Mineral Chemistry Studies on Pyroxenes in Fe Skarns in the West of Elazığ (Turkey); Their Role in the Skarn Mineralization Process
Abstract
1. Introduction
2. Materials and Methods
3. Results
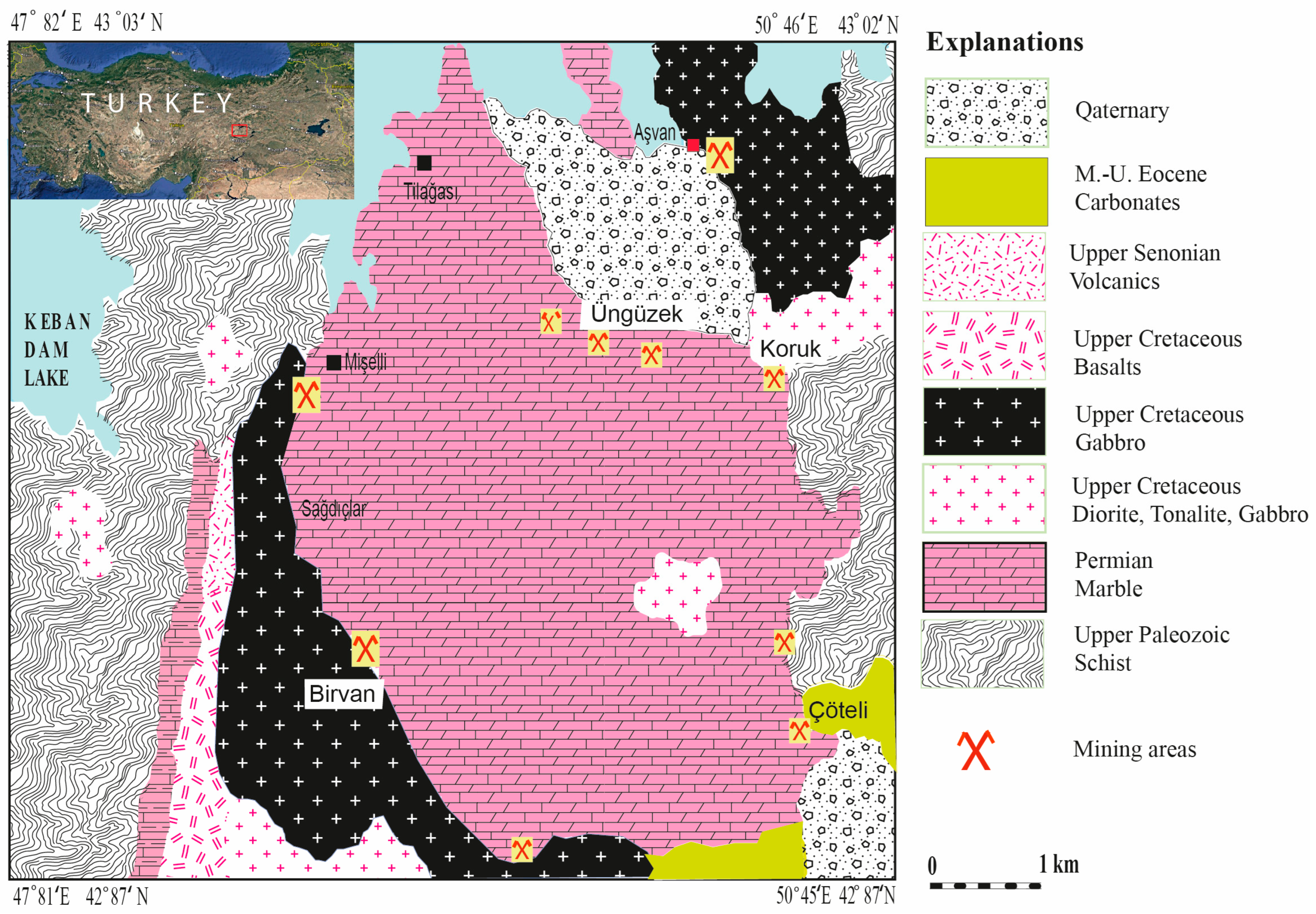
3.1. Geology
3.2. Mineralisation
3.3. Zoning
3.4. Geochemistry
4. Discussion
5. Conclusions
- This study presents the first detailed documentation of oscillatory and growth zoning in pyroxenes from polymetallic skarn zones in the Elazığ region, highlighting compositional variations from the core to the rim.
- Skarns develop along contacts between dioritic and granitic plutonic rocks and carbonate rocks, forming two main zones: a narrow endoskarn and a more extensive exoskarn. Endoskarns exhibit distinct mineralogical zoning, including pyroxene–garnet, pyroxene–scapolite, and epidote–garnet assemblages, whereas scapolite occurs in both endoskarn and exoskarn zones.
- Pyroxenes are predominantly composed of diopside, with cores enriched in SiO2 and MgO and rims enriched in FeO and CaO, reflecting magmatic differentiation and metasomatic processes. Minor serpentinized olivine within endoskarns is attributed to localized magnesium enrichment.
- Cu–Fe skarns show low Mn/Fe ratios (<0.1) and low Zn content (~200 ppm), Fe skarns exhibit high Mn/Fe ratios (>0.2) and elevated Zn (>200 ppm), and W-bearing skarns display intermediate Mn/Fe (~0.15) and high Zn (>500 ppm). These correlations demonstrate that Mn/Fe ratios and Zn content in pyroxenes are effective indicators of skarn-forming environment.
- Oscillatory and compositional zoning in pyroxenes provides key information on physicochemical conditions, fluid–rock interactions, and hydrothermal fluid evolution during skarn formation, offering a better understanding of magmatic and metasomatic processes controlling polymetallic skarn mineralization.
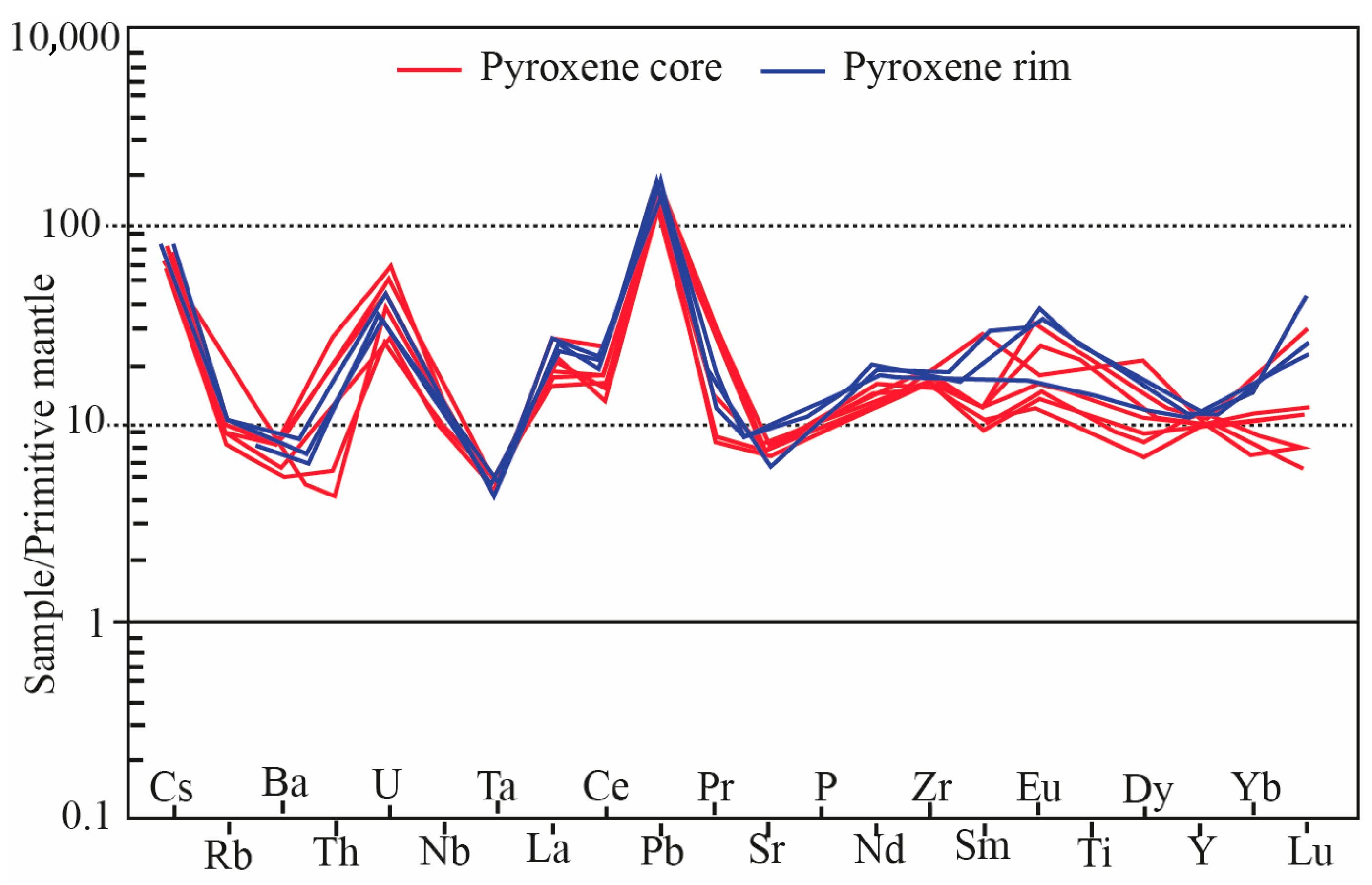
- The low uranium content in pyroxenes (0.01–0.24 ppm) and andradite–grossular composition of garnets indicate that skarn-forming fluids were relatively oxidized. This is consistent with the incorporation of Fe2+ into andradite and confirms that pyroxenes did not crystallize under reduced conditions.
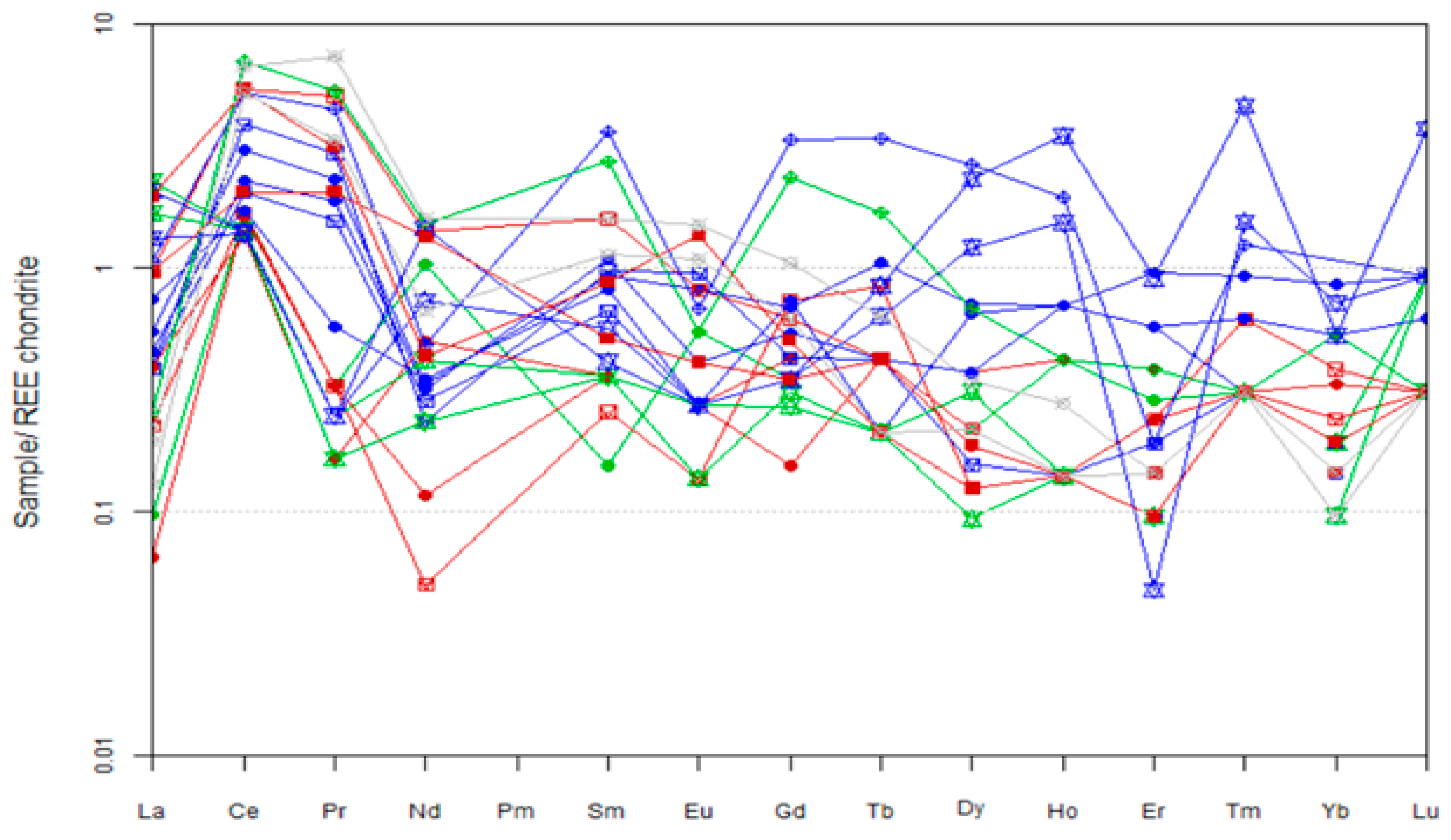
- Variations in ΣREE, Ce, Nd, and Eu anomalies reflect changes in fluid pH and temperature. The lower REE contents in pyroxene cores compared to rims suggest crystallization in a closed system for cores and an open system for rims, highlighting the influence of water/rock interaction on element mobility during skarn formation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meinert, L.D. Application of skarn deposit zonation models to mineral exploration. Explor. Min. Geol. 1997, 6, 185–208. [Google Scholar]
- Meinert, L.D.; Diple, G.M.; Nicolescu, S. World skarn deposits. In Economic Geology 100th Anniversary; Society of Economic Geologist, Inc.: Littleton, CO, USA, 2005; pp. 299–336. [Google Scholar]
- Sasmaz, A.; Sukach, V.; Bondarenko, S.; Aleksiienko, H.; Kaseb, H.E.; Sasmaz, B.; Kurylo, S.; Hrinchenko, O.; Somka, V.; Voudouris, P. Newly Identified Au-Ag-Bi-Te Mineralization in the Aydindere Skarn Fe and Cu Deposit, Giresun, NE Turkey: Implications of Gold Mineralization during Retrograde Skarn Evolution. J. Earth Sci. 2025, 36, 543–561. [Google Scholar] [CrossRef]
- Tagirov, B.R.; Baranova, N.N.; Zotov, A.V.; Akinfiev, N.N. Experimental study of garnet–fluid interaction in the system CaO–FeO–Al2O3–SiO3–H3O–Cl at 500 °C and 2 kbar: Implications for skarn formation. Geochim. Cosmochim. Acta 2002, 66, 1013–1030. [Google Scholar]
- Sağıroğlu, A. Pertek—Demürek (Tunceli) skarn tipi manyetit ve ilişkili bakır cevherleşmeleri. TJK Bült. 1992, 35, 63–70. [Google Scholar]
- Akgül, B.; Sasmaz, A. The pyrometasomatic formations and associated Fe-Ti mineralizations at the north of Elazığ. Geol. Bull. Turk. 1996, 39, 39–48. [Google Scholar]
- Ural, M.; Kurum, S. Microscopic and diffractometric studies inferred from skarn zonations between the Keban Metamorphites and Elazığ Magmatites, around Elazığ. Turk. J. Sci. Tech. 2009, 4, 87–102. [Google Scholar]
- Sasmaz, A.; Kılıç, A.D.; Akgul, B.; Sasmaz, B. A spectral approach on mineralogy and geochemistry of garnet skarns in Arc-Type granitoids. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 286, 122037. [Google Scholar] [CrossRef]
- Şasmaz, A.; Kılıç, A.D.; Konakçı, N. Chemical and Thermal Changes in Mg3Si2O5(OH)4 Polymorph Minerals and Importance as an Industrial Material. Appl. Sci. 2024, 14, 10298. [Google Scholar] [CrossRef]
- Yazgan, E. Geodynamics Evolution of the Eastern Taurus Region. In Geology of the Taurus Belt; International Symposıum Proceedings, 199–208; Tekeli, O., Göncüoğlu, M.C., Eds.; Mineral Research and Exploration Institute of Turkey (MTA): Ankara, Turkey, 1984. [Google Scholar]
- Yiğitbaş, E.; Yılmaz, Y. New evidence and solution to the Maden complex controversy of the the Southeast Anatolian orogenic belt (Turkey). Int. Geol. Rev. 1996, 38, 818–831. [Google Scholar] [CrossRef]
- Altunbey, M. Tuzbaşı-Kanatburun-Ayazpınar (Pertek-Tunceli) yöresindeki demir cevherleşmelerinin jeolojisi ve kökeni. Ph.D. Thesis, Fırat Üniversitesi, Elazığ, Turkey, 1996; 186p. [Google Scholar]
- Altunbey, M.; Sagiroglu, A. Skarn type ilmenite mineralization of the Tuzbasi Tunceli region. J. Asian Earth Sci. 2003, 21, 481–488. [Google Scholar] [CrossRef]
- Park, C.; Choi, W.; Kim, H.; Park, M.H.; Kang, I.M.; Lee, H.S.; Song, Y. Oscillatory zoning in skarn garnet: Implications for tungsten ore exploration. Ore Geol. Rev. 2017, 89, 1006–1018. [Google Scholar] [CrossRef]
- Ganguly, J.; Chakraborty, S. Compositional zoning and cation diffusion in garnets. In Diffusion, Atomic Ordering and Mass Transport. Advances in Physical Geochemistry; Ganguly, J., Ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1991; Volume 8, pp. 120–175. [Google Scholar]
- Pacey, A.; Wilkinson, J.J.; Cooke, D.R. Chlorite and epidote mineral chemistry in porphyry ore systems: A case study of the North Parkes district, New South Wales, Australia. Econ. Geol. 2020, 115, 701–727. [Google Scholar] [CrossRef]
- MTA. 1:500.000 ölçekli Türkiye Jeoloji Haritası Elazığ Paftası; MTA: Ankara, Turkey, 2012.
- Özgül, N. Geological characteristic of Taurids. TJK-Bull. 1976, 19, 65–78. [Google Scholar]
- Yazgan, E.; Chessex, R. Geology and tectonic evolution of the southeastern Taurides in the region of Malatya. Turk. Pet. Geol. 1991, 3, 1–42. [Google Scholar]
- Kaya, A.; Bozkaya, Ö. Triassic gypsum layers in Keban Metamorphics, eastern Tauride Belt, Southeastern Turkey: New data about Triassic rifting in northern Tethys margin. Int. Geol. Rev. 2023, 66, 1046–1066. [Google Scholar] [CrossRef]
- Asutay, H.J. The geology of Baskil (Elazığ) vicinity and petrology of Baskil magmatics. Bull. Miner. Res. Explor. 1988, 107, 46–72. [Google Scholar]
- Akgül, B. Petrographical and petrological features of magnetic rocks in the vicinity of Piran Village (Keban). F.U. Inst. Sci. 1993, 109, 11–18. [Google Scholar]
- Akgül, M. Baskil granitoyitinin petrografik ve petrolojik özellikleri. Yerbilim. Geosound 1991, 18, 67–78. [Google Scholar]
- Bingöl, A.F. Elazığ-Pertek-Kovancılar (Doğu Toroslar) yöresinin jeolojisi. In Toros Jeolojisi Uluslararası Sempozyumu, Tebliğler; Munzur Üniversitesi: Ankara, Turkey, 1984. [Google Scholar]
- Zheng, P.; Chen, K.; Zhang, J.-K.; Liu, Z.-F.; Li, Y.-S.; He, M.-P. The Fluid Evolution in the Skarn Stages of the Baoshan Skarn Cu-Polymetallic Deposit, South China. Minerals 2024, 14, 907. [Google Scholar] [CrossRef]
- Kamali, A.A.; Moayyed, M.; Saumur, B.M.; Fadaeian, M. Mineralogy and Mineral Chemistry of Dioritic Dykes, Quartz Diorite Enclaves and Pyroxene of the Sungun Cu-Mo Porphyry Deposit, East Azerbaijan, Iran. Minerals 2022, 12, 1218. [Google Scholar] [CrossRef]
- Agard, P.; Omrani, J.; Jolivet, L.; Whitechurch, H.; Vrielynck, B.; Spakman, W.; Monié, P.; Meyer, B.; Wortel, R. Zagros orogeny: A subduction-dominated process. Geol. Mag. 2011, 148, 692–725. [Google Scholar] [CrossRef]
- Arnason, J.; Bird, D.; Liou, J. Variables controlling epidote composition in hydrothermal and low-pressure regional metamorphic rocks. Abh. Geol. B. A. 1993, 49, 17–25. [Google Scholar]
- Bau, M. Rare-earth element mobility during hydrothermal and metamorphic fluid–rock interaction and the significance of the oxidation state of europium. Chem. Geol. 1991, 93, 219–230. [Google Scholar] [CrossRef]
- Plumlee, G.; Leach, D.; Hofstra, A.; Landis, G.; Rowan, E.; Viets, J. Chemical reaction path modeling of ore deposition in Mississippi Valley-type Pb-Zn deposits of the Ozark region, US Midcontinent. Econ. Geol. 1994, 89, 1361–1383. [Google Scholar] [CrossRef]
- Thompson, R.N. Magmatism of the British Tertiary Province. Scott. J. Geol. 1982, 18, 49–107. [Google Scholar] [CrossRef]
- Smith, M.P.; Henderson, P.; Jeffries, T.E.R.; Long, J.; Williams, C.T. The rare earth elements and uranium in garnets from the Beinn an Dubhaich aureole, Skye, Scotland, UK: Constraints on processes in a dynamic hydrothermal system. J. Petrol. 2004, 45, 457–484. [Google Scholar] [CrossRef]
- Peng, H.J.; Zhang, C.Q.; Mao, J.W.; Santosh, M.; Zhou, Y.M.; Hou, L. Garnets in porphyry–skarn systems: A LA–ICP–MS, fluid inclusion, and stable isotope study of garnets from the Hongniu-Hongshan copper deposit, Zhongdian area, NW Yunnan Province, China. J. Asian Earth Sci. 2015, 103, 229–251. [Google Scholar] [CrossRef]
- Gu, H.; Yang, X.; Nie, Z.; Deng, J.; Duan, L.; Hu, Q.; Shakoor, M.A.; Gao, E.; Hafiz, A.A.J. Study of late-Mesozoic magmatic rocks and their related copper-gold-polymetallic deposits in the Guichi ore-cluster district, Lower Yangtze River Metallogenic Belt, East China. Int. Geol. Rev. 2018, 60, 1404–1434. [Google Scholar] [CrossRef]
- Morimoto, N.; Fabries, J.; Ferguson, A.K.; Ginzburg, I.V.; Ross, M.; Seifert, F.A.; Zussman, J.; Aoki, K.; Gottardi, G. Nomenclature of pyroxenes. Am. Miner. 1988, 73, 1123–1133. [Google Scholar]
- Chiaradia, M.; Schaltegger, U.; Spikings, R.; Wotzlaw, J.F.; Ovtcharova, M. How accurately can we date the duration of magmatic-hydrothermal events in porphyry systems? Econ. Geol. 2013, 108, 565–584. [Google Scholar] [CrossRef]
- Clark, K.F.; Foster, C.T.; Damon, P.E. Cenozoic mineral deposits and subduction related magmatic arcs in Mexico. Geol. Soc. Am. Bull. 1982, 93, 533–544. [Google Scholar] [CrossRef]
- Öztunalı, Ö. Keban Maden Sahaları Durum Tespit Raporları 1985–1989; Etibank Maden Arama Müdürlüğü: Ankara, Turkey, 1989.
- Boynton, W.V. Cosmochemistry of the rare earth elements: Meteorite studies. In Rare Earth Element Geochemistry; Henderson, P., Ed.; Elsevier: Amsterdam, The Netherlands, 1984; pp. 63–114. [Google Scholar]
- Ray, G.E. A Review of Skarns in the Canadian Cordillera; British Columbia Ministry of Energy and Mines: Victoria, BC, Canada, 2013.
- Fei, X.; Zhang, Z.; Cheng, Z.; Santosh, M. Factors controlling the crystal morphology and chemistry of garnet in skarn deposits: A case study from the Cuihongshan polymetallic deposit, Lesser Xing’an Range, NE China. Am. Miner. 2019, 104, 1455–1468. [Google Scholar] [CrossRef]
- Garofalo, P.S.; Redi, D.; Malafeevskiy, N.; Schwarz, G.; Neff, C.; Keresztes Schmidt, P.; Günther, D. Failed genesis of a Fe-skarn deposit caused by redox states of intrusion and wall rocks (Torre di Rio, Island of Elba, Italy). Ore Geol. Rev. 2025, 177, 106446. [Google Scholar] [CrossRef]
- Nakano, T.; Yoshino, T.; Shimazaki, H.; Shimizu, M. Pyroxene composition as an indicator in the classification of skarn deposits. Econ. Geol. 1994, 89, 1567–1580. [Google Scholar] [CrossRef]
- Jamtveit, B. Oscillatory zonation patterns in hydrothermal grossular–andradite garnet: Nonlinear dynamics in regions of immiscibility. Am. Miner. 1991, 76, 1319–1327. [Google Scholar]
- Kılıç, A.D.; Konakci, N.; Sasmaz, A. Garnet Geochemistry of Pertek Skarns (Tunceli, Turkey) and U-Pb Age Findings. Minerals 2024, 14, 539. [Google Scholar] [CrossRef]
- Jiang, X.J.; Chen, X.; Zheng, Y.Y.; Gao, S.B.; Zhang, Z.L.; Zhang, Y.C.; Zhang, S.Z. Decoding the oxygen fugacity of ore-forming fluids from garnet chemistry, the Longgen skarn Pb-Zn deposit, Tibet. Ore Geol. Rev. 2020, 126, 103770. [Google Scholar] [CrossRef]
- Sun, S.S.; McDonough, W.F. Chemical and isotopic systematics of oceanic basalts: Implications for mantle composition and processes. Geol. Soc. Spec. Publ. 1989, 42, 313–345. [Google Scholar] [CrossRef]
- Bouabdellah, M.; El Ghazali, M.; Hidouci, K.; Kheyar, N.; Essaraj, S.; Sadki, O.; Poujol, M. Evolution of ore-forming fluids in the Azegour Mo-Cu-W skarn deposit, Anti-Atlas, Morocco: Constraints from mineral chemistry and fluid inclusions. Minerals 2023, 13, 1537. [Google Scholar]




| Sample No. | Px-1 | Px-2 | Px-3 | Px-4 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Position | rim | rim | core | core | rim | rim | core | core | rim | rim | rim | rim | core | core | rim | rim | core | core | rim | rim |
| SiO2 | 46.6 | 53.1 | 48.3 | 54.1 | 51.8 | 52.9 | 50.7 | 54.2 | 52.3 | 50.7 | 54.1 | 53.7 | 55.6 | 57.1 | 55.0 | 52.1 | 52.8 | 51.9 | 50.0 | 51.0 |
| Al2O3 | 8.09 | 3.15 | 6.66 | 0.88 | 4.40 | 2.74 | 3.73 | 2.24 | 5.13 | 5.17 | 1.65 | 2.42 | 0.61 | 0.85 | 1.54 | 3.22 | 2.95 | 2.22 | 4.61 | 5.33 |
| TiO2 | 0.81 | 0.40 | 0.39 | 0.14 | 0.38 | 0.40 | 0.25 | 0.08 | 0.27 | 0.45 | 0.07 | 0.07 | 0.00 | 0.00 | 0.06 | 0.07 | 0.14 | 0.05 | 0.27 | 0.34 |
| FeO | 5.69 | 2.45 | 4.57 | 1.11 | 3.67 | 2.12 | 3.55 | 2.67 | 4.04 | 3.19 | 2.09 | 2.47 | 0.67 | 0.68 | 2.16 | 2.84 | 3.36 | 2.14 | 4.35 | 4.59 |
| MgO | 13.3 | 16.6 | 13.21 | 18.07 | 15.6 | 16.6 | 15.9 | 16.2 | 14.6 | 14.3 | 17.3 | 16.6 | 17.7 | 17.1 | 16.2 | 16.1 | 16.1 | 16.7 | 15.3 | 14.4 |
| MnO | 0.06 | 0.07 | 0.05 | 0.08 | 0.11 | 0.10 | 0.06 | 0.09 | 0.09 | 0.10 | 0.05 | 0.05 | 0.04 | 0.01 | 0.07 | 0.08 | 0.10 | 0.06 | 0.07 | 0.07 |
| CaO | 24.6 | 25.0 | 24.7 | 25.3 | 24.8 | 25.2 | 25.0 | 24.9 | 24.7 | 24.8 | 24.8 | 25.3 | 24.7 | 24.4 | 24.9 | 25.1 | 24.9 | 25.4 | 25.3 | 25.2 |
| Na2O | 0.00 | 0.00 | 0.01 | 0.00 | 0.01 | 0.01 | 0.00 | 0.01 | 0.01 | 0.02 | 0.03 | 0.01 | 0.11 | 0.08 | 0.02 | 0.01 | 0.07 | 0.08 | 0.00 | 0.01 |
| Cr2O3 | 0.04 | 0.01 | 0.02 | 0.01 | 0.00 | 0.00 | 0.01 | 0.00 | 0.11 | 1.01 | 0.00 | 0.01 | 0.41 | 0.45 | 0.00 | 0.02 | 0.00 | 0.01 | 0.00 | 0.22 |
| Total | 102 | 101 | 97.4 | 99.7 | 100 | 100 | 99.3 | 100 | 101 | 99.7 | 99.2 | 100 | 99.8 | 100 | 99.9 | 99.5 | 100 | 98.5 | 99.9 | 101 |
| Number of cations | ||||||||||||||||||||
| Si | 1.74 | 1.88 | 1.85 | 1.97 | 1.82 | 1.90 | 1.90 | 1.92 | 1.80 | 1.91 | 1.95 | 1.92 | 2.01 | 2.01 | 1.93 | 1.90 | 1.90 | 1.95 | 1.85 | 1.88 |
| Al | 0.29 | 0.14 | 0.24 | 0.03 | 0.17 | 0.13 | 0.18 | 0.11 | 0.21 | 0.21 | 0.97 | 0.12 | 0.03 | 0.02 | 0.05 | 0.13 | 0.11 | 0.07 | 0.21 | 0.22 |
| Ti | 0.02 | 0.01 | 0.01 | 0.00 | 0.01 | 0.01 | 0.01 | 0.00 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 |
| Fe2+ | 0.18 | 0.08 | 0.14 | 0.04 | 0.11 | 0.07 | 0.11 | 0.08 | 0.13 | 0.09 | 0.05 | 0.06 | 0.01 | 0.01 | 0.08 | 0.09 | 0.09 | 0.10 | 0.11 | 0.16 |
| Fe3+ | 2.86 | 2.96 | 2.80 | 2.84 | 2.90 | 2.82 | 2.83 | 2.85 | 2.86 | 2.88 | 2.88 | 2.89 | 2.90 | 2.87 | 2.91 | 2.83 | 2.81 | 2.82 | 2.84 | 2.77 |
| Mn | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Cr | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 | 0.00 | 0.00 | 0.01 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 |
| K | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Mg | 0.71 | 0.90 | 0.79 | 0.96 | 0.85 | 0.90 | 0.88 | 0.87 | 0.81 | 0.78 | 0.93 | 0.89 | 0.96 | 0.91 | 0.88 | 0.87 | 0.84 | 0.91 | 0.84 | 0.75 |
| Ca | 0.95 | 0.94 | 0.95 | 0.97 | 0.97 | 0.96 | 0.97 | 0.95 | 0.95 | 0.96 | 0.94 | 0.96 | 0.96 | 0.95 | 0.96 | 0.96 | 0.91 | 1.00 | 1.01 | 0.98 |
| Fe3+/Fe2+ | 16 | 37 | 20 | 71 | 26 | 40 | 26 | 36 | 22 | 32 | 58 | 48 | 290 | 287 | 36 | 31 | 31 | 28 | 26 | 17 |
| Trace elements (ppm) | ||||||||||||||||||||
| Ba | 0.19 | 0.13 | 0.22 | 1.01 | 0.20 | 0.51 | 0.15 | 1.02 | 0.15 | 0.11 | 0.11 | 0.23 | 0.21 | 0.88 | 0.49 | 0.35 | 0.34 | 0.90 | 0.17 | 0.31 |
| Rb | 0.07 | 0.08 | 0.05 | 0.09 | 0.10 | 0.08 | 0.09 | 0.14 | 0.16 | 0.13 | 0.11 | 0.07 | 0.06 | 0.13 | 0.11 | 0.04 | 0.03 | 0.08 | 0.14 | 0.06 |
| Cs | 0.01 | 0.02 | 0.02 | 0.03 | 0.04 | 0.01 | 0.01 | 0.04 | 0.02 | 0.35 | 0.03 | 0.01 | 0.02 | 0.06 | 0.02 | 0.03 | 0.01 | 0.03 | 0.02 | 0.02 |
| V | 7.52 | 10.7 | 7.59 | 5.00 | 7.30 | 5.44 | 9.97 | 6.10 | 6.52 | 6.88 | 5.77 | 4.79 | 6.54 | 8.02 | 11.1 | 6.60 | 10.1 | 10.5 | 5.78 | 9.92 |
| Co | 0.84 | 0.85 | 0.92 | 0.90 | 0.90 | 0.84 | 1.51 | 1.59 | 0.89 | 1.22 | 0.84 | 0.90 | 1.56 | 1.45 | 0.88 | 0.69 | 1.31 | 1.37 | 1.08 | 0.94 |
| Sr | 19.0 | 20.6 | 15.2 | 20.1 | 15.5 | 15.9 | 18.3 | 19.7 | 22.3 | 15.3 | 15.1 | 15.1 | 16.1 | 15.1 | 16.1 | 17.1 | 20.5 | 22.6 | 20.3 | 18.2 |
| Th | 0.04 | 0.02 | 0.04 | 0.03 | 0.02 | 0.06 | 0.07 | 0.31 | 0.02 | 0.14 | 0.02 | 0.01 | 0.01 | 0.01 | 0.07 | 0.01 | 0.01 | 0.01 | 0.03 | 0.03 |
| U | 0.01 | 0.01 | 0.01 | 0.00 | 0.02 | 0.01 | 0.16 | 0.08 | 0.01 | 0.04 | 0.01 | 0.02 | 0.06 | 0.09 | 0.02 | 0.02 | 0.06 | 0.01 | 0.02 | 0.09 |
| Y | 0.64 | 0.90 | 0.17 | 0.18 | 0.91 | 1.58 | 0.17 | 0.17 | 0.29 | 1.28 | 0.13 | 0.29 | 0.78 | 0.45 | 1.20 | 0.27 | 0.30 | 0.14 | 0.11 | 0.37 |
| Cu | 3.11 | 2.57 | 2.60 | 2.51 | 2.60 | 3.01 | 2.33 | 2.77 | 2.94 | 2.56 | 3.02 | 3.01 | 2.39 | 2.50 | 2.82 | 2.91 | 3.08 | 3.15 | 3.17 | 2.72 |
| Zn | 216 | 233 | 227 | 229 | 239 | 233 | 221 | 215 | 219 | 221 | 222 | 241 | 215 | 225 | 211 | 218 | 220 | 220 | 233 | 225 |
| Pb | 0.69 | 0.78 | 0.33 | 0.39 | 0.40 | 1.12 | 0.54 | 1.01 | 0.29 | 0.30 | 0.36 | 0.31 | 0.60 | 0.78 | 0.35 | 0.23 | 0.48 | 1.25 | 0.29 | 1.02 |
| Mo | 6.70 | 6.51 | 5.97 | 6.59 | 6.11 | 6.19 | 5.49 | 5.41 | 6.88 | 6.60 | 6.50 | 6.91 | 5.90 | 6.00 | 7.01 | 6.50 | 7.23 | 5.85 | 6.58 | 5.70 |
| Ga | 1.22 | 1.20 | 1.39 | 1.60 | 1.45 | 1.32 | 1.40 | 1.25 | 0.60 | 0.70 | 0.66 | 0.59 | 1.30 | 1.82 | 0.75 | 0.70 | 1.74 | 1.78 | 1.50 | 1.63 |
| Ag | 0.02 | 0.03 | 0.03 | 0.04 | 0.03 | 0.04 | 0.05 | 0.06 | 0.07 | 0.07 | 0.07 | 0.06 | 0.04 | 0.05 | 0.04 | 0.06 | 0.05 | 0.05 | 0.06 | 0.04 |
| LILE | 20.0 | 21.6 | 15.9 | 21.7 | 16.3 | 17.7 | 19.3 | 22.0 | 22.9 | 16.4 | 15.74 | 15.73 | 17.1 | 17.0 | 17.2 | 17.8 | 21.4 | 24.9 | 21.0 | 19.6 |
| Sr/Y | 29.68 | 22.88 | 89.41 | 111.6 | 17.03 | 10.06 | 107.6 | 116 | 79.89 | 11.95 | 116 | 52.06 | 20.64 | 33.55 | 13.41 | 63.3 | 68.33 | 161 | 185 | 49.18 |
| Rare earth elements (ppm) | ||||||||||||||||||||
| La | 0.03 | 0.02 | 0.06 | 0.11 | 0.14 | 0.17 | 0.12 | 0.30 | 0.12 | 0.22 | 0.52 | 0.68 | 0.78 | 0.35 | 0.39 | 0.61 | 0.54 | 0.65 | 0.05 | 0.06 |
| Ce | 1.34 | 1.29 | 1.34 | 3.10 | 1.80 | 2.52 | 1.65 | 4.39 | 1.10 | 1.34 | 1.14 | 1.12 | 5.69 | 4.20 | 1.10 | 1.11 | 1.63 | 4.39 | 4.45 | 5.88 |
| Pr | 0.03 | 0.03 | 0.03 | 0.34 | 0.22 | 0.21 | 0.18 | 0.67 | 0.02 | 0.07 | 0.02 | 0.01 | 0.66 | 0.57 | 0.03 | 0.02 | 0.17 | 0.40 | 0.41 | 0.89 |
| Sm | 0.02 | 0.07 | 0.05 | 0.19 | 0.20 | 0.18 | 0.13 | 0.34 | 0.06 | 0.14 | 0.08 | 0.08 | 0.67 | 0.75 | 0.07 | 0.10 | 0.10 | 0.14 | 0.25 | 0.51 |
| Nd | 0.59 | 0.07 | 0.02 | 0.11 | 0.19 | 0.20 | 0.17 | 0.95 | 0.30 | 0.21 | 0.30 | 0.17 | 0.89 | 0.30 | 0.90 | 0.41 | 0.77 | 0.24 | 0.36 | 0.85 |
| Eu | 0.05 | 0.02 | 0.02 | 0.07 | 0.03 | 0.06 | 0.02 | 0.05 | 0.02 | 0.03 | 0.02 | 0.01 | 0.04 | 0.05 | 0.03 | 0.02 | 0.03 | 0.09 | 0.07 | 0.10 |
| Dy | 0.06 | 0.11 | 0.06 | 0.04 | 0.11 | 0.20 | 0.07 | 0.06 | 0.05 | 0.22 | 0.03 | 0.10 | 0.20 | 0.31 | 0.70 | 0.38 | 0.06 | 0.04 | 0.07 | 0.09 |
| Tb | 0.02 | 0.02 | 0.03 | 0.02 | 0.02 | 0.05 | 0.02 | 0.02 | 0.02 | 0.03 | 0.01 | 0.01 | 0.07 | 0.12 | 0.03 | 0.03 | 0.02 | 0.02 | 0.01 | 0.03 |
| Gd | 0.10 | 0.10 | 0.16 | 0.10 | 0.12 | 0.19 | 0.09 | 0.17 | 0.04 | 0.19 | 0.06 | 0.07 | 0.59 | 0.60 | 0.09 | 0.11 | 0.09 | 0.14 | 0.20 | 0.31 |
| Ho | 0.03 | 0.03 | 0.01 | 0.01 | 0.05 | 0.05 | 0.01 | 0.01 | 0.01 | 0.05 | 0.01 | 0.01 | 0.03 | 0.14 | 0.25 | 0.11 | 0.01 | 0.01 | 0.01 | 0.01 |
| Tm | 0.01 | 0.01 | 0.02 | 0.01 | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.04 | 0.12 | 0.05 | 0.01 | 0.01 | 0.01 | 0.01 |
| Er | 0.05 | 0.06 | 0.05 | 0.02 | 0.10 | 0.20 | 0.03 | 0.02 | 0.05 | 0.11 | 0.02 | 0.02 | 0.07 | 0.02 | 0.19 | 0.01 | 0.02 | 0.03 | 0.02 | 0.04 |
| Yb | 0.03 | 0.07 | 0.07 | 0.03 | 0.13 | 0.16 | 0.04 | 0.05 | 0.04 | 0.12 | 0.02 | 0.04 | 0.10 | 0.14 | 0.11 | 0.15 | 0.04 | 0.03 | 0.02 | 0.03 |
| Lu | 0.03 | 0.01 | 0.01 | 0.01 | 0.02 | 0.03 | 0.01 | 0.01 | 0.01 | 0.02 | 0.03 | 0.01 | 0.01 | 0.03 | 0.14 | 0.03 | 0.01 | 0.01 | 0.01 | 0.02 |
| ΣREE | 2.39 | 1.91 | 1.93 | 4.16 | 3.15 | 4.24 | 2.55 | 7.05 | 1.85 | 2.76 | 2.27 | 2.34 | 9.81 | 7.26 | 4.42 | 3.14 | 3.50 | 6.16 | 5.94 | 8.83 |
| ΣLREE | 2.06 | 1.51 | 1.52 | 3.92 | 2.58 | 3.34 | 2.27 | 6.70 | 1.62 | 2.01 | 2.08 | 2.07 | 8.73 | 6.22 | 2.52 | 2.27 | 3.24 | 5.91 | 5.59 | 8.29 |
| ΣHREE | 0.33 | 0.40 | 0.41 | 0.24 | 0.57 | 0.90 | 0.28 | 0.35 | 0.23 | 0.75 | 0.19 | 0.27 | 1.08 | 1.04 | 1.90 | 0.87 | 0.26 | 0.25 | 0.35 | 0.54 |
| LREE/HREE | 6.24 | 3.77 | 3.70 | 16.3 | 4.52 | 3.71 | 8.10 | 19.14 | 7.04 | 2.68 | 10.9 | 7.66 | 8.08 | 5.98 | 1.32 | 2.60 | 12.46 | 23.6 | 15.9 | 15.3 |
| LaN/YbN | 0.66 | 0.18 | 0.55 | 2.62 | 0.70 | 0.62 | 2.13 | 4.40 | 1.87 | 1.40 | 16.1 | 11.5 | 0.51 | 1.51 | 2.54 | 2.86 | 7.87 | 12.09 | 1.14 | 1.56 |
| Eu/Eu* | 1.94 | 0.67 | 0.37 | 1.36 | 0.46 | 0.91 | 0.59 | 0.75 | 1.09 | 0.42 | 0.61 | 0.56 | 0.19 | 0.18 | 0.75 | 0.47 | 0.99 | 1.87 | 1.04 | 0.82 |
| Ce/Ce* | 8.51 | 11.24 | 5.67 | 3.70 | 2.39 | 2.37 | 2.60 | 2.37 | 5.22 | 2.71 | 2.03 | 2.49 | 5.93 | 2.36 | 2.61 | 2.00 | 1.46 | 2.16 | 7.89 | 5.53 |
| Diopside | 99.7 | 99.6 | 99.5 | 100.0 | 99.7 | 100.0 | 100.0 | 99.9 | 99.8 | 99.8 | 99.7 | 99.7 | 99.3 | 99.5 | 99.8 | 99.8 | 99.5 | 99.4 | 99.9 | 99.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koprubasi, N.; Kiliç, A.D.; Sasmaz, A. Mineral Chemistry Studies on Pyroxenes in Fe Skarns in the West of Elazığ (Turkey); Their Role in the Skarn Mineralization Process. Appl. Sci. 2025, 15, 12277. https://doi.org/10.3390/app152212277
Koprubasi N, Kiliç AD, Sasmaz A. Mineral Chemistry Studies on Pyroxenes in Fe Skarns in the West of Elazığ (Turkey); Their Role in the Skarn Mineralization Process. Applied Sciences. 2025; 15(22):12277. https://doi.org/10.3390/app152212277
Chicago/Turabian StyleKoprubasi, Necla, Ayşe Didem Kiliç, and Ahmet Sasmaz. 2025. "Mineral Chemistry Studies on Pyroxenes in Fe Skarns in the West of Elazığ (Turkey); Their Role in the Skarn Mineralization Process" Applied Sciences 15, no. 22: 12277. https://doi.org/10.3390/app152212277
APA StyleKoprubasi, N., Kiliç, A. D., & Sasmaz, A. (2025). Mineral Chemistry Studies on Pyroxenes in Fe Skarns in the West of Elazığ (Turkey); Their Role in the Skarn Mineralization Process. Applied Sciences, 15(22), 12277. https://doi.org/10.3390/app152212277







