Abstract
Post-occlusive reactive hyperemia (PORH) is widely used to assess microvascular reactivity, but its systemic impact on contralateral neurovascular function remains unclear. This study quantified bilateral synchrony and asymmetry of cutaneous signals during unilateral PORH in healthy subjects using a novel multidimensional framework of inter-limb coherence. Twelve young adults underwent a standard suprasystolic occlusion (5 min at 200 mmHg) on the upper limb, while photoplethysmography (PPG), skin temperature, and electrodermal activity (EDA) were recorded bilaterally in the fingers. Coherence was characterized by profile similarity (Cross-Signal Similarity Index, CSSI), temporal lag (τ*), magnitude asymmetry (Bilateral Magnitude Difference Index, BDMI), directional concordance (Signal Direction Index, SDI; Directional Concordance Index, DCI), and integrated indices (IBIL, IBIS). At baseline, all signals showed high bilateral synchrony (CSSI ≈ 0.9; τ* < 20 ms). Occlusion markedly reduced CSSI for blood flow (0.89 to 0.07, p = 0.002) and temperature (0.93 to −0.03, p = 0.06), while EDA coherence remained preserved (0.95 to 0.82). Integrated indices decreased significantly (IBIL 0.84 to 0.17, p = 0.005; IBIS 0.84 to 0.18, p = 0.004) and recovered only partially during hyperemia (IBIL 0.20, p = 0.003). Directional concordance was heterogeneous: during hyperemia, 9 of 12 subjects showed concordant EDA changes but only 7 of 12 for perfusion. BDMI was largest for perfusion (≈0.8), moderate for temperature (≈0.5), and minimal for EDA (≈0.3). Unilateral PORH thus induces a marked loss of bilateral coherence in microvascular signals, whereas sympathetic-driven responses remain strongly synchronized. This dissociation reveals that occlusion evokes systemic autonomic adjustments beyond local hemodynamics. The proposed framework captures hidden aspects of neurovascular integration and may provide new markers for autonomic imbalance or perfusion asymmetry.
1. Introduction
Regulation of cardiovascular function is complex and depends on the interplay of feedback and feedforward systems that coordinate the activity of multiple tissues and organs to maintain homeostasis [1,2]. Organ perfusion relies directly on cardiac and vascular function, which in turn are modulated by neural and endocrine outputs. Although several autonomic and sensory neural pathways have been identified as determinants of vascular tone, the specific circuits involved and their interaction with higher neural centers remain poorly understood [3,4]. An emerging concept from recent human and animal studies is that acute perturbations in one limb, whether upper or lower, can induce measurable changes in the contralateral limb. Unilateral stimuli such as suprasystolic occlusion [5], limb dependency [6,7], and superficial massage [8,9] have all been shown to elicit contralateral perfusion changes. These observations suggest that the vascular system is not regulated solely at a local level but can be influenced by systemic neural integration, possibly involving both spinal and supraspinal mechanisms. Despite consistent descriptions of such contralateral effects, their physiological significance and clinical relevance remain unclear, partly because quantitative methods to characterize bilateral coherence are lacking.
Reactive hyperemia offers a robust experimental platform to investigate this phenomenon. Traditionally, reactive hyperemia has been used to assess the ability of the endothelium to release vasodilators, which can be elicited by transient suprasystolic arterial occlusion [10]. Depending on the technique, reactive hyperemia can be assessed in conduit arteries by ultrasonography, also termed flow-mediated dilation (FMD) [11], or in the skin, subcutaneous tissue, or skeletal muscle, termed post-occlusive reactive hyperemia (PORH). While FMD is standardized for clinical research, PORH is limited by reduced reproducibility, influenced by spatial heterogeneity, technical variability, and differences in occlusion time [10,12]. Nevertheless, blunted PORH or FMD responses have been consistently associated with cardiovascular and metabolic risk and disease, supporting their role as markers of endothelial dysfunction [13,14,15,16].
The physiological mechanisms underlying PORH are multifactorial, encompassing endothelial release of nitric oxide and prostaglandins, accumulation of metabolic vasodilators (adenosine, carbon dioxide, lactate, hydrogen ions), and release of vasodilators from afferent nerve terminals [17,18,19,20,21,22,23]. This physiological richness makes PORH not only a tool to assess local vascular reactivity but also an opportunity to explore new avenues regarding the systemic neurovascular integration. We hypothesize that contralateral responses elicited during PORH may provide a window into systemic neurovascular control. By simultaneously recording signals of different biophysical origin, cutaneous perfusion, skin temperature and perspiration, it becomes possible to quantify both synchrony and asymmetry between limbs. However, no quantitative framework has yet been proposed to assess bilateral responses in such a multidimensional way. Addressing this gap requires tools capable of integrating heterogeneous signals into indices of bilateral coherence.
The present study aimed to quantify bilateral synchrony and asymmetry of cutaneous physiological signals during PORH in healthy subjects, and to introduce composite indices that integrate different aspects of inter-limb coherence. This exploratory approach proposes a novel multidimensional framework to capture hidden aspects of systemic neurovascular integration beyond local vascular reactivity.
2. Materials and Methods
2.1. Subjects
A convenience sample of twelve young and healthy subjects (21.6 ± 1.9 years old; 6 females) was recruited among the faculty students and gave informed written consent prior to any experimental procedures (Table 1). Male and female subjects had comparable age, BMI, and blood pressure. Nine subjects were right-handed, and three were left-handed. At the time of their participation students had not been diagnosed with any psychiatric, neurological and/or cardiovascular diseases. Subjects were non-smokers and did not take any regular medications and/or supplements. Female subjects had regular menstrual cycles, were not taking any contraceptives, and were in the first week of their menstrual cycle. Subjects were asked not to consume caffeinated beverages and not to perform physical activity in the 12 h before the experiment. Subjects were asked to fast for a minimum of 2 h, and to avoid taking a heavy meal before the experiments to minimize any potential effects on sympathetic drive and endothelial activity [24,25,26]. Finally, subjects were asked to empty their bladders before the experiments. The study was approved by the local ethics committee (no. 11/2024) and followed the recommendations of the Declaration of Helsinki and subsequent amendments for studies conducted in human subjects [27].

Table 1.
Characteristics of subjects (means ± standard deviations).
2.2. Procedure
Subjects performed a standard suprasystolic arterial occlusion procedure on a randomly chosen arm. The occlusion protocol in resting conditions lasted 25 min and was divided in three phases—baseline (minutes 1 to 10), occlusion (minutes 11 to 15) and hyperemia (minutes 16 to 25). The occlusion was carried out by rapidly inflating (~5 s) a blood pressure cuff on the arm to 200 mmHg and holding it for 5 min, after which it was rapidly deflated (~5 s). All experiments were conducted in a room with controlled temperature and humidity conditions (22–24 °C, 40–65%). Subjects acclimatized to the room conditions for 20 min before initiating procedures.
2.3. Technologies
Several biosignals were acquired noninvasively with sensors placed on the fingers of both hands. All sensors were connected to a BITalino Revolution Plugged board (PLUX Biosignals, Lisbon, Portugal). On the distal phalanx of the second fingers of both hands, reflection photoplethysmography (PPG) sensors were used (530 nm wavelength) to measure blood volume pulse. Skin blood flow was estimated as the amplitude of the PPG waveform (i.e., value at the systolic peak of a given pulse wave minus the value at the onset point of the following pulse wave) and was expressed in arbitrary units (AU). Pulse (min−1) was calculated as the number of PPG pulse waves per minute from the PPG signal of the hand contralateral to the cuff side. On the middle phalanx of the same fingers negative temperature coefficient thermistors were placed to measure skin temperature, expressed in degrees Celsius. On the distal phalanx of the third and fourth fingers a pair of sensors were placed to record the skin resistance signal. Electrodermal activity (EDA) was defined as the average value of the tonic component of the skin resistance signal and was expressed in microSiemens (µS). The temperature and EDA values were initially acquired by analog-to-digital converters and converted to their respective units according to the manufacturer’s specifications. All signals were acquired at a 100 Hz sampling rate directly to the OpenSignals (r)evolution (https://support.pluxbiosignals.com/knowledge-base/introducing-opensignals-revolution/, accessed on 11 November 2025) (PLUX Biosignals, Lisbon, Portugal) dedicated software, before being uploaded to Matlab 2015a (Mathworks, Natick, MA, USA) for further analysis.
2.4. Signal Analysis
To characterize inter-limb similarity, we derived several novel parameters from paired raw signals of PPG, skin temperature, and EDA (Table 2). No preprocessing was applied, in order to preserve the full physiological content of the recordings.

Table 2.
Description of the parameters calculated in the study.
2.4.1. Cross-Signal Similarity Index (CSSI)
For each modality (i.e., signal) m and protocol phase P (baseline, occlusion, hyperemia), the normalized cross-correlation between test (T) and contralateral (C) signals was computed within a lag window (∣l∣ ≤ Ll):
where Tm[n] and Cm[n] are the signals from the test and contralateral arms, respectively, and are their means over the interval [n1, n2], l is the lag (in samples), n is the discrete sample index, and [n1, n2] define the temporal window (start and end of the analyzed segment). Correlations were computed within a physiologically plausible lag window (∣l∣ ≤ Ll), corresponding to ±2 s at the 100 Hz sampling rate. The variable l represents the lag in number of samples (e.g., l = 100 corresponds to a 1 s delay of the contralateral signal). This ±2 s range was chosen to encompass physiologically plausible bilateral delays, as vasomotor and sensory-axon reflex responses in the skin occur within seconds of stimulus onset [28,29]. Given the near-synchronous sympathetic activation of homologous regions [30], larger lags would likely reflect slow systemic drifts rather than genuine bilateral coupling.
The CSSI was defined as the maximum correlation within this window:
If either signal was constant (variance = 0), CSSI was set to 0.
2.4.2. Lag at Maximum Correlation (τ*)
The lag corresponding to the CSSI maximum was recorded as l*, converted to milliseconds as:
where fs is the sampling rate. By convention, a negative τ* indicates that the response of the test arm anticipates the contralateral response, whereas a positive τ* indicates the opposite.
2.4.3. Signal Direction Index (SDI)
To assess directional concordance, we calculated the sign of the mean changes (Δ) from baseline for each modality m in each phase P:
where μ denotes the mean signal amplitude. The SDI was then defined as:
With
Thus, SDI takes values in {−1, 0, +1}, representing opposite, null, or concordant directional changes between arms. Thus, SDI = +1 if both responses occur in the same direction, −1 if they change in opposite directions, and 0 if no change occurred.
2.4.4. Integrated Bilateral Index of Similarity (IBIL)
To summarize bilateral similarity across all three modalities, CSSI values for PPG, temperature, and EDA were averaged within each phase:
2.4.5. Bilateral Magnitude Difference Index (BMDI)
To quantify inter-limb asymmetry in response magnitude, the mean signal in each phase was computed, and the change from baseline (Δ) determined. The BMDI for modality m in phase P was then defined as:
with ε = 10−6 to avoid division by zero. BMDI ranges from 0 (symmetric) to 1 (maximal asymmetry). By definition, baseline deltas () are zero. Therefore, BMDI was computed only for occlusion and hyperemia.
2.4.6. Integrated Bilateral Index with Sign (IBIS)
To provide a more stringent measure, IBIS integrates CSSI values across modalities but only when SDI = +1 (directional concordance):
where M+ is the set of modalities with SDI = +1 in phase P, and N+ its cardinality.
2.4.7. Directional Concordance Index (DCI)
Finally, multimodal directionality was quantified using the cosine similarity between inter-arm delta vectors:
DCI takes values in [−1, +1] where −1 denotes perfect opposite multimodal directionality and +1 denotes perfect alignment across modalities.
2.5. Statistical Analysis
For statistical analysis, three time intervals characterized by signal stability and minimal motion artefacts were selected: 7–10 min (baseline), 11–14 min (occlusion), and 15–18 min (hyperemia). For each interval, the median and 95% confidence intervals were calculated for all measured variables and for the novel parameters. For the measured variables, the variation rate between occlusion and baseline (ΔII-I) and between hyperemia and baseline (ΔIII-I) were expressed as a percentage and calculated as:
All statistical analyses were performed using IBM SPSS Statistics version 21.0 (IBM Corp., Armonk, NY, USA). The distribution of each variable was visually inspected using histograms and Q–Q plots and formally tested with the Shapiro–Wilk test. Because most variables did not follow a normal distribution, non-parametric tests were used throughout. Within-subject comparisons between protocol phases (baseline, occlusion, hyperemia) and between-limb comparisons (test vs. contralateral) were conducted using the Wilcoxon test for paired samples. All tests were two-tailed, and statistical significance was set at α = 0.05. Given the exploratory and proof-of-concept nature of the study and the small sample size, no correction for multiple comparisons was applied. Results are expressed as medians with 95% confidence intervals, unless otherwise stated.
3. Results
Table 3 presents the medians and 95% confidence intervals for the main PORH parameters, and Figure 1 shows a representative recording. At baseline, no significant differences were observed between limbs in skin blood flow, temperature, or EDA, confirming equivalent starting conditions. As expected, occlusion caused a marked reduction in blood flow in the test hand (−96.4%, p = 0.003). In the contralateral hand, the decrease was smaller in amplitude but still significant (−8.0%, p = 0.006). During hyperemia, blood flow rose markedly in both limbs. In the test hand, hyperemic flow exceeded the occlusion level by a large margin, showing a clear reactive overshoot. Skin temperature did not change significantly during occlusion, although a downward trend was observed; during hyperemia, however, values were significantly lower than baseline in both limbs (p = 0.005 for each). EDA increased significantly in both limbs during occlusion (test: +32.2%, p = 0.003; contralateral: +29.9%, p = 0.005) and remained elevated above baseline during hyperemia (T: p = 0.013; C: p = 0.026). Finally, pulse showed no significant changes across phases.

Table 3.
Medians and the limits of the 95% confidence intervals of the variables of the procedure during the baseline, occlusion and reactive hyperemia phases (N = 12). Statistical comparison is presented (Wilcoxon-signed rank test for paired samples, * p < 0.05).
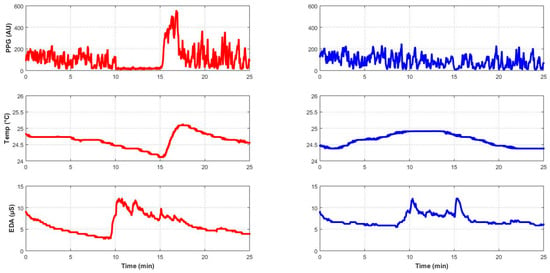
Figure 1.
Profile of skin blood flow (PPG), skin temperature (Temp) and electrodermal activity (EDA) of a representative subject (subject #2, male, 21 years old), with colors denoting limb assignment (red for the test limb and blue for the control limb).
Table 4 and Table 5 summarize the synchronicity parameters and their 95% confidence intervals. At baseline, all signals showed very high CSSI values and negligible temporal lag (τ* ≈ 0 ms for skin temperature and EDA, 15 ms for skin blood flow), indicating strong bilateral synchrony under stable perfusion conditions. These near-zero τ* values should not be interpreted as physiological response latencies, but rather as the relative inter-limb offset between homologous signals recorded simultaneously. Because temperature and electrodermal activity vary slowly, their bilateral waveforms evolve quasi-monotonically, yielding maximal correlation at zero lag.

Table 4.
Medians and 95% confidence intervals of the synchrony parameters newly defined for this study (N = 12). Statistical comparison used the Wilcoxon-signed rank test for paired samples (*—p < 0.05). CSSI = Cross-Signal Similarity Index; τ* = lag at maximum correlation (ms); BDMI = Bilateral Magnitude Difference Index; SDI = Signal Direction Index, denoting the absolute number of subjects showing concordant (+1), null (0), or opposite (−1) bilateral changes relative to baseline.

Table 5.
Medians and the limits of the 95% confidence intervals of the IBIL, DCI and IBIS parameters newly defined for this study (N = 12). Statistical comparison is presented (Wilcoxon test for paired and independent samples, *—p < 0.05). IBIL = Integrated Bilateral Index; DCI = Directional Concordance Index; IBIS = Integrated Bilateral Index with Sign.
In this context, τ* ≈ 0 ms simply indicates that no measurable inter-limb delay was detected within the ±2 s search window, a range chosen to encompass plausible vasomotor or sensitive-axon reflex delays in skin blood flow. Moreover, given the 100 Hz sampling rate (10 ms period, ~5 ms timing uncertainty), such values lie within the temporal resolution of the method. Occlusion caused a significant decrease in CSSI for skin blood flow (p = 0.002), reflecting a loss of similarity, whereas the decrease observed for skin temperature and EDA were not statistically significant. During hyperemia, CSSI remained significantly lower than baseline for both blood flow (p = 0.002) and temperature (p = 0.002). For EDA, CSSI decreased progressively but without significant differences across phases. No significant changes in τ* were detected, indicating temporal synchronization within the ±2 s interval. DCI did not change significantly between occlusion and hyperemia. DCI values remained within the same range across phases, indicating no detectable directional dominance. Regarding magnitude asymmetry, BDMI was highest for blood flow, consistent with the marked reduction in the test hand compared with the contralateral hand, moderate for temperature, and small for EDA. BDMI did not change significantly between phases, suggesting that the relative amplitude of responses remained globally equivalent across transitions. IBIL was high at baseline, again confirming global bilateral similarity, but decreased significantly during occlusion (p = 0.005), largely driven by asymmetric perfusion. The reduction persisted during hyperemia compared with baseline (p = 0.003), indicating no full recovery of initial bilateral coherence. Global directionality was more consistent during occlusion than during hyperemia, which elicited a less coherent response. IBIS, which integrates similarity and directionality, also decreased significantly during occlusion (p = 0.004) and remained below baseline during hyperemia (p = 0.002). Finally, SDI indicated that no subject showed null responses (SDI = 0) for blood flow or temperature; most subjects exhibited concordant bilateral changes (SDI = +1), whereas a minority showed opposite responses (SDI = −1).
4. Discussion
Reactive hyperemia is a classic procedure to assess endothelial reactivity in large arteries and in microcirculation alike. This response has been extensively studied in both the upper and lower limbs [5,31] with different quantification techniques [5,32,33,34,35]. However, variability in technique and protocol contributes to poor reproducibility and hinders clinical translation. One important source of variability is occlusion time, which has been reported from 30 s to 10 min [36], with our present results closely matching those from our previous work using 3 min [5]. Occlusion above systolic pressure abolishes arterial inflow and venous return, markedly reducing skin blood flow. Because blood carries heat, skin temperature also decreased. In contrast, the contralateral neurovascular response to PORH remains underexplored. Our results showed that occlusion significantly decreases contralateral skin blood flow, which is in line with our previous observations [5] and that it significantly increases EDA bilaterally, indicating a systemic sympathetic activation triggered by the ischemic and/or pain stimuli. Suprasystolic occlusion produces a transient accumulation of metabolites and local hypoxia, which activate afferent nerve fibers. These afferents elicit reflex sympathetic outflow via medullary centers, increasing sudomotor activity and vascular tone even in contralateral regions. This supports the concept of a neurogenic, rather than purely local, component to the PORH response.
In order to better characterize the bilateral/systemic nature of the PORH protocol, we propose several novel parameters that quantitatively assess the shape symmetry and temporal synchronization between signals from different limbs, the directionality and magnitude of the physiological responses to occlusion, and parameters to assess the integrated (i.e., collective) responses. The magnitude of these parameters is sufficiently informative to appreciate the underlying physiological conditioning, as evidenced by Figure 2. At baseline, skin blood flow, skin temperature, and EDA all showed high bilateral similarity (CSSI) and temporal synchrony (τ*). The appropriateness of the ±2 s lag window was confirmed empirically: the median lag (τ*) at which the maximum cross-correlation occurred was < 2 s across all modalities and phases, and 95% of all values fell within ± 1.8 s. Only isolated cases reached the window limits, supporting that the ±2 s range adequately encompassed physiologically relevant bilateral delays without truncating correlation peaks. Occlusion, however, differentially affected these signals. Skin blood flow displayed a marked drop in bilateral similarity, with partial re-attainment of baseline values during the hyperemia phase. Despite this partial hyperemia, BMDI revealed persistent magnitude asymmetries, reflecting the hyperemic overshoot limited to the occluded arm. Directionally, perfusion decreased bilaterally in 9 out of 12 subjects during occlusion and in 7 out of 12 during hyperemia. Considering that occlusion decreased perfusion in all test limbs, this result highlights the heterogeneity of contralateral responses. Skin temperature showed a different pattern. Although SDI indicated that both arms generally changed in the same direction (9 of 12 subjects), CSSI assumed negative values during occlusion, reflecting asynchronous dynamics. This discrepancy illustrates the slow, dampened kinetics of thermal responses: coherent in direction but divergent in temporal pattern. EDA behaved more consistently. CSSI and SDI remained high across phases, supporting bilateral and synchronous sympathetic sudomotor activity. Nevertheless, a significant CSSI reduction occurred during occlusion, which may reflect distal skin changes under reduced perfusion, such as cooling or altered epidermal resistance, although a direct mechanical effect of occlusion on the median nerve cannot be excluded. Magnitude asymmetries were small (BDMI ~0.3), consistent with a strong systemic drive to sudomotor activity even under asymmetric vascular manipulation. Directionality was highly consistent, with 11/12 of subjects showing bilateral increases during occlusion and 10/12 during hyperemia, both of which are consistent with an acute stress response.
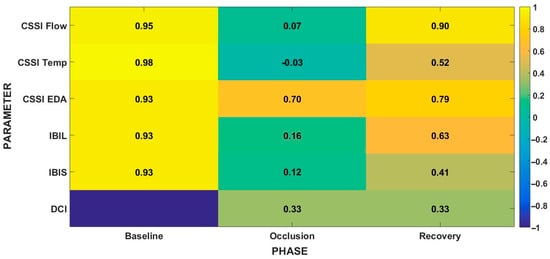
Figure 2.
Heatmap of indices across the phases of the PORH protocol (Baseline, Occlusion, Hyperemia). Cells show median values across subjects. CSSI = Cross-Signal Similarity Index; IBIL = Integrated Bilateral Index; IBIS = Integrated Bilateral Index with Sign; DCI = Directional Concordance Index. Color scale for CSSI/IBIL/IBIS ranges from −1 (low/negative similarity) to +1 (high similarity). DCI ranges from −1 to +1; DCI = 0 indicates no net directional change, DCI > 0 indicates aligned directions across modalities, DCI < 0 indicates opposite directions.
Taken together, these findings indicate that microvascular (perfusion and temperature) and autonomic (EDA) signals respond differently to occlusion and to hyperemia, again reinforcing the continuous physiological interest of studying this vascular adaptation to short-term ischemia. They also highlight the strong nature of occlusion, such that hyperemia was not fully attained in the present experimental conditions, as clearly shown in Figure 3. Each parameter captured a distinct aspect of the bilateral response, but their integration provided a more comprehensive view. CSSI reflected profile similarity, SDI directional alignment, BDMI magnitude asymmetries, and IBIL/IBIS integrated across modalities. Together, these indices revealed that global multimodal coherence collapsed during occlusion and recovered only partially afterwards. The DCI further confirmed that only a subset of signals aligned directionally, underscoring heterogeneous bilateral regulation under vascular challenge. Our findings highlight the dual contribution of local hemodynamics and systemic autonomic drive. This interpretation stems from the observation that suprasystolic occlusion, although applied unilaterally, produced bilateral changes in skin blood flow and electrodermal activity. Such contralateral effects cannot be accounted for by purely local mechanisms and are best explained by a reflex sympathetic activation triggered by ischemia, pain and venous pooling. The accumulation of metabolites and stimulation of group III–IV afferents during occlusion are known to elicit medullary sympathetic outflow, which modulates both vascular tone and sudomotor activity in distant regions. This supports the notion that local vascular challenges engage systemic autonomic feedback loops to preserve hemodynamic stability. In addition, our approach quantitatively demonstrates the systemic impact of occlusion. The neurovascular response here observed was mostly “consensual” (i.e., same direction), suggesting a centrally mediated sympathetic adjustment to changes in perfusion conditions, likely a compensatory response to prevent a fall in arterial pressure secondary to venous pooling and reduced effective circulating volume. However, the small number of discordant observations raise the need to better explore these atypical findings.
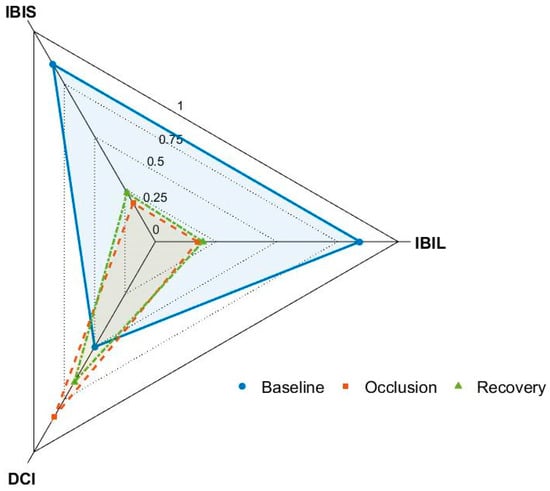
Figure 3.
Radar plot of three integrated indices (IBIL, DCI, and IBIS) displaying the median values for all phases of the protocol. IBIL = Integrated Bilateral Index; DCI = Directional Concordance Index; IBIS = Integrated Bilateral Index with Sign. DCI quantifies multimodal directional alignment (−1 to +1) and is represented here on a 0–1 radial scale using the transform (1 + DCI)/2. Accordingly, a value of 0.5 corresponds to DCI = 0 (no net directional change), values closer to 1 indicate stronger directional alignment, and values closer to 0 indicate opposite directions.
This study should be regarded as a proof-of-concept conducted in a small cohort of healthy young subjects, warranting validation in larger and patient populations. Few studies have examined the synchronicity of skin blood flow [37], skin temperature [38] and EDA [39]. However, only a limited number have proposed quantitative parameters to characterize such synchronicity, and, to the authors’ knowledge, no previous work has attempted to develop integrated indices combining bilateral perfusion, temperature, and EDA signals. By combining CSSI (profile similarity), τ* (temporal synchrony), BDMI (magnitude asymmetry), and SDI (directional alignment), together with composite indices such as IBIL and IBIS, we propose a multidimensional framework of bilateral coherence. This framework opens new avenues for probing neurovascular crosstalk and centrally mediated reflex pathways in human physiology. It also broadens the physiological interpretation of PORH, offering a non-invasive framework to probe systemic neurovascular integration. Beyond research applications, it may serve as a screening tool for conditions characterized by autonomic imbalance or perfusion asymmetry. For instance, EDA coherence might be reduced in diabetic neuropathy due to impaired autonomic conduction, BDMI may capture the perfusion asymmetries typical of peripheral artery disease, and DCI could decline in dysautonomia, where central reflex integration is disrupted. Overall, this multidimensional framework uncovered hidden aspects of bilateral coherence during PORH, revealing both signal-specific and integrated responses. While exploratory, it provides a foundation for translational research and could help identify subtle bilateral perfusion asymmetries in neuropathies, vascular diseases, and autonomic dysfunction.
5. Conclusions
This study highlights the often-overlooked contralateral component of the PORH response. We introduce new indices of inter-limb synchronization that proved sensitive to the hemodynamic and neural changes imposed by arterial occlusion. Occlusion markedly disrupted the bilateral coherence of perfusion and temperature signals, with EDA being less affected. Notably, considerable asymmetries persisted in the hyperemic phase, underscoring the need for further investigation of its underlying mechanisms. These indices captured physiological phenomena not revealed by traditional parameters. While this work represents a proof-of-concept in young healthy subjects, the proposed framework shows promise as a research tool and as a potential marker for translational studies in conditions characterized by autonomic or perfusion asymmetry. Importantly, the assessment of bilateral synchrony and asymmetry may have clinical relevance for the early detection of microvascular dysfunction, longitudinal monitoring of endothelial health, and stratification of cardiometabolic risk in populations prone to vascular impairment.
6. Limitations
This study has several limitations. First, it was conducted as proof-of-concept in a small, controlled cohort of healthy young subjects, which limits the generalizability of the findings. Second, despite careful control of procedural conditions (fasting duration, menstrual cycle phase, bladder emptying, sensor placement), residual factors may have contributed to response heterogeneity. One such factor was handedness, which was markedly unbalanced and not controlled in this sample. Given the predominance of right-handed subjects, lateralized sympathetic outflow may have contributed to subtle baseline asymmetries. Future studies should include balanced recruitment or subgroup analyses to control for this potential bias. Third, blood pressure was not measured continuously, precluding precise control of hemodynamic variables during the procedure. Finally, no preprocessing (e.g., filtering, detrending, normalization) was applied to the raw signals in order to preserve their full physiological richness. While this choice allowed the capture of unaltered responses, it may also have increased susceptibility to noise, particularly affecting CSSI, which is highly sensitive to signal variability and motion artifacts and may therefore underestimate true inter-limb similarity. Future sensitivity analyses will evaluate how different preprocessing approaches (e.g., band-pass filtering and drift correction) influence the robustness and reproducibility of the proposed indices.
Author Contributions
Conceptualization, H.S.; methodology, H.S.; software, H.S.; validation, H.S. and H.A.F.; formal analysis, H.S.; investigation, H.S. and N.L.; resources, H.S.; data curation, H.S.; writing—original draft preparation, H.S.; writing—review and editing, H.S. and H.A.F.; visualization, H.S.; supervision, H.S.; project administration, H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Faculty of Pharmacy of the University of Lisbon (11/2024; 11 October 2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The Research Institute for Medicines (iMed.ULisboa) acknowledges the financial support of Fundação para a Ciência e Tecnologia (FCT), I.P., through the R&D unit UID/04138/2025 (https://doi.org/10.54499/UID/04138/2025, accessed on 11 November 2025). The authors would like to thank all the volunteers who participated in this study. During the preparation of this manuscript, the authors used ChatGPT-5 (OpenAI, San Francisco, CA, USA) for text proofing and language refinement. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Manuel, J.; Färber, N.; Gerlach, D.A.; Heusser, K.; Jordan, J.; Tank, J.; Beissner, F. Deciphering the neural signature of human cardiovascular regulation. eLife 2020, 9, e55316. [Google Scholar] [CrossRef] [PubMed]
- Gordan, R.; Gwathmey, J.K.; Xie, L.H. Autonomic and endocrine control of cardiovascular function. World J. Cardiol. 2015, 7, 204. [Google Scholar] [CrossRef]
- Furlan, J.C.; Fehlings, M.G.; Shannon, P.; Norenberg, M.D.; Krassioukov, A.V. Descending vasomotor pathways in humans: Correlation between axonal preservation and cardiovascular dysfunction after spinal cord injury. J. Neurotrauma 2003, 20, 1351–1363. [Google Scholar] [CrossRef]
- Partida, E.; Mironets, E.; Hou, S.; Tom, V.J. Cardiovascular dysfunction following spinal cord injury. Neural Regen. Res. 2016, 11, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.; Rezendes, C.; Pinto, P.C. Enhancing the quantification of post-occlusive reactive hyperemia: A multimodal optical approach. Pflügers Arch.-Eur. J. Physiol. 2025, 477, 1213–1224. [Google Scholar] [CrossRef]
- Silva, H.; Ferreira, H.A.; da Silva, H.P.; Monteiro Rodrigues, L. The venoarteriolar reflex significantly reduces contralateral perfusion as part of the lower limb circulatory homeostasis in vivo. Front. Physiol. 2018, 9, 1123. [Google Scholar] [CrossRef]
- Silva, H.; Rezendes, C. Revisiting the venoarteriolar reflex—Further insights from upper limb dependency in healthy subjects. Biology 2024, 13, 715. [Google Scholar] [CrossRef]
- Monteiro Rodrigues, L.; Rocha, C.; Andrade, S.; Granja, T.; Gregório, J. The acute adaptation of skin microcirculatory perfusion in vivo does not involve a local response but rather a centrally mediated adaptive reflex. Front. Physiol. 2023, 14, 1177583. [Google Scholar] [CrossRef]
- Rodrigues, L.M.; Rocha, C.; Ferreira, H.; Silva, H. Different lasers reveal different skin microcirculatory flowmotion—Data from the wavelet transform analysis of human hindlimb perfusion. Sci. Rep. 2019, 9, 16951. [Google Scholar] [CrossRef] [PubMed]
- Rosenberry, R.; Nelson, M.D. Reactive hyperemia: A review of methods, mechanisms, and considerations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R605–R618. [Google Scholar] [CrossRef]
- Thijssen, D.H.J.; Black, M.A.; Pyke, K.E.; Padilla, J.; Atkinson, G.; Harris, R.A.; Parker, B.; Widlansky, M.E.; Tschakovsky, M.E.; Green, D.J. Assessment of flow-mediated dilation in humans: A methodological and physiological guideline. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H2–H12. [Google Scholar] [CrossRef]
- Coccarelli, A.; Nelson, M.D. Modeling reactive hyperemia to better understand and assess microvascular function: A review of techniques. Ann. Biomed. Eng. 2023, 51, 479–492. [Google Scholar] [CrossRef] [PubMed]
- López-Galán, E.; Montoya-Pedrón, A.; Barrio-Deler, R.; Sánchez-Hechavarría, M.E.; Muñoz-Bustos, M.E.; Muñoz-Bustos, G.A. Reactive hyperemia and cardiovascular autonomic neuropathy in type 2 diabetic patients: A systematic review of randomized and nonrandomized clinical trials. Medicina 2023, 59, 770. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.L.; Silver, A.E.; Shvenke, E.; Schopfer, D.W.; Jahangir, E.; Titas, M.A.; Shpilman, A.; Menzoian, J.O.; Watkins, M.T.; Raffetto, J.D.; et al. Predictive value of reactive hyperemia for cardiovascular events in patients with peripheral arterial disease undergoing vascular surgery. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2113–2119. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Ishigami, Y.; Otaki, Y.; Izumi, M.; Hiraoka, K.; Inoue, T.; Takamitsu, Y. Impairment of vascular responses to reactive hyperemia and nitric oxide in chronic renal failure. Nephron 2002, 92, 529–535. [Google Scholar] [CrossRef]
- Merino, J.; Megias-Rangil, I.; Ferré, R.; Plana, N.; Girona, J.; Rabasa, A.; Aragonés, G.; Cabré, A.; Bonada, A.; Heras, M.; et al. Body weight loss by very-low-calorie diet program improves small artery reactive hyperemia in severely obese patients. Obes. Surg. 2013, 23, 17–23. [Google Scholar] [CrossRef]
- Tran, N.; Garcia, T.; Aniqa, M.; Ali, S.; Ally, A.; Nauli, S.M. Endothelial nitric oxide synthase (eNOS) and the cardiovascular system: In physiology and in disease states. Am. J. Biomed. Sci. Res. 2022, 15, 153–177. [Google Scholar] [CrossRef]
- Tóth, A.; Pal, M.; Intaglietta, M.; Johnson, P.C. Contribution of anaerobic metabolism to reactive hyperemia in skeletal muscle. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H2643–H2653. [Google Scholar] [CrossRef][Green Version]
- Davis, M.J. Perspective: Physiological role(s) of the vascular myogenic response. Microcirculation 2012, 19, 99–114. [Google Scholar] [CrossRef]
- Lorenzo, S.; Minson, C.T. Human cutaneous reactive hyperaemia: Role of BKCa channels and sensory nerves. J. Physiol. 2007, 585, 295–303. [Google Scholar] [CrossRef]
- McGarr, G.W.; Cheung, S.S. Effects of sensory nerve blockade on cutaneous microvascular responses to ischemia-reperfusion injury. Microvasc. Res. 2022, 144, 104091. [Google Scholar] [CrossRef]
- Tagawa, T.; Imaizumi, T.; Endo, T.; Shiramoto, M.; Harasawa, Y.; Takeshita, A. Role of nitric oxide in reactive hyperemia in human forearm vessels. Circ. Res. 1994, 74, 376–382. [Google Scholar] [CrossRef]
- Roddie, I. Circulation to skin and adipose tissue. In Handbook of Physiology: The Cardiovascular System; American Physiological Society: Bethesda, MD, USA, 1983; pp. 285–317. [Google Scholar]
- Silva, H.; Šorli, J.; Lenasi, H. Oral glucose load and human cutaneous microcirculation: An insight into flowmotion assessed by wavelet transform. Biology 2021, 10, 953. [Google Scholar] [CrossRef]
- Muntzel, M.S.; Anderson, E.A.; Johnson, A.K.; Mark, A.L. Mechanisms of insulin action on sympathetic nerve activity. Clin. Exp. Hypertens. 1995, 17, 39–50. [Google Scholar] [CrossRef]
- Rajapakse, N.W.; Chong, A.L.; Zhang, W.Z.; Kaye, D.M. Insulin-mediated activation of the L-arginine nitric oxide pathway in man, and its impairment in diabetes. PLoS ONE 2013, 8, e61840. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.M.; Kellogg, D.L. Mechanisms and modulators of temperature regulation: Local thermal control of the human cutaneous circulation. J. Appl. Physiol. 2010, 109, 1229–1238. [Google Scholar] [CrossRef]
- Minson, C.T.; Berry, L.T.; Joyner, M.J. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J. Appl. Physiol. 2001, 91, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Krupatkin, A.I. The influence of sympathetic innervation on the skin microvascular tone and blood flow oscillations. Hum. Physiol. 2006, 32, 584–592. [Google Scholar] [CrossRef]
- Silva, H.; Ferreira, H.; Bujan, M.J.; Rodrigues, L.M. Regarding the quantification of peripheral microcirculation—Comparing responses evoked in vivo by postural changes, suprasystolic occlusion and oxygen breathing. Microvasc. Res. 2015, 99, 110–117. [Google Scholar] [CrossRef]
- Kishimoto, S.; Matsumoto, T.; Maruhashi, T.; Iwamoto, Y.; Kajikawa, M.; Oda, N.; Matsui, S.; Hashimoto, H.; Hidaka, T.; Kihara, Y.; et al. Reactive hyperemia–peripheral arterial tonometry is useful for assessment of not only endothelial function but also stenosis of the digital artery. Int. J. Cardiol. 2018, 260, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Hendrick, E.; Jamieson, A.; Chiesa, S.T.; Hughes, A.D.; Jones, S. A short review of application of near-infrared spectroscopy (NIRS) for the assessment of microvascular post-occlusive reactive hyperaemia (PORH) in skeletal muscle. Front. Physiol. 2024, 15, 1480720. [Google Scholar] [CrossRef]
- De Mul, F.F.M.; Morales, F.; Smit, A.J.; Graaff, R. A model for post-occlusive reactive hyperemia as measured with laser-Doppler perfusion monitoring. IEEE Trans. Biomed. Eng. 2005, 52, 184–190. [Google Scholar] [CrossRef]
- Roustit, M.; Blaise, S.; Millet, C.; Cracowski, J.L. Reproducibility and methodological issues of skin post-occlusive and thermal hyperemia assessed by single-point laser Doppler flowmetry. Microvasc. Res. 2010, 79, 102–108. [Google Scholar] [CrossRef]
- Jasperse, J.L.; Shoemaker, J.K.; Gray, E.J.; Clifford, P.S. Positional differences in reactive hyperemia provide insight into the initial phase of exercise hyperemia. J. Appl. Physiol. 2015, 119, 569–575. [Google Scholar] [CrossRef]
- Tankanag, A.V.; Grinevich, A.A.; Tikhonova, I.V.; Chaplygina, A.V.; Chemeris, N.K. Phase synchronization of skin blood flow oscillations in humans under asymmetric local heating. Biophysics 2017, 62, 629–635. [Google Scholar] [CrossRef]
- Crivelli, D.; Polimeni, E.; Crotti, D.; Bottini, G.; Salvato, G. Bilateral skin temperature drop and warm sensibility decrease following modulation of body part ownership through mirror-box illusion. Cortex 2021, 135, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.V.T.; Toska, K.; Wesche, J. Rapid, large, and synchronous sweat and cardiovascular responses upon minor stimuli in healthy subjects: Dynamics and reproducibility. Front. Neurol. 2020, 11, 51. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).