Physical Inactivity and Sedentary Behavior Negatively Impact Postural Balance and Gait
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Outcome Measures and Assessment Procedure
- Physical activity and sedentary behavior: The International Physical Activity Questionnaire (IPAQ) [13], a 27-item self-reported questionnaire, was used to assess participants’ physical activity levels pertaining to time spent on work, transportation, home activities, recreation, sport, leisure, and sitting in a typical week.
- Body motion (using inertial measurement units): The APDM monitoring inertial sensor system (APDM Inc., Portland, OR, USA) [17,18,19], a wireless wearable device for comprehensive analysis of balance and gait, was used to assess body motions. The device’s sensors were attached to seven anatomical sites: forehead (middle of the frontal bone, approximately 2.5 cm above the nasal bone), sternum (body of sternum superior to the xyphoid process), fifth lumbar vertebra, left and right wrists (immediately superior to the radio-ulnar joint), and left and right first feet (on the metatarsals, directly superior to the metatarsophalangeal joint). These sensors were used to collect and analyze balance and gait parameters, resulting in different dimensions of 33 and 194 variables, respectively.
- Static balance: The modified clinical test of sensory interaction on balance (MCTSIB) was used to assess participants’ balance using the Airex foam pad (Airex AG, Sins, Switzerland). This test identifies the sensory contributions of visual, somatosensory, and vestibular systems for postural balance. The MCTSIB has four conditions: (1) eyes open (EO) on stable support (SS); (2) eyes closed (EC) on SS; (3) EO on foam surface (FS), and (4) EC on FS [18]. Balance was assessed for each condition for 30 s with the APDM sensors in situ as the participants stood upright as stable as possible with arms by their sides.
2.4. Procedure
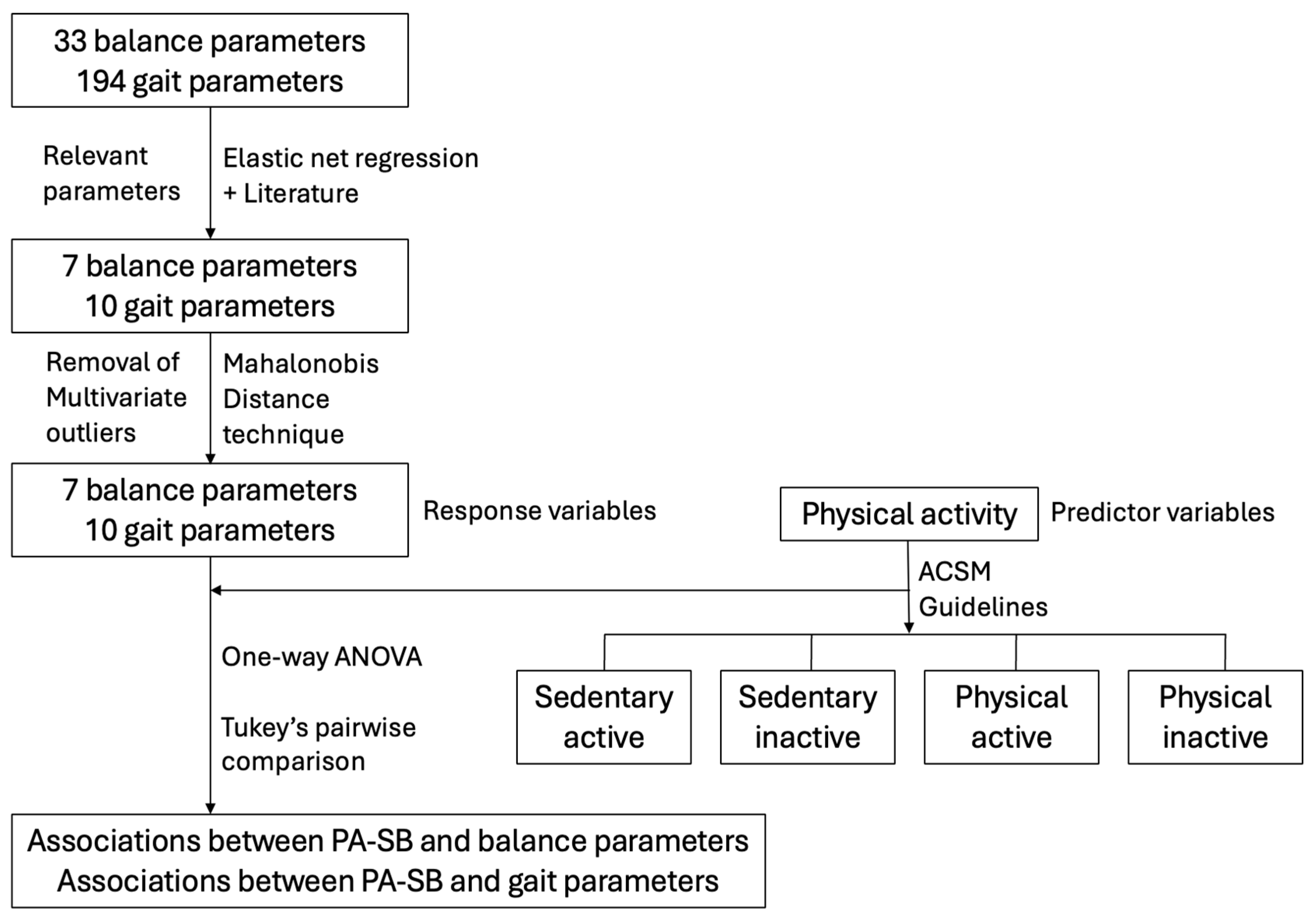
2.5. Data Management and Analysis
3. Results
3.1. Outliers Detection
3.2. Demographics
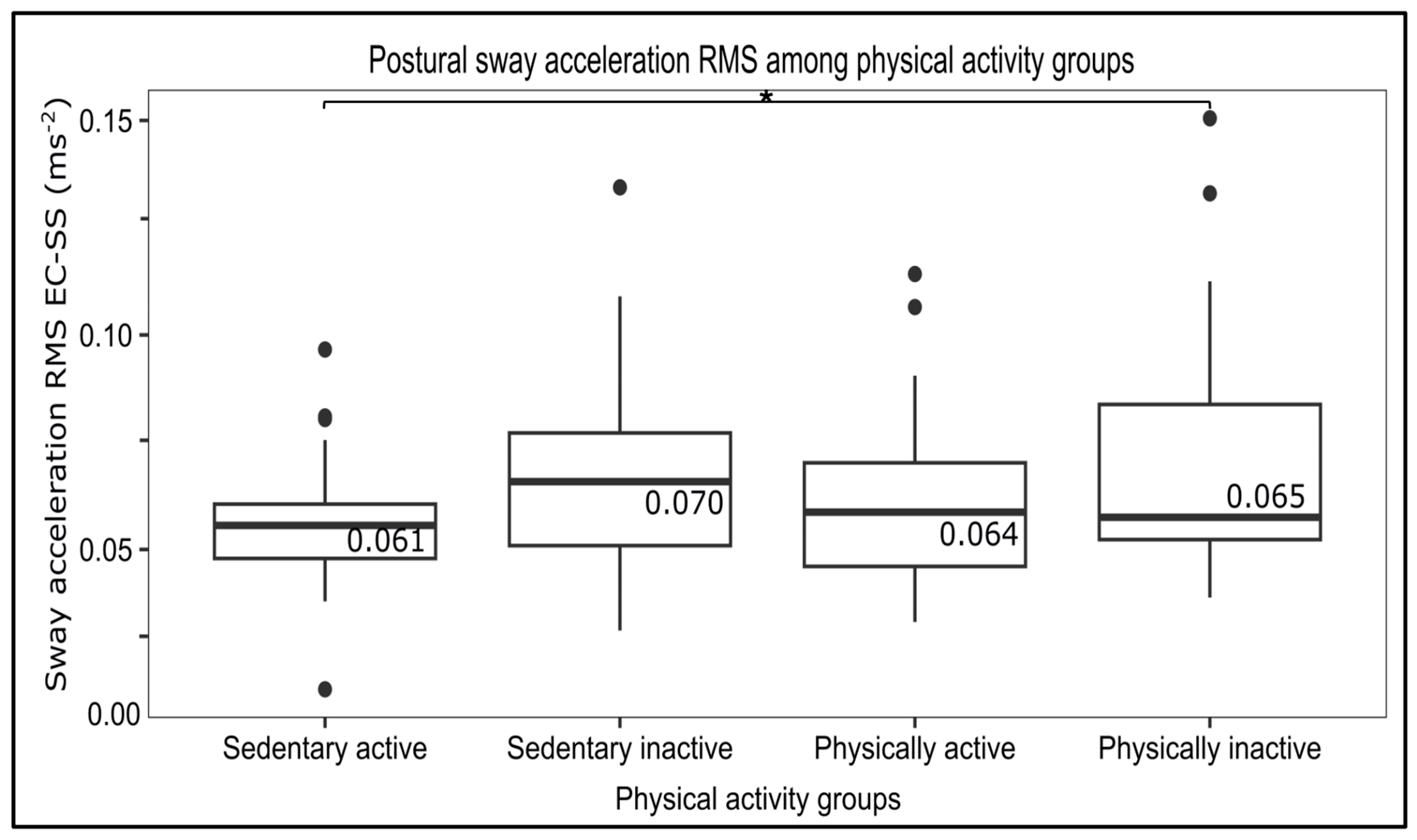
3.3. Influence of PA–SB on Postural Control
3.4. Influence of PA–SB on Gait
4. Discussion
4.1. Influence of PA–SB Interplay on Postural Balance
4.2. Influence of PA–SB Interplay on Gait
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PA | Physical activity |
| SB | Sedentary behavior |
| PA-SB | Physical activity–sedentary behavior interplay |
| APA | Anticipatory postural adjustment |
| RMS | Root mean squared |
| MET | Metabolic equivalent of energy |
| ACSM | American College of Sports Medicine |
| IPAQ | International Physical Activity Questionnaire |
| MCTSIB | Modified clinical test of sensory interaction on balance |
| EO | Eyes open |
| EC | Eyes closed |
| SS | Stable support |
| FS | Foam support |
| APDM | Ambulatory Parkinson’s Disease Monitoring |
| ANOVA | Analysis of variance |
References
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126. [Google Scholar]
- Piggin, J. The Politics of Physical Activity; Routledge: Oxfordshire, UK, 2019. [Google Scholar]
- US Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd ed.; U.S. Department of Health & Human Services: Washington, DC, USA, 2018.
- Carlson, S.A.; Adams, E.K.; Yang, Z.; Fulton, J.E. Peer reviewed: Percentage of deaths associated with inadequate physical activity in the United States. Prev. Chronic Dis. 2018, 15, E38. [Google Scholar] [CrossRef]
- Carlson, S.A.; Fulton, J.E.; Pratt, M.; Yang, Z.; Adams, E.K. Inadequate physical activity and health care expenditures in the United States. Prog. Cardiovasc. Dis. 2015, 57, 315–323. [Google Scholar] [CrossRef]
- Lee, I.M.; Shiroma, E.J.; Lobelo, F.; Puska, P.; Blair, S.N.; Katzmarzyk, P.T. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet 2012, 380, 219–229. [Google Scholar] [CrossRef]
- Dawe, R.J.; Leurgans, S.E.; Yang, J.; Bennett, J.M.; Hausdorff, J.M.; Lim, A.S.; Gaiteri, C.; Bennett, D.A.; Buchman, A.S. Association between quantitative gait and balance measures and total daily physical activity in community-dwelling older adults. J. Gerontol. Ser. A 2018, 73, 636–642. [Google Scholar] [CrossRef]
- Anderson, E.; Durstine, J.L. Physical activity, exercise, and chronic diseases: A brief review. Sports Med. Health Sci. 2019, 1, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Ekelund, U.; Steene-Johannessen, J.; Brown, W.J.; Fagerland, M.W.; Owen, N.; Powell, K.E.; Bauman, A.; Lee, I.-M.; Lancet Physical Activity Series 2 Executive Committe; Lancet Sedentary Behaviour Working Group. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet 2016, 388, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Pau, M.; Leban, B.; Collu, G.; Migliaccio, G.M. Effect of light and vigorous physical activity on balance and gait of older adults. Arch. Gerontol. Geriatr. 2014, 59, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Ciprandi, D.; Bertozzi, F.; Zago, M.; Ferreira, C.L.P.; Boari, G.; Sforza, C.; Galvani, C. Study of the association between gait variability and physical activity. Eur. Rev. Aging Phys. Act. 2017, 14, 19. [Google Scholar] [CrossRef]
- Brach, J.S.; Berlin, J.E.; VanSwearingen, J.M.; Newman, A.B.; Studenski, S.A. Too much or too little step width variability is associated with a fall history in older persons who walk at or near normal gait speed. J. Neuroeng. Rehabil. 2005, 2, 21. [Google Scholar] [CrossRef]
- van Poppel, M.N.; Chinapaw, M.J.; Mokkink, L.B.; Van Mechelen, W.; Terwee, C.B. Physical activity questionnaires for adults: A systematic review of measurement properties. Sports Med. 2010, 40, 565–600. [Google Scholar] [CrossRef]
- Du, Y.; Liu, B.; Sun, Y.; Snetselaar, L.G.; Wallace, R.B.; Bao, W. Trends in adherence to the physical activity guidelines for Americans for aerobic activity and time spent on sedentary behavior among US adults, 2007 to 2016. JAMA Netw. Open 2019, 2, e197597. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Li, Y.; Wang, B.; Zhao, C.; Wu, T.; Liu, T.; Sun, F. Objectively measured physical activity is associated with static balance in young adults. Int. J. Environ. Res. Public Health 2021, 18, 10787. [Google Scholar] [CrossRef]
- Brinkerhoff, S.A.; Sánchez, N.; Roper, J.A. Habitual exercise evokes fast and persistent adaptation during split-belt walking. PLoS ONE 2023, 18, e0286649. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; El-Gohary, M.; Pearson, S.; McNames, J.; Schlueter, H.; Nutt, J.G.; King, L.A.; Horak, F.B. Continuous monitoring of turning in Parkinson’s disease: Rehabilitation potential. NeuroRehabilitation 2015, 37, 3–7. [Google Scholar] [CrossRef]
- Mancini, M.; King, L.; Salarian, A.; Holmstrom, L.; McNames, J.; Horak, F.B. Mobility lab to assess balance and gait with synchronized body-worn sensors. J. Bioeng. Biomed. Sci. 2011, 7. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC4062543/ (accessed on 19 October 2025).
- El-Gohary, M.; Pearson, S.; McNames, J.; Mancini, M.; Horak, F.; Mellone, S.; Chiari, L. Continuous monitoring of turning in patients with movement disability. Sensors 2013, 14, 356–369. [Google Scholar] [CrossRef]
- Zou, H.; Hastie, T. Regularization and variable selection via the elastic net. J. R. Stat. Soc. Ser. B Stat. Methodol. 2005, 67, 301–320. [Google Scholar] [CrossRef]
- Chen, D.; Rust, S.; Lin, E.J.D.; Lin, S.; Nelson, L.; Alfano, L.; Lowes, L.P. Prediction of Clinical Outcomes of Spinal Muscular Atrophy Using Motion Tracking Data and Elastic Net Regression. In Proceedings of the 2018 ACM International Conference on Bioinformatics, Computational Biology, and Health Informatics, Washington, DC, USA, 29 August–1 September 2018; pp. 474–481. [Google Scholar]
- Sell, T.C. An examination, correlation, and comparison of static and dynamic measures of postural stability in healthy, physically active adults. Phys. Ther. Sport 2012, 13, 80–86. [Google Scholar] [CrossRef]
- Field, A. Discovering Statistics Using IBM SPSS Statistics; Sage Publications Limited: London, UK, 2024. [Google Scholar]
- Saunders, N.W. Reliability and Validity of an Accelerometer-Based Balance Assessment for Fall Risk Screening; The Ohio State University: Columbus, OH, USA, 2013. [Google Scholar]
- Neville, C.; Ludlow, C.; Rieger, B. Measuring postural stability with an inertial sensor: Validity and sensitivity. Med. Devices Evid. Res. 2015, 8, 447–455. [Google Scholar] [CrossRef]
- Howe, T.E.; Rochester, L.; Neil, F.; Skelton, D.A.; Ballinger, C. Exercise for improving balance in older people. Cochrane Database Syst. Rev. 2011, 2011, CD004963. [Google Scholar] [CrossRef]
- Vestibular Disorders Association The Human Balance System. 2021. Available online: https://vestibular.org/article/what-is-vestibular/the-human-balance-system/the-human-balance-system-how-do-we-maintain-our-balance/ (accessed on 6 September 2024).
- Horak, F.B. Postural orientation and equilibrium: What do we need to know about neural control of balance to prevent falls? Age Ageing 2006, 35 (Suppl. S2), ii7–ii11. [Google Scholar] [CrossRef]
- Peterka, R.J. Sensorimotor integration in human postural control. J. Neurophysiol. 2002, 88, 1097–1118. [Google Scholar] [CrossRef] [PubMed]
- Patla, A.E. Strategies for dynamic stability during adaptive human locomotion. IEEE Eng. Med. Biol. Mag. 2003, 22, 48–52. [Google Scholar] [CrossRef]
- Duarte, M.B.; da Silva Almeida, G.C.; Costa, K.H.A.; Garcez, D.R.; de Athayde Costa e Silva, A.; da Silva Souza, G.; de Melo-Neto, J.S.; Callegari, B. Anticipatory postural adjustments in older versus young adults: A systematic review and meta-analysis. Syst. Rev. 2022, 11, 251. [Google Scholar] [CrossRef] [PubMed]
- Bouisset, S.; Richardson, J.; Zattara, M. Are amplitude and duration of anticipatory postural adjustments identically scaled to focal movement parameters in humans? Neurosci. Lett. 2000, 278, 153–156. [Google Scholar] [CrossRef]
- Page, A.; Peeters, G.; Merom, D. Adjustment for physical activity in studies of sedentary behaviour. Emerg. Themes Epidemiol. 2015, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Geuze, R.H. Anticipatory postural adjustments in children with developmental coordination disorder. Dev. Med. Child Neurol. 2010, 52, 789. [Google Scholar] [CrossRef]
- Baghurst, T.; Bounds, E.; Boolani, A.; Betts, N. Comparison between perceived and actual physical activity of physical education teacher education students. PHEnex J. 2018, 9. Available online: https://ojs.acadiau.ca/index.php/phenex/article/view/1794 (accessed on 19 October 2025).

| Postural Balance | Gait |
|---|---|
| Sway pathlength during eyes closed on foam surface | Stride length |
| Sway RMS during eyes closed on stable surface | Gait speed |
| Sway velocity during eyes open on stable surface | Double support duration |
| Sway jerk during eyes closed on stable surface | Turn velocity |
| Sway jerk during eyes open on foam surface | Anticipatory postural adjustment duration |
| Sway jerk during eyes closed on foam surface | Arm swing velocity |
| Trunk/lumbar rotation during eyes open on foam surface | Asymmetry toe out angle |
| Stance duration | |
| Swing duration | |
| Gait cycle duration |
| Physical Activity vs. Sitting Duration | ≥150 min/Week (n, %) | <150 min/Week (n, %) |
|---|---|---|
| >6 h/day | Sedentary active (28, 22.0%) | Sedentary inactive (35, 27.6%) |
| ≤6 h/day | Physically active (35, 27.6%) | Physically inactive (29, 22.8%) |
| Variable | Sedentary Active (n = 28) | Sedentary Inactive (n= 35) | Physically Active (n = 35) | Physically Inactive (n = 29) | p-Value |
|---|---|---|---|---|---|
| Age (yrs) | 24.71 ± 5.94 | 24.95 ± 7.22 | 25.17 ± 5.69 | 26.72 ± 10.49 | 0.731 |
| Sex (M/F) | 10/18 | 13/22 | 12/23 | 10/19 | 0.995 |
| Height (m) | 1.71 ± 0.08 | 1.74 ± 0.09 | 1.73 ± 0.08 | 1.72 ± 0.10 | 0.931 |
| Weight (kg) | 70.39 ± 12.83 | 74.91 ± 16.55 | 74.91 ± 12.88 | 73.37 ± 13.18 | 0.558 |
| BMI (kg/m2) | 24.07 ± 4.02 | 24.63 ± 4.73 | 24.93 ± 4.44 | 24.55 ± 2.80 | 0.458 |
| Balance Parameter | All Subjects | Sedentary Active | Sedentary Inactive | Physically Active | Physically Inactive | F Value | p-Value |
|---|---|---|---|---|---|---|---|
| Sway pathlength coronal (m) | |||||||
| EO-SS | 2.51 ± 1.30 2.32–2.70 | 2.30 ± 0.81 2.08–2.53 | 2.45 ± 1.17 2.09–2.80 | 2.44 ± 1.19 2.07–2.81 | 2.86 ± 1.85 2.35–3.37 | 0.995 | 0.397 |
| EC-SS | 2.52 ± 1.08 2.36–3.68 | 2.83 ± 0.88 2.59–3.08 | 2.57 ± 1.13 2.23–2.91 | 2.49 ± 0.94 2.20–2.78 | 2.59 ± 1.39 2.20–2.98 | 0.136 | 0.938 |
| EO-FS | 3.65 ± 1.26 3.47–3.83 | 3.64 ± 1.02 3.36–3.92 | 3.54 ± 1.20 3.17–3.90 | 3.72 ± 1.24 3.34–4.10 | 3.70 ± 1.57 3.26–4.13 | 0.138 | 0.937 |
| EC-FS | 5.63 ± 2.22 5.30–5.95 | 5.63 ± 1.82 5.12–6.13 | 5.85 ± 2.69 5.03–6.67 | 5.45 ± 1.64 4.94–5.96 | 5.57 ± 2.64 4.84–6.30 | 0.191 | 0.902 |
| Sway velocity (m/s) | |||||||
| EO-SS | 0.13 ± 0.14 0.11–0.15 | 0.10 ± 0.04 0.09–0.11 | 0.14 ± 0.16 0.09–0.19 | 0.11 ± 0.08 0.09–0.14 | 0.17 ± 0.22 0.11–0.23 | 1.694 | 0.172 |
| EC-SS | 0.11 ± 0.06 0.10–0.12 | 0.10 ± 0.03 0.09–0.10 | 0.12 ± 0.07 0.10–0.14 | 0.10 ± 0.04 0.09–0.11 | 0.13 ± 0.09 0.10–0.16 | 1.527 | 0.211 |
| EO-FS | 0.14 ± 0.07 0.13–0.15 | 0.14 ± 0.08 0.11–0.16 | 0.13 ± 0.05 0.11–0.15 | 0.13 ± 0.06 0.11–0.15 | 0.15 ± 0.08 0.13–0.17 | 0.712 | 0.547 |
| EC-FS | 0.19 ± 0.11 0.17–0.21 | 0.18 ± 0.08 0.16–0.20 | 0.18 ± 0.07 0.16–0.20 | 0.20 ± 0.14 0.16–0.24 | 0.21 ± 0.12 0.18–0.24 | 0.517 | 0.671 |
| Sway RMS acceleration (m/s2) | |||||||
| EO-SS | 0.06 ± 0.05 0.05–0.07 | 0.05 ± 0.01 0.05–0.05 | 0.07 ± 0.08 0.05–0.09 | 0.05 ± 0.02 0.04–0.06 | 0.08 ± 0.07 0.06–0.10 | 2.010 | 0.116 |
| EC-SS | 0.06 ± 0.02 0.06–0.06 | 0.05 ± 0.01 0.05–0.05 | 0.06 ± 0.02 0.05–0.07 | 0.06 ± 0.01 0.06–0.06 | 0.07 ± 0.02 0.06–0.07 | 2.596 | 0.055 * |
| EO-FS | 0.07 ± 0.02 0.06–0.07 | 0.07 ± 0.02 0.06–0.08 | 0.06 ± 0.01 0.06–0.06 | 0.07 ± 0.01 0.07–0.07 | 0.07 ± 0.02 0.06–0.07 | 1.313 | 0.273 |
| EC-FS | 0.11 ± 0.03 0.11–0.11 | 0.11 ± 0.03 0.10–0.11 | 0.11 ± 0.03 0.10–0.11 | 0.11 ± 0.03 0.10–0.12 | 0.12 ± 0.04 0.11–0.13 | 0.993 | 0.399 |
| Sway jerk (sagittal) (m2/s5) | |||||||
| EO-SS | 1.73 ± 1.54 1.51–1.96 | 1.76 ± 4.60 0.48–3.03 | 1.50 ± 1.21 1.13–1.87 | 1.70 ± 1.90 1.10–2.29 | 1.95 ± 1.91 1.41–2.49 | 1.54 | 0.478 |
| EC-SS | 1.23 ± 1.00 1.08–1.38 | 1.03 ± 0.64 0.85–1.20 | 1.42 ± 0.93 1.14–1.70 | 1.09 ± 0.94 0.80–1.38 | 1.36 ± 1.35 0.99–1.74 | 1.19 | 0.316 |
| EO-FS | 1.73 ± 1.54 1.51–1.96 | 1.77 ± 1.00 1.50–2.05 | 1.51 ± 1.22 1.14–1.88 | 1.73 ± 1.86 1.15–2.30 | 1.95 ± 1.90 1.42–2.48 | 0.423 | 0.737 |
| EC-FS | 5.03 ± 4.36 4.39–5.67 | 5.44 ± 4.70 4.13–6.75 | 4.72 ± 3.20 3.74–5.70 | 4.41 ± 4.14 3.12–5.70 | 5.74 ± 5.45 4.23–7.26 | 0.618 | 0.604 |
| Gait Parameter | All Subjects | Sedentary Active | Sedentary Inactive | Physically Active | Physically Inactive | F Value | p-Value |
|---|---|---|---|---|---|---|---|
| Anticipatory postural adjustment duration (s) | 0.51 ± 0.26 0.47–0.55 | 0.44 ± 0.13 0.40–0.48 | 0.44 ± 0.19 0.38–050 | 0.64 ± 0.35 0.53–0.75 | 0.48 ± 0.23 0.42–0.51 | 3.94 | 0.010 * |
| Stride length (std; m) | 0.03 ± 0.01 0.02–0.03 | 0.04 ± 0.01 0.04–0.04 | 0.03 ± 0.01 0.03–0.03 | 0.04 ± 0.01 0.04–0.04 | 0.03 ± 0.01 0.03–0.03 | 1.37 | 0.272 |
| Gait speed (std; m/s) | 0.05 ± 0.01 0.05–0.05 | 0.05 ± 0.02 0.04–0.06 | 0.04 ± 0.01 0.04–0.04 | 0.05 ± 0.01 0.05–0.05 | 0.05 ± 0.01 0.05–0.05 | 0.95 | 0.421 |
| Gait cycle duration (s) | 1.14 ± 0.08 1.13–1.15 | 1.14 ± 0.08 1.12–1.16 | 1.14 ± 0.08 1.12–1.16 | 1.15 ± 0.09 1.12–1.18 | 1.11 ± 0.08 1.09–1.13 | 1.32 | 0.272 |
| Stance duration (std; s) | 0.39 ± 0.12 0.37–0.40 | 0.39 ± 0.10 0.36–0.41 | 0.42 ± 0.12 0.38–0.46 | 0.40 ± 0.16 0.35–0.45 | 0.37 ± 0.09 0.35–0.40 | 0.69 | 0.562 |
| Swing duration (std; s) | 1.27 ± 0.67 1.17–1.37 | 1.07 ± 0.46 0.94–1.20 | 1.39 ± 0.59 1.21–1.57 | 1.30 ± 0.67 1.10–1.50 | 1.28 ± 0.75 1.07–1.49 | 1.42 | 0.242 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Appiah-Kubi, K.O.; Senarathna, D.; Mondal, S.; Boolani, A. Physical Inactivity and Sedentary Behavior Negatively Impact Postural Balance and Gait. Appl. Sci. 2025, 15, 12058. https://doi.org/10.3390/app152212058
Appiah-Kubi KO, Senarathna D, Mondal S, Boolani A. Physical Inactivity and Sedentary Behavior Negatively Impact Postural Balance and Gait. Applied Sciences. 2025; 15(22):12058. https://doi.org/10.3390/app152212058
Chicago/Turabian StyleAppiah-Kubi, Kwadwo O., Dinushani Senarathna, Sumona Mondal, and Ali Boolani. 2025. "Physical Inactivity and Sedentary Behavior Negatively Impact Postural Balance and Gait" Applied Sciences 15, no. 22: 12058. https://doi.org/10.3390/app152212058
APA StyleAppiah-Kubi, K. O., Senarathna, D., Mondal, S., & Boolani, A. (2025). Physical Inactivity and Sedentary Behavior Negatively Impact Postural Balance and Gait. Applied Sciences, 15(22), 12058. https://doi.org/10.3390/app152212058






