Assessing Short-Term Temporal Variability of CO2 Emission and Soil O2 Influx in Tropical Pastures and Regenerating Forests
Abstract
1. Introduction
2. Materials and Methods
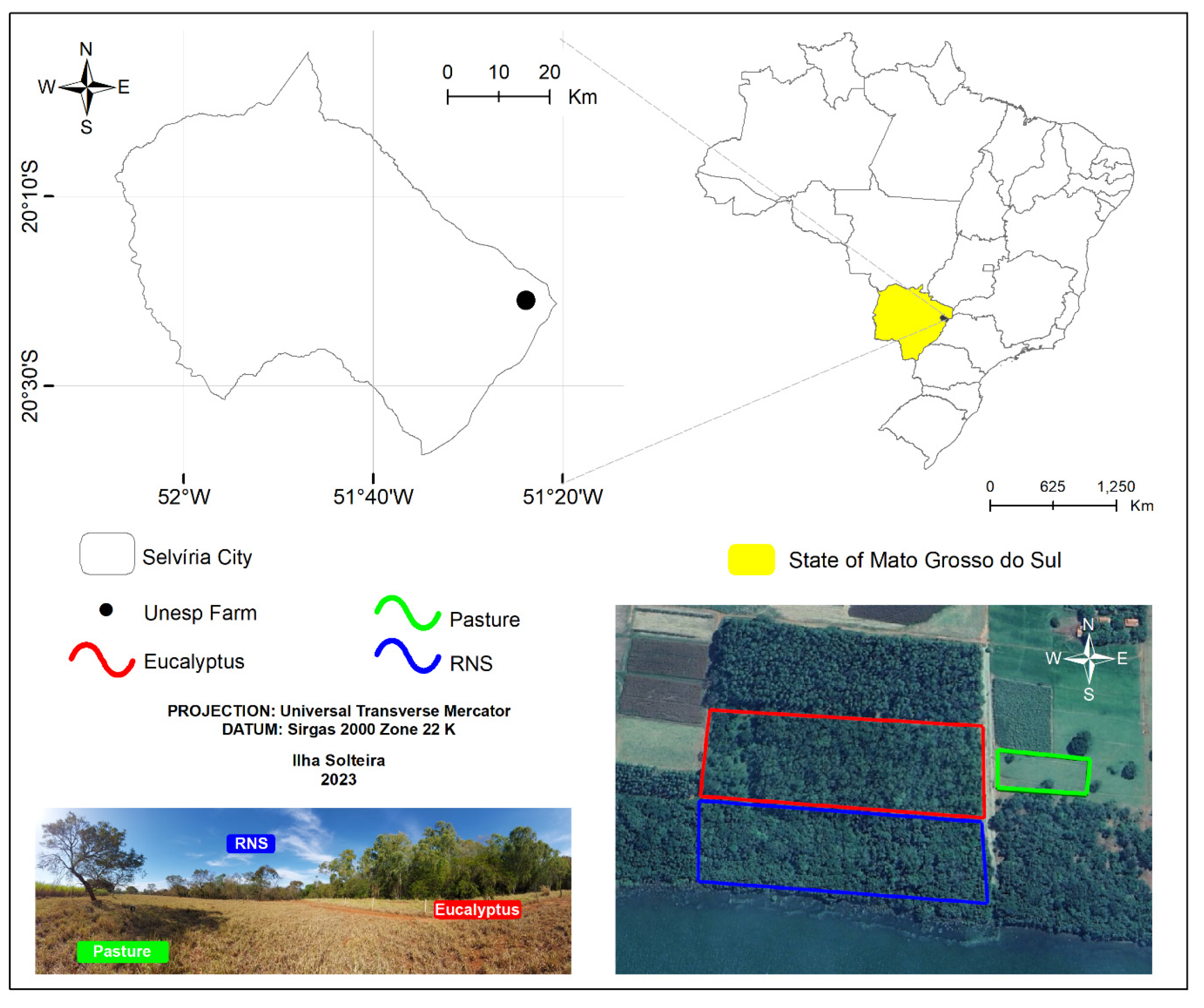
2.1. Study Area and Land Uses
2.2. Soil CO2 Emission, Soil Moisture and Temperature
2.3. Soil O2 Influx
2.4. Characterization of Soil Attributes in Agroecosystems
2.5. Data Analysis
2.5.1. Temporal Variability of Soil CO2 Emission, Soil O2 Influx, Soil Moisture and Soil Temperature
2.5.2. Analysis of Soil Attributes Under Different Uses
2.5.3. Principal Component Analysis with Clusters
3. Results
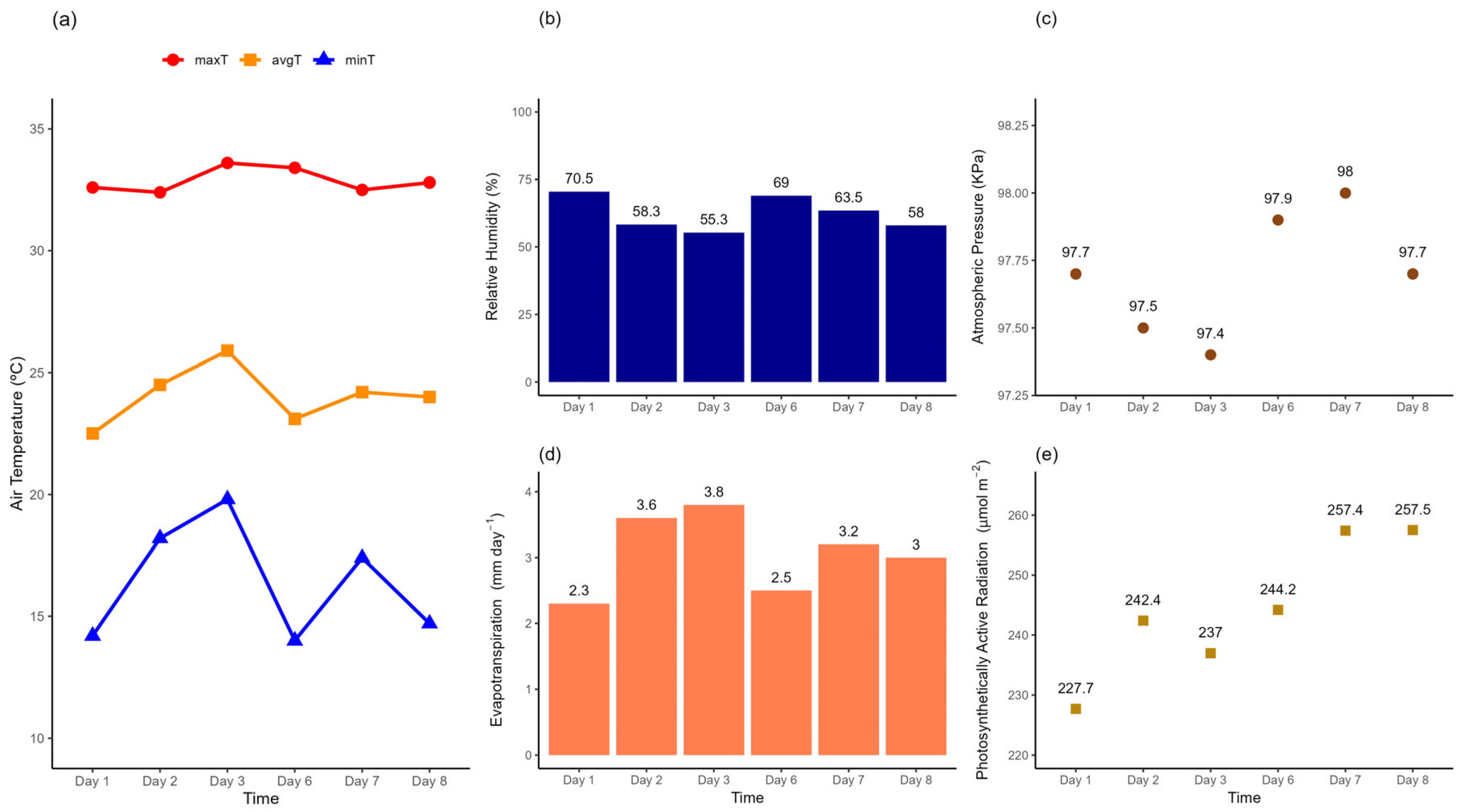
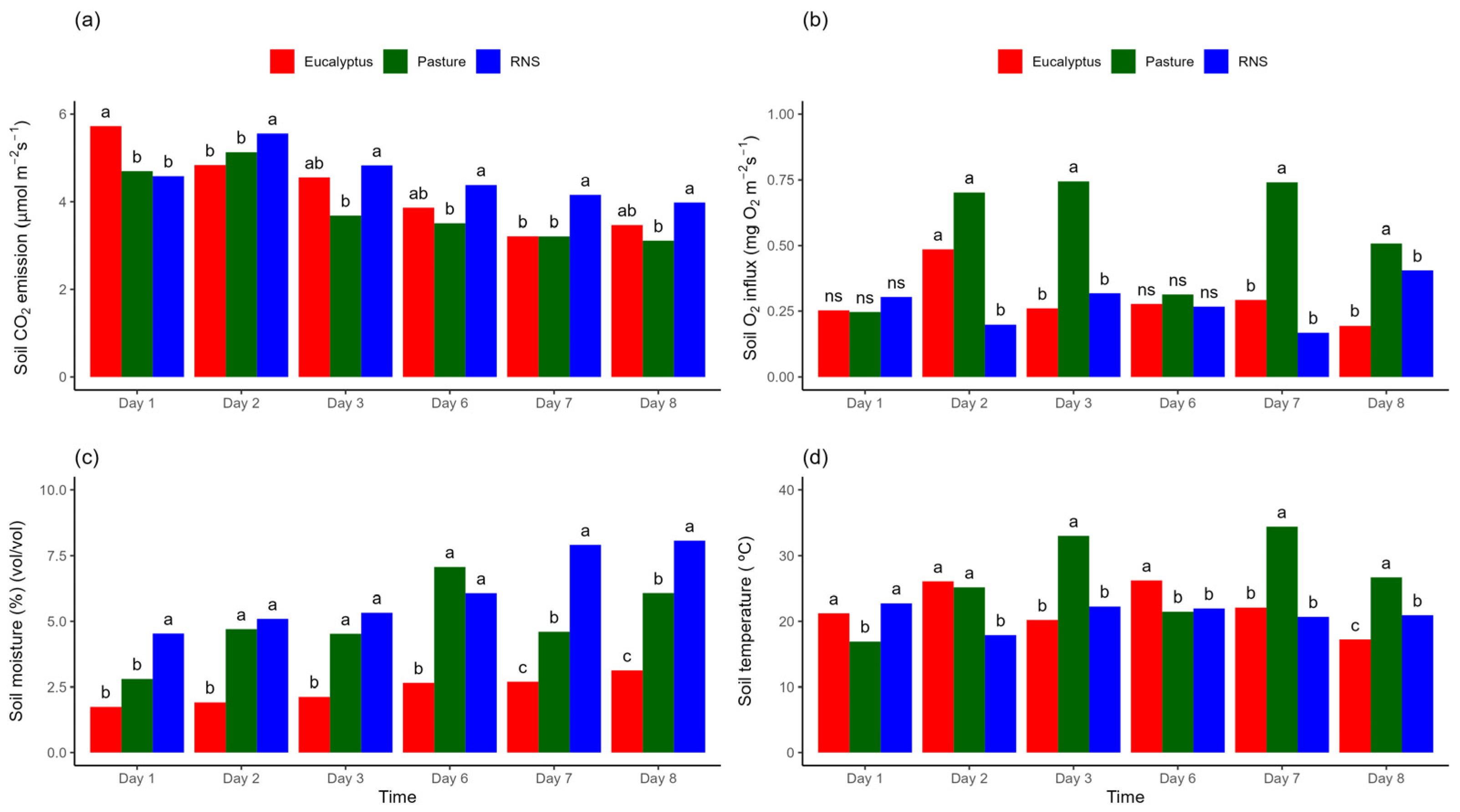
3.1. Temporal Variability of Soil CO2 Emission, Soil O2 Influx, Soil Moisture and Temperature
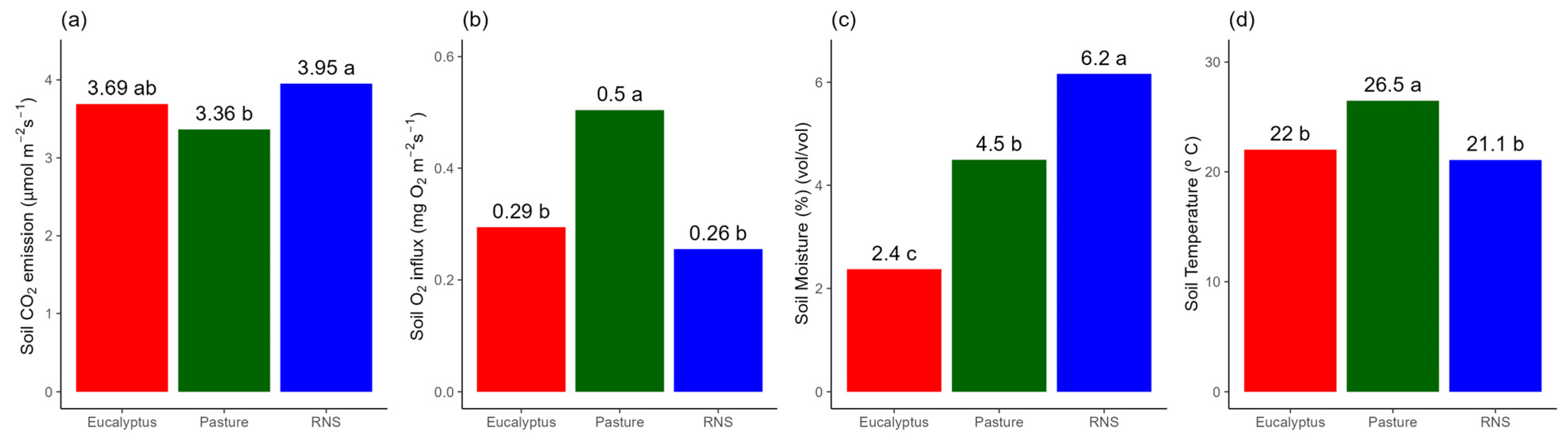
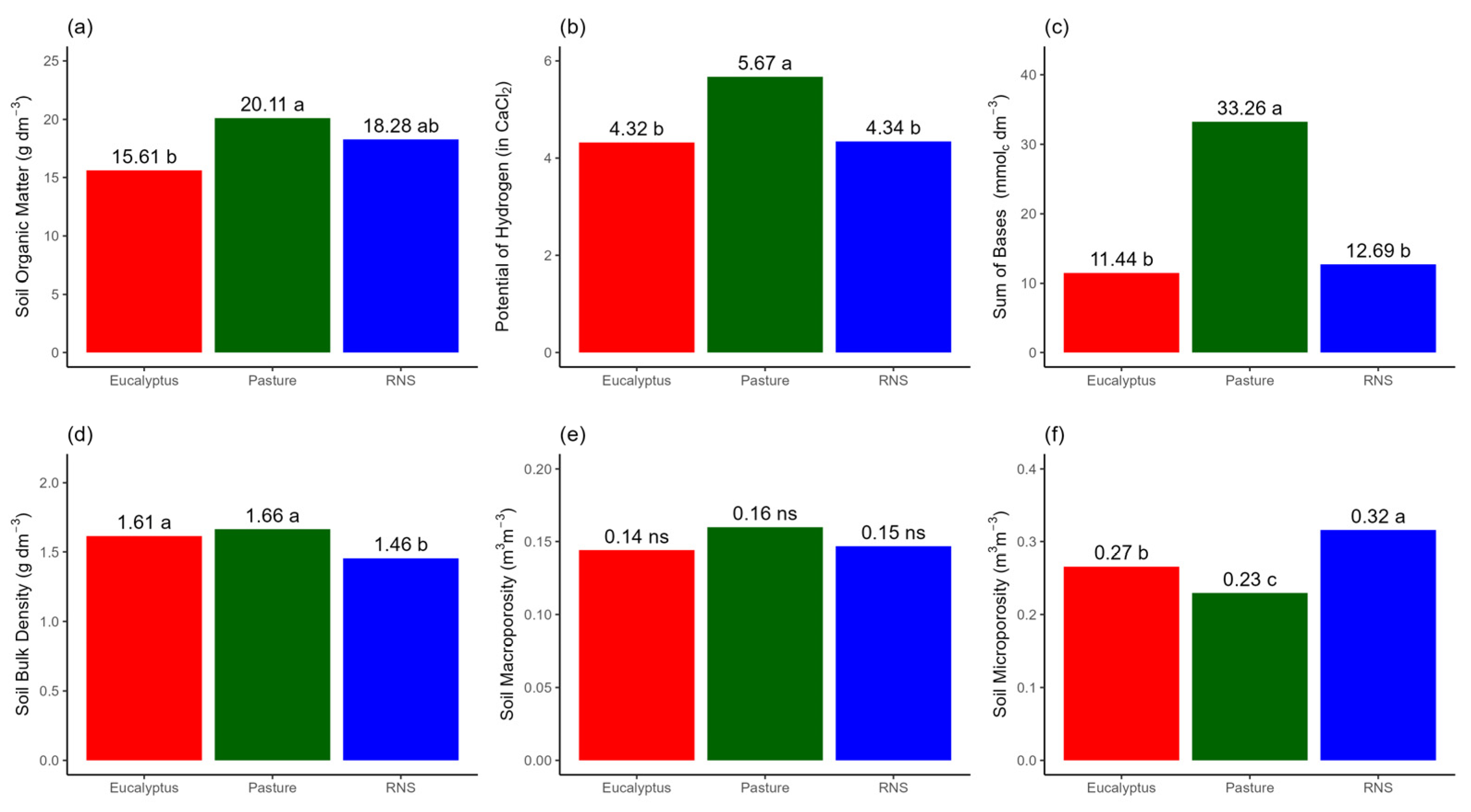
3.2. Soil Attributes in the Agroecosystems
3.3. Principal Component Analysis for Different Land Uses
4. Discussion
4.1. Temporal Variability of FCO2, iO2, SM and ST
4.2. Effect of Different Agroecosystems on Soil Attributes
4.3. Analysis of Principal Components and Clusters
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Intergovernmental Panel on Climate Change—IPCC. Synthesis Report; Sixth Assessment Report; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2023; 85p. [Google Scholar]
- Moss, R.; Edmonds, J.; Hibbard, K.; Manning, M.R.; Rose, S.K.; Van Vuuren, D.P.; Carter, T.R.; Emori, S.; Kainuma, M.; Kram, T. The next generation of scenarios for climate change research and assessment. Nature 2010, 463, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Richardson, K.; Steffen, W.; Lucht, W.; Bendtsen, J.; Cornell, S.E.; Donges, J.F.; Drüke, M.; Fetzer, I.; Bala, G.; von Bloh, W.; et al. Earth beyond six of nine planetary boundaries. Sci. Adv. 2023, 9, eadh2458. [Google Scholar] [CrossRef] [PubMed]
- Cerri, C.E.P.; Abbruzzini, T.F.; Carvalho, J.L.N.; Cherubin, M.R.; Frazão, L.A.; Maia, S.M.F.; Oliveira, D.M.S. Matéria orgânica do solo e o equilíbrio global de carbono. In Entendendo a Matéria Orgânica do Solo em Ambientes Tropical e Subtropical; Bettiol, W., Silva, C.A., Cerri, C.E.P., Martin-Neto, L., Andrade, C.A., Orgs.; Embrapa: Brasília, Brazil, 2023; pp. 211–254. [Google Scholar]
- Canteral, K.F.F.; Vicentini, M.E.; De Lucena, W.B.; Moraes, M.L.T.; Montanari, R.; Ferraudo, A.S.; Peruzzi, N.J.; La Scala, N., Jr.; Panosso, A.R. Machine learning for prediction of soil CO2 emission in tropical forests in the Brazilian Cerrado. Environ. Sci. Pollut. Res. 2023, 30, 61052–61071. [Google Scholar] [CrossRef]
- SEEG—Sistema de Estimativas de Emissões de Gases de Efeito Estufa. 2022. Available online: https://plataforma.seeg.eco.br/total_emission (accessed on 16 September 2023).
- Luo, Y.; Zhou, X. Soil Respiration and the Environment; Academic Press: San Diego, CA, USA, 2006; 316p. [Google Scholar]
- Moitinho, M.R.; Padovan, M.P.; Panosso, A.R.; La Scala, N., Jr. Efeito do preparo do solo e resíduo da colheita de cana-de-açúcar sobre a emissão de CO2. Rev. Bras. De Cienc. Do Solo 2013, 37, 1720–1728. [Google Scholar] [CrossRef]
- Lal, R. Challenges and opportunities in soil organic matter research. Eur. J. Soil Sci. 2009, 60, 158–169. [Google Scholar] [CrossRef]
- Lal, R. Reducing carbon footprints of agriculture and food systems. Carbon Footpr. 2022, 1, 3. [Google Scholar] [CrossRef]
- De Lucena, W.B.; Vicentini, M.E.; Santos, G.A.A.D.; Silva, B.O.; Costa, D.V.M.; Canteral, K.F.F.; Neira Román, J.A.; Rolim, G.S.; Panosso, A.R.; La Scala, N., Jr. Temporal variability of the CO2 emission and the O2 influx in a tropical soil in contrasting coverage conditions. J. South Am. Earth Sci. 2023, 121, 104120. [Google Scholar] [CrossRef]
- Almeida, R.F.; Teixeira, D.B.; Montanari, R.; Bolonhezi, A.C.; Teixeira, E.B.; Moitinho, M.R.; Panosso, A.R.; Spokas, K.A.; La Scala, N., Jr. Ratio of CO2 and O2 as index for categorising soil biological activity in sugarcane areas under contrasting straw management regimes. Soil Res. 2018, 56, 373–381. [Google Scholar] [CrossRef]
- Almeida, R.F.; Spokas, K.A.; Teixeira, D.B.; La Scala, N., Jr. Biochar insights from laboratory incubations monitoring O2 consumption and CO2 production. Biochar 2019, 1, 249–258. [Google Scholar] [CrossRef]
- Vicentini, M.E.; Pinotti, C.R.; Hirai, W.Y.; de Moraes, M.L.T.; Montanari, R.; Teixeira Filho, M.C.M.; Milori, D.M.B.P.; La Scala, N., Jr.; Panosso, A.R. CO2 emission and its relation to soil temperature, moisture, and O2 absorption in the reforested areas of Cerrado biome, Central Brazil. Plant Soil 2019, 444, 193–211. [Google Scholar] [CrossRef]
- Jensen, J.L.; Schjønning, P.; Watts, C.W.; Christensen, B.T.; Peltre, C.; Munkholm, L.J. Relating soil C and organic matter fractions to soil structural stability. Geoderma 2019, 337, 834–843. [Google Scholar] [CrossRef]
- De Lucena, W.B.; Tavares, M.A.; Carmo, P.H.O.; Perônico, B.W.C.; Santos, J.M.; Ferreira, G.B. Agricultura sustentável e atributos biológicos do solo: Uma breve revisão sobre os indicadores da qualidade agrícola-ambiental. In Ensaios nas Ciências Agrárias e Ambientais, 1st ed.; Oliveira, A.C., Ed.; Ponta Grossa: Atena, Greece, 2018; pp. 45–52. [Google Scholar] [CrossRef]
- Lisboa, B.B.; Vargas, L.K.; Silveira, A.O.D.; Martins, A.F.; Selbach, P.A. Indicadores microbianos de qualidade do solo em diferentes sistemas de manejo. Rev. Bras. De Cienc. Do Solo 2012, 36, 33–44. [Google Scholar] [CrossRef]
- Dias-Filho, M.B. Degradação de Pastagens: O que é e Como Evitar; Embrapa: Brasília, Brazil, 2017; 19p. [Google Scholar]
- Guarda, V.D.A.; Campos, L.J.M. Bases Ecofisiológicas da Assimilação de Carbono e Suas Implicações na Produção de Forragem; Embrapa Pesca e Aquicultura: Palmas, Spain, 2014; 48p. [Google Scholar]
- Campos, M.O.; Cerri, C.E.P.; La Scala, N., Jr. Atmospheric CO2, soil carbon stock and control variables in managed and degraded pastures in central Brazil. Remote Sens. Appl. Soc. Environ. 2022, 28, 100848. [Google Scholar] [CrossRef]
- Parente, L.; Mesquita, V.V.; Miziara, F.; Baumann, L.R.F.; Ferreira, L. Assessing the pasturelands and livestock dynamics in Brazil, from 1985 to 2017: A novel approach based on high spatial resolution imagery and Google Earth Engine cloud computing. Remote Sens. Environ. 2019, 232, 111301. [Google Scholar] [CrossRef]
- Moitinho, M.R.; Ferraudo, A.S.; Panosso, A.R.; Bicalho, E.d.S.; Teixeira, D.D.B.; Barbosa, M.d.A.; Tsai, S.M.; Borges, B.M.F.; Cannavan, F.S.; de Souza, J.A.M.; et al. Effects of burned and unburned sugarcane harvesting systems on soil CO2 emission and soil physical, chemical, and microbiological attributes. Catena 2021, 196, 104903. [Google Scholar] [CrossRef]
- Huang, C.-H.; Ouyang, Y.; Zhang, J.-E. Effects of a compact layer on soil O2. Geoderma 2006, 135, 224–232. [Google Scholar] [CrossRef]
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.Á.; Araujo Filho, J.C.; Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos, 5th ed; Embrapa: Brasília, Brazil, 2018; 356p. [Google Scholar]
- Soil Survey Staff. Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys, 12th ed; Natural Resources Conservation Service. U.S. Department of Agriculture Handbook 436; U.S. Department of Agriculture: Washington, DC, USA, 2014. [Google Scholar]
- Andrade, G.C. Eucalipto. Embrapa Florestas. 2021. Available online: https://www.embrapa.br/en/agencia-de-informacao-tecnologica/cultivos/eucalipto/pre-producao/aspectos-ambientais/agua#:~:text=Dados%20referentes%20a%20taxas%20de,2%20litros%20de%20%C3%A1gua%2F%C3%A1rvore (accessed on 29 September 2023).
- Thornthwaite, C.W. An approach toward a Rational Classification of Climate. Geogr. Rev. 1948, 38, 55–94. Available online: https://www.jstor.org/stable/210739 (accessed on 29 September 2023). [CrossRef]
- Rolim, G.S.; Camargo, M.B.P.; Lania, D.G.; Moraes, J.F.L. Classificação climática de Köppen e de Thornthwaite e sua aplicabilidade na determinação de zonas agroclimáticas para o Estado de São Paulo. Bragantia 2007, 66, 711–720. [Google Scholar] [CrossRef]
- Ramos, J.C.A. Emissão de CO2, Quantidade e Qualidade do Carbono do Solo em Sistemas Agrícolas na Região do Cerrado do Mato Grosso do Sul. Master’s Thesis, Faculdade de Engenharia (Fies), Universidade Estadual Paulista, Ilha Solteira, São Paulo, Brazil, 2018; 83p. [Google Scholar]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration Guidelines for Computing Crop Water Requirements; FAO-Irrigation and Drainage Paper 56; Food and Agriculture Organization: Rome, Italy, 1998. [Google Scholar]
- Lima, E.F.; Silva Filho, J.P.; Araújo, A.F.S. Dicionário dos Termos Usados em Ecologia; EMATER: Parnaíba, Brazil, 2016; 180p. [Google Scholar]
- Cavenage, A.; Moraes, M.L.T.; Alves, M.C.; Carvalho, M.A.C.; Freitas, M.L.M.; Buzetti, S. Alterações nas propriedades físicas de um Latossolo Vermelho-Escuro sob diferentes culturas. Rev. Bras. De Cienc. Do Solo 1999, 23, 997–1003. [Google Scholar] [CrossRef]
- Souza, Z.M.; Alves, M.C. Propriedades químicas de um latossolo vermelho distrófico de cerrado sob diferentes usos e manejos. Rev. Bras. De Cienc. Do Solo 2003, 27, 133–139. [Google Scholar] [CrossRef]
- Wolińska, A.; Stepniewska, Z.; Szafranek-Nakonieczna, A. Effect of selected physical parameters on respiration activities in common Polish mineral soils. Pol. J. Environ. Stud. 2011, 20, 1075–1082. [Google Scholar]
- Angert, A.; Yakir, D.; Rodeghiero, M.; Preisler, Y.; Davidson, E.A.; Weiner, T. Using O2 to study the relationships between soil CO2 efflux and soil respiration. Biogeosciences 2015, 12, 2089–2099. [Google Scholar] [CrossRef]
- Gastwirth, J.; Gel, Y.; Hui, W.; Lyubchich, V.; Miao, W.; Noguchi, K. lawstat: Tools for Biostatistics, Public Policy and Law. R Package Version 3.4. 2020. Available online: https://CRAN.R-project.org/package=lawstat (accessed on 10 October 2022).
- Jassal, R.S.; Black, T.A.; Nesic, Z.; Gaumont-Guay, D. Using automated non-steady-state chamber systems for making continuous long-term measurements of soil CO2 efflux in forest ecosystems. Agric. For. Meteorol. 2012, 161, 57–65. [Google Scholar] [CrossRef]
- Raij, B.V.; Andrade, J.C.; Cantarella, H.; Quaggio, J.A. Análise Química Para Avaliação da Fertilidade de Solos Tropicais; Instituto Agronômico: Campinas, Brazil, 2001; 285p. [Google Scholar]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solo, 3rd ed.; Embrapa: Brasília, Brazil, 2017; 574p. [Google Scholar]
- Mendiburu, F. agricolae: Statistical Procedures for Agricultural Research. R Package Version 1.3-5. 2021. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 10 October 2022).
- Tukey, J.W. Comparing Individual Means in the Analysis of Variance. Biometrics 1949, 5, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Littell, R.C.; Henry, P.R.; Ammerman, C.B. Statistical analysis of repeated measures using SAS procedures. J. Anim. Sci. 1998, 76, 1216–1231. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed; Springer: New York, NY, USA, 2002. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Bartlett, M.S. Properties of Sufficiency and Statistical Tests. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1937, 160, 268–282. Available online: http://www.jstor.org/stable/96803 (accessed on 9 August 2022).
- Cecon, P.A.; Silva, F.F.; Ferreira, A.; Ferrão, R.G.; Carneiro, A.P.S.; Detmann, E.; Faria, P.N.; Morais, T.S.S. Análise de medidas repetidas na avaliação de clones de café Conilon. Pesqui. Agropecu. Bras. 2008, 43, 1171–1176. [Google Scholar] [CrossRef]
- Canteri, M.G.; Althaus, R.A.; Virgens Filho, J.S.; Giglioti, E.A.; Cláudia, V.; Godoy, C.V. Sistema para análise e separação de médias em experimentos agrícolas pelos métodos Scott-Knott, Tukey e Duncan. Rev. Bras. De Agrocom-Putação 2001, 1, 18–24. [Google Scholar]
- La Scala, N., Jr.; Marques, J.; Pereira, G.; Corá, J. Carbon dioxide emission related to chemical properties of a tropical bare soil. Soil Biol. Biochem. 2000, 32, 1469–1473. [Google Scholar] [CrossRef]
- Silva, D.A.P.; Campos, M.C.C.; Mantovanelli, B.C.; Santos, L.A.C.; Soares, M.D.R.; Cunha, J.M. Variabilidade espacial da emissão de CO2, temperatura e umidade do solo em área de pastagem na região Amazônica, Brasil. Rev. De Ciências Agroveterinárias 2019, 18, 119–126. [Google Scholar] [CrossRef]
- Schneider, V.M. Análise Multivariada Aplicada no Estudo de Dentifrícios Comercializados no Brasil. Master’s Thesis, Centro de Ciências Naturais e Exatas, Federal University of Santa Maria, Santa Maria, Brazil, 2018; 78p. [Google Scholar]
- Kaiser, H.F. The varimax criterion for analytic rotation in factor analysis. Psychometrika 1958, 23, 187–200. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 10 October 2022).
- Ferreira, E.B.; Cavalcanti, P.P.; Nogueira, D.A. ExpDes.pt: Pacote Experimental Designs (Portuguese). R Package Version 1.2.0. 2018. Available online: https://CRAN.Rproject.org/package=ExpDes.pt (accessed on 10 October 2022).
- Kassambara, A.; Mundt, F. factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.7. 2020. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 10 October 2022).
- Wickham, H.; Bryan, J. readxl: Read Excel Files. R Package Version 1.4.0. 2022. Available online: https://CRAN.R-project.org/package=readxl (accessed on 10 October 2022).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; Mcgowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Kassambara, A. rstatix: Pipe-Friendly Framework for Basic Statistical Tests. R Package Version 0.7.0. 2021. Available online: https://CRAN.R-project.org/package=rstatix (accessed on 10 October 2022).
- Pedersen, T.L. patchwork: The Composer of Plots. R Package Version 1.1.1. 2020. Available online: https://CRAN.R-project.org/package=patchwork (accessed on 10 October 2022).
- Gross, J.; Ligges, U. Nortest: Tests for Normality. R Package Version 1.0-4. Available online: https://CRAN.R-project.org/package=nortest (accessed on 10 October 2022).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 2nd ed; Springer: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Chen, S.; Zou, J.; Yao, X.; Wang, J.; Hu, Z.; Lu, Y. A biophysical model to simulate seasonal variations of soil respiration in agroecosystems in China. Agric. For. Meteorol. 2023, 338, 109524. [Google Scholar] [CrossRef]
- Nogueira, D.C.S. Variabilidade da Emissão de CO2 e Atributos do solo em Pastagem e Sistema Silvipastoril. Doctoral Thesis, Faculdade de Ciências Agrárias e Veterinárias (FCAV), Universidade Estadual Paulista, Jaboticabal, São Paulo, Brazil, 2021; 164p. [Google Scholar]
- Silva, B.O. Emissão de CO2, Estoque e Humificação do Carbono do solo em Áreas Convertidas do Bioma Cerrado, Brasil Central. Ph.D. Thesis, Faculdade de Ciências Agrárias e Veterinárias (FCAV), Universidade Estadual Paulista, Jaboticabal, São Paulo, Brazil, 2021; 89p. [Google Scholar]
- Zanchi, F.B.; Waterloo, M.J.; Kruijt, B.; Kesselmeier, J.; Luizão, F.J.; Manzi, A.O.; Dolman, A.J. Soil CO2 efflux in Central Amazonia: Environmental and methodological effects. Acta Amaz. 2012, 42, 173–184. [Google Scholar] [CrossRef]
- Chen, X.; Liu, M.; Xu, Z.; Wei, H. Influences of temperature and moisture on abiotic and biotic soil CO2 emission from a subtropical forest. Carbon Balance Manag. 2021, 16, 18. [Google Scholar] [CrossRef]
- Bhattacharyya, S.S.; Ros, G.H.; Furtak, K.; Iqbal, H.M.N.; Parra-Saldívar, R. Soil carbon sequestration—An interplay between soil microbial community and soil organic matter dynamics. Sci. Total. Environ. 2022, 815, 152928. [Google Scholar] [CrossRef]
- Giacomo, G.; Angelo, F.; Fabio, B.; Stefano, B.; Riccardo, M. Measurements of soil carbon dioxide emissions from two maize agroecosystems at harvest under different tillage conditions. Sci. World J. 2014, 2014, 141345. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R. Elements of Nature and Soils Properties (Portuguese Version), 3rd ed.; Bookman: Porto Alegre, Brazil, 2013; 704p. [Google Scholar]
- Moreira, F.M.S.; Siqueira, J.O. Microbiologia e Bioquímica do Solo, 2nd ed.; Editora da UFLA: Lavras, Brazil, 2006; 729p. [Google Scholar]
- Feng, H.; Guo, J.; Malghani, S.; Han, M.; Cao, P.; Sun, J.; Xu, X.; Xu, X.; Wang, W. Effects of soil moisture and temperature on microbial regulation of methane fluxes in a poplar plantation. Forests 2021, 12, 407. [Google Scholar] [CrossRef]
- Liu, J.; Tang, W.; Chen, G.; Lu, Y.; Feng, C.; Tu, X.M. Correlation and agreement: Overview and clarification of competing concepts and measures. Shanghai Arch. Psychiatry 2016, 28, 115–120. [Google Scholar]
- Santos, G.A.A.; Moitinho, M.R.; Silva, B.O.; Xavier, C.V.; Teixeira, D.B.; Corá, J.E.; La Scala, N., Jr. Effects of long-term no-tillage systems with different succession cropping strategies on the variation of soil CO2 emission. Sci. Total. Environ. 2019, 686, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Davidson, E.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Neira, J.; Ortiz, M.; Morales, L.; Acevedo, E. Oxygen diffusion in soils: Understanding the factors and processes needed for modeling. Chil. J. Agric. Res. 2015, 75, 35–44. [Google Scholar] [CrossRef]
- Dias, J.A. Fluxo de CO2 Proveniente da Respiração do Solo em Áreas de Floresta Nativa da Amazônia. Ph.D. Thesis, Escola Superior de Agricultura Luiz de Queiroz (Esalq), Universidade de São Paulo, Piracicaba, São Paulo, Brazil, 2006; 87p. [Google Scholar]
- Holanda, A.C.; Feliciano, A.L.P.; Marangon, L.C.; Freire, F.J.; Holanda, E.M. Decomposição da serapilheira foliar e respiração edáfica em um remanescente de Caatinga na Paraíba. Rev. Arvore 2015, 39, 245–254. [Google Scholar] [CrossRef]
- Primavesi, A.M. Cartilha do Solo: Como Reconhecer e Sanar Seus Problemas; Movimento dos Trabalhadores Rurais Sem Terra (MST): São Paulo, Brazil, 2009. [Google Scholar]
- Nabinger, C. Eficiência do uso de pastagens: Disponibilidade e perdas de forragem. In Fundamentos do Pastoreio Rotacionado; Peixoto, A.M., Moura, J.C., Faria, V.P., Orgs.; FEALQ: Piracicaba, Brazil, 1997; pp. 213–251. [Google Scholar]
- Silveira, M.C.T.; Perez, N.B. Informações Sobre Plantas Forrageiras C4 Para Cultivo em Condições de Deficiência de Drenagem e Tolerância a Frio; Embrapa Pecuária Sul: Bagé, Brazil, 2014; 32p. [Google Scholar]
- Zhang, X.; Gregory, A.S.; Whalley, W.E.; Coleman, K.; Neal, A.L.; Bacq-Labreuil, A.; Mooney, S.J.; Crawford, J.W.; Soga, K.; Illangasekare, T.H. Relationship between soil carbon sequestration and the ability of soil aggregates to transport dissolved oxygen. Geoderma 2021, 403, 115370. [Google Scholar] [CrossRef]
- Caron, B.O.; Souza, V.Q.; Costa, E.C.; Eloyi, E.; Behling, A.; Trevisan, R. Interceptação da radiação luminosa pelo dossel de espécies florestais e sua relação com o manejo das plantas daninhas. Cienc. Rural. 2012, 42, 75–82. [Google Scholar] [CrossRef]
- Vital, M.H.F. Impacto Ambiental de Florestas de Eucalipto. Revista do BNDES. 2007. Available online: https://web.bndes.gov.br/bib/jspui/handle/1408/12554 (accessed on 8 November 2022).
- Juhász, C.E.P.; Cursi, P.T.; Cooper, M.; Oliveira, T.C.; Rodrigues, R.R. Dinâmica físico-hídrica de uma toposseqüência de solos sob savana florestada (cerradão) em Assis, SP. Rev. Bras. De Cienc. Do Solo 2006, 30, 401–412. [Google Scholar] [CrossRef]
- Vicentini, M.E.; Silva, P.A.; Canteral, K.F.F.; De Lucena, W.B.; Moraes, M.L.T.; Montanari, R.; Teixeira Filho, M.C.M.; Peruzzi, N.J.; La Scala, N., Jr.; Rolim, G.D.S.; et al. Artificial neural networks and adaptive neuro-fuzzy inference systems for prediction of soil respiration in forested areas southern Brazil. Environ. Monit. Assess. 2023, 195, 1074. [Google Scholar] [CrossRef]
- Braz, S.P.; Urquiaga, S.; Alves, B.J.R.; Boddey, R.M. Degradação de Pastagens, Matéria Orgânica do solo e a Recuperação do Potencial Produtivo em Sistemas de Baixo “input” Tecnológico na Região dos Cerrados; Embrapa: Seropédica, Brazil, 2004. [Google Scholar]
- Briedis, C.; Sá, J.C.M.; Caires, E.F.; Navarro, J.F.; Inagaki, T.M.; Ferreira, A.O. Carbono do solo e atributos de fertilidade em resposta à calagem superficial em plantio direto. Pesqui. Agropecu. Bras. 2012, 47, 1007–1014. [Google Scholar] [CrossRef]
- Mendonça, J.F.B. Solo: Substrato da Vida, 2nd ed; Embrapa: Brasília, Brazil, 2010; 129p. [Google Scholar]
- Iamaguti, J.L.; Moitinho, M.R.; Teixeira, D.B.; Bicalho, E.d.S.; Panosso, A.R.; Junior, N.L.S. Preparo do solo e emissão de CO2, temperatura e umidade do solo em área canavieira. Rev. Bras. De Eng. Agricola E Ambient. 2015, 19, 497–504. [Google Scholar] [CrossRef]
- Carneiro, J.S.S.; Santos, A.C.M.; Fidelis, R.R.; Silva Neto, S.P.; Santos, A.C.; Silva, R.R. Diagnóstico e manejo da variabilidade espacial da fertilidade do solo no Cerrado do Piauí. Rev. De Ciências Agroambientais 2016, 2, 14. [Google Scholar]
- Sousa, D.M.G.; Lobato, E. Cerrado: Correção do Solo e Adubação, 2nd ed; Embrapa Informação Tecnológica: Brasília, Brazil, 2004; 416p. [Google Scholar]
- Resende, J.M.A.; Marques Júnior, J.; Martins Filho, M.V.; Dantas, J.S.; Siqueira, D.S.; Teixeira, D.B. Variabilidade espacial de atributos de solos coesos do leste maranhense. Rev. Bras. De Cienc. Do Solo 2014, 38, 1077–1090. [Google Scholar] [CrossRef]
- Silva, B.O.; Moitinho, M.R.; Santos, G.A.A.; Teixeira, D.B.; Fernandes, C.; La Scala, N., Jr. Soil CO2 emission and short-term soil pore class distribution after tillage operations. Soil Tillage Res. 2019, 186, 224–232. [Google Scholar] [CrossRef]
- Stolf, R.; Thurler, A.M.; Bacchi, O.O.S.B.; Reichardt, K. Method to estimate soil macroporosity and microporosity based on sand content and bulk density. Rev. Bras. De Cienc. Do Solo 2011, 35, 447–459. [Google Scholar] [CrossRef]
- Hakansson, I.; Lipiec, J. A review of the usefulness of relative bulk density values in studies of soil structure and compaction. Soil Tillage Res. 2000, 53, 71–85. [Google Scholar] [CrossRef]
- Singh, J.S.; Gupta, V.K. Soil microbial biomass: A key soil driver in management of ecosystem functioning. Sci. Total. Environ. 2018, 634, 497–500. [Google Scholar] [CrossRef]
- Ronquim, C.C. Conceitos de Fertilidade do Solo e Manejo Adequado Para as Regiões Tropicais, 2nd ed.; Embrapa Territorial: Campinas, Brazil, 2020; 34p. [Google Scholar]
- Bini, D.; Santos, C.A.; Carmo, K.B.; Kishino, N.; Andrade, G.; Zangaro, W.; Nogueira, M.A. Effects of land use on soil organic carbon and microbial processes associated with soil health in southern Brazil. Eur. J. Soil Biol. 2013, 55, 117–123. [Google Scholar] [CrossRef]
- Six, J.; Frey, S.D.; Thies, R.K.; Batten, K.M. Bacterial and fungal contributions to carbon sequestration in agroecosystems. Soil Sci. Soc. Am. J. 2006, 70, 555–569. [Google Scholar] [CrossRef]
- Fang, C.; Moncrieff, J. A model for soil CO2 production and transport 1: Model development. Agric. For. Meteorol. 1999, 95, 225–236. [Google Scholar] [CrossRef]
- Wink, C. Estoque de Carbono em Plantações de Eucalyptuus sp. Implantados em Campo Nativo. Santa Maria. Master’s Thesis, Centro de Ciências Rurais, Federal University of Santa Maria, Santa Maria, Brazil, 2009; 130p. [Google Scholar]
- Oikawa, P.; Sturtevant, C.; Knox, S.; Verfaillie, J.; Huang, Y.; Baldocchi, D. Revisiting the partitioning of net ecosystem exchange of CO2 into photosynthesis and respiration with simultaneous flux measurements of 13CO2 and CO2, soil respiration and a biophysical model, CANVEG. Agric. For. Meteorol. 2017, 234–235, 149–163. [Google Scholar] [CrossRef]
- Kuyah, S.; Buleti, S.; Dimobe, K.; Nkurunziza, L.; Moussa, S.; Muthuri, C.; Öborn, I. Farmer-managed natural regeneration in Africa: Evidence for climate change mitigation and adaptation in drylands. In Agroforestry for Sustainable Intensification of Agriculture in Asia and Africa; Dagar, J.C., Gupta, S.R., Sileshi, G.W., Eds.; Sustainability Sciences in Asia and Africa; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
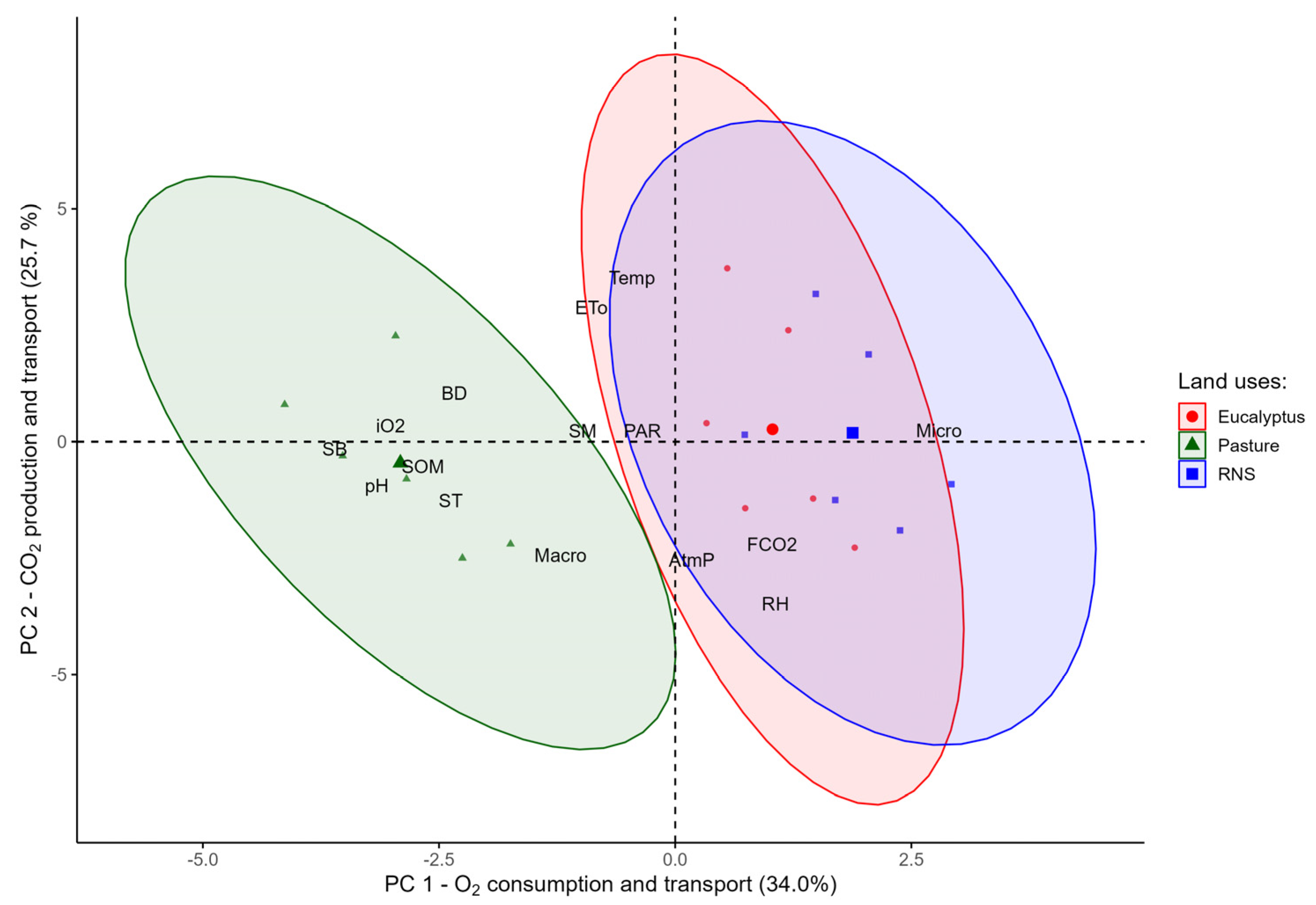
| Variables | PC 1 | PC 2 |
|---|---|---|
| FCO2 | 0.34 | −0.53 * |
| iO2 | −0.78 * | 0.01 |
| MS | −0.33 | −0.02 |
| MS | −0.72 * | −0.27 |
| SOM | −0.73 * | −0.24 |
| pH | −0.93 * | −0.18 |
| SB | −0.95 * | −0.13 |
| SbD | −0.71 * | 0.20 |
| Macro | −0.28 | −0.59 * |
| Micro | 0.83 * | 0.15 |
| Temp | −0.18 | 0.90 * |
| RH | 0.24 | −0.88 * |
| PAR | −0.15 | −0.02 |
| AtmP | 0.10 | −0.79 * |
| ETo | −0.19 | 0.89 * |
| Eigenvectors | 5.1 | 3.9 |
| Explained Variance (%) | 34.0 | 25.7 |
| Cumulative Explained Variance (%) | 34.0 | 59.7 |
| Interpretation of the formed processes | O2 consumption and transport | CO2 production and transport |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Lucena, W.B.; Canteral, K.F.F.; Vicentini, M.E.; Zulian, D.F.; Lima, R.P.D.; Moraes, M.L.T.D.; Cherubin, M.R.; Cerri, C.E.P.; Panosso, A.R.; La Scala Jr., N. Assessing Short-Term Temporal Variability of CO2 Emission and Soil O2 Influx in Tropical Pastures and Regenerating Forests. Appl. Sci. 2025, 15, 12302. https://doi.org/10.3390/app152212302
De Lucena WB, Canteral KFF, Vicentini ME, Zulian DF, Lima RPD, Moraes MLTD, Cherubin MR, Cerri CEP, Panosso AR, La Scala Jr. N. Assessing Short-Term Temporal Variability of CO2 Emission and Soil O2 Influx in Tropical Pastures and Regenerating Forests. Applied Sciences. 2025; 15(22):12302. https://doi.org/10.3390/app152212302
Chicago/Turabian StyleDe Lucena, Wanderson Benerval, Kleve Freddy Ferreira Canteral, Maria Elisa Vicentini, Daniele Fernanda Zulian, Renato Paiva De Lima, Mario Luiz Teixeira De Moraes, Maurício Roberto Cherubin, Carlos Eduardo Pellegrino Cerri, Alan Rodrigo Panosso, and Newton La Scala Jr. 2025. "Assessing Short-Term Temporal Variability of CO2 Emission and Soil O2 Influx in Tropical Pastures and Regenerating Forests" Applied Sciences 15, no. 22: 12302. https://doi.org/10.3390/app152212302
APA StyleDe Lucena, W. B., Canteral, K. F. F., Vicentini, M. E., Zulian, D. F., Lima, R. P. D., Moraes, M. L. T. D., Cherubin, M. R., Cerri, C. E. P., Panosso, A. R., & La Scala Jr., N. (2025). Assessing Short-Term Temporal Variability of CO2 Emission and Soil O2 Influx in Tropical Pastures and Regenerating Forests. Applied Sciences, 15(22), 12302. https://doi.org/10.3390/app152212302









