Detection of Brain Tumors Using UWB Antennas in a High-Fidelity Phantom Model
Abstract
1. Introduction
2. UWB Array Antenna Model
2.1. Vivaldi Antenna Element
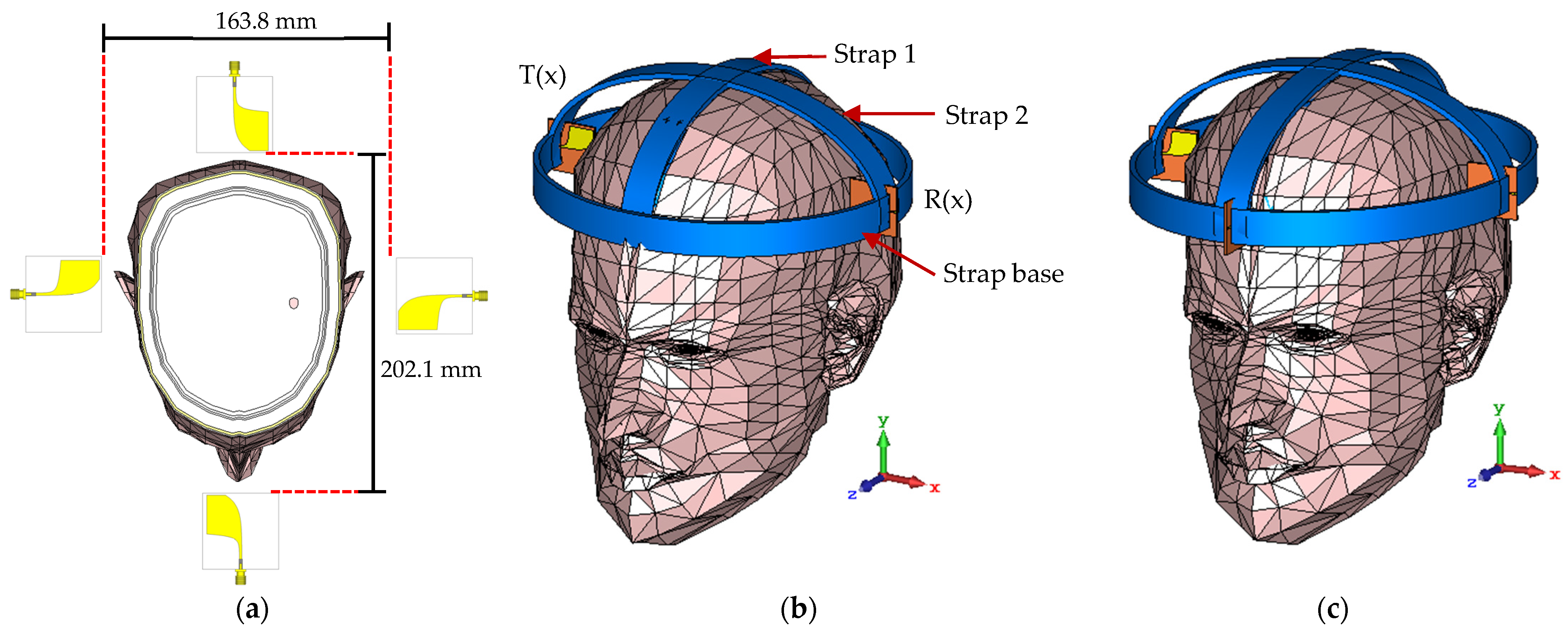
2.2. Phantom Model
2.3. UWB Array Antennas with Phantom
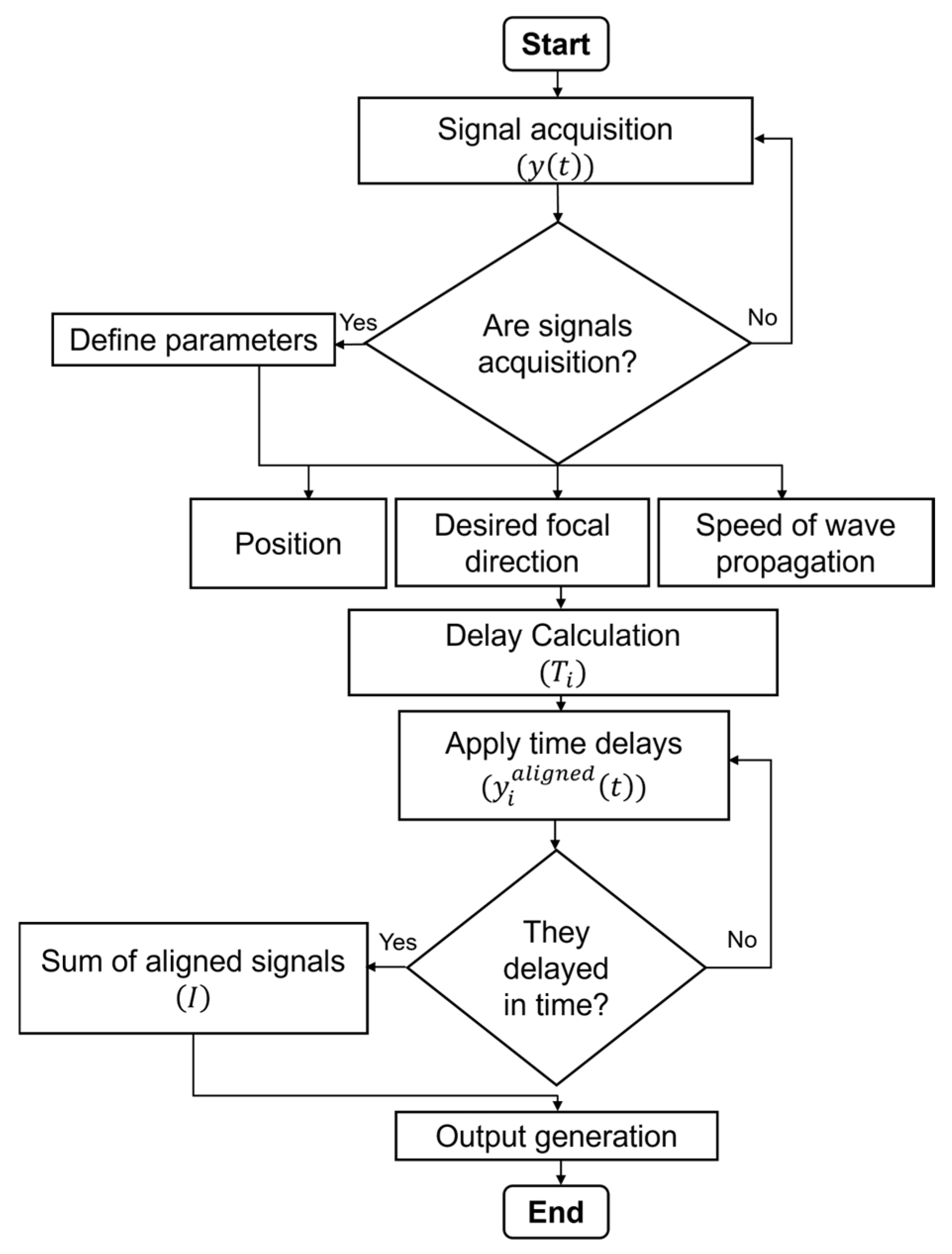
3. Imaging Method
4. Simulation Results
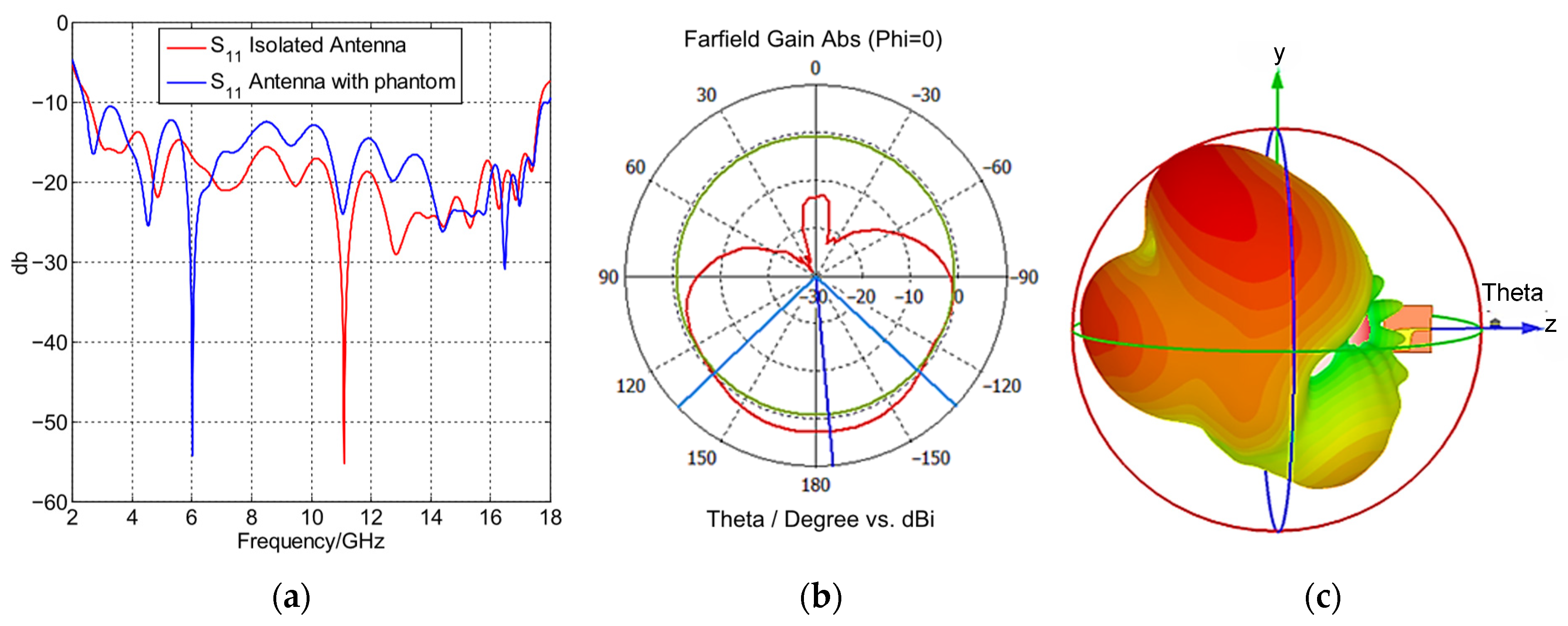
4.1. Single Antenna Element Performance
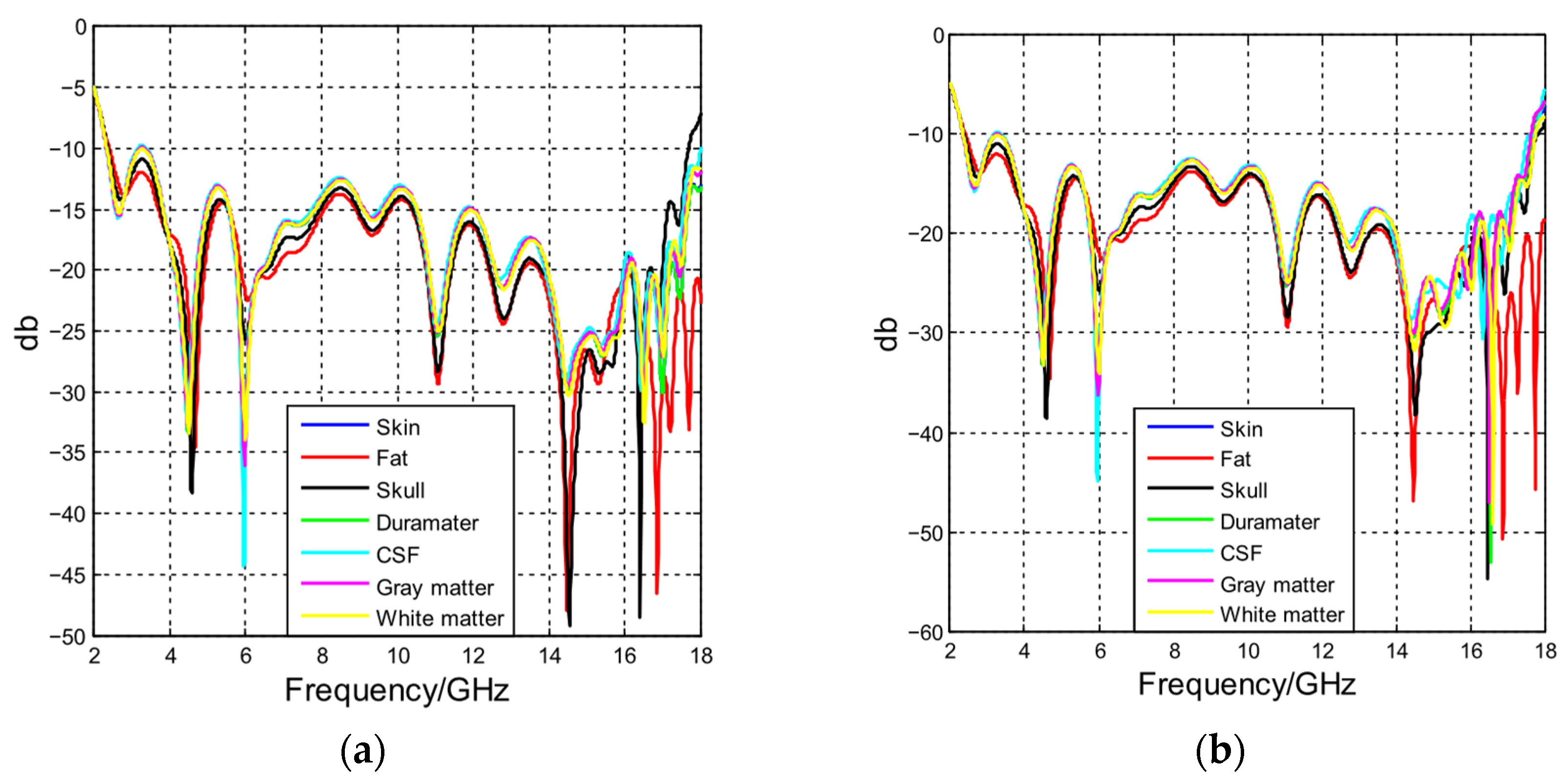
4.2. Antenna Performance with Phantoms Composed by Different Materials
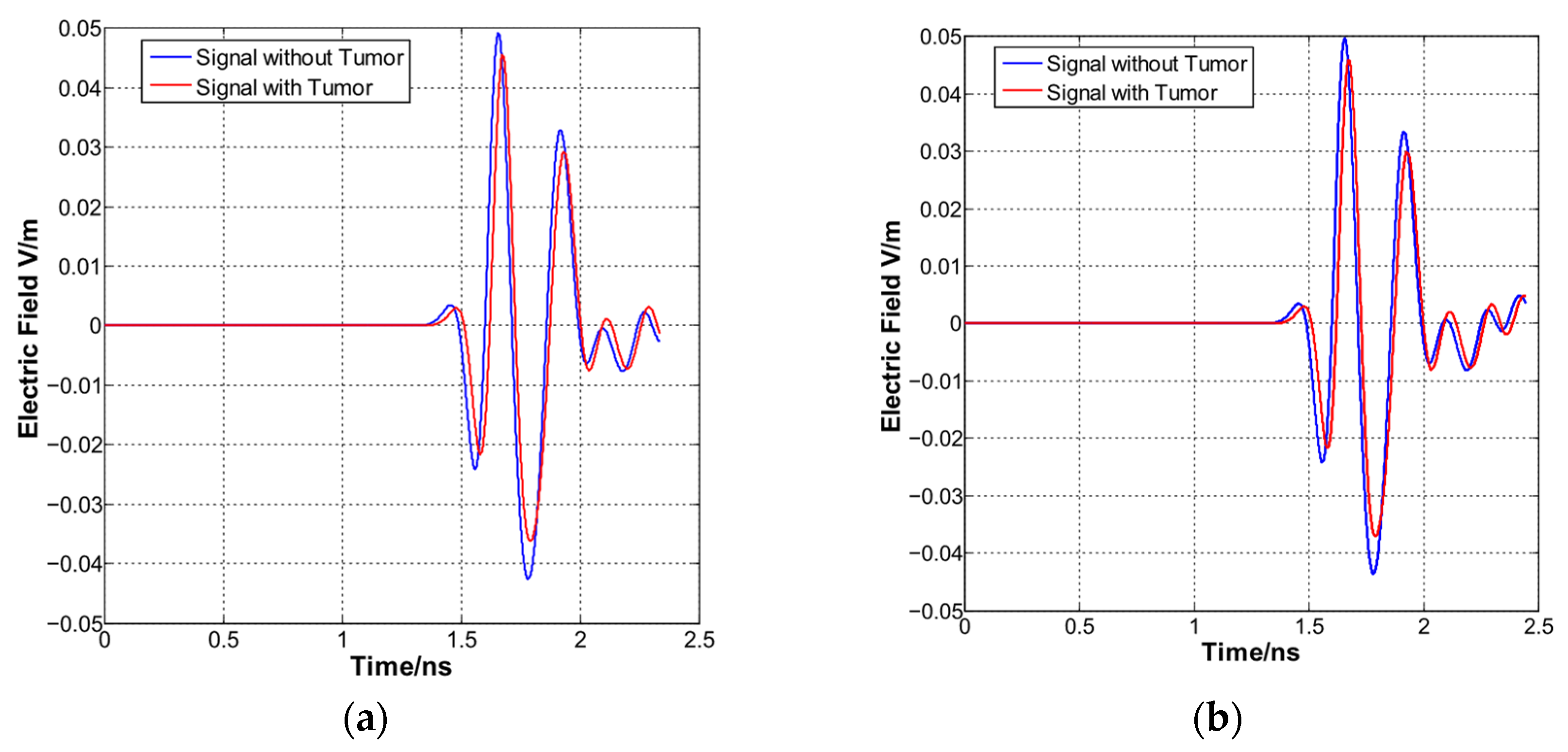
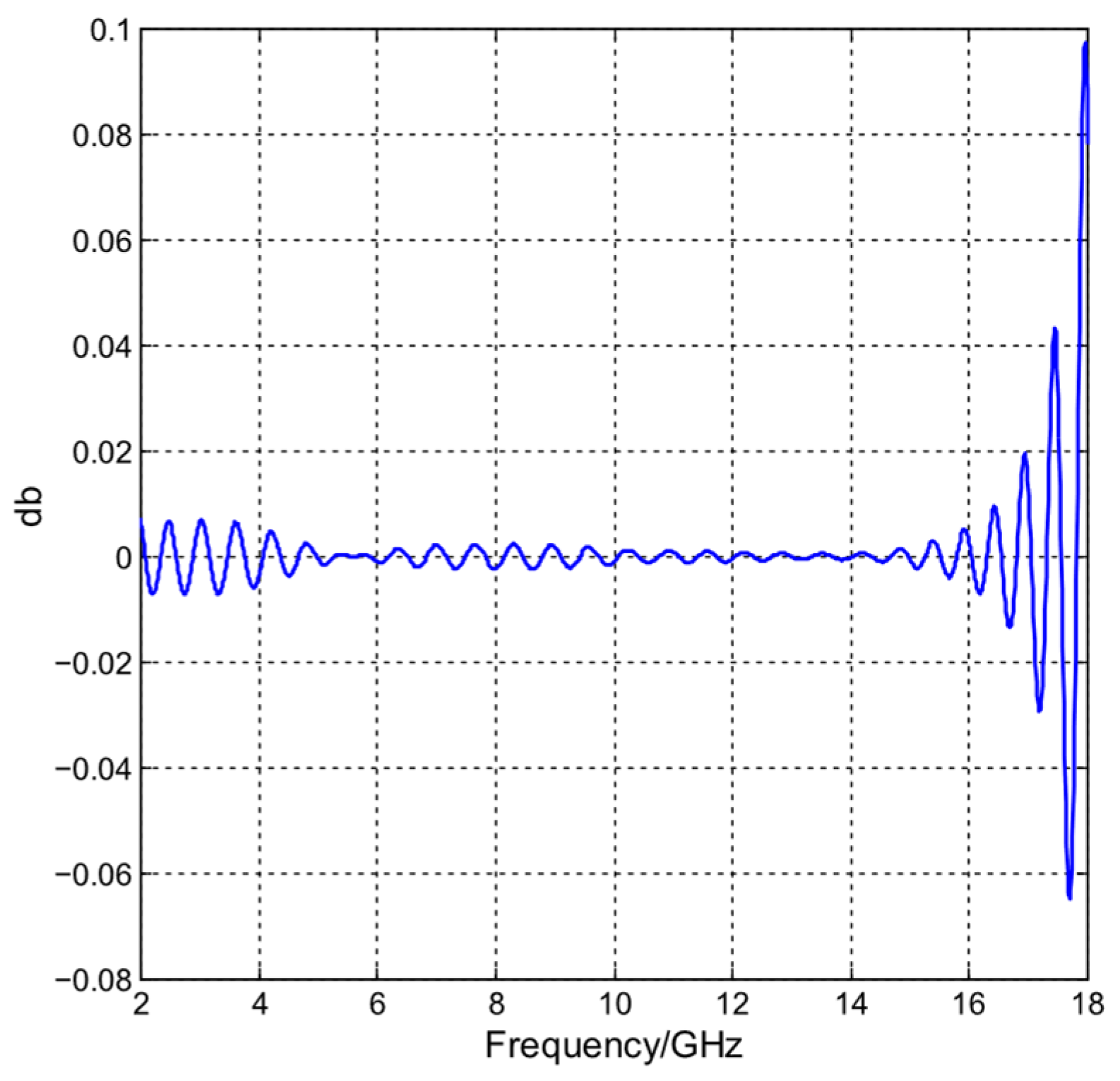
4.3. Time Signals and Image Resolution
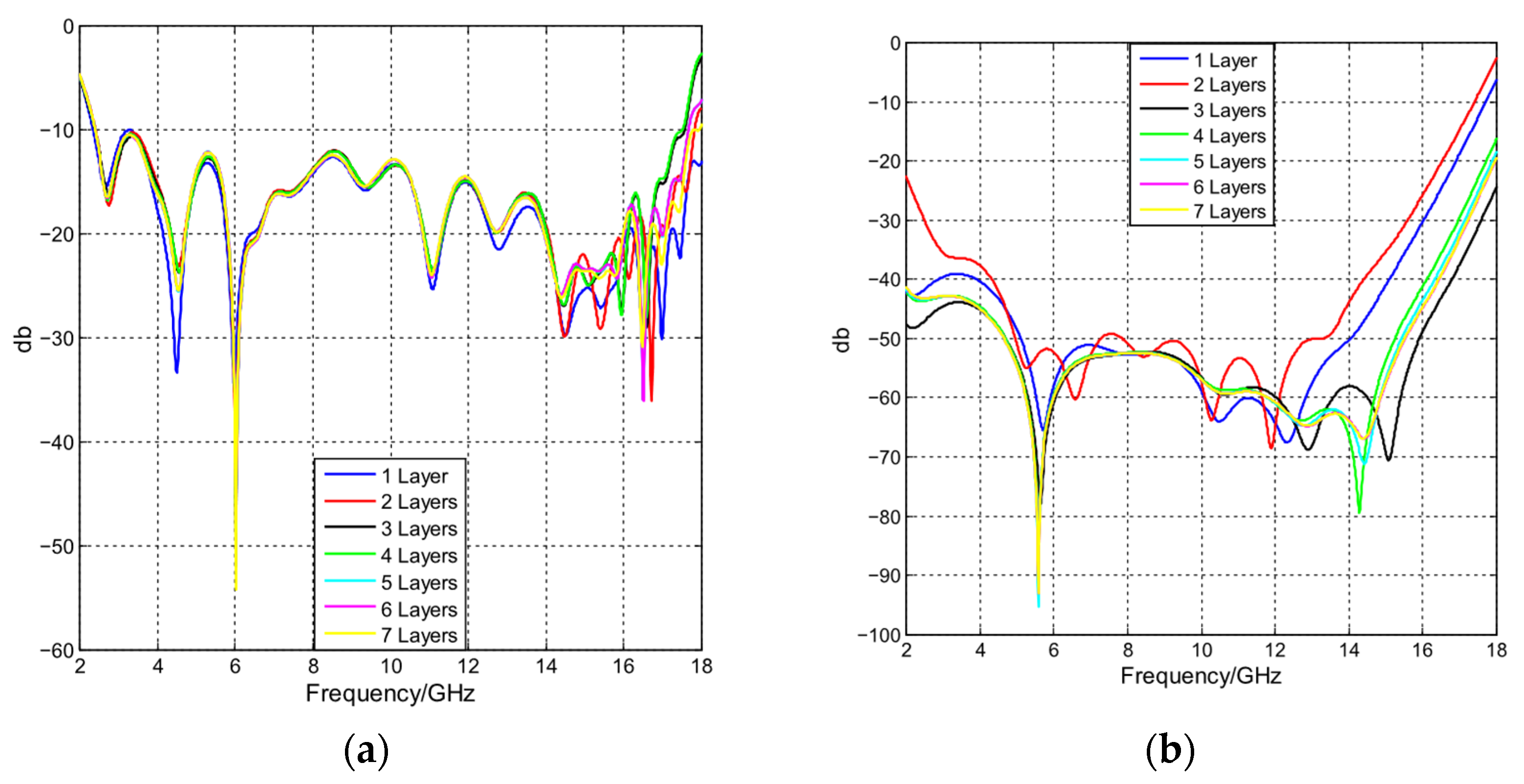
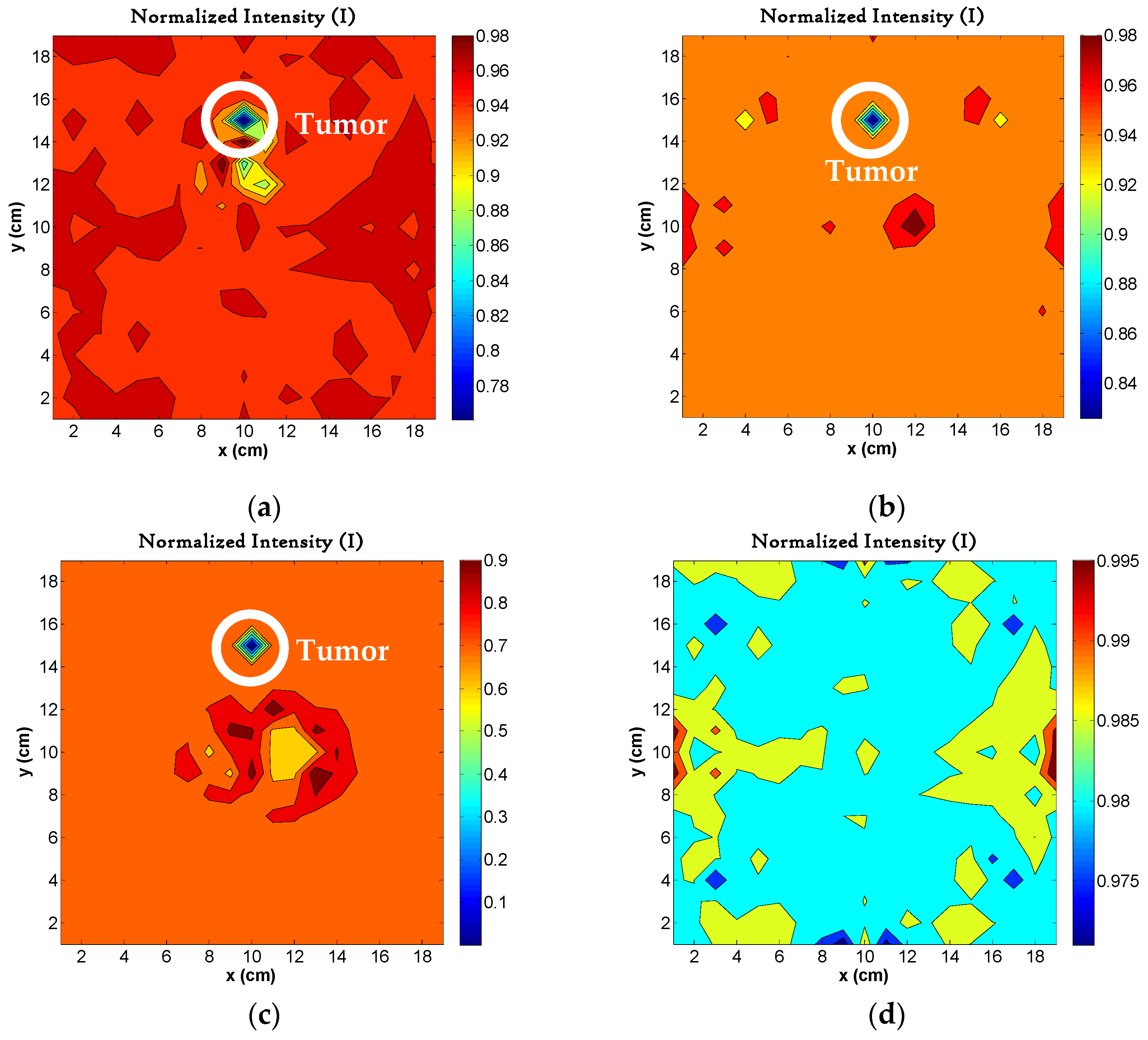
4.4. Image Reconstruction with Different Layers in Phantom
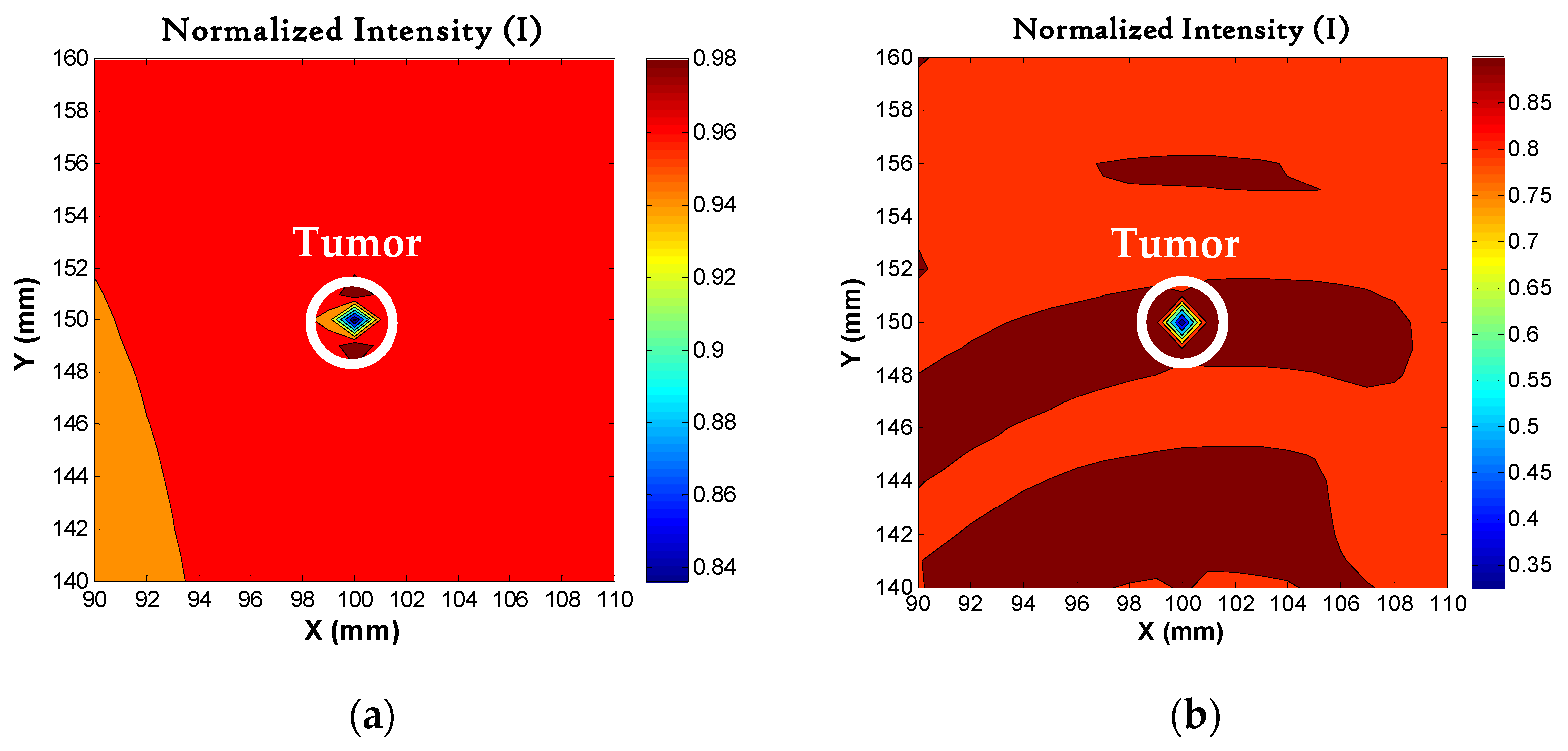
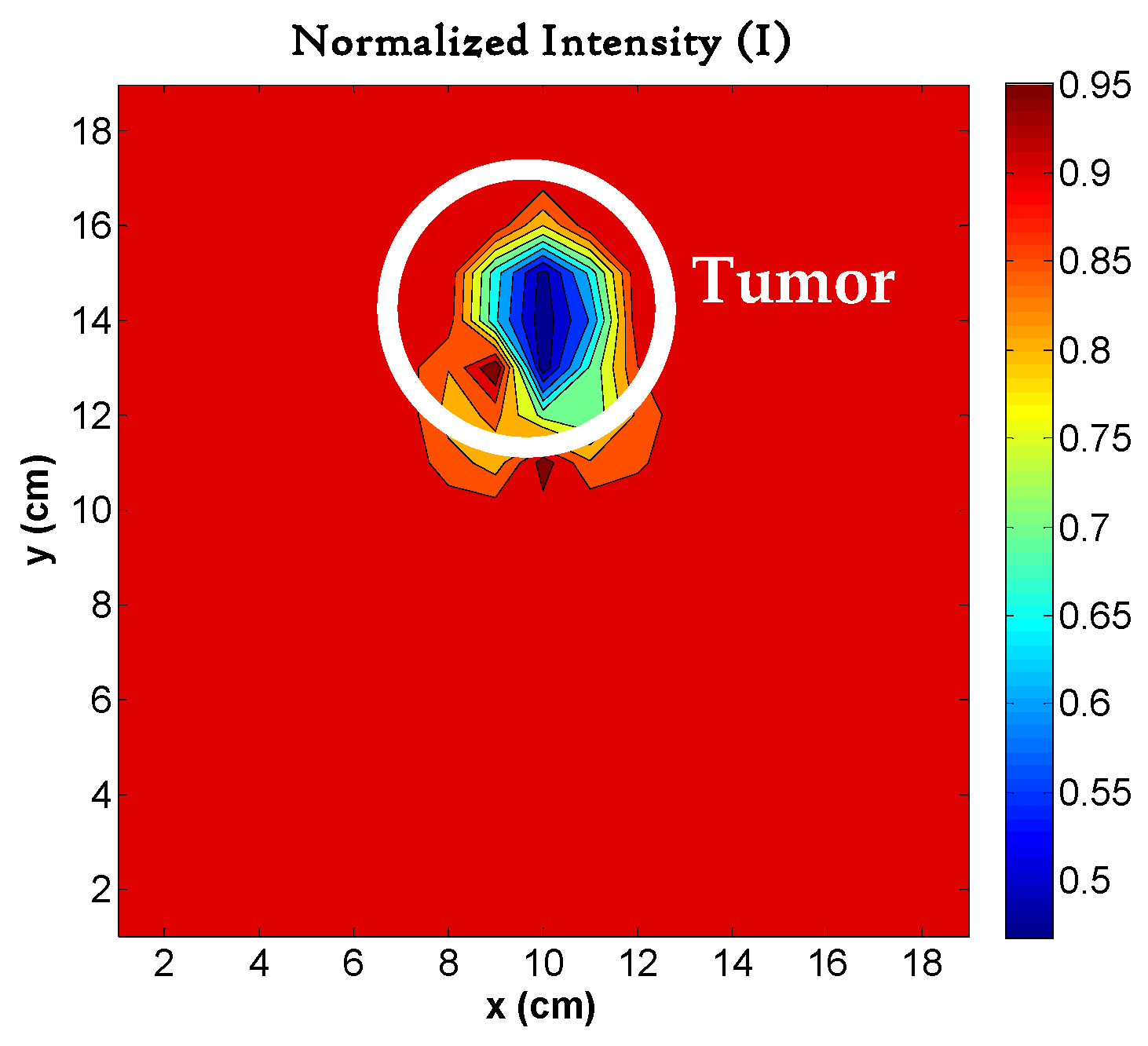
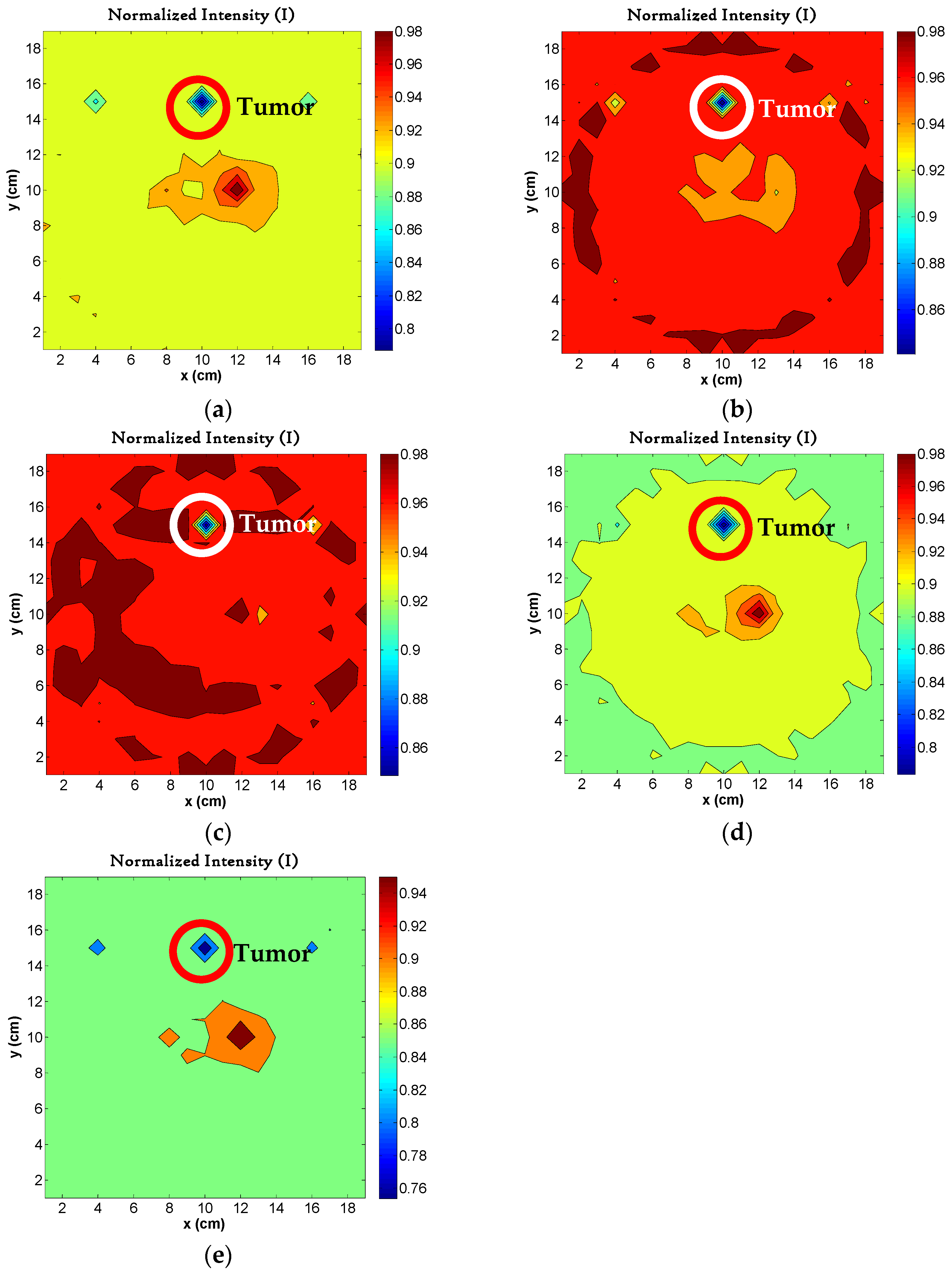
4.5. Image Reconstruction for Different Sizes of Tumors
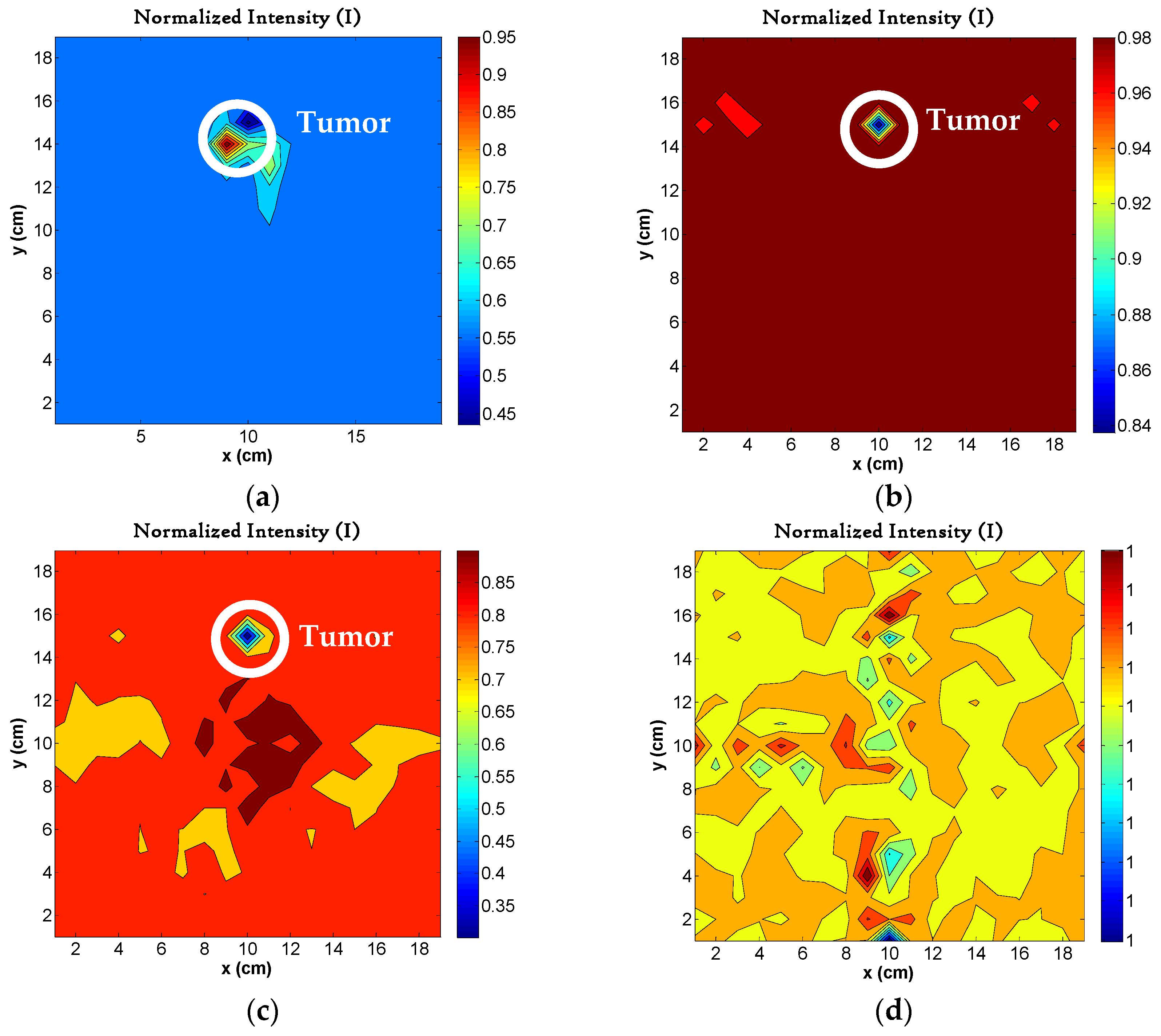
4.6. Image Reconstruction for Variations in Permittivity
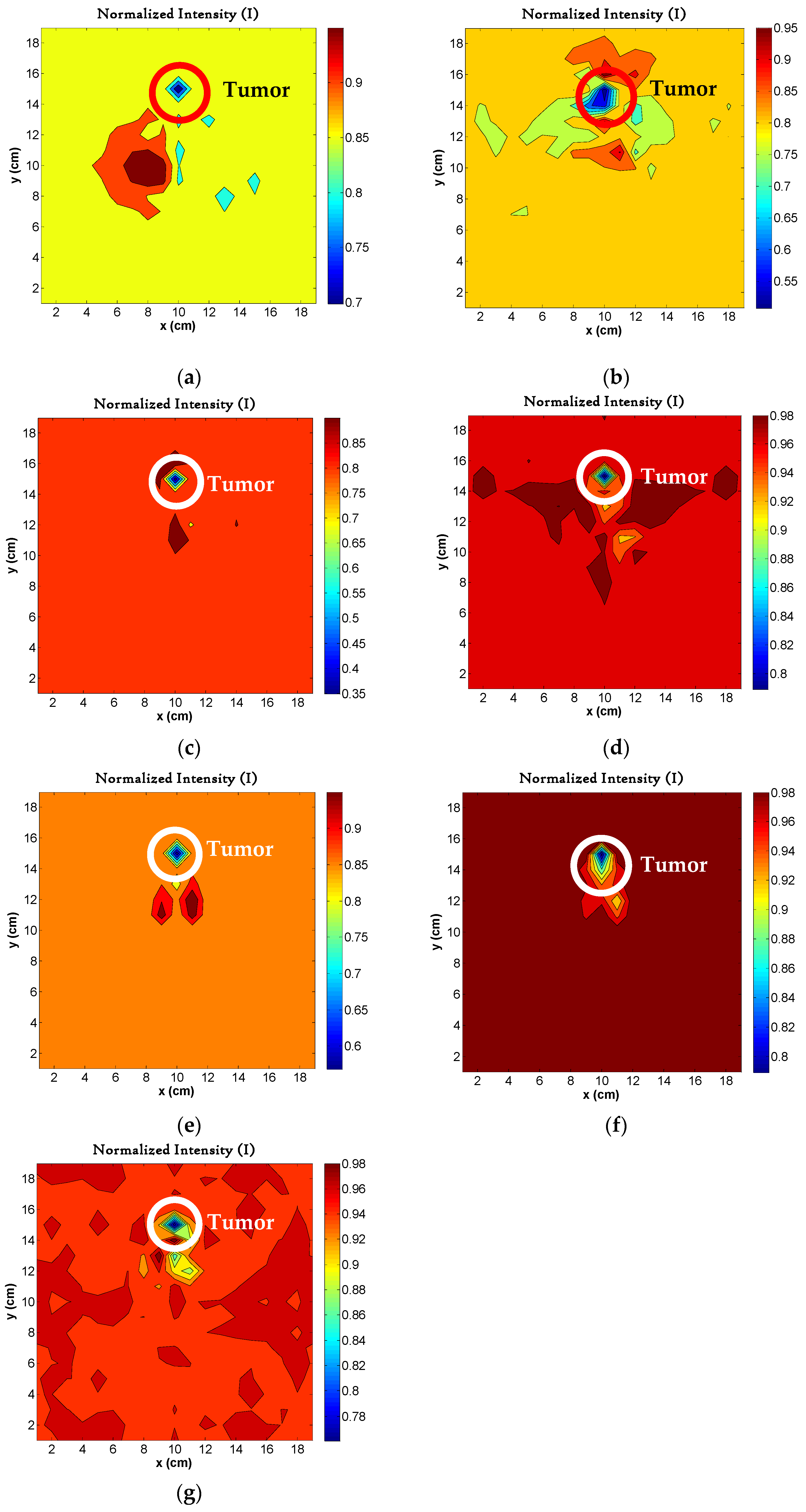
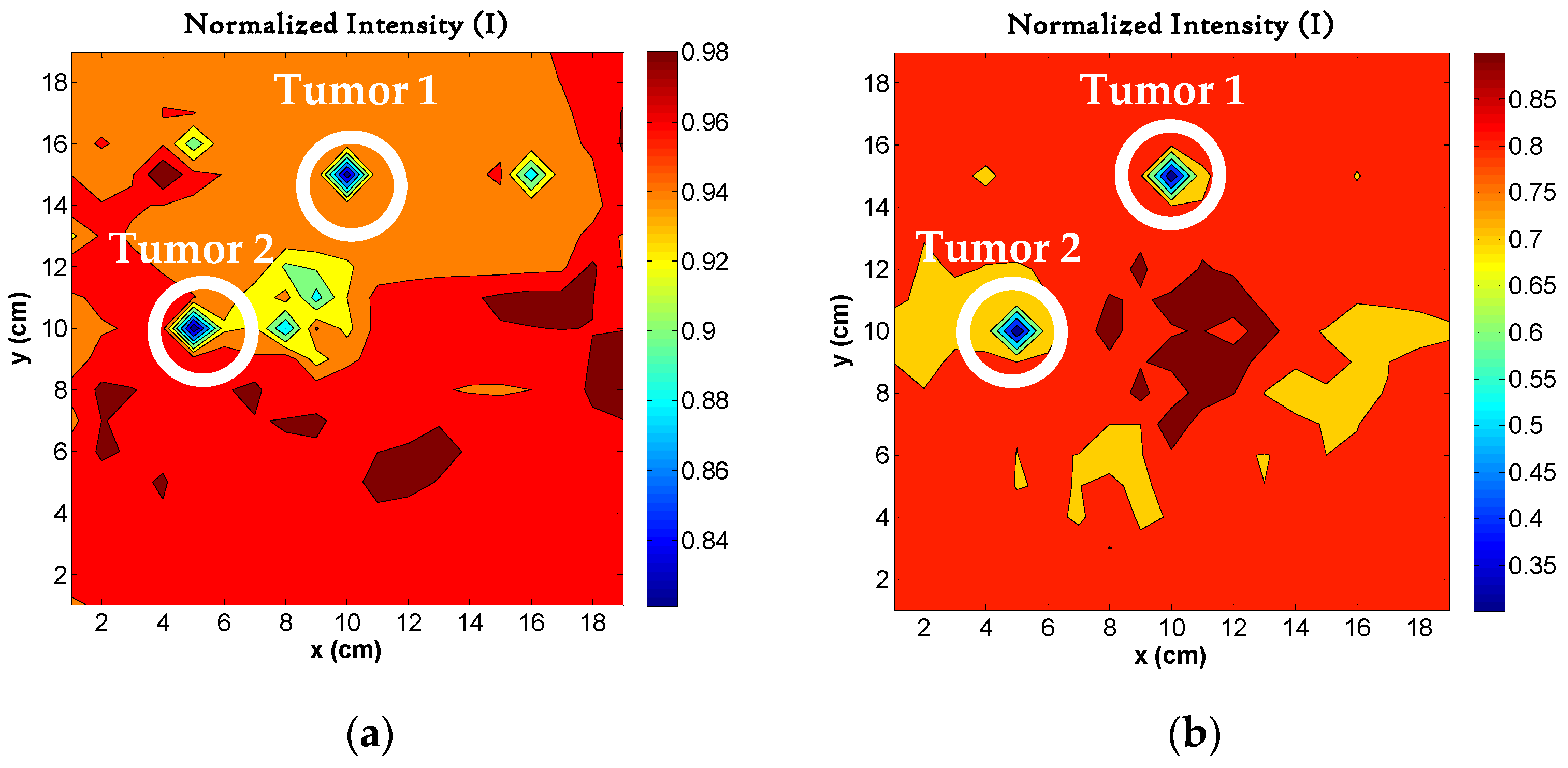
4.7. Image Reconstruction for Different Number of Tumors
4.8. Image Quality Metrics
4.9. Results Comparison with Similar Works
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mayo Clinic. Tumor Cerebral. Prensa de Mayo Clinic, 15 October 2025. [Google Scholar]
- Haskell, M.W.; Nielsen, J.-F.; Noll, D.C. Off-resonance artifact correction for magnetic resonance imaging: A review. NMR Biomed. 2023, 36, e4867. [Google Scholar] [CrossRef] [PubMed]
- Cancer Council NSW. Understanding Brain Tumors; IVE Group: Sydney, Australia, 2024. [Google Scholar]
- Narita, C.; Clements, W.; Varma, D. Assessing the necessity of intravenous contrast for computed tomography in the acute undifferentiated abdomen. J. Med. Imaging Radiat. Sci. 2023, 54, 123–130. [Google Scholar] [CrossRef]
- THANC Guide. Tomografía por Emisión de Positrones (PET); THANC Foundation: New York, NY, USA, 2025. [Google Scholar]
- Aljubran, A.H.; Badran, A.; Alshaer, O.; Alhashem, H.; Omar, A.; Eldali, A. Pattern of use of positron emission tomography/computed tomography (PET/CT) scan in non-colorectal gastrointestinal cancers at KFSHRC, Riyadh, Saudi Arabia. Egypt. J. Radiol. Nucl. Med. 2020, 50, 60. [Google Scholar] [CrossRef]
- Lasemiimeni, Z.; Atlasbaf, Z.; Karbaschi, N. Dual-Functional Ultrawideband Antenna With High Fidelity Factor for Body Area Networks and Microwave Imaging Systems. IEEE Access 2021, 9, 112930–112941. [Google Scholar] [CrossRef]
- Alhawari, A.R.H.; Almawgani, A.H.M.; Hindi, A.T.; Alghamdi, H.; Saeidi, T. Metamaterial-based wearable flexible elliptical UWB antenna for WBAN and breast imaging applications. AIP Adv. 2021, 11, 015128. [Google Scholar] [CrossRef]
- Fiser, O.; Hruby, V.; Vrba, J.; Drizdal, T.; Tesarik, J.; Vrba, J., Jr.; Vrba, D. UWB Bowtie Antenna for Medical Microwave Imaging Applications. IEEE Trans. Antennas Propag. 2022, 70, 5357–5372. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.; Kong, Y.; Zhou, C. Flexible Dual-Polarized UWB Antenna Sensors for Breast Tumor Detection. IEEE Sens. J. 2022, 22, 13648–13658. [Google Scholar] [CrossRef]
- Sediq, H.T. Tumor detection concepts using eagle-shaped UWB antenna signals for medical purposes. Sens. Actuators A Phys. 2023, 362, 114653. [Google Scholar] [CrossRef]
- Danjuma, I.M.; Akinsolu, M.O.; See, C.H.; Abd-Alhameed, R.A.; Liu, B. Design and optimization of a slotted monopole antenna for ultra-wide band body centric imaging applications. IEEE J. Electromagn. RF Microw. Med. Biol. 2020, 4, 140–147. [Google Scholar] [CrossRef]
- Subramanian, S.; Sundarambal, B.; Nirmal, D. Investigation on Simulation-Based Specific Absorption Rate in Ultra-Wideband Antenna for Breast Cancer Detection. IEEE Sens. J. 2018, 18, 10002–10009. [Google Scholar] [CrossRef]
- Särestöniemi, M.; Sonkki, M.; Myllymäki, S.; Pomalaza-Raez, C. Wearable Flexible Antenna for UWB On-Body and Implant Communications. Telecom 2021, 2, 285–301. [Google Scholar] [CrossRef]
- Hammouch, N.; Ammor, H.; Himdi, M. A Compact Flexible UWB Antenna for Biomedical Applications: Especially for Breast Cancer Detection. In Proceedings of the 6th International Conference on Wireless Technologies, Embedded, and Intelligent Systems, Fez, Morocco, 14–16 October 2020; Springer: Singapore, 2020; pp. 1061–1072. [Google Scholar]
- Naghavi, A.H.; Hassani, H.R.; Oloumi, D. Investigation and Analysis of EM Pulse Propagation Inside Human Head for High-Resolution UWB Elliptical SAR Imaging. IEEE J. Electromagn. RF Microw. Med. Biol. 2022, 6, 485–493. [Google Scholar] [CrossRef]
- Shin, G.; Kim, W.; Kim, M.C.; Kim, J.; Chung, J.Y.; Nah, J.; Yoon, I.J. A Deionized Water-Infilled Dual-Layer Insulator-Applied Brain-Implanted UWB Antenna for Wireless Biotelemetry Applications. IEEE Trans. Antennas Propag. 2022, 70, 6469–6478. [Google Scholar] [CrossRef]
- Gazit, E. Improved design of the Vivaldi antenna. IEE Proc. 1988, 135, 89–92. [Google Scholar] [CrossRef]
- Sasikala, S.; Karthika, K.; Arunkumar, S.; Anusha, K.; Adithya, S.; Al-Gburi, A.J.A. Design and Analysis of a Low-Profile Tapered Slot UWB Vivaldi Antenna for Breast Cancer Diagnosis. Prog. Electromagn. Res. M 2024, 124, 43–51. [Google Scholar] [CrossRef]
- Van Veen, B.D.; Buckley, K.M. Beamforming: A versatile approach to spatial filtering. IEEE ASSP Mag. 1988, 5, 4–24. [Google Scholar] [CrossRef]
- Nilavalan, R.; Gbedemah, A.; Craddock, I.; Li, X.; Hagness, S. Numerical investigation of breast tumour detection using multi-static radar. Electron. Lett. 2003, 39, 1787–1789. [Google Scholar] [CrossRef]
- Institutos Nacionales de Salud de EE.UU. ImageJ—Image Processing and Analysis in Java; Institutos Nacionales de Salud de EE.UU: Bethesda, MD, USA, 2024. [Google Scholar]
- Lasemi, Z.; Atlasbaf, Z. Impact of Fidelity Factor on Breast Cancer Detection. IEEE Antennas Wirel. Propag. Lett. 2020, 19, 1649–1653. [Google Scholar] [CrossRef]
- Vanegas Cárdenas, D.N. Estudio de Una Antena UWB: Diseño, Caracterización y Simulación de Una Antena UWB Para Sistemas de Detección de Cáncer. Bachelor’s Thesis, La Universidad de Los Andes, Bogotá, Colombia, 2018. [Google Scholar]
- Li, R.; Guo, Y. A Conformal UWB Dual-Polarized Antenna for Wireless Capsule Endoscope Systems. IEEE Antennas Wirel. Propag. Lett. 2021, 20, 483–487. [Google Scholar] [CrossRef]
- Inum, R.; Rana, M.M.; Shushama, K.N.; Quader, M.A. EBGBased Microstrip Patch Antenna for Brain Tumor Detection via Scattering Parameters in Microwave Imaging System. Int. J. Biomed. Imaging 2018, 2018, 8241438. [Google Scholar] [CrossRef] [PubMed]
- Dishali, M.J.; Kumar, K.M.; Nawaz, S.M. Design of Microstrip Patch Antenna for Brain Cancer Detection. ICTACT J. Microelectron. 2019, 5, 731–737. [Google Scholar]
- Yousaf, M.; Ben Mabrouk, I.; Zada, M.; Akram, A.; Amin, Y.; Nedil, M.; Yoo, H. An Ultra-Miniaturized Antenna With Ultra-Wide Bandwidth Characteristics for Medical Implant Systems. IEEE Access 2021, 9, 40086–40097. [Google Scholar] [CrossRef]
- Li, X.; Yan, J.; Jalilvand, M.; Zwick, T. A Compact Double-Elliptical Slot-Antenna for Medical Applications. In Proceedings of the 6th European Conference on Antennas and Propagation (EUCAP), Prague, Czech Republic, 26–30 March 2012. [Google Scholar]
- Hossain, A.; Islam, M.T.; Beng, G.K.; Kashem, S.B.A.; Soliman, M.S.; Misran, N.; Chowdhury, M.E.H. Microwave brain imaging system to detect brain tumor using metamaterial loaded stacked antenna array. Nat. Sci. Rep. 2022, 12, 16478. [Google Scholar] [CrossRef] [PubMed]



| Tissues | Dielectric Permittivity (εr) | Tangent Loss (δ) | Thickness (mm) |
|---|---|---|---|
| Skin | 40.847 | 0.297 | 1 |
| Fat | 5.125 | 0.160 | 2 |
| Skull | 10.532 | 0.310 | 10 |
| Dura mater | 40.096 | 0.307 | 1.5 |
| CSF | 63.73 | 0.366 | 2 |
| Gray matter | 46.58 | 0.298 | 3.7 |
| White matter | 34.478 | 0.278 | 70.52 |
| Tumor [19] | 54.9 | 4.0 | 10 |
| Object | x (mm) | y (mm) | z (mm) |
|---|---|---|---|
| Strap 1 | 15 | 89.23 | 272.1 |
| Strap 2 | 233.8 | 89.23 | 15 |
| Strap base | 233.8 | 89.23 | 272.1 |
| Frequency | Absorbed Power Ratio () | |
|---|---|---|
| Seven Layers | One Gray Matter Layer | |
| 3.92 GHz | 65.39% | 64.19% |
| 5.79 GHz | 81.39% | 75.95% |
| 7.91 GHz | 88.75% | 92.80% |
| 9.67 GHz | 81.47% | 86.62% |
| 11.28 GHz | 93.64% | 99.36% |
| Specific Absorption Rate | |||||
|---|---|---|---|---|---|
| Frequency | 3.92 GHz | 5.79 GHz | 7.91 GHz | 9.67 GHz | 11.28 GHz |
| Phantom with seven layers of materials | 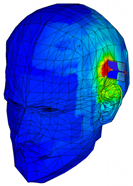 | 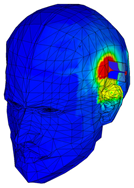 |  | 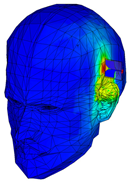 | 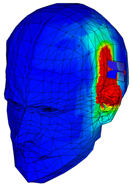 |
| SAR (W/Kg) | 1.05 | 1.68 | 1.29 | 1.37 | 1.79 |
| 0.92 | 1.61 | 1.43 | 1.55 | 1.96 | |
| Phantom of one layer of gray matter | 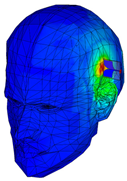 |  | 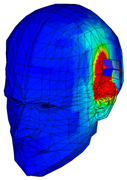 | 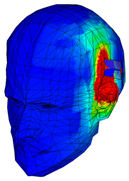 | 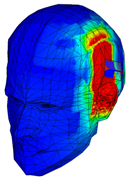 |
| Tissues | Original (εr) | Test 1 (εr) | Test 2 (εr) | Test 3 (εr) | Test 4 (εr) | Test 5 (εr) |
|---|---|---|---|---|---|---|
| Skin | 40.847 | 41.0085 | 40.4085 | 40.6585 | 40.4685 | 40.5085 |
| Fat | 5.125 | 5.1637 | 5.1437 | 5.1037 | 5.1737 | 5.0737 |
| Skull | 10.532 | 10.5167 | 10.5067 | 10.5867 | 10.5967 | 10.4667 |
| Dura mater | 40.096 | 40.3450 | 40.4250 | 39.7950 | 40.0050 | 40.2050 |
| CSF | 63.73 | 63.4427 | 63.9627 | 63.9227 | 63.2927 | 63.2427 |
| Gray matter | 46.58 | 46.5742 | 47.0142 | 46.4242 | 46.6642 | 46.3242 |
| White matter | 34.478 | 34.4532 | 34.4832 | 34.4732 | 34.4432 | 34.4632 |
| Tumor | 54.9 | 55.4010 | 54.9510 | 54.5010 | 54.5110 | 54.6310 |
| Two Antennas | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tumor (mm) | Tumor | Tumor | Clutter | Clutter | Contrast | Noise | SNR | CNR | Contrast Resolution |
| 10.0 | 127.5 | 1.732 | 57.75 | 1.258 | 69.75 | 2.445 | 73.6 | 28.5 | 38% |
| 2.5 | 101.5 | 1.732 | 59.5 | 1.291 | 42 | 2.459 | 58.6 | 17.1 | 26% |
| 0.625 | 127.5 | 1.291 | 148.5 | 1 | 21 | 2.141 | 98.8 | 9.81 | 8% |
| 0.3125 | 48 | 0.816 | 49.5 | 1.291 | 1.5 | 2.053 | 58.8 | 0.73 | 2% |
| Four Antennas | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tumor (mm) | Tumor | Tumor | Clutter | Clutter | Contrast | Noise | SNR | CNR | Contrast Resolution |
| 10.0 | 118.027 | 22.064 | 74.919 | 0.954 | 43.108 | 6.785 | 5.35 | 6.35 | 22% |
| 2.5 | 73.111 | 18.347 | 48.556 | 1.236 | 24.555 | 6.258 | 3.98 | 3.92 | 20% |
| 0.625 | 169.462 | 2.783 | 160.981 | 3.633 | 8.481 | 3.582 | 60.9 | 2.37 | 3% |
| 0.3125 | 43.417 | 1.975 | 42.833 | 0.389 | 0.584 | 2.174 | 22 | 0.27 | 1% |
| Design | N° Antennas | Freq. (GHz) | Size (mm) | Material | Application | Layers of Phantom | ⌀ Phantom (mm) | r Tumor (mm) | Imaging Method |
|---|---|---|---|---|---|---|---|---|---|
| Micro strip Antenna [7] | 12 | 3.0–12.0 | 16 × 20 × 1.6 | FR-4 | Breast | 1 | 100 | 3 | DAS Algorithm |
| Ultra-Miniaturized Antenna [28] | 1 | 2.45 | 7 × 7 × 0.2 | Rogers ULTRALAM | Implant | 1 | 100 | Not specified | Not specified |
| Dual-Polarized Antenna [25] | 2 | 2.15–14.75 | 21.7 × 14.8 × 0.8 | Roger 6010 | Endoscope | 1 | 60 | Not specified | Not specified |
| Flexible elliptical Antenna [8] | 12 | 6.5–35.0 | 10 × 10 × 0.7 | Textile | Breast | 2 | 50 | 2 | Time-reversal Algorithm |
| Bow-Tie Antenna [9] | 8 | 1.0–6.0 | 60 × 60 × 50 | Rogers RO4003C | Brain | 3 | 160 | 20 | MT Algorithm |
| Dual-Polarized Antenna [10] | 8 | 3.9–19.0 | 30 × 30 × 1.6 | Kapton polyimide | Breast | 3 | 100 | 5 | DAS Algorithm |
| Micro strip Antenna [11] | 2 | 2.79–18.0 | 22 × 26 × 2.6 | Roger 5880 | Breast and finger | 3 | 120 | 6.5 | SAR and PLD Method |
| Coplanar Antenna [12] | 2 | 3.0–11.0 | 33.14 × 14.9 × 0.84 | FR-4 | Breast | 3 | 120 | 2.5 | DAS Algorithm |
| Micro strip Antenna [13] | 2 | 3.0–15.0 | 27.0 × 29.0 × 1.6 | FR-4 | Breast | 3 | 120 | 5 | SAR Method |
| Micro strip Antenna [23] | 12 | 2.55–12.0 | 20.0 × 28.0 × 1.6 | FR-4 | Breast | 3 | 100 | 2 | DAS Algorithm |
| Micro strip Antenna [23] | 12 | 2.95–12.0 | 16.0 × 22.0 × 1.6 | FR-4 | Breast | 3 | 100 | 2 | DAS Algorithm |
| Double-elliptical slot antenna with feed network [29] | 1 | 1.2–9.0 | 40.0 × 40.0 × 1.2 | Rogers RT6010 | Human Body (Breast) | 3 | 52 | Not specified | Not specified |
| Coplanar Antenna [24] | 2 | 2.0–10.0 | 45.0 × 39.0 × 1.6 | FR-4 Epoxi | Breast | 3 | 163 | Not specified | Not specified |
| Metamaterial [30] | 9 | 1.37–3.16 | 50 × 40 × 8.66 | Rogers RT5880 and RO4350B | Brain | 3 | Not Specified | Not Specified | IC-CF-DMAS imaging algorithm |
| On-body flexible Antenna [14] | 1 | 2.0–11.0 | 20 × 30 | Rogers XT8100 | Hand and Abdomen | 4 | 78 | Not specified | Not specified |
| Flexible Microstrip Antenna [15] | 2 | 3.64–12.11 | 21 × 14 × 1.6 | Rogers RT-5880 | Breast | 4 | N/A | Not specified | Not specified |
| Coplanar Antenna [16] | 12 | 0.8–2.8 | 30 × 24 × 1.6 | FR-4 | Brain | 6 | 98 | 5 | E-SAR Method |
| Microstrip Antenna. Electromagnetic Band Gap (EBG) [26] | 1 | 6.3–7.4 | 14.5 × 8.9 × 0.7 | Rogers R03003 | Brain | 6 | 180 | 5 | Monostatic radar-based confocal |
| Microstrip Antenna [27] | 1 | 2.4–2.4835 | 60 × 60 × 1.56 | FR-4 | Brain | 6 | Not Specified | Not Specified | Return Loss and SAR |
| Micro strip Antenna [17] | 2 | 3.0–5.0 | 10 × 11 × 0.954 | TRF-43 | Brain Biotelemetry | 7 | 144.4 | Not specified | SAR Method |
| Antipoda Vivaldi Antenna [This Work] | 2 and 4 | 2.4–17.7 | 45 × 45 × 1.6 | FR-4 | Brain | 7 | 182.1 | 0.3125 | DAS Algorithm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Román, L.E.; Reyna, A.; Balderas, L.I.; Panduro, M.A. Detection of Brain Tumors Using UWB Antennas in a High-Fidelity Phantom Model. Appl. Sci. 2025, 15, 12275. https://doi.org/10.3390/app152212275
Román LE, Reyna A, Balderas LI, Panduro MA. Detection of Brain Tumors Using UWB Antennas in a High-Fidelity Phantom Model. Applied Sciences. 2025; 15(22):12275. https://doi.org/10.3390/app152212275
Chicago/Turabian StyleRomán, Luis E., Alberto Reyna, Luz I. Balderas, and Marco A. Panduro. 2025. "Detection of Brain Tumors Using UWB Antennas in a High-Fidelity Phantom Model" Applied Sciences 15, no. 22: 12275. https://doi.org/10.3390/app152212275
APA StyleRomán, L. E., Reyna, A., Balderas, L. I., & Panduro, M. A. (2025). Detection of Brain Tumors Using UWB Antennas in a High-Fidelity Phantom Model. Applied Sciences, 15(22), 12275. https://doi.org/10.3390/app152212275





