Hillslope Morphological Features Modulate Soil Microbial Communities and Chestnut Ink Disease via Clay and Water Redistribution
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Experimental Design
2.2. Soil Physicochemical Analyses
2.3. Soil Biochemical and Enzyme Analyses
2.4. DNA Extraction and Taxonomic Assignment
2.5. Indices Calculation and Statistical Analyses
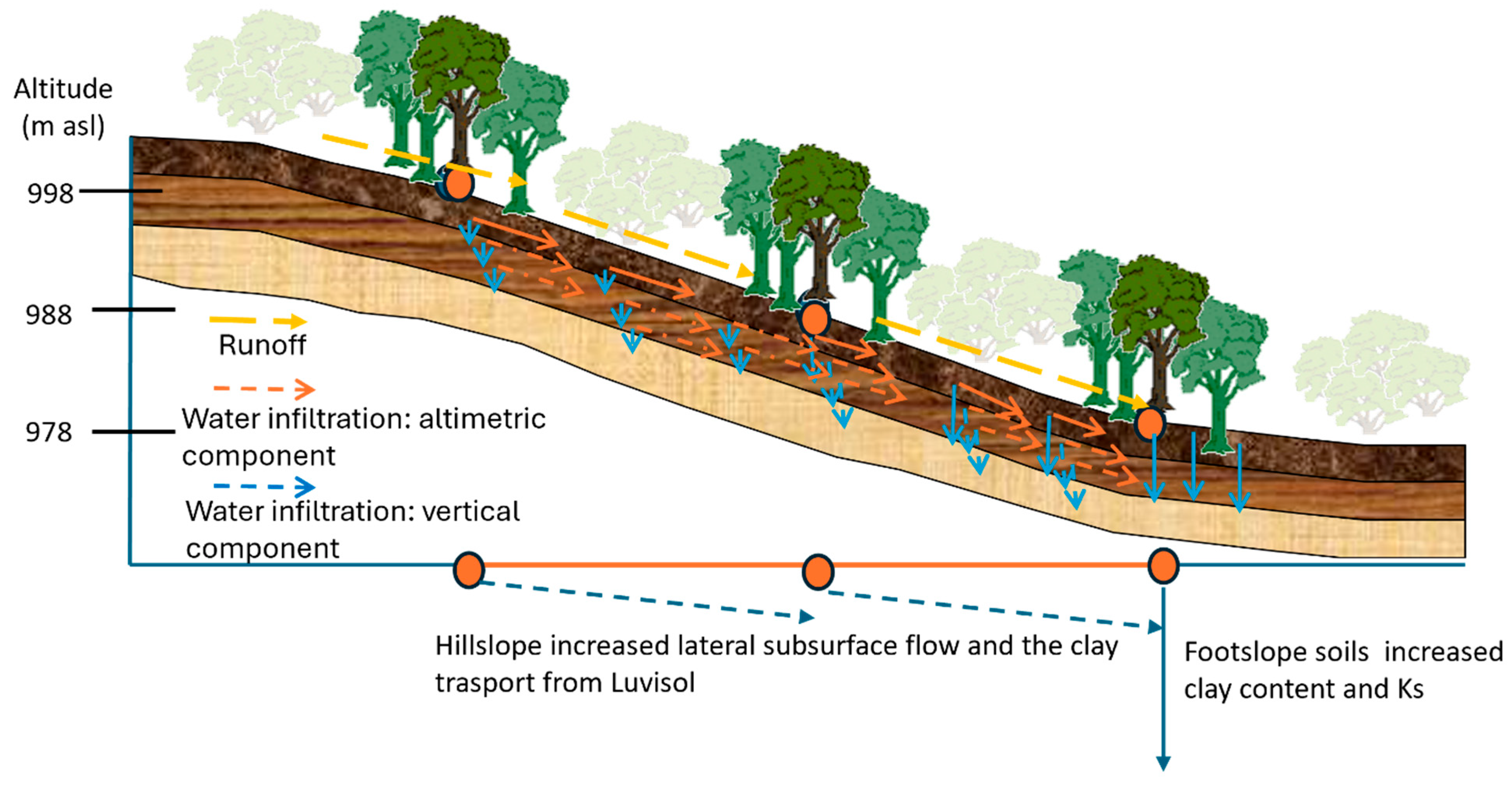
3. Results
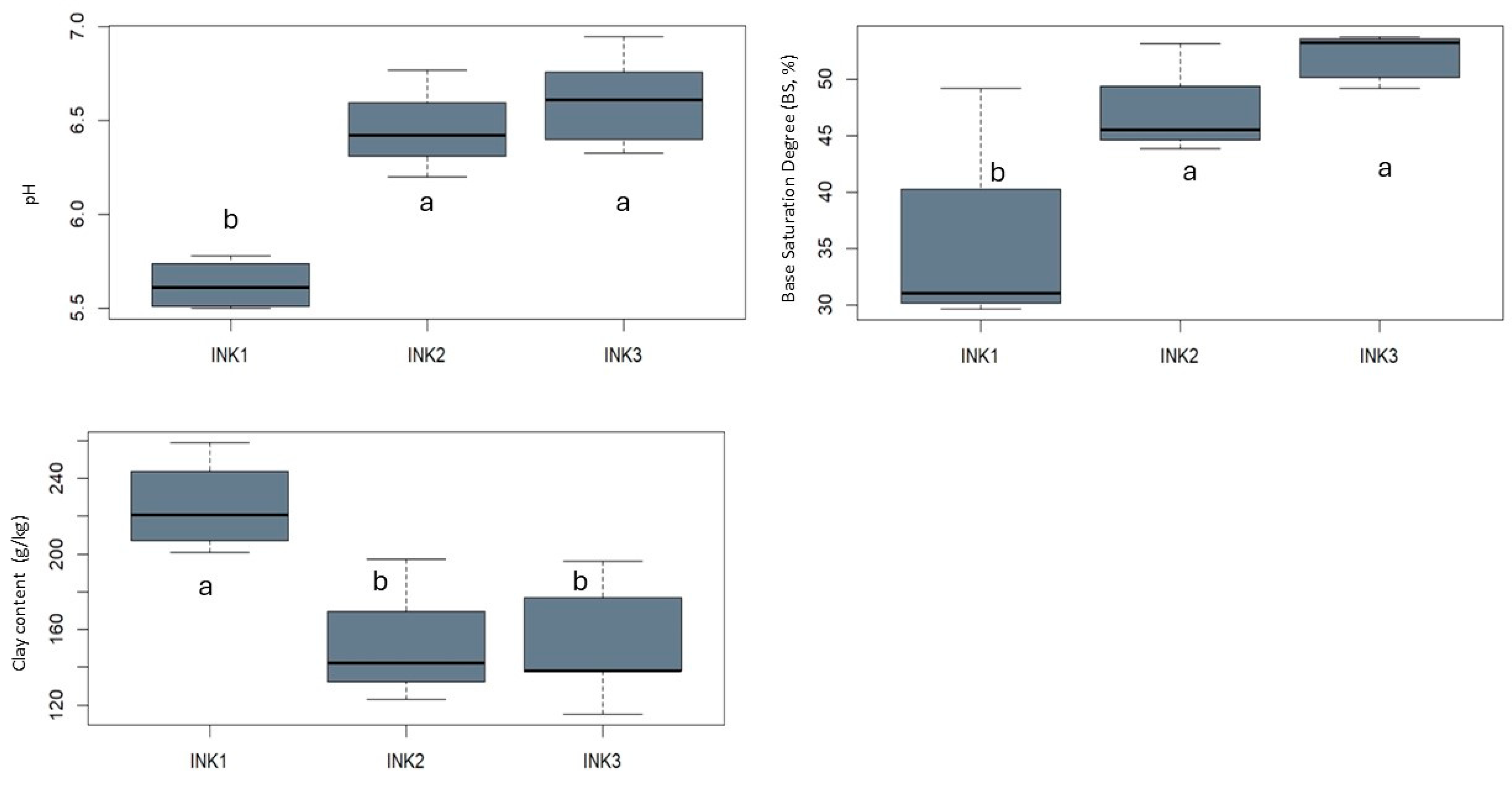
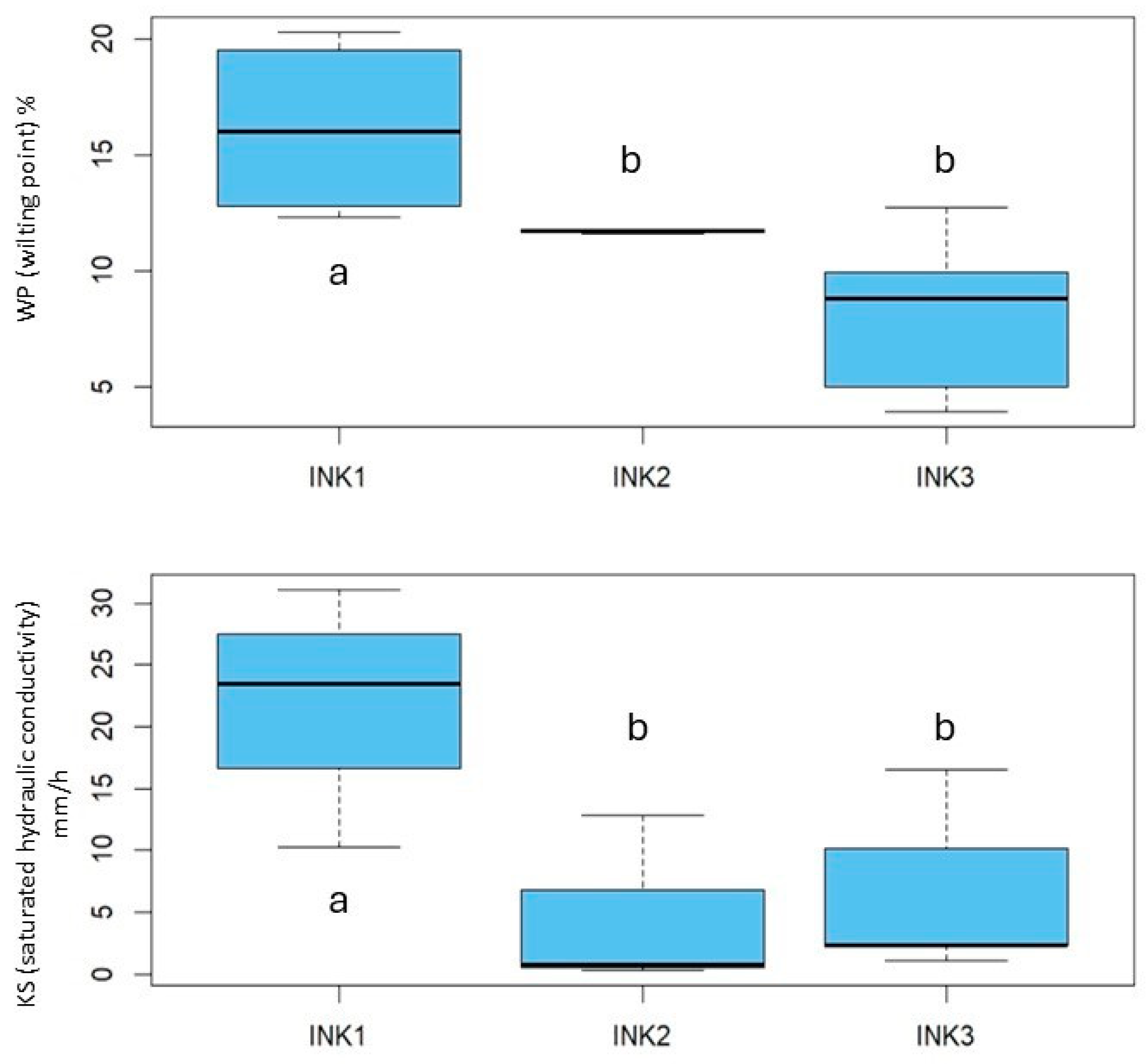
3.1. Soil Classification, Physicochemical and Hydraulic Properties
3.2. Soil Organic Matter, Related Properties and Enzyme Activities
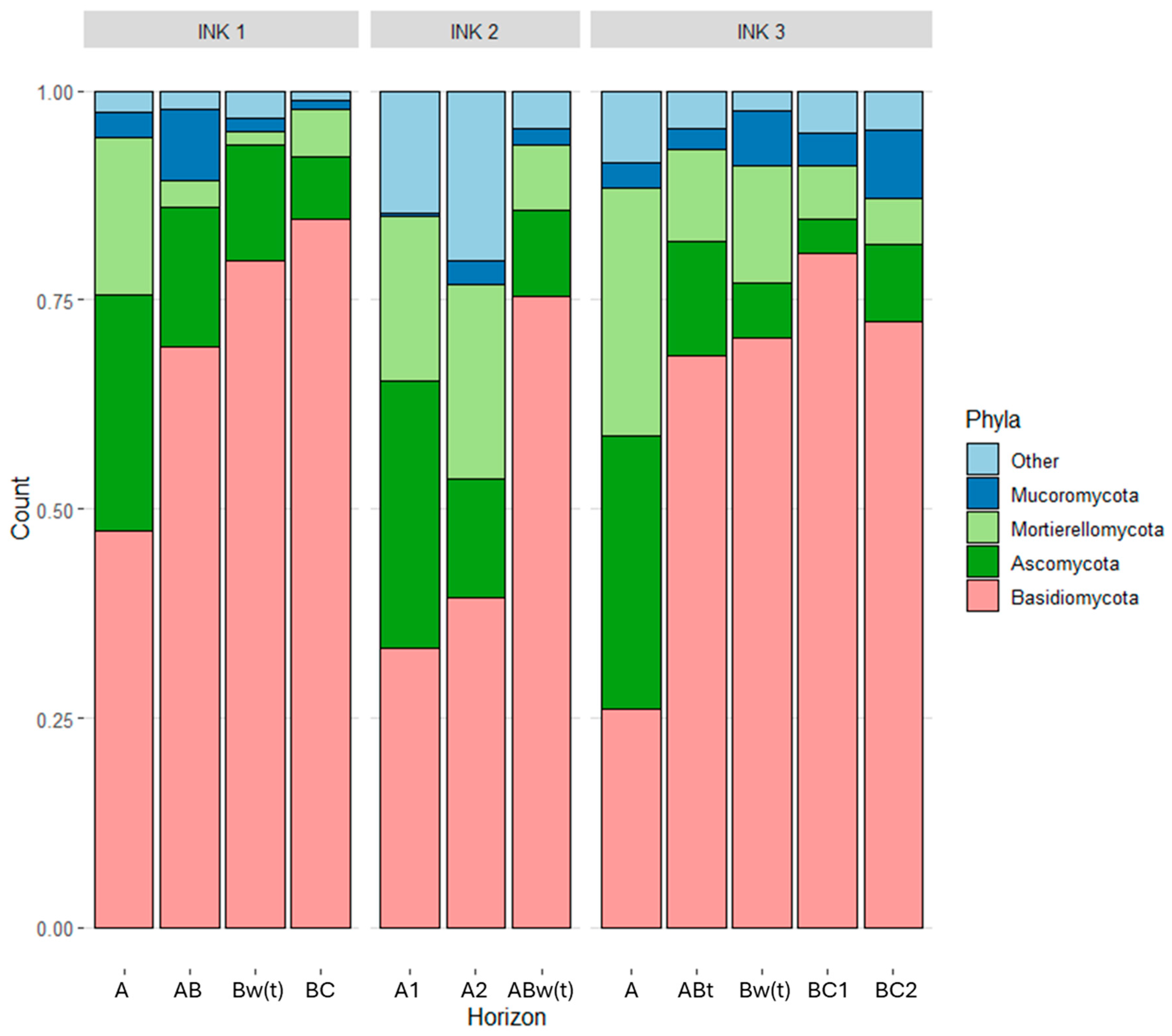
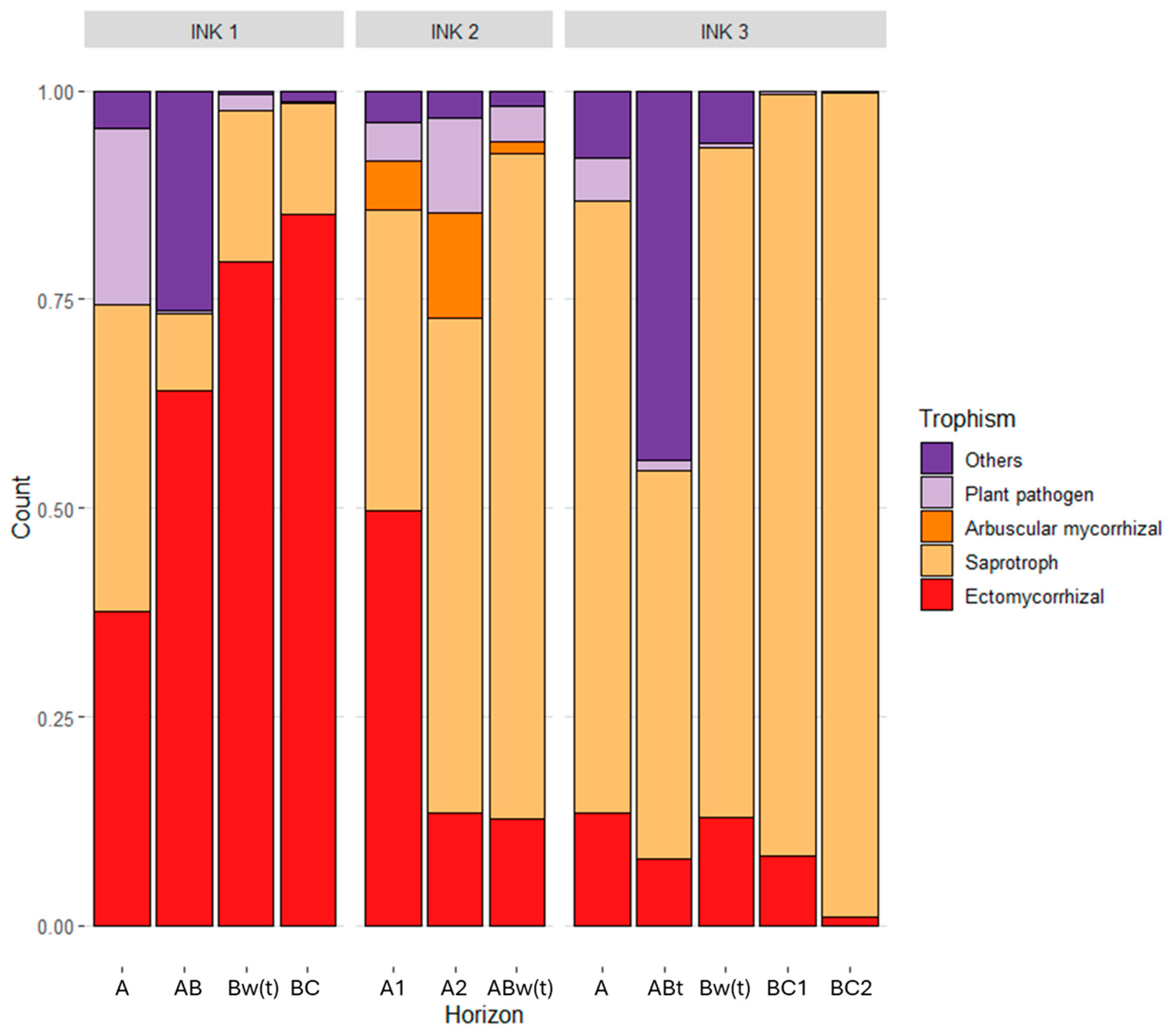
3.3. Biodiversity of the Fungal and Bacterial Communities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Soil Physicochemical Analyses
References
- Corona, P.; Calvani, P.; Mugnozza, G.S.; Pompei, E. Modelling natural forest expansion on a landscape level by multinomial logistic regression. Plant Biosyst. 2008, 142, 509–517. [Google Scholar] [CrossRef]
- Venanzi, R.; Picchio, R.; Piovesan, G. Silvicultural and logging impact on soil characteristics in Chestnut (Castanea sativa Mill.) Mediterranean coppice. Ecol. Eng. 2016, 92, 82–89. [Google Scholar] [CrossRef]
- Fernandes, P.; Colavolpe, M.B.; Serrazina, S.; Costa, R.L. European and American chestnuts: An overview of the main threats and control efforts. Front. Plant. Sci. 2022, 13, 951844. [Google Scholar] [CrossRef]
- EC. Interpretation Manual of European Union Habitats. 1 January 2007. Available online: https://eunis.eea.europa.eu/references/1271 (accessed on 16 January 2025).
- Buckley, P. Coppice restoration and conservation: A European perspective. J. For. Res. 2020, 2, 125–133. [Google Scholar] [CrossRef]
- Gullino, P.; Mellano, M.G.; Beccaro, G.L.; Devecchi, M.; Larcher, F. Strategies for the management of traditional chestnut landscapes in Pesio Valley, Italy: A participatory approach. Land 2020, 9, 536. [Google Scholar] [CrossRef]
- Maresi, G.; Oliveira Longa, C.M.; Turchetti, T. Brown rot on nuts of Castanea sativa Mill: An emerging disease and its causal agent. IForest 2013, 6, 294–301. [Google Scholar] [CrossRef]
- Murolo, S.; Bertoldi, D.; Pedrazzoli, F.; Mancini, M.; Romanazzi, G.; Maresi, G. New symptoms in Castanea sativa stands in Italy: Chestnut mosaic virus and nutrient deficiency. Forests 2022, 13, 1894. [Google Scholar] [CrossRef]
- Prospero, S.; Heinz, M.; Augustiny, E.; Chen, Y.Y.; Engelbrecht, J.; Fonti, M.; Hoste, A.; Ruffner, B.; Sigrist, R.; van den Berg, N.; et al. Distribution, causal agents, and infection dynamic of emerging ink disease of sweet chestnut in Southern Switzerland. Environ. Microbiol. 2023, 25, 2250–2265. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.; Pérez-Sierra, A.; Durán, A.; Jung, M.H.; Balci, Y.; Scanu, B. Canker and decline diseases caused by soil- and airborne Phytophthora species in forests and woodlands. Persoonia Mol. Phylogeny Evol. Fungi 2018, 40, 182–220. [Google Scholar] [CrossRef]
- Brasier, C.M.; Robredo, F.; Ferraz, J.F.P. Evidence for Phytophthora cinnamomi involvement in Iberian oak decline. Plant Pathol. 1993, 42, 140–145. [Google Scholar] [CrossRef]
- Davison, E.M. The role of waterlogging and Phytophthora cinnamomi in the decline and death of Eucalyptus marginata in Western Australia. GeoJournal 1988, 17, 239–244. [Google Scholar] [CrossRef]
- Jung, T.; Blaschke, H. Phytophthora root rot in declining forest trees. Phyton 1996, 36, 95–102. [Google Scholar]
- Jung, T.; Vettraino, A.M.; Cech, T.; Vannini, A. The impact of invasive Phytophthora species on European forests. In Phytophthora: A Global Perspective; CABI: Wallingford, UK, 2013; pp. 146–158. [Google Scholar]
- Peterson, E.; Hansen, E.; Hulbert, J. Source or sink? The role of soil and water borne inoculum in the dispersal of Phytophthora ramorum in Oregon tanoak forests. For. Ecol. Manag. 2014, 322, 48–57. [Google Scholar] [CrossRef]
- Vannini, A.; Natili, G.; Thomidis, T.; Belli, C.; Morales-Rodriguez, C. Anthropogenic and landscape features are associated with ink disease impact in Central Italy. For. Pathol. 2021, 51, e12722. [Google Scholar] [CrossRef]
- Anselmi, N.; Vettraino, A.M.; Franco, S.; Chiarot, E.; Vannini, A. Recrudescenze del “Mal dell’Inchiostro” del castagno in Italia: Nuove acquisizioni e suggerimenti di lotta. Linea Ecol. 1999, 28, 53–58. (In Italian) [Google Scholar]
- Martins, L.; Castro, J.; Macedo, W.; Marques, C.; Abreu, C. Assessment of the spread of chestnut Ink disease using remote sensing and geostatistical methods. Eur. J. Plant Pathol. 2007, 119, 159–164. [Google Scholar] [CrossRef]
- Fonseca, T.F.; Abreu, C.G.; Parresol, B.R. Soil compaction and chestnut ink disease. For. Pathol. 2004, 34, 273–283. [Google Scholar] [CrossRef]
- Martins, L.M.; Oliveira, M.T.; Abre, C.G. Soils and climatic characteristic of chestnut stands that differ in the presence of the Ink disease. Acta Hortic. 1999, 494, 447–449. [Google Scholar] [CrossRef]
- Liang, W.; Li, S.; Hung, F. Analysis of the contributions of topographic, soil, and vegetation features on the spatial distributions of surface soil moisture in a steep natural forested headwater catchment. Hydrol. Process. 2017, 31, 3796–3809. [Google Scholar] [CrossRef]
- Ribolzi, O.; Patin, J.; Bresson, L.; Latsachack, K.; Mouche, E.; Sengtaheuanghoung, O.; Silvera, N.; Thiébaux, J.P.; Valentin, C. Impact of slope gradient on soil surface features and infiltration on steep slopes in northern Laos. Geomorphology 2011, 127, 53–63. [Google Scholar] [CrossRef]
- Davison, E.M. How do Phytophthora spp. de Bary kill trees. N. Z. J. For. Sci. 2011, 41, 25–37. [Google Scholar]
- Corcobado, T.; Solla, A.; Madeira, M.A.; Moreno, G. Combined effects of soil properties and Phytophthora cinnamomi infections on Quercus ilex decline. Plant Soil 2013, 373, 403–413. [Google Scholar] [CrossRef]
- Lin, H.S.; Kogelmann, W.; Walker, C.; Bruns, M.A. Soil moisture patterns in a forested catchment: A hydropedological perspective. Geoderma 2006, 131, 345–368. [Google Scholar] [CrossRef]
- Tromp-van Meerveld, H.J.; McDonnell, J.J. On the interrelations between topography, soil depth, soil moisture, transpiration rates and species distribution at the hillslope scale. Adv. Water Resour. 2006, 29, 293–310. [Google Scholar] [CrossRef]
- Ribeiro, O.K. A source book of the genus Phytophthora. Mycologia 1979, 71, 460. [Google Scholar] [CrossRef]
- Tsao, P.H. Why many phytophthora root rots and crown rots of tree and horticultural crops remain undetected. EPPO Bull. 1990, 20, 11–17. [Google Scholar] [CrossRef]
- Marzocchi, G.; Maresi, G.; Luchi, N.; Pecori, F.; Gionni, A.; Longa, C.M.O.; Pezzi, G.; Ferretti, F. 85 Years counteracting an invasion: Chestnut ecosystems and landscapes survival against Ink disease. Biol. Invasions 2024, 26, 2049–2062. [Google Scholar] [CrossRef]
- Jiao, S.; Chen, W.; Wang, J.; Du, N.; Li, Q.; Wei, G. Soil microbiomes with distinct assemblies through vertical soil profiles drive the cycling of multiple nutrients in reforested ecosystems. Microbiome 2018, 6, 146. [Google Scholar] [CrossRef]
- Mendes, R.; Kruijt, M.; de Bruijn, I.; Dekkers, E.; van der Voort, M.; Schneider, J.H.M.; Piceno, Y.M.; DeSantis, T.Z.; Andersen, G.L.; Bakker, P.A.H.M.; et al. Deciphering the Rhizosphere Microbiome for Disease-Suppressive Bacteria. Science 2011, 332, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska-Długosz, A.; Długosz, J.; Frąc, M.; Gryta, A.; Breza-Boruta, B. Enzymatic activity and functional diversity of soil microorganisms along the soil profile—A matter of soil depth and soil-forming processes. Geoderma 2022, 416, 115779. [Google Scholar] [CrossRef]
- Banerjee, S.; van der Heijden, M.G.A. Soil microbiomes and one health. Nat. Rev. Microbiol. 2023, 21, 6–20. [Google Scholar] [CrossRef]
- Kirk, J.L.; Beaudette, L.A.; Hart, M.; Moutoglis, P.; Klironomos, J.N.; Lee, H.; Trevors, J.T. Methods of studying soil microbial diversity. J. Microbiol. Methods 2004, 58, 169–188. [Google Scholar] [CrossRef]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Zuccarini, P.; Asensio, D.; Ogaya, R.; Sardans, J.; Peñuelas, J. Effects of seasonal and decadal warming on soil enzymatic activity in a P-deficient Mediterranean shrubland. Glob. Change Biol. 2020, 26, 3698–3714. [Google Scholar] [CrossRef]
- Fanin, N.; Mooshammer, M.; Sauvadet, M.; Meng, C.; Alvarez, G.; Bernard, L.; Bertrand, I.; Blagodatskaya, E.; Bon, L.; Fontaine, S.; et al. Soil enzymes in response to climate warming: Mechanisms and feedbacks. Funct. Ecol. 2022, 36, 1378–1395. [Google Scholar] [CrossRef]
- Bhaduri, D.; Sihi, D.; Bhowmik, A.; Verma, B.C.; Munda, S.; Dari, B. A review on effective soil health bio-indicators for ecosystem restoration and sustainability. Front. Microbiol. 2022, 13, 938481. [Google Scholar] [CrossRef]
- Vázquez, E.; Benito, M.; Espejo, R.; Teutscherova, N. Response of soil properties and microbial indicators to land use change in an acid soil under Mediterranean conditions. Catena 2020, 189, 104486. [Google Scholar] [CrossRef]
- Moscatelli, M.C.; Secondi, L.; Marabottini, R.; Papp, R.; Stazi, S.R.; Mania, E.; Marinari, S. Assessment of soil microbial functional diversity: Land use and soil properties affect CLPP-MicroResp and enzymes responses. Pedobiologia 2018, 66, 36–42. [Google Scholar] [CrossRef]
- Wang, M.; Ji, L.; Shen, F.; Meng, J.; Wang, J.; Shan, C.; Yang, L. Differential responses of soil extracellular enzyme activity and stoichiometric ratios under different slope aspects and slope positions in Larix olgensis plantations. Forests 2022, 13, 845. [Google Scholar] [CrossRef]
- Huang, Y.-M.; Liu, D.; An, S.-S. Effects of slope aspect on soil nitrogen and microbial properties in the Chinese Loess region. Catena 2015, 125, 135–145. [Google Scholar] [CrossRef]
- Li, T.; Liang, J.; Chen, X.; Wang, H.; Zhang, S.; Pu, Y.; Xu, X.; Li, H.; Xu, J.; Wu, X.; et al. The interacting roles and relative importance of climate, topography, soil properties and mineralogical composition on soil potassium variations at a national scale in China. Catena 2021, 196, 104875. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, G.; Zhang, X.; Ge, J.; He, N.; Wang, Q.; Wang, D. The variations in soil microbial communities, enzyme activities and their relationships with soil organic matter decomposition along the northern slope of Changbai Mountain. Appl. Soil Ecol. 2015, 86, 19–29. [Google Scholar] [CrossRef]
- Rete Rurale Nazionale: La Corona di Matilde. Alto Reno. Terra di Castagni. 2023. Available online: https://www.reterurale.it/flex/cm/pages/ServeBLOB.php/L/IT/IDPagina/23098 (accessed on 16 January 2025). (In Italian).
- Vittori Antisari, L.; Trenti, W.; Buscaroli, A.; Falsone, G.; Vianello, G.; De Feudis, M. Pedodiversity and organic matter stock of soils developed on sandstone formations in the Northern Apennines (Italy). Land 2023, 12, 79. [Google Scholar] [CrossRef]
- Schoeneberger, P.J.; Wysocki, D.A.; Benham, E.C. Soil Survey Staff: Field Book for Describing and Sampling Soils, Version 3.0; US Department of Agriculture, Natural Resources Conservation Service, National Soil Survey Center: Lincoln, NE, USA, 2012.
- Cambardella, C.A.; Moorman, T.B.; Novak, J.M.; Parkin, T.B.; Karlen, D.L.; Turco, R.F.; Konopka, A.E. Field-scale variability of soil properties in Central Iowa soils. Soil Sci. Soc. Am. J. 1994, 58, 1501–1511. [Google Scholar] [CrossRef]
- Pätzold, S.; Mertens, F.M.; Bornemann, L.; Koleczek, B.; Franke, J.; Feilhauer, H.; Welp, G. Soil heterogeneity at the field scale: A challenge for precision crop protection. Precis. Agric. 2008, 9, 367–390. [Google Scholar] [CrossRef]
- Türkeş, M.; Dede, V.; Dengiz, O.; Şenol, H.; Serin, S. Periglacial landforms and soil formation on summit of the mount lda (Kaz Dağı), Biga peninsula-Turkey. Phys. Geogr. 2023, 44, 531–580. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Liao, A.; Liu, C.; Du, M.; Huang, A.; Guo, J. Estimation of variability in soil water content in a forested critical-zone experimental catchment in Eastern China. J. Contam. Hydrol. 2022, 248, 104022. [Google Scholar] [CrossRef]
- Thompson, S.E.; Katul, G.G.; Porporato, A. Role of microtopography in rainfall-runoff partitioning: An analysis using idealized geometry. Water Resour. Res. 2010, 46, W07520. [Google Scholar] [CrossRef]
- Oueslati, I.; Allamano, P.; Bonifacio, E.; Claps, P. Vegetation and topographic control on spatial variability of soil organic carbon. Pedosphere 2013, 23, 48–58. [Google Scholar] [CrossRef]
- Seibert, J.; Stendahl, J.; Sørensen, R. Topographical influences on soil properties in boreal forests. Geoderma 2007, 141, 139–148. [Google Scholar] [CrossRef]
- Vittori Antisari, L.; Papp, R.; Vianello, G.; Marinari, S. Effects of Douglas fir stand age on soil chemical properties, nutrient dynamics, and enzyme activity: A case study in Northern Apennines, Italy. Forests 2018, 9, 641. [Google Scholar] [CrossRef]
- Gee, G.W.; Bauder, J.W. Methods of Soil Analysis: Part 1—Physical and Mineralogical Methods; SSSA Book Series; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1986. [Google Scholar]
- De Feudis, M.; Falsone, G.; Vianello, G.; Vittori Antisari, L. The conversion of abandoned chestnut forests to managed ones does not affect the soil chemical properties and improves the soil microbial biomass activity. Forests 2020, 11, 786. [Google Scholar] [CrossRef]
- Marx, M.C.; Wood, M.; Jarvis, S.C. A microplate fluorimetric assay for the study of enzyme diversity in soils. Soil Biol. Biochem. 2001, 33, 1633–1640. [Google Scholar] [CrossRef]
- Vepsäläinen, M.; Kukkonen, S.; Vestberg, M.; Sirviö, H.; Maarit Niemi, R. Application of soil enzyme activity test kit in a field experiment. Soil Biol. Biochem. 2001, 33, 1665–1672. [Google Scholar] [CrossRef]
- García-López, J.D.; Barbieri, F.; Baños, A.; Madero, J.M.G.; Gardini, F.; Montanari, C.; Tabanelli, G. Use of two autochthonous bacteriocinogenic strains as starter cultures in the production of salchichónes, a type of Spanish fermented sausages. Curr. Res. Food. Sci. 2023, 7, 100615. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. 1990, 18, 315–322. [Google Scholar]
- Wood, D.E.; Lu, J.; Langmead, B. Improved metagenomic analysis with Kraken 2. Genome Biol. 2019, 20, 257. [Google Scholar] [CrossRef]
- Lu, J.; Breitwieser, F.P.; Thielen, P.; Salzberg, S.L. Bracken: Estimating species abundance in metagenomics data. PeerJ Comput. Sci. 2017, 3, e104. [Google Scholar] [CrossRef]
- Põlme, S.; Abarenkov, K.; Henrik Nilsson, R.; Lindahl, B.D.; Clemmensen, K.E.; Kauserud, H.; Nguyen, N.; Kjøller, R.; Bates, S.T.; Baldrian, P.; et al. FungalTraits: A user-friendly traits database of fungi and fungus-like stramenopiles. Fungal Divers. 2020, 105, 1–16. [Google Scholar] [CrossRef]
- Burgess, T.I.; White, D.; Sapsford, S.J. Comparison of primers for the detection of Phytophthora (and Other Oomycetes) from environmental samples. J. Fungi 2022, 8, 980. [Google Scholar] [CrossRef]
- Carini, P.; Marsden, P.J.; Leff, J.W.; Morgan, E.E.; Strickland, M.S.; Fierer, N. Relic DNA is abundant in soil and obscures estimates of soil microbial diversity. Nat. Microbiol. 2017, 2, 16242. [Google Scholar] [CrossRef]
- Levy-Booth, D.J.; Campbell, R.G.; Gulden, R.H.; Hart, M.M.; Powell, J.R.; Klironomos, J.N.; Pauls, K.P.; Swanton, C.J.; Trevors, J.T.; Dunfield, K.E. Cycling of extracellular DNA in the soil environment. Soil Biol. Biochem. 2007, 39, 2977–2991. [Google Scholar] [CrossRef]
- Szabó, B.; Weynants, M.; Weber, T.K.D. Updated European hydraulic pedotransfer functions with communicated uncertainties in the predicted variables (euptfv2). Geosci. Model Dev. 2021, 14, 151–175. [Google Scholar] [CrossRef]
- Naseri, M.; Peters, A.; Durner, W.; Iden, S.C. Effective hydraulic conductivity of stony soils: General effective medium theory. Adv. Water Resour. 2020, 146, 103765. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2022.
- Quénard, L.; Samouëlian, A.; Laroche, B.; Cornu, S. Lessivage as a major process of soil formation: A revisitation of existing data. Geoderma 2011, 167, 135–147. [Google Scholar] [CrossRef]
- Krevh, V.; Groh, J.; Weihermüller, L.; Filipović, L.; Defterdarović, J.; Kovač, Z.; Magdić, I.; Lazarević, B.; Baumgartl, T.; Filipović, V. Investigation of hillslope vineyard soil water dynamics using field measurements and numerical modeling. Water 2023, 15, 820. [Google Scholar] [CrossRef]
- Zambon, N.; Johannsen, L.L.; Strauss, P.; Dostal, T.; Zumr, D.; Cochrane, T.A.; Klik, A. Splash erosion affected by initial soil moisture and surface conditions under simulated rainfall. Catena 2021, 196, 104827. [Google Scholar] [CrossRef]
- Berg, B.; McClaugherty, C. What factors may influence the accumulation of humus layers? In Plant Litter; Springer International Publishing: Cham, Switzerland, 2020; pp. 247–272. [Google Scholar]
- Jobbágy, E.G.; Jackson, R.B. Patterns and mechanisms of soil acidification in the conversion of grasslands to forests. Biogeochemistry 2003, 64, 205–229. [Google Scholar] [CrossRef]
- Slessarev, E.W.; Lin, Y.; Bingham, N.L.; Johnson, J.E.; Dai, Y.; Schimel, J.P.; Chadwick, O.A. Water balance creates a threshold in soil pH at the global scale. Nature 2016, 540, 567–569. [Google Scholar] [CrossRef]
- Minasny, B.; McBratney, A.B. Limited effect of organic matter on soil available water capacity. Eur. J. Soil Sci. 2018, 69, 39–47. [Google Scholar] [CrossRef]
- Hudson, B.D. Soil organic matter and available water capacity. J. Soils Water Conserv. 1994, 49, 189–194. [Google Scholar] [CrossRef]
- de Jonge, L.W.; Moldrup, P.; Jacobsen, O.H.; Schjønning, P. Soil infrastructure, interfaces, and translocation processes. Vadose Zone J. 2009, 8, 651–656. [Google Scholar]
- Vannini, A.; Natili, G.; Anselmi, N.; Montaghi, A.; Vettraino, A. Distribution and gradient analysis of ink disease in chestnut forests. For. Pathol. 2010, 40, 73–86. [Google Scholar] [CrossRef]
- Doro, L.; Jones, C.; Williams, J.R.; Norfleet, M.L.; Izaurralde, R.C.; Wang, X.; Jeong, J. The variable saturation hydraulic conductivity method for improving soil water content simulation in EPIC and APEX models. Vadose Zone J. 2017, 16, 1–14. [Google Scholar] [CrossRef]
- Mohanty, B.P.; Mousli, Z. Saturated hydraulic conductivity and soil water retention properties across a soil-slope transition. Water Resour. Res. 2000, 36, 3311–3324. [Google Scholar] [CrossRef]
- Gyeltshen, J.; Dunstan, W.A.; Grigg, A.H.; Burgess, T.I.; Giles, G.E. The influence of time, soil moisture and exogenous factors on the survival potential of oospores and chlamydospores of Phytophthora cinnamomi. For. Pathol. 2021, 51, e12637. [Google Scholar] [CrossRef]
- Rhoades, C.C.; Brosi, S.L.; Dattilo, A.J.; Vincelli, P. Effect of soil compaction and moisture on incidence of phytophthora root rot on American chestnut (Castanea dentata) seedlings. For. Ecol. Manag. 2003, 184, 47–54. [Google Scholar] [CrossRef]
- Kunadiya, M.B.; Burgess, T.I.; Dunstan, W.; White, D.; Giles, G.E. Persistence and degradation of Phytophthora cinnamomi DNA and RNA in different soil types. Environ. DNA 2021, 3, 92–104. [Google Scholar] [CrossRef]
- Lanoue, A.; Burlat, V.; Henkes, G.J.; Koch, I.; Schurr, U.; Röse, U.S.R. De novo biosynthesis of defense root exudates in response to Fusarium attack in barley. New Phytol. 2010, 185, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Meier, I.C.; Avis, P.G.; Phillips, R.P. Fungal communities influence root exudation rates in pine seedlings. FEMS Microbiol. Ecol. 2013, 83, 585–595. [Google Scholar] [CrossRef]
- Preece, C.; Yang, K.; Llusià, J.; Winkler, J.B.; Schnitzler, J.P.; Peñuelas, J. Combined effects of drought and simulated pathogen attack on root exudation rates of tomatoes. Plant Soil 2024, 497, 629–645. [Google Scholar] [CrossRef]
- Dinis, L.T.; Peixoto, F.; Zhang, C.; Martins, L.; Costa, R.; Gomes-Laranjo, J. Physiological and biochemical changes in resistant and sensitive chestnut (Castanea) plantlets after inoculation with Phytophthora cinnamomi. Physiol. Mol. Plant Pathol. 2011, 75, 146–156. [Google Scholar] [CrossRef]
- Lovat, C.A.; Donnelly, D.J. Mechanisms and metabolomics of the host–pathogen interactions between Chestnut (Castanea species) and Chestnut blight (Cryphonectria parasitica). For. Pathol. 2019, 49, e12562. [Google Scholar] [CrossRef]
- Christ, M.J.; David, M.B. Temperature and moisture effects on the production of dissolved organic carbon in a Spodosol. Soil Biol. Biochem. 1996, 28, 1191–1199. [Google Scholar] [CrossRef]
- Fröberg, M.; Berggren, D.; Bergkvist, B.; Bryant, C.; Mulder, J. Concentration and fluxes of dissolved organic carbon (DOC) in three Norway spruce stands along a climatic gradient in Sweden. Biogeochemistry 2006, 77, 1–23. [Google Scholar] [CrossRef]
- Kaiser, K.; Kaupenjohann, M.; Zech, W. Sorption of dissolved organic carbon in soils: Effects of soil sample storage, soil-to-solution ratio, and temperature. Geoderma 2001, 99, 317–328. [Google Scholar] [CrossRef]
- Jansson, S.L.; Persson, J. Mineralization and immobilization of soil nitrogen. Nitrogen Agric. Soils 2015, 22, 229–252. [Google Scholar]
- D’Angelo, T.; Goordial, J.; Lindsay, M.R.; McGonigle, J.; Booker, A.; Moser, D.; Stepanauskus, R.; Orcutt, B.N. Replicated life-history patterns and subsurface origins of the bacterial sister phyla Nitrospirota and Nitrospinota. ISME J. 2023, 17, 891–902. [Google Scholar] [CrossRef]
- Kalam, S.; Basu, A.; Ahmad, I.; Sayyed, R.Z.; El-Enshasy, H.A.; Dailin, D.J.; Suriani, N.L. Recent understanding of soil Acidobacteria and their ecological significance: A critical review. Front. Microbiol. 2020, 11, 2712. [Google Scholar] [CrossRef]
- Maitra, P.; Hrynkiewicz, K.; Szuba, A.; Jagodziński, A.M.; Al-Rashid, J.; Mandal, D.; Mucha, J. Metabolic niches in the rhizosphere microbiome: Dependence on soil horizons, root traits and climate variables in forest ecosystems. Front. Plant Sci. 2024, 15, 1344205. [Google Scholar] [CrossRef]
- Naylor, D.; McClure, R.; Jansson, J. Trends in microbial community composition and function by soil depth. Microorganisms 2022, 10, 540. [Google Scholar] [CrossRef]
- Carteron, A.; Beigas, M.; Joly, S.; Turner, B.L.; Laliberté, E. Temperate forests dominated by arbuscular or ectomycorrhizal fungi are characterized by strong shifts from saprotrophic to mycorrhizal fungi with increasing soil depth. Microb. Ecol. 2021, 82, 377–390. [Google Scholar] [CrossRef]
- Dukunde, A.; Schneider, D.; Schmidt, M.; Veldkamp, E.; Daniel, R. Tree species shape soil bacterial community structure and function in temperate deciduous forests. Front. Microbiol. 2019, 10, 1519. [Google Scholar] [CrossRef]
- López-Mondéjar, R.; Voříšková, J.; Větrovský, T.; Baldrian, P. The bacterial community inhabiting temperate deciduous forests is vertically stratified and undergoes seasonal dynamics. Soil Biol. Biochem. 2015, 87, 43–50. [Google Scholar] [CrossRef]
- Marinari, S.; Radicetti, E.; Petroselli, V.; Allam, M.; Mancinelli, R. Microbial indices to assess soil health under different tillage and fertilization in potato (Solanum tuberosum L.) Crop. Agriculture 2022, 12, 415. [Google Scholar] [CrossRef]
- Coonan, E.C.; Kirkby, C.A.; Kirkegaard, J.A.; Amidy, M.R.; Strong, C.L.; Richardson, A.E. Microorganisms and nutrient stoichiometry as mediators of soil organic matter dynamics. Nutr. Cycl. Agroecosyst. 2020, 117, 273–298. [Google Scholar] [CrossRef]
- Qu, Y.; Tang, J.; Li, Z.; Zhou, Z.; Wang, J.; Wang, S.; Cao, Y. Soil enzyme activity and microbial metabolic function diversity in soda Saline–Alkali rice paddy fields of northeast China. Sustainability 2020, 12, 10095. [Google Scholar] [CrossRef]
- Trasar-Cepeda, C.; Leirós, M.C.; Gil-Sotres, F. Hydrolytic enzyme activities in agricultural and forest soils. Some implications for their use as indicators of soil quality. Soil Biol. Biochem. 2008, 40, 2146–2155. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Renella, G.; Puglisi, E.; Ceccanti, B.; Masciandaro, G.; Fornasier, F.; Moscatelli, M.C.; Marinari, S. Soil enzymology: Classical and molecular approaches. Biol. Fertil. Soils 2012, 48, 743–762. [Google Scholar] [CrossRef]
- Frey, S.D.; Ollinger, S.; Nadelhoffer, K.; Bowden, R.; Brzostek, E.; Burton, A.; Caldwell, B.A.; Crow, S.; Goodale, C.L.; Grandy, A.S.; et al. Chronic nitrogen additions suppress decomposition and sequester soil carbon in temperate forests. Biogeochemistry 2014, 121, 305–316. [Google Scholar] [CrossRef]
- Castaño, C.; Alday, J.G.; Parladé, J.; Pera, J.; Martínez de Aragón, J.; Bonet, J.A. Seasonal dynamics of the ectomycorrhizal fungus Lactarius vinosus are altered by changes in soil moisture and temperature. Soil Biol. Biochem. 2017, 115, 253–260. [Google Scholar] [CrossRef]
- Kilpeläinen, J.; Barbero-López, A.; Vestberg, M.; Heiskanen, J.; Lehto, T. Does severe soil drought have after-effects on arbuscular and ectomycorrhizal root colonisation and plant nutrition? Plant Soil 2017, 418, 377–386. [Google Scholar] [CrossRef]
- Branzanti, M.B.; Rocca, E.; Pisi, A. Effect of ectomycorrhizal fungi on chestnut ink disease. Mycorrhiza 1999, 9, 103–109. [Google Scholar] [CrossRef]
- Corcobado, T.; Vivas, M.; Moreno, G.; Solla, A. Ectomycorrhizal symbiosis in declining and non-declining Quercus ilex trees infected with or free of Phytophthora cinnamomi. For. Ecol. Manag. 2014, 324, 72–80. [Google Scholar] [CrossRef]
- Scattolin, L.; Dal Maso, E.; Mutto Accordi, S.; Sella, L.; Montecchio, L. Detecting asymptomatic ink-diseased chestnut trees by the composition of the ectomycorrhizal community. For. Pathol. 2012, 42, 501–509. [Google Scholar] [CrossRef]
- Agerer, R. Exploration types of ectomycorrhizae. Mycorrhiza 2001, 11, 107–114. [Google Scholar] [CrossRef]
- Defrenne, C.E.; Philpott, T.J.; Guichon, S.H.A.; Roach, W.J.; Pickles, B.J.; Simard, S.W. Shifts in ectomycorrhizal fungal communities and exploration types relate to the environment and fine-root traits across interior douglas-fir forests of western Canada. Front. Plant. Sci. 2019, 10, 643. [Google Scholar] [CrossRef]
- Hobbie, E.A.; Agerer, R. Nitrogen isotopes in ectomycorrhizal sporocarps correspond to belowground exploration types. Plant Soil 2010, 327, 71–83. [Google Scholar] [CrossRef]
- Garten, C.T., Jr. Variability in soil properties at a landscape scale. Soil Sci. Soc. Am. J. 1993, 57, 1693–1700. [Google Scholar]
- Conant, R.T.; Paustian, K.; Elliott, E.T. Quantifying and reporting uncertainty in estimates of soil organic carbon stocks and changes. Soil Biol. Biochem. 2003, 35, 19–30. [Google Scholar]
- Orsini, L.; Rémy, J. Utilisation du chlorure de cobaltihexamine pour la détermination simultanée de la capacité d’échange et des bases échangeables des sols. Bull. l’Assoc. Fr. d’Etude Sol 1976, 4, 269–279. [Google Scholar]
- Ciesielski, H.; Sterckeman, T. Determination of cation exchange capacity and exchangeable cations in soils by means of cobalt hexamine trichloride. Effects of experimental conditions. Agronomie 1997, 17, 1–7. [Google Scholar]





| Nutrient | Unit | INK1 | INK2 | INK3 |
|---|---|---|---|---|
| N | % | 2.1 a | 2.5 b | 2.7 c |
| Al | g kg−1 | 0.12 a | 0.21 b | 0.12 a |
| Fe | g kg−1 | 0.15 a | 0.27 b | 0.16 a |
| Mn | g kg−1 | 0.41 a | 0.27 b | 0.27 b |
| K | g kg−1 | 7.1 a | 7.8 b | 10.7 c |
| Ca | g kg−1 | 6.8 a | 7.6 b | 10.1 c |
| Mg | g kg−1 | 2.2 | 2.0 | 2.0 |
| P | g kg−1 | 1.8 c | 1.6 a | 1.7 b |
| B | mg kg−1 | 2.7 a | 20.2 b | 20.2 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trenti, W.; De Feudis, M.; Marinari, S.; Murolo, S.; Tabanelli, G.; Puliga, F.; Marabottini, R.; Zambonelli, A.; Gardini, F.; Vittori Antisari, L. Hillslope Morphological Features Modulate Soil Microbial Communities and Chestnut Ink Disease via Clay and Water Redistribution. Appl. Sci. 2025, 15, 11695. https://doi.org/10.3390/app152111695
Trenti W, De Feudis M, Marinari S, Murolo S, Tabanelli G, Puliga F, Marabottini R, Zambonelli A, Gardini F, Vittori Antisari L. Hillslope Morphological Features Modulate Soil Microbial Communities and Chestnut Ink Disease via Clay and Water Redistribution. Applied Sciences. 2025; 15(21):11695. https://doi.org/10.3390/app152111695
Chicago/Turabian StyleTrenti, William, Mauro De Feudis, Sara Marinari, Sergio Murolo, Giulia Tabanelli, Federico Puliga, Rosita Marabottini, Alessandra Zambonelli, Fausto Gardini, and Livia Vittori Antisari. 2025. "Hillslope Morphological Features Modulate Soil Microbial Communities and Chestnut Ink Disease via Clay and Water Redistribution" Applied Sciences 15, no. 21: 11695. https://doi.org/10.3390/app152111695
APA StyleTrenti, W., De Feudis, M., Marinari, S., Murolo, S., Tabanelli, G., Puliga, F., Marabottini, R., Zambonelli, A., Gardini, F., & Vittori Antisari, L. (2025). Hillslope Morphological Features Modulate Soil Microbial Communities and Chestnut Ink Disease via Clay and Water Redistribution. Applied Sciences, 15(21), 11695. https://doi.org/10.3390/app152111695











