1. Introduction
Acrylic denture base resin, composed of poly(methyl methacrylate) (PMMA), remains the benchmark material for removable prostheses. It has a translucent pink hue that mimics gingival tissue; it can be processed economically (by compression moulding, injection, or CAD/CAM milling); and it offers a clinically acceptable balance of flexural strength, impact toughness, and colour stability [
1]. Chronic exposure to trace metals (e.g., cadmium, nickel) released from denture polymers has been linked to cytotoxicity and allergic reactions [
2,
3]. Incomplete polymerisation and subsequent hydrolytic degradation can also leach residual monomer, additives, and pigments, compromising biocompatibility [
2,
4]. Even sub-cytotoxic levels of released nickel may provoke oral hypersensitivity in sensitised individuals [
3].
The European Medical Device Regulation (MDR 2017/745) requires manufacturers to demonstrate chemical safety through a structured risk-management process. ISO 10993-18 provides an analytical framework for quantitatively identifying organic and inorganic constituents that may be released under clinically relevant conditions [
5]. Beyond toxicological considerations, leachate analysis is also a sensitive indicator of material degradation that can precede a loss of mechanical integrity.
Advances in polymer chemistry have transformed denture fabrication from conventional chairside heat-cured PMMA doughs to computer-aided design/computer-aided manufacturing (CAD/CAM) milled blanks and photopolymerised three-dimensional (3D)-printed resins. Comparative, multi-element leaching data covering all three manufacturing routes under clinically relevant pH conditions remain scarce. Most prior studies focused on organic residuals or single ions, often at neutral pH, leaving a critical evidence gap that directly affects material selection in daily prosthodontic practice.
CAD/CAM denture base blanks are polymerised at ≥130 °C under >200 MPa pressure, achieving a degree of conversion near 99% and leaving negligible residual monomer [
6,
7,
8]. 3D-printed denture resins rely on photo-curing and require meticulous post-processing. Sequential isopropanol washes and high-intensity post-curing can remove ~80% of unreacted oligomers and increase conversion by ~10–15% [
9,
10,
11]. Nonetheless, any residual photoinitiator or oligomer may leach out if curing is incomplete, especially after mechanical abrasion or hydrolytic ageing [
10,
12]. A systematic review confirmed that CAD/CAM-milled PMMAs consistently reach higher conversion than conventionally cured resins, although long-term chemical stability data for 3D-printed materials remain limited [
13]. When properly post-cured, 3D-printed denture base and occlusal-splint resins can attain flexural strength and hardness comparable to or exceeding those of heat-cured PMMA [
14,
15].
Hydrolytic scission of PMMA chains and enzymatic attack by oral microflora have been implicated in the loss of flexural strength and the formation of microcracks that predispose acrylic prostheses to fracture [
16,
17]. For context, normal human saliva contains about 6–10 mg L
−1 of calcium; accordingly, even the highest calcium release observed in our study (~3 mg L
−1) would have a negligible impact on overall oral calcium levels. Similar ageing-related declines in mechanical properties have been observed in resin-based composites used for long-term interim prostheses [
18]. The oral environment is chemically dynamic: dietary acids, bacterial metabolism, and reduced salivary flow can intermittently lower pH to <5.5, whereas salivary buffering can raise it to >8.0 [
19,
20]. Sustained acidity accelerates PMMA ester hydrolysis and increases the solubility of metallic pigments, leading to colour changes and surface roughening that facilitate microbial adhesion [
21,
22,
23,
24,
25]. This surface deterioration is clinically relevant, as it promotes
Candida albicans and mixed-species biofilm formation associated with denture stomatitis [
25,
26,
27]. Recent in vitro work comparing CAD/CAM-milled, 3D-printed and conventional denture base resins has demonstrated that milled materials exhibit the lowest *C. albicans* adhesion and the highest flexural strength [
26]. A systematic review likewise concluded that CAD/CAM-milled resins reduce microbial adhesion relative to conventional and 3D-printed resins, whereas the latter show a pronounced tendency for *C. albicans* colonisation [
28]. We did not perform microbial adhesion testing in this study; these statements summarise prior reports and are noted as a direction for future work. These findings underscore that differences in microstructure and surface properties, rather than polymer chemistry alone, govern biofilm formation; thus, selecting materials with both low ion release and smooth surfaces may confer clinical benefits.
Dental polymers often contain colourants (e.g., ferric oxide and, historically, cadmium-based pigments, though Cd use is now restricted) [
29]. Catalyst residues (such as zinc or cobalt salts) and bactericidal additives (e.g., silver-containing nanomaterials) can also leach into the oral cavity [
3,
30]. In addition, functional fillers meant to improve aesthetics or impart antimicrobial activity (for instance, Mn-doped alumina pigments or bioactive glass) can alter the ionic profile of eluates [
31,
32]. In addition to conventional pigments and catalyst residues, reinforcing fillers such as calcium β-pyrophosphate have been incorporated into PMMA to improve mechanical performance. PMMA composites containing 0.5–1% β-CPP that were ball-milled for 6 h showed a significant increase in flexural strength compared with unmodified PMMA [
33]. Similarly, adding bioactive glass fillers to acrylic resins was reported to decrease C. albicans adhesion and increase hardness [
32]. Such compositional modifications may not only enhance mechanical and thermal properties but also alter the spectrum of ions released during ageing and reduce microbial colonisation. Elevated ion release under acidic conditions has been documented in orthodontic acrylic appliances as well [
34].
Patients with persistently low salivary pH (e.g., poorly controlled diabetics) may face an elevated risk of denture polymer degradation and mucosal inflammation [
19,
20]. Yet, comprehensive data comparing conventional, CAD/CAM-milled, and 3D-printed denture resins across clinically relevant pH ranges and time spans are lacking. The present study aimed to (i) quantify the release of selected ions (Ca, Na, K, Mg, Zn, Ni, Cu, Cr, Fe, Ti, Cd, Pb) from denture base polymers made via conventional heat-curing, CAD/CAM milling, and 3D printing, and (ii) assess the influence of material type, immersion pH, and immersion duration on ion release. The null hypotheses were that (1) ion release would not differ between materials, and (2) neither pH nor immersion duration would significantly affect ion concentrations.
4. Discussion
Previous investigations have proposed that bioactive glass and fluoride-releasing fillers inhibit fungal adhesion while providing sustained release of Na
+ and Ca
2+ that elevates pH and creates an environment unfavourable to oral microbiota [
32]. Incorporating such fillers into denture base materials could therefore mitigate ion depletion while conferring antimicrobial benefits. The interplay between ion release, pH changes, and biofilm formation requires systematic evaluation.
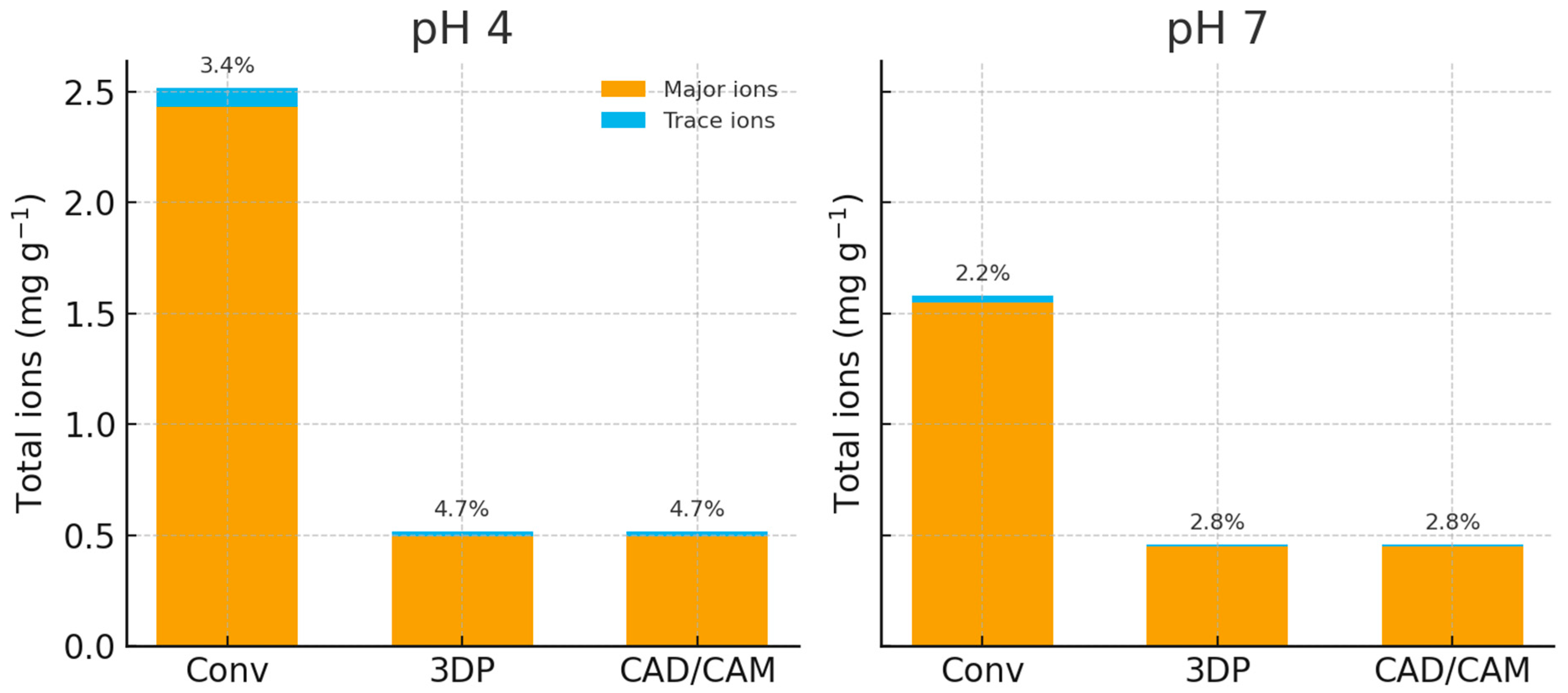
Overview of findings. In this study, we quantified inorganic ion release from three denture base materials (conventional heat-cured, CAD/CAM-milled, and 3D-printed) under two pH conditions and two immersion durations. The null hypotheses were only partially supported. Material type and pH had significant effects on ion release (
p < 0.01), whereas prolonging immersion from 24 h to 30 d had only a minimal effect (significant differences arose only for Na). The CAD/CAM-milled resin leached far fewer ions than the conventional heat-cured acrylic, consistent with the notion that a highly polymerised, dense polymer network is more inert in aqueous environments [
7]. The 3D-printed resin showed intermediate behaviour, resembling the conventional acrylic for certain ions (e.g., Ca, Mg) but aligning with the CAD/CAM resin for others, reflecting differences in the bulk elemental compositions of the materials (
Table 2). Across conditions, cumulative release followed the hierarchy conventional > 3D-printed > CAD/CAM; acidic pH selectively increased Ca, Cr, Ni, and Zn, and the effect of time was minimal except for a small but significant increase in Na.
Figure 1 compares the total ion release across materials and pH conditions. The conventional heat-cured PMMA released substantially more total ions than either the 3D-printed or CAD/CAM resin. Calcium, Mg, K, and Na dominated the conventional PMMA’s eluate, with trace metals contributing <10%. The CAD/CAM material released only about one-fifth of the total ion mass of the conventional, underscoring how a nearly fully cured, dense polymer network limits leaching.
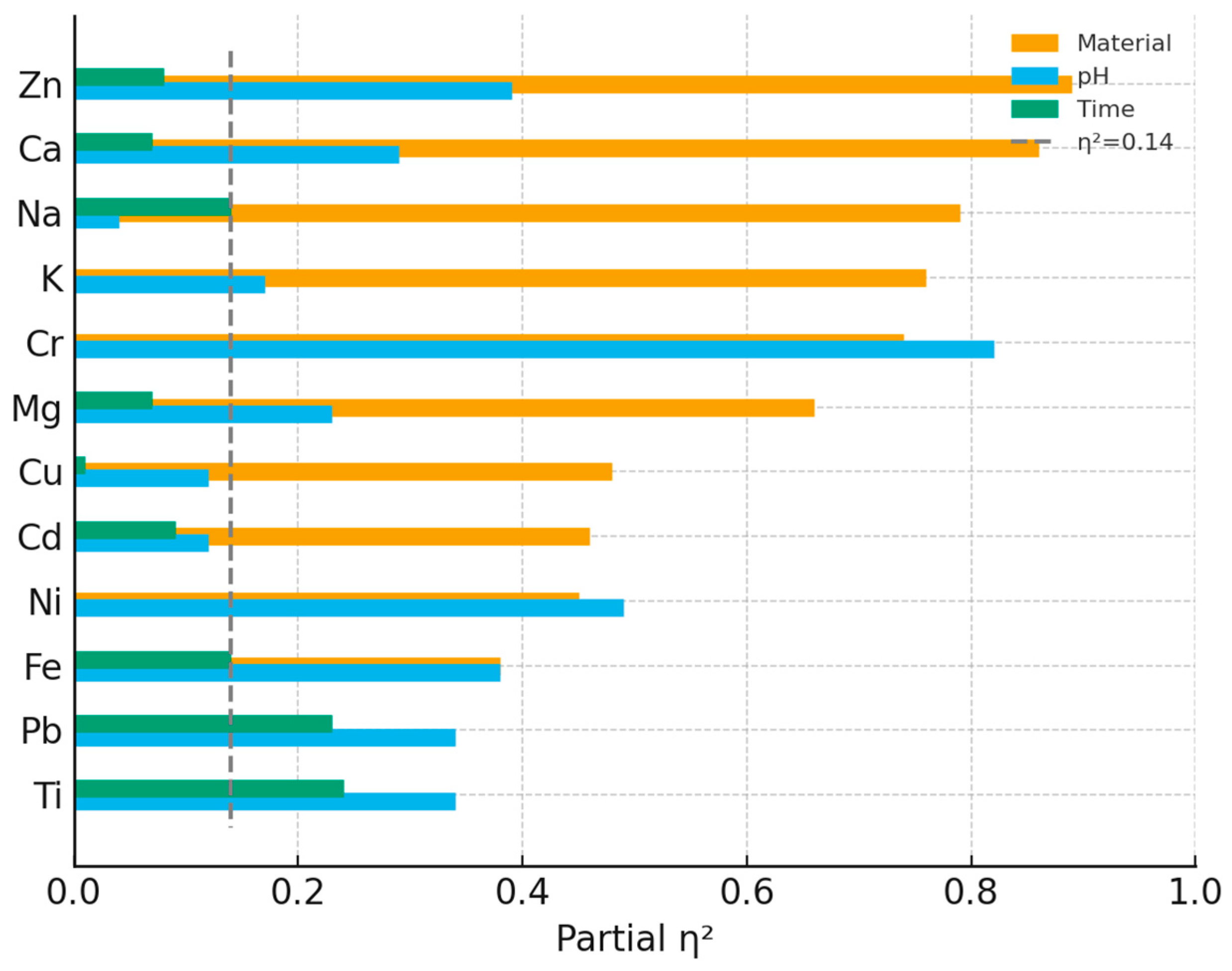
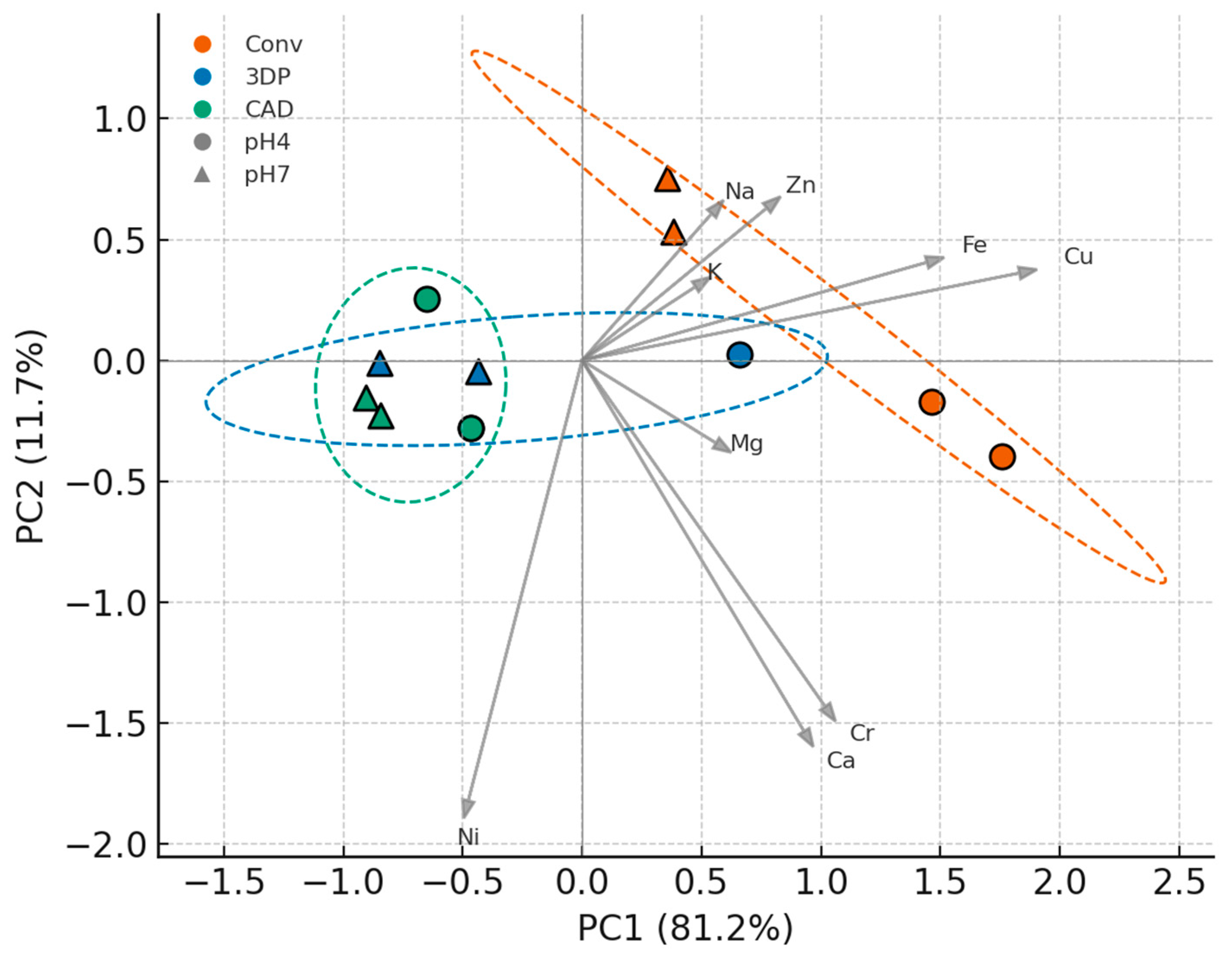
Figure 5 (PCA) further shows that material composition (rather than pH) was the primary driver of multivariate differences: conventional PMMA samples clustered at negative PC1 (high Ca/Mg), CAD/CAM samples at positive PC1 (elevated Ni, K, Na), and 3D-printed samples in between. The 95% confidence ellipses indicated a tight cluster for the CAD/CAM group and a more dispersed cluster for the 3D-printed group, implying greater inherent variability in the latter. An effect-size analysis (
Figure 3) reinforced this pattern: material formulation accounted for the largest share of variance in every element except Pb and Ti, whereas pH and time contributed comparatively little.
Acidic (pH 4) conditions increased total ion release by roughly 40% overall, driven largely by specific ions (especially Ca, Cr, Ni, Zn). The volcano plot (
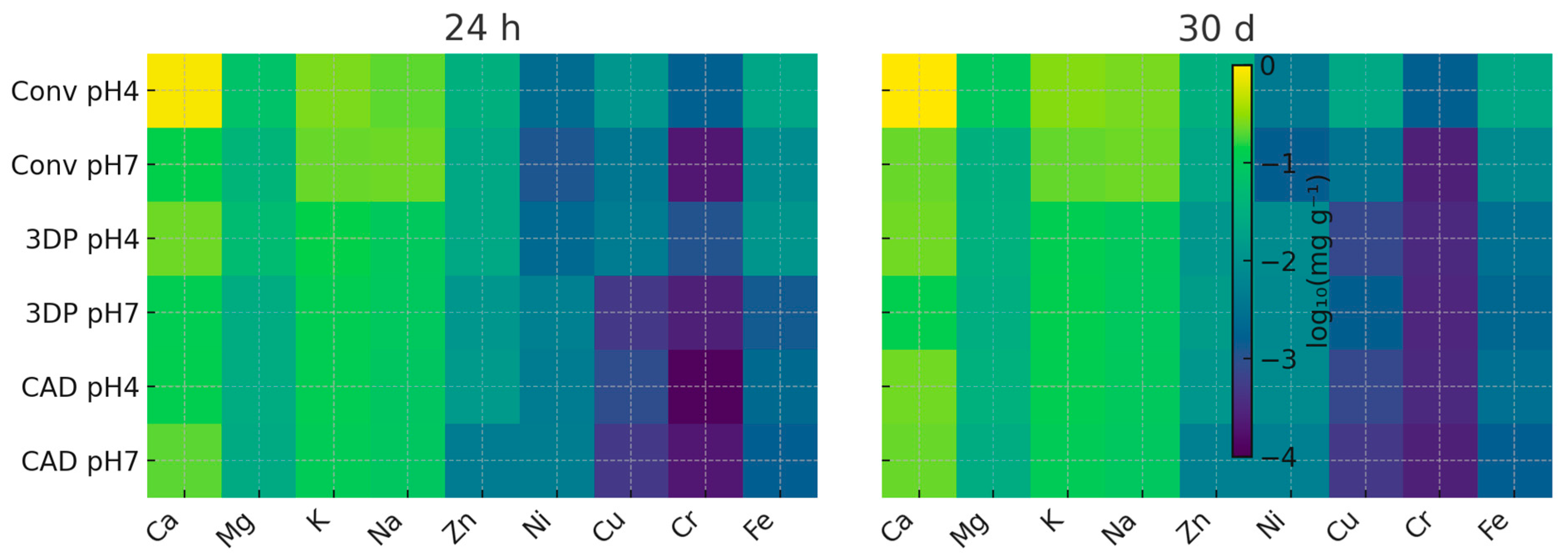
Figure 2) highlights Ca, Cr, Ni, and Zn as the most pH-sensitive ions, whereas Na and K changed little between pH 7 and pH 4. Immersion time had only a limited effect: the heat maps (
Figure 3) show that the ion release profile at 24 h was nearly identical to that at 30 d, indicating that most leaching occurred within the first day (a “burst release”). The minimal time effect (
Figure 3) reinforces this, suggesting that a one-day pre-soak in water could remove the majority of leachable species. Even under pH 4 conditions, Ni and other trace-metal levels remained several orders of magnitude below cytotoxic thresholds [
5].
Material formulation was the dominant factor controlling ion leaching from these denture resins. Ion release varied significantly with material type and pH (rejecting the first null hypothesis and the pH portion of the second), whereas extending immersion beyond 24 h had negligible impact (supporting the time portion of the second hypothesis). Conventional heat-cured PMMA released the greatest total ion load, the 3D-printed resin an intermediate amount, and the highly converted CAD/CAM blank the least. Acidic pH 4 selectively elevated the release of Ca, Ni, Cr, and Zn, whereas other ions were scarcely affected. Approximately 90% of the total ion release occurred within the first 24 h, with only marginal increases up to 30 d. All metal levels stayed in the microgram-per-litre range; even the highest nickel concentration was several orders of magnitude below in vitro cytotoxic thresholds. Thus, the cumulative ion burden is well below biocompatibility risk thresholds [
5].
Table 5 compares the worst-case ion concentrations from this study to normal salivary levels and toxicological benchmarks. For example, the highest calcium concentration (~3 mg L
−1) was about half the 6–10 mg L
−1 typical of resting saliva. The peak nickel level (0.008 mg L
−1) fell within the background range reported for stainless steel orthodontic appliances and was well below in vitro cytotoxic concentrations reported for nickel in dental materials. The sodium release (0.25 mg L
−1) exceeded that of a laboratory S-PRG-filled resin but remained far below any level of concern. Strontium, cadmium, and lead were not detected at all, reflecting the high purity of the filler-free, pigment-free PMMA blanks. Collectively, these observations indicate that inorganic ion release from contemporary denture bases poses minimal systemic or local risk, although nickel-hypersensitive patients may still react to even trace Ni.
These results indicate that ion release is largely governed by the material’s composition and chemical binding state rather than by diffusion kinetics alone, consistent with previous reports for acrylic appliances and pigment/filler effects [
2,
29,
31,
32,
34]. For example, the conventional resin’s higher Ca and Zn contents corresponded to greater Ca
2+ and Zn
2+ leaching, whereas the CAD/CAM blank, which contained trace Ni, released slightly more Ni despite its otherwise inert network. The 3D-printed resin, with moderate Ca but very low trace-metal content, showed intermediate release. There was a strong correlation between each material’s bulk elemental inventory and its 30-day cumulative leachate (Pearson r ≈ 0.8 across elements,
p < 0.001). The fraction of each element released likely reflects its chemical form and the polymer’s microstructure (e.g., porosity and cross-link density). We did not quantify porosity/density in this study; future work will map porosity by micro-CT and measure density by Archimedes/helium pycnometry, and relate these metrics to ion release. For instance, the 3D-printed resin contained ~20-fold more Ti than the other materials, yet its Ti release was only ~4-fold higher, consistent with TiO
2 pigment particles remaining largely trapped within the highly cross-linked urethane dimethacrylate network [
1,
10]. The conventional PMMA (whose powder is lubricated with Zn- and Ca-stearates [
36] exhibited disproportionately high leaching of those alkaline-earth ions after 24 h. The Ca-rich CAD/CAM blank released comparatively little Ca
2+, indicating that most Ca was bound in poorly soluble salts formed during its high-temperature, high-pressure polymerisation [
18]. The modest but significant Ni release observed in the CAD/CAM group can be attributed to trace Ni contamination in the blank (below the detectable level in bulk composition), plausibly introduced via milling-tool abrasion or pigment impurities. These findings underscore that both elemental makeup and chemical binding state dictate ion mobility, helping explain why modern industrially cured resins outperform chairside acrylics in chemical stability [
7]. This interpretation is consistent with differences in degree of conversion: heat-cured denture acrylics typically retain some residual monomer and porosity that can harbour soluble salts, whereas industrially polymerised CAD/CAM blanks are nearly fully cured and void-free [
7]. The 3D-printed resin, although post-cured, may still contain unreacted multifunctional monomers and photoinitiator residues that can leach or facilitate ion diffusion upon ageing. Post-curing parameters influence DC and leaching behaviour, so using a single protocol without DC quantification may have affected the observed release profile [
8,
9,
10,
11,
12].
Biocompatibility perspective: The absolute quantities of ions released were small. Under the worst-case condition (pH 4, 30 d), calcium in eluates was ~3 mg L−1, which is roughly half of typical salivary levels (6–10 mg L−1) and negligible relative to dietary intake (~1000 mg day−1). Nickel peaked at ~0.008 mg L−1, which is more than 100-fold below commonly cited in vitro cytotoxic thresholds (~1 mg L−1). Cadmium and lead were not detected. These values indicate very large margins of safety for systemic exposure. Nickel hypersensitivity remains possible in sensitised patients, so clinical screening is advisable.
Potentially toxic metals (Ni, Cr, Cd, Pb) were either not detected or found only in trace quantities. Nickel was measurable in the low µg L
−1 range, peaking at ~0.008 mg L
−1 from the CAD/CAM resin, but this is over two orders of magnitude below in vitro cytotoxic levels (>1 mg L
−1) [
3]. Nickel remains one of the most common oral allergens; thus, even microgram-level Ni exposure could elicit mucosal reactions in sensitised individuals [
3]. Cadmium and lead were essentially absent (<0.002 mg L
−1), easing historical concerns from the days when denture plastics were pigmented with cadmium compounds [
2]. All three tested resins are unlikely to cause adverse systemic or local effects via inorganic ion release.
Clinical note: Even microgram-per-litre levels of nickel could provoke contact stomatitis in highly Ni-sensitised patients. Clinicians are advised to inquire about Ni allergies and, if present, to consider using CAD/CAM-milled or 3D-printed denture bases. In our study, these materials released ≤0.008 mg L
−1 Ni under worst-case conditions, well below any known threshold for mucosal reactions [
5].
Even under the most aggressive test condition (conventional PMMA at pH 4 for 30 d), the concentrations of Ni, Cr, and Cu remained <0.05 mg g
−1, well below toxicologically concerning thresholds for systemic exposure. The consistent non-detection of Pb and Cd underscores the high raw-material purity of contemporary denture polymers, in contrast to early reports of cadmium leaching from older denture plastics [
2,
29]. Comparative ion release data from the present study and key previous investigations are summarised in
Table 6.
Comparative insights: Earlier work focused almost exclusively on cadmium leaching from conventional denture polymers, yielding only microgram quantities even under strongly acidic conditions [
2,
29]. The present study is the first to evaluate a broad spectrum of inorganic ions released from both traditional and modern denture base materials. Our findings indicate that contemporary polymers release similarly small absolute amounts, heavy metals in the µg L
−1 range and benign ions like Ca in a few mg L
−1. The CAD/CAM blank yielded no detectable Cd or Pb, highlighting modern raw-material purity.
Nickel from our CAD/CAM samples (~0.008 mg L
−1) is >100-fold below the in vitro cytotoxic threshold of ≈1 mg L
−1.3 and comparable to salivary Ni levels reported for orthodontic appliances (a few µg L
−1.3). Nevertheless, clinicians should screen highly Ni-sensitised patients; these patients, when indicated, should be managed with CAD/CAM or 3D-printed bases, which still released ≤0.008 mg L
−1 Ni, well below the mucosal reaction threshold in ISO 10993-18 [
5].
The current results equal or surpass historical performance, confirming that today’s industrially polymerised denture bases contribute negligibly to systemic metal exposure while maintaining excellent biocompatibility.
Material differences: The conventional acrylic consistently leached more divalent cations (Ca
2+, Mg
2+, Zn
2+) than the other materials. One explanation is that the powder component of conventional denture PMMA often contains zinc stearate (as a processing lubricant and polymerisation accelerator) and other additives that could release Zn and Ca salts [
36]. Titanium or zinc oxide may also be present as white pigments, along with other colourants that incorporate metal oxides [
1,
13,
31]. The high-temperature, high-pressure curing process for the CAD/CAM disc likely binds these additives tightly, greatly reducing their release. The 3DP resin’s intermediate Ca/Mg release may derive from filler particles or Ca-based stabilisers in its formulation. The CAD/CAM resin’s higher Ni content (and slightly higher K/Na) might reflect trace metal catalysts or pigments used during its industrial polymerisation or metal impurities from milling tools [
3]. Even though the 3DP resin is a “cold” polymerised system (UV-cured), it did not release more residual initiator metals (such as Cu or Fe from photoinitiators) than the others: Fe and Cu levels were uniformly low across all groups, implying negligible leaching of any photoinitiator-derived metals.
Effect of pH: The pronounced pH effect for ions like Ca and Mg (approximately four-fold higher Ca at pH 4 vs. 7) is likely due to the presence of acid-soluble salts or fillers (e.g., CaCO
3, MgO) in the resin matrix that dissolve at low pH [
34]. Traces of metallic pigments could explain the pH-dependent increase in Cr and Ni as well, consistent with reports that acidic conditions promote metal ion release from acrylic appliances [
34]. Clinically, patients with very acidic oral conditions (e.g., high dietary acid intake or poor hygiene leading to acidic plaque) might experience marginally higher ion leaching. Although the absolute amounts released are negligible toxicologically, over long periods the selective loss of Ca/Zn could subtly affect the material (for instance, slight surface chalkiness or colour change due to mineral loss) [
20,
33].
Effect of time. The lack of a substantial time effect beyond the initial 24 h indicates a classic burst release: most readily soluble species (residual salts, unreacted surface additives) leach out during day 1, after which the release rate plateaus. The small Na increase (partial η
2 ≈ 0.23;
p = 0.025) is consistent with gradual diffusion of highly soluble Na-containing species. The condition-specific Ca rise in the conventional resin at pH 4 plausibly reflects slow dissolution of acid-soluble Ca-bearing additives (e.g., stearates/pigments) from sub-surface layers; across all conditions, the Time main effect for Ca was not significant (
p = 0.074;
Table 4). This pattern mirrors reports for organic leachates (e.g., residual monomer) [
5]. In practice, the minimal additional release after day 1 suggests these denture materials do not continuously shed inorganic components; a 24 h water pre-soak likely removes the majority of initial leachables.
Limitations. This in vitro study has several limitations. Pooling the weekly fractions sacrifices kinetic resolution and may obscure short-lived transients. This was a deliberate choice to improve sensitivity for cumulative totals; models that include intermediate pH values (5.5 to 8.0), periodic intake, or continuous flow should be tested. Each experimental condition contained only three specimens (n = 3 per cell; total n = 72 per element), which reduces statistical power and may obscure small effects. The observed effects were large, in particular for the Material factor (partial η
2 approximately 0.38 to 0.89, negligible only for Pb and Ti), which permitted detection of group differences. The 30-day accelerated immersion approximates about six months of intraoral service but may not capture slower degradation. The use of disc specimens with a high surface area to volume ratio may exaggerate leaching compared with complete dentures. Porosity/density and microstructure were not quantified; future studies should include micro-CT, water sorption and pycnometry to relate microstructural features to ion release. The study quantified only inorganic ions; potential organic leachates (residual monomers, additives, pigments) and changes in mechanical properties and surface morphology were outside the scope but remain clinically relevant. In addition, only one post-curing protocol was applied to the 3D-printed resin and the degree of conversion (DC) was not measured; future studies should quantify DC and compare alternative post-curing schedules [
8,
9,
10,
11,
12]. The substitution of values below the limit of detection with one-half of the LOD, while a standard conservative practice, may overestimate mean concentrations of elements that were essentially absent (e.g., Cd, Pb). All materials tested came from a single manufacturer; the experimental 3D-printed resin is a prototype, and its formulation may not represent commercially available printed denture bases. The results for this single material should therefore not be over-generalised. From a regulatory perspective, the measured ion releases are consistent with ISO 10993-18 and MDR 2017/745 considerations for mucosal devices, and the cumulative 30-day values are well below tolerable intake thresholds, supporting the clinical adoption of CAD/CAM and 3D-printed analogues. Nevertheless, future studies should employ larger sample sizes, include multiple commercially available materials, evaluate longer ageing periods (>6 months), incorporate mechanical and biofilm stressors, and examine the relationships between chemical leaching, surface roughness, mechanical performance and microbial adhesion under simulated oral conditions.
Clinical implications: All three tested materials released only trace levels of ions, well below harmful thresholds. The CAD/CAM-milled resin demonstrated the least ion release, aligning with its reported superior physical properties and biostability [
14]. This could translate to better long-term performance in the oral cavity (less water sorption and component leaching). The conventional PMMA, while releasing more ions, has a long history of safe use; the levels observed here do not raise safety alarms but do reflect its more porous, less fully cured nature. The 3D-printed resin performed reasonably, though its intermediate ion release suggests it still contains more leachable components than the CAD/CAM counterpart. As printed denture materials improve in degree of conversion and purity, their chemical stability should approach that of milled resins. For patients, there is no direct evidence that these minute ion releases cause any clinical symptoms. From a materials-science perspective, lower ion release indicates a more stable polymer matrix, which could correlate with less water sorption, less surface staining, and possibly reduced biofilm adherence over time [
24,
25,
26,
27]. The enhanced chemical stability of CAD/CAM and next-generation 3D-printed denture bases supports their clinical use by minimising leachable components and potential long-term degradation. Further studies could investigate whether the observed ion release correlates with changes in the mechanical properties or colour of dentures over time and should integrate standardised microbial adhesion/biofilm challenges.
These trends are visualised in the conceptual overview presented in
Figure 6, which highlights the dominant influence of material type, the accelerating effect of acidic pH, and the fact that >90% of ions are released during the first day while remaining below toxicological limits.
The conventional heat-cured PMMA showed the highest cumulative ion release, the 3D-printed resin showed an intermediate amount, and the CAD/CAM-milled PMMA the lowest. Acidic pH 4 markedly increased the elution of Ca, Ni, Cr and Zn from all materials. More than 90% of the total ion load was liberated within the first 24 h, after which further release was minimal. Although all detected ion concentrations remained below established cytotoxic thresholds, measurable Ni warrants continued scrutiny. The highly converted polymer networks of digitally manufactured resins confer superior chemical stability, and a 24 h water pre-soak of new dentures may help remove initial leachables.