The Scent of Emotion: A Pilot Study on Olfactory Perception Beyond Visual Cues
Abstract
1. Introduction
2. Materials and Methods
2.1. Spices
2.2. Study Procedure
2.3. Psychophysiological Assessment
2.4. ECG Acquisition and Processing
2.5. GSR Acquisition and Processing
2.6. Study Population
2.7. Statistical Analysis
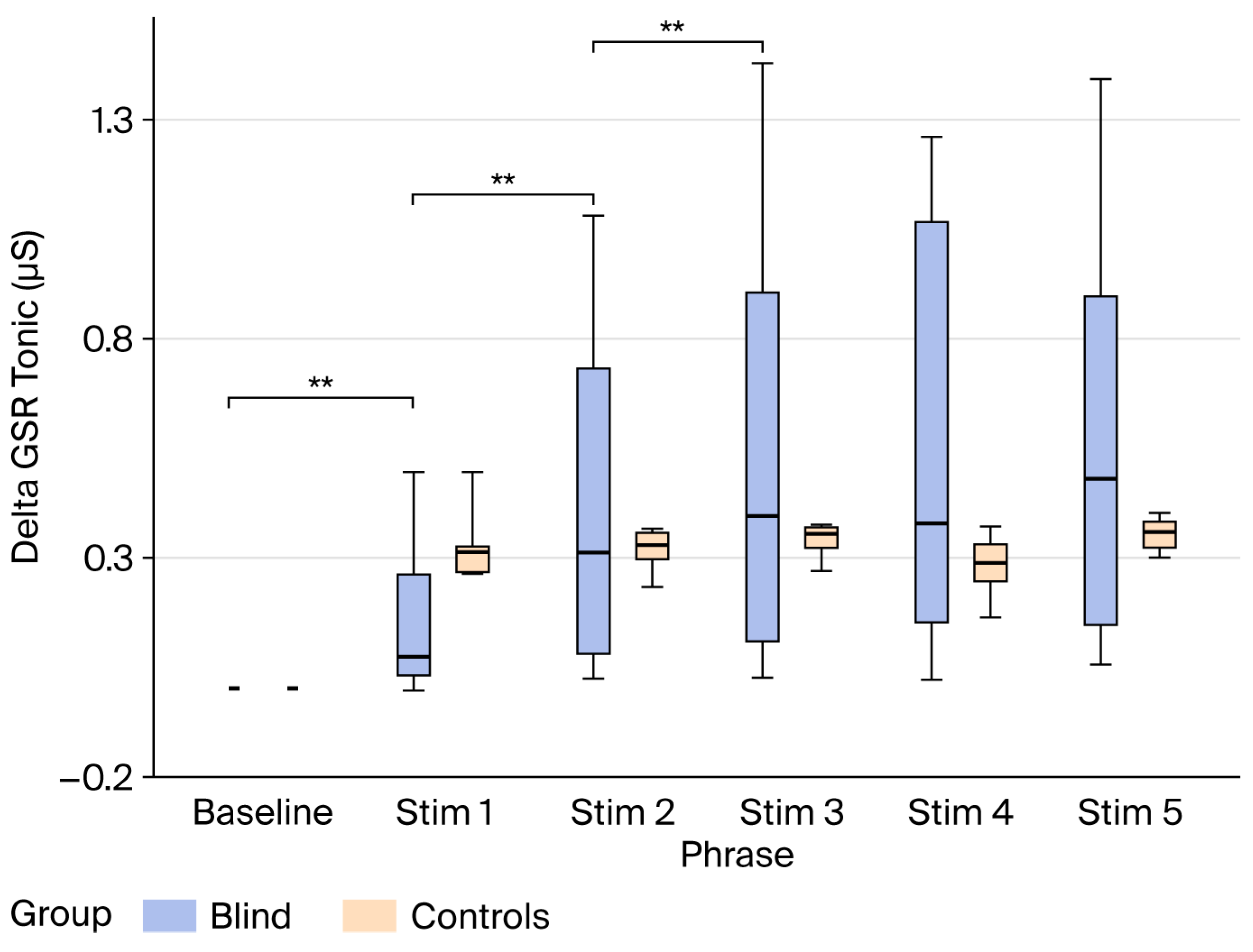
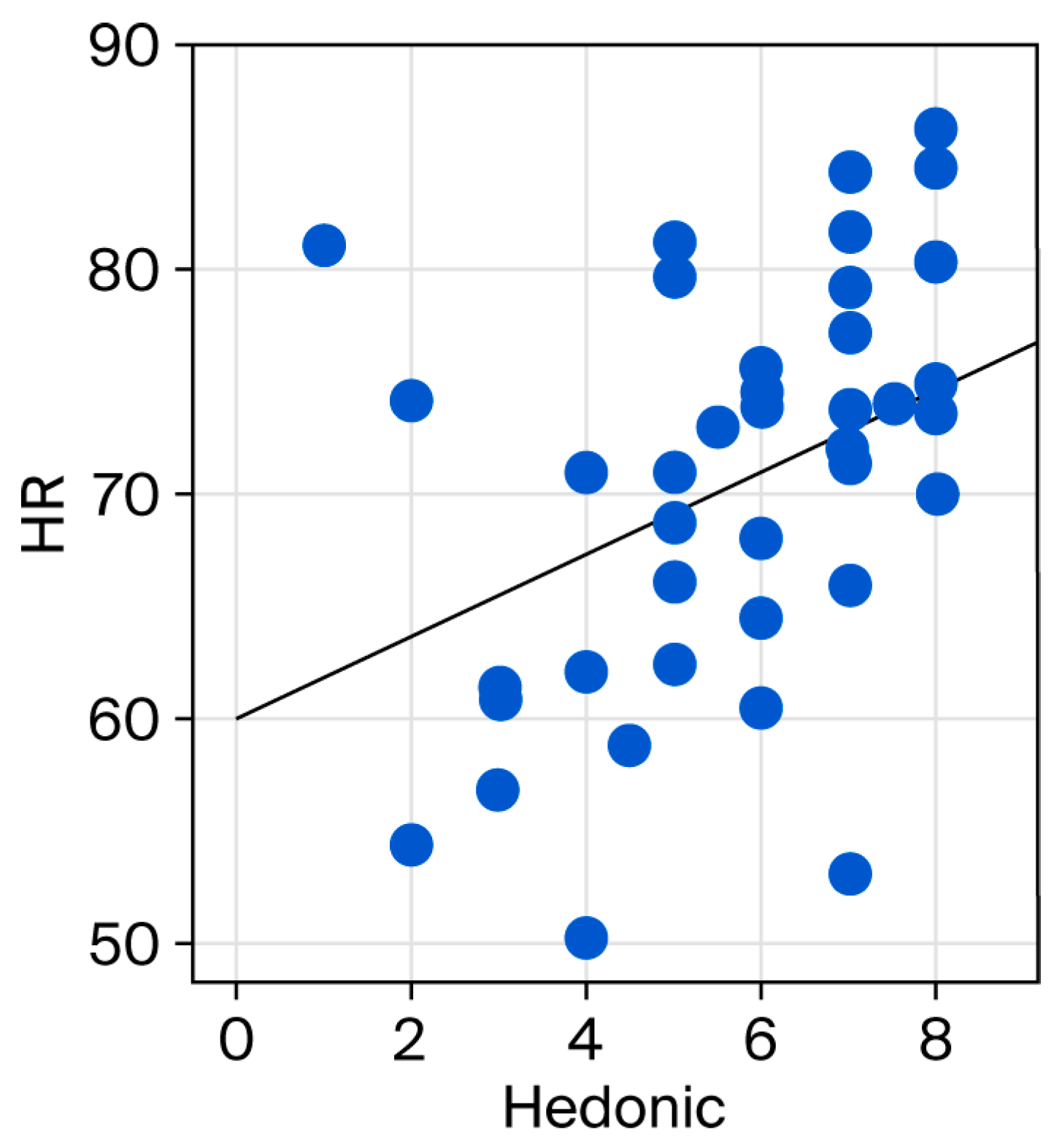
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANS | Autonomic Nervous System |
| CSI | Cardiac Sympathetic Index |
| CVI | Cardiac Vagal Index |
| ECG | Electrocardiogram |
| EEG | Electroencephalogram |
| GSR | Galvanic Skin Response |
| HR | Heart Rate |
| HRV | Heart Rate Variability |
| LF/HF | Low-to-High-Frequency Component Ratio |
| NS | Not Significant |
| pNN50 | Percentage of Normal R-R Intervals Differing by More Than 50 ms |
| RH | Relative Humidity |
| SDNN | Standard Deviation of the Normal R-R Intervals |
| SH | Spices and Aromatic Herbs |
References
- Brandt, T.; Huppert, D. The mysterious sense of smell: Evolution, historical perspectives, and neurological disorders. Front. Hum. Neurosci. 2025, 19, 1588935. [Google Scholar] [CrossRef]
- Kupers, R.; Beaulieu-Lefebvre, M.; Schneider, F.C.C.; Kassuba, T.; Paulson, O.B.; Siebner, H.R.; Ptito, M. Neural correlates of olfactory processing in congenital blindness. Neuropsychologia 2011, 49, 2037–2044. [Google Scholar] [CrossRef]
- Rombaux, P.; Huart, C.; De Volder, A.G.; Cuevas, I.; Renier, L.; Duprez, T.; Grandin, C. Increased olfactory bulb volume and olfactory function in early blind subjects. NeuroReport 2010, 21, 1069–1073. [Google Scholar] [CrossRef]
- Mazal, P.P.; Haehner, A.; Hummel, T. Relation of the volume of the olfactory bulb to psychophysical measures of olfactory function. Eur. Arch. Otorhinolaryngol. 2016, 273, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hinojosa-Aguayo, I.; Garcia-Burgos, D.; Catena, A.; González, F. Implicit and explicit measures of the sensory and hedonic analysis of beer: The role of tasting expertise. Food Res. Int. 2022, 152, 110873. [Google Scholar] [CrossRef] [PubMed]
- Lagast, S.; Gellynck, X.; Schouteten, J.J.; De Herdt, V.; De Steur, H. Consumers’ emotions elicited by food: A systematic review of explicit and implicit methods. Trends Food Sci. Technol. 2017, 69, 172–189. [Google Scholar] [CrossRef]
- Cuevas, I.; Plaza, P.; Rombaux, P.; De Volder, A.G.; Renier, L. Odour discrimination and identification are improved in early blindness. Neuropsychologia 2009, 47, 3079–3083. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, L.; Ismaili, A.R.A.; Ptito, M.; Kupers, R. Superior orthonasal but not retronasal olfactory skills in congenital blindness. PLoS ONE 2015, 10, e0122567. [Google Scholar] [CrossRef]
- Dodo, N.; Hashimoto, R. Autonomic nervous system activity during a speech task. Front. Neurosci. 2019, 13, 406. [Google Scholar] [CrossRef]
- Herzig, D.; Eser, P.; Omlin, X.; Riener, R.; Wilhelm, M.; Achermann, P. Reproducibility of heart rate variability is parameter and sleep stage dependent. Front. Physiol. 2018, 8, 1100. [Google Scholar] [CrossRef]
- Amancharla, A.; Shanbhag, A.A. Analysis of EEG and ECG time series in response to olfactory and cognitive tasks. Procedia Comput. Sci. 2024, 235, 745–756. [Google Scholar] [CrossRef]
- Oleszkiewicz, A.; Heyne, L.; Sienkiewicz-Oleszkiewicz, B.; Cuevas, M.; Haehner, A.; Hummel, T. Odours count: Human olfactory ecology appears to be helpful in the improvement of the sense of smell. Sci. Rep. 2021, 11, 16888. [Google Scholar] [CrossRef]
- Tang, B.; Zhu, M.; Wu, Y.; Guo, G.; Hu, Z.; Ding, Y. Autonomic responses associated with olfactory preferences of fragrance consumers: Skin conductance, respiration, and heart rate. Sensors 2024, 24, 5604. [Google Scholar] [CrossRef] [PubMed]
- Torrico, D.D.; Mehta, A.; Borssato, A.B. New methods to assess sensory responses: A brief review of innovative techniques in sensory evaluation. Curr. Opin. Food Sci. 2023, 49, 100978. [Google Scholar] [CrossRef]
- Hutmacher, F. Why is there so much more research on vision than on any other sensory modality? Front. Psychol. 2019, 10, 2246. [Google Scholar] [CrossRef] [PubMed]
- Kupers, R.; Ptito, M. Compensatory plasticity and cross-modal reorganization following early visual deprivation. Neurosci. Biobehav. Rev. 2014, 41, 36–52. [Google Scholar] [CrossRef]
- Iversen, K.D.; Ptito, M.; Møller, P.; Kupers, R. Enhanced chemosensory detection of negative emotions in congenital blindness. Neural Plast. 2015, 2015, 469750. [Google Scholar] [CrossRef]
- Gamond, L.; Vecchi, T.; Ferrari, C.; Merabet, L.B.; Cattaneo, Z. Emotion processing in early blind and sighted individuals. Neuropsychology 2017, 31, 516–524. [Google Scholar] [CrossRef]
- Giraud, M.; Marelli, M.; Nava, E. Embodied language of emotions: Predicting human intuitions with linguistic distributions in blind and sighted individuals. Heliyon 2023, 9, e17425. [Google Scholar] [CrossRef]
- Ahulló-Fuster, M.A.; Sánchez-Sánchez, M.L.; Varela-Donoso, E.; Ortiz, T. Early attentional processing and cortical remapping strategies of tactile stimuli in adults with an early and late-onset visual impairment: A cross-sectional study. PLoS ONE 2024, 19, e0306478. [Google Scholar] [CrossRef]
- Taglieri, I.; Tonacci, A.; Flamini, G.; Díaz-Guerrero, P.; Ascrizzi, R.; Bachi, L.; Venturi, F. Novel perspectives for sensory analysis applied to Piperaceae and aromatic herbs: A pilot study. Foods 2025, 14, 110. [Google Scholar] [CrossRef]
- Díaz-Guerrero, P.; Panzani, S.; Sanmartin, C.; Muntoni, C.; Taglieri, I.; Venturi, F. “Pepper”: Different spices, one name—Analysis of sensory and biological aspects. Molecules 2025, 30, 1891. [Google Scholar] [CrossRef]
- Bell, B.; Adhikari, K.; Chambers, E., IV; Alavi, S.; King, S.; Haub, M. Spices in a product affect emotions: A study with an extruded snack product. Foods 2017, 6, 70. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, S.; Wu, Q.; Ren, M. You see what you eat: Effects of spicy food on emotion perception. Curr. Psychol. 2024, 43, 3275–3291. [Google Scholar] [CrossRef]
- Douglas, M.; Heyes, J.; Smallfield, B. Herbs, Spices and Essential Oils: Post-Harvest Operations in Developing Countries; FAO: Rome, Italy, 2005; Available online: https://openknowledge.fao.org/handle/20.500.14283/AD420E (accessed on 20 September 2025).
- Mangalakumari, C.K.; Sreedharan, V.P.; Mathew, A.G. Studies on blackening of pepper (Piper nigrum, Linn) during dehydration. J. Food Sci. 1983, 48, 604–606. [Google Scholar] [CrossRef]
- Ahmad, H.; Khera, R.A.; Hanif, M.A.; Ayub, M.A. Cubeb. In Medicinal Plants of South Asia; Elsevier: Amsterdam, The Netherlands, 2020; pp. 149–164. [Google Scholar]
- Maiti, S.; Kumari, P. Cultivation of Long Pepper; National Research Centre for Medicinal and Aromatic Plants: Gujarat, India, 2002; Available online: https://www.dmapr.org.in/Publications/bulletine/Cultivation%20of%20long%20pepper.pdf (accessed on 1 October 2025).
- de Carvalho, R.O.; Machado, M.B.; Lopes, R.S.; Scherer, V.S.; Cruz, W.A. Agroindustry for drying pink pepper (Schinus terebinthifolius). Agric. Eng. Int. CIGR J. 2015, 3, 177–180. [Google Scholar]
- Solar, H.; Fernández, E.; Tartarisco, G.; Pioggia, G.; Cvetković, B.; Kozina, S.; Lampe, J. A non-invasive, wearable sensor platform for multi-parametric remote monitoring in CHF patients. Health Technol. 2013, 3, 99–109. [Google Scholar] [CrossRef]
- Sansone, F.; Sanmartin, C.; Taglieri, I.; Tonacci, A.; Venturi, F. Consumer Wearables in the Digital Health Ecosystem: Reliability and Bias in Biometric Measurements During Sensory Analysis; IEEE CINTI: Budapest, Hungary, 2025. [Google Scholar]
- Malik, M.; Bigger, J.T.; Camm, A.J.; Kleiger, R.E.; Malliani, A.; Moss, A.J.; Schwartz, P.J. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef]
- Tonacci, A.; Scalzini, G.; Díaz-Guerrero, P.; Sanmartin, C.; Taglieri, I.; Ferroni, G.; Venturi, F. Chemosensory analysis of emotional wines: Merging of explicit and implicit methods to measure emotions aroused by red wines. Food Res. Int. 2024, 190, 114611. [Google Scholar] [CrossRef]
- Tonacci, A.; Billeci, L.; Sansone, F.; Masci, A.; Pala, A.P.; Domenici, C.; Conte, R. An innovative, unobtrusive approach to investigate smartphone interaction in nonaddicted subjects based on wearable sensors: A pilot study. Medical 2019, 55, 37. [Google Scholar] [CrossRef]
- Benedek, M.; Kaernbach, C. A continuous measure of phasic electrodermal activity. J. Neurosci. Methods 2010, 190, 80–91. [Google Scholar] [CrossRef]
- Billeci, L.; Sanmartin, C.; Tonacci, A.; Taglieri, I.; Bachi, L.; Ferroni, G.; Venturi, F. Wearable sensors to evaluate autonomic response to olfactory stimulation: The influence of short, intensive sensory training. Biosensors 2023, 13, 478. [Google Scholar] [CrossRef]
- Ptito, M.; Desgent, S. Sensory input-based adaptation and brain architecture. In Lifespan Development and the Brain; Cambridge University Press: Cambridge, UK, 2006; pp. 111–123. [Google Scholar]
- Desgent, S.; Ptito, M. Cortical GABAergic interneurons in cross-modal plasticity following early blindness. Neural Plast. 2012, 2012, 590725. [Google Scholar] [CrossRef]
- Kupers, R.; Pietrini, P.; Ricciardi, E.; Ptito, M. The nature of consciousness in the visually deprived brain. Front. Psychol. 2011, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Chouinard-Leclaire, C.; Manescu, S.; Collignon, O.; Lepore, F.; Frasnelli, J. Altered morphological traits along central olfactory centres in congenitally blind subjects. Eur. J. Neurosci. 2022, 56, 4486–4500. [Google Scholar] [CrossRef]
- Kupers, R.; Chebat, D.R.; Madsen, K.H.; Paulson, O.B.; Ptito, M. Neural correlates of virtual route recognition in congenital blindness. Proc. Natl. Acad. Sci. USA 2010, 107, 12716–12721. [Google Scholar] [CrossRef]
- Beaulieu-Lefebvre, M.; Schneider, F.C.; Kupers, R.; Ptito, M. Odor perception and odor awareness in congenital blindness. Brain Res. Bull. 2011, 84, 206–209. [Google Scholar] [CrossRef]
- Renier, L.; Cuevas, I.; Grandin, C.B.; Dricot, L.; Plaza, P.; Lerens, E.; Rombaux, P.; De Volder, A.G. Right occipital cortex activation correlates with superior odor processing performance in the early blind. PLoS ONE 2013, 8, e71907. [Google Scholar] [CrossRef]
- Araneda, R.; Renier, L.A.; Rombaux, P.; Cuevas, I.; De Volder, A.G. Cortical plasticity and olfactory function in early blindness. Front. Syst. Neurosci. 2016, 10, 75. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; Ginsberg, J.P. An overview of heart rate variability metrics and norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Bensafi, M.; Rouby, C.; Farget, V.; Bertrand, B.; Vigouroux, M.; Holley, A. Autonomic nervous system responses to odours: The role of pleasantness and arousal. Chem. Senses 2002, 27, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Sorokowska, A.; Sorokowski, P.; Karwowski, M.; Larsson, M.; Hummel, T. Olfactory perception and blindness: A systematic review and meta-analysis. Psychol. Res. 2019, 83, 1595–1611. [Google Scholar] [CrossRef] [PubMed]
- Frasnelli, J.; Collignon, O.; Voss, P.; Lepore, F. Crossmodal plasticity in sensory loss. Prog. Brain Res. 2011, 191, 233–249. [Google Scholar] [PubMed]
| Botanical Name | Family | Distribution Area | Common Name | Edible Organs | Production Methods |
|---|---|---|---|---|---|
| Piper nigrum L. | Piperaceae | Hainan, Yunnan, and Guangdong in China and Europe | (Eng) black pepper, (Fra) poivre noir, (Esp) pimienta negra, (Deu) schwarzer pfeffer, (Ita) pepe nero | Fruit and bark | Berries are harvested at early ripening when they turn yellow. Then, berries are washed in hot water, and finally, they are sun-dried or dried by artificial methods [25,26] |
| Piper cubeba L.f | Piperaceae | Sri Lanka, Sumatra, Malaysia, Southern Borneo, and Java | (Eng) cubeb pepper, (Fra) poivre cubèbe, (Esp) pimienta cubeba, (Deu) kubeben pfeffer, (Ita) pepe cubebe | Fruit | The fruits are harvested by hand when ripe and separated from spikes. Then, the berries can be directly dried or immersed in water to remove the pericarp, and afterwards, they are dried for 3–4 days [27] |
| Piper longum L. | Piperaceae | India, Malaysia, Indonesia, Singapore, Sri Lanka | (Eng) long pepper, (Fra) poivre long, (Esp) pimienta larga, (Deu) langer pfeffer, (Ita) pepe lungo | Dried infructescence and leaves | The infructescence is harvested before ripening when the color is blackish green. Subsequently, the berries are dried in the sun for about 4–5 days [28] |
| Schinus terebinthifolius Raddi | Anacardiacee | Central and North America, Europe, Asia, and Africa | (Eng) pink pepper or false pepper, (Fra) faux poivrier, (Esp) pimienta de brasil o pimienta rosada, (Deu) rosa pfeffer, (Ita) pepe rosa | Fruit | The berries are harvested manually once they have reached maturity and then dried [29] |
| Pimenta dioica (L.) Merrill | Myrtaceae | West Indies (Jamaica) and Central America (Cuba, Mexico, Brazil, Honduras, Guatemala, Belize) | (Eng) Jamaica pepper or allspice, (Fra) poivre de la jamaïque, (Esp) pimienta de jamaica, (Deu) jamaika pfeffer, (Ita) pepe garofanato | Fruit and leaves | The harvested berries are left for up to 5 days in sacks to ferment. Then, they are dried for 5 to 10 days, depending on the weather, until the moisture content is about 12% [25] |
| Spices’ Common Name | Blind Individuals (Mean ± SD) | Controls (Mean ± SD) | p-Value |
|---|---|---|---|
| Black pepper | 5.46 ± 1.62 | 6.00 ± 1.41 | NS |
| Cubeb pepper | 5.50 ± 1.83 | 4.86 ± 1.75 | NS |
| Long pepper | 6.83 ± 1.64 | 6.64 ± 1.74 | NS |
| Pink pepper | 6.29 ± 1.84 | 5.93 ± 2.02 | NS |
| Jamaica pepper | 5.96 ± 2.16 | 6.43 ± 2.10 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonacci, A.; Sanmartin, C.; Taglieri, I.; Sansone, F.; Panzani, S.; Venturi, F. The Scent of Emotion: A Pilot Study on Olfactory Perception Beyond Visual Cues. Appl. Sci. 2025, 15, 12307. https://doi.org/10.3390/app152212307
Tonacci A, Sanmartin C, Taglieri I, Sansone F, Panzani S, Venturi F. The Scent of Emotion: A Pilot Study on Olfactory Perception Beyond Visual Cues. Applied Sciences. 2025; 15(22):12307. https://doi.org/10.3390/app152212307
Chicago/Turabian StyleTonacci, Alessandro, Chiara Sanmartin, Isabella Taglieri, Francesco Sansone, Sofia Panzani, and Francesca Venturi. 2025. "The Scent of Emotion: A Pilot Study on Olfactory Perception Beyond Visual Cues" Applied Sciences 15, no. 22: 12307. https://doi.org/10.3390/app152212307
APA StyleTonacci, A., Sanmartin, C., Taglieri, I., Sansone, F., Panzani, S., & Venturi, F. (2025). The Scent of Emotion: A Pilot Study on Olfactory Perception Beyond Visual Cues. Applied Sciences, 15(22), 12307. https://doi.org/10.3390/app152212307







