Integration of a Fluoride- and Mint-Based Spray in Nighttime Aligner Therapy: Effects on Salivary Concentration and Biofilm
Abstract
1. Introduction
- (1)
- measuring the salivary fluoride concentration over time with a fluoride ion-selective electrode (ISE);
- (2)
- assessing the biofilm formation in aligners with a Scanning Electron Microscopy (SEM).
2. Materials and Methods
2.1. Study Design and Participants Selection
- NEW aligner: a freshly delivered aligner that had never been worn;
- USED aligner: an aligner previously worn by the same subject for 14 consecutive nights.
2.2. Spray Formulation and Application Protocol
2.3. Saliva Sampling and Fluoride Measurement (ISE Analysis)
- Baseline: before applying the spray to the aligners;
- T0: immediately after the insertion of sprayed aligners;
- T15, T30 and T45: 15, 30, and 45 min after spray application.
2.4. SEM Analysis of Biofilm on NOXI Aligners
- Aligner A (new control);
- Aligner B (used 14 nights without spray);
- Aligner C (used 14 nights with nightly spray application).
- A.
- Increased deposition was defined as continuous organic layers, confluent bacterial aggregates, or dense clusters covering the underlying polymer texture.
- B.
- Reduced deposition was defined as sparse bacterial micro-colonies, isolated clusters, or largely clean regions with visible polymer texture.
2.5. Statistical Analysis
3. Results
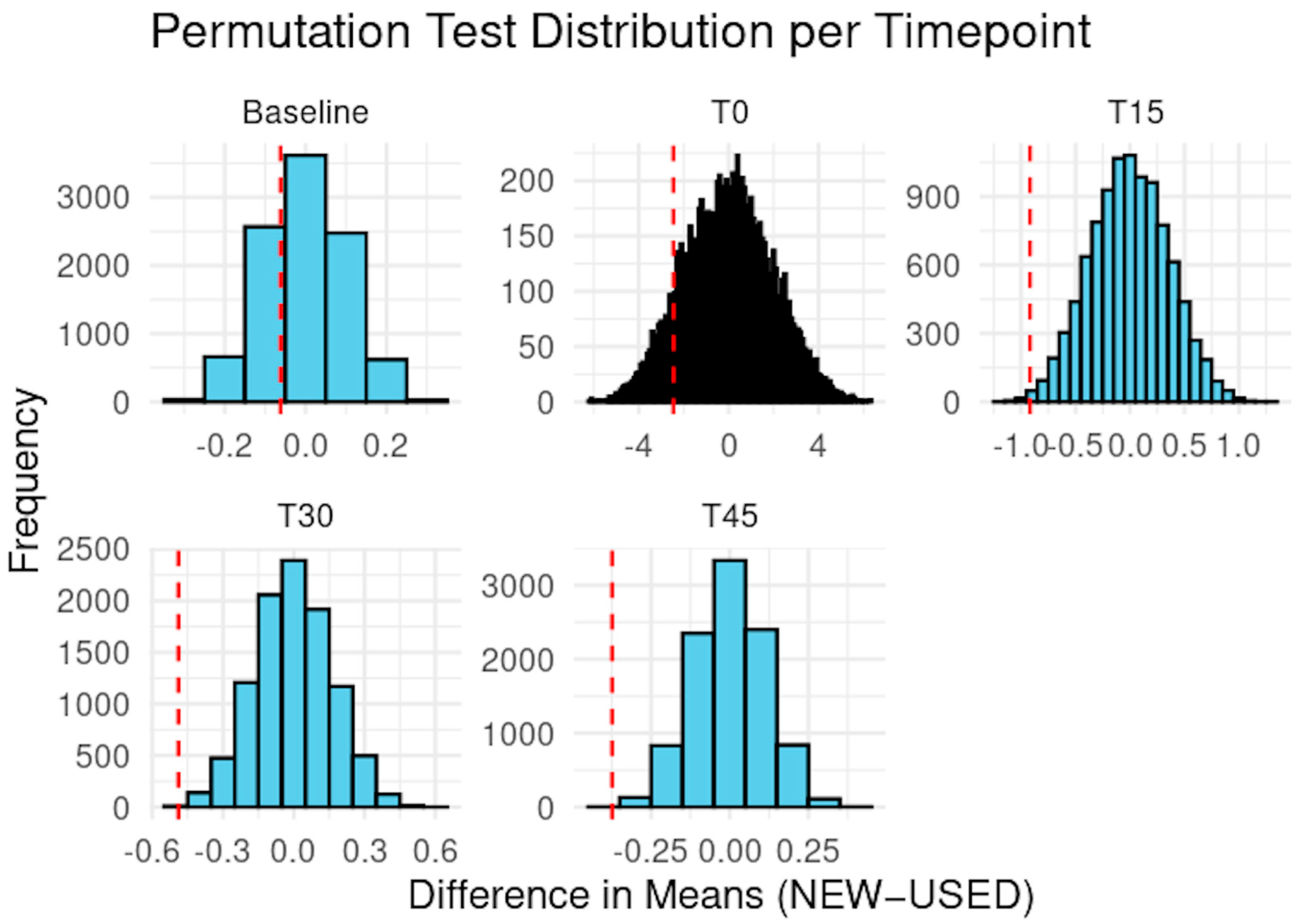
3.1. Salivary Fluoride Concentration (ISE)
3.1.1. Intragroup Analysis
NEW Aligner
USED Aligner
3.1.2. Intergroup Analysis
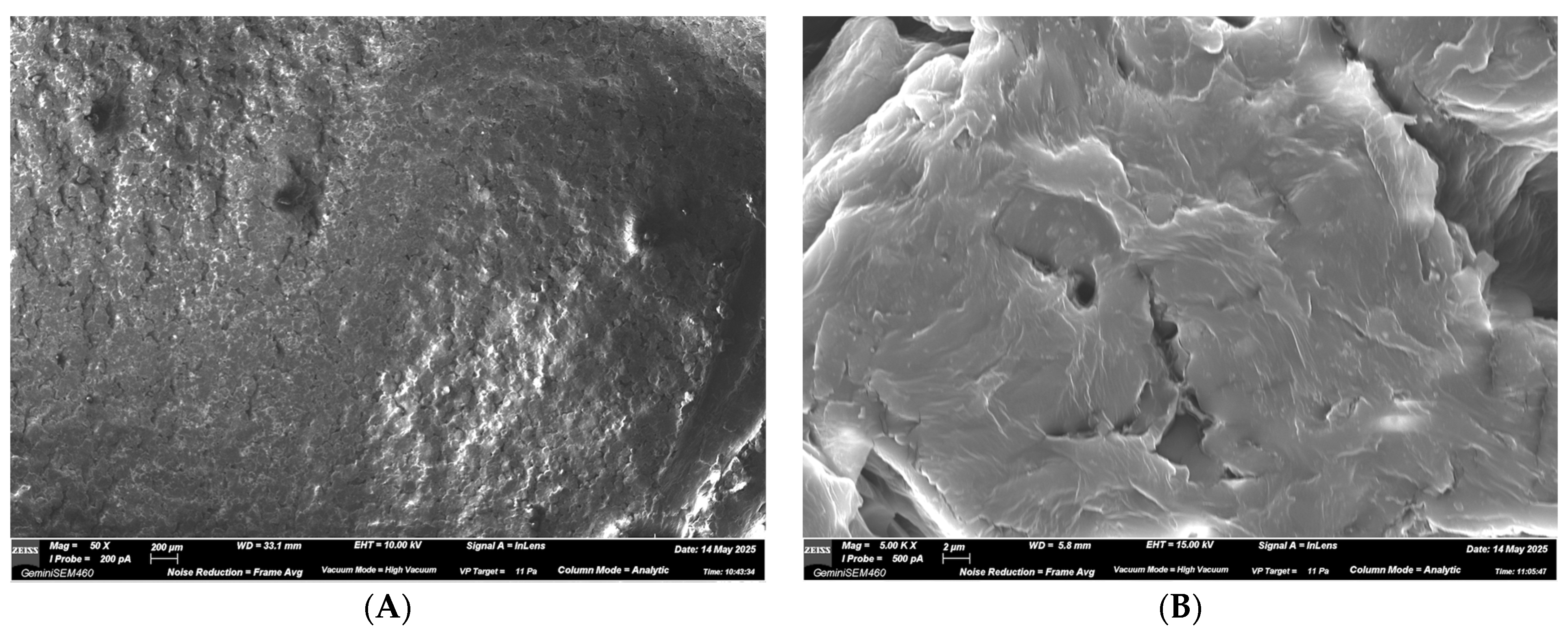
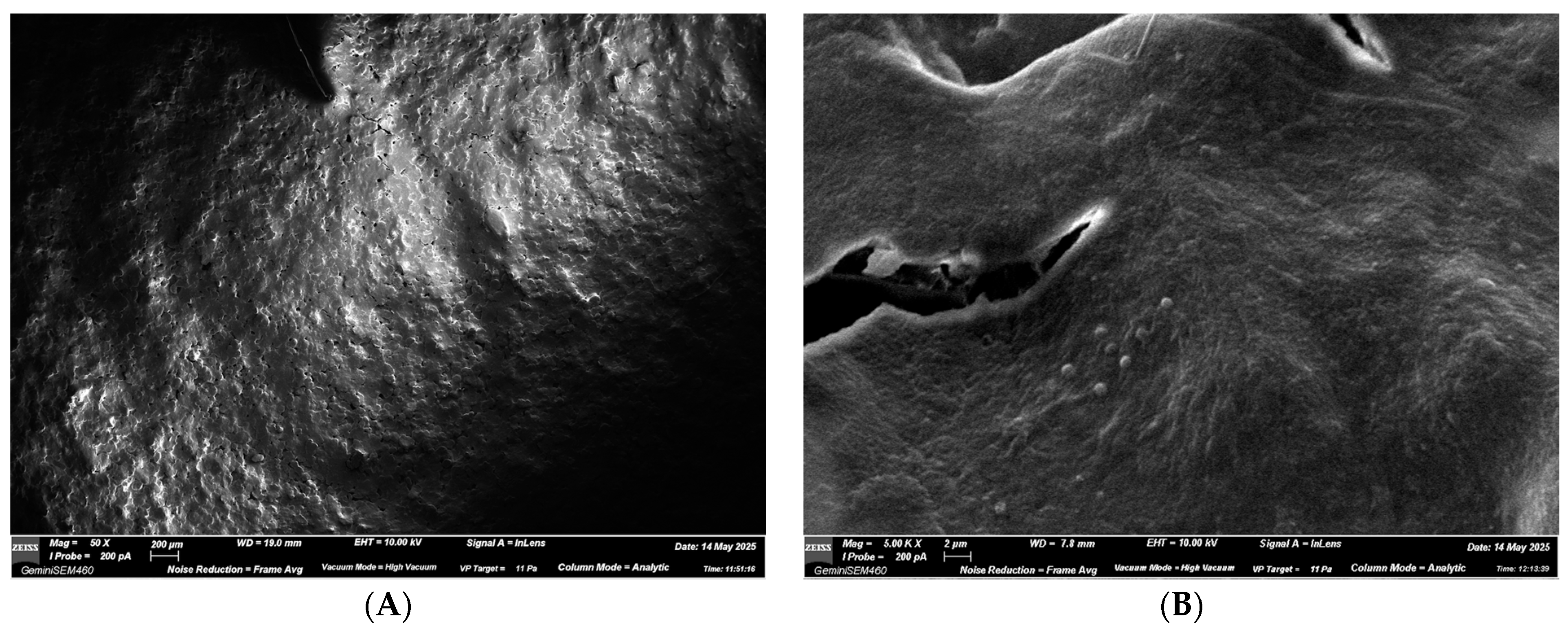
3.2. SEM Observations
- NEW aligner (unused);
- USED aligner worn for 14 nights without spray;
- USED aligner worn for 14 nights with spray.
3.2.1. ALIGNER A—NEW Aligner
3.2.2. ALIGNER B—USED Aligner Without Spray
3.2.3. ALIGNER C—USED Aligner with Spray
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ISE | Ion-selective Electrode |
| SEM | Scanning Electron Microscope |
| TISAB IV | Total Ionic Strength Adjustment Buffer (version IV) |
| PA | Aliphatic polyamide |
Appendix A
| Comparison | Statistic | p-Value | p-Bonferroni |
|---|---|---|---|
| Baseline vs. T0 | 0 | 0.002 ** | 0.02 * |
| Baseline vs. T15 | 1 | 0.013 * | 0.129 |
| Baseline vs. T30 | 18 | 0.359 | 1.0 |
| Baseline vs. T45 | 22 | 0.625 | 1.0 |
| T0 vs. T15 | 55 | 0.006 ** | 0.059 |
| T0 vs. T30 | 55 | 0.002 ** | 0.02 * |
| T0 vs. T45 | 55 | 0.002 ** | 0.02 * |
| T15 vs. T30 | 55 | 0.002 ** | 0.02 * |
| T15 vs. T45 | 55 | 0.002 ** | 0.02 * |
| T30 vs. T45 | 39 | 0.058 | 0.58 |
| Comparison | Statistic | p-Value | p-Bonferroni |
|---|---|---|---|
| Baseline vs. T0 | 0 | 0.002 ** | 0.02 * |
| Baseline vs. T15 | 0 | 0.002 ** | 0.02 * |
| Baseline vs. T30 | 0 | 0.002 ** | 0.02 * |
| Baseline vs. T45 | 0 | 0.002 ** | 0.02 * |
| T0 vs. T15 | 55 | 0.002 ** | 0.02 * |
| T0 vs. T30 | 55 | 0.002 ** | 0.02 * |
| T0 vs. T45 | 55 | 0.002 ** | 0.02 * |
| T15 vs. T30 | 55 | 0.002 ** | 0.02 * |
| T15 vs. T45 | 55 | 0.002 ** | 0.02 * |
| T30 vs. T45 | 44 | 0.013 | 0.129 |
References
- Rosvall, M.D.; Fields, H.W.; Ziuchkovski, J.; Rosenstiel, S.F.; Johnston, W.M. Attractiveness, acceptability, and value of orthodontic appliances. Am. J. Orthod. Dentofacial Orthop. 2009, 135, 276.e1–276.e12; discussion 276–277. [Google Scholar] [CrossRef]
- Soygun, K.; Bolayir, G.; Boztug, A. Mechanical and thermal properties of polyamide versus reinforced PMMA denture base materials. J. Adv. Prosthodont. 2013, 5, 153–160. [Google Scholar] [CrossRef]
- Cremonini, F.; Brucculeri, L.; Pepe, F.; Palone, M.; Lombardo, L. Comparison of stress relaxation properties between 3-dimensional printed and thermoformed orthodontic aligners: A pilot study of in vitro simulation of two consecutive 8-hours force application. APOS Trends Orthod. 2024, 14, 225–234. [Google Scholar] [CrossRef]
- Palone, M.; Longo, M.; Arveda, N.; Nacucchi, M.; Pascalis, F.; Spedicato, G.A.; Siciliani, G.; Lombardo, L. Micro-computed tomography evaluation of general trends in aligner thickness and gap width after thermoforming procedures involving six commercial clear aligners: An in vitro study. Korean J. Orthod. 2021, 51, 135–141. [Google Scholar] [CrossRef]
- Proffit, W.R.; Henry, W.F.; David, M.S.; James, L.A. Contemporary Orthodontics, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Hahn, W.; Dathe, H.; Fialka-Fricke, J.; Fricke-Zech, S.; Zapf, A.; Kubein-Meesenburg, D.; Sadat-Khonsari, R. Influence of thermoplastic appliance thickness on the magnitude of force delivered to a maxillary central incisor during tipping. Am. J. Orthod. Dentofacial Orthop. 2009, 136, 12.e1–12.e7; discussion 12–13. [Google Scholar] [CrossRef]
- Levrini, L.; Novara, F.; Margherini, S.; Tenconi, C.; Raspanti, M. Scanning electron microscopy analysis of the growth of dental plaque on the surfaces of removable orthodontic aligners after the use of different cleaning methods. Clin. Cosmet. Investig. Dent. 2015, 7, 125–131. [Google Scholar] [CrossRef]
- Pardo, A.; Signoriello, A.; Zangani, A.; Messina, E.; Gheza, S.; Faccioni, P.; Albanese, M.; Lombardo, G. Home Biofilm Management in Orthodontic Aligners: A Systematic Review. Dent. J. 2024, 12, 335. [Google Scholar] [CrossRef] [PubMed]
- Wegehaupt, F.; Menghini, G. Update Fluorid [Fluoride Update]. Swiss Dent. J. 2020, 130, 677–683. (In German) [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Guan, L.; Guo, J.; Chuan, A.; Tong, J.; Ban, J.; Tian, T.; Jiang, W.; Wang, S. Application of fluoride disturbs plaque microecology and promotes remineralization of enamel initial caries. J. Oral. Microbiol. 2022, 14, 2105022. [Google Scholar] [CrossRef]
- Satou, R.; Yamagishi, A.; Takayanagi, A.; Iwasaki, M.; Kamijo, H.; Sugihara, N. Improved Enamel Acid Resistance by Highly Concentrated Acidulated Phosphate Sodium Monofluorophosphate Solution. Materials 2022, 15, 7298. [Google Scholar] [CrossRef] [PubMed]
- Yudaev, P.; Tupikov, A.; Chistyakov, E. Organocyclophosphazenes and Materials Based on Them for Pharmaceuticals and Biomedicine. Biomolecules 2025, 15, 262. [Google Scholar] [CrossRef]
- Gaudioso, C.; Hao, J.; Martin-Eauclaire, M.F.; Gabriac, M.; Delmas, P. Menthol pain relief through cumulative inactivation of voltage-gated sodium channels. Pain 2012, 153, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Henley-Smith, C.J.; Kok, A.M.; Botha, F.S.; Baker, C.; Lall, N. The effect of a poly-herbal plant extract on the adhesion of Streptococcus mutans to tooth enamel. BMC Complement. Med. Ther. 2024, 24, 402. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Rajula, M.P.; Rao, K.S.; Ravishankar, P.L.; Albar, D.H.; Bahammam, M.A.; Alamoudi, A.; Alzahrani, K.J.; Alsharif, K.F.; Halawani, I.F.; et al. Antimicrobial Efficacy of Blended Essential Oil and Chlorhexidine against Periodontal Pathogen (P. gingivalis)-An In Vitro Study. Niger. J. Clin. Pract. 2023, 26, 625–629. [Google Scholar] [CrossRef]
- Landeo-Villanueva, G.E.; Salazar-Salvatierra, M.E.; Ruiz-Quiroz, J.R.; Zuta-Arriola, N.; Jarama-Soto, B.; Herrera-Calderon, O.; Pari-Olarte, J.B.; Loyola-Gonzales, E. Inhibitory Activity of Essential Oils of Mentha spicata and Eucalyptus globulus on Biofilms of Streptococcus mutans in an In Vitro Model. Antibiotics 2023, 12, 369. [Google Scholar] [CrossRef]
- Alexa, V.T.; Obistioiu, D.; Dumitrescu, R.; Cretescu, I.; Hulea, A.; Bolchis, V.; Balean, O.; Jumanca, D.; Galuscan, A. In Vitro Evaluation of Biofilm Formation by Oral Microorganisms on Clear Aligner Materials: Influence of Mouthwash Exposure. J. Funct. Biomater. 2025, 16, 424. [Google Scholar] [CrossRef]
- Panagiotou, A.; Rossouw, P.E.; Michelogiannakis, D.; Javed, F. Role of Essential Oil-Based Mouthwashes in Controlling Gingivitis in Patients Undergoing Fixed Orthodontic Treatment. A Review of Clinical Trials. Int. J. Environ. Res. Public. Health 2021, 18, 10825. [Google Scholar] [CrossRef]
- Patil, K.B.; Gulve, N.D.; Patil, R.K. Effectiveness of an Essential Oil Mouthwash in Maintaining Proper Oral Health in Orthodontic Patients—An In Vivo Study. J. Indian. Orthod. Soc. 2023, 57, 75–83. [Google Scholar] [CrossRef]
- Araujo Marcelo, W.B.; Charles, C.A.; Weinstein, R.B.; McGuire, J.A.; Parikh-Das, A.M.; Du, Q.; Zhang, J.; Berlin, J.A.; Gunsolley, J.C. Meta-analysis of the effect of an essential oil–containing mouthrinse on gingivitis and plaque. J. Am. Dent. Assoc. 2015, 146, 610–622. [Google Scholar] [CrossRef]
- Naumova, E.A.; Dickten, C.; Jung, R.; Krauss, F.; Rübesamen, H.; Schmütsch, K.; Sandulescu, T.; Zimmer, S.; Arnold, W.H. Dynamics of Fluoride Bioavailability in the Biofilms of Different Oral Surfaces after Amine Fluoride and Sodium Fluoride Application. Sci. Rep. 2016, 6, 18729. [Google Scholar] [CrossRef]
- Yan, D.; Liu, Y.; Che, X.; Mi, S.; Jiao, Y.; Guo, L.; Li, S. Changes in the Microbiome of the Inner Surface of Clear Aligners After Different Usage Periods. Curr. Microbiol. 2021, 78, 566–575. [Google Scholar] [CrossRef]
- Moradinezhad, M.; Abbasi Montazeri, E.; Hashemi Ashtiani, A.; Pourlotfi, R.; Rakhshan, V. Biofilm formation of Streptococcus mutans, Streptococcus sanguinis, Staphylococcus epidermidis, Staphylococcus aureus, Lactobacillus casei, and Candida albicans on 5 thermoform and 3D printed orthodontic clear aligner and retainer materials at 3 time points: An in vitro study. BMC Oral. Health 2024, 24, 1107. [Google Scholar] [CrossRef]
- Reshetnyak, V.Y.; Nesterova, O.V.; Admakin, O.I.; Dobrokhotov, D.A.; Avertseva, I.N.; Dostdar, S.A.; Khakimova, D.F. Evaluation of free and total fluoride concentration in mouthwashes via measurement with ion-selective electrode. BMC Oral. Health 2019, 19, 251. [Google Scholar] [CrossRef]
- Eriwati, Y.; Putriani, D.; Geraldine, K.; Hermansyah, H. Peppermint flavor oil in fluoride varnishes enhances fluoride release. Sci. Dent. J. 2021, 5, 133. [Google Scholar] [CrossRef]
- Pessan, J.P.; Toumba, K.J.; Buzalaf, M.A.R. Topical use of fluorides for caries control. Monogr. Oral. Sci. 2011, 22, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chen, J.; Bai, D.; Wang, P.; Shu, R. Effects of clear aligners and traditional removable appliances on oral microbiome in mixed dentition: A comparative study. BMC Oral. Health 2024, 24, 1276. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, L.; Martini, M.; Cervinara, F.; Spedicato, G.A.; Oliverio, T.; Siciliani, G. Comparative SEM analysis of nine F22 aligner cleaning strategies. Prog. Orthod. 2017, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Nazzal, H.; Duggal, M.S.; Kowash, M.B.; Kang, J.; Toumba, K.J. Comparison of residual salivary fluoride retention using amine fluoride toothpastes in caries-free and caries-prone children. Eur. Arch. Paediatr. Dent. 2016, 17, 165–169. [Google Scholar] [CrossRef]
- Albahrani, M.M.; Alyahya, A.; Qudeimat, M.A.; Toumba, K.J. Salivary fluoride concentration following toothbrushing with and without rinsing: A randomised controlled trial. BMC Oral. Health 2022, 22, 53. [Google Scholar] [CrossRef]
- Duckworth, R.M.; Morgan, S.N. Oral fluoride retention after use of fluoride dentifrices. Caries Res. 1991, 25, 123–129. [Google Scholar] [CrossRef]
- Naumova, E.A.; Weber, L.; Pankratz, V.; Czenskowski, V.; Arnold, W.H. Bacterial viability in oral biofilm after tooth brushing with amine fluoride or sodium fluoride. Arch. Oral. Biol. 2019, 97, 91–96. [Google Scholar] [CrossRef]
- Marinho, V.C.; Higgins, J.P.; Sheiham, A.; Logan, S. Combinations of topical fluoride (toothpastes, mouthrinses, gels, varnishes) versus single topical fluoride for preventing dental caries in children and adolescents. Cochrane Database Syst. Rev. 2004, 2004, CD002781. [Google Scholar] [CrossRef]
- Talwar, M.; Tewari, A.; Chawla, H.S.; Sachdev, V.; Sharma, S. Fluoride Concentration in Saliva following Professional Topical Application of 2% Sodium Fluoride Solution. Contemp. Clin. Dent. 2019, 10, 423–427. [Google Scholar] [CrossRef]
- Marinho, V.C.; Higgins, J.P.; Sheiham, A.; Logan, S. Fluoride toothpastes for preventing dental caries in children and adolescents. Cochrane Database Syst. Rev. 2003, 2003, CD002278. [Google Scholar] [CrossRef] [PubMed]
- Polat Sagsoz, N.; Orhan, F.; Baris, O.; Sagsoz, O. In Vitro Evaluation of Plant Antimicrobials Against Candida albicans Biofilm on Denture Base Materials: A Comparison with Chemical Denture Cleansers. Polymers 2025, 17, 2869. [Google Scholar] [CrossRef] [PubMed]
- Kiatsirirote, K.; Sitthisettapong, T.; Phantumvanit, P.; Chan, D.C.N. Fluoride-Releasing Effect of a Modified Resin Denture Containing S-PRG Fillers on Salivary Fluoride Retention: A Randomized Clinical Study. Caries Res. 2019, 53, 137–144. [Google Scholar] [CrossRef]
- Dagli, N.; Dagli, R.; Mahmoud, R.; Baroudi, K. Essential oils, their therapeutic properties, and implication in dentistry: A review. J. Int. Soc. Prev. Community Dent. 2015, 5, 335–340. [Google Scholar] [CrossRef]
- Abdelrahman, S.M.; El Samak, M.; El-Baz, L.M.F.; Hanora, A.M.S.; Satyal, P.; Dosoky, N.S. Effects of Mint Oils on the Human Oral Microbiome: A Pilot Study. Microorganisms 2024, 12, 1538. [Google Scholar] [CrossRef] [PubMed]
- Dobler, D.; Runkel, F.; Schmidts, T. Effect of essential oils on oral halitosis treatment: A review. Eur. J. Oral. Sci. 2020, 128, 476–486. [Google Scholar] [CrossRef]

| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Age > 18 years | Systemic diseases or medications affecting salivary flow |
| Ongoing orthodontic treatment with nighttime 3D-printed polyamide aligners | Untreated active caries, gingivitis or periodontitis in acute phase |
| Good general hygiene | Use of fluoride-containing products during observation |
| Normal salivary flow | Known allergy to fluorides or peppermint essential oil |
| No use of fluoride products in the previous 24 h | Pregnancy or breastfeeding |
| Ability and willingness to follow the protocol | Poor compliance with study requirements |
| Signed informed consent |
| Time Point | NEW Aligner (Mean ± SD) | USED Aligner (Mean ± SD) |
|---|---|---|
| Baseline | 0.76 ± 0.14 a | 0.83 ± 0.27 a |
| T0 | 5.96 ± 4.41 b | 8.42 ± 4.05 b |
| T15 | 1.37 ± 0.71 c * | 2.29 ± 0.59 c * |
| T30 | 0.91 ± 0.19 c * | 1.40 ± 0.30 c * |
| T45 | 0.81 ± 0.17 ac * | 1.18 ± 0.17 ac * |
| Time Point | Z Statistic | p-Value | p-Bonferroni | p-FDR |
|---|---|---|---|---|
| Baseline | −0.595 | 0.582 | 1.000 | 0.582 |
| T0 | −1.30 | 0.222 | 1.000 | 0.278 |
| T15 | −2.51 | 0.0035 | 0.0175 * | 0.00583 ** |
| T30 | −2.82 | 0.0017 | 0.0085 ** | 0.005 ** |
| T45 | −2.84 | 0.002 | 0.010 ** | 0.005 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cremonini, F.; Bernardi, A.; Bernardi, A.; Lombardo, L. Integration of a Fluoride- and Mint-Based Spray in Nighttime Aligner Therapy: Effects on Salivary Concentration and Biofilm. Appl. Sci. 2025, 15, 12435. https://doi.org/10.3390/app152312435
Cremonini F, Bernardi A, Bernardi A, Lombardo L. Integration of a Fluoride- and Mint-Based Spray in Nighttime Aligner Therapy: Effects on Salivary Concentration and Biofilm. Applied Sciences. 2025; 15(23):12435. https://doi.org/10.3390/app152312435
Chicago/Turabian StyleCremonini, Francesca, Anna Bernardi, Alberto Bernardi, and Luca Lombardo. 2025. "Integration of a Fluoride- and Mint-Based Spray in Nighttime Aligner Therapy: Effects on Salivary Concentration and Biofilm" Applied Sciences 15, no. 23: 12435. https://doi.org/10.3390/app152312435
APA StyleCremonini, F., Bernardi, A., Bernardi, A., & Lombardo, L. (2025). Integration of a Fluoride- and Mint-Based Spray in Nighttime Aligner Therapy: Effects on Salivary Concentration and Biofilm. Applied Sciences, 15(23), 12435. https://doi.org/10.3390/app152312435






