Abstract
Traumatic brain injury (TBI) is a significant public health concern and its rising prevalence in road traffic accidents underscores the need for deeper understanding and tailored investigation. This study explores the feasibility of employing the female finite element head model (FeFEHM) to analyse biomechanical responses in two distinct road traffic accident scenarios, focusing on strain and stress distribution in critical brain structures. Two collision scenarios from the German In-Depth Accident Study (GIDAS) were reconstructed using validated Total Human Model for Safety (THUMS) simulations. The extracted skull kinematics were applied to the FeFEHM in ABAQUS to compute maximum principal strain, von Mises stress, and intracranial pressure across key brain regions, including the corpus callosum and pituitary gland. Simulations revealed strain concentrations in the parietal and temporal lobes, while the mid-body region was the most affected in the corpus callosum. Pituitary gland deformation was minimal under both loading conditions. Our findings align qualitatively with reported injury sites and injury risk was consistent with those observed in the real-world crashes. The findings highlight the potential of integrating sex-specific biomechanical models into crash biomechanics workflows. Future work should extend this approach across larger datasets and impact scenarios to support its implementation in regulatory and engineering contexts, since the actual sample size prevents conclusions regarding sex-specific biomechanics.
1. Introduction
Traumatic brain injury (TBI) represents a significant public health concern, particularly in road traffic accidents, which account for approximately 20% of cases, according to the Centers for Disease Control and Prevention (CDC). Between 2007 and 2010, emergency visits for TBI among women increased 49% with mild to severe TBI, predominantly characterised by concussions, representing 60% of all head injuries sustained in vehicular accidents [1,2,3]. The consequences of TBI extend beyond immediate physical injuries, as they can result in long-term cognitive, emotional, and psychosocial impairments, with up to 30% of individuals who have sustained a concussion experiencing persistent deficits [4].
Recent years have seen a growing recognition of the impact TBI on female individuals. However, there remains a notable lack of female representation in clinical studies, with research historically focusing predominantly on male subjects, resulting in a significant gap in understanding the unique variables affecting female individuals [5,6,7]. For instance, studies have shown that female patients with TBI may experience different outcomes compared to their male counterparts, with evidence suggesting that females often have higher levels of certain biomarkers associated with neurodegenerative diseases following TBI [7,8]. Moreover, the scarcity of finite element head models (FEHMs) specifically designed for females hinders our ability to accurately simulate and study these outcomes [9].
Emerging research links repeated head impacts and the development of neurodegenerative diseases, such as Chronic Traumatic Encephalopathy (CTE), characterised by a progressive degeneration of brain tissue [10,11]. The combined effects of such injuries can result in debilitating conditions, including depression and cognitive decline, which is a particularly salient issue given the rising incidence of TBI in both male and female populations [12].
Finite element analysis (FEA) offers a robust framework for simulating brain tissue responses to mechanical loads during impact events. While this tool can not directly predict biological or clinical outcomes, it enables insights to better understand the dynamics of injury mechanisms, such as the regions most susceptible to mechanical loading under various crash conditions [13,14,15]. This computational approach not only aids in elucidating the immediate consequences of TBI but also facilitates the exploration of long-term consequences in future studies that integrate biomechanical perspectives.
This study demonstrates the feasibility of employing a female FEHM, the Female Finite Element Head Model (FeFEHM), in two road traffic accident case studies from the German In-depth Accident Study [16], provided by the Federal Highway Research and Transport Institute [17]. The input kinematic data (linear acceleration and angular velocity), derived from Total Human Model for Safety (THUMS) reconstructions [18], were applied to the FeFEHM to analyse strain and stress distribution over key brain structures, including the corpus callosum (CC) and the pituitary gland (PG). Established thresholds were assessed to correlate the applied injury criteria to brain injuries. Therefore, our main objective was to integrate a female-specific FEHM into accident reconstructions, highlighting the model’s qualitative and quantitative outputs, rather than establish sex-specific injury mechanism.
2. Methods
2.1. Framework of the Employed FEHM
This research employed a female head model, the FeFEHM, developed by Carmo et al. [19], based on subject-specific female anthropological data from computed tomography (CT) scans and magnetic resonance imaging (MRI) for the segmentation of the skull and soft tissues. The principal cerebral regions under analysis included the gyri and sulci, the CC, and the PG, as illustrated in Figure 1.
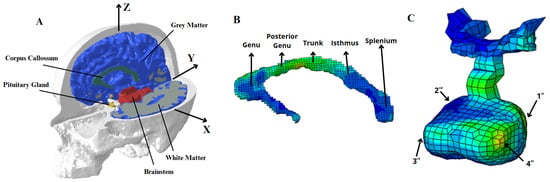
Figure 1.
Overview of the Female Finite Element Head Model (FeFEHM). (A) Sagittal and axial cross section view of the FeFEHM, where the CSF is supressed for a clear view of the brain regions. (B) The regions under analysis in the CC and (C) in the PG.
The model comprises hexahedral and tetrahedral elements across structures including the white matter (WM), grey matter (GM), CC, PG, cerebellum, cerebrospinal fluid (CSF), and skull, where the work of Menichetti et al. [20] was employed to characterise the brain tissues. The material properties were derived from micro-indentation at dynamic rates in specific brain sections, and the response of the components was described as a quasi-linear viscoelastic framework [21]. The non-linear elasticity was characterised by a Neo-hookean constitutive law, employed as a strain energy function. For the CSF, the work of Gilchrist [22] was considered, based on the Mooney–Rivlin constitutive law. In the absence of experimental data on the PG, the work of Bouchonville et al. [23] led to the assumption of a linear elastic formulation.
The validation of the FeFEHM involves a comparison of the simulated results with a post-mortem specimen experiment. The chosen experiment was carried out by [24] since the three-dimensional deformation of the brain made it possible to obtain displacements in all three anatomical planes, with particular consideration of female subjects, making it suitable for model comparison. The method used is sonomicrometry, which uses ultrasound pulses to determine the distance between a piezoelectric crystal inserted into the brain and the inside of the skull.
Model validation was conducted by comparing to the experimental data [24], employing sonomicrometry to determine the distance between a piezoelectric crystal inserted into the brain and the inside of the skull. The precise coordinates of each crystal relative to the center of gravity (COG) were obtained through CT scans, thus enabling the validation simulation with the receivers 9, 16, and 31. The 20 rad/s and 60 ms acceleration profile was selected for analysis. In ABAQUS, triaxial displacements were reproduced by assigning reference points (RPs) on the crystals’ coordinates, tied to the COG to ensure no motion in relation to the skull. Simulated results were compared to experimental data to validate the model. The detailed validation process is presented in Carmo et al.’s [19] work.
Regarding the mesh quality, the implementation of a hexahedral mesh primarily prevented previous anomalies, notably the locking phenomenon. As mentioned by Gomes et al. [25], the element type C3D8R was determined to be the most suitable, and the selected number of elements has been demonstrated to improve the computational cost and accuracy when compared to the experimental results.
2.2. Brain Regions Analysis
The analysis is performed with the use of commonly employed thresholds to define a correlation between the numerical results and the actual instances of trauma in real life. The selected criteria for this study includes the maximum principal strain (MPS), von Mises stress, and pressure [26,27]. It is know that these threshold values vary across models, due to differences in mesh resolution, element type, and constitutive properties. Therefore, thresholds were applied to the qualitative analysis of the presented brain injuries rather than as a definitive predictive tool. Although quantitative results were obtained, these only imply potential causes of injury. Thus, potential neurological and endocrine sequelae following TBI, mainly related to the CC and PG, were considered strictly in a biomechanical context.
These examinations focused on the four cerebral lobes GM, WM, and the brainstem. In consideration of the GM, it was decided to select the finite elements belonging to the sulcus region, given that these areas exhibit an irregular geometry and varying densities and stiffness, thus providing a suitable basis for investigating the concentration of stresses. Moreover, the accumulation of hyperphosphorylated tau (p-tau) in the depths of the sulci has been associated with the initial stages of CTE, motivating further research using integrated biomechanical–clinical frameworks [28]. For the WM, the region of the corona radiata (CR) was selected, given its role in cortical communication. The medulla oblongata and pons were examined due to vulnerability to mechanical loads along WM tracts.
Corpus Callosum and Pituitary Gland Overview
The methodology employed for the analysis of the CC entailed the selection of five regions of finite elements within the structure and four regions in the PG, reducing the likelihood of numerical overestimation or underestimation. All elements of each CC region were averaged for the analysis, and the five partitions are distinguished as follows: the genu, posterior to genu, the midbody or trunk, the isthmus, and the splenium, as illustrated in Figure 1B. On the PG, the surrounding finite elements of the selected points located at the vertices of the anterior and posterior lobes are averaged, as observed in Figure 1C. The examination of these elements is essential for assessing the PG’s mechanical vulnerability, as the posterior lobe relates to oxytocin and vasopressin release. Conversely, the anterior lobe is responsible for the secretion of other significant hormones, including the growth hormone, prolactin, and thyroid-stimulating hormone, among others.
2.3. Data Collection and Car Crash Scenarios
The Federal Highway Research and Transport Institute [17] provided relevant data from three GIDAS cases for the present study. Of the three candidate cases, two were selected based on female occupant involvement. Data included accident reports and photos, parameters of the collision, and medical information on the occupants.
A previous collaboration between the BASt and the Toyota Motor Corporation [18] involved the use of the THUMS to recreate accident scenarios in LS-DYNA, with the resulting dataset comprising linear acceleration and angular velocity values for each case. This procedure is visualised in Figure 2. The THUMS is scaled as necessary for each case, based on the height and weight of the individual occupant, rather than involving case-specific CT or MRI scans of the actual occupants. The human model is installed within the vehicle in the estimated seat position, thus creating the final setting alongside the documented vehicle positions and the overview of the accident situation, ready for the simulation. Finally, the results are analysed to validate the final vehicle positions and their deformation state, determining the part causing the head injury and its location.
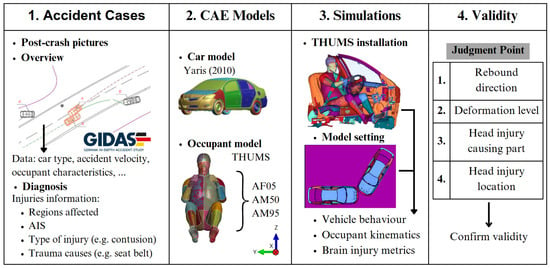
Figure 2.
The selected flow to conduct accident reconstructions by using accident information. Adapted from [18].
In both reconstructed cases, the same FeFEHM geometry, mesh resolution, and material parameters were employed to ensure consistency between simulations. The only variation between cases resided in the imposed boundary conditions (BCs), corresponding to the distinct linear and angular kinematic profiles derived from each THUMS reconstruction. No geometric scaling of the head model was performed, as both female occupants had anthropometric values within 7% of the FeFEHM reference subject (165 cm, 83 kg).
2.3.1. Case 1 Scenario
The accident was caused by car A leaving its own traffic lane and colliding with car B. Figure 3A,B illustrate the accident situation, in which, following the crash, car A moved diagonally rightwards and came to a stop at the edge of the road, while car B was thrown forwards out of the lane.
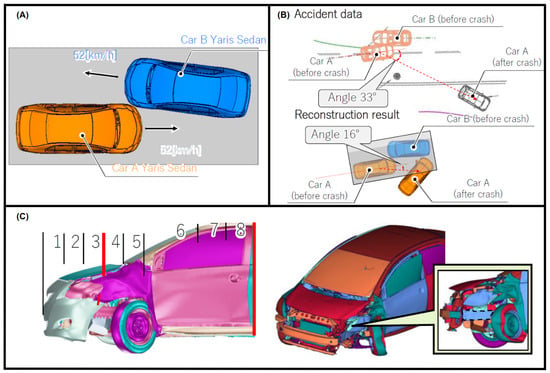
Figure 3.
Accident situation in Case 1: collision occurs after car A runs out of own traffic lane. (A) Travelling speed of each vehicle, (B) THUMS reconstruction results comparison with the accident data, and (C) maximum impact zone (left side) and side member unaffected by the collision (right side). Provided by BASt [17].
The sole occupant of car A, an adult female, was travelling at 52 km/h at the time of the accident, a velocity similar to that of vehicle B, which was occupied by two other individuals. The car A occupant sustained an abrasion to the head, laceration to the upper body, and a fractured chest, as seen in Table 1. She lost consciousness when she hit her head on the headrest, suffering a TBI. Therefore, this individual was selected for the numerical simulations in the THUMS’ study [18] and in this study with the FeFEHM.

Table 1.
Occupant characteristics and injury information in Case 1.
As the car B individuals did not sustain any head injuries, they were not considered for further study. Therefore, the linear acceleration and angular velocity data that was provided as input for the simulation with the FeFEHM was based solely on the occupant that suffered a brain injury. This kinematic data was then induced at the head model’s COG.
Affected impact zones from the THUMS simulation thoroughly replicated the damage in car A, as seen in Figure 3C, according to the GIDAS accident report. To further validate it, as in the real collision, the reconstructed result demonstrates that the side member did not suffer deformation—Figure 3C (right side).
The imposed BCs consisted of translational accelerations and rotational velocities applied to a reference node located at the model’s COG, where the rigid skull is fully linked to prevent body drift. Peak acceleration and velocity values are presented in Table 2. The analyses were conducted within a total time of 0.10 s, using the dynamic ABAQUS/Explicit 2017 solver with double precision. Output request was established at every 0.5 ms. This 100 ms time window corresponds to a segment of the full acceleration time history, presented in Figure A1 in Appendix A, where the instant of peak resultant acceleration was defined as the central point of the loading event, and a 50 ms interval was extracted around this peak.

Table 2.
Peak values of linear acceleration and rotational velocity of the simulated cases.
2.3.2. Case 2 Scenario
The accident occurred in a manner analogous to the previous case: car A crossed its own traffic lane and collided with car B. Figure 4A,B depict the accident situation in which car A has advanced, being turned into the opposite lane. Conversely, the trajectory of car B was maintained, resulting in forward motion.
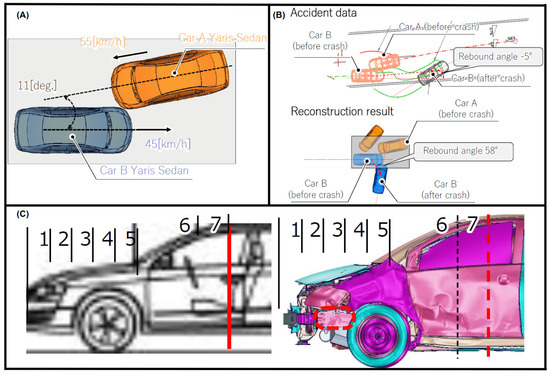
Figure 4.
Similar collision to Case 2: car A run out of own traffic lane. (A) Travelling speed of each vehicle, (B) THUMS reconstruction results comparison with the accident data, and (C) affected contact zone (left side) and side member undeformed by the collision (right side). Provided by BASt [17].
car B, which had five occupants, was travelling at 45 km/h at the time of the collision, while car A’s sole occupant impacted at 55 km/h. The driver B, a senior female individual, sustained the most severe injuries, including haematomas to the upper extremity and a cervical sprain due to the movement of her body, as a result of colliding with the door trim. Upon impact with the headrest, the patient sustained a scalp laceration. Additionally, the deployment of the driver airbag (DAB) resulted in a concussion and cranial nerve injury, which were classified as a brain injury. These factors, presented in Table 3, determined this individual as the one selected for the simulation.

Table 3.
Occupant characteristics and injury information in Case 2.
Deformation level was compared in Figure 4C, where the damage zone ends similarly in both the accident data report and the reconstruction results, and the side member in the simulation also did not deform (right image).
Values of peak acceleration and velocity induced at the FeFEHM’s COG from the selected simulation time are presented in Table 2. The corresponding full-time history plot of the input signals are provided in Figure A2 in Appendix A.
3. Results
3.1. Case 1
The FeFEHM simulation employed translational and rotational kinematics directly extracted from the THUMS reconstruction as BCs applied to the model’s COG. Therefore, both models demonstrate identical head motion trajectories by definition. The objective of the simulation was evaluating the resulting internal strain and stress propagation within the brain tissue under the THUMS’ predicted head kinematics, rather than reproducing the external motion itself. As demonstrated in Figure 5A, the THUMS reconstruction indicated a lateral head contact with the roof side rail, consistent with the anterior–lateral deformation direction observed in the FeFEHM in Figure 6A, confirming that the prescribed kinematics generated strain-wave propagation aligned with the reconstructed impact direction. However, the THUMS reconstruction yielded minor discrepancies with the contact location of the accident report, possibly resulting from the steering wheel and pedals’ interaction upon collision.
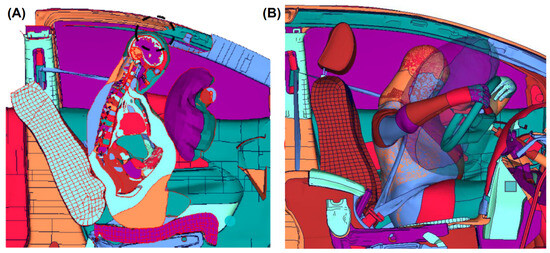
Figure 5.
(A) Contact between the head and the roof side rail. (B) The accident reconstruction demonstrates the head being restrained by the DAB. Reconstructed and provided using the LS-DYNA software by the Toyota Motor Corporation [29].
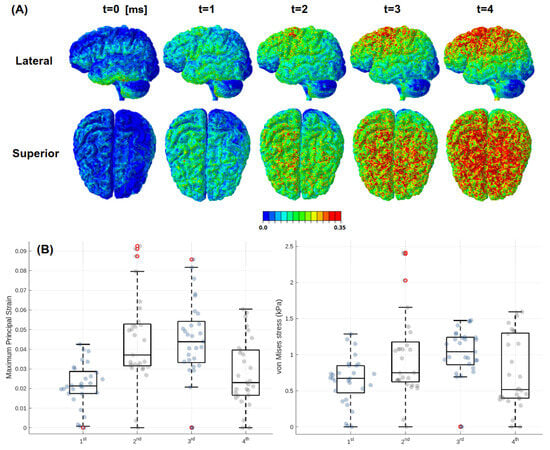
Figure 6.
Strain and stress distribution in the FeFEHM during Case 1 impact. (A) MPS analysis of a series of frame slices from the moment of impact and (B) box plots analyses of the MPS and von Mises stress conducted on the four ends of the PG.
The resulting strain distribution was concentrated in the upper brain regions, particularly at the parietal and temporal lobes, observed in Figure 6. Qualitatively, a 0.35 MPS threshold was established to reveal potential injury risks from regions surpassing it, as demonstrated in some FEHMs [30]. Yet, at 0.5 MPS, no significant alterations were observed. Peak values of the selected criteria are presented in Table 4, where MPS values reached 0.52 in the parietal lobe, followed by 0.45 in the temporal lobe. Frontal and occipital lobes revealed lower deformation: 0.31 and 0.19 strain, respectively. Quantitative results also reflected the predominant anterior–lateral loading direction. Von Mises stresses remained below 20 kPa in all lobes, while intracranial pressure peaked at 38 kPa. CR WM deformation was up to 0.28 and the stresses were over 7 kPa. The brainstem was less affected, with low pressure (2.2 kPa) and stress (1.5 kPa) values, though localized strains have been observed (0.24). The four extremities of the PG are analysed in Figure 6B, revealing minimal deformation, with none of the finite elements exceeding 0.1 strain or 3 kPa stress.

Table 4.
Maximum value reached for the injury criteria in the cerebral lobes, WM, and brainstem for Case 1.
3.2. Case 2
In Takahira et al.’s [18] study, the accident reconstruction accurately reproduced the contacts reported in the accident report, including the head restraint by the deployment of the DAB, observed in Figure 5B. The FeFEHM simulation closely matched the contact sequence reported in the GIDAS case and replicated in the THUMS reconstruction. Qualitatively, in Figure 7A, it is observed that in the upper brain area of the frontal lobe, the head is no longer in contact with the DAB but is subject to the rebound phase and contact with the headrest.
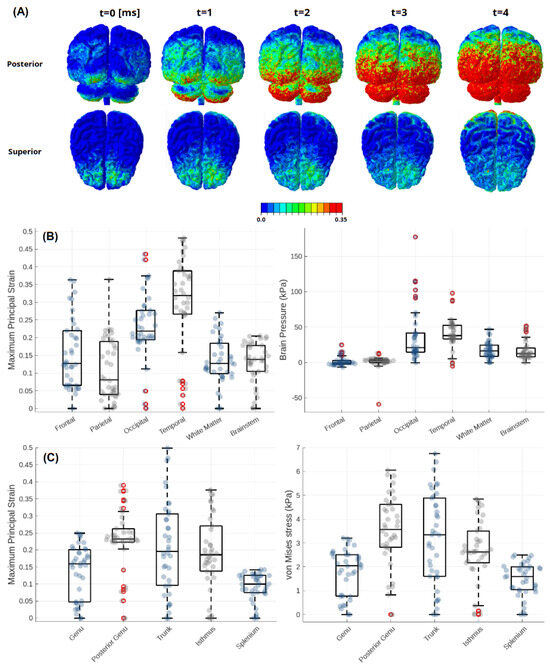
Figure 7.
Results obtained for Case 2: (A) Sequence of different perspectives on the MPS. (B) Box plots analyses of the MPS and pressure conducted on several brain regions and (C) analysis of the MPS and von Mises stress in the selected sections of the CC.
Peak criteria values are set forth in Table 5 and Figure 7B. It was found that the temporal and occipital lobe sulcus exhibited the most significant impact, with values reaching 0.48 and 0.44 strain, respectively. Frontal and parietal lobes reached 0.36 strain, while the brainstem and WM were the least affected, at 0.21 and 0.27, respectively. Pressure was highest in the occipital lobe (177 kPa), reflecting the rebound contact with the headrest, but decreasing to the anterior regions, with frontal lobe peak at 25 kPa and parietal lobe at 13 kPa.

Table 5.
Maximum value reached for the injury criteria in the cerebral lobes, WM, and brainstem for Case 2.
Regarding the CC, as depicted in Figure 7C, it was revealed that notable mechanical loading was present across most regions, except the splenium. The trunk reached the highest MPS at 0.5, while the posterior genu and isthmus recorded 0.39 and 0.38, respectively. Von Mises stress remained below 7 kPa in all regions.
4. Discussion
This study employed the FeFEHM to the kinematic input of two THUMS reconstructed vehicular accidents, involving female occupants, enabling evaluation of brain injury mechanisms under real-world loading conditions. The primary objective relies on the feasibility and practicality of using the current female head model in crash biomechanics studies, instead of determining clinical injury outcomes or any statistically sex-based difference.
The time interval under analysis (100 ms) is derived from the maximum value of the acceleration vector calculation. For instance, in Case 2, this initial peak was found to correspond to the moment of rebound and collision with the headrest of the individual in car B, immediately following the impact with the DAB. It must be noted that while the simulation represents the highest recorded acceleration value, this does not indicate that this was the primary cause of the brain injuries presented, which included a concussion and cranial nerve injury; a sequence of head motions likely contributed to injury. These include a variety of head movements, including forward motion towards the airbag following the initial collision, primary impact with the airbag, rebound motion moving backwards, and headrest contact. It is possible that the combined effects of multiple impacts may be the cause of the brain injuries.
Across the simulations, the FeFEHM did not produce strain and stress patterns qualitatively consistent with the Case 1 clinical report, since the simulated kinematics slightly diverged from the accident report, likely due to steering wheel or pedal interaction, with the simulation suggesting a potential head impact against the roof side rail. The result of the FEHM analysis was anticipated, given that linear acceleration and angular velocity inputs were derived from the THUMS simulation. Thus, it also presented an impact against the roof side rail, as revealed by the strain pattern in Figure 6A. Deformation in the PG of Case 1 was minimal, with peak strain remaining below 0.1. These values may reflect the anterior–lateral loading path and the PG’s central location, protected within the skull base. Despite its modelling, no injury criteria thresholds were established at the PG for the current knowledge of the authors, rejecting any attempt to quantitatively compare it to other brain regions’ thresholds. Conversely, Case 2 simulation closely matched the reported injury mechanism, replicating the brain injuries mechanism caused by the deployment of the DAB. The vehicle’s momentum caused forward motion into the airbag, followed by a rebound. This backward rebound could result in notable strain and stress in the posterior regions (occipital lobe and cerebellum), as the brain shifts towards the back of the skull due to the inertia, with another impact occurring with the headrest, which led to a scalp laceration in this region, as evidenced by the GIDAS accident injury report. This dual-phase motion contributed to high MPS in the left temporal lobe and occipital lobes region, corroborating the rebound motion and impact with the headrest. Notably, pressure levels in the occipital lobe and brainstem were considerably higher than Case 1, peaking at 177 kPa and 51 kPa, respectively. This likely corresponds to the rapid deceleration and rebound phase, as the brain compresses against the posterior skull, following initial anterior acceleration. Within the same Case 2, the CC demonstrated higher strains to the mid section of this component, with the trunk reaching 0.5 MPS and posterior genu 0.39, while the splenium remained relatively unaffected. The distribution suggests axial stretch and shear concentration in the mid regions, indicating its vulnerability under rotational and rebound conditions.
Wu et al. [31] evaluated brain injury metrics with an existing database of 31 National Football League (NFL) head impact reconstruction cases, where 20 concerned players were diagnosed with mild TBI (mTBI). From another database, 22 subjects were selected from 335 sled tests, in which 50 non-injurious cases were simulated. The results reported that a 50% risk of mTBI occurred at a MPS95 (applying a 95th percentile filter) of 0.27, whereas severe TBI (sTBI) was typically associated with MPS95 values exceeding 0.40. In the present simulations, the MPS peaked at 0.52 in the parietal lobe (Case 1) and 0.48 in the temporal lobe (Case 2), which fall within the strain range reported for high-severity brain loading. These values represent peak strain, as the 95th percentile filtering used to mitigate localized numerical overestimation was not applied in this study. Therefore, the actual mean tissue deformation would likely be slightly lower. Comparable strain magnitudes were also reported by Carlsen et al. [14], whose FEA indicated that head rotations of 50–60 rad/s produce MPS levels of 0.25–0.45, depending on impact direction and duration. Given that the angular velocities in the current cases were 50.2 rad/s (Case 1) and 60.5 rad/s (Case 2), the observed strain responses are consistent with those predicted in previous studies.
In addition to strain-based metrics, intracranial pressure levels have the capacity to provide complementary insight into injury severity. Earlier findings of cadaveric and computational investigations indicated that mTBI has been shown to occur typically at peak pressures of up to 173 kPa, with more severe injuries being associated with pressures exceeding 200 kPa [26,27]. In Case 1 presented in Table 4, pressure magnitudes in all brain regions remained below 39 kPa, thus falling within the range of non-injurious to mTBI thresholds. In Case 2, shown in Table 5, the pressure values indicated a peak of 177.27 kPa in the occipital lobes, which is consistent with the expected location of coup injury, given that the simulation is based on the headrest impact. This value also corroborates the possibility of mTBI by the 0.44 strain threshold. However, Figure 7B demonstrates that this pressure peak is punctual and that interquartile limits range from 25 to 70 kPa.
Despite the application of identical head geometry and constitutive parameters in both cases, age-related differences in actual brain tissue stiffness may influence the mechanical response. Experimental magnetic resonance elastography (MRE) research has indicated that the shear modulus of brain tissue has a tendency to decrease with age, typically by 11 to 15 Pa per year in healthy brains, attributed to microstructural degeneration of axonal and vascular networks, and reduced myelination [32,33]. This suggests that the 60-year-old subject in Case 2 might experience higher local strains than, for instance, the younger 58-year-old subject in Case 1, if exposed to identical acceleration inputs, despite the FeFEHM (parameterized for a 65-year-old female) predicting similar results for both.
Taken together, the results of both cases highlight the potential value of integrating the FeFEHM into accident reconstruction workflows, providing regional mechanical responses to head kinematics that qualitatively aligned with reported injury mechanisms and spatial stress and stress patterns. This suggests that the current female-specific model may enhance the contextual understanding of female head biomechanics when subject-specific imaging data are not available.
However, it is important to emphasize that no male head was simulated under the same conditions, and thus no conclusions can be drawn about sex-specific differences in mechanical response. Furthermore, while strain values were compared to thresholds from other studies, the mentioned differences in mesh density, element formulation, and material modelling limit this applicability. Importantly, this study does not attempt to correlate model outputs with actual injury occurrence beyond basic qualitative trends.
The present study demonstrates the potential of the FeFEHM to predict head dynamics and injury risk during the two vehicular accidents impacts. While our results supports its feasibility, there are limitations that must be taken into consideration. Only two crash cases involving female occupants were analysed. While these were selected based on relevance to the study, the sample size is insufficient to draw generalised conclusions about injury risks or sex-specific biomechanical responses. Broader statistical comparisons, crucially including male–female simulations, are necessary to explore these differences meaningfully. Additionally, this study was confined to the utilisation of the FeFEHM, which comprises age-specific data pertaining to the skull’s anthropometry and the brain tissue properties. The employment of more female FEHMs, with the capacity to be age-differentiated, has a potential advantage in enhancing the precision of anatomical representation across age-specific groups. While the employment of the FeFEHM is consistent with the objective of the study, a male head model under identical conditions would be necessary to ascertain whether differences are attributable to sex-specific biomechanical properties or merely anthropometric variations. Regarding the material characterisation, the employed CSF material, represented by a Mooney–Rivlin formulation, results in a higher shear modulus magnitude order than the stiffness of the physiological CSF, although it provided stable numerical coupling between the skull and brain. Therefore, the model likely overestimates shear-wave transmission and local intracranial pressures. Consequently, some authors have opted for the smoothed-particle hydrodynamics (SPH) method [34,35]. While hyperelastic simplification ensures a good compromise between accuracy and efficiency, such mesh-free methods increase computational demand but capture the fluid interaction more accurately.
For the numerical results, current comparisons to the literature criteria were used in a qualitative and comparative manner to contextualize the mechanical responses. This was due to the fact that direct quantitative strain and pressure results cannot be assumed until the current model is calibrated.
The THUMS reconstruction methodology employed a Toyota Yaris (2010) for the selected simulations, available on the National Highway Traffic Safety Administration’s (NHTSA) website [36], a different model from the accidents situation. Accordingly, while THUMS included head scaling, no CT or MRI data from the actual occupants were available. As such, applied anthropometry is approximate and does not account for individual variability. Although chosen as the most suitable for the study, such differences could affect the occupant’s kinematic and injury responses.
5. Conclusions
Despite the development of safer technologies, TBI remains a significant concern in road traffic accidents. The present study demonstrated the feasibility of employing a female-specific head model, the FeFEHM, to analyse the biomechanical responses under two real-world road traffic accident scenarios, using kinematic data derived from the THUMS validated full-body model. Simulations provided brain regional mechanical responses that aligned with strain and stress patterns from the THUMS reconstruction and accident report.
While these results highlight the potential of female-specific models in accident analysis and injury biomechanics research, they do not establish sex-based differences in injury response or clinical outcomes, as it is also limited by its small sample size and lack of subject-specific anatomical data.
Future work should expand the current dataset to include more case studies, preferentially incorporating male–female simulations, to pursue more validation against experimental and clinical data. Additionally, quantitative calibration of the FeFEHM against experimental data to establish model-specific injury thresholds would improve predicted accuracy, as would the additional integration of more injury metrics, such as the Cumulative Strain Damage Model (CSDM) and BrIC. While the calibration is not established, a parametric sensitivity study of the input signals would enhance the robustness of the model’s qualitative response. With such developments, sex-specific head models may contribute to our understanding of brain injury mechanisms and the long-term goal of refinement of safety mechanisms and public health.
Author Contributions
C.G.S.C.: Writing—original draft and editing, Methodology, Investigation, Validation, Software; A.E.: Resources, Writing—review, Supervision; M.W.: Resources, Writing—review, Supervision; F.A.O.F.: Project administration, Supervision; R.J.A.d.S.: Project administration, Writing—review, Supervision, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
University of Aveiro authors acknowledge the support given by the Portuguese Science Foundation under grants PTDC/EME-EME/1239/2021, 2023.11365.PEX and UIDP 00481 Centre for Mechanical Technology and Automation; and CENTRO-01-0145-FEDER-022083—Portugal Regional Operational Programme (Centro2020) under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The kinematic data used in this study are not openly available to protect the privacy of participants. The corresponding author may make this data available upon request.
Acknowledgments
The authors express their gratitude to Toyota Motor Corporation for providing the head acceleration data derived from the THUMS simulations.
Conflicts of Interest
The authors report no financial conflicts of interest in regard to the research reported herein.
Appendix A
In this appendix, we present the acceleration and velocity data prescribed at the FeFEHM’s COG to replicate the THUMS reconstruction simulation (Figure A1 and Figure A2).
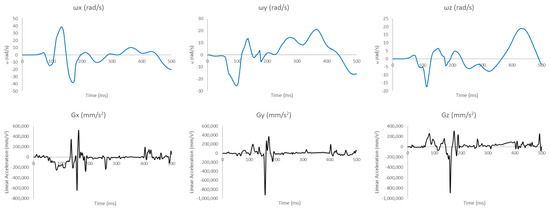
Figure A1.
Linear accelerations and angular velocities acquired from the THUMS reconstruction for Case 1.
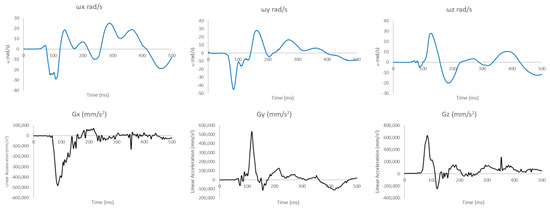
Figure A2.
Linear accelerations and angular velocities acquired from the THUMS reconstruction for Case 2.
References
- Hughes, N.; Williams, W.H.; Chitsabesan, P.; Walesby, R.C.; Mounce, L.T.A.; Clasby, B. The prevalence of traumatic brain injury among young offenders in custody. J. Head Trauma Rehabil. 2015, 30, 94–105. [Google Scholar] [CrossRef]
- Lavoie, S.; Sechrist, S.; Quach, N.; Ehsanian, R.; Duong, T.; Gotlib, I.H.; Isaac, L. Depression in men and women one year following traumatic brain injury (TBI): A TBI model systems study. Front. Psychol. 2017, 8, 634. [Google Scholar] [CrossRef]
- Viano, D.C.; Parenteau, C.S. Concussion, Diffuse Axonal Injury, and AIS4+ Head Injury in Motor Vehicle Crashes. Traffic Inj. Prev. 2015, 16, 747–753. [Google Scholar] [CrossRef]
- Carroll, L.J.; Cassidy, J.D.; Cancelliere, C.; Côté, P.; Hincapié, C.A.; Kristman, V.L.; Holm, L.W.; Borg, J.; Boussard, C.N.-D.; Hartvigsen, J. Systematic review of the prognosis after mild traumatic brain injury in adults: Cognitive, psychiatric, and mortality outcomes: Results of the international collaboration on mild traumatic brain injury prognosis. Arch. Phys. Med. Rehabil. 2014, 95, S152–S173. [Google Scholar] [CrossRef]
- Mazure, C.M.; Jones, D.P. Twenty years and still counting: Including women as participants and studying sex and gender in biomedical research. BMC Women’s Health 2015, 15, 94. [Google Scholar] [CrossRef]
- Blaya, M.O.; Raval, A.P.; Bramlett, H.M. Traumatic brain injury in women across lifespan. Neurobiol. Dis. 2022, 164, 105613. [Google Scholar] [CrossRef]
- Valera, E.M.; Joseph, A.-L.C.B.; Snedaker, K.L.; Breiding, M.J.; Robertson, C.L.; Colantonio, A.; Levin, H.; Pugh, M.J.; Yurgelun-Todd, D.; Mannix, R.; et al. Understanding Traumatic Brain Injury in Females: A State-of-the-Art Summary and Future Directions. J. Head Trauma Rehabil. 2021, 36, E1–E17. [Google Scholar] [CrossRef] [PubMed]
- Sass, D.; Guedes, V.A.; Smith, E.G.; Vorn, R.; Devoto, C.; Edwards, K.A.; Mithani, S.; Hentig, J.; Lai, C.; Wagner, C.; et al. Sex differences in behavioral symptoms and the levels of circulating gfap, tau, and nfl in patients with traumatic brain injury. Front. Pharmacol. 2021, 12, 746491. [Google Scholar] [CrossRef] [PubMed]
- Bah, M. Racial and gender disparities in traumatic brain injury clinical trial enrollment. Neurosurg. Focus 2023, 55, E11. [Google Scholar] [CrossRef]
- McKee, A.C.; Mez, J.; Abdolmohammadi, B.; Butler, M.; Huber, B.R.; Uretsky, M.; Babcock, K.; Cherry, J.D.; Alvarez, V.E.; Martin, B.; et al. Neuropathologic and Clinical Findings in Young Contact Sport Athletes Exposed to Repetitive Head Impacts. JAMA Neurol. 2023, 80, 1037–1050. [Google Scholar] [CrossRef] [PubMed]
- Fesharaki-Zadeh, A. Chronic Traumatic Encephalopathy: A Brief Overview. Front. Neurol. 2019, 10, 713. [Google Scholar] [CrossRef]
- Munivenkatappa, A.; Agrawal, A.; Shukla, D.P.; Kumaraswamy, D.; Devi, B.I. Traumatic brain injury: Does gender influence outcomes? Int. J. Crit. Illn. Inj. Sci. 2016, 6, 70. [Google Scholar] [CrossRef]
- Eagle, S.R.; Pease, M.; Nwachuku, E.; Deng, H.; Okonkwo, D.O. Prognostic models for traumatic brain injury have good discrimination but poor overall model performance for predicting mortality and unfavorable outcomes. Neurosurgery 2022, 92, 137–143. [Google Scholar] [CrossRef]
- Carlsen, R.W.; Fawzi, A.L.; Wan, Y.; Kesari, H.; Franck, C. A quantitative relationship between rotational head kinematics and brain tissue strain from a 2-D parametric finite element analysis. Brain Multiphys. 2021, 2, 100024. [Google Scholar] [CrossRef]
- Perkins, R.A.; Bakhtiarydavijani, A.; Ivanoff, A.E.; Jones, M.; Hammi, Y.; Prabhu, R.K. Assessment of brain injury biomechanics in soccer heading using finite element analysis. Brain Multiphys. 2022, 3, 100052. [Google Scholar] [CrossRef]
- GIDAS (German In-Depth Accident Study). 2025. Available online: https://www.gidas.org/start-en.html (accessed on 27 October 2025).
- BASt. Federal Highway Research and Transport Institute (Bundesanstalt für Straßenwesen). 1951. Available online: https://www.bast.de/EN/BASt/BASt_node.html (accessed on 27 October 2025).
- Takahira, Y.; Koshizako, K.; Hayashi, S.; Imai, H. Prediction of Brain Injury in Vehicle Accident Reconstruction using THUMS. In Proceedings of the IRCOBI Pre-Conference Workshop, Stockholm, Sweden, 10 September 2024. [Google Scholar]
- Carmo, G.P.; Dymek, M.; Ptak, M.; Alves-de-Sousa, R.J.; Fernandes, F.A. Development, validation and a case study: The female finite element head model (FeFEHM). Comput. Methods Programs Biomed. 2023, 231, 107430. [Google Scholar] [CrossRef] [PubMed]
- Menichetti, A.; MacManus, D.B.; Gilchrist, M.D.; Depreitere, B.; Sloten, J.V.; Famaey, N. Regional characterization of the dynamic mechanical properties of human brain tissue by microindentation. Int. J. Eng. Sci. 2020, 155, 103355. [Google Scholar] [CrossRef]
- Puso, M.A.; Weiss, J.A. Finite Element Implementation of Anisotropic Quasi-Linear Viscoelasticity Using a Discrete Spectrum Approximation. J. Biomech. Eng. 1998, 120, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, M.D. Modelling and Accident Reconstruction of Head Impact Injuries. Key Eng. Mater. 2003, 245–246, 417–432. [Google Scholar] [CrossRef]
- Bouchonville, N.; Meyer, M.; Gaude, C.; Gay, E.; Ratel, D.; Nicolas, A. AFM mapping of the elastic properties of brain tissue reveal kPa/-gradients of rigidity. Soft Matter 2016, 12, 6232–6239. [Google Scholar] [CrossRef] [PubMed]
- Alshareef, A.; Giudice, J.S.; Forman, J.; Salzar, R.S.; Panzer, M.B. A novel method for quantifying human in situ whole brain deformation under rotational loading using sonomicrometry. J. Neurotrauma 2018, 35, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.S.; Carmo, G.P.; Ptak, M.; Fernandes, F.A.O.; de Sousa, R.J.A. Accuracy and efficiency of finite element head models: The role of finite element formulation and material laws. Int. J. Numer. Methods Biomed. Eng. 2024, 40, e3851. [Google Scholar] [CrossRef]
- Fernandes, F.A.; de Sousa, R.J.A. Head injury predictors in sports trauma—A state-of-the-art review. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2015, 229, 592–608. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.A.O.; Alves de Sousa, R.J.; Ptak, M. Finite Element Head Modelling and Head Injury Predictors. In Head Injury Simulation in Road Traffic Accidents; SpringerBriefs in Applied Sciences and Technology; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Katz, D.I.; Bernick, C.; Dodick, D.W.; Mez, J.; Mariani, M.L.; Adler, C.H.; Alosco, M.L.; Balcer, L.J.; Banks, S.J.; Barr, W.B.; et al. National Institute of Neurological Disorders and Stroke Consensus Diagnostic Criteria for Traumatic Encephalopathy Syndrome. Neurology 2021, 96, 848–863. [Google Scholar] [CrossRef]
- Corporation, Toyota Motor. Total Human Model for Safety (THUMS): Revolutionizing Crash Simulation to Support Safe Mobility for All. 2021. Available online: https://www.toyota.co.jp/thums/ (accessed on 16 December 2024).
- Takhounts, E.G.; Craig, M.J.; Moorhouse, K.; McFadden, J.; Hasija, V. Development of brain injury criteria (BrIC). Stapp Car Crash J. 2013, 57, 243–266. [Google Scholar] [CrossRef]
- Wu, T.; Hajiaghamemar, M.; Giudice, J.S.; Alshareef, A.; Margulies, S.S.; Panzer, M.B. Evaluation of Tissue-Level Brain Injury Metrics Using Species-Specific Simulations. J. Neurotrauma 2021, 38, 1879–1888. [Google Scholar] [CrossRef]
- Arani, A.; Murphy, M.C.; Glaser, K.J.; Manduca, A.; Lake, D.S.; Kruse, S.A.; Jack, C.R.; Ehman, R.L.; Huston, J. Measuring the effects of aging and sex on regional brain stiffness with MR elastography in healthy older adults. NeuroImage 2015, 111, 59–64. [Google Scholar] [CrossRef]
- Sack, I.; Beierbach, B.; Wuerfel, J.; Klatt, D.; Hamhaber, U.; Papazoglou, S.; Martus, P.; Braun, J. The impact of aging and gender on brain viscoelasticity. NeuroImage 2009, 46, 652–657. [Google Scholar] [CrossRef]
- Wilhelm, J.; Ptak, M.; Fernandes, F.A.O.; Kubicki, K.; Kwiatkowski, A.; Ratajczak, M.; Sawicki, M.; Szarek, D. Injury biomechanics of a child’s head: Problems, challenges and possibilities with a new aHEAD finite element model. Appl. Sci. 2020, 10, 4467. [Google Scholar] [CrossRef]
- Toma, M.; Nguyen, P.D. Fluid–structure interaction analysis of cerebrospinal fluid with a comprehensive head model subject to a rapid acceleration and deceleration. Brain Inj. 2018, 32, 1576–1584. [Google Scholar] [CrossRef]
- National Highway Traffic Safety Administration. Crash Simulation Vehicle Models. U.S. Department of Transportation. Available online: https://www.nhtsa.gov/crash-simulation-vehicle-models (accessed on 12 June 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).