Abstract
Idiopathic normal-pressure hydrocephalus (iNPH) is clinically characterized by the Hakim–Adams triad: gait disturbance, cognitive decline, and urinary incontinence. Cognitive impairment in iNPH predominates as a subcortical syndrome, with deficits in executive functions, psychomotor slowing, and memory inefficiency. However, the cognitive profile is heterogeneous and often overlaps with other neurodegenerative conditions, complicating differential diagnosis. This study investigated the cognitive features of iNPH using the Rey Auditory Verbal Learning Test (RAVLT), comparing 29 iNPH patients to 28 healthy controls. Demographic and neuroimaging parameters—such as Evans Index and Callosal Angle—were assessed. Results indicated significantly lower Montreal Cognitive Assessment (MoCA) and RAVLT scores in iNPH patients compared to controls. Analysis of serial position effects revealed that, whereas healthy individuals exhibited typical primacy and recency effects in verbal memory, iNPH patients demonstrated a selective impairment of the primacy effect, likely reflecting hippocampal dysfunction. These findings underline the importance of detailed neuropsychological evaluation in differentiating iNPH from other dementias and suggest that damage to medial temporal lobe structures plays a prominent role in the verbal memory deficit observed in iNPH.
1. Introduction
Normal-pressure hydrocephalus (NPH) represents a complex neuropsychiatric syndrome, with the fundamental distinction based on the identification of an underlying etiology. NPH is characterized by the Hakim–Adams [1] clinical triad comprising gait disturbances, cognitive impairment, and urinary incontinence, associated with ventriculomegaly in the presence of normal cerebrospinal fluid pressure. Idiopathic normal-pressure hydrocephalus (iNPH) represents the primary form of the condition, characterized by the absence of an identifiable underlying cause. According to the Japanese guidelines [2], the criteria for iNPH include the following: presence of at least one symptom of the clinical triad; absence of neurological diseases that could fully explain the symptoms; and exclusion of pre-existing pathologies that could cause ventricular dilatation. Idiopathic normal-pressure hydrocephalus (iNPH) is a progressive disorder observed typically in the elderly population, with a peak of prevalence in males over 65 years old. Epidemiological data are still unclear due to studies’ selection bias, mainly because of the difficulties faced during the diagnosis process. iNPH worldwide prevalence is estimated to be 1–3% of the general population [3,4], which rose to 11.6% according to a 2015 systematic review [4]. As its definition suggests, patients present common features at neuroimaging: ventriculomegaly [5]; disproportionately enlarged subarachnoid-space hydrocephalus (DESH) features; reduced Callosal Angle (CA); and increased Evans Index (EI). Although there is no clear evidence of increased intracranial pressure (ICP).
iNPH clinically presents with a triad of symptoms including gait disturbance, cognitive decline, and urinary incontinence, known as the Hakim–Adams triad [1]. Gait disturbance is usually the first clinical symptom manifesting with imbalance, difficulties in changing direction, and shuffling gait. Urinary disorders present initially as urinary urgency, leading to incontinence due to loss of bladder control. Cognitive impairment presents with executive functions deficits, difficulties focusing, attention and short-term memory deficits, sometimes even with behavioral disorders [6,7,8]. iNPH cognitive dysfunction is associated with abnormal cerebrospinal fluid dynamics, neuromodulation, frontostriatal circuits, entorhinal–hippocampal dysfunction, presence of β-amyloid, and tau-pathology, even if some mechanisms are still unclear [9]. There is also a temporary impairment of the Default Mode Network (DMN) associated with executive function and poor verbal memory. Moreover, the gray matter volumes of the putamen, globus pallidus, nucleus accumbens, and caudate nucleus in iNPH patients are greatly reduced compared to healthy individuals [10].
Cognitive deficits in iNPH are frequent but present a heterogeneous profile, often of a “subcortical” type, with impairment of executive functions, psychomotor slowing, and memory inefficiency. Differential diagnosis is complex due to symptomatic overlap with other neurodegenerative diseases, such as Alzheimer’s disease (AD), vascular dementia (VaD), dementia with Lewy bodies (LBD), progressive supranuclear palsy (PSP), and corticobasal degeneration (CBD) [11]. For example, compared to individuals with AD, patients with iNPH tend to experience less impairment in orientation and memory but exhibit more significant impairment in executive functioning and a greater slowing of cognitive processing speed [12]. Nevertheless, the specific impacted cognitive domains and the degree of dysfunction can vary widely among individuals with iNPH [13].
Given the symptomatic heterogeneity of iNPH, neuropsychological assessment should go beyond general or screening tests such as the Mini-Mental State Examination (MMSE) and should include comprehensive cognitive batteries that allow for the distinction between iNPH and other forms of dementia [11,14]. In detail, we focus our attention on the Rey Auditory Verbal Learning Test (RAVLT) that assesses verbal learning and short- and long-term memory through tasks involving list learning, recall, and recognition. Indeed, the RAVLT is often used to assess verbal memory and learning abilities in patients with cognitive impairment. Several studies have used this test in patients with iNPH to explore cognitive profiles and to evaluate the effectiveness of therapeutic interventions. The study by Kanno et al. (2021) [15] showed that DMN connectivity was positively correlated with RAVLT immediate recall scores. Patients with lower connectivity showed more severe memory deficits and less improvement after shunting. Peterson’s (2016) [16] meta-analysis, however, shows that after shunt surgery, significant improvements in verbal memory are observed, especially in total and delayed recall outcomes, but with some heterogeneity. The RAVLT appears to be among the most sensitive tests for detecting cognitive changes. It has also been demonstrated that the RAVLT can also have a predictive role in cognitive recovery after shunting in iNPH patients; in a prospective study by McGovern et al. (2019) [17] on 52 patients with pre- and post-operative neuropsychological assessment, it was demonstrated that the RAVLT was the only test to show statistically significant improvement after both lumbar drainage and shunting. Early improvement on the RAVLT predicts further post-operative progress. Other studies comparing different pathologies such as AD have shown that both iNPH and Alzheimer’s disease (AD) patients perform significantly worse than healthy controls on the RAVLT. In the learning trials, both groups show similar difficulties. In delayed recall, iNPH patients typically perform better than those with AD. This suggests that more pronounced impairments in delayed recall is characteristic of AD, while relatively preserved performance in these areas may be indicative of iNPH [18,19,20]. However, specific evidence on the utility of the RAVLT to distinguish Alzheimer’s from PSP or distinguish PSP from iNPH is limited. The aim of our observational study is to demonstrate that both the primacy effect and the recency effect of the verbal memory RAVLT are impaired in iNPH patients compared to healthy subjects. These findings highlight the necessity for precise and sensitive neuropsychological assessments, such as the RAVLT, to accurately characterize the cognitive profile of iNPH patients and to distinguish them from other dementia subtypes with overlapping symptoms. However, despite growing evidence supporting the use of the RAVLT in both clinical and research contexts, uncertainties remain regarding its capacity to detect specific patterns of verbal memory impairment—particularly those involving the primacy and recency effects—in iNPH compared to healthy individuals. Therefore, the aim of our observational study is to demonstrate that both the primacy effect and the recency effect of the verbal memory at RAVLT test are impaired in iNPH patients compared to healthy subjects.
2. Materials and Methods
2.1. Participants
A total of 57 people were enlisted for the study, out of which 28 were diagnosed with iNPH, and the remaining 29 subjects were healthy volunteers (MoCA correct total score > 18.29) [21]. The study received approval from the local ethics committee in accordance with the Declaration of Helsinki. Additionally, all participants provided their informed permission by signing the informed consent.
Table 1 presents a summary of the sociodemographic characteristics and provides the total raw and correct scores of the MoCA for each group.

Table 1.
Summary of sociodemographic features and MoCA scores of subsamples.
Diagnostic Criteria of iNPH
All patients underwent brain MRI using a 3-T GE MR750 scanner (General Electric, Milwaukee, WI, USA) with an 8-channel head coil, employing a protocol that included three sequences: a sagittal 3D T1-weighted volumetric spoiled gradient echo (TR/TE = 9.2/3.7 ms, slice thickness = 1.0 mm, matrix = 256 × 256, flip angle = 12°, FOV = 25.6 mm), an axial T2-weighted fast spin echo (TR/TE = 5462/85 ms, slice thickness = 4.0 mm, matrix = 512 × 256, FOV = 24 mm), and an axial T2-weighted fluid-attenuated inversion recovery sequence (TR/TE/TI = 9500/100/2250 ms, slice thickness = 4.0 mm, matrix = 512 × 256), with motion restraint achieved through cushions and adhesive medical tape to minimize artifacts.
The trained rater manually assessed the Evans Index (EI) and Callosal Angle (CA) in all study participants following established protocols; the EI was calculated on a T1-weighted bi-commissural axial slice as the ratio of the maximum left-to-right width of the frontal horns of the lateral ventricles to the maximum inner skull diameter, while the CA was measured on a coronal plane perpendicular to the anterior–posterior commissure line as the angle formed between the superior borders of the lateral ventricles at the level of the posterior commissure. Based on prior research, EI values greater than 0.32 and CA values less than 120° were classified as abnormal [22]. Descriptive statistics of EI and CA are shown in Table 2.

Table 2.
Descriptive statistics of EI and CA.
2.2. Neuropsychological Assessment
All participants underwent MoCA and RAVLT.
The Montreal Cognitive Assessment (MoCA) [23,24] is a brief cognitive screening tool designed to detect mild cognitive impairment, administered over approximately 15 min. It evaluates several cognitive domains through specific tasks. Visuospatial abilities are assessed using a three-dimensional cube copying task and a clock-drawing task, while executive functions are measured with the Trail Making B task. Language skills are evaluated through a three-item confrontation naming task with unfamiliar animals, repetition of two syntactically complex sentences, and a verbal fluency task. Short-term memory is tested with two learning trials of five nouns, followed by delayed recall after approximately five minutes. Attention, concentration, and working memory are assessed via a sustained attention task requiring target detection with tapping, alongside a serial subtraction exercise. Finally, orientation to time and place is evaluated. The main purpose of the test is to detect the presence of mild cognitive impairment in patients. For the MoCA test [24], normative data from Santangelo et al. (2014) [21] were used. Healthy participants with a correct total score <18.29 were excluded from the analysis (correct total score < 15.5 = cognitive decline; correct total score between 15.51, and 18.28 = mild cognitive impairment).
The Rey Auditory Verbal Learning Test (RAVLT; Version 1) [25,26,27,28] is a verbal memory assessment used to evaluate verbal learning and memory through both immediate and delayed recall measures. The test involves presenting a list of 15 words over five consecutive learning trials, during which the participant is asked to recall as many words as possible after each trial. After a 15 min delay following the final trial, delayed recall is assessed by asking the participant to recall the same list again. The score for RAVLT was determined by how many times each word was recalled in both immediate and delayed recall, and the serial sequence of recall in each word list was used to determine the presence of primacy and recency effects and to draw attention to the differences between the control and iNPH groups.
2.3. Statistical Analysis
All statistical analyses were performed in the R statistical environment [29].
To evaluate differences between the control group and iNPH patient, a series of T-tests were performed, evaluating differences in MoCA, RAVLT immediate recall total score, and RAVLT delayed recall score.
Initial descriptive statistics were conducted, specifically focusing on the percentage of accurately remembered words (PARW) during immediate and delayed recall.
Indeed, the PARW is dependent on the serial position of the item that must be recalled; to estimate this dependence, the specific PARW of the single word in each repetition was calculated. A graphical investigation of bimodal distribution of both groups was made to evidence the difference in recency and primacy effects.
In terms of inferential statistics, a Generalized Linear Model was used to calculate the probability of recalling a particular word in a specific serial position. The dependent variable in this model was dichotomous (1 word recalled, or 0), and the predictors included groups, serial position, repetition, and the interaction between the group and serial position. The repetition variable was included in the model as a random intercept.
3. Results
Regarding the differences in MoCA, iNPH patients displayed lower scores in both sum scores (raw total score, t = 5.47, p < 0.001; correct total score, t = 4.6, p < 0.001).
Also, the difference between iNPH and healthy subjects in RAVLT immediate recall total score and delayed recall total score highlighted lower scores in the iNPH group (immediate total score, t = 8.04, p < 0.001;delayed total score t = 8.84, p < 0.001; see Figure 1).
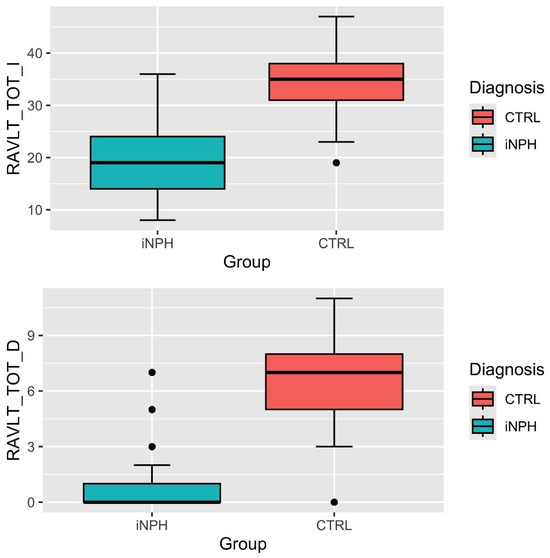
Figure 1.
RAVLT immediate and delayed total score distributions. Note: RAVLT_TOT_I = total score of RAVLT immediate recall; RAVLT_TOT_D = total score of RAVLT delayed recall; CTRL = control group; iNPH = idiopathic normal-pressure hydrocephalus patients.
Observing the PARW of the five immediate recalls, the iNPH group displayed recency effects that fall in delayed recall (see Figure 2).
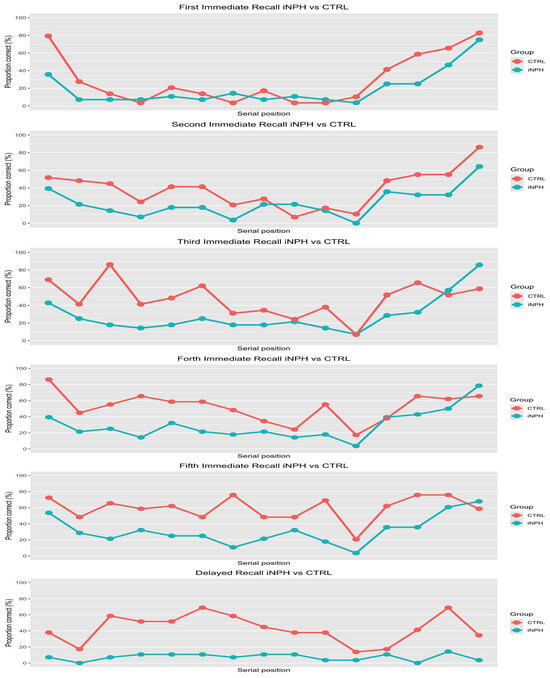
Figure 2.
Proportion of correct words recalled depending on serial position. Note: The red line is the path of PARW of the control group, while the blue line is the visualization of iNPH’s PARW. The points represent the single words; on the y-axis is displayed the PARW, while on x-axis is the serial position, from left to right.
Regarding the primacy effect, it seems absent. Indeed, the distribution of PARW is close to uni-modal distribution instead of bi-modal, regardless of learning stage. Lastly, the PARW indicates that the iNPH group in the delayed recall task exhibits a floor effect. This floor effect in the delayed recall and the PARW of the single words did not increase with repetition, indicating a likely lack of learning, in contrast to the CTRL group. The descriptive analysis, summarized in Figure 2, was supported by inferential statistics; the results suggested that all fixed effects were significant indeed. First, the iNPH patient group as a predictor show a negative coefficient (β = −1.27, p < 0.001); this result was confirmed by post hoc analysis over the level of “Serial position of the itemi”, suggesting that the control group had 3.22 times the odds of remembering a word than iNPH group (Figure 3).
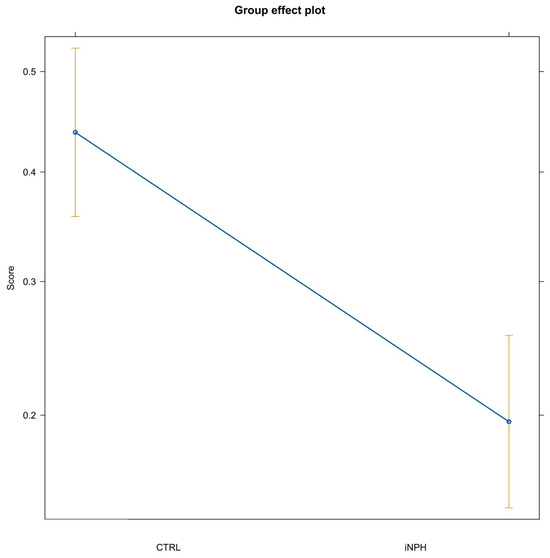
Figure 3.
Group effect plot. Note: CTRL = control group; iNPH = idiopathic normal-pressure hydrocephalus. Difference across groups of the expected probability of correct recall over the levels of serial position and repetition.
According to the primacy effect, the coefficient associated with the first item in terms of serial position was positive over the level of group (β = 0.69, p = 0.003), suggesting a major probability to recall it than the “central items” (items with central position in the list).
The same results were observed for the last two items in terms of serial position (Item 14 β = 0.56, p = 0.01; Item 15 β = 0.61, p = 0.008). The effect was confirmed by post hoc comparisons (contrast between the odds for recalling items); for example, in the control group, the odds ratio between Item 1 and Item 9 was 6.57 (p < 0.001).
However, the most interesting result regards the interaction between group and serial position. On one hand, the odds ratio between Item1 of the control group and iNPH were 3.59 (p < 0.001); on the other, the odds ratio between Item 15 of the control group and iNPH were 1.08 (p = 1). A summary of the interaction effect between serial position and group is shown in Figure 4.
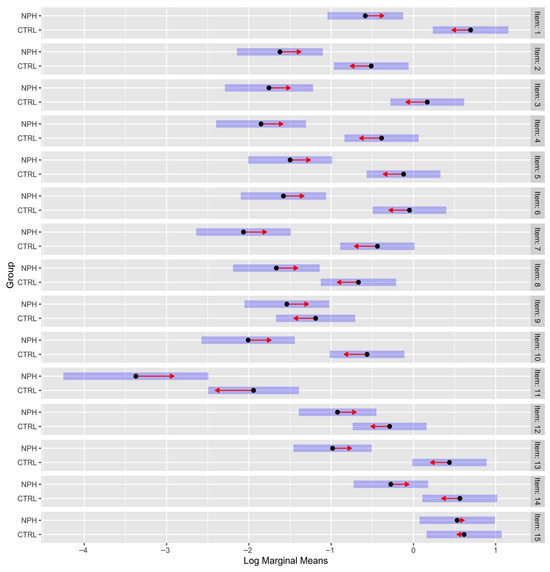
Figure 4.
Contrast between groups (control vs. iNPH). Note: Distance between red arrows is the difference between log marginal means. If the red arrows overlap, the odd ratio is ~1; namely, there is no difference between odds. The bars around the arrows are the confidence intervals. The order of the items is linked to serial position (from Item 1 in the first word in terms of serial position, to Item 15 in the last one). The log marginal means provide a fairer comparison across groups (control group and iNPH) and conditions (the serial position of the word that must be recalled) over the levels of repetition.
4. Discussion
In our observational study, we administered the MoCA to select the sample and ensure the cognitive integrity of the healthy control group [30]. The RAVLT was then applied to both healthy participants and patients to investigate differences in the primacy and recency effects in individuals with iNPH [31]. In our research, serial position analysis demonstrated a loss of the primacy effect in iNPH, with recall probability for initial list items markedly reduced, while the recency effect was relatively spared. A floor effect was observed in delayed recall for iNPH patients, indicating limited learning across repetitions. Statistical modeling revealed that controls had more than three times the odds of recalling words than iNPH patients, especially for early serial positions. The interaction analysis highlighted selective deficits in long-term memory processes in iNPH, reflecting hippocampal dysfunction.
According to the scientific literature, the primacy and recency effects are associated with specific anatomical regions. The inferior parietal lobule, angular gyrus, and parahippocampal and hippocampal gyri are particularly involved in the primacy effect, while both primacy and recency effects appear to correlate with parietal and frontal activation [32,33]. As previously discussed, patients with iNPH exhibit damage to the parahippocampal and hippocampal gyri [33,34], thus we expected that both the primacy and recency effects would be impaired in iNPH patients compared to healthy controls.
However, our findings indicate that on the immediate recall portion of the RAVLT, when compared to the control group, the primacy effect is significantly impaired in iNPH patients, whereas the recency effect shows only a slight difference between the two groups. This pattern suggests that impairments in iNPH may predominantly affect the neural circuits underlying long-term encoding processes, typically associated with the primacy effect, while short-term recall mechanisms related to the recency effect appear to be relatively preserved. These results provide further insight into the selective vulnerabilities of memory processes in iNPH and underscore the importance of distinguishing between different aspects of verbal memory in clinical assessment.
Our model estimates the probability of recalling each word from the fifteen-word list used in the RAVLT. In healthy controls, we observed the expected pattern: the probability of recalling the first (primacy) and last (recency) words is higher compared to the words in the middle of the list, reflecting robust primacy and recency effects as documented in the literature. Specifically, the typical U-shaped serial position curve demonstrates enhanced memory performance for initial and final list positions in healthy individuals.
However, our findings for iNPH patients tell a different story. In the iNPH group, the primacy effect appears to be absent—patients show a similar probability of recalling both the initial and central list items. In contrast, the recency effect seems preserved, as these patients tend to recall the final words of the list better than both the initial and middle words. When directly comparing the two groups, healthy controls have a higher recall probability for the first list items than iNPH patients, while the recall probability for the last items does not significantly differ between the groups. Both groups show comparable performance in the recall of words from the end (recency positions), but only healthy controls display a clear primacy advantage.
These results suggest that in iNPH, the cognitive mechanisms underlying the primacy effect—often related to long-term encoding and possibly hippocampal function—are selectively impaired [19,35].
Patients with iNPH have been shown to be significantly impaired in episodic memory tests based on the Alzheimer’s Disease Assessment Scale (ADAS), which include free recall of word lists [36]. For example, in the study by Saito and colleagues [36], the mean true recall score on the ADAS word list was 12.9 ± 4.0 in iNPH patients, compared to 21.5 ± 2.8 in healthy controls. This substantial difference demonstrates a marked deficit in initial learning and recall abilities in iNPH, strongly supporting the presence of encoding and retrieval dysfunctions in this population.
These results further corroborate the validity of using list learning paradigms, such as the RAVLT, to sensitively detect and quantify the verbal memory deficits characteristic of iNPH. The pronounced impairment in initial acquisition and recall provides objective evidence of the specific cognitive profile associated with this condition and highlights the clinical utility of verbal learning tests in both diagnostic workup and the evaluation of treatment outcomes in iNPH.
Meanwhile, the recency effect, linked more closely to immediate memory or attention, remains relatively unaffected. This selective disruption may reflect the distinct neural substrate vulnerabilities present in iNPH and emphasizes the importance of detailed serial position analysis in differentiating memory impairments across neurocognitive disorders. Our findings reinforce the need to consider both the qualitative pattern and quantitative probabilities of recall when evaluating verbal memory deficits in clinical populations.
5. Conclusions
This study confirms that patients with iNPH exhibit significant impairments in verbal memory, as evidenced by their performance on the RAVLT. Specifically, iNPH patients show a marked reduction in both immediate and delayed recall scores compared to healthy controls, highlighting pervasive deficits in both acquisition and retention of verbal information. The serial position analysis reveals that while healthy controls display a classic U-shaped recall curve characterized by robust primacy and recency effects, iNPH patients present with an absent primacy effect and a relatively preserved recency effect. Statistical modeling corroborates these observations, demonstrating a significantly lower probability of recalling items from the beginning of the list among iNPH patients, but no significant difference in the recall of final list items compared to controls. These findings suggest that iNPH predominantly affects neural mechanisms related to long-term encoding, likely involving hippocampal and parahippocampal regions, while short-term memory and attentional processes remain less impacted. Our results underscore the clinical utility of detailed serial position analysis and list learning tasks in distinguishing the specific memory deficits associated with iNPH and support the integration of such paradigms in the neuropsychological assessment and monitoring of these patients. Finally, the RAVLT may also be relevant for other forms and subtypes of hydrocephalus and can assist in differential diagnosis as well as in the characterization of distinct cognitive syndromes.
Among the limitations of this study, the small sample size must be highlighted, primarily due to the rarity of the disorder and the persistent difficulty in recruiting patients, which is compounded by challenges in differential diagnosis from both neurological and psychological perspectives. Furthermore, our patient sample included only individuals with advanced age, reflecting limited access to younger iNPH patients who may present with different clinical characteristics. For future studies, efforts will be made to increase the sample size of both the patient group and healthy participants, where feasible.
Author Contributions
Conceptualization, M.G.V., M.L.M., R.G. and A.Q. (Andrea Quattrone); methodology, R.G., M.L.M. and M.G.V.; software, R.G.; validation, E.P., D.L.T., A.Q. (Aldo Quattrone) and A.Q. (Andrea Quattrone); formal analysis, R.G.; investigation, M.G.V., D.L.T., E.P., A.Q. (Andrea Quattrone) and A.Q. (Aldo Quattrone); resources, A.Q. (Aldo Quattrone); data curation, R.G., M.L.M. and M.G.V.; writing—original draft preparation, M.L.M., M.G.V., R.G. and E.P.; writing—review and editing, D.L.T., A.Q. (Aldo Quattrone) and A.Q. (Andrea Quattrone); supervision, D.L.T., A.Q. (Aldo Quattrone) and M.G.V.; project administration, A.Q. (Aldo Quattrone) All authors have read and agreed to the published version of the manuscript.
Funding
Roberto Giorgini and Marialucia Maiuolo are funded by ‘Italian NRRP, Mission 4, Component 2, Investment 1.5, call for the creation and strengthening of Innovation Ecosystems, building Territorial R&D Leaders (Directorial Decree n. 2021/3277)—project Tech4You—Technologies for climate change adaptation and quality of life improvement, n. ECS0000009’.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Local Ethics Committee of Calabria (Comitato Etico della Regione Calabria) (Co n.359; 22 October 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
Raw data and analysis script are available from the corresponding author (M.G.V) upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| iNPH | idiopathic normal-pressure hydrocephalus |
| DESH | disproportionately enlarged subarachnoid-space hydrocephalus |
| CA | Callosal Angle |
| EI | Evans Index |
| DMN | Default Mode Network |
| AD | Alzheimer’s disease |
| VaD | vascular dementia |
| LBD | dementia with Lewy bodies |
| PSP | progressive supranuclear palsy |
| CBD | corticobasal degeneration |
| MMSE | Mini-Mental State Examination |
| RAVLT | Rey Auditory Verbal Learning Test |
| PARW | percentage of accurately remembered words |
| ADAS | Alzheimer’s Disease Assessment Scale |
References
- Hakim, S.; Adams, R.D. The special clinical problem of symptomatic hydrocephalus with normal cerebrospinal fluid pressure: Observations on cerebrospinal fluid hydrodynamics. J. Neurol. Sci. 1965, 2, 307–327. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Yamada, S.; Miyajima, M.; Ishii, K.; Kuriyama, N.; Kazui, H.; Kanemoto, H.; Suehiro, T.; Yoshiyama, K.; Kameda, M.; et al. Guidelines for Management of Idiopathic Normal Pressure Hydrocephalus (Third Edition): Endorsed by the Japanese Society of Normal Pressure Hydrocephalus. Neurol. Med. Chir. 2021, 61, 63–97. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Yamaguchi, S.; Ishikawa, H.; Ishii, H.; Meguro, K. Prevalence of possible idiopathic normal-pressure hydrocephalus in Japan: The Osaki-Tajiri project. Neuroepidemiology 2009, 32, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, K.; Meguro, K.; Mori, E. Prevalence of idiopathic normal-pressure hydrocephalus in the elderly population of a Japanese rural community. Neurol. Med. Chir. 2008, 48, 197–200. [Google Scholar] [CrossRef]
- Martín-Láez, R.; Caballero-Arzapalo, H.; López-Menéndez, L.Á.; Arango-Lasprilla, J.C.; Vázquez-Barquero, A. Epidemiology of idiopathic normal pressure hydrocephalus: A systematic review of the literature. World Neurosurg. 2015, 84, 2002–2009. [Google Scholar] [CrossRef]
- Bianco, M.G.; Quattrone, A.; Sarica, A.; Vescio, B.; Buonocore, J.; Vaccaro, M.G.; Aracri, F.; Calomino, C.; Gramigna, V.; Quattrone, A. Cortical atrophy distinguishes idiopathic normal-pressure hydrocephalus from progressive supranuclear palsy: A machine learning approach. Park. Relataed Disord. 2022, 103, 7–14. [Google Scholar] [CrossRef]
- Andrén, K.; Wikkelsø, C.; Tisell, M.; Hellström, P. Natural course of idiopathic normal pressure hydrocephalus. J. Neurol. Neurosurg. Psychiatry 2014, 85, 806–810. [Google Scholar] [CrossRef]
- Kiefer, M.; Unterberg, A. The differential diagnosis and treatment of normal-pressure hydrocephalus. Dtsch. Ärzteblatt Int. 2012, 109, 15–22. [Google Scholar] [CrossRef]
- Xiao, H.; Hu, F.; Ding, J.; Ye, Z. Cognitive impairment in idiopathic normal pressure hydrocephalus. Neurosci. Bull. 2022, 38, 1085–1096. [Google Scholar] [CrossRef]
- Peterson, K.A.; Mole, T.B.; Keong, N.C.; DeVito, E.E.; Savulich, G.; Pickard, J.D.; Sahakian, B.J. Structural correlates of cognitive impairment in normal pressure hydrocephalus. Acta Neurol. Scand. 2019, 139, 305–312. [Google Scholar] [CrossRef]
- Langheinrich, T.; Chen, C.; Thomas, O. Update on the Cognitive Presentations of iNPH for Clinicians. Front. Neurol. 2022, 13, 894617. [Google Scholar] [CrossRef] [PubMed]
- Ogino, A.; Kazui, H.; Miyoshi, N.; Hashimoto, M.; Ohkawa, S.; Tokunaga, H.; Ikejiri, Y.; Takeda, M. Cognitive impairment in patients with idiopathic normal pressure hydrocephalus. Dement. Geriatr. Cogn. Disord. 2006, 21, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Picascia, M.; Minafra, B.; Zangaglia, R.; Gracardi, L.; Pozzi, N.G.; Sinforiani, E.; Pacchetti, C. Spectrum of cognitive disorders in idiopathic normal pressure hydrocephalus. Funct. Neurol. 2016, 31, 143. [Google Scholar] [CrossRef] [PubMed]
- Niermeyer, M.; Gaudet, C.; Malloy, P.; Piryatinsky, I.; Salloway, S.; Klinge, P.; Lee, A. Frontal Behavior Syndromes in Idiopathic Normal Pressure Hydrocephalus as a Function of Alzheimer’s Disease Biomarker Status. J. Int. Neuropsychol. Soc. 2020, 26, 883–893. [Google Scholar] [CrossRef]
- Kanno, S.; Ogawa, K.I.; Kikuchi, H.; Toyoshima, M.; Abe, N.; Sato, K.; Miyazawa, K.; Oshima, R.; Ohtomo, S.; Arai, H.; et al. Reduced default mode network connectivity relative to white matter integrity is associated with poor cognitive outcomes in patients with idiopathic normal pressure hydrocephalus. BMC Neurol. 2021, 21, 353. [Google Scholar] [CrossRef]
- Peterson, K.A.; Savulich, G.; Jackson, D.; Killikelly, C.; Pickard, J.D.; Sahakian, B.J. The effect of shunt surgery on neuropsychological performance in normal pressure hydrocephalus: A systematic review and meta-analysis. J. Neurol. 2016, 263, 1669–1677. [Google Scholar] [CrossRef]
- McGovern, R.A.; Nelp, T.B.; Kelly, K.M.; Chan, A.K.; Mazzoni, P.; Sheth, S.A.; Honig, L.S.; Teich, A.F.; McKhann, G.M. Predicting cognitive improvement in normal pressure hydrocephalus patients using preoperative neuropsychological testing and cerebrospinal fluid biomarkers. Neurosurgery 2019, 85, E662–E669. [Google Scholar] [CrossRef]
- Gainotti, G.; Marra, C.; Villa, G.; Parlato, V.; Chiarotti, F. Sensitivity and specificity of some neuropsychological markers of Alzheimer dementia. Alzheimer Dis. Assoc. Disord. 1998, 12, 152–162. [Google Scholar] [CrossRef]
- Juhlin, F.; Mellqvist, J.; Eckerström, M.; Hellström, P. Rey Auditory Verbal Learning Test in idiopathic normal pressure hydrocephalus and Alzheimer’s disease. Clin. Neuropsychol. 2024, 38, 202–218. [Google Scholar] [CrossRef]
- Laera, R.; Gorgoglione, M.L.; Curcio, A.; Marzano, G.; Caruso, G.; Caffo, M.; Germano, A. Idiopathic normal pressure hydrocephalus diagnosis: Quantitative and qualitative score predicting outcome of extended lumbar drainage. Heliyon 2024, 10, e31004. [Google Scholar] [CrossRef]
- Santangelo, G.; Siciliano, M.; Pedone, R.; Vitale, C.; Falco, F.; Bisogno, R.; Siano, P.; Barone, P.; Grossi, D.; Santangelo, F.; et al. Normative data for the Montreal Cognitive Assessment in an Italian population sample. Neurol. Sci. 2015, 36, 585–591. [Google Scholar] [CrossRef]
- Park, H.Y.; Kim, M.; Suh, C.H.; Lee, D.H.; Shim, W.H.; Kim, S.J. Diagnostic performance and interobserver agreement of the callosal angle and Evans’ index in idiopathic normal pressure hydrocephalus: A systematic review and meta-analysis. Eur. Radiol. 2021, 31, 5300–5311. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Pirani, A.; Nasreddine, Z.S.; Tulipani, C.; Neri, M. Montreal Cognitive Assessment (MOCA): Uno strumento rapido per lo screening del Mild Cognitive Impairment. In Dati Preliminari Della Versione Italiana; Atti IV Congresso Regionale Associazione Italiana Psicogeriatria: Bologna, Italy, 2007. [Google Scholar]
- Rey, A. L’examen Clinique en Psychologie; Presses Universitaries de France: Paris, France, 1958. [Google Scholar]
- Caltagirone, C.; Gainotti, G.; Carlesimo, G.A.; Parnetti, L. Batteria per la valutazione del deterioramento mentale: I. Descrizione di uno strumento di diagnosi neuropsicologica [The Mental Deterioration Battery: I. Description of a neuropsychological diagnostic instrument]. Arch. Psicol. Neurol. Psichiatr. 1995, 56, 461–470. [Google Scholar]
- Carlesimo, G.A.; Sabbadini, M.; Fadda, L.; Caltagirone, C. Different components in wordlist forgetting of pure amnesics, degenerative demented and healthy subjects. Cortex 1995, 31, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Carlesimo, G.A.; Caltagirone, C.; Gainotti, G.; Fadda, L.; Gallassi, R.; Lorusso, S.; Marfia, G.; Marra, C.; Nocentini, U.; Parnetti, L. The mental deterioration battery: Normative data, diagnostic reliability, and qualitative analyses of cognitive impairment. Eur. Neurol. 1996, 36, 378–384. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 30 July 2025).
- Dapor, C.; Devita, M.; Iannizzi, P.; Arbia, E.; Bruzzano, A.; Dessì, M.; Lupi, D.; Rolandino, G.M.; Rossi, M.; Saccomano, A.; et al. The Montreal cognitive assessment (MoCA) 8.1 version, including the memory index score (MoCA-MIS): Italian norms. Neurol. Sci. 2025, 46, 2581–2589. [Google Scholar] [CrossRef]
- Consonni, M.; Rossi, S.; Cerami, C.; Marcone, A.; Iannaccone, S.; Francesco Cappa, S.; Perani, D. Executive dysfunction affects word list recall performance: Evidence from amyotrophic lateral sclerosis and other neurodegenerative diseases. J. Neuropsychol. 2017, 11, 74–90. [Google Scholar] [CrossRef]
- Hong, Y.J.; Yoon, B.; Shim, Y.S.; Cho, A.H.; Lim, S.C.; Ahn, K.J.; Yang, D.W. Differences in microstructural alterations of the hippocampus in Alzheimer disease and idiopathic normal pressure hydrocephalus: A diffusion tensor imaging study. Am. J. Neuroradiol. 2010, 31, 1867–1872. [Google Scholar] [CrossRef]
- Golomb, J.; de Leon, M.J.; George, A.E.; Kluger, A.; Convit, A.; Rusinek, H.; de Santi, S.; Litt, A.; Foo, S.H.; Ferris, S.H. Hippocampal atrophy correlates with severe cognitive impairment in elderly patients with suspected normal pressure hydrocephalus. J. Neurol. Neurosurg. Psychiatry 1994, 57, 590–593. [Google Scholar] [CrossRef]
- Froula, J.M.; Hastings, S.D.; Krook-Magnuson, E. The little brain and the seahorse: Cerebellar-hippocampal interactions. Front. Syst. Neurosci. 2023, 17, 1158492. [Google Scholar] [CrossRef]
- Burkart, M.; Heun, R.; Benkert, O. Serial position effects in dementia of the Alzheimer type. Dement. Geriatr. Cogn. Disord. 1998, 9, 130–136. [Google Scholar] [CrossRef]
- Saito, M.; Nishio, Y.; Kanno, S.; Uchiyama, M.; Hayashi, A.; Takagi, M.; Kykuchi, H.; Yamasaky, H.; Shimomura, T.; Iizuka Mori, E. Cognitive profile of idiopathic normal pressure hydrocephalus. Dement. Geriatr. Cogn. Disord. Extra 2011, 1, 202–211. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).