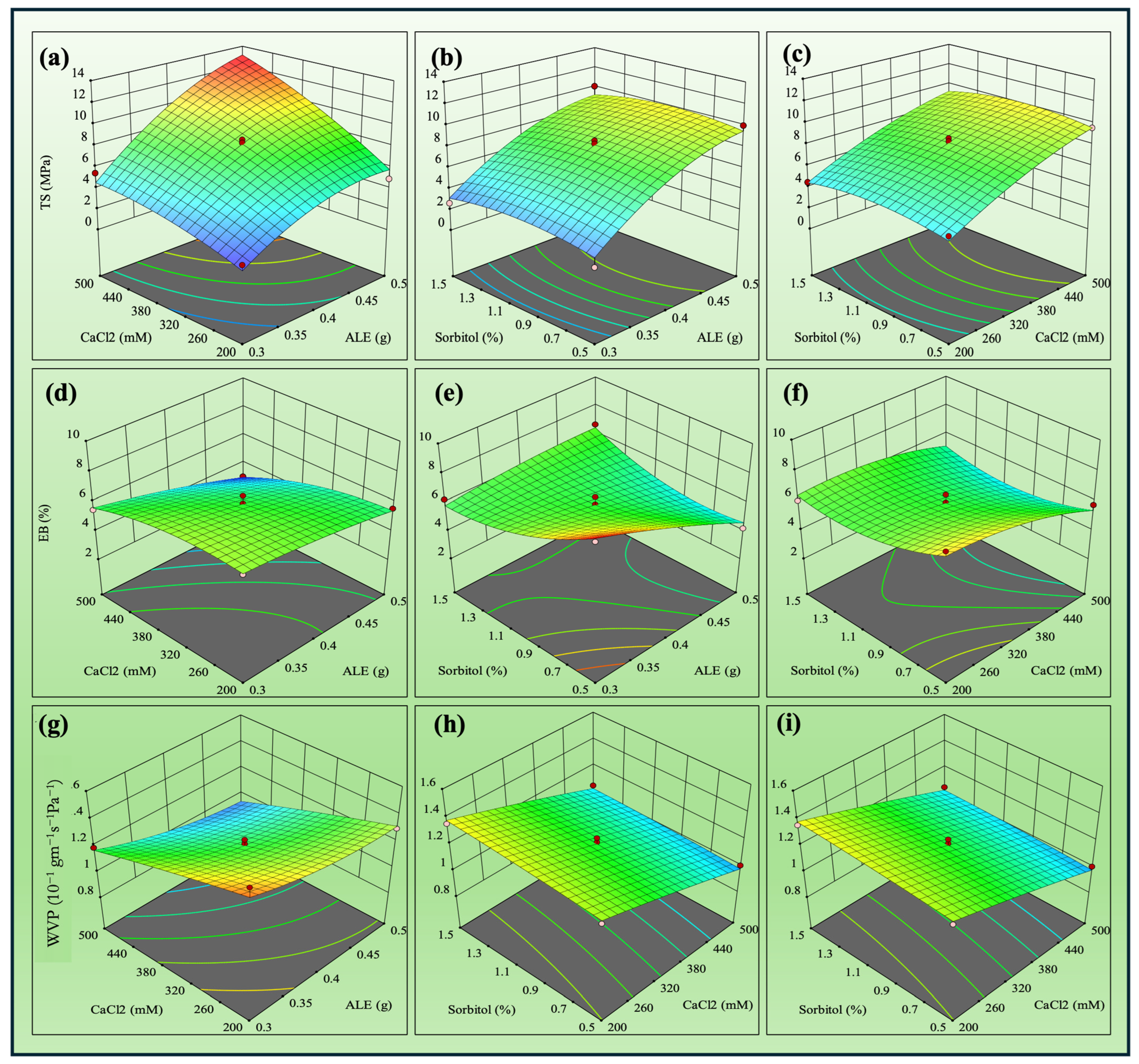
3.1. Film Optimization
| Response | Source | F-Value | p-Value | R2 |
|---|---|---|---|---|
| TS | Model | 16.29 | 0.0034 | 0.9670 |
| Lack of fit | 7.52 | 0.1197 | ||
| EB | Model | 10.41 | 0.0095 | 0.9493 |
| Lack of fit | 1.02 | 0.5295 | ||
| WVP | Model | 7.91 | 0.0174 | 0.9343 |
| Lack of fit | 2.53 | 0.2957 |
| Sample | TS | EB | WVP | Reference |
|---|---|---|---|---|
| Alfalfa lignocellulose | 11.2 ± 0.7 | 5.8 ± 0.9 | 1.2 ± 0.2 | This Study |
| Alfalfa cellulose | 16.9 | 10.1 | 0.47 | [44] |
| Avocado peel fiber | 7.2–15.7 | 5.2–13.6 | 2.4–2.5 | [51] |
| Banana peel fiber | 16.3–31.3 | 4.9–13.0 | 2.4–3.6 × 103 | [52] |
| Carboxymethyl cellulose | 2.1–22.0 | 6.7–45.1 | 48.6–214.0 | [53] |
| Cellulose | 0.3–22.4 | 4.3–13.2 | 0.6–12.2 | [46] |
| Cellulose acetate | 0.1–3.2 | 0.2–9.5 | 47.2–233.3 | [54] |
| Corncob cellulose | 4.7 | 15.4 | 1.8 | [48] |
| Cow dung cellulose | 2.2–4.2 | 9.7–11.0 | 1.0–1.5 | [55] |
| Grapevine cellulose | 15.4–18.2 | 6.1–8.6 | 0.7–1.4 | [56] |
| Palm sprout peel | 4.0–11.2 | 4.8–80.4 | 0.1–9.2 × 105 | [57] |
| Soyhull cellulose | 6.3 | 30.2 | 0.9 | [49] |
| Soyhull lignocellulose | 9.3 | 8.8 | 0.3 | [43] |
| Soyhull lignocellulose extract | 16.8 | 14.7 | 0.2 | [50] |
| Spent coffee grounds lignocellulose | 8.4–26.8 | 3.8–7.9 | 0.8–1.8 | [58] |
| Switchgrass lignocellulose | 9.9–14.7 | 3.4–4.7 | 0.1–0.2 | [59] |
| Switchgrass lignocellulosic extract | 8.9–12.7 | 2.2–2.4 | 0.2–0.3 | [60] |
| Wheat straw fiber | 5.3–6.6 | 16.4–27.3 | 1.9–2.4 | [61] |
3.2. Optimized Film Characterization
3.2.1. Optical Properties
3.2.2. Antioxidant Property
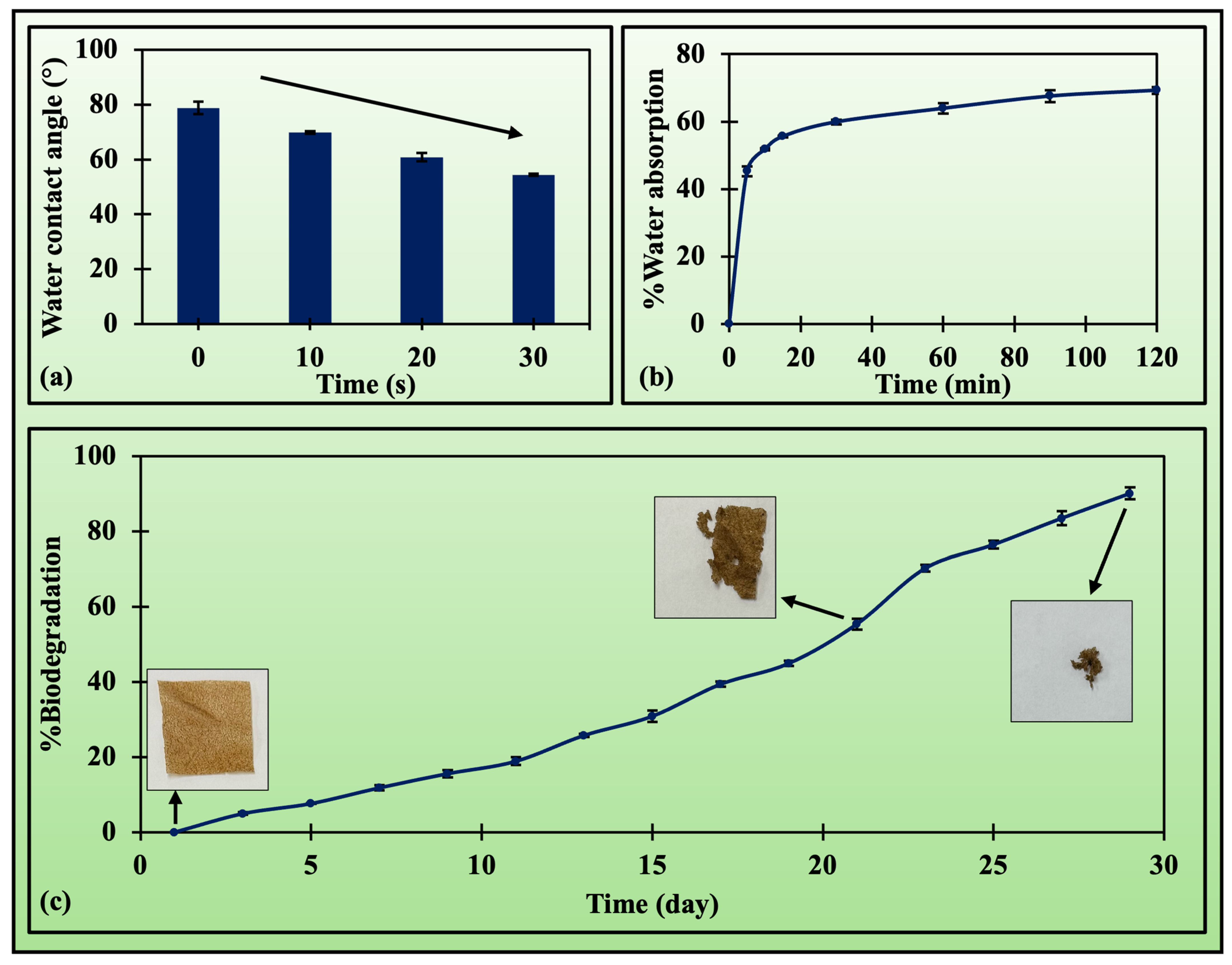
3.2.3. Hydration Properties
3.2.4. Soil Biodegradation
3.3. Recovery of Chemicals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Regmi, S.; Paudel, S.; Janaswamy, S. Packaging through the Ages: A Heuristic Journey from Leaves to Plastics to Cellulosic Fibers and Smart Technologies. Int. J. Biol. Macromol. 2025, 330, 147885. [Google Scholar] [CrossRef] [PubMed]
- Guillard, V.; Gaucel, S.; Fornaciari, C.; Angellier-Coussy, H.; Buche, P.; Gontard, N. The next Generation of Sustainable Food Packaging to Preserve Our Environment in a Circular Economy Context. Front. Nutr. 2018, 5, 121. [Google Scholar] [CrossRef] [PubMed]
- Kour, H.; Towseef Wani, N.A.; Malik, A.; Kaul, R.; Chauhan, H.; Gupta, P.; Bhat, A.; Singh, J. Advances in Food Packaging—A Review. Stewart Postharvest Rev. 2013, 9, 1–7. [Google Scholar] [CrossRef]
- Statista Research Department Annual Production of Plastics Worldwide from 1950 to 2023. Available online: https://www.statista.com/statistics/282732/global-production-of-plastics-since-1950/ (accessed on 22 October 2025).
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, 3–8. [Google Scholar] [CrossRef]
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. Environmental Impact of Food Packaging Materials: A Review of Contemporary Development from Conventional Plastics to Polylactic Acid Based Materials. Materials 2020, 13, 4994. [Google Scholar] [CrossRef]
- Rhodes, C.J. Plastic Pollution and Potential Solutions. Sci. Prog. 2018, 101, 207–260. [Google Scholar] [CrossRef]
- Aravanitoyannis, I.S.; Bosnea, L. Migration of Substances from Food Packaging Materials to Foods. Crit. Rev. Food Sci. Nutr. 2004, 44, 63–76. [Google Scholar] [CrossRef]
- Heidbreder, L.M.; Bablok, I.; Drews, S.; Menzel, C. Tackling the Plastic Problem: A Review on Perceptions, Behaviors, and Interventions. Sci. Total Environ. 2019, 668, 1077–1093. [Google Scholar] [CrossRef] [PubMed]
- Narancic, T.; O’Connor, K.E. Plastic Waste as a Global Challenge: Are Biodegradable Plastics the Answer to the Plastic Waste Problem? Microbiology 2019, 165, 129–137. [Google Scholar] [CrossRef]
- Bucci, K.; Tulio, M.; Rochman, C.M. What Is Known and Unknown about the Effects of Plastic Pollution: A Meta-analysis and Systematic Review. Ecol. Appl. 2020, 30, e02044. [Google Scholar] [CrossRef]
- Roman, L.; Schuyler, Q.; Wilcox, C.; Hardesty, B.D. Plastic Pollution Is Killing Marine Megafauna, but How Do We Prioritize Policies to Reduce Mortality? Conserv. Lett. 2021, 14, e12781. [Google Scholar] [CrossRef]
- Darrah, P. The Fascinating World of Marine Animals. Available online: https://www.gviusa.com/blog/smb-the-fascinating-world-of-marine-animals/ (accessed on 22 October 2025).
- Zhao, X.; You, F. Microplastic Human Dietary Uptake from 1990 to 2018 Grew across 109 Major Developing and Industrialized Countries but Can Be Halved by Plastic Debris Removal. Environ. Sci. Technol. 2024, 58, 8709–8723. [Google Scholar] [CrossRef] [PubMed]
- Codrington, J.; Varnum, A.A.; Hildebrandt, L.; Pröfrock, D.; Bidhan, J.; Khodamoradi, K.; Höhme, A.L.; Held, M.; Evans, A.; Velasquez, D.; et al. Detection of Microplastics in the Human Penis. Int. J. Impot. Res. 2024, 37, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Jenner, L.C.; Rotchell, J.M.; Bennett, R.T.; Cowen, M.; Tentzeris, V.; Sadofsky, L.R. Detection of Microplastics in Human Lung Tissue Using ΜFTIR Spectroscopy. Sci. Total Environ. 2022, 831, 154907. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and Quantification of Plastic Particle Pollution in Human Blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef] [PubMed]
- Montano, L.; Giorgini, E.; Notarstefano, V.; Notari, T.; Ricciardi, M.; Piscopo, M.; Motta, O. Raman Microspectroscopy Evidence of Microplastics in Human Semen. Sci. Total Environ. 2023, 901, 165922. [Google Scholar] [CrossRef]
- Ragusa, A.; Notarstefano, V.; Svelato, A.; Belloni, A.; Gioacchini, G.; Blondeel, C.; Zucchelli, E.; De Luca, C.; D’avino, S.; Gulotta, A.; et al. Raman Microspectroscopy Detection and Characterisation of Microplastics in Human Breastmilk. Polymers 2022, 14, 2700. [Google Scholar] [CrossRef]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First Evidence of Microplastics in Human Placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef]
- Vethaak, A.D.; Legler, J. Microplastics and Human Health. Science 2021, 371, 672–674. [Google Scholar] [CrossRef]
- Nihart, A.J.; Garcia, M.A.; El Hayek, E.; Liu, R.; Olewine, M.; Kingston, J.D.; Castillo, E.F.; Gullapalli, R.R.; Howard, T.; Bleske, B.; et al. Bioaccumulation of Microplastics in Decedent Human Brains. Nat. Med. 2025, 31, 1114–1119. [Google Scholar] [CrossRef]
- Sun, A.; Wang, W.-X. Human Exposure to Microplastics and Its Associated Health Risks. Environ. Health 2023, 1, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.; Baek, J.Y.; Koo, J.; Park, S.; Ryu, Y.-K.; Kim, K.-S.; Zhang, S.; Chung, C.; Dogan, R.; Choi, H.-S.; et al. Maternal Exposure to Polystyrene Nanoplastics Causes Brain Abnormalities in Progeny. J. Hazard. Mater. 2022, 426, 127815. [Google Scholar] [CrossRef]
- Feng, Y.; Tu, C.; Li, R.; Wu, D.; Yang, J.; Xia, Y.; Peijnenburg, W.J.G.M.; Luo, Y. A Systematic Review of the Impacts of Exposure to Micro- and Nano-Plastics on Human Tissue Accumulation and Health. Eco-Environ. Health 2023, 2, 195–207. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Edgar, K.J.; Buchanan, C.M.; Debenham, J.S.; Rundquist, P.A.; Seiler, B.D.; Shelton, M.C.; Tindall, D. Advances in Cellulose Ester Performance and Application. Prog. Polym. Sci. 2001, 26, 1605–1688. [Google Scholar] [CrossRef]
- Cui, B.; Liu, L.; Li, S.; Wang, W.; Tan, L.; Liu, C.; Wang, W. Bio-Inspired, UV-Blocking, Water-Stable and Antioxidant Lignin/Cellulose Films Combining High Strength, Toughness and Flexibility. Mater. Chem. Front. 2023, 7, 897–905. [Google Scholar] [CrossRef]
- Janaswamy, S.; Yadav, M.P.; Hoque, M.; Bhattarai, S.; Ahmed, S. Cellulosic Fraction from Agricultural Biomass as a Viable Alternative for Plastics and Plastic Products. Ind. Crops Prod. 2022, 179, 114692. [Google Scholar] [CrossRef]
- Girijappa, Y.G.T.; Mavinkere Rangappa, S.; Parameswaranpillai, J.; Siengchin, S. Natural Fibers as Sustainable and Renewable Resource for Development of Eco-Friendly Composites: A Comprehensive Review. Front. Mater. 2019, 6, 226. [Google Scholar] [CrossRef]
- Hadidi, M.; Orellana Palacios, J.C.; McClements, D.J.; Mahfouzi, M.; Moreno, A. Alfalfa as a Sustainable Source of Plant-Based Food Proteins. Trends Food Sci. Technol. 2023, 135, 202–214. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L. Advances in Basic Biology of Alfalfa (Medicago sativa L.): A Comprehensive Overview. Hortic. Res. 2025, 12, uhaf081. [Google Scholar] [CrossRef] [PubMed]
- Diatta, A.A.; Min, D.; Jagadish, S.V.K. Drought Stress Responses in Non-Transgenic and Transgenic Alfalfa—Current Status and Future Research Directions. In Advances in Agronomy; Sparks, D.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 35–100. [Google Scholar]
- IMARC Alfalfa Hay Market Size, Share, Trends and Forecast by Type, Application, End User, and Region, 2025–2033. Available online: https://www.imarcgroup.com/alfalfa-hay-market (accessed on 22 October 2025).
- Jarek, K. Determining the Value of Standing Alfalfa in 2022. Available online: https://dodge.extension.wisc.edu/files/2022/05/Determining-the-Value-of-Standing-Alfalfa-in-2022-Statewide-Final-5-9-22-2.pdf (accessed on 1 September 2025).
- Langsdorf, A.; Volkmar, M.; Holtmann, D.; Ulber, R. Material Utilization of Green Waste: A Review on Potential Valorization Methods. Bioresour. Bioprocess. 2021, 8, 19. [Google Scholar] [CrossRef]
- Ma, J.; Huangfu, W.; Yang, X.; Xu, J.; Zhang, Y.; Wang, Z.; Zhu, X.; Wang, C.; Shi, Y.; Cui, Y. “King of the Forage”—Alfalfa Supplementation Improves Growth, Reproductive Performance, Health Condition and Meat Quality of Pigs. Front. Vet. Sci. 2022, 9, 1025942. [Google Scholar] [CrossRef] [PubMed]
- USDA. USDA South Dakota Direct Hay Report. Available online: https://www.ams.usda.gov/mnreports/ams_3183.pdf (accessed on 22 October 2025).
- He, Y.; Ye, H.; Li, H.; Cui, F.; Xu, F.; You, T. Multifunctional Films with Superior Mechanical Performance, Transparency, Antibacterial Properties Enabled by a Physical and Chemical Dual Crosslinking Network Construction. Chem. Eng. J. 2024, 479, 147546. [Google Scholar] [CrossRef]
- Dai, Q.; Bai, Y.; Fu, B.; Yang, F. Multifunctional Bacterial Cellulose Films Enabled by Deep Eutectic Solvent-Extracted Lignin. ACS Omega 2023, 8, 7430–7437. [Google Scholar] [CrossRef]
- Bello, F.; Peresin, M.S. Multifunctional Pectin and Lignin-Containing Cellulose Nanofiber Films with Improved UV Resistance and Mechanical Properties. Food Hydrocoll. 2024, 157, 110378. [Google Scholar] [CrossRef]
- Huang, H.; Zheng, C.; Zhang, Z.; Huang, C. Adding Lignin to Bagasse Cellulose-Based Materials Increases the Wet Strength and Reduces Micro-Nano Scale Wet Low-Temperature Defects. Ind. Crops Prod. 2024, 222, 119750. [Google Scholar] [CrossRef]
- Regmi, S.; Paudel, S.; Janaswamy, S. Development of Eco-Friendly Packaging Films from Soyhull Lignocellulose: Towards Valorizing Agro-Industrial Byproducts. Foods 2024, 13, 4000. [Google Scholar] [CrossRef]
- Paudel, S.; Janaswamy, S. Use of Alfalfa Cellulose for Formulation of Strong, Biodegradable Film to Extend the Shelf Life of Strawberries. Int. J. Biol. Macromol. 2025, 290, 139004. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, C.; Rosswurm, K.; Yao, T.; Janaswamy, S. A Facile Route to Prepare Cellulose-Based Films. Carbohydr. Polym. 2016, 149, 274–281. [Google Scholar] [CrossRef]
- Paudel, S.; Regmi, S.; Janaswamy, S. Effect of Glycerol and Sorbitol on Cellulose-Based Biodegradable Films. Food Packag. Shelf Life 2023, 37, 101090. [Google Scholar] [CrossRef]
- Suyatma, N.E.; Tighzert, L.; Copinet, A.; Coma, V. Effects of Hydrophilic Plasticizers on Mechanical, Thermal, and Surface Properties of Chitosan Films. J. Agric. Food Chem. 2005, 53, 3950–3957. [Google Scholar] [CrossRef] [PubMed]
- Paudel, S.; Janaswamy, S. Corncob-Derived Biodegradable Packaging Films: A Sustainable Solution for Raspberry Post-Harvest Preservation. Food Chem. 2024, 454, 139749. [Google Scholar] [CrossRef]
- Regmi, S.; Janaswamy, S. Biodegradable Films from Soyhull Cellulosic Residue with UV Protection and Antioxidant Properties Improve the Shelf-Life of Post-Harvested Raspberries. Food Chem. 2024, 460, 140672. [Google Scholar] [CrossRef]
- Regmi, S.; Janaswamy, S. Biodegradable Packaging Films from the Alkali-Extracted Lignocellulosic Residue of Soyhulls Extend the Shelf Life of Strawberries. Food Biosci. 2025, 65, 106016. [Google Scholar] [CrossRef]
- Ahmed, S.; Janaswamy, S. Strong and Biodegradable Films from Avocado Peel Fiber. Ind. Crops Prod. 2023, 201, 116926. [Google Scholar] [CrossRef]
- Hoque, M.; Janaswamy, S. Biodegradable Packaging Films from Banana Peel Fiber. Sustain. Chem. Pharm. 2024, 37, 101400. [Google Scholar] [CrossRef]
- Mukherjee, S.; Sengupta, A.; Preetam, S.; Das, T.; Bhattacharya, T.; Thorat, N. Effects of Fatty Acid Esters on Mechanical, Thermal, Microbial, and Moisture Barrier Properties of Carboxymethyl Cellulose-Based Edible Films. Carbohydr. Polym. Technol. Appl. 2024, 7, 100505. [Google Scholar] [CrossRef]
- Barman, A.R.; Dutta, P.P.; Kapila, K.; Bania, K.K.; Haloi, D.J. Synergistic Effect of Glycerol and Calcium Chloride on the Properties of Cellulose Acetate Film. J. Appl. Polym. Sci. 2024, 142, e56615. [Google Scholar] [CrossRef]
- Paudel, S.; Regmi, S.; Janaswamy, S. Valorization of Biowaste to Biopackaging: Development of Biodegradable Films from Cow Dung-Derived Cellulose. Biomass Bioenergy 2026, 204, 108443. [Google Scholar] [CrossRef]
- Paudel, S.; Regmi, S.; Bhattarai, S.; Fennell, A.; Janaswamy, S. Valorization of Grapevine Agricultural Waste into Transparent and High-Strength Biodegradable Films for Sustainable Packaging. Sustain. Food Technol. 2025, 3, 1218–1231. [Google Scholar] [CrossRef]
- Rajeshwari, E.R.; Sathanya, P.S.; Vignesh, S.; Chandrasekar, V.; Baskaran, N. Valorization of Borassus Flabellifer Sprout Peel: Synthesis and Characterization of Carboxymethyl Cellulose for Biodegradable Packaging. Biomass Convers. Biorefinery 2025, 15, 17829–17852. [Google Scholar] [CrossRef]
- Bhattarai, S.; Janaswamy, S. Biodegradable, UV-Blocking, and Antioxidant Films from Lignocellulosic Fibers of Spent Coffee Grounds. Int. J. Biol. Macromol. 2023, 253, 126798. [Google Scholar] [CrossRef]
- Bhattarai, S.; Janaswamy, S. Biodegradable Films from the Lignocellulosic Residue of Switchgrass. Resour. Conserv. Recycl. 2024, 201, 107322. [Google Scholar] [CrossRef]
- Bhattarai, S.; Janaswamy, S. Biodegradable, UV-Blocking, and Antioxidant Films from Alkali-Digested Lignocellulosic Residue Fibers of Switchgrass. Chemosphere 2024, 359, 142393. [Google Scholar] [CrossRef]
- Ahmed, S.; Janaswamy, S.; Yadav, M.P. Biodegradable Films from the Lignocellulosic Fibers of Wheat Straw Biomass and the Effect of Calcium Ions. Int. J. Biol. Macromol. 2024, 264, 130601. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Ragauskas, A. Lignin as a UV Light Blocker—A Review. Polymers 2020, 12, 1134. [Google Scholar] [CrossRef]
- Lee, J.W.; Son, S.M.; Hong, S.I. Characterization of Protein-Coated Polypropylene Films as a Novel Composite Structure for Active Food Packaging Application. J. Food Eng. 2008, 86, 484–493. [Google Scholar] [CrossRef]
- Riahi, Z.; Khan, A.; Ebrahimi, M.; Rhim, J.; Shin, G.H.; Kim, J.T. Exploring Sustainable Carbon Dots as UV-blocking Agents for Food Preservation. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70192. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Chen, C.; Yao, Y.; Li, J.; He, S.; Zhou, Y.; Li, T.; Pan, X.; Yao, Y.; Hu, L. A Strong, Biodegradable and Recyclable Lignocellulosic Bioplastic. Nat. Sustain. 2021, 4, 627–635. [Google Scholar] [CrossRef]
- Liu, Y.; Kim, H.-J. Fourier Transform Infrared Spectroscopy (FT-IR) and Simple Algorithm Analysis for Rapid and Non-Destructive Assessment of Developmental Cotton Fibers. Sensors 2017, 17, 1469. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ma, N.L.; Zhang, W.; Wu, Y.; Ma, Y.; Chen, G.; Sun, Y.; Wang, J.; Sun, C. Highly Efficient Conversion of Cellulose and Hemicellulose Using an Innovative Ternary Deep Eutectic Solvent: Achieving the Green and Complete Use of Lignocellulose. Ind. Crops Prod. 2025, 230, 121091. [Google Scholar] [CrossRef]
- Dalmis, R.; Kilic, G.B.; Seki, Y.; Koktas, S.; Keskin, O.Y. Characterization of a Novel Natural Cellulosic Fiber Extracted from the Stem of Chrysanthemum Morifolium. Cellulose 2020, 27, 8621–8634. [Google Scholar] [CrossRef]
- Maache, M.; Bezazi, A.; Amroune, S.; Scarpa, F.; Dufresne, A. Characterization of a Novel Natural Cellulosic Fiber from Juncus effusus L. Carbohydr. Polym. 2017, 171, 163–172. [Google Scholar] [CrossRef]
- Kong, W.; Chang, M.; Zhang, C.; Liu, X.; He, B.; Ren, J. Preparation of Xylan-g-/P(AA-Co-AM)/GO Nanocomposite Hydrogel and Its Adsorption for Heavy Metal Ions. Polymers 2019, 11, 621. [Google Scholar] [CrossRef] [PubMed]
- Abidi, N.; Cabrales, L.; Haigler, C.H. Changes in the Cell Wall and Cellulose Content of Developing Cotton Fibers Investigated by FTIR Spectroscopy. Carbohydr. Polym. 2014, 100, 9–16. [Google Scholar] [CrossRef]
- Longaresi, R.H.; de Menezes, A.J.; Pereira-da-Silva, M.A.; Baron, D.; Mathias, S.L. The Maize Stem as a Potential Source of Cellulose Nanocrystal: Cellulose Characterization from Its Phenological Growth Stage Dependence. Ind. Crops Prod. 2019, 133, 232–240. [Google Scholar] [CrossRef]
- Pancholi, M.J.; Khristi, A.; M, A.K.; Bagchi, D. Comparative Analysis of Lignocellulose Agricultural Waste and Pre-Treatment Conditions with FTIR and Machine Learning Modeling. Bioenergy Res. 2023, 16, 123–137. [Google Scholar] [CrossRef]
- Ding, Q.; Han, W.; Li, X.; Jiang, Y.; Zhao, C. New Insights into the Autofluorescence Properties of Cellulose/Nanocellulose. Sci. Rep. 2020, 10, 21387. [Google Scholar] [CrossRef]
- Rabyk, M.; Galisova, A.; Jiratova, M.; Patsula, V.; Srbova, L.; Loukotova, L.; Parnica, J.; Jirak, D.; Stepanek, P.; Hruby, M. Mannan-Based Conjugates as a Multimodal Imaging Platform for Lymph Nodes. J. Mater. Chem. B 2018, 6, 2584–2596. [Google Scholar] [CrossRef] [PubMed]
- Geminiani, L.; Campione, F.; Corti, C.; Luraschi, M.; Motella, S.; Recchia, S.; Rampazzi, L. Differentiating between Natural and Modified Cellulosic Fibres Using ATR-FTIR Spectroscopy. Heritage 2022, 5, 4114–4139. [Google Scholar] [CrossRef]
- Kılınç, A.Ç.; Köktaş, S.; Seki, Y.; Atagür, M.; Dalmış, R.; Erdoğan, Ü.H.; Göktaş, A.A.; Seydibeyoğlu, M.Ö. Extraction and Investigation of Lightweight and Porous Natural Fiber from Conium Maculatum as a Potential Reinforcement for Composite Materials in Transportation. Compos. Part B Eng. 2018, 140, 1–8. [Google Scholar] [CrossRef]
- Klapiszewski, Ł.; Jamrozik, A.; Strzemiecka, B.; Koltsov, I.; Borek, B.; Matykiewicz, D.; Voelkel, A.; Jesionowski, T. Characteristics of Multifunctional, Eco-Friendly Lignin-Al2O3 Hybrid Fillers and Their Influence on the Properties of Composites for Abrasive Tools. Molecules 2017, 22, 1920. [Google Scholar] [CrossRef] [PubMed]
- Vârban, R.; Crișan, I.; Vârban, D.; Ona, A.; Olar, L.; Stoie, A.; Ștefan, R. Comparative FT-IR Prospecting for Cellulose in Stems of Some Fiber Plants: Flax, Velvet Leaf, Hemp and Jute. Appl. Sci. 2021, 11, 8570. [Google Scholar] [CrossRef]
- Shi, Z.; Xu, G.; Deng, J.; Dong, M.; Murugadoss, V.; Liu, C.; Shao, Q.; Wu, S.; Guo, Z. Structural Characterization of Lignin from D. Sinicus by FTIR and NMR Techniques. Green. Chem. Lett. Rev. 2019, 12, 235–243. [Google Scholar] [CrossRef]
- Alekseeva, O.V.; Noskov, A.V.; Agafonov, A.V. Structure, Physicochemical Properties, and Adsorption Performance of the Ethyl Cellulose/Bentonite Composite Films. Cellulose 2022, 29, 3947–3961. [Google Scholar] [CrossRef]
- Suryanto, H.; Marsyahyo, E.; Irawan, Y.S.; Soenoko, R. Morphology, Structure, and Mechanical Properties of Natural Cellulose Fiber from Mendong Grass (Fimbristylis Globulosa). J. Nat. Fibers 2014, 11, 333–351. [Google Scholar] [CrossRef]
- Belwal, T.; Giri, L.; Bhatt, I.D.; Rawal, R.S.; Pande, V. An Improved Method for Extraction of Nutraceutically Important Polyphenolics from Berberis Jaeschkeana C.K. Schneid. Fruits. Food Chem. 2017, 230, 657–666. [Google Scholar] [CrossRef]
- Belwal, T.; Dhyani, P.; Bhatt, I.D.; Rawal, R.S.; Pande, V. Optimization Extraction Conditions for Improving Phenolic Content and Antioxidant Activity in Berberis Asiatica Fruits Using Response Surface Methodology (RSM). Food Chem. 2016, 207, 115–124. [Google Scholar] [CrossRef]
- Li, K.; Xu, H.; Liu, Y.; Zhong, W.; Jin, Y.; Wu, W. Exploring the Relationship between Lignin Structure and Antioxidant Property Using Lignin Model Compounds. Int. J. Biol. Macromol. 2024, 282, 136786. [Google Scholar] [CrossRef]
- Sravanthi, G.B.; Thakur, R.; Giri, S.; Dissanayake, T.; Janaswamy, S.; Mekonnen, T.; Bandara, N.; Pal, K.; Sarkar, P. Xyloglucan Films from Tamarind Kernels Reinforced with Chemically Modified Cellulose Nanospheres. Int. J. Biol. Macromol. 2025, 331, 148411. [Google Scholar] [CrossRef]
- Huang, H.; Xu, C.; Zhu, X.; Li, B.; Huang, C. Lignin-Enhanced Wet Strength of Cellulose-Based Materials: A Sustainable Approach. Green. Chem. 2023, 25, 4995–5009. [Google Scholar] [CrossRef]
- Wang, W.; Xue, Y.; Wang, X.; Zheng, X.; Quan, J.; Wang, Z.; Zhang, J.; Yao, J.; Xue, B. Lignin-Enhanced Wet Strength and UV-Blocking Properties of Nanocellulose-Based Composite Films Obtained by Casting/Hot-Pressing Methods. Eur. Polym. J. 2024, 213, 113109. [Google Scholar] [CrossRef]
- Farris, S.; Introzzi, L.; Biagioni, P.; Holz, T.; Schiraldi, A.; Piergiovanni, L. Wetting of Biopolymer Coatings: Contact Angle Kinetics and Image Analysis Investigation. Langmuir 2011, 27, 7563–7574. [Google Scholar] [CrossRef]
- Karbowiak, T.; Debeaufort, F.; Champion, D.; Voilley, A. Wetting Properties at the Surface of Iota-Carrageenan-Based Edible Films. J. Colloid. Interface Sci. 2006, 294, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Modaressi, H.; Garnier, G. Mechanism of Wetting and Absorption of Water Droplets on Sized Paper: Effects of Chemical and Physical Heterogeneity. Langmuir 2002, 18, 642–649. [Google Scholar] [CrossRef]
- Kokoszka, S.; Debeaufort, F.; Hambleton, A.; Lenart, A.; Voilley, A. Protein and Glycerol Contents Affect Physico-Chemical Properties of Soy Protein Isolate-Based Edible Films. Innov. Food Sci. Emerg. Technol. 2010, 11, 503–510. [Google Scholar] [CrossRef]
- Ahmed, S.; Janaswamy, S. Green Fabrication of Biodegradable Films: Harnessing the Cellulosic Residue of Oat Straw. Int. J. Biol. Macromol. 2025, 303, 140656. [Google Scholar] [CrossRef]

| Run | Independent Variable | Response | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Coded | Actual | ||||||||
| A | B | C | ALE | CaCl2 | Sorbitol | TS | EB | WVP | |
| ALE1 | −1 | −1 | 0 | 0.3 | 200 | 1 | 2.4 ± 0.4 | 6.7 ± 0.9 | 1.6 ± 0.1 |
| ALE2 | 1 | −1 | 0 | 0.5 | 200 | 1 | 4.9 ± 0.8 | 5.6 ± 0.5 | 1.3 ± 0.3 |
| ALE3 | −1 | 1 | 0 | 0.3 | 500 | 1 | 5.4 ± 0.1 | 5.5 ± 1.3 | 1.2 ± 0.1 |
| ALE4 | 1 | 1 | 0 | 0.5 | 500 | 1 | 12.6 ± 0.3 | 2.9 ± 0.7 | 0.9 ± 0.1 |
| ALE5 | −1 | 0 | −1 | 0.3 | 350 | 0.5 | 2.2 ± 0.7 | 9.1 ± 0.1 | 1.3 ± 0.1 |
| ALE6 | 1 | 0 | −1 | 0.5 | 350 | 0.5 | 10.0 ± 2.6 | 4.2 ± 1.0 | 1.1 ± 0.2 |
| ALE7 | −1 | 0 | 1 | 0.3 | 350 | 1.5 | 2.6 ± 0.4 | 6.3 ± 1.4 | 1.3 ± 0.1 |
| ALE8 | 1 | 0 | 1 | 0.5 | 350 | 1.5 | 10.1 ± 1.0 | 6.6 ± 1.8 | 1.2 ± 0.1 |
| ALE9 | 0 | −1 | −1 | 0.4 | 200 | 0.5 | 4.8 ± 1.6 | 8.1 ± 1.2 | 1.3 ± 0.3 |
| ALE10 | 0 | 1 | −1 | 0.4 | 500 | 0.5 | 9.6 ± 0.8 | 5.8 ± 0.1 | 1.0 ± 0.1 |
| ALE11 | 0 | −1 | 1 | 0.4 | 200 | 1.5 | 4.5 ± 0.5 | 6.0 ± 0.6 | 1.4 ± 0.1 |
| ALE12 | 0 | 1 | 1 | 0.4 | 500 | 1.5 | 8.9 ± 1.6 | 4.8 ± 1.0 | 1.1 ± 0.1 |
| ALE13 | 0 | 0 | 0 | 0.4 | 350 | 1 | 8.6 ± 1.1 | 5.3 ± 1.7 | 1.1 ± 0.2 |
| ALE14 | 0 | 0 | 0 | 0.4 | 350 | 1 | 7.7 ± 0.1 | 5.9 ± 1.8 | 1.2 ± 0.2 |
| ALE15 | 0 | 0 | 0 | 0.4 | 350 | 1 | 8.3 ± 1.5 | 6.4 ± 1.9 | 1.2 ± 0.1 |
| Model | a | b | c | R2 | RMSE |
|---|---|---|---|---|---|
| Water absorption | |||||
| Peleg (a as K1 and b as K2) | 0.0606 | 0.0141 | - | 0.9990 | 0.0179 |
| Gornicki | 0.3671 | 0.3551 | 0.1938 | 0.9898 | 0.0082 |
| Pilosof | 0.3671 | 0.3551 | 14.5354 | 0.9898 | 0.0082 |
| Czel and Czigany (b as m) | 0.3815 | 0.1277 | - | 0.9829 | 0.0107 |
| Peppas (a as K1 and b as K2) | −0.0216 | 0.3045 | - | 0.9073 | 0.1495 |
| Singh | −3.4892 | 13.6014 | 3.2728 | 0.8982 | 0.0258 |
| Garcia Pascual | 0.5919 | 1.2782 | - | 0.3414 | 0.2668 |
| Vega-Galvez | 1.2408 | 0.7426 | - | 0 | 0.0808 |
| Weibull (a as α and b as β) | 53,687,092.2 | 11.2069 | - | ND | ND |
| Biodegradation | |||||
| First-order (a as m) | 0.1084 | - | 1.6557 | 0.9808 | 0.1740 |
| Second-order | 1.1031 | 0.2011 | −0.0029 | 0.9969 | 0.0493 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paudel, S.; Janaswamy, S. Green Valorization of Alfalfa into Sustainable Lignocellulosic Films for Packaging Applications. Appl. Sci. 2025, 15, 11889. https://doi.org/10.3390/app152211889
Paudel S, Janaswamy S. Green Valorization of Alfalfa into Sustainable Lignocellulosic Films for Packaging Applications. Applied Sciences. 2025; 15(22):11889. https://doi.org/10.3390/app152211889
Chicago/Turabian StylePaudel, Sandeep, and Srinivas Janaswamy. 2025. "Green Valorization of Alfalfa into Sustainable Lignocellulosic Films for Packaging Applications" Applied Sciences 15, no. 22: 11889. https://doi.org/10.3390/app152211889
APA StylePaudel, S., & Janaswamy, S. (2025). Green Valorization of Alfalfa into Sustainable Lignocellulosic Films for Packaging Applications. Applied Sciences, 15(22), 11889. https://doi.org/10.3390/app152211889








