1. Introduction
M-type hexaferrites, with the general formula AFe
12O
19 (A = Ba, Sr, or Pb), are among the most important classes of hard magnetic materials due to their high magnetocrystalline anisotropy, large coercivity (H
C), high Curie temperature, and excellent chemical stability [
1,
2,
3]. These properties make them highly attractive for permanent magnet applications such as motors, loudspeakers, microwave devices, and magnetic recording media. Sr or Ba hexaferrites have been widely studied and utilized because of their superior hard magnetic properties and ease of synthesis.
The SrFe
12O
19 (SrM) compound possesses a crystal structure composed of alternating spinel (S, Fe
6O
8) and hexagonal (R, SrFe
6O
11) blocks, arranged in the sequence RSR* S*, where the asterisk denotes a 180° rotation around the hexagonal c-axis [
4]. Within one unit cell, twenty-four Fe
3+ ions occupy five distinct crystallographic sites in the oxygen lattice: one tetrahedral (4
f1), three octahedral (12
k, 2
a, and 4
f2), and one trigonal bipyramidal (2
b) site. The magnetic structure originates from superexchange interactions between Fe
3+ and O
2− ions, resulting in parallel spin alignment along the c-axis for Fe
3+ ions at the 12
k, 2
a, and 2
b sites, and antiparallel alignment for those at the 4
f1 and 4
f2 sites. Consequently, the net magnetic moment per formula unit of SrM (half of the unit cell) is 20 μ
B at 0 K. However, this theoretical value essentially defines the upper limit of the saturation magnetization (M
S) of SrM. Although SrM exhibits relatively high M
S and strong magnetocrystalline anisotropy, which make it suitable for permanent magnet applications, its intrinsic magnetic properties remain inadequate for advanced applications, particularly in automotive traction motors, high-efficiency BLDC (Blushless DC) motors, and energy-harvesting or power-generation systems.
To address these limitations, extensive studies have focused on modifying the intrinsic magnetic behavior of M-type hexaferrites through the substitution of rare-earth (RE) ions at either the A-site (Ba, Sr) or Fe-site positions [
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20]. Early systematic investigations by Grossinger et al. revealed that RE ions such as La, Ce, and Nd can substantially enhance magnetocrystalline anisotropy and H
C by inducing lattice distortion within the hexagonal magnetoplumbite framework [
5]. More recently, Lobo Guerrero et al. confirmed through a comprehensive review that RE substitution (La, Ce, Gd, Sm) tends to increase H
C and thermal stability while simultaneously improving grain homogeneity in both BaM and SrM systems [
6]. Furthermore, complementary experiments by Todkar et al. demonstrated that Ce–Dy co-doping in BaM nanoparticles yields remarkably high H
C suitable for microwave absorber applications, illustrating the technological significance of RE incorporation for permanent magnet design [
7].
Additionally, several mechanistic studies have clarified how RE and transition-metal co-substitution modify magnetic anisotropy and exchange coupling. Ueda et al. reported that La–Co substitution in SrM enhances the anisotropy field through spin–orbit coupling between Co
2+ and Fe
2+ ions [
8], while Cao et al. highlighted that partial Ce
3+ ion substitution in SrM stabilizes the M-type phase but can also lead to secondary Ce-rich phases at higher concentrations [
9]. Similarly, Huang et al. revealed that Gd–Zn co-doping in Ba–Sr ferrites improves M
S and suppresses abnormal grain growth during sol–gel synthesis [
10]. Furthermore, Islam et al. demonstrated that Cu–Gd co-substitution in SrM increases both H
C and magnetization simultaneously due to synergistic modifications of Fe–O superexchange pathways [
11]. Despite these advances, most RE substitution studies have primarily focused on single-site doping or RE–transition-metal co-doping, whereas systematic investigations combining rare-earth and alkaline-earth-modifying cations such as Mg
2+ remain limited. Therefore, exploring RE–Mg co-substitution offers a promising approach to simultaneously enhance magnetocrystalline anisotropy and refine the microstructure, paving the way for environmentally benign, Co-free hard ferrite magnets optimized for high-performance applications. In addition, Mg
2+ plays a crucial role in controlling grain growth and refining the microstructure, which is expected to improve the hard magnetic properties further.
In this context, the present study investigates the synthesis and characterization of La–Mg-substituted SrM prepared via a conventional ceramic route. Systematic substitutions at both the Sr and Fe sites were carried out to optimize the balance between remanence and HC. Furthermore, various sintering additives, including SiO2, CaO, CaCO3, Al2O3, Cr2O3, SrCO3, and Bi2O3, were examined to improve densification and microstructural uniformity. The correlations among substitution composition, processing parameters, microstructure, and the resulting hard magnetic properties are discussed in detail, providing valuable insights for the design of advanced hexaferrite permanent magnets suitable for high-performance applications.
2. Materials and Methods
M-type hexaferrites with the chemical compositions Sr1−xLaxFe12−xMgxO19 (x = 0, 0.05, 0.1, 0.15) were synthesized via the conventional solid-state reaction method. The precursor and sintering additive powders—Fe2O3 (99.5%, industrial use), SiO2 (99.0%, industrial use), SrCO3, La2O3, MgO, CaO, CaCO3, Al2O3, Cr2O3, CuO (all 99.9% purity, Kojundo Chemical Lab. Co., Ltd., Sakado, Japan), and Bi2O3 (99.9% purity, Aldrich, St. Louis, MO, USA) —were weighed according to the desired cation ratios. Each powder mixture (50.00 g) was placed in a polypropylene bottle with yttria-stabilized ZrO2 balls and deionized (DI) water and ball-milled at a rotation speed of 100 rpm for 24 h. Then, the mixed powder was dried in an oven, placed in an alumina crucible, and calcined at a temperature of 1100 °C in air for 4 h. The calcined powders were ground, sieved, ball-milled again for 20 h, and dried. The ball-milled powders were pressed in a disk-shaped mold with a diameter of 30.0 mm at a pressure of approximately 15 MPa to produce green compact pellets. The pelletized samples were sintered in air at a temperature of 1230 °C for 2 h.
The sample density was calculated based on the weight and geometric dimensions of the disk-shaped samples. X-ray diffraction (XRD; D2 Phaser, Bruker, Billerica, MA, USA) was conducted for phase identification using a Cu-Kα radiation source (λ = 0.15406 nm) over a 2θ range of 20–70°, with a step size of 0.0197° and a total acquisition time of 18 min per sample. Field-emission scanning electron microscopy (FE-SEM; JSM-7610F, JEOL, Akishima, Japan) was used for phase identification. The average grain size was determined from the SEM micrographs using the line-intercept method. The magnetic hysteresis curves were measured at room temperature using a vibrating-sample magnetometer (VSM; Lakeshore 7410, Westerville, OH, USA) and a B–H loop tracer (BH-5501, Denshijiki Industry, Tokyo, Japan). For the VSM measurements, rectangular sintered samples (~2.0 mm × ~1.5 mm × ~0.8 mm) were tested under magnetic fields ranging from −23 to 23 kOe. The B–H loop tracer measurements were conducted on disk-shaped samples (diameter ~24 mm, thickness ~6 mm) by applying a maximum field of 20 kOe and then decreasing it to −20 kOe to obtain the demagnetization curves.
3. Results and Discussion
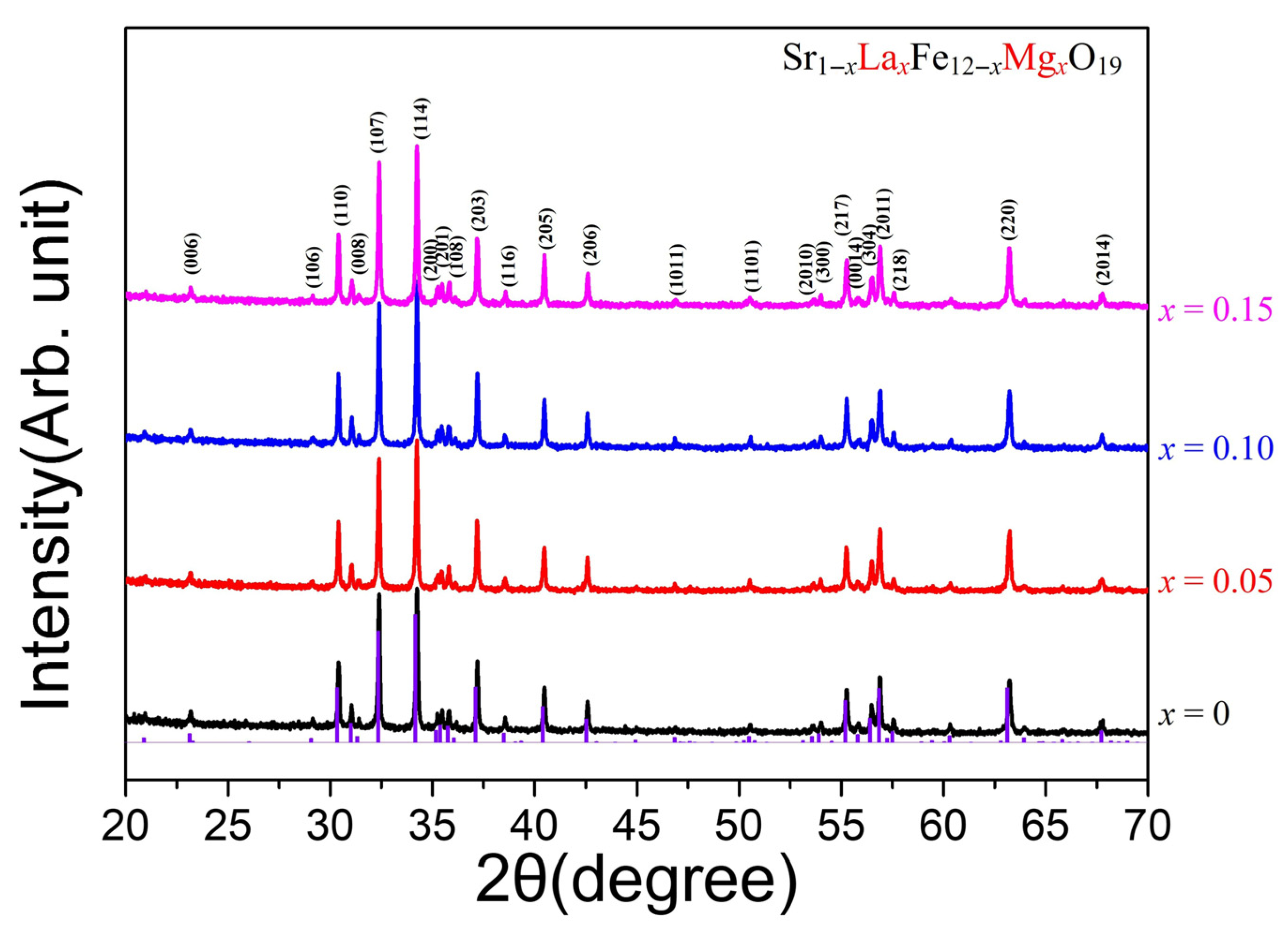
Figure 1 shows the powder XRD patterns of the Sr
1−xLa
xFe
12−xMg
xO
19 (x = 0, 0.05, 0.1, 0.15) samples sintered at 1230 °C. The diffraction peaks for the M-type hexaferrites were indexed based on hexagonal magnetoplumbite crystal structures with space group
P6
3/mmc. All the diffraction peaks are well matched with the standard data from the International Center for Diffraction Data (ICDD, PDF no. 00-33-1340), and no secondary phases were detected, confirming the formation of a single-phase M-type hexaferrite structure in all samples. This result indicates that La and Mg ions were successfully incorporated into the Sr and Fe sites, respectively. However, no significant XRD peak shift was observed with varying x values; therefore, the variation in lattice parameters was not determined.
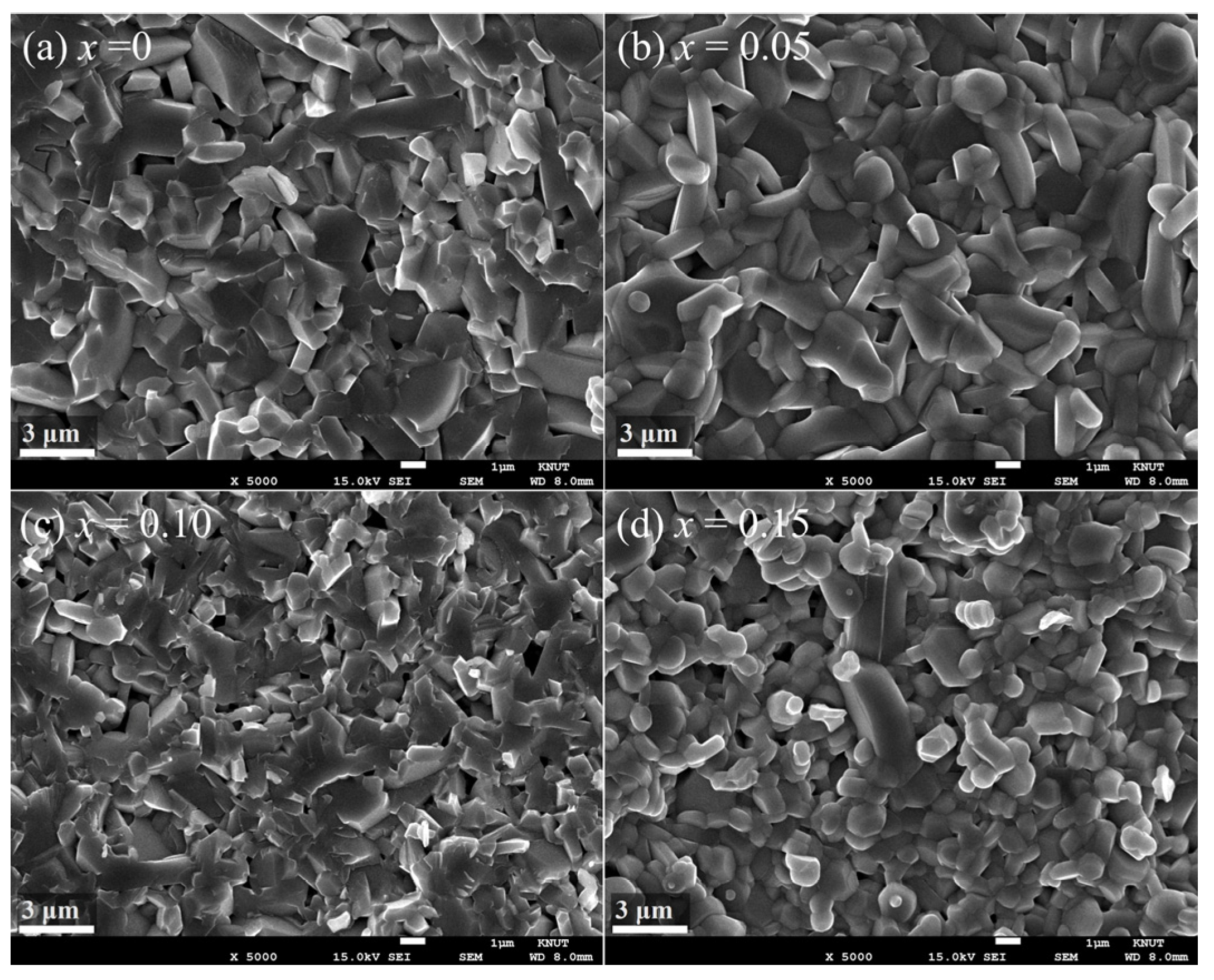
Figure 2a–d show the SEM micrographs of Sr
1−xLa
xFe
12−xMg
xO
19 (x = 0, 0.05, 0.1, 0.15) samples, illustrating the microstructural evolution with varying La–Mg substitution levels. All samples were sintered at 1230 °C with the addition of 0.5 wt% SiO
2 and 1 wt% CaCO
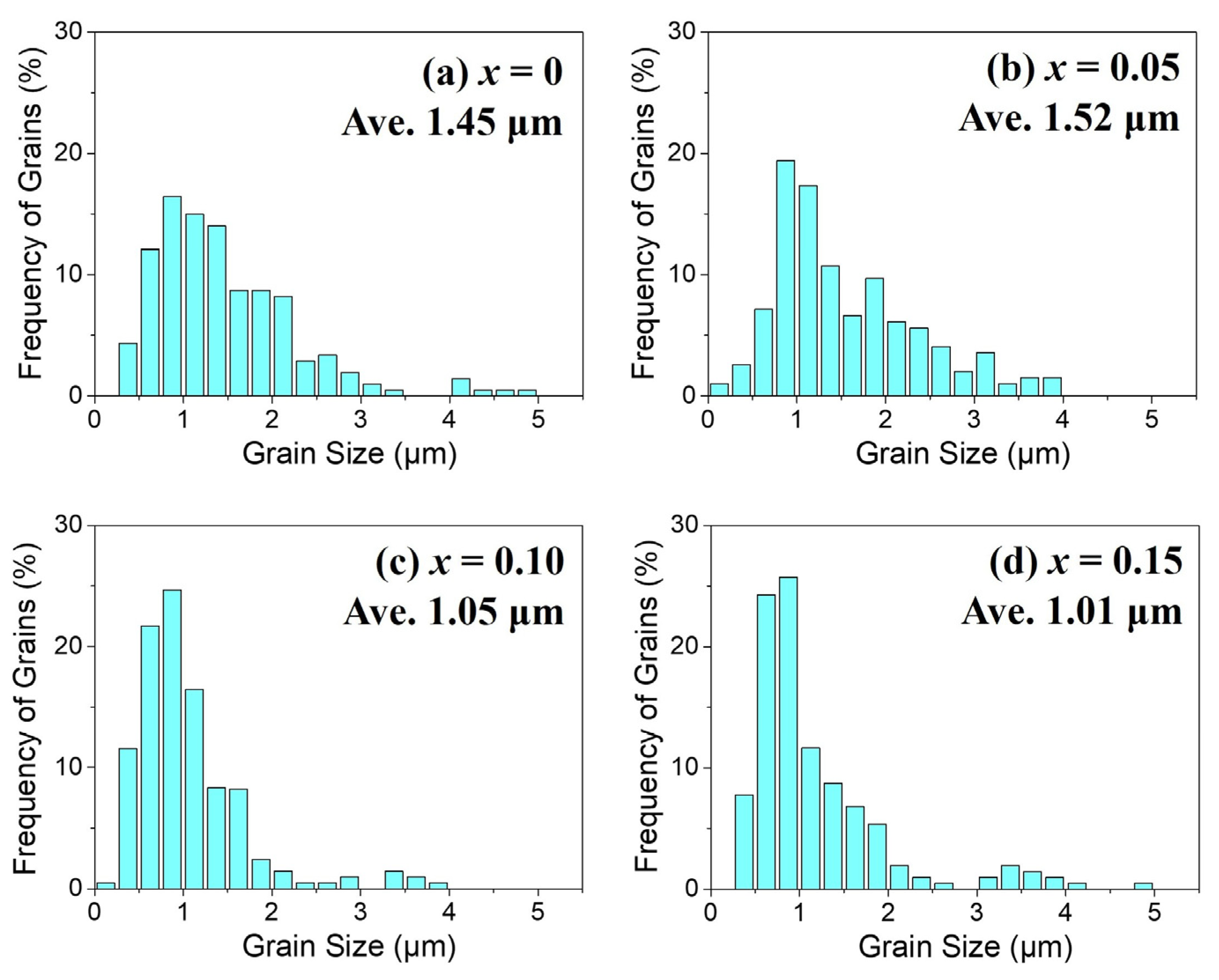
3 as sintering aids. The grain size distributions of each sample are presented as histograms in
Figure 3a–d. In the case of the unsubstituted SrFe
12O
19 sample (
Figure 2a), the average grain size was 1.45 μm. With La–Mg substitutions, the average grain size slightly increased to about 1.5 μm at x = 0.05, while it was refined to around 1.0 μm at higher substitution levels of x = 0.1 and 0.15.
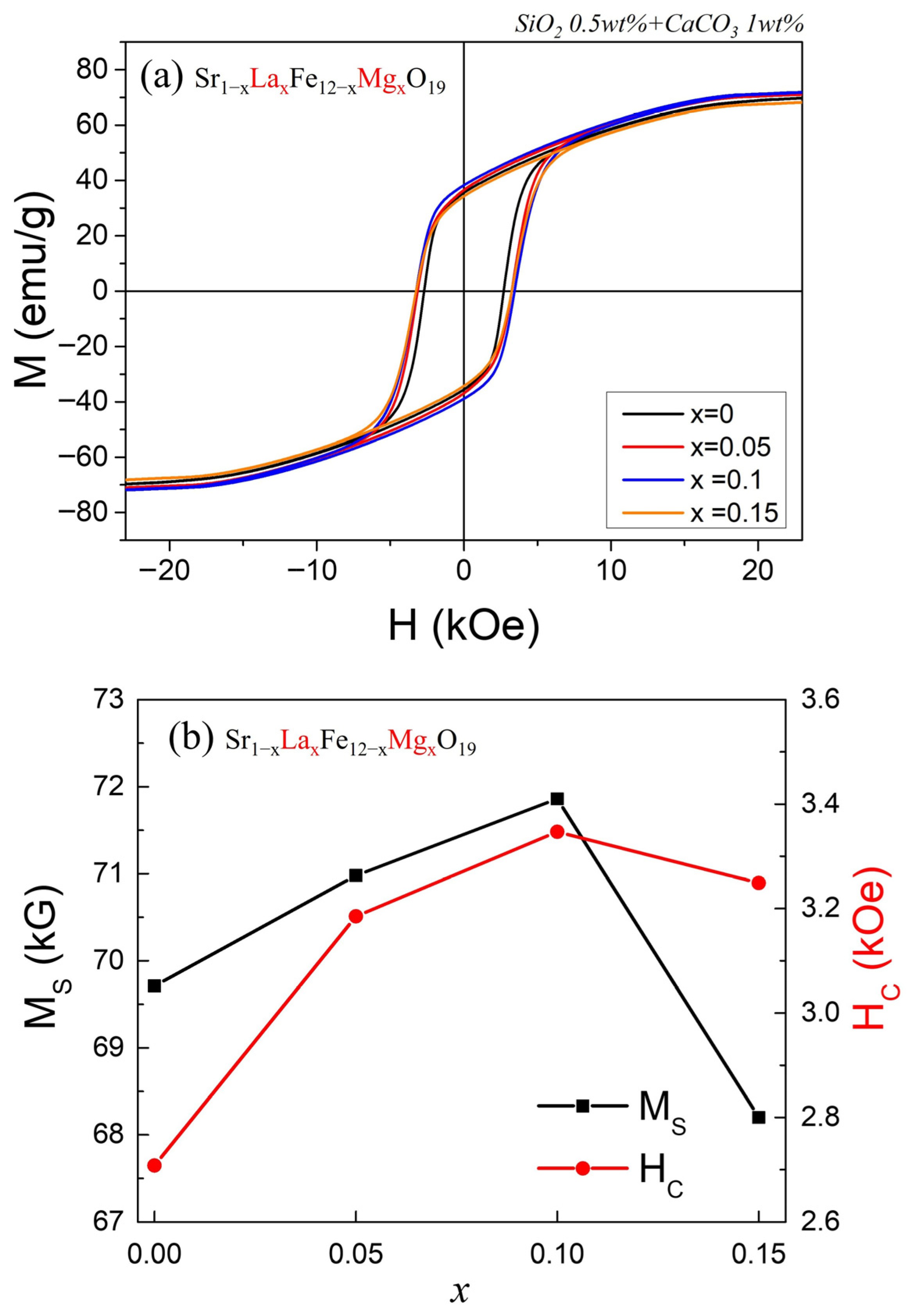
Figure 4a shows the M–H curves of Sr
1−xLa
xFe
12−xMg
xO
19 (x = 0, 0.05, 0.1, 0.15) samples. The specimens used for the VSM measurements were rectangular sintered bodies (~2.0 mm × ~1.5 mm × ~0.8 mm), and the magnetization (M) values represent the magnetic moment measured in emu normalized by the sample mass. In the VSM measurements, attention was focused on the intrinsic property of each cation-substituted sample—specifically, the variation in the M
S per unit mass. The compositional dependence of M
S and Hc values with increasing substitution level x is shown in
Figure 4b, and the numerical values are summarized in
Table 1.
Both M
S and H
C increased as x increased from 0 to 0.1, but decreased simultaneously at x = 0.15. As confirmed in
Figure 1, all four samples consist of a single M-type phase; therefore, the change in M
S can be attributed to variations in the net spin moment of Fe
3+ ions contributing to M
S. Since La
3+ substitutes for Sr
2+ and Mg
2+ substitutes for Fe
3+ simultaneously, the valence state of the remaining Fe
3+ ions is not expected to change. Under this assumption, the enhanced M
S observed at x = 0.1 indicates that the nonmagnetic Mg
2+ ions preferentially occupy the down-spin Fe
3+ sites in the M-type hexaferrite structure.
Such an increase in M
S due to selective cation substitution has also been reported for minor La–Zn and Mn–Zn substitutions in M-type hexaferrites. In the case of La–Zn substitution, the highest M
S was obtained at x = 0.3 [
13], whereas for Mn–Zn substitution, the maximum M
S occurred at x = 0.1 [
14]. However, when the substitution level of nonmagnetic ions exceeds a certain limit, M
S inevitably decreases, as also observed for the La–Mg system, where M
S begins to decrease beyond x = 0.15.
Generally, when M
S increases, the H
C tends to decrease because of the weakening of the magnetic anisotropy. However, in the La–Mg substituted samples, both M
S and H
C increased simultaneously from x = 0.05 to x = 0.1, which is a noteworthy result. Since H
C is also influenced by extrinsic factors such as the average grain size and its distribution, these effects should be considered together. The H
C is known to follow the relationship:
where D
cri is the critical grain size for a single domain [
15]. The fact that the x = 0.05 sample exhibited a higher H
C value despite having a larger average grain size than the unsubstituted sample suggests that the La–Mg substitution enhanced the intrinsic hard magnetic properties, such as the magnetocrystalline anisotropy constant (K
u), of the M-type hexaferrite. Because H
C is proportional to the anisotropy field (H
ani), which is related to M
S by H
ani = 2K
u/M
S, a simultaneous increase without decreasing M
S implies that the K
u increased with La–Mg substitution, resulting in enhanced H
ani and consequently higher H
C.
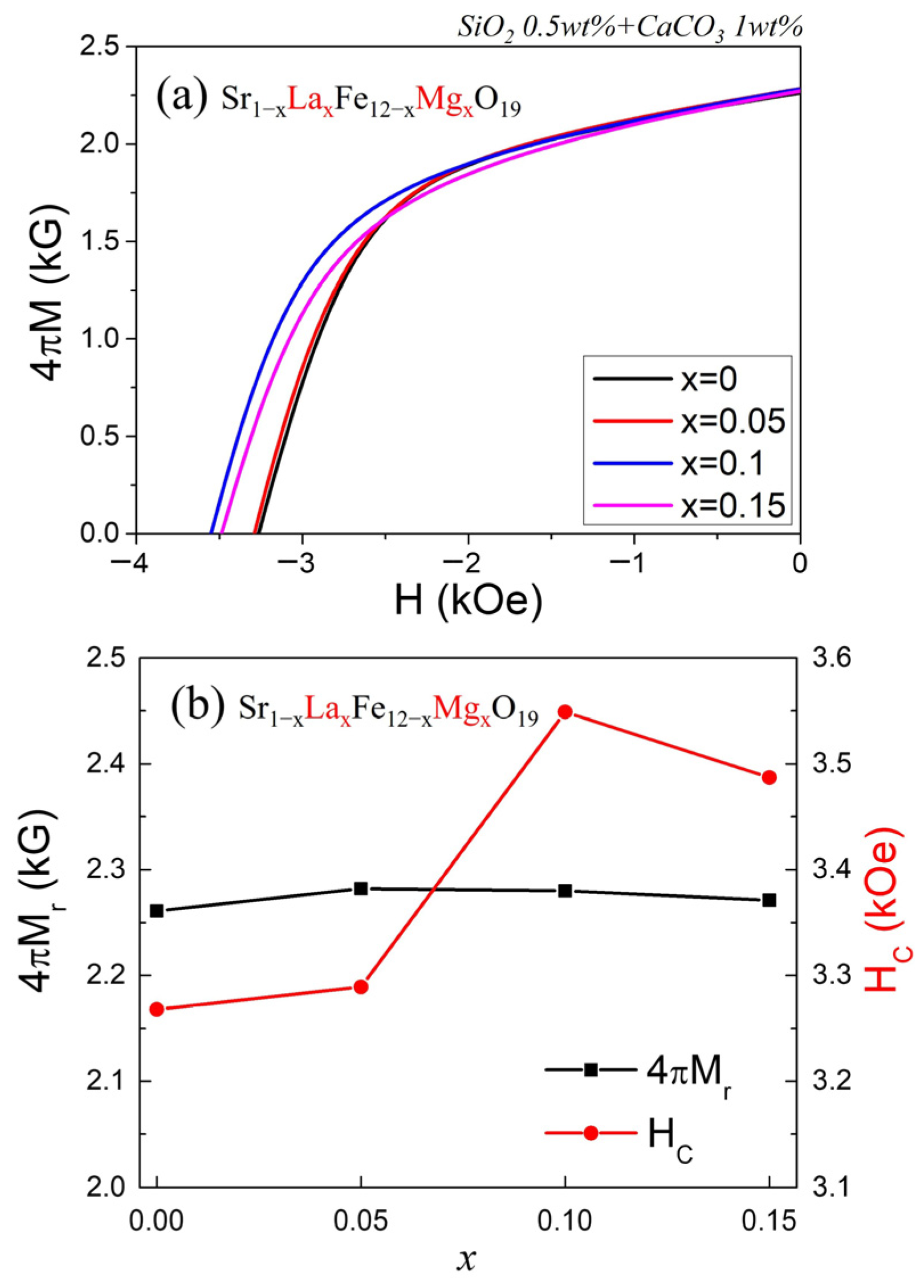
Figure 5a presents the demagnetization curves of sintered Sr
1−xLa
xFe
12−xMg
xO
19 (x = 0, 0.05, 0.1, 0.15) samples, measured using a B–H loop tracer. While the M–H curves in
Figure 4 were obtained by a VSM using small specimens (~2.0 mm × ~1.5 mm × ~0.8 mm) under vibration, the demagnetization curves in
Figure 5 were acquired from disk-shaped samples with a much larger volume (diameter ~24 mm, thickness ~6 mm). In this measurement, a varying magnetic field was applied to the specimen, and the magnetic flux density change was detected through a J-coil. Thus, the results in
Figure 5 more closely represent the actual magnetic performance of isotropic sintered magnets.
Figure 5b show the variations in remanent flux density (4πM
r) and H
C as a function of the substitution level x. The trend of 4πM
r with increasing x is consistent with that of the VSM-derived results in
Figure 4b. However, while M
S obtained from the VSM represents an intrinsic magnetic property (magnetization per unit mass), 4πM
r is influenced by extrinsic factors such as sample density and crystallographic alignment.
Practically, the 4πM
r value (in units of Gauss) obtained from the B–H loop tracer can be related to the M
S (in emu/g) measured by the VSM through the following equation [
20]:
Here,
ρ represents the density of the sintered sample (in g/cm
3), and
S denotes the grain orientation factor, which takes a value of 1 when all grains are perfectly aligned along the c-axis and 0.5 when they are completely randomly oriented.
fi is the volumetric fraction of nonmagnetic impurities. Since the present samples are randomly oriented isotropic magnets,
S value is expected to be close to 0.5 [
2]. For the La–Mg substituted samples, in which no secondary phase was detected, the
fi value can be regarded as zero (
fi ≈ 0).
The dependence of H
C on x also follows a similar trend to that shown in
Figure 4b,c, although the absolute values differ slightly. Because the B–H loop tracer data are obtained from specimens with a volume roughly 1000 times larger than those used in the VSM, they are considered more representative of the macroscopic magnetic behavior and compositional dependence. The apparent sintered density of the disk samples, along with the 4πM
r and H
C values obtained from the B–H loop tracer measurements, are also presented in
Table 1. Considering all results, the Sr
0.
9La
0.
1Fe
11.
9Mg
0.
1O
19 sample (x = 0.1) exhibited the most superior hard magnetic properties, with the highest M
S of 71.9 emu/g, a remanent flux density of 2280 G, and a H
C of 3549 Oe.
Finally, to further optimize the hard magnetic properties, additional B–H loop tracer measurements were performed on Sr
0.
9La
0.
1Fe
11.
9Mg
0.
1O
19 samples fabricated using various sintering additives, as shown in
Figure 6a. Among the sintering additives, SiO
2 and Al
2O
3 are known to effectively suppress grain growth, while CaCO
3, CaO, SrCO
3, Bi
2O
3, and CuO promote densification and grain coarsening [
21,
22,
23,
24].
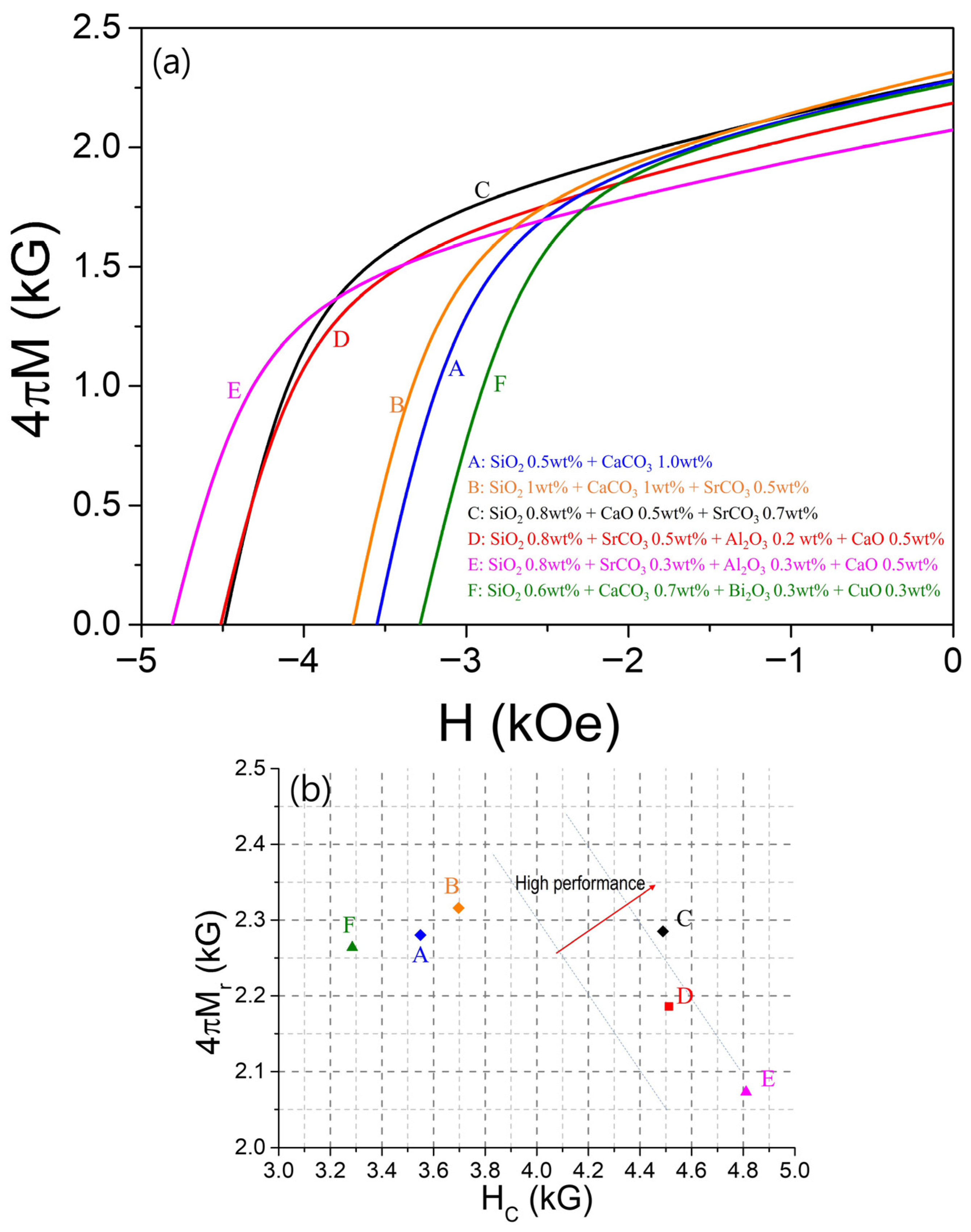
Figure 6a presents the demagnetization curves for samples prepared with six different combinations of sintering aids. Curve A represents the sample sintered with the original additives, 0.5 wt% SiO
2 and 1.0 wt% CaCO
3. When 0.5 wt% SiO
2 and 0.5 wt% SrCO
3 were additionally introduced, as shown in curve B, both 4πM
r and H
C improved simultaneously. In curve C, 4πM
r slightly decreased compared with B, while H
C increased significantly (when 1 wt% of CaCO
3 was replaced by 0.5 wt% of CaO, and the SiO
2 and SrCO
3 contents were adjusted to 0.8 wt% and 0.7 wt%, respectively). For the samples containing Al
2O
3 (curves D and E), high H
C values were obtained; however, this was accompanied by a significant reduction in 4πM
r. Among them, the sample sintered with 0.8 wt% SiO
2, 0.3 wt% SrCO
3, 0.3 wt% Al
2O
3, and 0.5 wt% CaO exhibited the highest H
C = 4811 Oe (curve E). On the other hand, when Bi
2O
3 or CuO was used instead of SrCO
3, the sintered density increased most significantly, but H
C decreased considerably.
Figure 6b summarizes these results by plotting 4πM
r versus H
C. Since these two parameters generally exhibit a trade-off relationship, samples located toward the upper-right region of the graph are considered to have superior hard magnetic performance. Accordingly, the sample corresponding to curve C, prepared with 0.8 wt% SiO
2, 0.5 wt% CaO, and 0.7 wt% SrCO
3, demonstrated the best isotropic magnetic properties, achieving H
C = 4489 Oe and 4πM
r = 2285 G. Furthermore, if this composition were subjected to magnetic field alignment before sintering—thereby increasing the grain orientation factor
S in Equation (2) to approximately 0.95—and further improving the sintered density, it is expected that an anisotropic magnet with 4πM
r ≈ 4500 G could be fabricated, albeit with a slight decrease in H
C. The values of 4πM
r and H
C are also presented in
Table 2. These results demonstrate a remarkable enhancement in magnetic properties achieved without the use of Co, which is typically considered an essential element for high-performance ferrite fabrication, indicating the great potential of this approach.