Microplastics in Humans: A Critical Review of Biomonitoring Evidence and Immune–Metabolic Associations
Abstract
1. Introduction
2. Scope and Approach
2.1. Inclusion Criteria
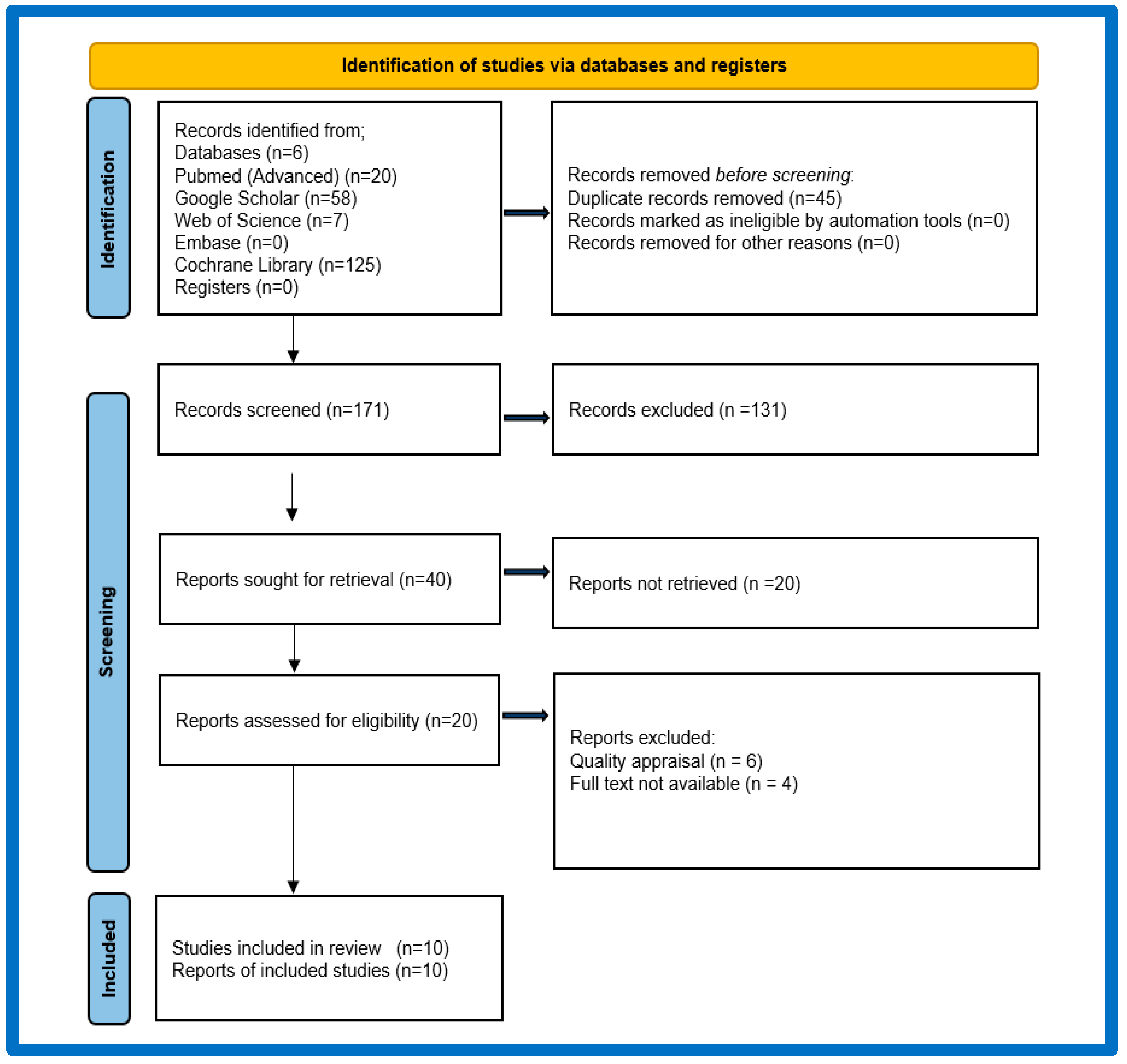
2.2. Selection Process
3. Results and Discussion
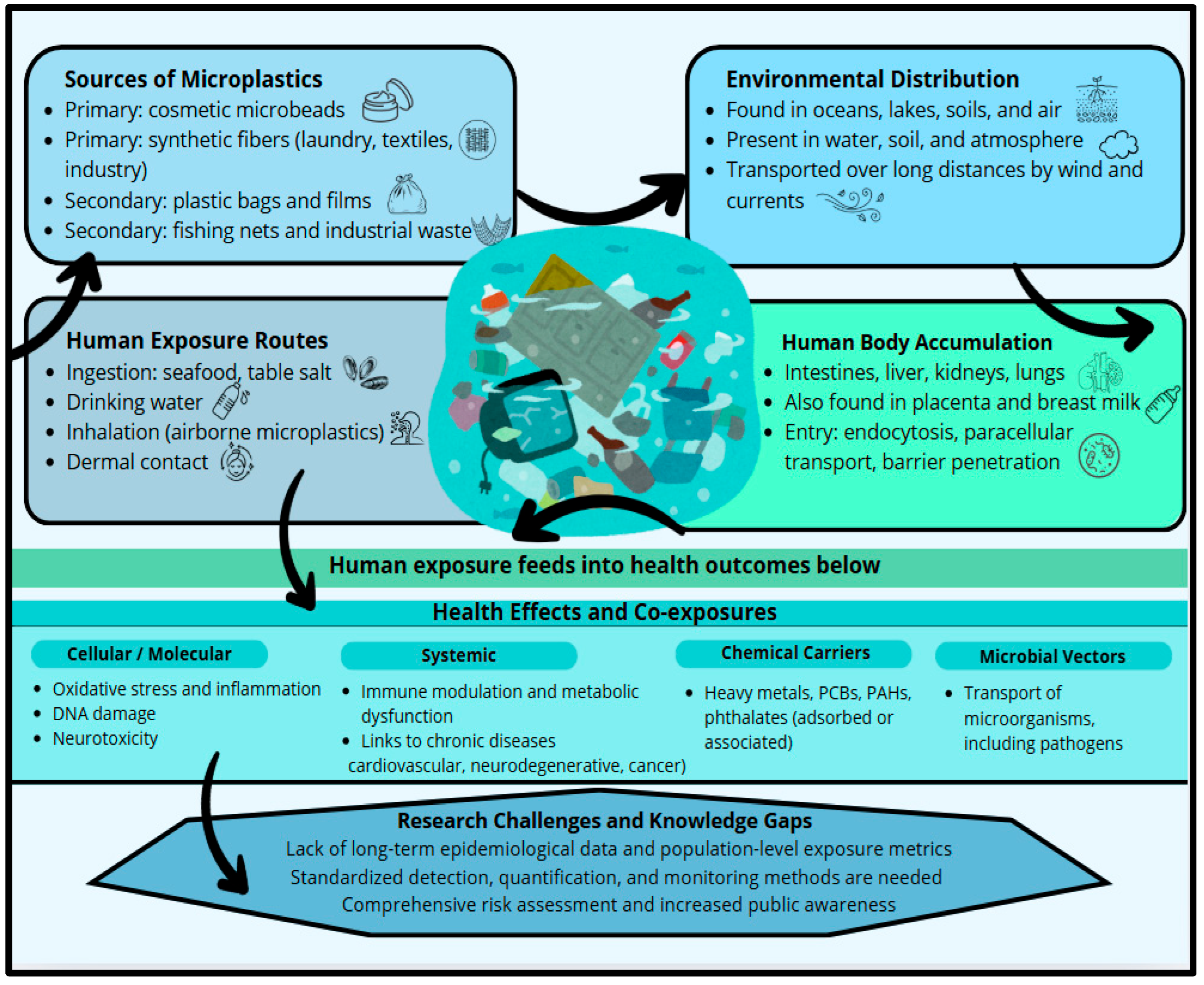
3.1. Accumulation of Microplastics in Human Tissues
3.2. Effects on Immune Regulation
3.3. Impact on Metabolic Functions
3.4. Analytical Challenges and Perspectives
3.5. Microplastic Pollution of Aquatic Environments: Ecological Implications and Human Exposure Pathways
3.6. Limitations
4. Conclusions
- How does chronic exposure to microplastics affect immune system regulation and inflammatory responses?
- What are the long-term metabolic consequences of microplastic accumulation in humans?
- Which molecular pathways are disrupted by MPs and their associated chemical additives?
- How can exposure levels to microplastics be accurately quantified in different populations and environments?
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABS | acrylonitrile butadiene styrene |
| CRP | c-reactive protein |
| EDCs | endocrine-disrupting chemicals |
| EPS | expanded polystyrene |
| FTIR-ATR | Fourier transform infrared attenuated total reflectance |
| IL-6 | interleukin-6 |
| IUGR | intrauterine growth restriction |
| LOQ | limit of quantification |
| MAPK | mitogen-activated protein kinases |
| NAFLD | non-alcoholic fatty liver disease |
| NF-κB | nuclear factor-kappa b |
| NO | nitric oxide |
| PAHs | polycyclic aromatic hydrocarbons |
| PCBs | polychlorinated biphenyls |
| PE | polyethene |
| PET | polyethene terephthalate |
| PMMA | polymethyl methacrylate |
| PP | polypropylene |
| PPROM | preterm premature rupture of membranes |
| PRISMA | preferred reporting items for systematic reviews and meta-analyses |
| PS | polystyrene |
| PT | prothrombin time |
| PVC | polyvinyl chloride |
| Py-GC/MS | pyrolytic gas chromatography coupled with mass spectrometry |
| ROS | reactive oxygen species |
| SCFA | short-chain fatty acids |
| TNF- α | tumor necrosis factor alpha |
References
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.; McGonigle, D.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef]
- Wu, P.; Huang, J.; Zheng, Y.; Yang, Y.; Zhang, Y.; He, F.; Chen, H.; Quan, G.; Yan, J.; Li, T.; et al. Environmental occurrences, fate, and impacts of microplastics. Ecotoxicol. Environ. Saf. 2019, 184, 109612. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, Y.; Zhang, T.; Zhang, F.; Ren, H.; Zhang, Y. Analysis of microplastics in human feces reveals a correlation between fecal microplastics and inflammatory bowel disease status. Environ. Sci. Technol. 2022, 56, 414–421. [Google Scholar] [CrossRef]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of microplastic on shorelines worldwide: Sources and sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef]
- Duis, K.; Coors, A. Microplastics in the aquatic and terrestrial environment: Sources (with a specific focus on personal care products), fate and effects. Environ. Sci. Eur. 2016, 28, 2. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, L. Microplastic migration and transformation pathways and exposure health risks. Environ. Pollut. 2025, 368, 125700. [Google Scholar] [CrossRef]
- Wright, S.L.; Kelly, F.J. Plastic and human health: A micro issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Manna, C.; Padha, S.; Verma, A.; Sharma, P.; Dhar, A.; Ghosh, A.; Bhattacharya, P. Micro(nano)plastics pollution and human health: How plastics can induce carcinogenesis to humans? Chemosphere 2022, 298, 134267. [Google Scholar] [CrossRef] [PubMed]
- Akdogan, Z.; Guven, B. Microplastics in the environment: A critical review of current understanding and identification of future research needs. Environ. Pollut. 2019, 254, 113011. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.Y.; Guo, J.Y.; Wang, X.Y.; Chang, X. Sources and distributions of microplastics and the hazards to plants, animals, and human health: A review. Ying Yong Sheng Tai Xue Bao J. Appl. Ecol. 2024, 35, 2301–2312. [Google Scholar]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Bakir, A.; Rowland, S.J.; Thompson, R.C. Enhanced desorption of persistent organic pollutants from microplastics under simulated physiological conditions. Environ. Pollut. 2014, 185, 16–23. [Google Scholar] [CrossRef]
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the “plastisphere”: Microbial communities on plastic marine debris. Environ. Sci. Technol. 2013, 47, 7137–7146. [Google Scholar] [CrossRef]
- Galloway, T.S.; Cole, M.; Lewis, C. Interactions of microplastic debris throughout the marine ecosystem. Nat. Ecol. Evol. 2017, 1, 116. [Google Scholar] [CrossRef]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of various microplastics in human stool: A prospective case series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Donisi, I.; Colloca, A.; Anastasio, C.; Balestrieri, M.L.; D’Onofrio, N. Micro(nano)plastics: An emerging burden for human health. Int. J. Biol. Sci. 2024, 20, 5779–5792. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, C.; Duan, X.; Liang, B.; Xu, G.; Huang, Z. Micro- and nanoplastics: A new cardiovascular risk factor? Environ. Int. 2023, 171, 107662. [Google Scholar] [CrossRef]
- Allouzi, M.M.A.; Tang, D.Y.Y.; Chew, K.W.; Rinklebe, J.; Bolan, N.; Allouzi, S.M.A.; Show, P.L. Micro(nano)plastic pollution: The ecological influence on soil-plant system and human health. Sci. Total Environ. 2021, 788, 147815. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Kang, Y.; Ma, M.; Wu, Z.; Zhang, L.; Hu, R.; Xu, Q.; Zhu, J.; Gu, X.; An, L. Tissue accumulation of microplastics and potential health risks in humans. Sci. Total Environ. 2024, 915, 170004. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.; Ehrlich, L.; Henrich, W.; Koeppel, S.; Lomako, I.; Schwabl, P.; Liebmann, B. Detection of microplastic in human placenta and meconium in a clinical setting. Pharmaceutics 2021, 13, 921. [Google Scholar] [CrossRef]
- Xue, J.; Xu, Z.; Hu, X.; Lu, Y.; Zhao, Y.; Zhang, H. Microplastics in maternal amniotic fluid and their associations with gestational age. Sci. Total Environ. 2024, 920, 171044. [Google Scholar] [CrossRef]
- Amereh, F.; Amjadi, N.; Mohseni-Bandpei, A.; Isazadeh, S.; Mehrabi, Y.; Eslami, A.; Naeiji, Z.; Rafiee, M. Placental plastics in young women from general population correlate with reduced foetal growth in IUGR pregnancies. Environ. Pollut. 2022, 314, 120174. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Halfar, J.; Čabanová, K.; Vávra, K.; Delongová, P.; Motyka, O.; Špaček, R.; Kukutschová, J.; Šimetka, O.; Heviánková, S. Microplastics and additives in patients with preterm birth: The first evidence of their presence in both human amniotic fluid and placenta. Chemosphere 2023, 343, 140301. [Google Scholar] [CrossRef] [PubMed]
- Salvia, R.; Cañaveras, M.; Rico, L.G.; Drozdowskyj, A.; Ward, M.D.; Jurado, R.; Gómez-Muñoz, L.; Vives-Pi, M.; Martínez-Cáceres, E.; Petriz, J. Prospective investigation of nanoplastic accumulation in healthy subjects, autoimmune diseases, hematological malignancies, lung cancer, and murine models. Microplastics 2025, 4, 1. [Google Scholar] [CrossRef]
- Hwang, J.; Choi, D.; Han, S.; Choi, J.; Hong, J. An assessment of the toxicity of polypropylene microplastics in human-derived cells. Sci. Total Environ. 2019, 684, 657–669. [Google Scholar] [CrossRef]
- Lee, D.W.; Jung, J.; Park, S.A.; Lee, Y.; Kim, J.; Han, C.; Kim, H.C.; Lee, J.H.; Hong, Y.C. Microplastic particles in human blood and their association with coagulation markers. Sci. Rep. 2024, 14, 30419. [Google Scholar] [CrossRef]
- Ali, N.; Katsouli, J.; Marczylo, E.L.; Gant, T.W.; Wright, S.; Bernardino de la Serna, J. The potential impacts of micro- and nano-plastics on various organ systems in humans. EBioMedicine 2024, 99, 104901. [Google Scholar] [CrossRef]
- Adler, M.Y.; Issoual, I.; Rückert, M.; Deloch, L.; Meier, C.; Tschernig, T.; Alexiou, C.; Pfister, F.; Ramsperger, A.F.; Laforsch, C.; et al. Effect of micro- and nanoplastic particles on human macrophages. J. Hazard Mater. 2024, 471, 134253. [Google Scholar] [CrossRef]
- Binatti, E.; Zoccatelli, G.; Zanoni, F.; Donà, G.; Mainente, F.; Chignola, R. Effects of combination treatments with astaxanthin-loaded microparticles and pentoxifylline on intracellular ROS and radiosensitivity of J774A.1 macrophages. Molecules 2021, 26, 5152. [Google Scholar] [CrossRef] [PubMed]
- Lopez, G.L.; Lamarre, A. The impact of micro- and nanoplastics on immune system development and functions: Current knowledge and future directions. Reprod. Toxicol. 2025, 135, 108951. [Google Scholar] [CrossRef]
- Gigault, J.; Halle, A.T.; Baudrimont, M.; Pascal, P.Y.; Gauffre, F.; Phi, T.L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef]
- Feng, Y.; Tu, C.; Li, R.; Wu, D.; Yang, J.; Xia, Y.; Peijnenburg, W.J.G.M.; Luo, Y. A systematic review of the impacts of exposure to micro- and nano-plastics on human tissue accumulation and health. Eco Environ. Health 2023, 2, 195–207. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, Y.; Du, F.; Cai, H.; Wang, G.; Shi, H. Microplastic fallout in different indoor environments. Environ. Sci. Technol. 2020, 54, 6530–6539. [Google Scholar] [CrossRef] [PubMed]
- Wolff, C.M.; Singer, D.; Schmidt, A.; Bekeschus, S. Immune and inflammatory responses of human macrophages, dendritic cells, and T-cells in presence of micro- and nanoplastics of different types and sizes. J. Hazard Mater. 2023, 459, 132194. [Google Scholar] [CrossRef] [PubMed]
- Dan, K.B.; Yoo, J.Y.; Min, H. The emerging threat of micro- and nanoplastics on the maturation and activity of immune cells. Biomol. Ther. 2025, 33, 95–105. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, M.; Wang, L.; Gu, W.; Li, X.; Han, Z.; Fu, X.; Wang, X.; Li, X.; Su, Z. Continuous oral exposure to micro- and nanoplastics induced gut microbiota dysbiosis, intestinal barrier, and immune dysfunction in adult mice. Environ. Int. 2023, 182, 108353. [Google Scholar] [CrossRef]
- Wang, K.; Zhu, L.; Rao, L.; Zhao, L.; Wang, Y.; Wu, X.; Zheng, H.; Liao, X. Nano- and micro-polystyrene plastics disturb gut microbiota and intestinal immune system in honeybee. Sci. Total Environ. 2022, 842, 156819. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Arroyo, C.; Tamargo, A.; Molinero, N.; Moreno-Arribas, M.V. The gut microbiota, a key to understanding the health implications of micro(nano)plastics and their biodegradation. Microb. Biotechnol. 2023, 16, 34–53. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Wang, C.; Adyel, T.M.; Wu, J.; Liu, Z.; You, G.; Meng, M.; Qu, H.; Huang, L.; Yu, Y.; et al. Microbial carbon metabolic functions of biofilms on plastic debris influenced by substrate types and environmental factors. Environ. Int. 2020, 143, 106007. [Google Scholar] [CrossRef]
- Allen, S.; Allen, D.; Karbalaei, S.; Maselli, V.; Walker, T.R. Micro(nano)plastics sources, fate, and effects: What we know after ten years of research. J. Hazard Mater. Adv. 2022, 6, 100057. [Google Scholar] [CrossRef]
- Zheng, Y.; Xu, S.; Liu, J.; Liu, Z. The effects of micro- and nanoplastics on the central nervous system: A new threat to humanity? Toxicology 2024, 504, 153457. [Google Scholar] [CrossRef]
- Jiménez, D.J.; Öztürk, B.; Wei, R.; Bugg, T.D.; Amaya Gomez, C.V.; Salcedo Galan, F.; Castro-Mayorga, J.L.; Saldarriaga, J.F.; Tarazona, N.M. Merging plastics, microbes, and enzymes: Highlights from an international workshop. Appl. Environ. Microbiol. 2022, 88, e0072122. [Google Scholar] [CrossRef]
- Ni, P.; Li, C.; Fu, Y.; Stover, N.A.; Li, L. Physiological and molecular responses to different sizes of polystyrene micro/nanoplastics in the model unicellular eukaryote Paramecium tetraurelia. J. Hazard Mater. 2025, 495, 138963. [Google Scholar] [CrossRef]
- Jiang, N.; Chang, X.; Huang, W.; Khan, F.U.; Fang, J.K.; Hu, M.; Xu, E.G.; Wang, Y. Physiological response of mussel to rayon microfibres and PCB exposure: Overlooked semi-synthetic micropollutant? J. Hazard Mater. 2024, 470, 134107. [Google Scholar] [CrossRef]
- Habumugisha, T.; Zhang, Z.; Uwizewe, C.; Yan, C.; Ndayishimiye, J.C.; Rehman, A.; Zhang, X. Toxicological review of micro- and nano-plastics in aquatic environments: Risks to ecosystems, food web dynamics and human health. Ecotoxicol. Environ. Saf. 2024, 278, 116426. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, W.X. Differential effects of foodborne and waterborne micro(nano)plastics exposure on fish liver metabolism and gut microbiota community. J. Hazard Mater. 2025, 488, 137471. [Google Scholar] [CrossRef]
| References | Type of Study | Participants/Tissues | Country | Methods | Results |
|---|---|---|---|---|---|
| Ragusa et al. (2021) [21] | observational, descriptive | - healthy pregnant women with physiological delivery (n = 6) - placenta | Italy | - spectroscopic method (Raman Microspectroscopy) - morphological analysis - comparison of spectrograms | - microplastics detected in human placenta—first report - various types and sizes of particles → mainly PP and PE - present in 66.7% of samples → penetration into foetal tissues - significantly higher incidence compared to the control |
| Zhu et al. (2024) [22] | observational (cross-sectional) study | - lungs, intestines, tonsils | China | - Laser Direct Infrared Spectroscopy (LDIR) method | - microplastics detected in human tissues, mainly in the lungs (dominant polymer: PVC) - significant differences in microplastic levels between different tissues - potential health risks associated with microplastic accumulation |
| Braun et al. (2021) [23] | observational (clinical) study | - women giving birth (n = 2) and newborns (n = 2) - placenta and meconium | Germany | - FTIR microspectroscopy method after chemical preparation of samples (separation of microplastics >50 µm) | - microplastics detected in the placenta and meconium → transfer to the mother’s and foetus’s organism - significant differences between test and control samples |
| Xue et al. (2024) [24] | observational clinical study | - pregnant women during pregnancy (n = 40) - amniotic fluid (n = 32) | China | - Laser Direct Infrared (LD-IR) - Spectroscopic analysis compared with a library of microplastic spectra | - microplastics detected in amniotic fluid in 80% of pregnant women → penetration into the prenatal environment - various types and sizes of particles; predominant: PE and PP - significant correlation between the presence of microplastics and gestational age - higher concentrations of microplastics may be associated with shorter gestation periods → potential impact on foetal development and newborn health |
| Amereh et al. (2022) [25] | observational clinical-analytical study | - pregnant women from the general population (n = 46), including those with IUGR (n = 13) and normal pregnancy (n = 30) - placenta | Iran | - Digital microscopy - Raman microscopy | - microplastics (<5 mm) detected in all IUGR placenta samples - particles <10 µm accounted for up to 64% of all detected particles. - fragments and fibres predominated in IUGR pregnancies; fragments predominated in normal pregnancies - dominant polymers: PE and PS - correlations in IUGR pregnancies: birth weight, body length, head circumference, Apgar 1 min |
| Leslie et al. (2022) [26] | empirical study—human biomonitoring | - healthy volunteers, blood donors from the general population (n = 22) - blood | Netherlands | - Double-shot Py-GC/MS method for quantitative detection of plastic particles (≥700 nm) in human whole blood | - four main types of polymers: PET (50%), PE (23%), styrene polymers (PS (36%)) and polymethyl methacrylate (PMMA) (5%) - in 77% of subjects, particle concentration >LOQ - three different polymers detected in one person - average concentration of microplastics in blood: 1.6 µg/mL |
| Halfar et al. (2023) [27] | observational clinical-analytical study | - pregnant women with physiological, single pregnancies complicated by PPROM (n = 10) - amniotic fluid and placenta | China | - FTIR-ATR method after KOH digestion | - higher microplastic levels in women with preterm birth vs. full-term pregnancies - microplastics and plastic additives detected - dominant polymers: PP and PET |
| Salvia et al. (2025) [28] | prospective cohort study | - adults and children (n = 262) with autoimmune and cancerous diseases (n = 196), healthy volunteers (n = 66) - lymph and peripheral blood | Poland/Europe | - a combination of Nile red fluorescence and nanocytometry (flow cytometry + high-resolution imaging) is used to detect and quantitatively determine nanoplastics in biological samples. | - higher levels of nanoplastics in lymph and blood in patients with autoimmune and haematological diseases - increased nanoplastic levels correlate with disease severity |
| Hwang et al. (2019) [29] | in vitro experimental study | - human cells (immune system and blood) | Germany | - in vitro experimental method with cell function analysis (cytokines, ROS, proliferation) | - exposure of human immune cells to PP microplastics → increase in cytotoxicity, ROS and oxidative stress markers, activation of IL-6 and TNF-α - potential risk of microplastic immunotoxicity |
| Lee et al. (2024) [30] | cross-sectional study | - healthy adults (n = 36) - blood | China | - spectroscopic methods, including analysis using Fourier transform spectroscopy in the infrared range (µ-FTIR) and Raman spectroscopy (µ-Raman) | - microplastics detected in 88.9% of blood samples - average microplastic concentration was 4.2 ± 1.1 particles/mL - correlation with prolonged prothrombin time (PT) and elevated C-reactive protein (CRP) levels |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusyk, D.; Górna, I.; Kowalówka, M.; Banaszak, M.; Jakubowski, K.; Drzymała-Czyż, S. Microplastics in Humans: A Critical Review of Biomonitoring Evidence and Immune–Metabolic Associations. Appl. Sci. 2025, 15, 12289. https://doi.org/10.3390/app152212289
Kusyk D, Górna I, Kowalówka M, Banaszak M, Jakubowski K, Drzymała-Czyż S. Microplastics in Humans: A Critical Review of Biomonitoring Evidence and Immune–Metabolic Associations. Applied Sciences. 2025; 15(22):12289. https://doi.org/10.3390/app152212289
Chicago/Turabian StyleKusyk, Dominika, Ilona Górna, Magdalena Kowalówka, Michalina Banaszak, Karol Jakubowski, and Sławomira Drzymała-Czyż. 2025. "Microplastics in Humans: A Critical Review of Biomonitoring Evidence and Immune–Metabolic Associations" Applied Sciences 15, no. 22: 12289. https://doi.org/10.3390/app152212289
APA StyleKusyk, D., Górna, I., Kowalówka, M., Banaszak, M., Jakubowski, K., & Drzymała-Czyż, S. (2025). Microplastics in Humans: A Critical Review of Biomonitoring Evidence and Immune–Metabolic Associations. Applied Sciences, 15(22), 12289. https://doi.org/10.3390/app152212289






