Breath Analysis by Mass Spectrometry-Based Technologies for Biomonitoring Environmental Exposures
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Study Selection
2.2. Data Collection
3. Results
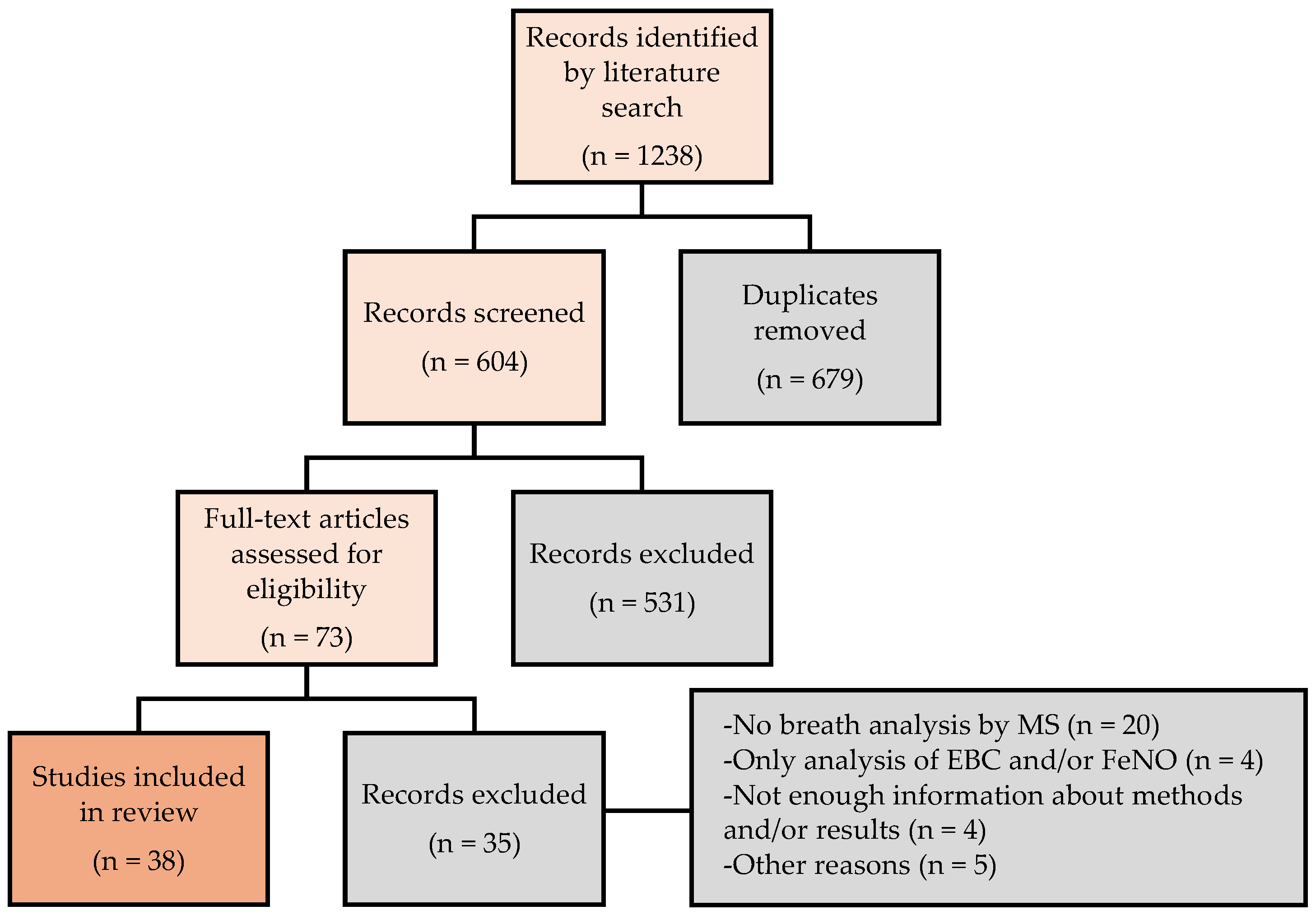
3.1. Study Selection
3.2. Data Collection
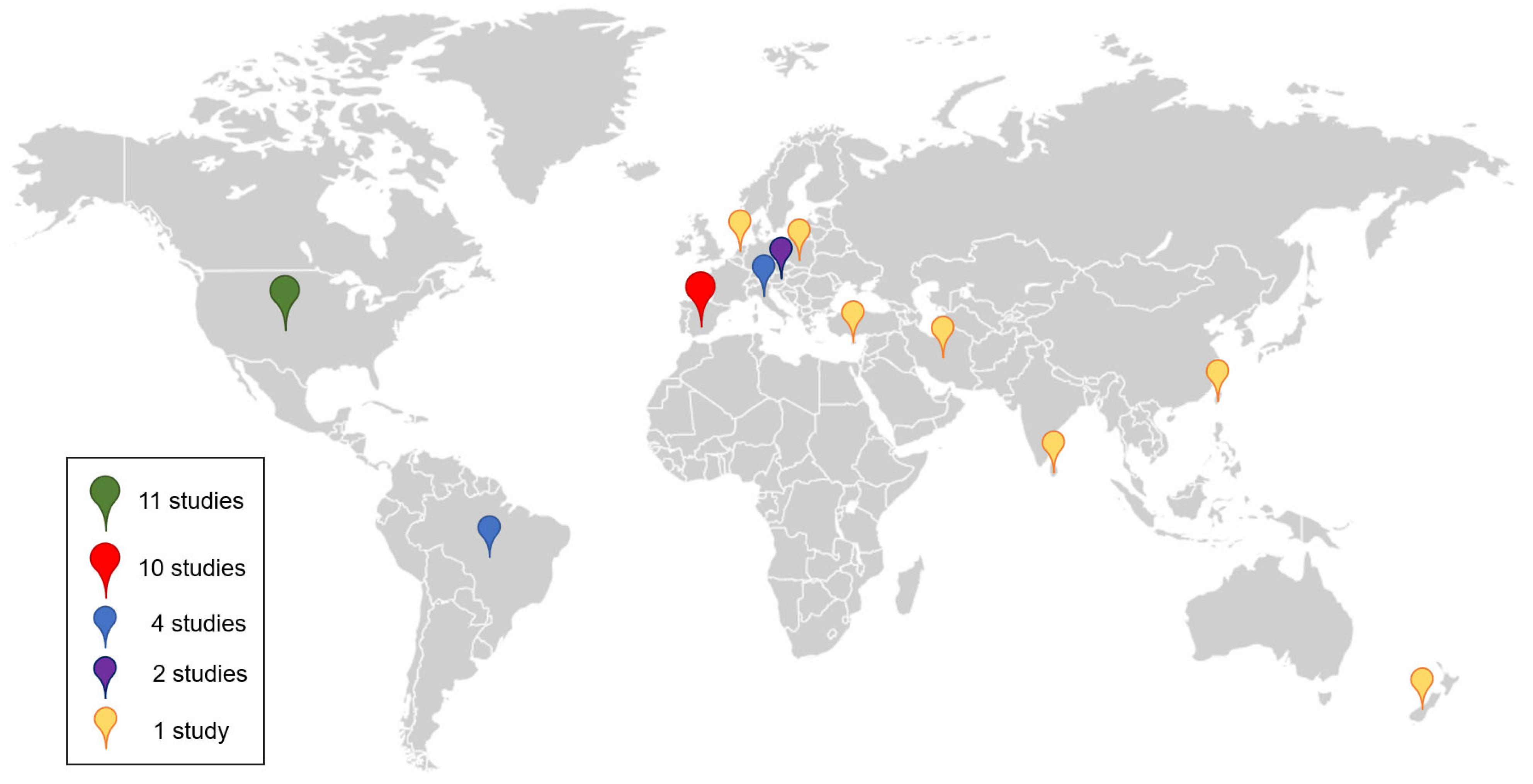
3.3. Environmental Exposures Assessed by Breath Analysis
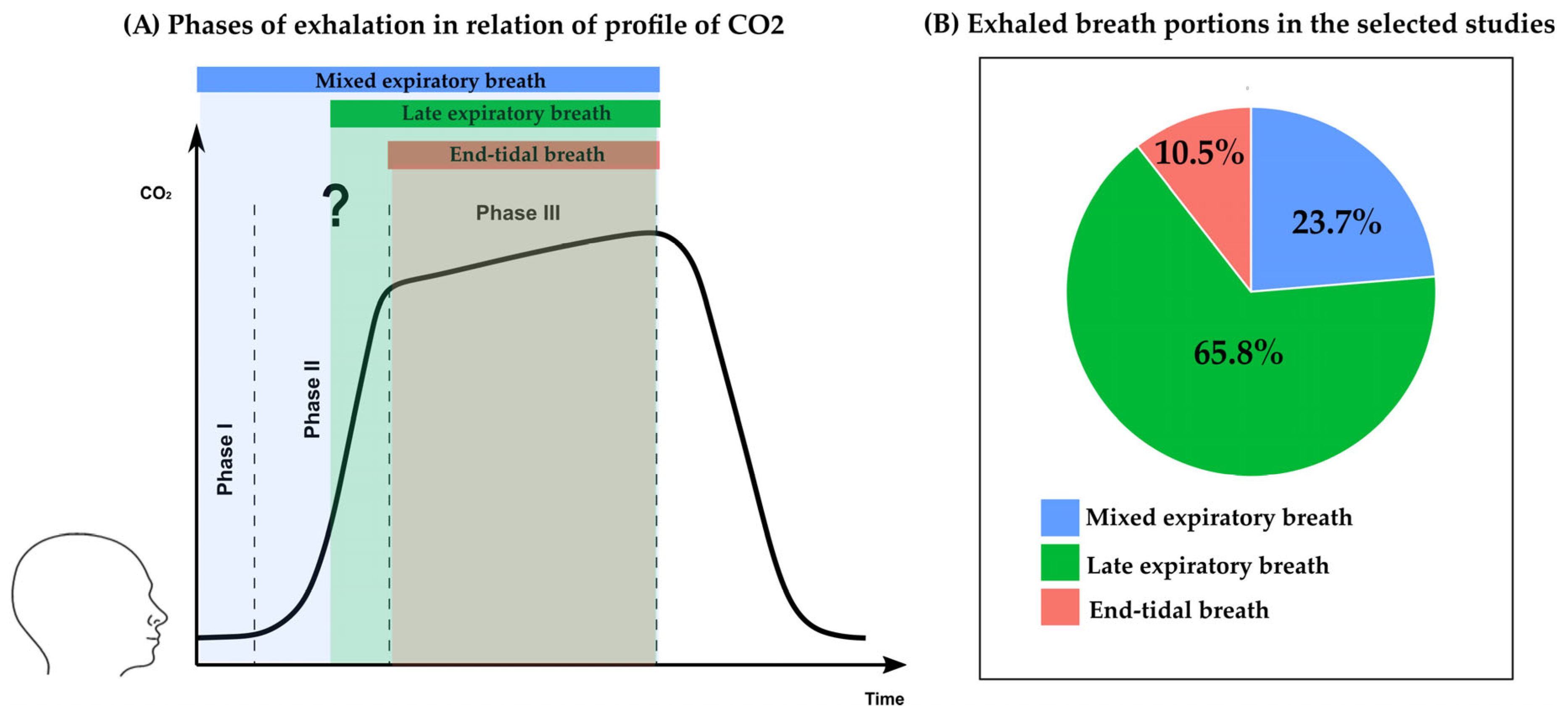
3.4. Collection and Analysis of Exhaled Breath
3.5. Statistical Analysis in Biomonitoring by Breath Analysis
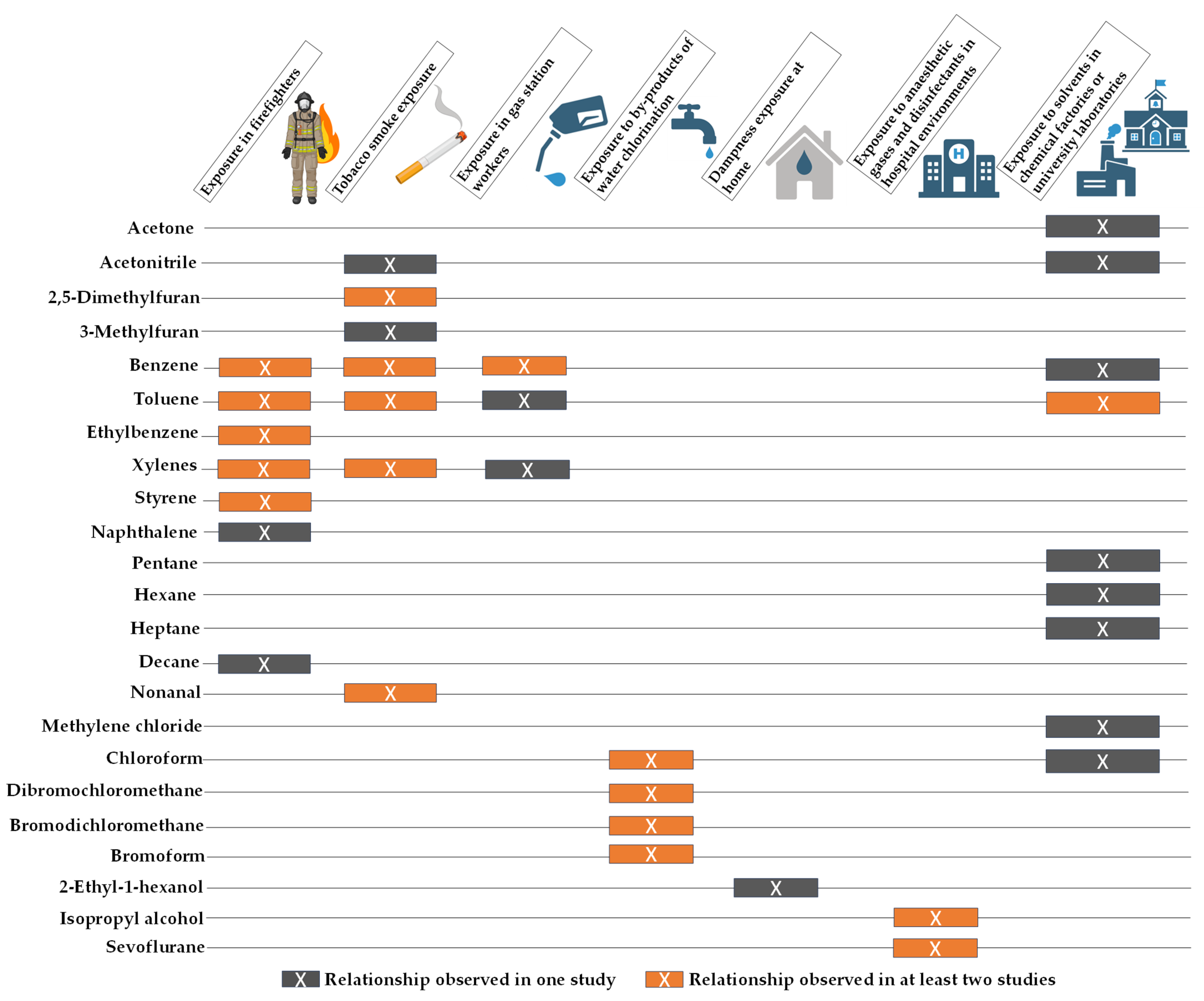
3.6. Selected VOCs for Environmental Exposure Monitoring by Breath Analysis
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pham, Y.L.; Beauchamp, J. Breath Biomarkers in Diagnostic Applications. Molecules 2021, 26, 5514. [Google Scholar] [CrossRef]
- Popov, T.A. Human exhaled breath analysis. Ann. Allergy Asthma Immunol. 2011, 106, 451–456. [Google Scholar] [CrossRef]
- Blanchet, L.; Smolinska, A.; Baranska, A.; Tigchelaar, E.; Swertz, M.; Zhernakova, A.; Dallinga, J.W.; Wijmenga, C.; van Schooten, F.J. Factors that influence the volatile organic compound content in human breath. J. Breath Res. 2017, 11, 016013. [Google Scholar] [CrossRef]
- Davis, M.D.; Fowler, S.J.; Montpetit, A.J. Exhaled breath testing—A tool for the clinician and researcher. Paediatr. Respir. Rev. 2019, 29, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Agapiou, A.; Amann, A.; Mochalski, P.; Statheropoulos, M.; Thomas, C.L.P. Trace detection of endogenous human volatile organic compounds for search, rescue and emergency applications. Trends Anal. Chem. 2015, 66, 158–175. [Google Scholar] [CrossRef]
- de Lacy Costello, B.; Amann, A.; Al-Kateb, H.; Flynn, C.; Filipiak, W.; Khalid, T.; Osborne, D.; Ratcliffe, N.M. A review of the volatiles from the healthy human body. J. Breath Res. 2014, 8, 014001. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.; Porto-Figueira, P.; Cavaco, C.; Taunk, K.; Rapole, S.; Dhakne, R.; Nagarajaram, H.; Câmara, J.S. Breath analysis as a potential and non-invasive frontier in disease diagnosis: An Overview. Metabolites 2015, 5, 3–55. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; van Berkel, J.J.B.N.; Dallinga, J.W.; Smolinska, A.; Wouters, E.F.; van Schooten, F.J. The versatile use of exhaled volatile organic compounds in human health and disease. J. Breath Res. 2012, 6, 027108. [Google Scholar] [CrossRef]
- Catino, A.; De Gennaro, G.; Di Gilio, A.; Facchini, L.; Galetta, D.; Palmisani, J.; Porcelli, F.; Varesano, N. Breath Analysis: A Systematic Review of Volatile Organic Compounds (VOCs) in Diagnostic and Therapeutic Management of Pleural Mesothelioma. Cancers 2019, 11, 831. [Google Scholar] [CrossRef]
- Bajo-Fernández, M.; Souza-Silva, É.A.; Barbas, C.; Rey-Stolle, M.F.; García, A. GC-MS-based metabolomics of volatile organic compounds in exhaled breath: Applications in health and disease. A review. Front. Mol. Biosci. 2023, 10, 1295955. [Google Scholar] [CrossRef]
- Azim, A.; Barber, C.; Dennison, P.; Riley, J.; Howarth, P. Exhaled volatile organic compounds in adult asthma: A systematic review. Eur. Respir. J. 2019, 54, 1900056. [Google Scholar] [CrossRef] [PubMed]
- Sola-Martínez, R.A.; Pastor Hernández, J.M.; Yanes Torrado, Ó.; Cánovas, M.; de Diego Puente, T.; Vinaixa Crevillent, M. Exhaled volatile organic compounds analysis in clinical pediatrics: A systematic review. Pediatr. Res. 2021, 89, 1352–1363. [Google Scholar] [CrossRef] [PubMed]
- Berna, A.Z.; Odom John, A.R. Breath Metabolites to Diagnose Infection. Clin. Chem. 2022, 68, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Kurada, S.; Alkhouri, N.; Fiocchi, C.; Dweik, R.; Rieder, F. Review article: Breath analysis in inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2015, 41, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Dixit, K.; Fardindoost, S.; Ravishankara, A.; Tasnim, N.; Hoorfar, M. Exhaled Breath Analysis for Diabetes Diagnosis and Monitoring: Relevance, Challenges and Possibilities. Biosensors 2021, 11, 476. [Google Scholar] [CrossRef]
- Kaloumenou, M.; Skotadis, E.; Lagopati, N.; Efstathopoulos, E.; Tsoukalas, D. Breath Analysis: A Promising Tool for Disease Diagnosis-The Role of Sensors. Sensors 2022, 22, 1238. [Google Scholar] [CrossRef]
- Sola-Martínez, R.A.; Lozano-Terol, G.; Gallego-Jara, J.; Cánovas, M.; de Diego Puente, T. Off-line breath analysis: Standardization of breath sampling and analysis using mass spectrometry and innovative algorithms. In Breath Analysis; Weigl, S., Ed.; Bioanalytical Reviews; Springer Nature: Cham, Switzerland, 2022; pp. 19–44. [Google Scholar]
- Li, Y.; Wei, X.; Zhou, Y.; Wang, J.; You, R. Research progress of electronic nose technology in exhaled breath disease analysis. Microsyst. Nanoeng. 2023, 9, 129. [Google Scholar] [CrossRef]
- Ibrahim, W.; Carr, L.; Cordell, R.; Wilde, M.J.; Salman, D.; Monks, P.S.; Thomas, P.; Brightling, C.E.; Siddiqui, S.; Greening, N.J. Breathomics for the Clinician: The use of volatile organic compounds in respiratory diseases. Thorax 2021, 76, 514–521. [Google Scholar] [CrossRef]
- Wallace, M.A.G.; Pleil, J.D. Evolution of clinical and environmental health applications of exhaled breath research: Review of methods and instrumentation for gas-phase, condensate, and aerosols. Anal. Chim. Acta 2018, 1024, 18–38. [Google Scholar] [CrossRef]
- Davidson, C.J.; Hannigan, J.H.; Bowen, S.E. Effects of inhaled combined Benzene, Toluene, Ethylbenzene, and Xylenes (BTEX): Toward an environmental exposure model. Environ. Toxicol. Pharmacol. 2021, 81, 103518. [Google Scholar] [CrossRef]
- Preuss, R.; Angerer, J.; Drexler, H. Naphthalene—An environmental and occupational toxicant. Int. Arch. Occup. Environ. Health 2003, 76, 556–576. [Google Scholar] [CrossRef]
- Amorim, L.C.A.; Cardeal, Z.d.L. Breath air analysis and its use as a biomarker in biological monitoring of occupational and environmental exposure to chemical agents. J. Chromatogr. B 2007, 853, 1–9. [Google Scholar] [CrossRef]
- Tang, Z.; Liu, Y.; Duan, Y. Breath analysis: Technical developments and challenges in the monitoring of human exposure to volatile organic compounds. J. Chromatogr. B 2015, 1002, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Carraro, S.; Ferraro, V.A.; Zanconato, S. Impact of air pollution exposure on lung function and exhaled breath biomarkers in children and adolescents. J. Breath Res. 2022, 16, 044002. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Eckhardt, C.M.; Baccarelli, A.A. Molecular mechanisms of environmental exposures and human disease. Nat. Rev. Genet. 2023, 24, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Vineis, P.; Robinson, O.; Chadeau-Hyam, M.; Dehghan, A.; Mudway, I.; Dagnino, S. What is new in the exposome? Environ. Int. 2020, 143, 105887. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Chen, H.; Wu, Z.; Hu, M.; Yao, M. Haze Air Pollution Health Impacts of Breath-Borne VOCs. Environ. Sci. Technol. 2022, 56, 8541–8551. [Google Scholar] [CrossRef]
- Billionnet, C.; Gay, E.; Kirchner, S.; Leynaert, B.; Annesi-Maesano, I. Quantitative assessments of indoor air pollution and respiratory health in a population-based sample of French dwellings. Environ. Res. 2011, 111, 425–434. [Google Scholar] [CrossRef]
- Jie, Y.; Isa, Z.M.; Jie, X.; Ju, Z.L.; Ismail, N.H. Urban vs. rural factors that affect adult asthma. Rev. Environ. Contam. Toxicol. 2013, 226, 33–63. [Google Scholar] [CrossRef]
- Cincinelli, A.; Martellini, T. Indoor Air Quality and Health. Int. J. Environ. Res. Public Health 2017, 14, 1286. [Google Scholar] [CrossRef]
- Lirk, P.; Bodrogi, F.; Deibl, M.; Kähler, C.M.; Colvin, J.; Moser, B.; Pinggera, G.; Raifer, H.; Rieder, J.; Schobersberger, W. Quantification of recent smoking behaviour using proton transfer reaction-mass spectrometry (PTR-MS). Wien. Klin. Wochenschr. 2004, 116, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Alonso, M.; Castellanos, M.; Sanchez, J.M. Evaluation of potential breath biomarkers for active smoking: Assessment of smoking habits. Anal. Bioanal. Chem. 2010, 396, 2987–2995. [Google Scholar] [CrossRef] [PubMed]
- Papaefstathiou, E.; Stylianou, M.; Andreou, C.; Agapiou, A. Breath analysis of smokers, non-smokers, and e-cigarette users. J. Chromatogr. B 2020, 1160, 122349. [Google Scholar] [CrossRef] [PubMed]
- Scheepers, P.T.J.; de Werdt, L.; van Dael, M.; Anzion, R.; Vanoirbeek, J.; Duca, R.C.; Creta, M.; Godderis, L.; Warnakulasuriya, D.T.D.; Devanarayana, N.M. Assessment of exposure of gas station attendants in Sri Lanka to benzene, toluene and xylenes. Environ. Res. 2019, 178, 108670. [Google Scholar] [CrossRef] [PubMed]
- Amorim, L.C.A.; Carneiro, J.P.; Cardeal, Z.L. An optimized method for determination of benzene in exhaled air by gas chromatography-mass spectrometry using solid phase microextraction as a sampling technique. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2008, 865, 141–146. [Google Scholar] [CrossRef]
- Menezes, H.C.; Amorim, L.C.A.; Cardeal, Z.L. Sampling of benzene in environmental and exhaled air by solid-phase microextraction and analysis by gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 2009, 395, 2583–2589. [Google Scholar] [CrossRef]
- Font-Ribera, L.; Kogevinas, M.; Schmalz, C.; Zwiener, C.; Marco, E.; Grimalt, J.O.; Liu, J.; Zhang, X.; Mitch, W.; Critelli, R.; et al. Environmental and personal determinants of the uptake of disinfection by-products during swimming. Environ. Res. 2016, 149, 206–215. [Google Scholar] [CrossRef]
- Espín-Pérez, A.; Font-Ribera, L.; Van Veldhoven, K.; Krauskopf, J.; Portengen, L.; Chadeau-Hyam, M.; Vermeulen, R.; Grimalt, J.O.; Villanueva, C.M.; Vineis, P.; et al. Blood transcriptional and microRNA responses to short-term exposure to disinfection by-products in a swimming pool. Environ. Int. 2018, 110, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Nuckols, J.R.; Ashley, D.L.; Lyu, C.; Gordon, S.M.; Hinckley, A.F.; Singer, P. Influence of tap water quality and household water use activities on indoor air and internal dose levels of trihalomethanes. Environ. Health Perspect. 2005, 113, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Sola-Martínez, R.A.; Lozano Terol, G.; Gallego-Jara, J.; Morales, E.; García-Marcos, L.; Noguera-Velasco, J.A.; Cánovas, M.; de Diego Puente, T. Influence of Home Indoor Dampness Exposure on Volatile Organic Compounds in Exhaled Breath of Mothers and Their Infants: The NELA Birth Cohort. Appl. Sci. 2022, 12, 6864. [Google Scholar] [CrossRef]
- Liu, S.; Yan, E.Z.; Turyk, M.E.; Srisai Katta, S.; Rasti, A.F.; Lee, J.H.; Alajlouni, M.; Wallace, T.E.; Catt, W.; Aikins, E.A. A pilot study characterizing tetrachloroethylene exposure with exhaled breath in an impacted community. Environ. Pollut. 2022, 297, 118756. [Google Scholar] [CrossRef] [PubMed]
- Scheepers, P.T.J.; Graumans, M.H.F.; van Dael, M.; de Werdt, L.; Pinckaers, N.; Beckmann, G.; Anzion, R. Intrusion of chlorinated hydrocarbons and their degradation products from contaminated soil. Measurement of indoor air quality and biomonitoring by analysis of end-exhaled air. Sci. Total Environ. 2019, 653, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Ulanowska, A.; Kowalkowski, T.; Trawińska, E.; Buszewski, B. The application of statistical methods using VOCs to identify patients with lung cancer. J. Breath Res. 2011, 5, 046008. [Google Scholar] [CrossRef] [PubMed]
- Dias, C.M.; Menezes, H.C.; Cardeal, Z.L. Use of exhaled air as an improved biomonitoring method to assess perchloroethylene short-term exposure. Environ. Res. 2017, 156, 108–112. [Google Scholar] [CrossRef] [PubMed]
- De Gennaro, G.; Dragonieri, S.; Longobardi, F.; Musti, M.; Stallone, G.; Trizio, L.; Tutino, M. Chemical characterization of exhaled breath to differentiate between patients with malignant plueral mesothelioma from subjects with similar professional asbestos exposure. Anal. Bioanal. Chem. 2010, 398, 3043–3050. [Google Scholar] [CrossRef] [PubMed]
- Ghimenti, S.; Tabucchi, S.; Bellagambi, F.G.; Lomonaco, T.; Onor, M.; Trivella, M.G.; Fuoco, R.; Di Francesco, F. Determination of sevoflurane and isopropyl alcohol in exhaled breath by thermal desorption gas chromatography–mass spectrometry for exposure assessment of hospital staff. J. Pharm. Biomed. Anal. 2015, 106, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.Y.; Chuang, H.C.; Shie, R.H.; Liao, W.H.; Hwang, Y.H. Pilot studies of VOC exposure profiles during surgical operations. Ann. Work Expo. Health 2019, 63, 173–183. [Google Scholar] [CrossRef]
- Castellanos, M.; Xifra, G.; Fernández-Real, J.M.; Sánchez, J.M. Breath gas concentrations mirror exposure to sevoflurane and isopropyl alcohol in hospital environments in non-occupational conditions. J. Breath Res. 2016, 10, 016001. [Google Scholar] [CrossRef]
- Storer, M.; Curry, K.; Squire, M.; Kingham, S.; Epton, M. Breath testing and personal exposure--SIFT-MS detection of breath acetonitrile for exposure monitoring. J. Breath Res. 2015, 9, 036006. [Google Scholar] [CrossRef]
- Sanchez, J.M. Air and breath analysis for the assessment of exposure to solvent emissions in university chemistry laboratories. Atmos. Pollut. Res. 2019, 10, 1795–1802. [Google Scholar] [CrossRef]
- Ghittori, S.; Alessio, A.; Negri, S.; Maestri, L.; Zadra, P.; Imbriani, M. A Field Method for Sampling Toluene in End-Exhaled Air, as a Biomarker of Occupational Exposure: Correlation with Other Exposure Indices. Ind. Health 2004, 42, 226–234. [Google Scholar] [CrossRef]
- Jalali, M.; Zare Sakhvidi, M.J.; Bahrami, A.; Berijani, N.; Mahjub, H. Oxidative stress biomarkers in exhaled breath of workers exposed to crystalline silica dust by SPME-GC-MS. J. Res. Health Sci. 2016, 16, 153–161. [Google Scholar] [PubMed]
- Pleil, J.D.; Stiegel, M.A.; Fent, K.W. Exploratory breath analyses for assessing toxic dermal exposures of firefighters during suppression of structural burns. J. Breath Res. 2014, 8, 037107. [Google Scholar] [CrossRef] [PubMed]
- Filipiak, W.; Ruzsanyi, V.; Mochalski, P.; Filipiak, A.; Bajtarevic, A.; Ager, C.; Denz, H.; Hilbe, W.; Jamnig, H.; Hackl, M.; et al. Dependence of exhaled breath composition on exogenous factors, smoking habits and exposure to air pollutants. J. Breath Res. 2012, 6, 036008. [Google Scholar] [CrossRef] [PubMed]
- Fent, K.W.; Eisenberg, J.; Snawder, J.; Sammons, D.; Pleil, J.D.; Stiegel, M.A.; Mueller, C.; Horn, G.P.; Dalton, J. Systemic Exposure to PAHs and Benzene in Firefighters Suppressing Controlled Structure Fires. Ann. Occup. Hyg. 2014, 58, 830–845. [Google Scholar] [CrossRef] [PubMed]
- Fent, K.W.; Evans, D.E.; Booher, D.; Pleil, J.D.; Stiegel, M.A.; Horn, G.P.; Dalton, J. Volatile organic compounds off-gassing from firefighters personal protective equipment ensembles after use. J. Occup. Environ. Hyg. 2015, 12, 404–414. [Google Scholar] [CrossRef]
- Wallace, M.; Pleil, J.D.; Oliver, K.D.; Whitaker, D.A.; Mentese, S.; Fent, K.W.; Horn, G.P. Targeted GC-MS analysis of firefighters’ exhaled breath: Exploring biomarker response at the individual level. J. Occup. Environ. Hyg. 2019, 16, 355–366. [Google Scholar] [CrossRef]
- Wallace, M.; Pleil, J.D.; Oliver, K.D.; Whitaker, D.A.; Mentese, S.; Fent, K.W.; Horn, G.P. Non-targeted GC/MS analysis of exhaled breath samples: Exploring human biomarkers of exogenous exposure and endogenous response from professional firefighting activity. J. Toxicol. Environ. Health Part A 2019, 82, 244–260. [Google Scholar] [CrossRef]
- Fent, K.W.; Toennis, C.; Sammons, D.; Robertson, S.; Bertke, S.; Calafat, A.M.; Pleil, J.D.; Wallace, M.A.G.; Kerber, S.; Smith, D.; et al. Firefighters’ absorption of PAHs and VOCs during controlled residential fires by job assignment and fire attack tactic. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 338–349. [Google Scholar] [CrossRef]
- Mayer, A.C.; Fent, K.W.; Wilkinson, A.; Chen, I.C.; Kerber, S.; Smith, D.L.; Kesler, R.M.; Horn, G.P. Characterizing exposure to benzene, toluene, and naphthalene in firefighters wearing different types of new or laundered PPE. Int. J. Hyg. Environ. Health 2022, 240, 113900. [Google Scholar] [CrossRef]
- Mayer, A.C.; Fent, K.W.; Wilkinson, A.F.; Chen, I.C.; Siegel, M.R.; Toennis, C.; Sammons, D.; Meadows, J.; Kesler, R.M.; Kerber, S.; et al. Evaluating Exposure to VOCs and Naphthalene for Firefighters Wearing Different PPE Configurations through Measures in Air, Exhaled Breath, and Urine. Int. J. Environ. Res. Public Health 2023, 20, 6057. [Google Scholar] [CrossRef]
- Gordon, S.M.; Brinkman, M.C.; Ashley, D.L.; Blount, B.C.; Lyu, C.; Masters, J.; Singer, P.C. Changes in Breath Trihalomethane Levels Resulting from Household Water-Use Activities. Environ. Health Perspect. 2005, 114, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Jareño-Esteban, J.J.; Muño-Lucas, M.A.; Carrillo-Aranda, B.; Maldonado-Sanz, J.Á.; de Granda-Orive, I.; Aguilar-Ros, A.; Civera-Tejuca, C.; Gutierrez-Ortega, C.; Callol-Sánchez, L.M. Volatile organic compounds in exhaled breath in a healthy population: Effect of tobacco smoking. Arch. Bronconeumol. 2013, 49, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Jareño-Esteban, J.J.; Muño-Lucas, M.A.; Gomez-Martin, O.; Utrilla-Trigo, S.; Gutierrez-Ortega, C.; Aguilar-Ros, A.; Collado-Yurrita, L.; Callol-Sánchez, L.M. Study of 5 Volatile Organic Compounds in Exhaled Breath in Chronic Obstructive Pulmonary Disease. Arch. Bronconeumol. 2017, 53, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Capone, S.; Tufariello, M.; Forleo, A.; Longo, V.; Giampetruzzi, L.; Radogna, A.V.; Casino, F.; Siciliano, P. Chromatographic analysis of VOC patterns in exhaled breath from smokers and nonsmokers. Biomed. Chromatogr. 2018, 32, e4132. [Google Scholar] [CrossRef]
- Dias, C.M.; Menezes, H.C.; Cardeal, Z.L. Environmental and biological determination of acrolein using new cold fiber solid phase microextraction with gas chromatography mass spectrometry. Anal. Bioanal. Chem. 2017, 409, 2821–2828. [Google Scholar] [CrossRef]
- van Drooge, B.L.; Marco, E.; Perez, N.; Grimalt, J.O. Influence of electronic cigarette vaping on the composition of indoor organic pollutants, particles, and exhaled breath of bystanders. Environ. Sci. Pollut. Res. 2019, 26, 4654–4666. [Google Scholar] [CrossRef]
- Castellanos, M.; Suñer, R.; Fernández-Real, J.M.; Sanchez, J.M. Original investigation 2,5-Dimethylfuran as a Validated Biomarker of Smoking Status. Nicotine Tob. Res. 2019, 21, 828–834. [Google Scholar] [CrossRef]
- Barros, B.; Oliveira, M.; Morais, S. Firefighters’ occupational exposure: Contribution from biomarkers of effect to assess health risks. Environ. Int. 2021, 156, 106704. [Google Scholar] [CrossRef]
- Srivastav, A.L.; Patel, N.; Chaudhary, V.K. Disinfection by-products in drinking water: Occurrence, toxicity and abatement. Environ. Pollut. 2020, 267, 115474. [Google Scholar] [CrossRef]
- Callahan-Lyon, P. Electronic cigarettes: Human health effects. Tob. Control 2014, 23, ii36–ii40. [Google Scholar] [CrossRef]
- Bruderer, T.; Gaisl, T.; Gaugg, M.T.; Nowak, N.; Streckenbach, B.; Müller, S.; Moeller, A.; Kohler, M.; Zenobi, R. On-Line Analysis of Exhaled Breath. Chem. Rev. 2019, 119, 10803–10828. [Google Scholar] [CrossRef] [PubMed]
- Sola-Martínez, R.A.; Zeng, J.; Awchi, M.; Gisler, A.; Arnold, K.; Singh, K.D.; Frey, U.; Cánovas, M.; de Diego Puente, T.; Sinues, P. Preservation of exhaled breath samples for analysis by off-line SESI-HRMS: Proof-of-concept study. J. Breath Res. 2024, 18, 011002. [Google Scholar] [CrossRef] [PubMed]
- Lawal, O.; Ahmed, W.M.; Nijsen, T.M.E.; Goodacre, R.; Fowler, S.J. Exhaled breath analysis: A review of ‘breath-taking’ methods for off-line analysis. Metabolomics 2017, 13, 110. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, J.D.; Miekisch, W. Breath sampling and standardization. In Breathborne Biomarkers and the Human Volatilome; Beauchamp, J., Davis, C., Pleil, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 23–40. ISBN 978-0-12-819967-1. [Google Scholar]
- Xie, M.; Liu, X.; Cao, X.; Guo, M.; Li, X. Trends in prevalence and incidence of chronic respiratory diseases from 1990 to 2017. Respir. Res. 2020, 21, 49. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.; Fan, M.; Harshman, S.W.; Garrison, C.E.; Dershem, V.L.; Phillips, J.B.; Grigsby, C.C.; Ott, D.K. Evaluation of Bio-VOC Sampler for Analysis of Volatile Organic Compounds in Exhaled Breath. Metabolites 2014, 4, 879. [Google Scholar] [CrossRef]
- Westphal, K.; Dudzik, D.; Waszczuk-Jankowska, M.; Graff, B.; Narkiewicz, K.; Markuszewski, M.J. Common Strategies and Factors Affecting Off-Line Breath Sampling and Volatile Organic Compounds Analysis Using Thermal Desorption-Gas Chromatography-Mass Spectrometry (TD-GC-MS). Metabolites 2023, 13, 8. [Google Scholar] [CrossRef]
- Beauchamp, J.; Herbig, J.; Gutmann, R.; Hansel, A. On the use of Tedlar® bags for breath-gas sampling and analysis. J. Breath Res. 2008, 2, 046001. [Google Scholar] [CrossRef]
- Skawinski, M.; van Schooten, F.J.; Smolinska, A. A comprehensive guide to volatolomics data analysis. J. Breath Res. 2024, 19, 015001. [Google Scholar] [CrossRef]
- Longo, V.; Forleo, A.; Giampetruzzi, L.; Siciliano, P.; Capone, S. Human Biomonitoring of Environmental and Occupational Exposures by GC-MS and Gas Sensor Systems: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 10236. [Google Scholar] [CrossRef]
- Liao, Q.; Du, R.; Ma, R.; Liu, X.; Zhang, Y.; Zhang, Z.; Ji, P.; Xiao, M.; Cui, Y.; Xing, X.; et al. Association between exposure to a mixture of benzene, toluene, ethylbenzene, xylene, and styrene (BTEXS) and small airways function: A cross-sectional study. Environ. Res. 2022, 212, 113488. [Google Scholar] [CrossRef]
- Weisel, C.P. Benzene exposure: An overview of monitoring methods and their findings. Chem. Biol. Interact. 2010, 184, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Kashtan, Y.; Nicholson, M.; Finnegan, C.J.; Lebel, E.D.; Michanowicz, D.R.; Shonkoff, S.B.C.; Nadeau, K.C.; Jackson, R.B. Exposure and health risks of benzene from combustion by gas stoves: A modelling approach in U.S. homes. J. Hazard. Mater. 2025, 492, 137986. [Google Scholar] [CrossRef] [PubMed]
- Rosting, C.; Olsen, R. Biomonitoring of the benzene metabolite s-phenylmercapturic acid and the toluene metabolite s-benzylmercapturic acid in urine from firefighters. Toxicol. Lett. 2020, 329, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Wesselink, A.K.; Hatch, E.E.; Wise, L.A.; Rothman, K.J.; Vieira, V.M.; Aschengrau, A. Exposure to tetrachloroethylene-contaminated drinking water and time to pregnancy. Environ. Res. 2018, 167, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Yasny, J.S.; White, J. Environmental Implications of Anesthetic Gases. Anesth. Prog. 2012, 59, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, N.; Wieczorek, T.; Drabińska, N.; Drabińska, N.; Gould, O.; Osborne, A.; De Lacy Costello, B. A mechanistic study and review of volatile products from peroxidation of unsaturated fatty acids: An aid to understanding the origins of volatile organic compounds from the human body. J. Breath Res. 2020, 14, 034001. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.; Godbeer, L.; Allsworth, M.; Boyle, B.; Ball, M.L. Progress and challenges of developing volatile metabolites from exhaled breath as a biomarker platform. Metabolomics 2024, 20, 72. [Google Scholar] [CrossRef]
- Steinle, S.; Reis, S.; Sabel, C.E.; Semple, S.; Twigg, M.M.; Braban, C.F.; Leeson, S.R.; Heal, M.R.; Harrison, D.; Lin, C.; et al. Personal exposure monitoring of PM2.5 in indoor and outdoor microenvironments. Sci. Total Environ. 2015, 508, 383–394. [Google Scholar] [CrossRef]
- Sola-Martínez, R.A.; Garcia Palomo, C.; Lozano Terol, G.; de Diego Puente, T. Monitorización de la exposición ocupacional mediante el análisis de compuestos orgánicos volátiles. In Salud Global: Ciencia, Sostenibilidad e Innovación; Martínez Lopez, S., Ed.; Salvadora Martínez López: Murcia, Spain, 2025; pp. 101–114. ISBN 9788409703142. [Google Scholar]
- Amann, A.; de Lacy Costello, B.; Miekisch, W.; Schubert, J.; Buszewski, B.; Pleil, J.; Ratcliffe, N.; Risby, T. The human volatilome: Volatile organic compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. J. Breath Res. 2014, 8, 034001. [Google Scholar] [CrossRef]
- Pleil, J.D.; Stiegel, M.A. Evolution of environmental exposure science: Using breath-borne biomarkers for “discovery” of the human exposome. Anal. Chem. 2013, 85, 9984–9990. [Google Scholar] [CrossRef]
- Gaude, E.; Nakhleh, M.K.; Patassini, S.; Boschmans, J.; Allsworth, M.; Boyle, B.; Van Der Schee, M.P. Targeted breath analysis: Exogenous volatile organic compounds (EVOC) as metabolic pathway-specific probes. J. Breath Res. 2019, 13, 032001. [Google Scholar] [CrossRef]
- Smolinska, A.; Hauschild, A.-C.; Fijten, R.R.R.; Dallinga, J.W.; Baumbach, J.; van Schooten, F.J. Current breathomics—A review on data pre-processing techniques and machine learning in metabolomics breath analysis. J. Breath Res. 2014, 8, 027105. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Bos, L.D.; van der Schee, M.P.; van Schooten, F.J.; Sterk, P.J. Exhaled Molecular Fingerprinting in Diagnosis and Monitoring: Validating Volatile Promises. Trends Mol. Med. 2015, 21, 633–644. [Google Scholar] [CrossRef] [PubMed]
| Ref. | Study Design | Environmental Exposure | Study Population | Samples | Exhaled Breath Portion/Breath Container | Analytical Platform | Statistical Methods | Summary of Findings Related to VOCs in Exhaled Breath |
|---|---|---|---|---|---|---|---|---|
| [32] | Target | Tobacco smoke exposure | 268 subjects (48 smokers and 220 non-smokers) | -EB -AA | -Mixed expiratory breath -Teflon bag | PTR-MS | Mann–Whitney test and ROC curves | Acetonitrile (smokers vs. non-smokers) |
| [33] | Target | Tobacco smoke exposure | 204 healthy subjects (100 smokers and 104 non-smokers) | -EB -AA | -Late expiratory breath -Tedlar bag | In-house capillary TD-GC/MS | Mann–Whitney test and Spearman correlation | 2,5-dimethylfuran, benzene, toluene, o-xylene, and m/p-xylene (smokers vs. non-smokers) 2,5-dimethylfuran (significant differences after more than 24 h without smoking) |
| [44] | Untarget | Tobacco smoke exposure | 137 patients with lung cancer and 143 healthy subjects (41 active smokers, 16 passive smokers, and 86 non-smokers) | -EB -AA | -End-tidal breath -Tedlar bag | SPME-GC/MS | Discriminant analysis and CHAID model | Higher concentration of acetonitrile, benzene, and furan derivatives in smokers without lung cancer Butyrolactone (passive smokers vs. non-smokers and smokers) 3-methylfuran and 2,5-dimethylfuran (smokers vs. non-smokers) Carbon disulfide (passive smokers and non-smokers) |
| [55] | Untarget | Tobacco smoke exposure | 115 subjects (47 smokers and 68 non-smokers) | -EB -EA | -End-tidal breath -Tedlar bag and multibed sorption tube | TD-GC/MS | Kruskal–Wallis test and linear regression | Aromatic compounds, furan derivatives, alkenes, alkynes, dienes, ketones, VNCs, and VSCs (smokers vs. non-smokers and ex-smokers) |
| [64] | Target | Tobacco smoke exposure | 89 healthy subjects (35 non-smokers, 24 ex-smokers, and 30 smokers) | -EB -AA | -Late expiratory breath -Bio-VOC and TD tubes (Tenax TA + graphitised carbon black + carbonized molecular sieve) | TD-GC/MS | Kruskal–Wallis test and Mann–Whitney test | Nonanal (smokers and ex-smokers vs. non-smokers) |
| [65] | Target | Tobacco smoke exposure | 100 healthy subjects (non-smokers, ex-smokers, and smokers) and 57 subjects with COPD | -EB -AA | -Late expiratory breath -Bio-VOC and TD tubes (Tenax TA + graphitised carbon black + carbonized molecular sieve) | TD-GC/MS | Logistic regression and odds ratio calculation | Hexanal (COPD patients vs. healthy controls) Nonanal (smokers and ex-smokers vs. non-smokers) |
| [66] | Untarget | Tobacco smoke exposure | 26 healthy subjects (16 smokers and 10 non-smokers) | -EB (in the morning before smoking—“blank smokers”) -EB (in the morning after 1 h abstinence after smoking) -EB (in the night after 1 h abstinence after smoking) | -Late expiratory breath -QUINTRON breath sampling system (discard bag and collecting bag) | SPME-GC/MS | Mann–Whitney test and predictive Probit model | Toluene, pyridine, and pyrrole (smokers vs. non-smokers) Nonane, 2,3-dimethyl (“blank smokers” vs. non-smokers) Toluene, pyridine, pyrrole, benzene, 2-butanone, 2-pentanone and 1-methyldecylamine (“blank smokers” vs. smokers after cigarette smoking) |
| [67] | Target | Tobacco smoke exposure in indoor environments | 20 smokers | -EB -AA of room contaminated by cigarette smoke | -Mixed expiratory breath | CF-SPME-GC/MS | ANOVA test, linear regression and Pearson correlation | Acrolein (VOC levels in exhaled breath and indoor air were correlated) |
| [68] | Untarget | E-cigarette smoke exposure | 5 non-vaping subjects | -EB from the day without vaping -EB from the vaping day -AA | -Late expiratory breath -Bio-VOC and TD tubes (Tenax TA) | TD-GC/MS | Higher concentrations of ethanol, ethyl acetate, and 1,4-Dichlorobenzene (vaping day vs. non-vaping day) | |
| [69] | Target | Tobacco smoke exposure | Training group: 377 subjects (174 smokers and 203 non-smokers) Validation group: 64 subjects (self-reported smoking) | -EB | -Late expiratory breath -Tedlar bag | In-house capillary TD-GC/MS | Mann–Whitney test, ROC curves, and Multivariate logistic regression analysis | 2,5-dimethylfuran, benzene, toluene, o-xylene, and m/p-xylene (smokers vs. non-smokers) 2,5-dimethylfuran (smoking status) |
| [34] | Untarget | Tobacco and e-cigarette smoke exposure | Training group: 48 healthy subjects (10 smokers, 18 vapers, and 20 non-smokers) Validation group: 4 smokers and 4 vapers | -EB after smoking or vaping -AA from breath sampling room | -Mixed expiratory breath -Tedlar bag | SPME-GC/MS | PCA with HCA and PLS-DA | Aromatic compounds, furan derivatives, VNCs, ketones, and alkenes are related to smokers Esters, terpenes, and oxygenated compounds (vapers vs. smokers) |
| [35] | Target | Occupational exposure: BTX exposure in gas station attendants | 29 gas station attendants and 16 office workers | -EB (pre- and post-shift samples) -AA (personal air sampling) | -Late expiratory breath -Bio-VOC and TD tubes (Carbograph 1TD and Carbograph 2TD) | TD-GC/MS | Wilcoxon signed-rank test, Mann–Whitney test, Kruskal–Wallis test, and Spearman correlation | Benzene, toluene, m/p-xylene, and o-xylene (post-shift vs. pre-shift) Benzene, toluene, and m/p-xylene (gas station attendants vs. controls) |
| [36] | Target | Occupational exposure: benzene in gas stations and in gasoline quality control laboratories | 25 subjects exposed to benzene in gasoline (workers in gas stations and in gasoline quality control laboratories) and 25 non-exposed subjects | -EB in the end of morning or in the middle of the work shift | -Late expiratory breath -SPME | SPME-GC/MS | ANOVA test and Brown–Forsythe’s Test | Benzene (exposed group vs. non-exposed group) |
| [37] | Target | Occupational exposure: benzene from gasoline in different workplaces | 15 workers exposed to low levels of benzene (employees of restaurants, coffee shops, offices, park guards, and teachers) and 30 workers in retail gasoline stations—“exposed group” | -EB -AA (urban air samples and ambient air of the workplace) | -Late expiratory breath -SPME | SPME-GC/MS | Mann–Whitney test, correlation analysis, and linear models constructed by the least squares method, weighted by the experimental variance | Benzene (VOC levels in exhaled breath and in ambient air were correlated) Benzene (exposed group vs. non-exposed group) |
| [38] | Target | Exposure to disinfection by-products in chlorinated swimming pools | 116 healthy and non-smoking subjects (non-professional swimmers) | -EB before and after swimming -AA from different locations of the swimming pool | -Late expiratory breath -Bio-VOC and TD tubes | TD-GC/MS | Kruskal–Wallis test, Spearman correlation, and linear regression models | Median level of exhaled total trihalomethanes (chloroform, dibromochloromethane, bromodichloromethane and bromoform) increased after swimming Dibromochloromethane, Bromodichloromethane, and bromoform (VOC levels in exhaled breath and in pool water were correlated) (VOC levels in exhaled breath and trichloramine in air were correlated) |
| [39] | Target | Exposure to disinfection by-products in chlorinated swimming pools | 43 healthy and non-smoking subjects (non-professional swimmers) | -EB before and after swimming -AA (room and swimming pool) | -Late expiratory breath -Bio-VOC and TD tubes (Tenax TA 35/60 mesh) | TD-GC/MS | Paired t-test | Trihalomethanes (chloroform, dibromochloromethane, bromodichloromethane and bromoform) (after swimming vs. before swimming) |
| [40] | Target | Residential exposure: by-products of water chlorination | 7 healthy subjects (4 in a residence with water with a high concentration of trihalomethanes and 3 in a residence with water with a low concentration of trihalomethanes) | -EB before all water use activities (baseline) and during or after water use activities -AA | -End-tidal breath -1 L Silcosteel stainless steel canisters | GC/MS | Higher concentration of chloroform in exhaled breath of participants from the site with water, with high concentration of trihalomethanes | |
| [63] | Target | Residential exposure: by-products of water chlorination | 7 healthy subjects that performed 12 common water-use activities in 2 residences | -EB before water use activities and 5 min after the end of the activity -EB during the shower event (n = 2) -AA | -End-tidal breath -1 L Silcosteel stainless steel canisters | GC/MS | Dixon’s outlier test, Mann–Whitney test, and Spearman correlation | Chloroform (after showering/bathing vs. before showering/bathing) Chloroform (VOC levels in exhaled breath and indoor air were correlated in showering) (VOC levels in exhaled breath and water were correlated in bathing) (VOC levels in exhaled breath and blood were correlated in showering and bathing) |
| [41] | Target | Residential exposure: indoor dampness | 337 women (86 with dampness in home and 251 without dampness in home) and 337 children (87 with dampness in home and 250 without dampness in home) | -EB -AA from breath sampling room | -Mixed expiratory breath -Tedlar bag (women)/ Tedlar bag + Quintron bag (children) and TD tubes (Tenax TA/carbograph 5td) | TD-GC/MS | Mann–Whitney test and Student’s t-test | 2-ethyl-hexanol (women with home dampness exposure vs. women without home dampness exposure) |
| [42] | Target | Residential exposure: chlorinated VOCs caused by groundwater contamination plumes | 38 healthy non-smokers from 26 residences located in different areas (12 on the superfund site, 11 on other plumes, and 3 outside any plumes) | -EB -AA at homes located on the superfund site | -Late expiratory breath -Tedlar bag | TD-GC/MS | ANOVA test and mixed linear models | Tetrachloroethylene (VOC levels in exhaled breath and in indoor air were correlated) |
| [43] | Target | Soil contamination with chlorinated hydrocarbons in a bookshop that was a dry-cleaning shop | 2 smoking workers in bookshops (shop owner who works 7 days per week and employee who works 3 days per week) | -EB (pre-shift and post-shift samples) -AA inside bookshop and outdoor sample close to ventilator air intake point (EB and AA in summer and in winter) | -Late expiratory breath -Bio-VOC and TD tubes (Carbograph 1 and Carbosieve SIII) | TD-GC/MS | Paired t-test | Tetrachloroethylene, benzene, and toluene (post-shift vs. pre-shift) |
| [45] | Target | Occupational exposure: tetrachloroethylene in different workplaces | 24 workers of dry cleaners, 1 worker in an electroplating industry, 1 worker of in a research laboratory, and 1 worker in an automotive paint preparation shop | -EB at the end of the work shift -AA (samples of the different workplaces) | -Mixed expiratory breath | CF-SPME-GC/MS | Linear regression and correlation analysis | Tetrachloroethylene (VOC levels in exhaled breath and in ambient air were correlated) |
| [46] | Untarget | Occupational exposure: long-term professional exposure to asbestos | 39 subjects (13 patients affected by malignant pleural mesothelioma (MPM), 13 subjects with long-term professional exposure to asbestos “exposed group”, and 13 healthy controls) | -EB | -Mixed expiratory breath -Tedlar bag and sorbent cartridges (Carboxen 1003, Carbopack B, and carbopackY) | TD-GC/MS | ANOVA test, PCA, DFA and CP-ANN | Cyclopentane, methyl-octane, and dimethyl-nonane (exposed group vs. MPM and healthy controls) Cyclopentane (long-term asbestos exposure) |
| [47] | Target | Occupational exposure: monitoring of sevoflurane exposure levels in hospital staff | 5 anesthesiologists working in different operating rooms | -EB (at beginning of first day of working week, at end of same day, and at end of working week) | -Mixed expiratory breath -Nalophan bag and TD tubes (60/80 mesh Tenax GR phase (70% Tenax TA, 2,6-diphenyl-p-phenylene oxide and 30% graphite)) | TD-GC/MS | Inconclusive results | |
| Occupational exposure: monitoring of isopropyl alcohol exposure levels in hospital staff | 9 nurses | -EB (before beginning of work shift, and 90 and 180 min later) -AA | Linear regression and correlation analysis | Isopropyl alcohol (VOC levels in exhaled breath and in ambient air were correlated) | ||||
| [48] | Untarget | Occupational exposure: VOCs in operating room personnel | 12 workers of surgical operations (surgeons, surgical assistants, or nurses) and 1 administration nurse | -EB (before and after surgery) -AA (during the surgeries in process) | -Mixed expiratory breath -Bottle-Vac | GC/MS | Wilcoxon signed rank test | Sevoflurane (after surgical operations vs. before surgical operations) |
| [49] | Target | Exposure to anesthetic gases and disinfectants in hospital environments | 100 subjects (24 hospital staff, 45 hospital visitors, and 31 external controls) | -EB | -Late expiratory breath -Tedlar bag | In-house microtrap-GC/MS | Mann–Whitney test | Isopropyl alcohol (hospital staff vs. external controls) and (hospital visitors vs. external controls) Sevoflurane (hospital staff vs. hospital visitors) 2,5-dimethylfuran (smoking status) |
| [50] | Target | Occupational exposure: acetonitrile in a university chemical laboratory | 14 healthy non-smokers (6 workers at chemistry department laboratory—“exposed group”; 8 workers at geography department—“non exposed group”) | -EB (in the morning and early and late afternoon) -AA | -Mixed expiratory breath -Tedlar bag | SIFT-MS | Acetonitrile (exposed group vs. non-exposed group) | |
| Acetonitrile exposure testing | 4 healthy non-smokers sat for 30 min in laboratory | -EB (before exposure, after exposure, and 30 min after exposure) | Higher concentration of acetonitrile after 30 min exposure | |||||
| [51] | Target | Occupational exposure: solvent emissions in university chemical laboratories | 76 subjects (55 researchers from 4 laboratories of chemistry department—“exposed group”; 21 non-exposed subjects) | -EB -AA (samples for each laboratory and for each sampling day) | -Late expiratory breath -Tedlar bag | In-house capillary TD-GC/MS | Kruskal–Wallis test and Mann–Whitney test | Diethyl ether, acetone, n-hexane, 2-methylpentane, methylene chloride, 3-methylpentane, methylcyclopentane, ethyl acetate, chloroform, n-pentane, n-heptane, benzene, and toluene (exposed group vs. non-exposed group) |
| [52] | Target | Occupational exposure: toluene used as solvent in a chemical factory | 36 workers of a chemical factory exposed to toluene | -EB (16 h after shift) -AA in breathing zone during work-shift with personal passive dosimeters | -Late expiratory breath -Glass vial (Tenax TA) and TD tube (Carbotrap 201) | TD-GC/MSD | Linear regression and correlation analysis | Toluene (VOC levels in exhaled breath and in environmental air were correlated) |
| [53] | Untarget | Occupational exposure: crystalline silica dust | 69 subjects (20 workers exposed to silica, 4 silicosis patient—“positive”, 20 healthy non-smokers, and 25 healthy smokers) | -EA -AA | -Late expiratory breath -Tedlar bag | SPME-GC/MS | Kruskal–Wallis test, ANOVA test, and Student’s t-test | Acetaldehyde, 2-propanol, decane, 1,3 butadiene, propanthiol, 3-hydroxy-2-butanone, hexanal, pentadecane, butanoic acid, and nonanal (exposed subjects vs. negative control groups) |
| [54] | Target | Occupational exposure: firefighters in two rounds of controlled structure burns | 18 firefighters | -EB before, shortly after, and 6 h after specific firefighting tasks (planned exposure) | -Late expiratory breath -Bio-VOC and TD tubes (Carbograph 2TD and Carbograph 1TD) | TD-GC/MS | Heatmap, within-subject and between-subject variance components, and ICC | Benzene, toluene, ethylbenzene, styrene, 1,3,5-trimethylbenzene, and naphthalene (post firefighting tasks vs. pre firefighting tasks) Benzene, ethylbenzene, m,p-xylene, styrene, and naphthalene (post firefighting tasks vs. 6 h after firefighting tasks) |
| [56] | Target | Occupational exposure: firefighters in two rounds of controlled structure burns | 18 non-smoking firefighters | -EB before, shortly after, and 6 h after the controlled burn -AA (personal air sampling) | -Late expiratory breath -Bio-VOC and TD tubes (Carbograph 2TD and Carbograph 1TD) | TD-GC/MS | Non-parametric sign tests and Spearman correlation | Benzene (post firefighting tasks vs. pre firefighting tasks) ΔBenzene (change in pre- to post-firefighting tasks) was correlated with personal air concentrations of polycyclic aromatic hydrocarbons in firefighters from round 2 |
| [57] | Target | Occupational exposure: VOCs off-gassing from personal protective equipment (PPE) of firefighters in controlled structure burns | 6 non-smoking firefighters | -EB before and shortly after controlled burn -AA inside structure (before each burn, during each burn, and during last burn) -Off-gas sampling from PPE Ensembles (before each burn and after each burn) | -Late expiratory breath -Bio-VOC and TD tubes (Carbograph 2TD and Carbograph 1TD) | TD-GC/MS | Paired t-tests, linear regression and Pearson correlation | Benzene, toluene, ethylbenzene, xylenes, and styrene (post-burn exhaled breath concentrations and off-gassing air concentrations from used PPE ensembles were correlated) |
| [58] | Target | Occupational exposure: firefighters with different tasks in controlled structure burns | 40 non-smoking firefighters | -EB before, immediately after, and 1 h after participation in controlled structure burns -AA | -Late expiratory breath -Bio-VOC and TD tubes (Carbograph 2TD and Carbograph 1TD) | TD-GC/MS | Student’s t-test | All firefighter samples: benzene and ethylbenzene (post-exposure vs. pre-exposure) Firefighters participating in attack and search: benzene, ethylbenzene, m/p-xylene, and o-xylene (post-exposure vs. pre-exposure) Firefighters participating in outside ventilation: benzene (post-exposure vs. pre-exposure) |
| [59] | Untarget | Occupational exposure: firefighters in controlled structure burns | 40 non-smoking firefighters | -EB before, immediately after, and 1 h after participation in controlled structure burns -AA | -Late expiratory breath -Bio-VOC and TD tubes (Carbograph 2TD and Carbograph 1TD) | TD-GC/MS | Student’s t-test and heatmap | Decane (post-exposure vs. pre-exposure) Trifluorobenzene and 1,2,4-trimethylbenzene (post-exposure vs. pre-exposure) |
| [60] | Target | Occupational exposure: firefighters with different tasks and fire attack tactics in controlled residential fires | 36 non-smoking firefighters | -EB before, immediately after, and 1 h after each fire | -Late expiratory breath -Bio-VOC and TD tubes (Carbograph 2TD and Carbograph 1TD) | TD-GC/MS | Mixed linear models | Firefighters assigned to attack and search: benzene, Ethylbenzene, and xylenes (post-exposure vs. pre-exposure) Firefighters assigned to outside vent: benzene (post-exposure vs. pre-exposure) Firefighters assigned to overhaul: benzene (post-exposure vs. pre-exposure) No difference in concentrations of exhaled benzene associated with tactic (interior attack vs. transitional attack) |
| [61] | Target | Occupational exposure: firefighters with three different PPE ensembles and two treatments (new/laudered) during fire exposure | 24 firefighters | -EB before and immediately after each fire -AA (personal air sampling of the outside and inside of the turnout jacket) -AA | -Late expiratory breath -Bio-VOC and TD tubes (Carbograph 2TD and Carbograph 1TD) | TD-GC/MS | Paired t-test, mixed models and Pearson correlation | Benzene (post-fire vs. pre-fire) There were no significant differences among 3 conditions Toluene (post-fire vs. pre-fire) in firefighters with new Nomex® knit hood, new turnout jacket, and new turnout pants Firefighters who did not wear new knit hood: ΔBenzene (change in pre- to post-fire) was correlated with outside and inside jacket personal air concentrations ΔBenzene (change in pre- to post-fire) was correlated with outside and inside jacket personal air concentrations in firefighters with laundered turnout jacket, pants, and particulate-blocking hoods |
| [62] | Target | Occupational exposure: firefighters with three PPE and base-layer configurations during fire exposure | 23 non-smoking firefighters | -EB before and immediately after removing the equipment after each burn scenario -AA (personal air sampling) -AA | -Late expiratory breath -Bio-VOC and TD tubes (Carbograph 2TD and Carbograph 1TD) | TD-GC/MS | Mixed models | Benzene and toluene (post-fire vs. pre-fire) with all PPE configurations and zip statuses. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sola-Martínez, R.A.; Porras-Guillén, A.; Lozano-Terol, G.; Martínez-Vivancos, A.; Gallego-Jara, J.; Ortega, Á.; de Diego Puente, T. Breath Analysis by Mass Spectrometry-Based Technologies for Biomonitoring Environmental Exposures. Appl. Sci. 2025, 15, 12220. https://doi.org/10.3390/app152212220
Sola-Martínez RA, Porras-Guillén A, Lozano-Terol G, Martínez-Vivancos A, Gallego-Jara J, Ortega Á, de Diego Puente T. Breath Analysis by Mass Spectrometry-Based Technologies for Biomonitoring Environmental Exposures. Applied Sciences. 2025; 15(22):12220. https://doi.org/10.3390/app152212220
Chicago/Turabian StyleSola-Martínez, Rosa A., Aurora Porras-Guillén, Gema Lozano-Terol, Adrián Martínez-Vivancos, Julia Gallego-Jara, Álvaro Ortega, and Teresa de Diego Puente. 2025. "Breath Analysis by Mass Spectrometry-Based Technologies for Biomonitoring Environmental Exposures" Applied Sciences 15, no. 22: 12220. https://doi.org/10.3390/app152212220
APA StyleSola-Martínez, R. A., Porras-Guillén, A., Lozano-Terol, G., Martínez-Vivancos, A., Gallego-Jara, J., Ortega, Á., & de Diego Puente, T. (2025). Breath Analysis by Mass Spectrometry-Based Technologies for Biomonitoring Environmental Exposures. Applied Sciences, 15(22), 12220. https://doi.org/10.3390/app152212220











