1. Introduction
Silver has long been recognized as a valuable and strategically important element, not only due to its scarcity but also because of its remarkable physicochemical properties, which render it indispensable across a wide range of scientific, industrial, and technological applications [
1,
2]. Occurring both in its native state and in various mineral forms, silver is particularly notable for possessing the highest electrical and thermal conductivity among all metals, attributes that are critical for its extensive use in modern electronics and precision technologies. Moreover, its inherent resistance to oxidation and corrosion significantly enhances its durability and reliability in harsh industrial environments, thereby ensuring long-term functionality [
3,
4]. Classified among the noble or precious metals—together with gold, platinum, and palladium—silver is extensively employed in diverse sectors [
5]. In electronics, it serves as an essential component in conductors, contacts, and circuit boards [
6]. In catalysis, it plays a pivotal role in facilitating oxidation reactions, while in the medical field, its potent antimicrobial properties are exploited in wound dressings, coatings, and sterilization products [
7]. These unique applications have fueled a steady and growing global demand for silver, particularly in the context of advancing technologies and healthcare innovations [
8]. However, this increasing demand has placed substantial pressure on primary silver resources, highlighting the urgent need to develop more sustainable and environmentally responsible sourcing strategies. In this regard, the recovery of silver from secondary resources—such as mining tailings, electronic waste, and industrial residues—has emerged as a central focus of research and technological development [
9]. Recovering silver from wastes provides a dual advantage: it mitigates the environmental degradation associated with conventional mining practices, while simultaneously improving resource efficiency through the reclamation of valuable materials from otherwise discarded wastes [
10,
11].
Implementing and optimizing recovery techniques not only contributes to the circular economy but also supports global sustainability goals by minimizing the ecological footprint of metal production [
12]. To this end, the development of innovative, cost-effective, and environmentally benign methods for silver recovery has become increasingly important. These methods aim to increase efficiency, reduce the use of hazardous chemicals, and lower energy consumption, thereby addressing both the environmental and economic challenges of traditional extraction processes [
13]. Eventually, enhancing the efficiency and sustainability of silver recovery from secondary sources is essential for meeting future demand while aligning with the principles of green chemistry and sustainable resource management [
14].
A wide range of complementary instrumental techniques has been developed and successfully applied for the determination of silver. Among these, flame atomic absorption spectrometry (FAAS) and electrothermal atomic absorption spectrometry (ETAAS) remain widely utilized owing to their operational simplicity, robustness, and proven reliability in trace metal analysis [
15,
16,
17]. More advanced plasma-based methods, including inductively coupled plasma optical emission spectrometry (ICP-OES) and inductively coupled plasma mass spectrometry (ICP-MS), provide superior analytical capabilities, offering lower detection limits, wide linear ranges, and simultaneous multi-element analysis, which make them particularly well-suited for the accurate quantification of silver in complex environmental matrices [
18,
19]. In parallel, UV–Vis spectrophotometry and fluorescence spectrometry have also been employed as complementary approaches, further expanding the analytical repertoire available for silver determination [
20,
21]. Despite the remarkable progress in instrumental technology, it is well established that reliable quantification in real-world samples continues to rely heavily on sample preparation and matrix isolation procedures, which remain indispensable for minimizing interferences and ensuring analytical accuracy [
22]. Consequently, a variety of preconcentration and separation techniques have been explored for trace silver determination, including liquid–liquid extraction (LLE), solid-phase extraction (SPE), cloud point extraction (CPE), and dispersive liquid–liquid microextraction (DLLME) [
23,
24,
25,
26]. Among these, solidified floating organic drop microextraction (SFODME) has emerged as a particularly attractive strategy, combining high enrichment factors with operational simplicity and environmental compatibility, thereby positioning itself as one of the most advantageous alternatives for silver preconcentration prior to detection [
27].
This work addresses the preconcentration of Silver from complex mine-waste matrices using DES-assisted SFODME coupled to FAAS. Prior studies predominantly relied on DLLME/CPE or SFODME without DES, were limited to model solutions, or required chelators, which increases solvent use. Here, a chloride-rich ChCl: urea DES is integrated as a benign phase-modifier to enhance transfer without toxic chelating agents, using only [200 µL] 1-dodecanol and a solidification step that obviates centrifugation. Unlike earlier reports, we validate on certified materials and real tailings from Au–Ag operations, demonstrating matrix tolerance and routine-lab compatibility. The method delivers low LOD, high precision and a notable enhancement factor with small solvent volumes under mild conditions. Collectively, these features fill a gap between green preconcentration and real-sample applicability in FAAS.
2. Materials and Methods
2.1. Reagents and Materials
This study exclusively utilized analytical reagent-grade chemicals. Deionized water, produced by a Barnstead Nanopure Diamond purification system (Los Angeles, CA, USA) and exhibiting a conductivity of 18.2 mΩ, served as the solvent for all aqueous solutions. The silver standard solution originated from a 1000 mg/L silver ion stock (Custom Grade Standard, Inorganic Ventures, Christiansburg, VA, USA), which was subsequently diluted to create working standards. The extraction solvent, 1-dodecanol, was obtained from Merck (Darmstadt, Germany). Choline chloride (C5H14NO·Cl, 97%) and urea (CH4N2O, 99%) were procured from Sigma-Aldrich (St. Louis, MO, USA). A buffer solution with a pH of 3 was formulated using potassium hydrogen phthalate and hydrochloric acid, both sourced from Sigma-Aldrich (St. Louis, MO, USA), with concentrations adjusted as required. Ethanol, supplied by Merck (Darmstadt, Germany), was employed as a diluent to reduce the viscosity of the organic extract and improve the efficiency of flame nebulization. Whatman® (Maidstone, UK) quantitative, ashless filter paper (Grade 589/3, pore size: <2 μm) was used for filtration. All laboratory glassware underwent a rigorous cleaning procedure involving an overnight soak in a 10% nitric acid solution, followed by copious rinsing with deionized water and drying in a controlled, dust-free atmosphere. The analytical methodology was validated using certified reference materials from Rocklabs (Auckland, New Zealand) and OREAS Gold-Silver Ore reference materials provided by ORE RESEARCH & EXPLORATION (Bayswater North, Australia).
2.2. Instrumentation
The determination of Ag(I) concentrations in standard and sample solutions was performed using a PerkinElmer A Analyst 800 Atomic Absorption Spectrophotometer (Hopkinton, MA, USA). This instrument is equipped with a deuterium background corrector and utilizes an air-acetylene flame. A silver hollow cathode lamp, operating at a specific wavelength of 212.6 nm and a slit width of 0.7 nm, served as the radiation source to ensure high precision in measurements. Absorbance values were recorded with air and acetylene flow rates maintained at 17.0 L/min and 1.8 L/min, respectively. To achieve maximum absorbance, both the nebulizer flow rate and burner height were optimized, with the analyte being introduced as an ethanolic solution.
2.3. Preparation of Real Mining Samples
The samples were weighed into 400 mL beakers and transported to the acidification room, where the fume hood fan and heating plate were activated. The heating plate temperature was set to 250 °C. A two-step acidification process was then performed on the samples. Initially, 30 mL of nitric acid (HNO3) was added to each beaker, resulting in observable gas formation. In the second stage, 90 mL of hydrochloric acid (HCl) was added, and the samples were placed on the preheated plate. Teflon rods were used for periodic mixing, roughly every 10 min, to enhance metal dissolution. As heating continued, the samples eventually began to boil, with mixing sustained every 10 min. The dissolution proceeded until the sample volumes reduced to approximately 100 mL. At this stage, the beakers were removed from the hot plate, and the inner walls were rinsed to bring the total volume to 250 mL. After thorough mixing with Teflon rods, the samples were left to settle for 6–8 h and then placed in plastic trays. Once settling was complete, 15 mL aliquots of the samples were transferred with a glass pipette into glass tubes held in a rack. To eliminate any remaining particulates, each 15 mL sample was filtered using blue ribbon filter paper after the settling period. The samples were now fully prepared for the SFODME procedure, ready for direct use.
2.4. Preparation of Deep Eutectic Solvent
The synthesis of the ChCl-based DES followed the method outlined in a prior publication [
28]. To prepare the DES, choline chloride (C
5H
14NO·Cl) and urea were mixed at a 1:2 molar ratio at 60 °C. The mixture was continuously stirred for one hour until a transparent, homogeneous solution was obtained, after which it was allowed to cool.
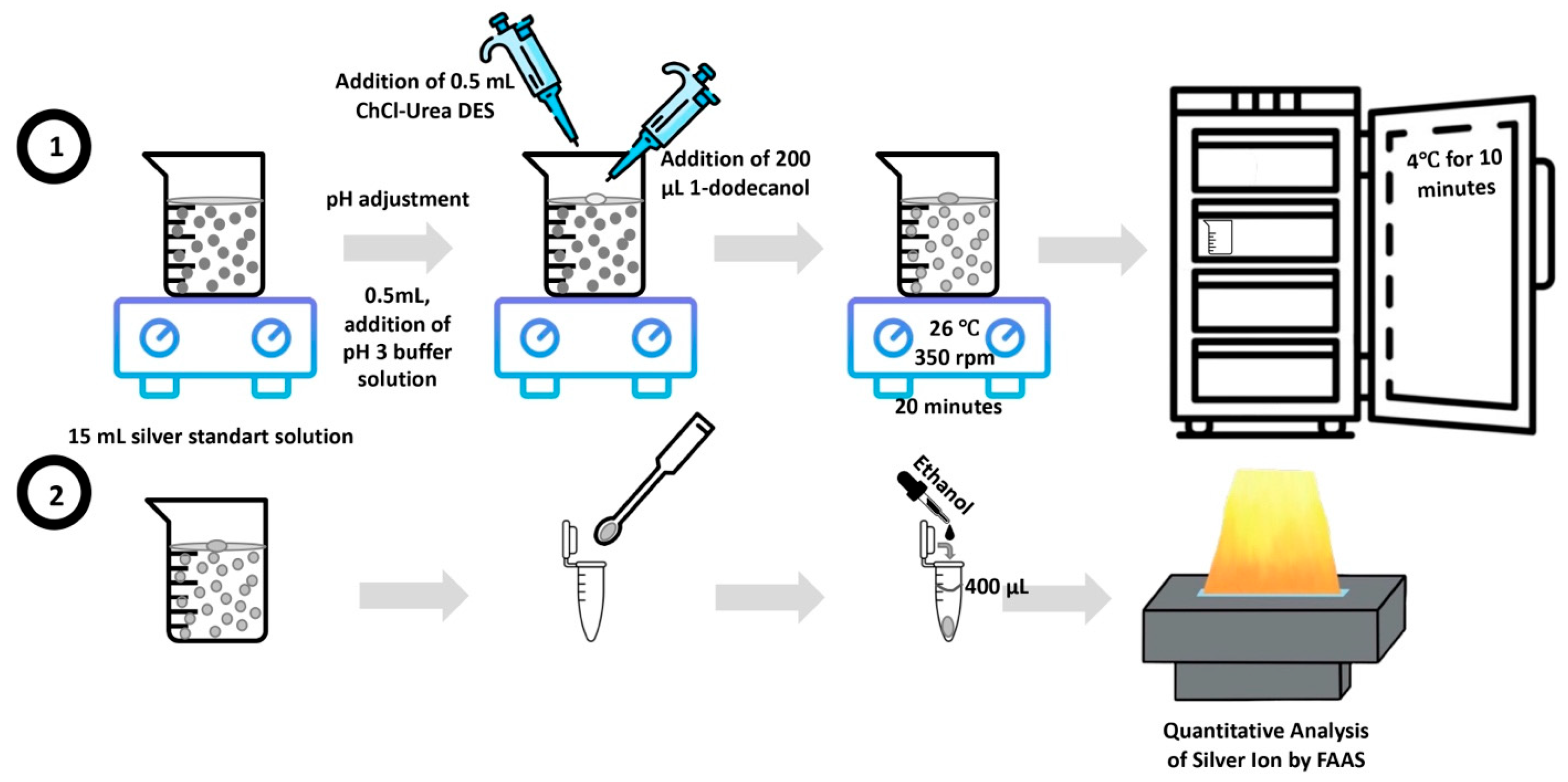
2.5. Procedure
In the SFODME procedure, 15 mL aliquots of either sample or standard silver solution were transferred to beakers containing magnetic stir bars. The pH was adjusted to 3 using 0.5 mL of a phthalate buffer, followed by the sequential addition of 0.5 mL of ChCl-Urea DES and 200 µL of 1-dodecanol. This mixture was stirred at 26 °C and 350 rpm for 20 min. During this stirring, a droplet of organic solvent floated on the aqueous phase, given its lower density, enabling efficient extraction of silver ions into the 1-dodecanol by the help of DES. Once extraction was complete, the beaker was placed in a refrigerator at 4 °C to solidify the organic drop. Since the melting point of 1-dodecanol is close to room temperature (around 24 °C), solidification of drop occurred within 10 min. The solidified droplet was then carefully collected with a mini spatula into a conical vial and diluted to 400 µL with ethanol. This prepared solution was subsequently introduced into the FAAS nebulizer, with ethanol serving as the carrier. The outlined procedure is depicted in
Figure 1.
3. Results
3.1. Optimization of pH and Added pH Buffer Amount
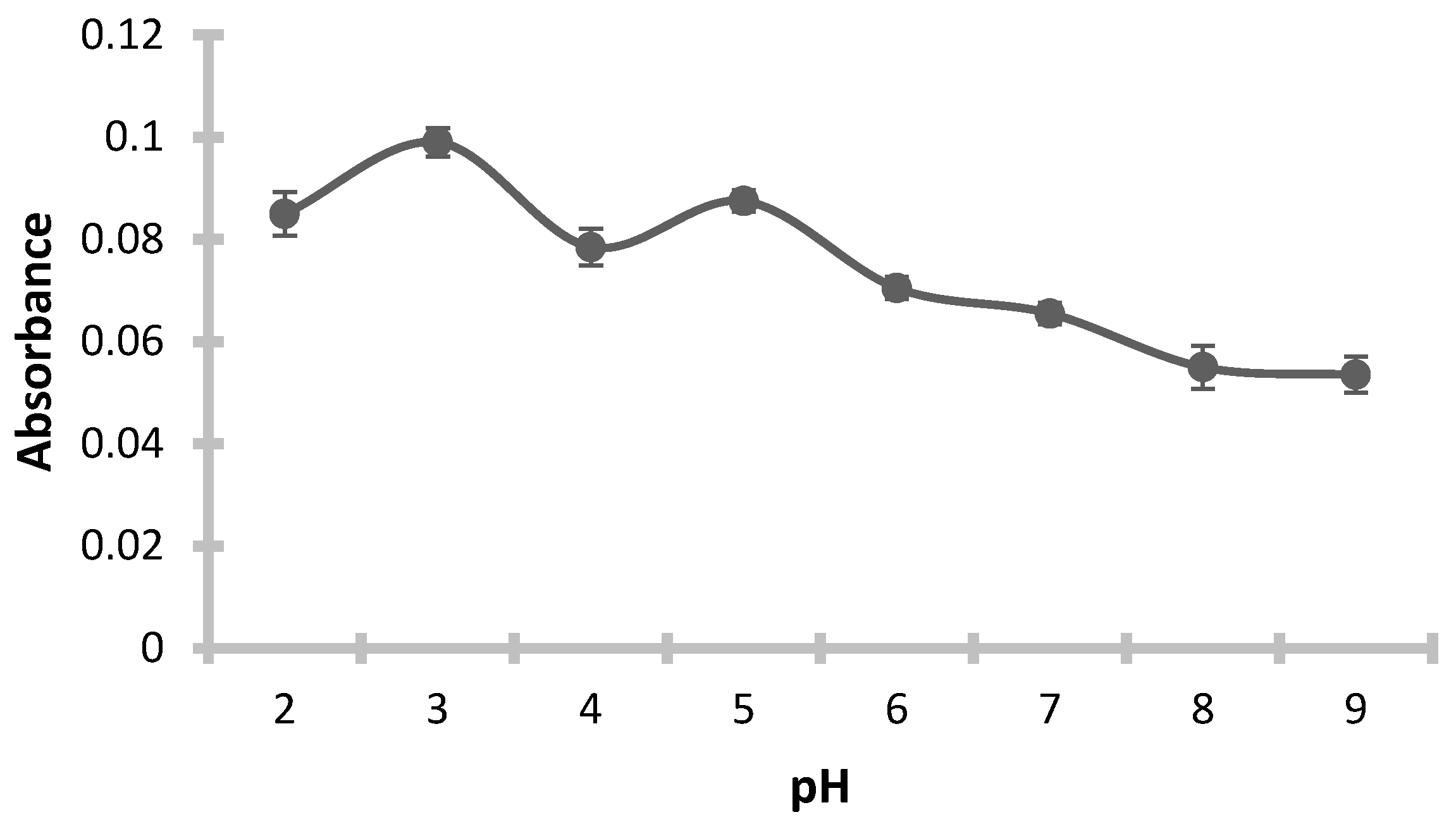
The pH plays a critical role in the formation and extraction of metal complexes. In this study, the effect of pH on the preconcentration of silver ions was assessed over a pH range of 2.0 to 9.0, as shown in
Figure 2. Different buffer solutions were employed to adjust the pH accordingly: a mixture of 0.1 mol/L potassium hydrogen phthalate and 0.1 mol/L hydrochloric acid for pH values between 2.0 and 4.0; a combination of 0.1 mol/L potassium hydrogen phthalate and 0.1 mol/L sodium hydroxide for the pH range of 4.0 to 6.0; 0.1 mol/L tris(hydroxymethyl)aminomethane with 0.2 mol/L hydrochloric acid for pH values of 7.0 and 8.0; and 0.025 mol/L sodium borate decahydrate with 0.1 mol/L sodium hydroxide for pH 9.0. The most effective extraction of the silver complex occurred at pH 3.0, which was therefore chosen as the optimum pH for subsequent procedures.
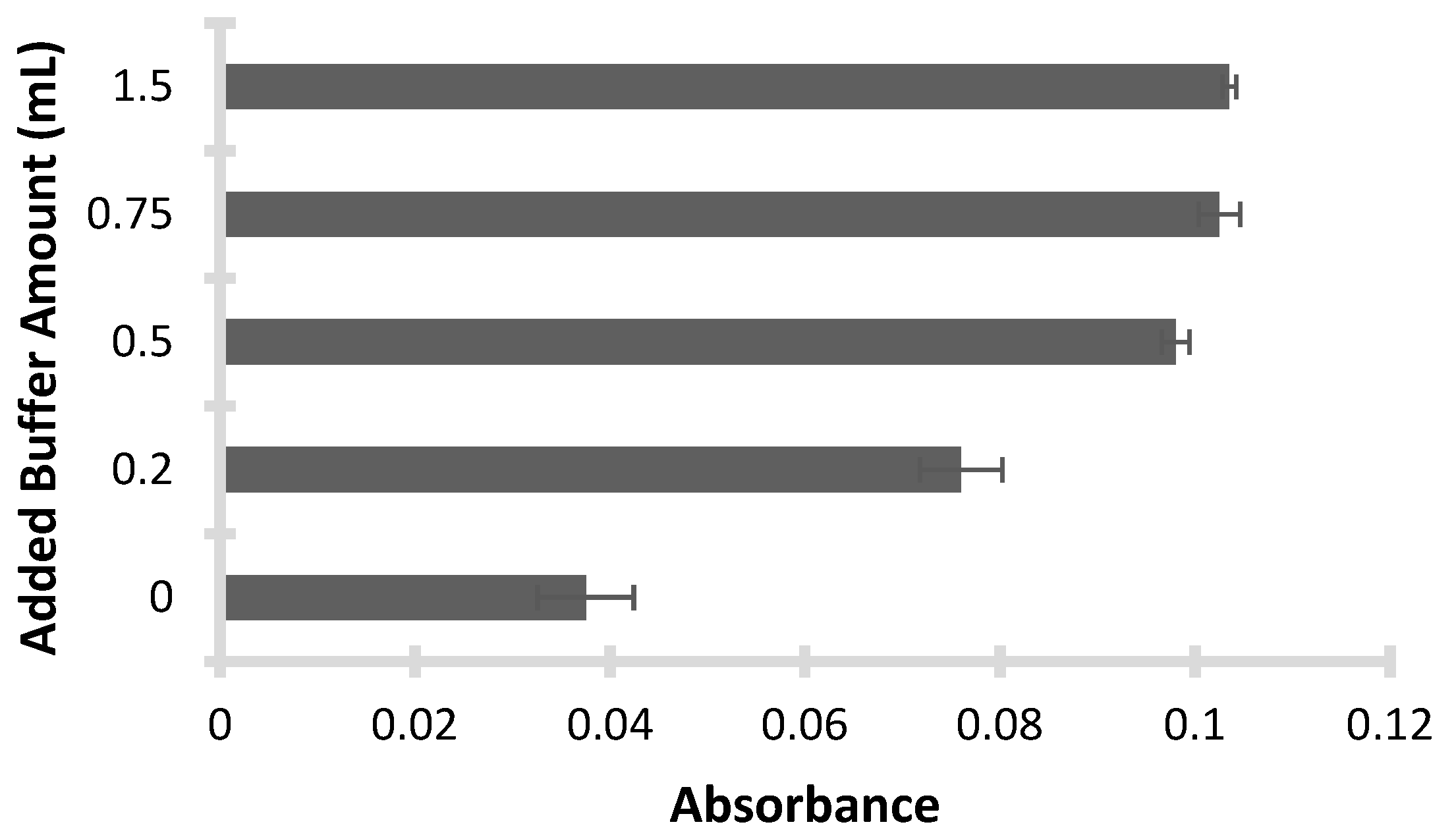
Moreover, the volume of buffer solution was a key parameter influencing the efficiency of silver ion preconcentration. To identify the most suitable volume, various amounts—0 mL, 0.2 mL, 0.5 mL, 0.75 mL, and 1.5 mL—were evaluated based on their ability to stabilize the target pH and enhance extraction efficiency. As illustrated in
Figure 3, the use of 0.5 mL of buffer provided the highest extraction yield by consistently maintaining the optimal pH of 3.0. In contrast, insufficient volumes such as 0.2 mL or no buffer (0 mL) led to pH instability, resulting in suboptimal extraction. Conversely, higher volumes (0.75 mL and 1.5 mL) offered no additional benefit and were less practical. Ultimately, 0.5 mL was selected as the optimal buffer volume for further experiments, as it ensured consistent pH control and efficient recovery of the Silver-DES complex.
Conditions: Added Buffer Amount: 0.5 mL; Added ChCl-Urea DES Amount: 0.5 mL; Added 1-Dodecanol Amount: 200 µL; Mixing Speed: 300 rpm; Mixing Temperature: 32 °C; Mixing Time: 15 min; Final Volume: 400 µL.
Conditions: pH: 3; Added ChCl–Urea DES Amount: 0.5 mL; Added 1-Dodecanol Amount: 200 µL; Mixing Speed: 300 rpm; Mixing Temperature: 32 °C; Mixing Time: 15 min; Final Volume: 400 µL.
3.2. Added Deep Eutectic Solvent Amount
Mechanistically, the ChCl–Urea DES enhances extraction of Silver through chloride-driven speciation, ion-pairing, and interfacial microenvironment effects. At pH ≈ 3, chloride from choline chloride shifts Ag(I) toward extractable chloroargentate species (e.g., [AgCl
2]
−/[AgCl
3]
2−), which form tight ion pairs with the permanently charged choline cation, yielding neutral or less-polar aggregates that preferentially partition into the floating 1-dodecanol drop. Concurrently, the urea-rich hydrogen-bonding network lowers interfacial tension and facilitates desolvation/solvation at the interface, accelerating mass transfer and increasing the distribution ratio. The rise in signal with DES dose up to ~500 µL reflects increasing availability of extractable ion pairs and improved interfacial transfer; the subsequent plateau indicates saturation of complexation sites and/or a slight increase in organic-phase polarity/viscosity or retention of Ag(I) within DES-rich microdomains, which limits further partitioning. Overall, the sequence, chloride speciation—choline ion-pairing—DES-assisted interfacial transfer and solvation in 1-dodecanol—accounts for the observed preconcentration gain (consistent with
Figure 4) [
29,
30,
31].
Conditions: pH: 3; Added Buffer Amount: 0.5 mL; Added 1-Dodecanol Amount: 200 µL; Mixing Speed: 300 rpm; Mixing Temperature: 32 °C; Mixing Time: 15 min; Final Volume: 400 µL.
3.3. Added Extraction Solvent Amount
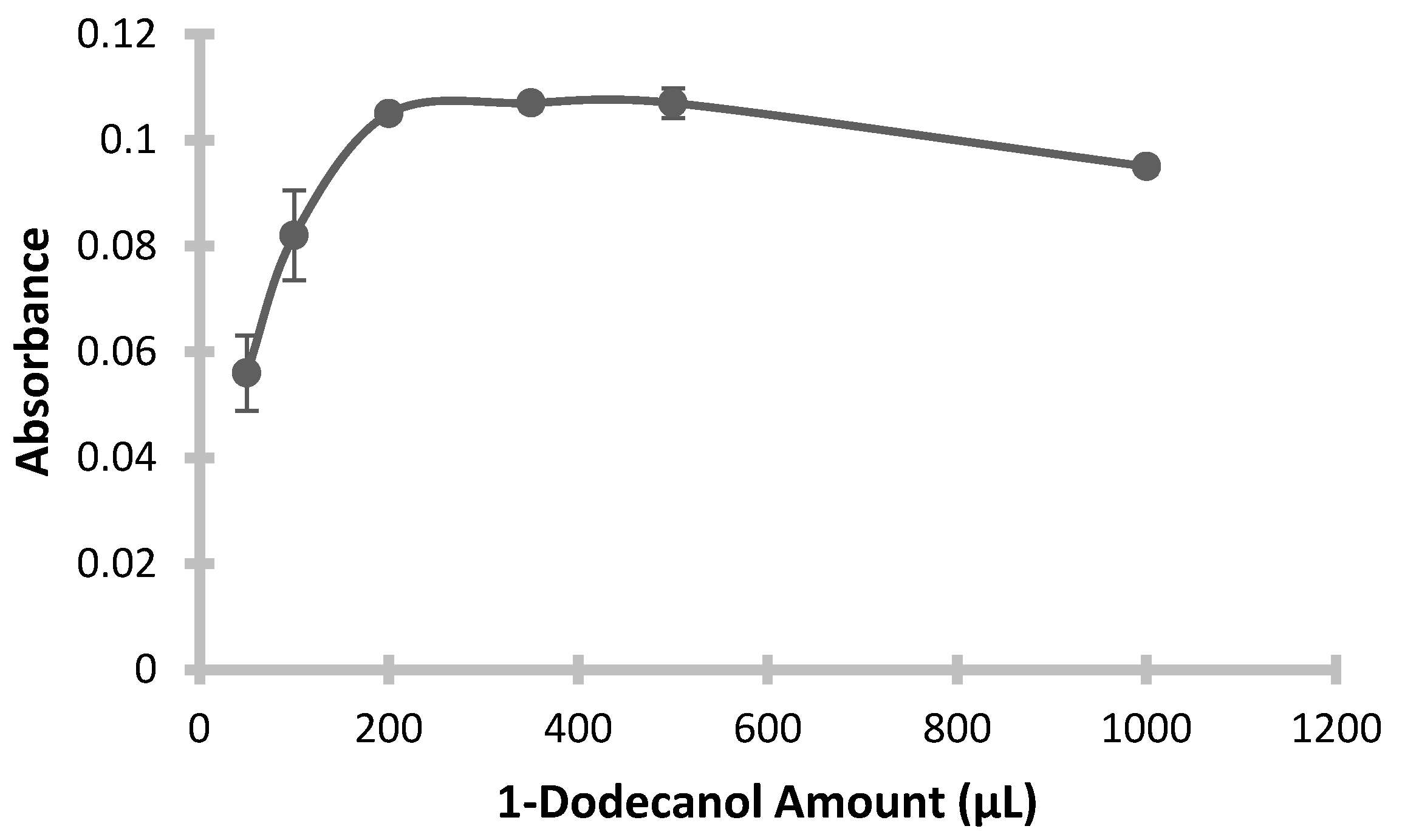
In this part of the experimental study, the optimal volume of extraction solvent was determined by varying the amount of 1-dodecanol added, ranging from 50 μL to 1000 μL, while all other parameters remained constant. The resulting absorbance values, plotted against the volume of dodecanol, are presented in
Figure 5. As shown in the graph, absorbance peaked at 200 μL of dodecanol, with no further increase and even a slight decrease at higher volumes. It was observed that smaller volumes did not yield sufficient extraction efficiency, likely due to inadequate solvent volume. Consequently, 200 μL of 1-dodecanol was identified as the optimal volume for this method.
Conditions: pH: 3; Added Buffer Amount: 0.5 mL; Added ChCl-Urea DES Amount: 0.5 mL; Mixing Speed: 300 rpm; Mixing Temperature: 32 °C; Mixing Time: 15 min; Final Volume: 400 µL.
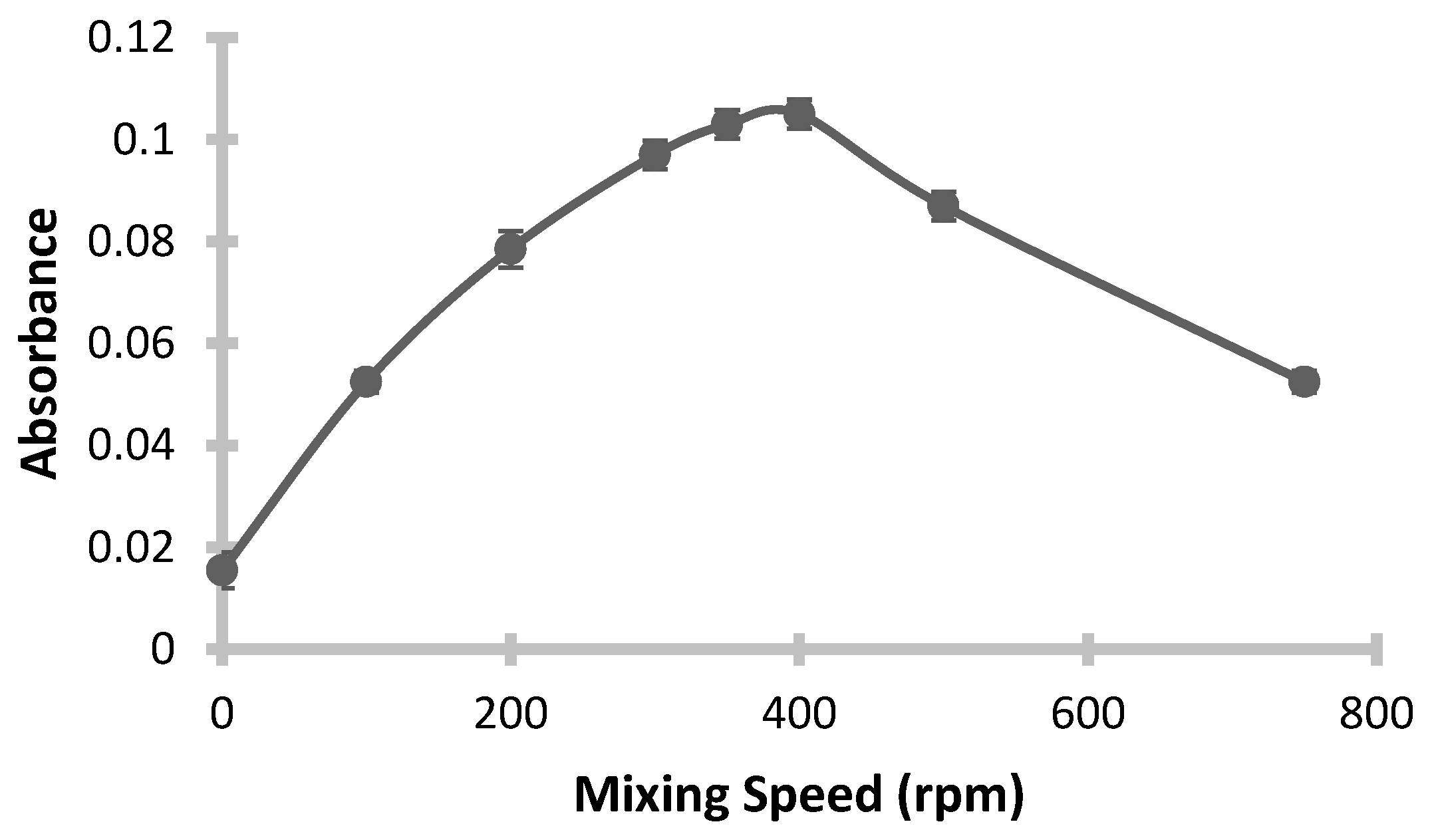
3.4. Effect of Stirring Rate
In microextraction methods, efficient mixing in the liquid phase is crucial, as it accelerates the transfer of analytes between the aqueous and organic phases, thereby reducing extraction time. However, excessive mixing speed can disrupt the stability of the organic droplet, causing it to disperse and degrade, which may negatively impact extraction efficiency. Thus, selecting an appropriate mixing speed is essential. In this study, the extraction was carried out at various mixing speeds, ranging from 0 to 750 rpm, while keeping other parameters constant. The absorbance values measured at each speed are shown in
Figure 6. The results indicate a linear increase in absorbance up to a speed of 350 rpm, beyond which a decrease is observed. This decrease is attributed to the dispersion and structural degradation of the extraction droplet. At speeds of 500 rpm and above, the droplet could no longer be fully recovered due to its loss of structure, rendering accurate absorbance measurement difficulty; hence, these data points were excluded from the graph. The optimal mixing speed was determined to be 350 rpm, as this speed provided the highest efficiency.
Conditions: pH: 3; Added Buffer Amount: 0.5 mL; Added ChCl-Urea DES Amount: 0.5 mL; Added 1-Dodecanol Amount: 200 µL; Mixing Temperature: 32 °C; Mixing Time: 15 min; Final Volume: 400 µL.
3.5. Effect of Extraction Temperature
To optimize the enrichment of silver ions via the solidified floating organic drop extraction (SFODME) technique, the impact of temperature on extraction efficiency was investigated. In SFODME, an organic phase with a high freezing point is essential to facilitate solidification post-extraction. If the temperature is too low, the organic phase solidifies prematurely, hindering mass transfer between phases and impeding efficient extraction. Consequently, the temperature scan commenced above the organic phase’s freezing point. At elevated temperatures, however, the solubility of the organic phase in water increases, reducing its volume and thereby negatively affecting extraction efficiency. The extraction process was conducted at varying solution temperatures, specifically at 26 °C, 32 °C, 44 °C, 56 °C, and 72 °C, by adjusting the solution temperature during each trial. As shown in
Figure 7, the highest absorbance was observed at 32 °C; however, the absorbance difference between 26 °C and 32 °C was minimal. Thus, the experiments proceeded at 26 °C without activating the magnetic stirrer’s heater, conserving energy and aligning with green chemistry principles, with only a slight reduction in absorbance. Accordingly, subsequent experiments were conducted at a constant temperature of 26 °C.
Conditions: pH: 3; Added Buffer Amount: 0.5 mL; Added ChCl-Urea DES Amount: 0.5 mL; Added 1-Dodecanol Amount: 200 µL; Mixing Speed: 350 rpm; Mixing Time: 15 min; Final Volume: 400 µL.
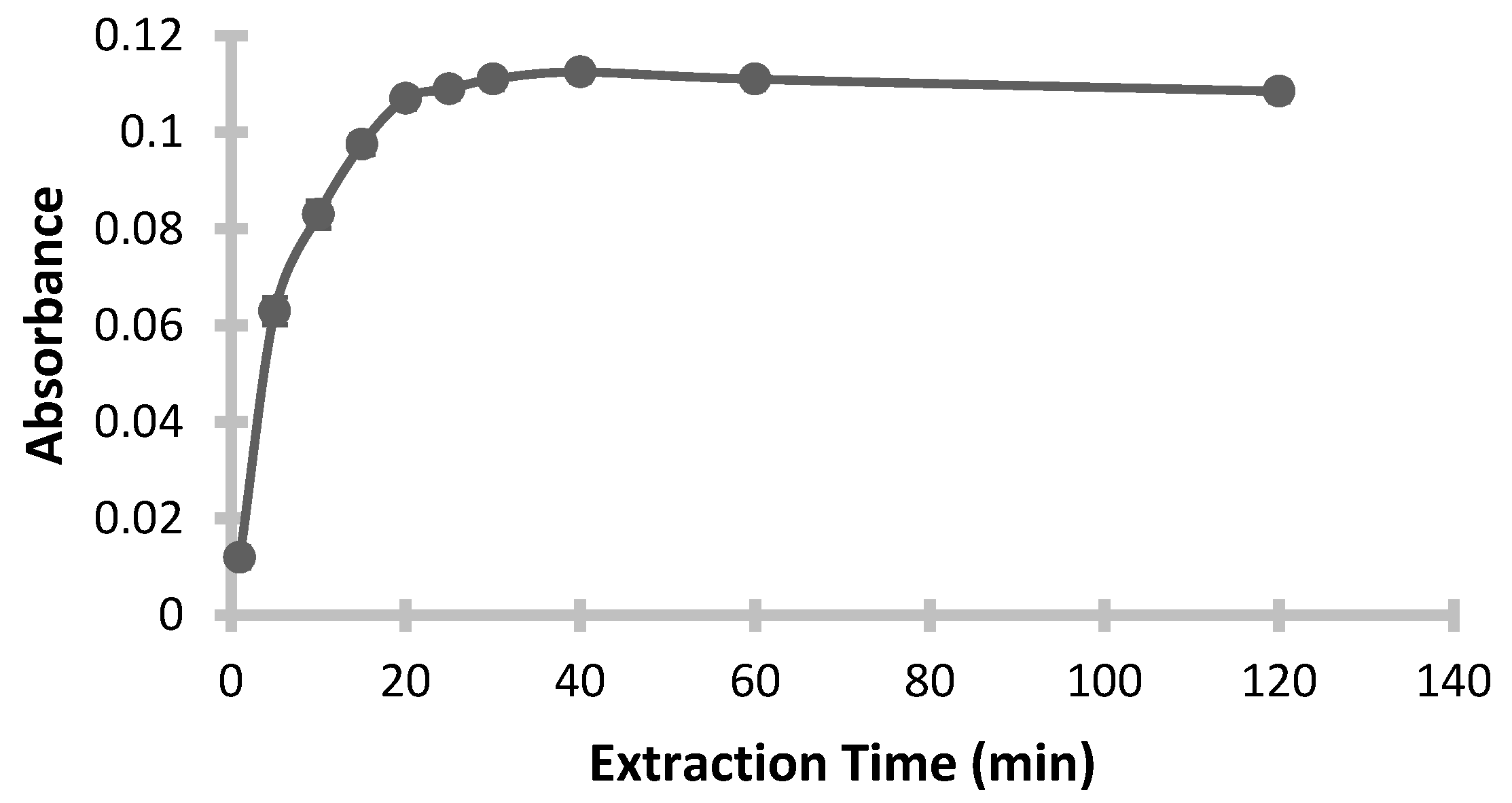
3.6. Effect of Extraction Time
In preconcentration processes, extraction time is crucial to achieving efficient completion of the extraction. Selecting an optimal extraction time is essential not only to enhance repeatability but also to establish equilibrium between the aqueous and organic phases. If the extraction time is too short, emulsification remains incomplete, preventing the system from reaching equilibrium, which results in low yield. Conversely, if extraction time is excessive, the method becomes impractical, and some of the extracted analyte may re-dissolve in the aqueous phase, or the ion pair complex may degrade, reducing overall efficiency. To evaluate the effect of extraction time on efficiency and determine the optimal duration, the extraction time was varied between 1 and 120 min, while other experimental conditions were held constant.
Figure 8 presents the plot of absorbance against extraction time. The results reveal a sharp increase in extraction efficiency and absorbance with time initially, reaching a peak at 20 min, after which the values stabilized with no significant change. Therefore, 20 min was selected as the optimal extraction time for maximum efficiency.
Conditions: pH: 3; Added Buffer Amount: 0.5 mL; Added ChCl-Urea DES Amount: 0.5 mL; Added 1-Dodecanol Amount: 200 µL; Mixing Temperature: 26 °C; Mixing Speed: 350 rpm; Final Volume: 400 µL.
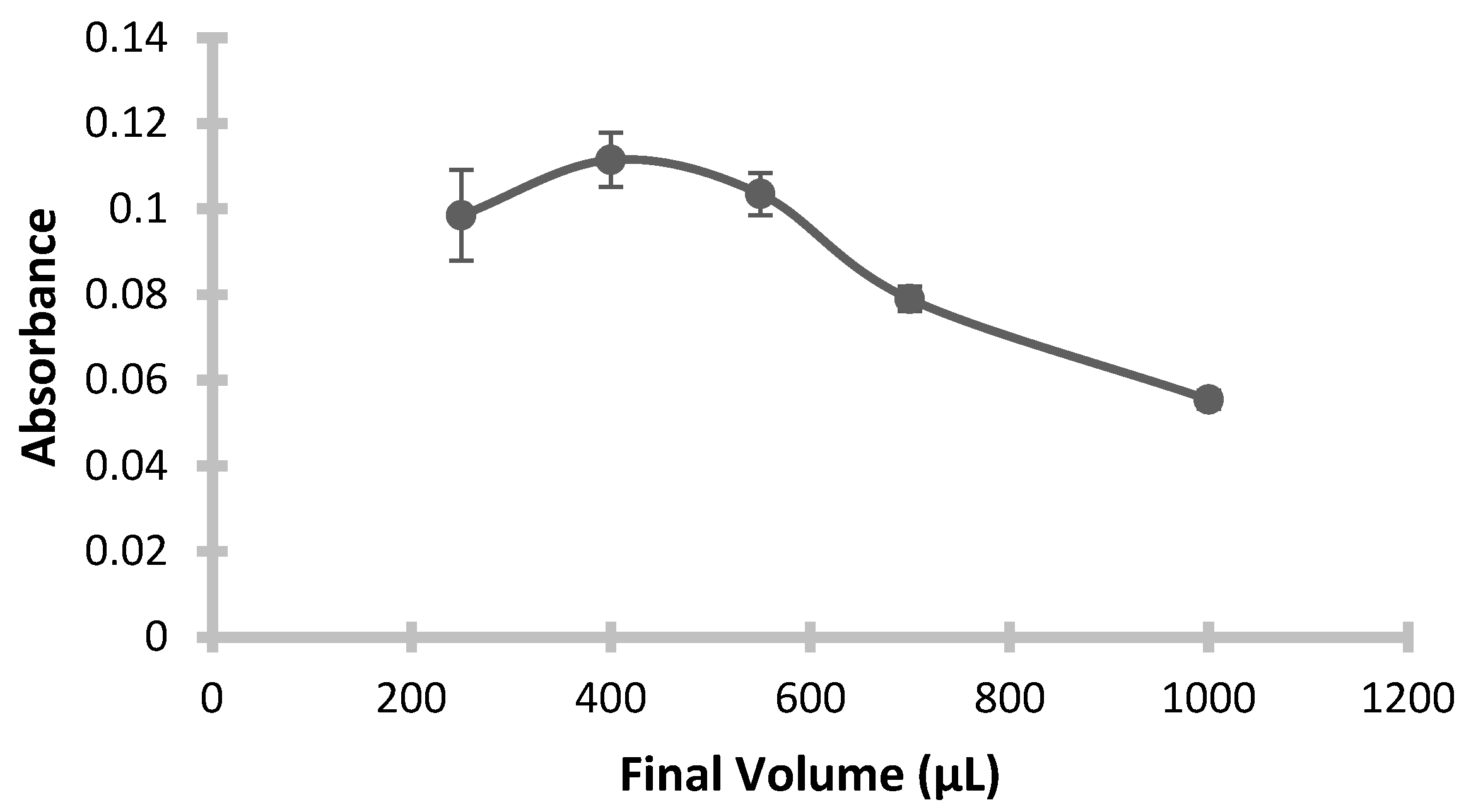
3.7. Effect of Final Volume
At this stage, determining the optimal final volume diluted by alcohol is essential and requires experimental evaluation to determine viscosity and obtain the optimum final volume. To investigate the effect of final dilution volume on extraction efficiency, the solidified organic drop was diluted with ethanol, and absorbance measurements were taken at final volumes ranging from 250 μL to 1000 μL. To maintain a high enhancement factor, smaller final volumes were prioritized. However, volumes below 400 μL proved impractical due to high viscosity, which led to partial blockages in the nebulizer (fogger-sampling unit) of the instrument, impeding accurate absorbance measurements. Consequently, reliable analyte signals could not be obtained below this volume, making scans below 400 μL untrustworthy.
Figure 9 illustrates these findings. The results indicate that maximum absorbance occurred at 400 μL, identifying it as the optimal volume. At higher volumes, the concentration of dissolved analyte naturally decreases, resulting in lower absorbance values.
Conditions: pH: 3; Added Buffer Amount: 0.5 mL; Added ChCl-Urea DES Amount: 0.5 mL; Added 1-Dodecanol Amount: 200 µL; Mixing Temperature: 26 °C; Mixing Speed: 350 rpm; Mixing Time: 20 min.
3.8. Effect of Interferences
To evaluate the feasibility of selectively recovering analyte ions in the presence of interfering ions, a protocol was conducted using a 15 mL solution containing 50 µg/L of silver, along with various concentrations of interfering ions. The tolerance limit was defined as the absorbance resulting from the added ion that produced less than a ±5% relative error in the determination of Ag(I). The maximum tolerance limits for the cations and anions analyzed are detailed in
Table 1.
3.9. Analytical Performance of the Proposed Method
Under optimized SFODME conditions, the calibration curves for silver exhibited good linearity across the concentration range of 0.0075–0.2 mg/L. The calibration equation was determined to be A = 1.79 C + 7 × 10−3, where A corresponds to the absorbance and C is the concentration of silver ions in mg/L. The correlation coefficient (R2) exceeded 0.995, indicating excellent linearity. In comparison, when FAAS was applied without preconcentration, the calibration curve followed the equation A = 6.42 × 10−2 C + 2.1 × 10−3 with an R2 value of 0.999, covering a linear range of 0.2–8 mg/L. The enhancement factor, calculated by comparing the slopes of the calibration plots with and without preconcentration, was found to be 27.9. The limit of detection (LOD), defined as three times the standard deviation (3σ) from ten replicate blank analyses, was calculated to be 0.0021 mg/L. The limit of quantification (LOQ), representing the lowest concentration quantifiable with acceptable accuracy and precision, was determined as ten times the standard deviation (10σ), yielding a value of 0.007 mg/L. For a silver concentration of 0.015 mg/L, the relative standard deviation (RSD) was ±2.32% based on eight replicates (n = 8), confirming the method’s precision.
3.10. Analysis of Certified Reference Materials
The proposed SFODME method was utilized on certified reference materials according to the sample preparation procedure described in the section titled ‘Preparation of Real Sample.’ Recovery rates ranging from 80% to 93% were obtained, and the findings are presented in
Table 2.
3.11. Analysis of Real Samples
Real samples were collected from various mining activities, specifically including waste material from an open-pit gold-silver mine and tailings derived from an underground gold-silver operation, both located in the Aegean region of Turkey. The developed DES-SFODME technique was applied to these samples following the established sample preparation procedure. After the preconcentration process, silver concentrations were determined by correlating the measured absorbance values with the previously constructed calibration curve (
Table 3).
4. Discussion
This research highlights the effective utilization of deep eutectic solvents (DES) in conjunction with solidified floating organic drop microextraction (SFODME) for extracting silver from real mining samples, with quantification by flame atomic absorption spectrometry (FAAS). The method demonstrated satisfactory analytical performance, including a high enhancement factor, low detection limit, good linearity, and excellent precision, all of which surpass values reported in much of the existing literature. A comprehensive comparison of the proposed method with other silver preconcentration techniques is provided in
Table 4, supporting the superior efficiency of the developed approach.
In addition to its analytical merits, the use of DES in this approach offers a greener alternative to traditional organic solvents, reducing toxicity and overall solvent consumption. The SFODME procedure further simplifies phase separation through solidification, eliminating the need for centrifugation and making the workflow faster and more cost-effective. These advantages enhance the method’s suitability for monitoring of silver in complex mining waste matrices, where robustness and reproducibility are critical.
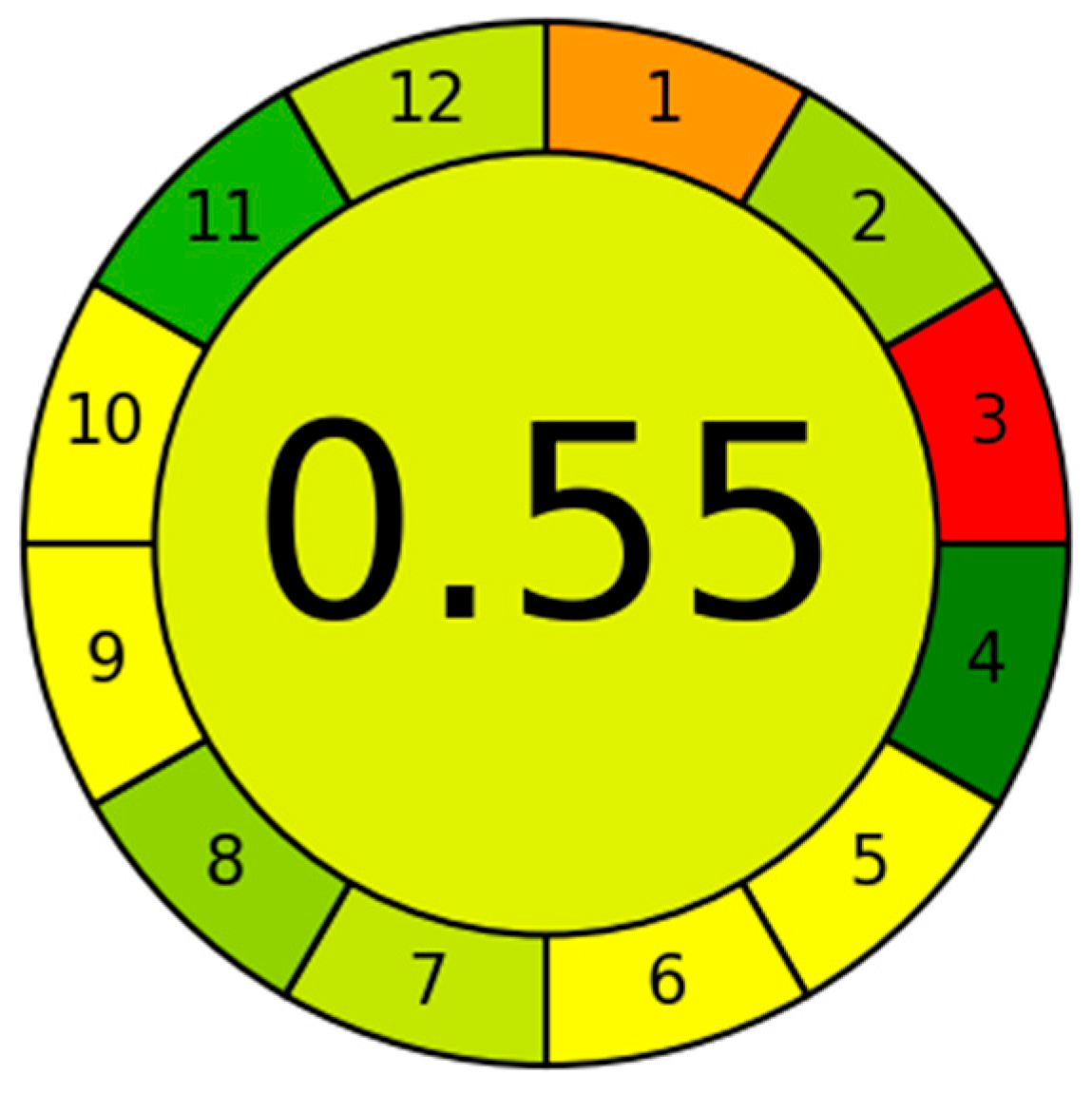
Furthermore, its favorable AGREE score confirms that the procedure supports green analytical chemistry and offers an environmentally responsible alternative for routine silver monitoring. The Analytical GREEnness (AGREE) tool, derived from the 12 principles of Green Analytical Chemistry, provides a comprehensive yet practical means of assessing the sustainability of analytical procedures [
38]. In this work, the developed DES-SFODME–FAAS method achieved an AGREE score of 0.55 (
Figure 10.), reflecting its low environmental burden and alignment with green chemistry objectives.
5. Conclusions
A novel and environmentally oriented method was successfully developed for the recovery and determination of trace silver from mining waste using deep eutectic solvent-assisted solidified floating organic drop microextraction (DES-SFODME) combined with FAAS. The method achieved low detection limits, high precision, good linearity, and a significant enhancement factor under mild experimental conditions. Its selectivity and robustness were confirmed by analyses of certified reference materials and real mining waste samples, consistently yielding recoveries above 90%.
The use of readily available reagents and the method’s compatibility with FAAS combination suggest that rapid, low-cost preconcentration is feasible for routine mining laboratory operations. The small solvent volumes, simplicity of operation, and potential for automation make this approach a promising candidate for sustainable monitoring of silver in complex matrices. Future work should explore pilot-scale implementation and techno-economic evaluation to support industrial adoption.