Low-Dose Narrowband UVB Exposure Modulates Systemic Metabolism in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. UVB Irradiation Device and Irradiating Conditions
2.3. Respiratory Exchange Ratio (RER), VO2, and Locomotor Activity
2.4. Food Intake Measurement
2.5. Body Surface Temperature Measurement
2.6. Tissue Collection and Real-Time Polymerase Chain Reaction
2.7. Capillary Electrophoresis Mass Spectrometry (CE-MS) Analysis
2.8. Statistical Analyses
3. Results
3.1. Low-Dose UVB Irradiation by the Customized Device Did Not Affect Feeding Behavior

3.2. UVB Impacts on Locomotor Activity and Systemic Metabolism
3.3. UVB Effects on Plasma Metabolome
3.4. Body Surface Temperature Is Increased by UVB Irradiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BAT | brown adipose tissue |
| ELISA | enzyme-linked immunosorbent assay |
| HPA | hypothalamic–pituitary–adrenal |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LED | light-emitting diode |
| NEFA | non-esterified fatty acids |
| Pparα | peroxisome proliferator-activated receptor alpha |
| REE | resting energy expenditure |
| RER | respiratory exchange ratio |
| RT-qPCR | reverse transcription quantitative polymerase chain reaction |
| Tbp | TATA box-binding protein |
| TSH | thyroid-stimulating hormone |
| UCP1 | uncoupling protein 1 |
| UVA | ultraviolet A |
| UVB | ultraviolet B |
| VO2 | oxygen consumption |
| ZT | zeitgeber time |
| Actβ | β-actin |
| Cidea | cell death-inducing DFFA-like effector a |
| PCA | principal component analysis |
| HFD | high-fat diet |
| CORT | corticosterone |
| iBAT | interscapular brown adipose tissue |
| TCA | tricarboxylic acid |
References
- Fleury, G.; Masís-Vargas, A.; Kalsbeek, A. Metabolic Implications of Exposure to Light at Night: Lessons from Animal and Human Studies. Obesity 2020, 28, S18–S28. [Google Scholar] [CrossRef]
- Masís-Vargas, A.; Ritsema, W.I.G.R.; Mendoza, J.; Kalsbeek, A. Metabolic Effects of Light at Night Are Time- and Wavelength-Dependent in Rats. Obesity 2020, 28, S114–S125. [Google Scholar] [CrossRef]
- Meng, J.-J.; Shen, J.-W.; Li, G.; Ouyang, C.-J.; Hu, J.-X.; Li, Z.-S.; Zhao, H.; Shi, Y.-M.; Zhang, M.; Liu, R.; et al. Light Modulates Glucose Metabolism by a Retina-Hypothalamus-Brown Adipose Tissue Axis. Cell 2023, 186, 398–412.e17. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Zhang, W.; Chen, S.; Liu, C. Chronic Exposure to Green Light Aggravates High-Fat Diet-Induced Obesity and Metabolic Disorders in Male Mice. Ecotoxicol. Environ. Saf. 2019, 178, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Opperhuizen, A.-L.; Stenvers, D.J.; Jansen, R.D.; Foppen, E.; Fliers, E.; Kalsbeek, A. Light at Night Acutely Impairs Glucose Tolerance in a Time-, Intensity- and Wavelength-Dependent Manner in Rats. Diabetologia 2017, 60, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Cajochen, C.; Münch, M.; Kobialka, S.; Kräuchi, K.; Steiner, R.; Oelhafen, P.; Orgül, S.; Wirz-Justice, A. High Sensitivity of Human Melatonin, Alertness, Thermoregulation, and Heart Rate to Short Wavelength Light. J. Clin. Endocrinol. Metab. 2005, 90, 1311–1316. [Google Scholar] [CrossRef]
- Münch, M.; Kobialka, S.; Steiner, R.; Oelhafen, P.; Wirz-Justice, A.; Cajochen, C. Wavelength-Dependent Effects of Evening Light Exposure on Sleep Architecture and Sleep EEG Power Density in Men. Am. J. Physiol. Integr. Comp. Physiol. 2006, 290, R1421–R1428. [Google Scholar] [CrossRef]
- Zubidat, A.E.; Nelson, R.J.; Haim, A. Spectral and Duration Sensitivity to Light-at-Night in ‘Blind’ and Sighted Rodent Species. J. Exp. Biol. 2011, 214, 3206–3217. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Zmijewski, M.A.; Plonka, P.M.; Szaflarski, J.P.; Paus, R. How UV Light Touches the Brain and Endocrine System Through Skin, and Why. Endocrinology 2018, 159, 1992–2007. [Google Scholar] [CrossRef]
- Parrish, J.A.; Jaenicke, K.F.; Anderson, R.R. Erythema and melanogenesis action spectra of normal human skin. Photochem. Photobiol. 1982, 36, 187–191. [Google Scholar] [CrossRef]
- Diffey, B.L. An Overview Analysis of the Time People Spend Outdoors: Time Spent Outdoors. Br. J. Dermatol. 2011, 164, 848–854. [Google Scholar] [CrossRef]
- Burns, A.C.; Saxena, R.; Vetter, C.; Phillips, A.J.K.; Lane, J.M.; Cain, S.W. Time Spent in Outdoor Light Is Associated with Mood, Sleep, and Circadian Rhythm-Related Outcomes: A Cross-Sectional and Longitudinal Study in over 400,000 UK Biobank Participants. J. Affect. Disord. 2021, 295, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Myers, E.; Kheradmand, S.; Miller, R. An Update on Narrowband Ultraviolet B Therapy for the Treatment of Skin Diseases. Cureus 2021, 13, e19182. [Google Scholar] [CrossRef]
- Geldenhuys, S.; Hart, P.H.; Endersby, R.; Jacoby, P.; Feelisch, M.; Weller, R.B.; Matthews, V.; Gorman, S. Ultraviolet Radiation Suppresses Obesity and Symptoms of Metabolic Syndrome Independently of Vitamin D in Mice Fed a High-Fat Diet. Diabetes 2014, 63, 3759–3769. [Google Scholar] [CrossRef] [PubMed]
- Fleury, N.; Feelisch, M.; Hart, P.H.; Weller, R.B.; Smoothy, J.; Matthews, V.B.; Gorman, S. Sub-Erythemal Ultraviolet Radiation Reduces Metabolic Dysfunction in Already Overweight Mice. J. Endocrinol. 2017, 233, 81–92. [Google Scholar] [CrossRef]
- Teng, S.; Chakravorty, L.; Fleury, N.; Gorman, S. Regular Exposure to Non-Burning Ultraviolet Radiation Reduces Signs of Non-Alcoholic Fatty Liver Disease in Mature Adult Mice Fed a High Fat Diet: Results of a Pilot Study. BMC Res. Notes 2019, 12, 78. [Google Scholar] [CrossRef] [PubMed]
- Skobowiat, C.; Postlethwaite, A.E.; Slominski, A.T. Skin Exposure to Ultraviolet B Rapidly Activates Systemic Neuroendocrine and Immunosuppressive Responses. Photochem. Photobiol. 2017, 93, 1008–1015. [Google Scholar] [CrossRef]
- Skobowiat, C.; Slominski, A.T. UVB Activates Hypothalamic–Pituitary–Adrenal Axis in C57BL/6 Mice. J. Investig. Dermatol. 2015, 135, 1638–1648. [Google Scholar] [CrossRef]
- Skobowiat, C.; Slominski, A.T. Ultraviolet B Stimulates Proopiomelanocortin Signalling in the Arcuate Nucleus of the Hypothalamus in Mice. Exp. Dermatol. 2016, 25, 120–123. [Google Scholar] [CrossRef]
- Parikh, S.; Parikh, R.; Michael, K.; Bikovski, L.; Barnabas, G.; Mardamshina, M.; Hemi, R.; Manich, P.; Goldstein, N.; Malcov-Brog, H.; et al. Food-Seeking Behavior Is Triggered by Skin Ultraviolet Exposure in Males. Nat. Metab. 2022, 4, 883–900. [Google Scholar] [CrossRef]
- Quan, Q.-L.; Kim, E.J.; Kim, S.; Kim, Y.K.; Chung, M.H.; Tian, Y.-D.; Shin, C.-Y.; Lee, D.H.; Chung, J.H. UV Irradiation Increases Appetite and Prevents Body Weight Gain through the Upregulation of Norepinephrine in Mice. J. Investig. Dermatol. 2024, 144, 2273–2284.e5. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuizen, A.G.; Rutters, F. The Hypothalamic-Pituitary-Adrenal-Axis in the Regulation of Energy Balance. Physiol. Behav. 2008, 94, 169–177. [Google Scholar] [CrossRef]
- Christiansen, J.J.; Djurhuus, C.B.; Gravholt, C.H.; Iversen, P.; Christiansen, J.S.; Schmitz, O.; Weeke, J.; Jørgensen, J.O.L.; Møller, N. Effects of Cortisol on Carbohydrate, Lipid, and Protein Metabolism: Studies of Acute Cortisol Withdrawal in Adrenocortical Failure. J. Clin. Endocrinol. Metab. 2007, 92, 3553–3559. [Google Scholar] [CrossRef]
- Cone, R.D. The Central Melanocortin System and Energy Homeostasis. Trends Endocrinol. Metab. 1999, 10, 211–216. [Google Scholar] [CrossRef]
- Butler, A.A. The Melanocortin System and Energy Balance. Peptides 2006, 27, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti-de-Albuquerque, J.P.; Bober, J.; Zimmer, M.R.; Dietrich, M.O. Regulation of Substrate Utilization and Adiposity by Agrp Neurons. Nat. Commun. 2019, 10, 311. [Google Scholar] [CrossRef]
- Dhamrait, G.K.; Panchal, K.; Fleury, N.J.; Abel, T.N.; Ancliffe, M.K.; Crew, R.C.; Croft, K.; Fernandez, B.O.; Minnion, M.; Hart, P.H.; et al. Characterising Nitric Oxide-Mediated Metabolic Benefits of Low-Dose Ultraviolet Radiation in the Mouse: A Focus on Brown Adipose Tissue. Diabetologia 2020, 63, 179–193. [Google Scholar] [CrossRef]
- Cannon, B.; Nedergaard, J. Brown Adipose Tissue: Function and Physiological Significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef]
- Cypess, A.M.; Cannon, B.; Nedergaard, J.; Kazak, L.; Chang, D.C.; Krakoff, J.; Tseng, Y.-H.; Schéele, C.; Boucher, J.; Petrovic, N.; et al. Emerging Debates and Resolutions in Brown Adipose Tissue Research. Cell Metab. 2025, 37, 12–33. [Google Scholar] [CrossRef]
- Zhang, W.; Bi, S. Hypothalamic Regulation of Brown Adipose Tissue Thermogenesis and Energy Homeostasis. Front. Endocrinol. 2015, 6, 136. [Google Scholar] [CrossRef] [PubMed]
- Orava, J.; Nuutila, P.; Lidell, M.E.; Oikonen, V.; Noponen, T.; Viljanen, T.; Scheinin, M.; Taittonen, M.; Niemi, T.; Enerbäck, S.; et al. Different Metabolic Responses of Human Brown Adipose Tissue to Activation by Cold and Insulin. Cell Metab. 2011, 14, 272–279. [Google Scholar] [CrossRef]
- Bartelt, A.; Bruns, O.T.; Reimer, R.; Hohenberg, H.; Ittrich, H.; Peldschus, K.; Kaul, M.G.; Tromsdorf, U.I.; Weller, H.; Waurisch, C.; et al. Brown Adipose Tissue Activity Controls Triglyceride Clearance. Nat. Med. 2011, 17, 200–205. [Google Scholar] [CrossRef]
- Whitehead, A.; Krause, F.N.; Moran, A.; MacCannell, A.D.V.; Scragg, J.L.; McNally, B.D.; Boateng, E.; Murfitt, S.A.; Virtue, S.; Wright, J.; et al. Brown and Beige Adipose Tissue Regulate Systemic Metabolism through a Metabolite Interorgan Signaling Axis. Nat. Commun. 2021, 12, 1905. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P. Brown Adipose Tissue—A Therapeutic Target in Obesity? Front. Physiol. 2018, 9, 1672. [Google Scholar] [CrossRef] [PubMed]
- Allemann, T.S.; Dhamrait, G.K.; Fleury, N.J.; Abel, T.N.; Hart, P.H.; Lucas, R.M.; Matthews, V.B.; Gorman, S. Low-Dose UV Radiation before Running Wheel Access Activates Brown Adipose Tissue. J. Endocrinol. 2020, 244, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Hohlbaum, K.; Bert, B.; Dietze, S.; Palme, R.; Fink, H.; Thöne-Reineke, C. Severity Classification of Repeated Isoflurane Anesthesia in C57BL/6JRj Mice—Assessing the Degree of Distress. PLoS ONE 2017, 12, e0179588. [Google Scholar] [CrossRef]
- TLVs and BEIs: Threshold Limit Values for Chemical Substances and Physical Agents & Biological Exposure Indices, 2024th ed.; ACGIH TLVs and BEIs Committees, ACGIH: Cincinnati, OH, USA, 2024.
- Lai, T.H.; Hwang, J.S.; Ngo, Q.N.; Lee, D.-K.; Kim, H.J.; Kim, D.R. A Comparative Assessment of Reference Genes in Mouse Brown Adipocyte Differentiation and Thermogenesis in Vitro. Adipocyte 2024, 13, 2330355. [Google Scholar] [CrossRef]
- Mawatari, K.; Koike, N.; Nohara, K.; Wirianto, M.; Uebanso, T.; Shimohata, T.; Shikishima, Y.; Miura, H.; Nii, Y.; Burish, M.J.; et al. The Polymethoxyflavone Sudachitin Modulates the Circadian Clock and Improves Liver Physiology. Mol. Nutr. Food Res. 2023, 67, 2200270. [Google Scholar] [CrossRef]
- Samet, J.M.; Spengler, J.D. Indoor Environments and Health: Moving into the 21st Century. Am. J. Public. Health 2003, 93, 1489–1493. [Google Scholar] [CrossRef]
- Mills, E.L.; Pierce, K.A.; Jedrychowski, M.P.; Garrity, R.; Winther, S.; Vidoni, S.; Yoneshiro, T.; Spinelli, J.B.; Lu, G.Z.; Kazak, L.; et al. Accumulation of Succinate Controls Activation of Adipose Tissue Thermogenesis. Nature 2018, 560, 102–106. [Google Scholar] [CrossRef]
- Xie, N.; Zhang, L.; Gao, W.; Huang, C.; Huber, P.E.; Zhou, X.; Li, C.; Shen, G.; Zou, B. NAD+ Metabolism: Pathophysiologic Mechanisms and Therapeutic Potential. Signal Transduct. Target. Ther. 2020, 5, 227. [Google Scholar] [CrossRef]
- Shafer, M.; Low, V.; Li, Z.; Blenis, J. The Emerging Role of Dysregulated Propionate Metabolism and Methylmalonic Acid in Metabolic Disease, Aging, and Cancer. Cell Metab. 2025, 37, 316–329. [Google Scholar] [CrossRef]
- Collins, S. β-Adrenergic Receptors and Adipose Tissue Metabolism: Evolution of an Old Story. Annu. Rev. Physiol. 2022, 84, 1–16. [Google Scholar] [CrossRef]
- Dong, S.; Jiang, M.; Sun, Q.; Xu, J.; Zhang, L.; Han, L.; Li, Y.; Zhou, Z.; Xu, Y. Aspartate Restrains Thermogenesis by Inhibiting the AMPK Pathway in Adipose Tissues. Food Funct. 2024, 15, 11564–11577. [Google Scholar] [CrossRef]
- Hasek, B.E.; Stewart, L.K.; Henagan, T.M.; Boudreau, A.; Lenard, N.R.; Black, C.; Shin, J.; Huypens, P.; Malloy, V.L.; Plaisance, E.P.; et al. Dietary Methionine Restriction Enhances Metabolic Flexibility and Increases Uncoupled Respiration in Both Fed and Fasted States. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2010, 299, R728–R739. [Google Scholar] [CrossRef]
- Cadet, J.; Douki, T.; Ravanat, J. Oxidatively Generated Damage to Cellular DNA by UVB and UVA Radiation. Photochem. Photobiol. 2015, 91, 140–155. [Google Scholar] [CrossRef]
- Santiago, J.L.; Muñoz-Rodriguez, J.R.; Cruz-Morcillo, M.A.D.L.; Villar-Rodriguez, C.; Gonzalez-Lopez, L.; Aguado, C.; Nuncia-Cantarero, M.; Redondo-Calvo, F.J.; Perez-Ortiz, J.M.; Galan-Moya, E.M. Characterization of Permeability Barrier Dysfunction in a Murine Model of Cutaneous Field Cancerization Following Chronic UV-B Irradiation: Implications for the Pathogenesis of Skin Cancer. Cancers 2021, 13, 3935. [Google Scholar] [CrossRef]
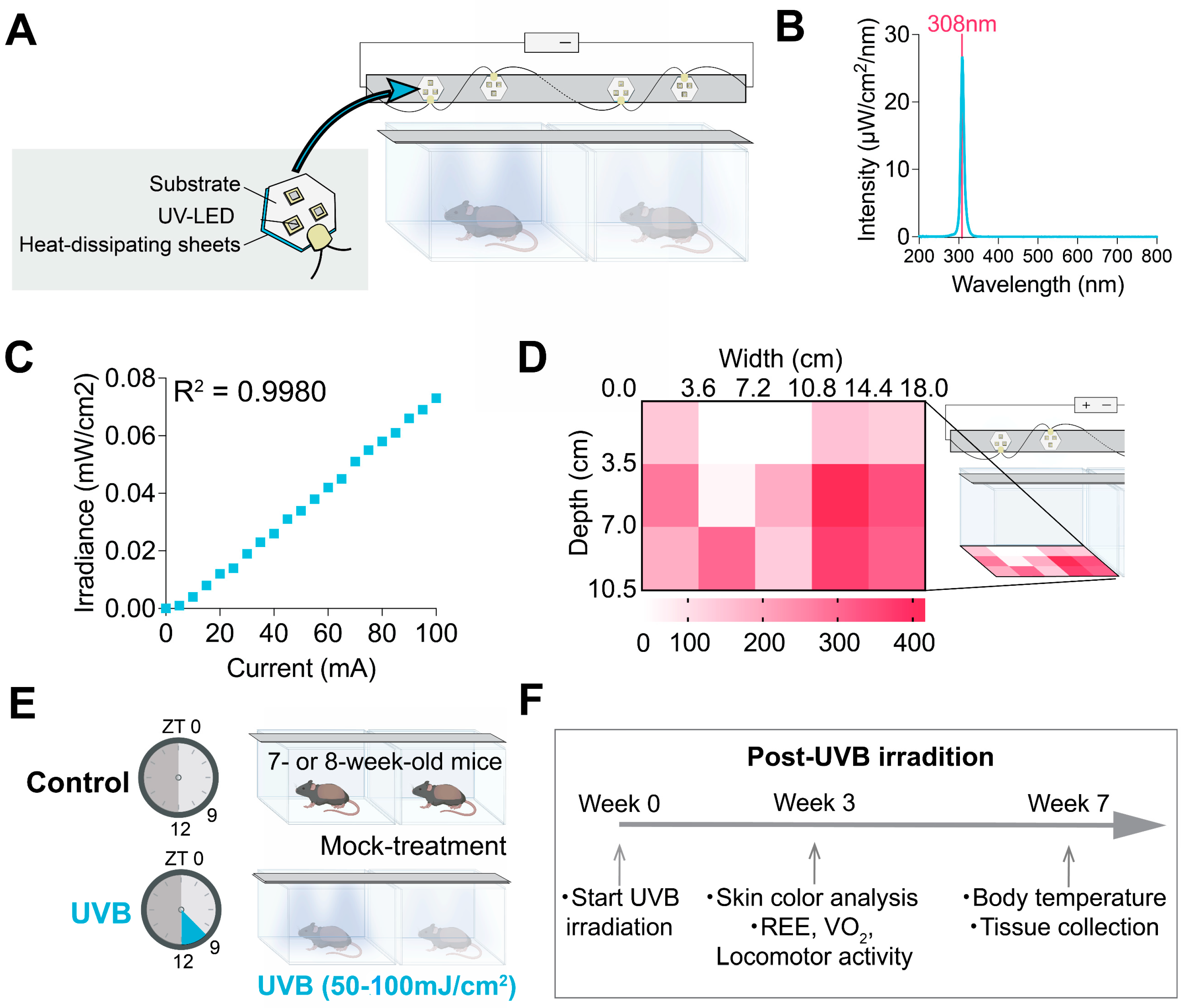

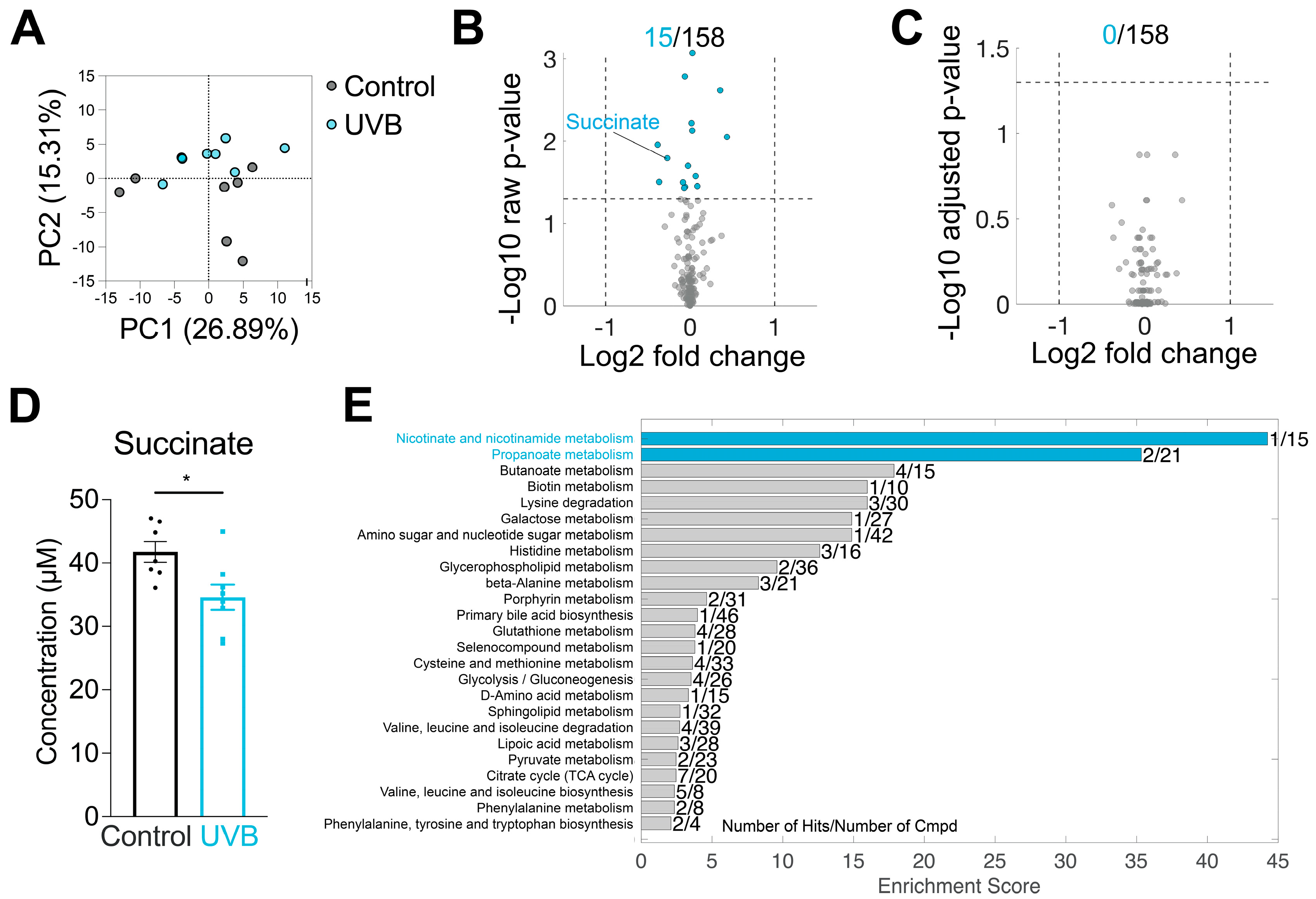
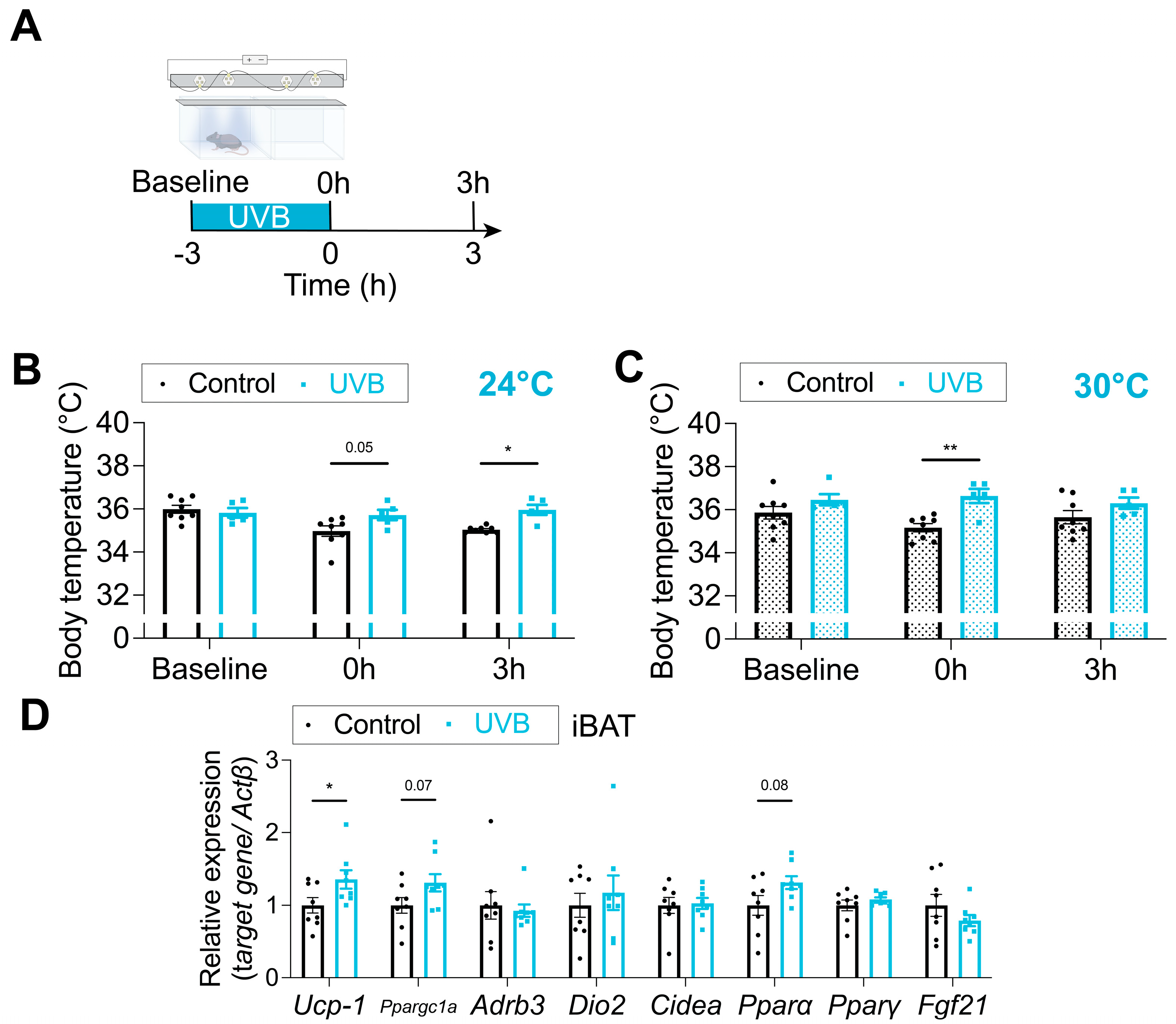
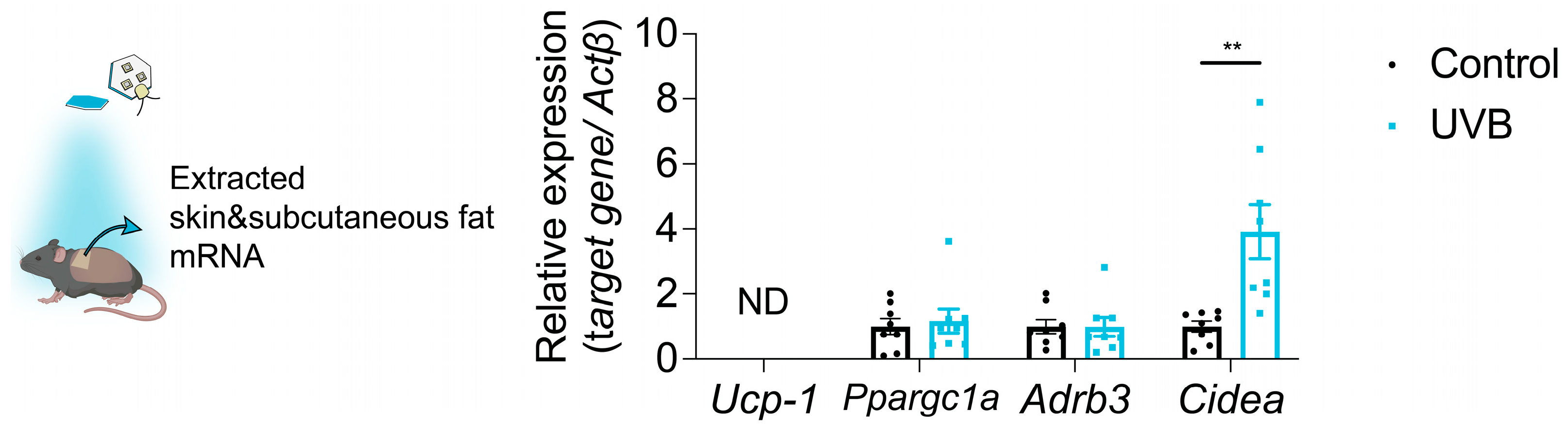
| Cohort No. | Groups (n, sex) | Assay/Measurement | Fluence (mJ/cm2) Period (Week) | Fluence (mJ/cm2·Day−1) |
|---|---|---|---|---|
| 1 | Control (n = 4, M) UVB (n = 4, M) | Food intake Skin color analysis | 50 1–3 | Figure S2 Figure 2A,B,F |
| 2 * | Control (n = 4, M; n = 4, F) UVB (n = 4, M; n = 4, F) | Food intake Tissue collection for RT-qPCR Blood collection for ELISA and metabolome | 50 1–7 | Figures S2–S4 Figure 2 Figure 4 Figure 5D Figure 6 |
| 3 | Control (n = 4, M) UVB (n = 4, M) | Food intake | 50 1 | Figure S2 Figure 2F |
| 4 # | Control (n = 8, M) UVB (n = 8, M) | Food intake Metabolic measurement Body temperature | 100 1–7 | Figure S2 Figure 2F Figure 3 Figure 5A–C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuki, S.; Mawatari, K.; Uebanso, T.; Takahashi, A.; Shiuchi, T. Low-Dose Narrowband UVB Exposure Modulates Systemic Metabolism in Mice. Appl. Sci. 2025, 15, 11869. https://doi.org/10.3390/app152211869
Yuki S, Mawatari K, Uebanso T, Takahashi A, Shiuchi T. Low-Dose Narrowband UVB Exposure Modulates Systemic Metabolism in Mice. Applied Sciences. 2025; 15(22):11869. https://doi.org/10.3390/app152211869
Chicago/Turabian StyleYuki, Shion, Kazuaki Mawatari, Takashi Uebanso, Akira Takahashi, and Tetsuya Shiuchi. 2025. "Low-Dose Narrowband UVB Exposure Modulates Systemic Metabolism in Mice" Applied Sciences 15, no. 22: 11869. https://doi.org/10.3390/app152211869
APA StyleYuki, S., Mawatari, K., Uebanso, T., Takahashi, A., & Shiuchi, T. (2025). Low-Dose Narrowband UVB Exposure Modulates Systemic Metabolism in Mice. Applied Sciences, 15(22), 11869. https://doi.org/10.3390/app152211869






