Diffusion Tensor Imaging in Degenerative Cervical Myelopathy: Clinical Translation Opportunities for Cause of Pain Detection and Potentially Early Diagnoses
Abstract
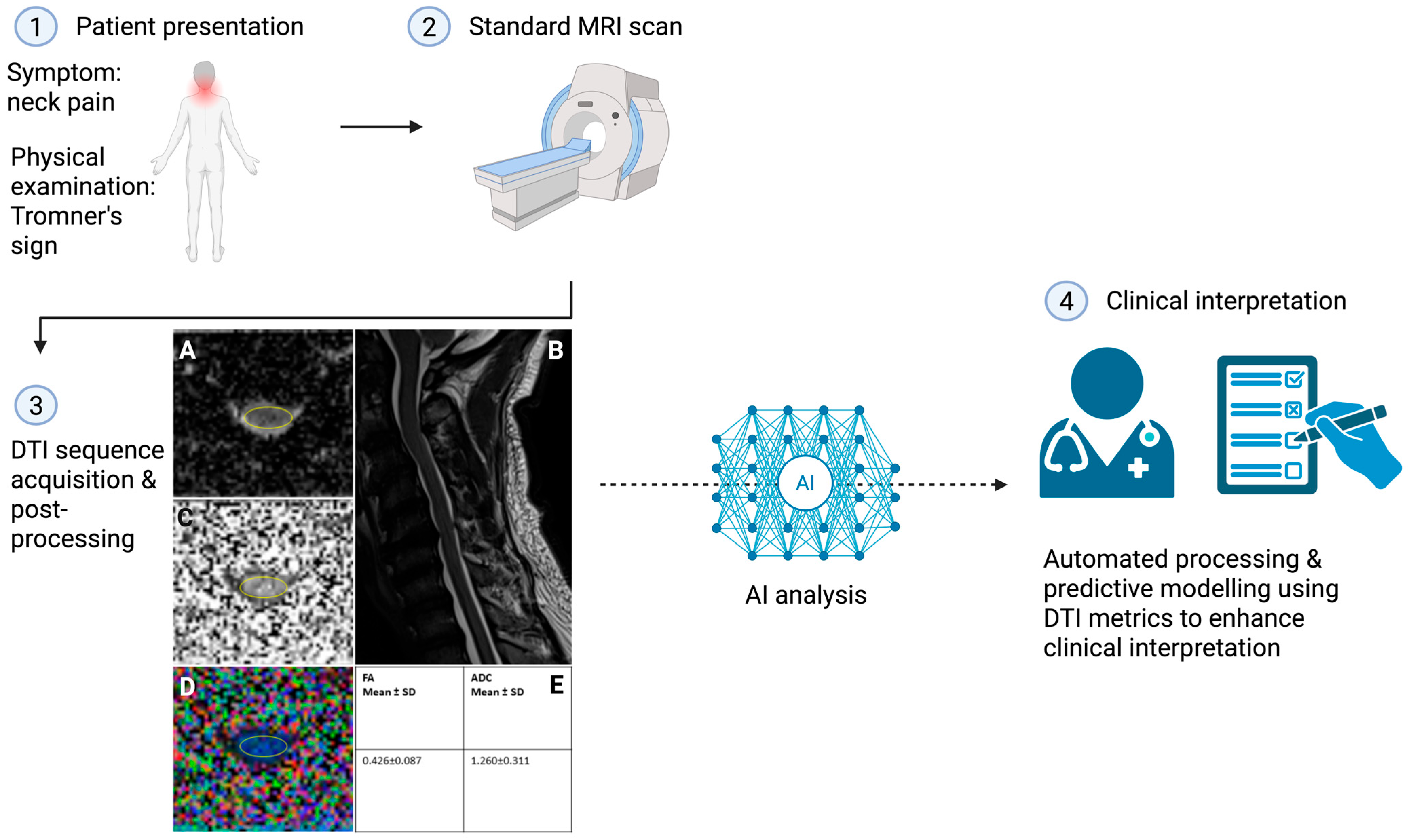
1. Introduction
2. Methods
3. Conventional MRI as the Diagnostic Standard for DCM
4. Diffusion Tensor Imaging in the Spinal Cord
5. Diagnostic and Prognostic Utility of DTI in DCM
6. Unexplored Relationships Between DTI Metrics and Pain
7. Methodological and Interpretative Barriers in DCM
8. Future Directions: DTI as a Pain—Specific Imaging Biomarker in DCM
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AD | Axial diffusivity |
| ADC | Apparent diffusion coefficient |
| AI | Artificial intelligence |
| AUC | Area under the curve |
| CSA | Cross-sectional area |
| CSM | Cervical spondylotic myelopathy |
| DCM | Degenerative cervical myelopathy |
| DeVa | Decay variance |
| DN4 | Douleur Neuropathique 4 |
| DTI | Diffusion tensor imaging |
| EMS | European Myelopathy Score |
| EQ-5D | EuroQol 5-Dimension Questionnaire |
| FA | Fractional anisotropy |
| JOA | Japanese Orthopaedic Association |
| JOACMEQ | Japanese Orthopaedic Association Cervical Myelopathy Evaluation Questionnaire |
| Kr | Radial kurtosis |
| MCC | Maximum canal compromise |
| MD | Mean diffusivity |
| MK | Mean kurtosis |
| MRI | Magnetic resonance imaging |
| MSCC | Maximum spinal cord compression |
| mJOA | Modified Japanese Orthopaedic Association |
| NDI | Neck Disability Index |
| OPLL | Ossification of the posterior longitudinal ligament |
| QOL | Quality of life |
| RD | Radial diffusivity |
| ROI | Region of interest |
| SD | Standard deviation |
| SF-12 | Short form-12 |
| SF-36 | Short form-36 |
| VAS | Visual Analogue Scale |
References
- Nouri, A.; Tetreault, L.; Singh, A.; Karadimas, S.K.; Fehlings, M.G. Degenerative Cervical Myelopathy: Epidemiology, Genetics, and Pathogenesis. Spine 2015, 40, E675–E693. [Google Scholar] [CrossRef]
- Akter, F.; Yu, X.; Qin, X.; Yao, S.; Nikrouz, P.; Syed, Y.A.; Kotter, M. The pathophysiology of degenerative cervical myelopathy and the physiology of recovery following decompression. Front. Neurosci. 2020, 14, 138. [Google Scholar]
- Sharma, S.; Sial, A.; Sima, S.; Aggarwal, A.; Yull, D.; Diwan, A. Making the diagnosis of degenerative cervical myelopathy in clinical practice: Essential evidence-based examination tools for healthcare practitioners. J. Clin. Neurosci. 2025, 137, 111297. [Google Scholar] [CrossRef] [PubMed]
- Davies, B.M.; Yang, X.; Khan, D.Z.; Mowforth, O.D.; Touzet, A.Y.; Nouri, A.; Harrop, J.S.; Aarabi, B.; Rahimi-Movaghar, V.; Kurpad, S.N.; et al. A minimum data set-Core outcome set, core data elements, and core measurement set-For degenerative cervical myelopathy research (AO Spine RECODE DCM): A consensus study. PLoS Med. 2024, 21, e1004447. [Google Scholar] [CrossRef]
- Sial, A.W.; Sima, S.; Narulla, R.; Najib, N.; Davies, M.; Diwan, A.D. Is neck pain a marker for something serious? Like myelopathy. Spinal Cord 2024, 62, 718–720. [Google Scholar] [CrossRef]
- Badhiwala, J.H.; Ahuja, C.S.; Akbar, M.A.; Witiw, C.D.; Nassiri, F.; Furlan, J.C.; Curt, A.; Wilson, J.R.; Fehlings, M.G. Degenerative cervical myelopathy—Update and future directions. Nat. Rev. Neurol. 2020, 16, 108–124. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sial, A.; Sima, S.; Diwan, A. Clinical signs and symptoms for degenerative cervical myelopathy: A scoping review of case-control studies to facilitate early diagnosis among healthcare professionals with stakeholder engagement. Spinal Cord 2025, 63, 171–180. [Google Scholar] [CrossRef]
- Davies, B.M.; Mowforth, O.D.; Smith, E.K.; Kotter, M.R. Degenerative cervical myelopathy. BMJ 2018, 360, k186. [Google Scholar] [CrossRef] [PubMed]
- Fehlings, M.G.; Tetreault, L.A.; Kurpad, S.; Brodke, D.S.; Wilson, J.R.; Smith, J.S.; Arnold, P.M.; Brodt, E.D.; Dettori, J.R. Change in functional impairment, disability, and quality of life following operative treatment for degenerative cervical myelopathy: A systematic review and meta-analysis. Glob. Spine J. 2017, 7, 53S–69S. [Google Scholar] [CrossRef]
- Karadimas, S.K.; Erwin, W.M.; Ely, C.G.; Dettori, J.R.; Fehlings, M.G. Pathophysiology and natural history of cervical spondylotic myelopathy. Spine 2013, 38, S21–S36. [Google Scholar] [CrossRef]
- Rodrigues-Pinto, R.; Montenegro, T.S.; Davies, B.M.; Kato, S.; Kawaguchi, Y.; Ito, M.; Zileli, M.; Kwon, B.K.; Fehlings, M.G.; Koljonen, P.A. Optimizing the application of surgery for degenerative cervical myelopathy [AO Spine RECODE-DCM Research Priority Number 10]. Glob. Spine J. 2022, 12, 147S–158S. [Google Scholar] [CrossRef]
- Tetreault, L.; Côté, P.; Kopjar, B.; Arnold, P.; Fehlings, M. AOSpine North America and International Clinical Trial Research Network. A clinical prediction model to assess surgical outcome in patients with cervical spondylotic myelopathy: Internal and external validations using the prospective multicenter AOSpine North American and international datasets of 743 patients. Spine J. 2015, 15, 388–397. [Google Scholar]
- Behrbalk, E.; Salame, K.; Regev, G.J.; Keynan, O.; Boszczyk, B.; Lidar, Z. Delayed diagnosis of cervical spondylotic myelopathy by primary care physicians. Neurosurg. Focus 2013, 35, E1. [Google Scholar] [CrossRef]
- Sadasivan, K.K.; Reddy, R.P.; Albright, J. The natural history of cervical spondylotic myelopathy. Yale J. Biol. Med. 1993, 66, 235. [Google Scholar]
- Nouri, A.; Martin, A.R.; Kato, S.; Reihani-Kermani, H.; Riehm, L.E.; Fehlings, M.G. The Relationship Between MRI Signal Intensity Changes, Clinical Presentation, and Surgical Outcome in Degenerative Cervical Myelopathy: Analysis of a Global Cohort. Spine 2017, 42, 1851–1858. [Google Scholar] [CrossRef]
- Sial, A.W.; Sima, S.; Chen, X.; Saulys, C.; Kuan, J.; Davies, M.; Diwan, A.D. Spinal column radiological factors associated with increased spinal cord intramedullary signal intensity—A study evaluating aging spinal cord’s relation to spinal disc degeneration. J. Clin. Neurosci. 2024, 126, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Harrop, J.S.; Naroji, S.; Maltenfort, M.; Anderson, D.G.; Albert, T.; Ratliff, J.K.; Ponnappan, R.K.; Rihn, J.A.; Smith, H.E.; Hilibrand, A.; et al. Cervical myelopathy: A clinical and radiographic evaluation and correlation to cervical spondylotic myelopathy. Spine 2010, 35, 620–624. [Google Scholar] [CrossRef]
- He, B.; Sheldrick, K.; Das, A.; Diwan, A. Clinical and Research MRI Techniques for Assessing Spinal Cord Integrity in Degenerative Cervical Myelopathy-A Scoping Review. Biomedicines 2022, 10, 2621. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Bian, B.; Wang, G.; Tian, C.; Lv, Z.; Shao, Z.; Li, D. Evaluation of microstructural changes in spinal cord of patients with degenerative cervical myelopathy by diffusion kurtosis imaging and investigate the correlation with JOA score. BMC Neurol. 2020, 20, 185. [Google Scholar] [CrossRef]
- Budde, M.D.; Kim, J.H.; Liang, H.F.; Schmidt, R.E.; Russell, J.H.; Cross, A.H.; Song, S.K. Toward accurate diagnosis of white matter pathology using diffusion tensor imaging. Magn. Reson. Med. 2007, 57, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Loy, D.N.; Liang, H.F.; Trinkaus, K.; Schmidt, R.E.; Song, S.K. Noninvasive diffusion tensor imaging of evolving white matter pathology in a mouse model of acute spinal cord injury. Magn. Reson. Med. 2007, 58, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, P.; Raj, D.; Liu, J.; Lam, C.; Yung, A.C.; Tetzlaff, W. Characterizing white matter damage in rat spinal cord with quantitative MRI and histology. J. Neurotrauma 2008, 25, 653–676. [Google Scholar] [CrossRef]
- Xie, M.; Wang, Q.; Wu, T.H.; Song, S.K.; Sun, S.W. Delayed axonal degeneration in slow Wallerian degeneration mutant mice detected using diffusion tensor imaging. Neuroscience 2011, 197, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Nouri, A.; Martin, A.R.; Mikulis, D.; Fehlings, M.G. Magnetic resonance imaging assessment of degenerative cervical myelopathy: A review of structural changes and measurement techniques. Neurosurg. Focus 2016, 40, E5. [Google Scholar] [CrossRef]
- Nagata, K.; Kiyonaga, K.; Ohashi, T.; Sagara, M.; Miyazaki, S.; Inoue, A. Clinical value of magnetic resonance imaging for cervical myelopathy. Spine 1990, 15, 1088–1096. [Google Scholar] [CrossRef]
- Sun, Q.; Hu, H.; Zhang, Y.; Li, Y.; Chen, L.; Chen, H.; Yuan, W. Do intramedullary spinal cord changes in signal intensity on MRI affect surgical opportunity and approach for cervical myelopathy due to ossification of the posterior longitudinal ligament? Eur. Spine J. 2011, 20, 1466–1473. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.M.; Yoo, W.K.; Yoo, J.H.; Kwak, Y.H.; Oh, J.K.; Song, J.S.; Kim, S.W. The functional relevance of diffusion tensor imaging in comparison to conventional MRI in patients with cervical compressive myelopathy. Skelet. Radiol. 2017, 46, 1477–1486. [Google Scholar] [CrossRef]
- Mastronardi, L.; Elsawaf, A.; Roperto, R.; Bozzao, A.; Caroli, M.; Ferrante, M.; Ferrante, L. Prognostic relevance of the postoperative evolution of intramedullary spinal cord changes in signal intensity on magnetic resonance imaging after anterior decompression for cervical spondylotic myelopathy. J. Neurosurg. Spine 2007, 7, 615–622. [Google Scholar] [CrossRef]
- Nouri, A.; Tetreault, L.; Côté, P.; Zamorano, J.J.; Dalzell, K.; Fehlings, M.G. Does Magnetic Resonance Imaging Improve the Predictive Performance of a Validated Clinical Prediction Rule Developed to Evaluate Surgical Outcome in Patients with Degenerative Cervical Myelopathy? Spine 2015, 40, 1092–1100. [Google Scholar] [CrossRef]
- Yagi, M.; Ninomiya, K.; Kihara, M.; Horiuchi, Y. Long-term surgical outcome and risk factors in patients with cervical myelopathy and a change in signal intensity of intramedullary spinal cord on Magnetic Resonance imaging. J. Neurosurg. Spine 2010, 12, 59–65. [Google Scholar] [CrossRef]
- Yukawa, Y.; Kato, F.; Yoshihara, H.; Yanase, M.; Ito, K. MR T2 image classification in cervical compression myelopathy: Predictor of surgical outcomes. Spine 2007, 32, 1675–1678. [Google Scholar] [CrossRef] [PubMed]
- Chatley, A.; Kumar, R.; Jain, V.K.; Behari, S.; Sahu, R.N. Effect of spinal cord signal intensity changes on clinical outcome after surgery for cervical spondylotic myelopathy: Clinical article. J. Neurosurg. Spine 2009, 11, 562–567. [Google Scholar] [CrossRef]
- Zhang, C.; Das, S.K.; Yang, D.J.; Yang, H.F. Application of magnetic resonance imaging in cervical spondylotic myelopathy. World J. Radiol. 2014, 6, 826–832. [Google Scholar] [CrossRef]
- Kato, F.; Yukawa, Y.; Suda, K.; Yamagata, M.; Ueta, T. Normal morphology, age-related changes and abnormal findings of the cervical spine. Part II: Magnetic resonance imaging of over 1200 asymptomatic subjects. Eur. Spine J. 2012, 21, 1499–1507. [Google Scholar] [CrossRef]
- Martin, A.R.; De Leener, B.; Cohen-Adad, J.; Cadotte, D.W.; Nouri, A.; Wilson, J.R.; Tetreault, L.; Crawley, A.P.; Mikulis, D.J.; Ginsberg, H.; et al. Can microstructural MRI detect subclinical tissue injury in subjects with asymptomatic cervical spinal cord compression? A prospective cohort study. BMJ Open 2018, 8, e019809. [Google Scholar] [CrossRef]
- Smith, S.S.; Stewart, M.E.; Davies, B.M.; Kotter, M.R.N. The Prevalence of Asymptomatic and Symptomatic Spinal Cord Compression on Magnetic Resonance Imaging: A Systematic Review and Meta-analysis. Glob. Spine J. 2021, 11, 597–607. [Google Scholar] [CrossRef]
- Chen, C.J.; Lyu, R.K.; Lee, S.T.; Wong, Y.C.; Wang, L.J. Intramedullary high signal intensity on T2-weighted MR images in cervical spondylotic myelopathy: Prediction of prognosis with type of intensity. Radiology 2001, 221, 789–794. [Google Scholar] [CrossRef]
- Nakashima, H.; Yukawa, Y.; Ito, K.; Machino, M.; Kanbara, S.; Morita, D.; Takahashi, H.; Imagama, S.; Ito, Z.; Ishiguro, N.; et al. Prediction of lower limb functional recovery after laminoplasty for cervical myelopathy: Focusing on the 10-s step test. Eur. Spine J. 2012, 21, 1389–1395. [Google Scholar] [CrossRef]
- Nouri, A.; Tetreault, L.; Zamorano, J.J.; Dalzell, K.; Davis, A.M.; Mikulis, D.; Yee, A.; Fehlings, M.G. Role of magnetic resonance imaging in predicting surgical outcome in patients with cervical spondylotic myelopathy. Spine 2015, 40, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.J.; Jin, B.H.; Kim, K.S.; Cho, Y.E.; Cho, W.H. Intramedullary high signal intensity and neurological status as prognostic factors in cervical spondylotic myelopathy. Acta Neurochir. 2010, 152, 1687–1694. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Crockard, H.A.; Platts, A.; Stevens, J. Clinical and radiological correlates of severity and surgery-related outcome in cervical spondylosis. J. Neurosurg. 2001, 94, 189–198. [Google Scholar] [CrossRef]
- Suri, A.; Chabbra, R.P.; Mehta, V.S.; Gaikwad, S.; Pandey, R.M. Effect of intramedullary signal changes on the surgical outcome of patients with cervical spondylotic myelopathy. Spine J. 2003, 3, 33–45. [Google Scholar] [CrossRef]
- Uchida, K.; Nakajima, H.; Sato, R.; Kokubo, Y.; Yayama, T.; Kobayashi, S.; Baba, H. Multivariate analysis of the neurological outcome of surgery for cervical compressive myelopathy. J. Orthop. Sci. 2005, 10, 564–573. [Google Scholar] [CrossRef]
- Vedantam, A.; Jonathan, A.; Rajshekhar, V. Association of magnetic resonance imaging signal changes and outcome prediction after surgery for cervical spondylotic myelopathy. J. Neurosurg. Spine 2011, 15, 660–666. [Google Scholar] [CrossRef]
- Wada, E.; Yonenobu, K.; Suzuki, S.; Kanazawa, A.; Ochi, T. Can intramedullary signal change on magnetic resonance imaging predict surgical outcome in cervical spondylotic myelopathy? Spine 1999, 24, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.M.; Salem, K.M.; Burget, F.; Bommireddy, R.; Klezl, Z. Cervical spondylotic myelopathy: The prediction of outcome following surgical intervention in 93 patients using T1- and T2-weighted MRI scans. Eur. Spine J. 2015, 24, 2930–2935. [Google Scholar] [CrossRef]
- Martin, A.R.; De Leener, B.; Cohen-Adad, J.; Cadotte, D.W.; Kalsi-Ryan, S.; Lange, S.F.; Tetreault, L.; Nouri, A.; Crawley, A.; Mikulis, D.J.; et al. A Novel MRI Biomarker of Spinal Cord White Matter Injury: T2*-Weighted White Matter to Gray Matter Signal Intensity Ratio. AJNR Am. J. Neuroradiol. 2017, 38, 1266–1273. [Google Scholar] [CrossRef]
- Okada, Y.; Ikata, T.; Yamada, H.; Sakamoto, R.; Katoh, S. Magnetic resonance imaging study on the results of surgery for cervical compression myelopathy. Spine 1993, 18, 2024–2029. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Lafage, V.; Ryan, D.J.; Shaffrey, C.I.; Schwab, F.J.; Patel, A.A.; Brodke, D.S.; Arnold, P.M.; Riew, K.D.; Traynelis, V.C.; et al. Association of myelopathy scores with cervical sagittal balance and normalized spinal cord volume: Analysis of 56 preoperative cases from the AOSpine North America Myelopathy study. Spine 2013, 38, S161–S170. [Google Scholar] [CrossRef] [PubMed]
- Tetreault, L.A.; Dettori, J.R.; Wilson, J.R.; Singh, A.; Nouri, A.; Fehlings, M.G.; Brodt, E.D.; Jacobs, W.B. Systematic review of magnetic resonance imaging characteristics that affect treatment decision making and predict clinical outcome in patients with cervical spondylotic myelopathy. Spine 2013, 38, S89–S110. [Google Scholar] [CrossRef]
- Martin, A.R.; Tetreault, L.; Nouri, A.; Curt, A.; Freund, P.; Rahimi-Movaghar, V.; Wilson, J.R.; Fehlings, M.G.; Kwon, B.K.; Harrop, J.S.; et al. Imaging and Electrophysiology for Degenerative Cervical Myelopathy [AO Spine RECODE-DCM Research Priority Number 9]. Glob. Spine J. 2022, 12, 130S–146S. [Google Scholar] [CrossRef]
- Zhang, L.; Zeitoun, D.; Rangel, A.; Lazennec, J.Y.; Catonné, Y.; Pascal-Moussellard, H. Preoperative evaluation of the cervical spondylotic myelopathy with flexion-extension magnetic resonance imaging: About a prospective study of fifty patients. Spine 2011, 36, E1134–E1139. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Chen, W.J.; Yang, B.; Zhao, H.P.; Huang, J.W.; Cai, M.J.; Dong, T.F.; Li, T.S. Diffusion tensor imaging in the cervical spinal cord. Eur. Spine J. 2011, 20, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Pierpaoli, C.; Jezzard, P.; Basser, P.J.; Barnett, A.; Di Chiro, G. Diffusion tensor MR imaging of the human brain. Radiology 1996, 201, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, C.; Allen, P.S. Determinants of anisotropic water diffusion in nerves. Magn. Reson. Med. 1994, 31, 394–400. [Google Scholar] [CrossRef]
- Martin, A.R.; Aleksanderek, I.; Cohen-Adad, J.; Tarmohamed, Z.; Tetreault, L.; Smith, N.; Cadotte, D.W.; Crawley, A.; Ginsberg, H.; Mikulis, D.J.; et al. Translating state-of-the-art spinal cord MRI techniques to clinical use: A systematic review of clinical studies utilizing DTI, MT, MWF, MRS, and fMRI. Neuroimage Clin. 2016, 10, 192–238. [Google Scholar] [CrossRef]
- Fehlings, M.G. Degenerative Cervical Myelopathy: From Basic Science to Clinical Practice; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Nanda, G.; Jain, P.; Suman, A.; Mahajan, H. Role of diffusion tensor imaging and tractography in spinal cord injury. J. Clin. Orthop. Trauma 2022, 33, 101997. [Google Scholar] [CrossRef]
- Basser, P.J.; Mattiello, J.; LeBihan, D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J. Magn. Reson. B 1994, 103, 247–254. [Google Scholar] [CrossRef]
- Mohammadi, M.; Roohollahi, F.; Farahbakhsh, F.; Mohammadi, A.; Mortazavi Mamaghani, E.; Kankam, S.B.; Moarrefdezfouli, A.; Ghamari Khameneh, A.; Mahmoudi, M.M.; Baghdasaryan, D.; et al. Diffusion Tensor Imaging in Diagnosing and Evaluating Degenerative Cervical Myelopathy: A Systematic Review and Meta-Analysis. Glob. Spine J. 2025, 15, 267–283. [Google Scholar] [CrossRef]
- Ellingson, B.M.; Salamon, N.; Grinstead, J.W.; Holly, L.T. Diffusion tensor imaging predicts functional impairment in mild-to-moderate cervical spondylotic myelopathy. Spine J. 2014, 14, 2589–2597. [Google Scholar] [CrossRef]
- Gao, S.J.; Yuan, X.; Jiang, X.Y.; Liu, X.X.; Liu, X.P.; Wang, Y.F.; Cao, J.B.; Bai, L.N.; Xu, K. Correlation study of 3T-MR-DTI measurements and clinical symptoms of cervical spondylotic myelopathy. Eur. J. Radiol. 2013, 82, 1940–1945. [Google Scholar] [CrossRef]
- Jones, J.G.; Cen, S.Y.; Lebel, R.M.; Hsieh, P.C.; Law, M. Diffusion tensor imaging correlates with the clinical assessment of disease severity in cervical spondylotic myelopathy and predicts outcome following surgery. AJNR Am. J. Neuroradiol. 2013, 34, 471–478. [Google Scholar] [CrossRef]
- Maki, S.; Koda, M.; Ota, M.; Oikawa, Y.; Kamiya, K.; Inada, T.; Furuya, T.; Takahashi, K.; Masuda, Y.; Matsumoto, K. Reduced field-of-view diffusion tensor imaging of the spinal cord shows motor dysfunction of the lower extremities in patients with cervical compression myelopathy. Spine 2018, 43, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Aleksanderek, I.; Cohen-Adad, J.; Cadotte, D.; Kalsi-Ryan, S.; Nugaeva, N. Next-generation MRI of the human spinal cord: A prospective longitudinal study in cervical spondylotic myelopathy (CSM) to develop quantitative imaging biomarkers. In Proceedings of the Congress of Neurological Surgeons Annual Meeting, New Orleans, LA, USA, 26–30 September 2015. [Google Scholar]
- Wen, C.Y.; Cui, J.L.; Liu, H.S.; Mak, K.C.; Cheung, W.Y.; Luk, K.D.; Hu, Y. Is diffusion anisotropy a biomarker for disease severity and surgical prognosis of cervical spondylotic myelopathy? Radiology 2014, 270, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Chen, Z.; Zhang, F.; Song, Q.; Hou, C.; Tang, Y.; Wang, J.; Chen, S.; Bian, Y.; Hao, Q.; et al. Evaluation of DTI Parameter Ratios and Diffusion Tensor Tractography Grading in the Diagnosis and Prognosis Prediction of Cervical Spondylotic Myelopathy. Spine 2017, 42, E202–E210. [Google Scholar] [CrossRef]
- Demir, A.; Ries, M.; Moonen, C.T.; Vital, J.M.; Dehais, J.; Arne, P.; Caillé, J.M.; Dousset, V. Diffusion-weighted MR imaging with apparent diffusion coefficient and apparent diffusion tensor maps in cervical spondylotic myelopathy. Radiology 2003, 229, 37–43. [Google Scholar] [CrossRef]
- Mamata, H.; Jolesz, F.A.; Maier, S.E. Apparent diffusion coefficient and fractional anisotropy in spinal cord: Age and cervical spondylosis-related changes. J. Magn. Reson. Imaging 2005, 22, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Uda, T.; Takami, T.; Tsuyuguchi, N.; Sakamoto, S.; Yamagata, T.; Ikeda, H.; Nagata, T.; Ohata, K. Assessment of cervical spondylotic myelopathy using diffusion tensor magnetic resonance imaging parameter at 3.0 tesla. Spine 2013, 38, 407–414. [Google Scholar] [CrossRef]
- Facon, D.; Ozanne, A.; Fillard, P.; Lepeintre, J.F.; Tournoux-Facon, C.; Ducreux, D. MR diffusion tensor imaging and fiber tracking in spinal cord compression. AJNR Am. J. Neuroradiol. 2005, 26, 1587–1594. [Google Scholar]
- Lee, S.; Lee, Y.H.; Chung, T.S.; Jeong, E.K.; Kim, S.; Yoo, Y.H.; Kim, I.S.; Yoon, C.S.; Suh, J.S.; Park, J.H. Accuracy of Diffusion Tensor Imaging for Diagnosing Cervical Spondylotic Myelopathy in Patients Showing Spinal Cord Compression. Korean J. Radiol. 2015, 16, 1303–1312. [Google Scholar] [CrossRef]
- Mostafa, N.S.A.-A.; Hasanin, O.A.M.; Al Yamani Moqbel, E.A.H.; Nagy, H.A. Diagnostic value of magnetic resonance diffusion tensor imaging in evaluation of cervical spondylotic myelopathy. Egypt. J. Radiol. Nucl. Med. 2023, 54, 175. [Google Scholar] [CrossRef]
- Ragaee, S.M.; Gawad, E.A.A.; Gamal, S.; Nageeb, M.M.; Ibrahim, A.S. Leverage of applying diffusion tensor imaging (DTI) indices in assessment of cervical spondylotic myelopathy. Egypt. J. Radiol. Nucl. Med. 2024, 55, 73. [Google Scholar] [CrossRef]
- Wu, W.; Yang, Z.; Zhang, T.; Ru, N.; Zhang, F.; Wu, B.; Liang, J. Microstructural Changes in Compressed Cervical Spinal Cord Are Consistent with Clinical Symptoms and Symptom Duration. Spine 2020, 45, E999–E1005. [Google Scholar] [CrossRef]
- Kerkovský, M.; Bednarík, J.; Dušek, L.; Sprláková-Puková, A.; Urbánek, I.; Mechl, M.; Válek, V.; Kadanka, Z. Magnetic resonance diffusion tensor imaging in patients with cervical spondylotic spinal cord compression: Correlations between clinical and electrophysiological findings. Spine 2012, 37, 48–56. [Google Scholar] [CrossRef]
- Grabher, P.; Mohammadi, S.; Trachsler, A.; Friedl, S.; David, G.; Sutter, R.; Weiskopf, N.; Thompson, A.J.; Curt, A.; Freund, P. Voxel-based analysis of grey and white matter degeneration in cervical spondylotic myelopathy. Sci. Rep. 2016, 6, 24636. [Google Scholar] [CrossRef]
- Valošek, J.; Labounek, R.; Horák, T.; Horáková, M.; Bednařík, P.; Keřkovský, M.; Kočica, J.; Rohan, T.; Lenglet, C.; Cohen-Adad, J.; et al. Diffusion magnetic resonance imaging reveals tract-specific microstructural correlates of electrophysiological impairments in non-myelopathic and myelopathic spinal cord compression. Eur. J. Neurol. 2021, 28, 3784–3797. [Google Scholar] [CrossRef]
- Ellingson, B.M.; Salamon, N.; Woodworth, D.C.; Yokota, H.; Holly, L.T. Reproducibility, temporal stability, and functional correlation of diffusion MR measurements within the spinal cord in patients with asymptomatic cervical stenosis or cervical myelopathy. J. Neurosurg. Spine 2018, 28, 472–480. [Google Scholar] [CrossRef]
- Martin, A.R.; De Leener, B.; Cohen-Adad, J.; Kalsi-Ryan, S.; Cadotte, D.W.; Wilson, J.R.; Tetreault, L.; Nouri, A.; Crawley, A.; Mikulis, D.J.; et al. Monitoring for myelopathic progression with multiparametric quantitative MRI. PLoS ONE 2018, 13, e0195733. [Google Scholar] [CrossRef]
- Chen, X.; Kong, C.; Feng, S.; Guan, H.; Yu, Z.; Cui, L.; Wang, Y. Magnetic resonance diffusion tensor imaging of cervical spinal cord and lumbosacral enlargement in patients with cervical spondylotic myelopathy. J. Magn. Reson. Imaging 2016, 43, 1484–1491. [Google Scholar] [CrossRef] [PubMed]
- Budzik, J.F.; Balbi, V.; Le Thuc, V.; Duhamel, A.; Assaker, R.; Cotten, A. Diffusion tensor imaging and fibre tracking in cervical spondylotic myelopathy. Eur. Radiol. 2011, 21, 426–433. [Google Scholar] [CrossRef]
- Rajasekaran, S.; Yerramshetty, J.S.; Chittode, V.S.; Kanna, R.M.; Balamurali, G.; Shetty, A.P. The assessment of neuronal status in normal and cervical spondylotic myelopathy using diffusion tensor imaging. Spine 2014, 39, 1183–1189. [Google Scholar] [CrossRef]
- Cui, J.L.; Li, X.; Chan, T.Y.; Mak, K.C.; Luk, K.D.; Hu, Y. Quantitative assessment of column-specific degeneration in cervical spondylotic myelopathy based on diffusion tensor tractography. Eur. Spine J. 2015, 24, 41–47. [Google Scholar] [CrossRef]
- David, G.; Vallotton, K.; Hupp, M.; Curt, A.; Freund, P.; Seif, M. Extent of Cord Pathology in the Lumbosacral Enlargement in Non-Traumatic versus Traumatic Spinal Cord Injury. J. Neurotrauma 2022, 39, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Seif, M.; David, G.; Huber, E.; Vallotton, K.; Curt, A.; Freund, P. Cervical Cord Neurodegeneration in Traumatic and Non-Traumatic Spinal Cord Injury. J. Neurotrauma 2020, 37, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Roohollahi, F.; Mahmoudi, M.M.; Mohammadi, A.; Mohamadi, M.; Kankam, S.B.; Ghamari Khameneh, A.; Baghdasaryan, D.; Farahbakhsh, F.; Martin, A.R.; et al. Correlation Between Pre-Operative Diffusion Tensor Imaging Indices and Post-Operative Outcome in Degenerative Cervical Myelopathy: A Systematic Review and Meta-Analysis. Glob. Spine J. 2024, 14, 1800–1817. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.; Binder, A.; Wasner, G. Neuropathic pain: Diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010, 9, 807–819. [Google Scholar] [CrossRef]
- Qiu, Z.; Liu, T.; Zeng, C.; Yang, M.; Xu, X. Local abnormal white matter microstructure in the spinothalamic tract in people with chronic neck and shoulder pain. Front. Neurosci. 2025, 18, 1485045. [Google Scholar] [CrossRef]
- Qi, X.; He, Y.; Wang, Q.; Ren, S.; Yao, H.; Cao, W.; Guan, L. Diffusion tensor and kurtosis imaging reveal microstructural changes in the trigeminal nerves of patients with trigeminal neuralgia. Eur. Radiol. 2023, 33, 8046–8054. [Google Scholar] [CrossRef]
- Zhang, Y.; Vakhtin, A.A.; Jennings, J.S.; Massaband, P.; Wintermark, M.; Craig, P.L.; Ashford, J.W.; Clark, J.D.; Furst, A.J. Diffusion tensor tractography of brainstem fibers and its application in pain. PLoS ONE 2020, 15, e0213952. [Google Scholar] [CrossRef]
- Wang, C.; Kutch, J.J.; Labus, J.S.; Yang, C.C.; Harris, R.E.; Mayer, E.A.; Ellingson, B.M. Reproducible Microstructural Changes in the Brain Associated with the Presence and Severity of Urologic Chronic Pelvic Pain Syndrome (UCPPS): A 3-Year Longitudinal Diffusion Tensor Imaging Study From the MAPP Network. J. Pain 2023, 24, 627–642. [Google Scholar] [CrossRef]
- Yacubian Fernandes, A.; Fernandes da Silva, F.E.; Hamamoto Filho, P.T.; Talamoni Fonoff, E. MR diffusion tensor imaging applied to the spinal cord of patients with neuropathic pain secondary to herpes zoster infection. J. Clin. Neurosci. 2024, 130, 110912. [Google Scholar] [CrossRef]
- Yoo, W.K.; Kim, T.H.; Hai, D.M.; Sundaram, S.; Yang, Y.M.; Park, M.S.; Kim, Y.C.; Kwak, Y.H.; Ohn, S.H.; Kim, S.W. Correlation of magnetic resonance diffusion tensor imaging and clinical findings of cervical myelopathy. Spine J. 2013, 13, 867–876. [Google Scholar] [CrossRef]
- Vallotton, K.; David, G.; Hupp, M.; Pfender, N.; Cohen-Adad, J.; Fehlings, M.G.; Samson, R.S.; Wheeler-Kingshott, C.; Curt, A.; Freund, P.; et al. Tracking White and Gray Matter Degeneration along the Spinal Cord Axis in Degenerative Cervical Myelopathy. J. Neurotrauma 2021, 38, 2978–2987. [Google Scholar] [CrossRef]
- Nukala, M.; Abraham, J.; Khandige, G.; Shetty, B.K.; Rao, A.P.A. Efficacy of diffusion tensor imaging in identification of degenerative cervical spondylotic myelopathy. Eur. J. Radiol. Open 2019, 6, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Liu, T.; Zeng, C.; Yang, M.; Yang, H.; Xu, X. Exploratory study on the ascending pain pathway in patients with chronic neck and shoulder pain based on combined brain and spinal cord diffusion tensor imaging. Front. Neurosci. 2025, 19, 1460881. [Google Scholar] [CrossRef] [PubMed]
- Vitzthum, H.E.; Dalitz, K. Analysis of five specific scores for cervical spondylogenic myelopathy. Eur. Spine J. 2007, 16, 2096–2103. [Google Scholar] [CrossRef] [PubMed]
- d’Avanzo, S.; Ciavarro, M.; Pavone, L.; Pasqua, G.; Ricciardi, F.; Bartolo, M.; Solari, D.; Somma, T.; de Divitiis, O.; Cappabianca, P.; et al. The Functional Relevance of Diffusion Tensor Imaging in Patients with Degenerative Cervical Myelopathy. J. Clin. Med. 2020, 9, 1828. [Google Scholar] [CrossRef] [PubMed]
- Stroman, P.W.; Wheeler-Kingshott, C.; Bacon, M.; Schwab, J.M.; Bosma, R.; Brooks, J.; Cadotte, D.; Carlstedt, T.; Ciccarelli, O.; Cohen-Adad, J. The current state-of-the-art of spinal cord imaging: Methods. Neuroimage 2014, 84, 1070–1081. [Google Scholar] [CrossRef]
- Huang, S.; Shao, H.; Liu, Q.; Liu, W.V.; Zhang, Q.; Deng, L.; Liu, C.; Omar, D.M.; Tang, X. Quantitative Assessment of Spinal Cord Injury in Cervical Spondylotic Myelopathy: A Comparison Study of MAGiC and MUSE-DTI. Eur. J. Radiol. 2025, 190, 112214. [Google Scholar] [CrossRef]
- Alomar, S.; Bakhaidar, M. Neuroimaging of neuropathic pain: Review of current status and future directions. Neurosurg. Rev. 2018, 41, 771–777. [Google Scholar] [CrossRef]
- Zhao, R.; Su, Q.; Chen, Z.; Sun, H.; Liang, M.; Xue, Y. Neural Correlates of Cognitive Dysfunctions in Cervical Spondylotic Myelopathy Patients: A Resting-State fMRI Study. Front. Neurol. 2020, 11, 596795. [Google Scholar] [CrossRef] [PubMed]
- Figley, C.R.; Uddin, M.N.; Wong, K.; Kornelsen, J.; Puig, J.; Figley, T.D. Potential Pitfalls of Using Fractional Anisotropy, Axial Diffusivity, and Radial Diffusivity as Biomarkers of Cerebral White Matter Microstructure. Front. Neurosci. 2021, 15, 799576. [Google Scholar] [CrossRef]
- Willis, W.D.; Westlund, K.N. Neuroanatomy of the pain system and of the pathways that modulate pain. J. Clin. Neurophysiol. 1997, 14, 2–31. [Google Scholar] [CrossRef]
- Jones, D.K.; Knösche, T.R.; Turner, R. White matter integrity, fiber count, and other fallacies: The do’s and don’ts of diffusion MRI. Neuroimage 2013, 73, 239–254. [Google Scholar] [CrossRef]
- Wheeler-Kingshott, C.A.; Cercignani, M. About “axial” and “radial” diffusivities. Magn. Reson. Med. 2009, 61, 1255–1260. [Google Scholar] [CrossRef]
- Drake-Pérez, M.; Boto, J.; Fitsiori, A.; Lovblad, K.; Vargas, M.I. Clinical applications of diffusion weighted imaging in neuroradiology. Insights Imaging 2018, 9, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Faiyaz, A.; Doyley, M.M.; Schifitto, G.; Uddin, M.N. Artificial intelligence for diffusion MRI-based tissue microstructure estimation in the human brain: An overview. Front. Neurol. 2023, 14, 1168833. [Google Scholar] [CrossRef]
- Yang, S.; Li, J.; Fei, N.; Li, G.; Hu, Y. A Deep-Learning-Based Diffusion Tensor Imaging Pathological Auto-Analysis Method for Cervical Spondylotic Myelopathy. Bioengineering 2025, 12, 806. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.T.; Zeevi, T.; Payabvash, S. Strategies to Improve the Robustness and Generalizability of Deep Learning Segmentation and Classification in Neuroimaging. BioMedInformatics 2025, 5, 20. [Google Scholar] [CrossRef]
- Sima, S.; Sial, A.; Sharma, S.; Ananthakrishnan, D.; Kuan, J.; Diwan, A. DeVa (Decay Variance): A Novel Score Calculated via Postprocessing the Changes in Signal Intensity of an Intervertebral Disc in a T2* Multi-Echo Magnetic Resonance Image Can Quantify Painful and Degenerate Lumbar Vertebral Discs. JOR Spine 2025, 8, e70056. [Google Scholar] [CrossRef]
| Author (Year) | Sample Size (DCM/Controls) | MRI Field Strength | Levels Assessment Strategy | Parameters | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| Demir (2003) [68] | 12/19 | 1.5T | MC | FA, ADC | FA: 72 ADC: — | FA: 50 ADC: — |
| Mamata (2005) [69] | 40 (mixed age-related and CSM) | 1.5T | MC | FA, ADC | FA: ~80 ADC: — | FA: ~60 ADC: — |
| Facon (2005) [71] | 11/15 | — | MC | FA, ADC | FA: 73.3 ADC: 13.4 | FA: 100 ADC: 80 |
| Keřkovský (2012) [76] | 13/52 | — | MC | FA, ADC | FA: 65 ADC: 70 | FA: 71.9 ADC: 75 |
| Uda (2013) [70] | 30/26 | 3.0T | MC | MD, FA | MD: 100 FA: — | MD: 75 FA: 76 |
| Ellingson (2014) [61] | 9/48 | 3.0T | MC | FA, RD | FA: 72 RD: — | FA: 75 RD: 89.4 |
| Lee (2015) [72] | 50/14 | 3.0T | MC | FA, MD, AD, RD | FA: 100 MD: 100 | FA: 27.6 MD: 44.8 |
| Wu (2020) [75] | 29/29 | — | Mean of several levels | FA, ADC | FA: 75.9 ADC: 96.6 | FA: 89.7 ADC: 72.4 |
| Mostafa (2023) [73] | 30/60 | — | Mean of several levels | FA, ADC | FA: 97 ADC: 88.1 | FA: 92.7 ADC: 98 |
| Ragaee (2024) [74] | 30 (DCM cohort only) | — | — | FA, ADC, AD, RD | FA: 83.9 ADC: 76.8 AD/RD: — | — |
| DTI Metric | Pathophysiological Interpretation | Key Clinical Correlate | Pain Outcome Used | Evidence Source/Level | Main Limitation |
|---|---|---|---|---|---|
| Fractional Anisotropy (FA) | Integrity and degree of coherence of white matter tracts | Reduced FA in dorsal columns linked to sensory pathway disruption and central sensitisation | VAS, NDI, SF-36 pain subscale | Mixed evidence—DCM-specific [61,66] and cross-condition extrapolation from neuropathic pain [92] | Non-directional; limited tract specificity due to crossing fibres; like other diffusion metrics, FA is sensitive but not specific to the underlying pathology (e.g., demyelination vs. axonal loss). |
| Mean Diffusivity (MD) | Overall water mobility; ↑ MD suggests oedema or chronic inflammation | Higher MD associated with poorer function and neuropathic pain features | VAS, DN4 | DCM-specific | Susceptible to ageing and comorbidity effects |
| Axial Diffusivity (AD) | Axonal integrity | Lower AD associated with dorsal column/spinothalamic tract injury | VAS, NDI | DCM-specific and experimental confirmation in animal models | Rarely stratified by pain phenotype |
| Radial Diffusivity (RD) | Myelin integrity; ↑ RD indicates demyelination | Elevated RD implicated in neuropathic pain mechanisms | VAS, DN4 | Cross-condition extrapolation (post-herpetic neuralgia, chronic pain models) | Limited pain-specific DCM research |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, S.; Sial, A.; Bright, G.E.; O’Hare Doig, R.; Diwan, A.D. Diffusion Tensor Imaging in Degenerative Cervical Myelopathy: Clinical Translation Opportunities for Cause of Pain Detection and Potentially Early Diagnoses. Appl. Sci. 2025, 15, 11607. https://doi.org/10.3390/app152111607
Sharma S, Sial A, Bright GE, O’Hare Doig R, Diwan AD. Diffusion Tensor Imaging in Degenerative Cervical Myelopathy: Clinical Translation Opportunities for Cause of Pain Detection and Potentially Early Diagnoses. Applied Sciences. 2025; 15(21):11607. https://doi.org/10.3390/app152111607
Chicago/Turabian StyleSharma, Suhani, Alisha Sial, Georgia E. Bright, Ryan O’Hare Doig, and Ashish D. Diwan. 2025. "Diffusion Tensor Imaging in Degenerative Cervical Myelopathy: Clinical Translation Opportunities for Cause of Pain Detection and Potentially Early Diagnoses" Applied Sciences 15, no. 21: 11607. https://doi.org/10.3390/app152111607
APA StyleSharma, S., Sial, A., Bright, G. E., O’Hare Doig, R., & Diwan, A. D. (2025). Diffusion Tensor Imaging in Degenerative Cervical Myelopathy: Clinical Translation Opportunities for Cause of Pain Detection and Potentially Early Diagnoses. Applied Sciences, 15(21), 11607. https://doi.org/10.3390/app152111607








