Effects of Tetracycline on Growth and Nutrient Removal by Lemna aoukikusa and Spirodela polyrhiza Under Short-Term Cultivation
Abstract
1. Introduction
2. Materials and Methods
2.1. Duckweed Species and Medium
2.2. Batch Cultivation Conditions
2.3. Analytical Procedures
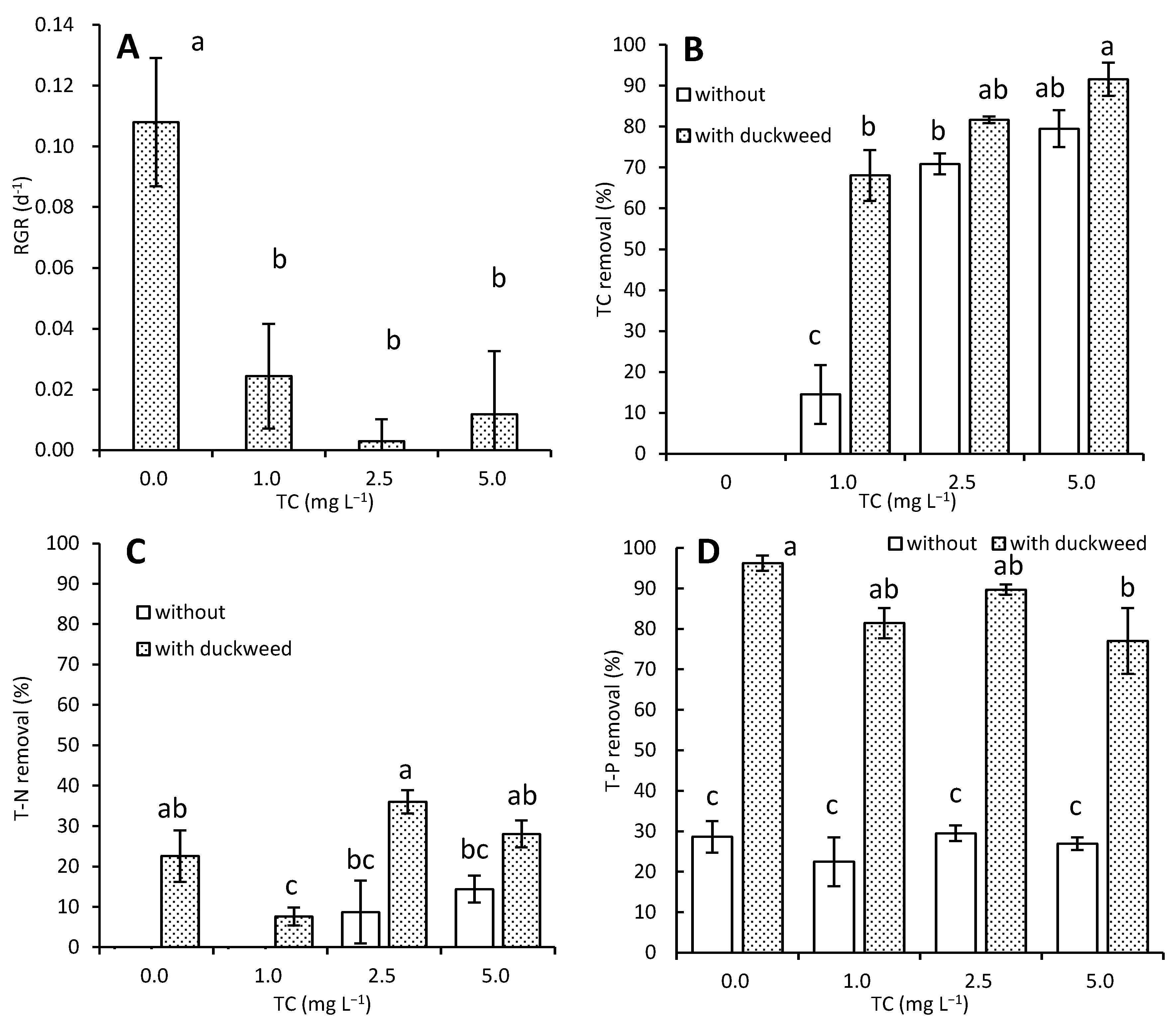
3. Results
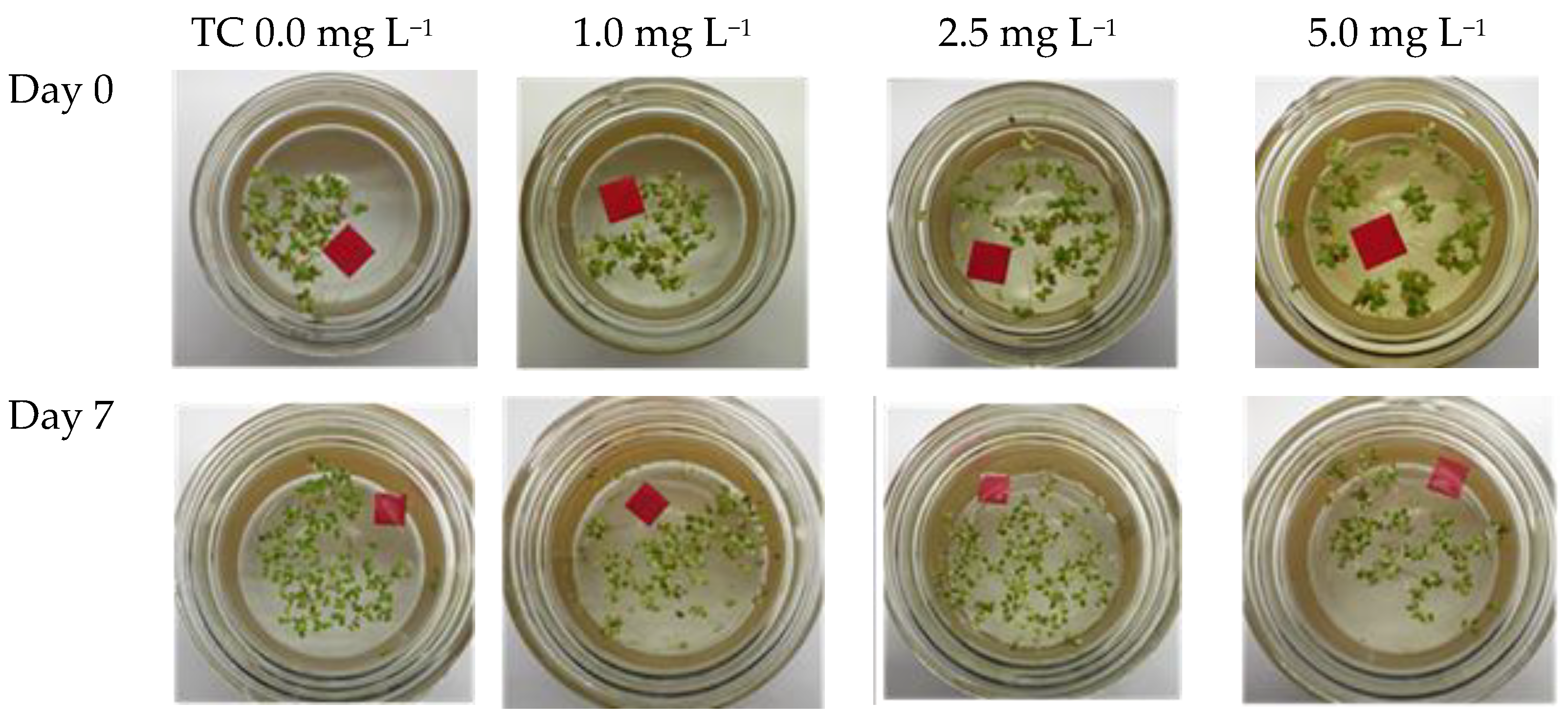
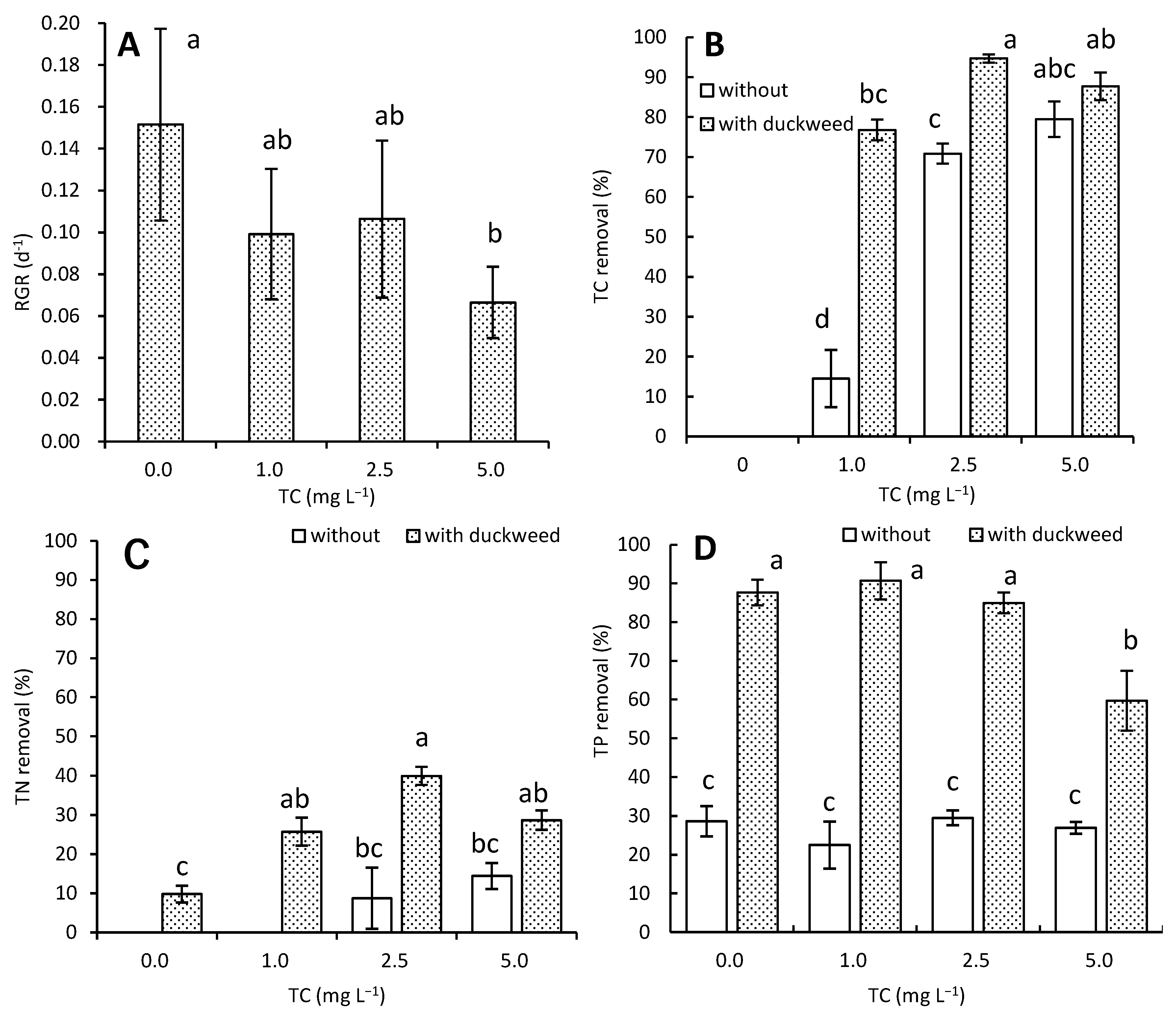
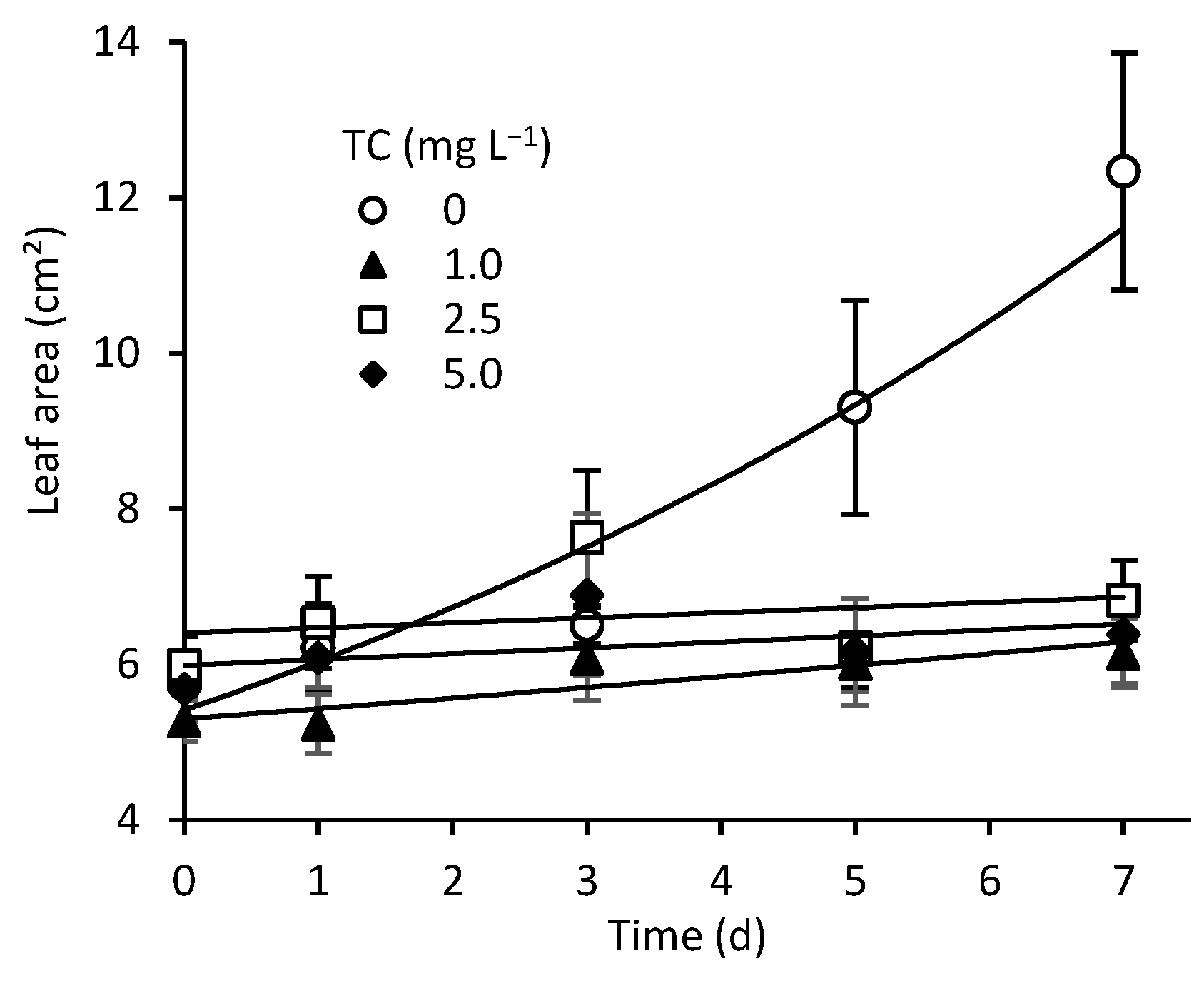
3.1. Effects of TC on Growth and Nutrient Removal by L. aoukikusa
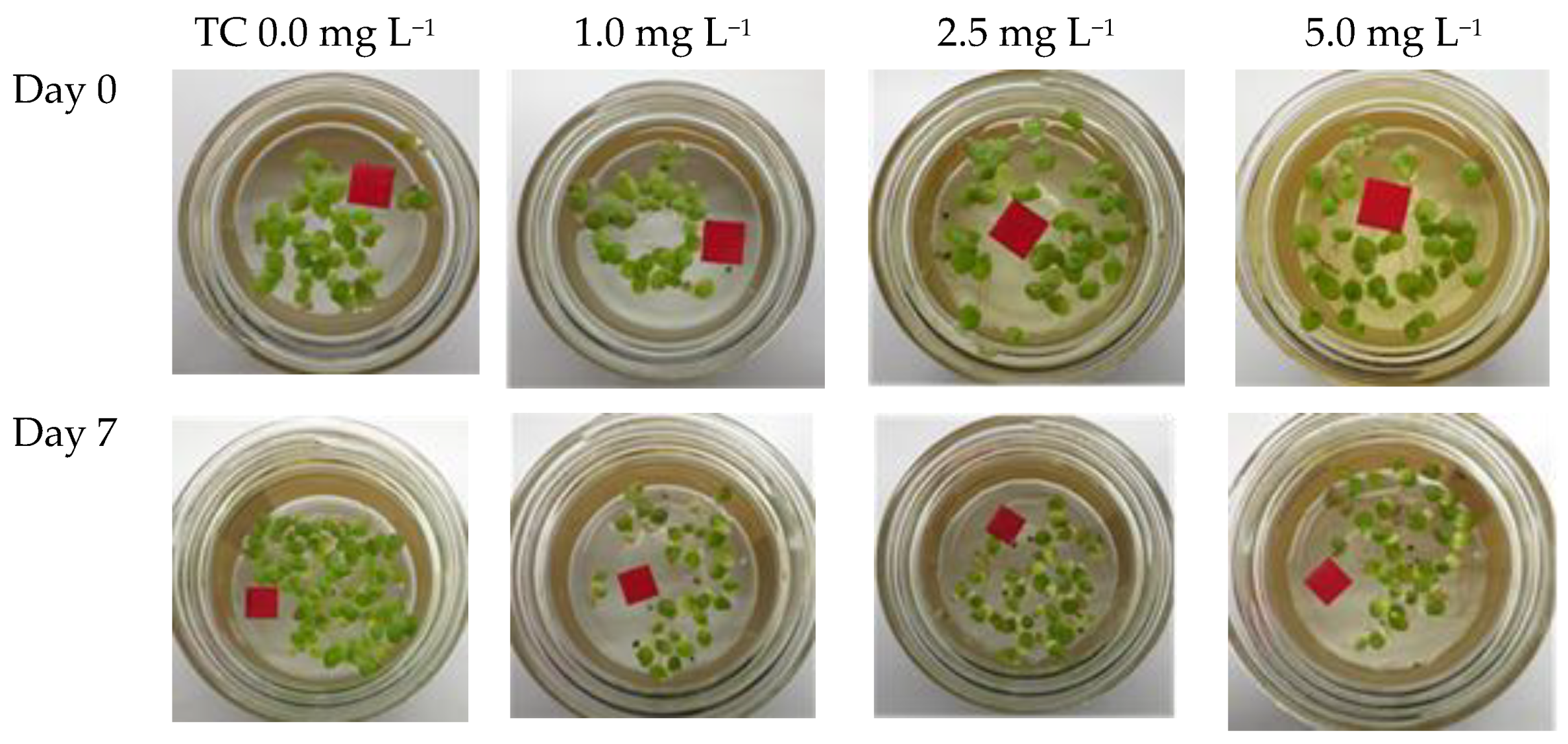
3.2. Effects of TC on Growth and Nutrient Removal by S. polyrhiza
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OTC | Oxytetracycline |
| RGR | Relative growth rate |
| TC | Tetracycline |
| TN | Total nitrogen |
| TP | Total phosphorus |
References
- Cheng, D.L.; Ngo, H.H.; Guo, W.S.; Chang, S.W.; Nguyen, D.D.; Liu, Y.W.; Wei, Q.; Wei, D. A critical review on antibiotics and hormones in swine wastewater: Water pollution problems and control approaches. J. Hazard. Mater. 2020, 387, 121682. [Google Scholar] [CrossRef] [PubMed]
- Topal, M.; Öbek, E.; Uslu Şenel, G.; Arslan Topal, E.I. Phytoremediation of tetracycline and degradation products from aqueous solutions. Pollution 2018, 4, 471–480. [Google Scholar] [CrossRef]
- Sánchez-Polo, M.; Velo-Gala, I.; López-Peñalver, J.J.; Rivera-Utrilla, J. Molecular imprinted polymer to remove tetracycline from aqueous solutions. Microporous Mesoporous Mater. 2015, 203, 32–40. [Google Scholar] [CrossRef]
- Campagnolo, E.R.; Johnson, K.R.; Karpati, A.; Rubin, C.S.; Kolpin, D.W.; Meyer, M.T.; Esteban, J.E.; Currier, R.W.; Smith, K.; Thu, K.M.; et al. Antimicrobial residues in animal waste and water resources proximal to large-scale swine and poultry feeding operations. Sci. Total Environ. 2002, 299, 89–95. [Google Scholar] [CrossRef]
- Bergmann, B.A.; Cheng, J.; Classen, J.; Stomp, A.M. Nutrient removal from swine lagoon effluent by duckweed. Trans. ASAE 2000, 43, 263–269. [Google Scholar] [CrossRef]
- Cheng, J.; Landesman, L.; Bergmann, B.A.; Classen, J.J.; Howard, J.W.; Yamamoto, Y. Nutrient removal from swine lagoon liquid by Lemna minor 8627. Trans. ASAE 2002, 45, 1003–1010. [Google Scholar] [CrossRef]
- Xu, J.; Shen, G. Effects of harvest regime and water depth on nutrient recovery from swine wastewater by growing Spirodela oligorrhiza. Water Environ. Res. 2011, 83, 2049–2056. [Google Scholar] [CrossRef]
- Mohedano, R.A.; Costa, H.R.; Tarares, A.; Filho, P.B. High nutrient removal rate from swine wastes and protein biomass production by full-scale duckweed ponds. Bioresour. Technol. 2012, 112, 98–104. [Google Scholar] [CrossRef]
- Sudiarto, S.I.A.; Renggaman, A.; Choi, H.L. Floating aquatic plants for total nitrogen and phosphorus removal from treated swine wastewater and their biomass characteristics. J. Environ. Manag. 2019, 231, 763–769. [Google Scholar] [CrossRef]
- Dinh, T.T.U.; Soda, S.; Nguyen, T.A.H.; Nakajima, J.; Cao, T.H. Nutrient removal by duckweed from anaerobically treated swine wastewater in lab-scale stabilization ponds in Vietnam. Sci. Total Environ. 2020, 772, 137854. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.A.H.; Bui, T.H.; Nguyen, T.T.; Dan, A.; Soda, S. Constructed wetlands for sustainable bioremediation of antibiotics from wastewater: Potential and challenge. In Nature-Based Solutions for Urban Sustainability; Lens, P., Thanh, B.X., Eds.; IWA-publishing: London, UK, 2025; pp. 61–95. [Google Scholar] [CrossRef]
- Iatrou, E.A.; Gatidou, G.; Damalas, D.; Thomaidis, N.S.; Stasinakis, A.S. Fate of antimicrobials in duckweed Lemna minor wastewater treatment systems. J. Hazard. Mater. 2017, 330, 116–126. [Google Scholar] [CrossRef]
- Dinh, T.T.U.; Semba, S.; Nakajima, J.; Soda, S. Image analysis for estimation of biomass and nutrient removal of duckweed Lemna aoukikusa, Spirodela polyrhiza, and Wolffia globosa in lab-scale cultivation. Jpn. J. Water Treat. Biol. 2022, 58, 35–43. [Google Scholar] [CrossRef]
- Pan, M.; Chu, L.M. Phytotoxicity of veterinary antibiotics to seed germination and root elongation of crops. Ecotoxicol. Environ. Saf. 2016, 126, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Brain, R.A.; Johnson, D.J.; Richards, S.M.; Sanderson, H.; Sibley, P.K.; Solomon, K.R. Effects of 25 pharmaceutical compounds to Lemna gibba using a seven-day static-renewal test. Environ. Toxicol. Chem. 2004, 23, 371–382. [Google Scholar] [CrossRef]
- Baciak, M.; Sikorski, L.; Piotrowicz-Cieslak, A.I.; Adomos, B. Content of biogenic amines in Lemna minor (common duckweed) growing in medium contaminated with tetracycline. Aquat. Toxicol. 2016, 180, 95–102. [Google Scholar] [CrossRef]
- Krupka, M.; Michalczyk, D.J.; Žaltauskaitė, J.; Sujetovienė, G.; Glowacka, K.; Grajek, H.; Wierzbicka, M.; Piotrowicz-Cieślak, A.I. Physiological and biochemical parameters of common duckweed Lemna minor after the exposure to tetracycline and the recovery from this stress. Molecules 2021, 26, 6765. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhou, Q.; Li, X.; Lou, W.; Du, C.; Teng, Q.; Zhang, D.; Liu, H.; Zhong, Y.; Yang, C. Phytoremediation of anaerobically digested swine wastewater contaminated by oxytetracycline via Lemna aequinoctialis: Nutrient removal, growth characteristics and degradation pathways. Bioresour. Technol. 2019, 291, 121853. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Li, X.; Wu, S.; Lou, W.; Yang, C. Effects of long-term exposure to oxytetracycline on phytoremediation of swine wastewater via duckweed systems. J. Hazard. Mater. 2021, 414, 125508. [Google Scholar] [CrossRef]
- APHA/AWWA/WEF. Standard methods for the examination of water and wastewater. In Standard Methods; APHA/AWWA/WEF: Washington, DC, USA, 2012; p. 541. [Google Scholar]
- Ciarlone, A.E.; Fry, B.W.; Ziemer, D.M. Some observations on the adsorption of tetracyclines to glass and plastic labware. Microchem. J. 1990, 42, 250–255. [Google Scholar] [CrossRef]
- Sawada, K.; Hojo, Y.; Shimizu, T.; Soda, S. Soil adsorption, photodegradation, and removal of antibiotics from water by duckweed: Case studies examining erythromycin, lincomycin, and others. J. Environ. Conserv. Eng. 2023, 52, 296–304. (In Japanese) [Google Scholar] [CrossRef]
- Yamaga, F.; Washio, K.; Morikawa, M. Sustainable biodegradation of phenol by Acinetobacter calcoaceticus P23 isolated from the rhizosphere of duckweed Lemna aoukikusa. Environ. Sci. Technol. 2010, 44, 6470–6474. [Google Scholar] [CrossRef]
- Ishizawa, H.; Kuroda, M.; Morikawa, M.; Ike, M. Evaluation of environmental bacterial communities as a factor affecting the growth of duckweed Lemna minor. Biotechnol. Biofuels 2017, 10, 62. [Google Scholar] [CrossRef]
- Langbehn, R.K.; Michels, C.; Soares, H.M. Tetracyclines lead to ammonium accumulation during nitrification process. J. Environ. Sci. Health A 2020, 55, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Hu, Y.; Cheng, C.; Cheng, J.; Chen, Y. Simultaneous degradation of tetracycline and denitrification by a novel bacterium, Klebsiella sp. SQY5. Chemosphere 2018, 209, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Lei, A.; Yang, S.; Chen, H.; Liu, X.; Liu, L.; Kang, X. Biodegradation and bioaugmentation of tetracycline by Providencia stuartii TX2: Performance, degradation pathway, genetic background, key enzymes, and application risk assessment. J. Hazard. Mater. 2024, 477, 135231. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinh, U.T.T.; Nakagawa, S.; Shimizu, T.; Soda, S. Effects of Tetracycline on Growth and Nutrient Removal by Lemna aoukikusa and Spirodela polyrhiza Under Short-Term Cultivation. Appl. Sci. 2025, 15, 11621. https://doi.org/10.3390/app152111621
Dinh UTT, Nakagawa S, Shimizu T, Soda S. Effects of Tetracycline on Growth and Nutrient Removal by Lemna aoukikusa and Spirodela polyrhiza Under Short-Term Cultivation. Applied Sciences. 2025; 15(21):11621. https://doi.org/10.3390/app152111621
Chicago/Turabian StyleDinh, Uyen Thi To, Shoki Nakagawa, Toshiyuki Shimizu, and Satoshi Soda. 2025. "Effects of Tetracycline on Growth and Nutrient Removal by Lemna aoukikusa and Spirodela polyrhiza Under Short-Term Cultivation" Applied Sciences 15, no. 21: 11621. https://doi.org/10.3390/app152111621
APA StyleDinh, U. T. T., Nakagawa, S., Shimizu, T., & Soda, S. (2025). Effects of Tetracycline on Growth and Nutrient Removal by Lemna aoukikusa and Spirodela polyrhiza Under Short-Term Cultivation. Applied Sciences, 15(21), 11621. https://doi.org/10.3390/app152111621





