European Consumer and Regulatory Trends in Medicinal Plant Food Supplements and Their Functional Properties: The Road from Farm to Fork
Abstract
1. Introduction
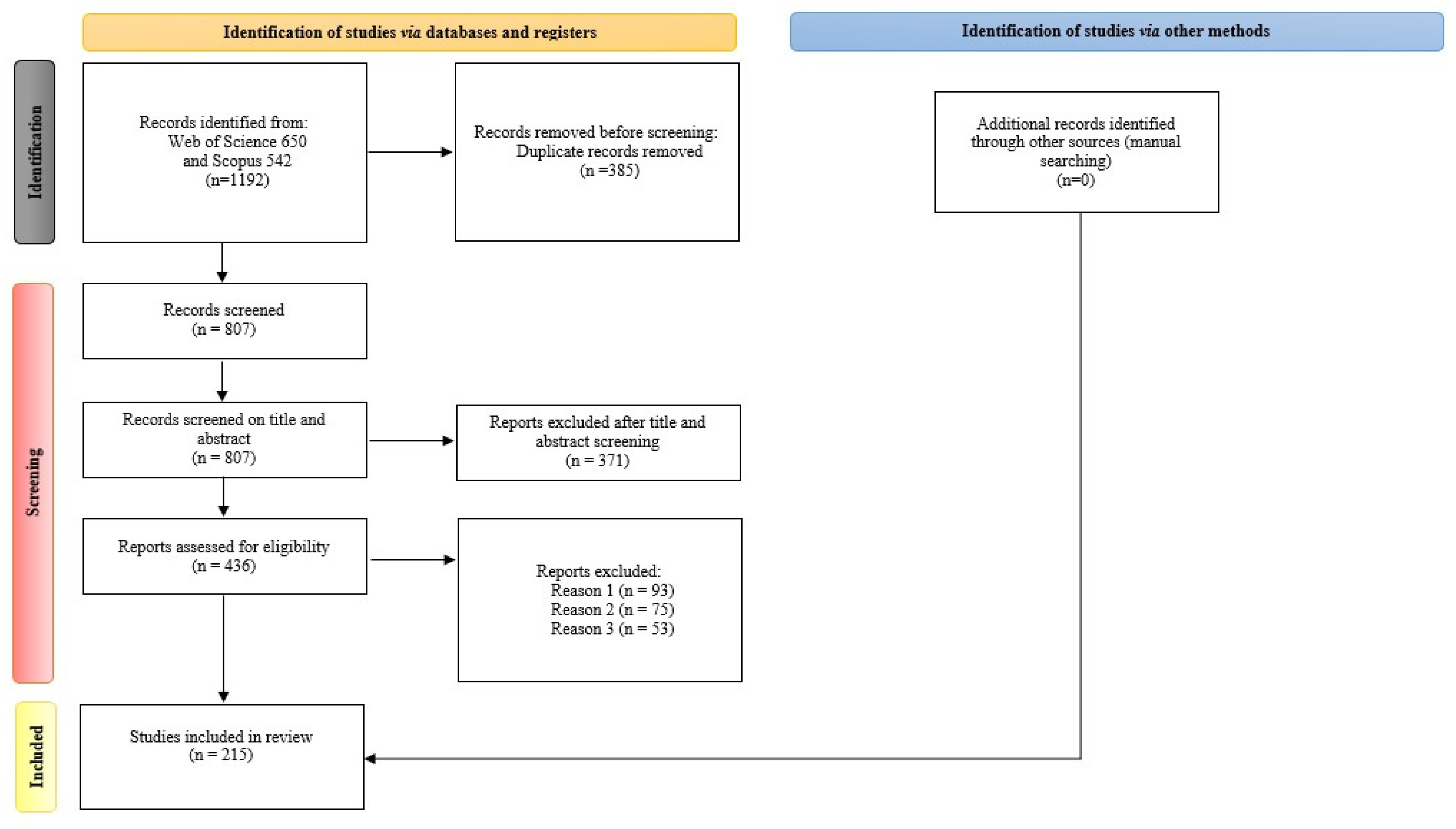
2. Methodology
3. Results and Discussions
3.1. Overview of Medicinal Plant Supplements
3.2. Traditional Roots and Modern Validation of Medicinal Plants
| Medicinal Plant | Botanical Name | Traditional Uses | Clinical Validation | Active Compounds | Refs. |
|---|---|---|---|---|---|
| Ginger | Zingiber officinale |
| effectiveness against nausea and vomiting in pregnancy and chemotherapy |
| [61] |
| Turmeric | Curcuma longa |
| anti-inflammatory and antioxidant agent against various diseases |
| [62] |
| Echinacea | Echinacea purpurea |
| efficacy in reducing the duration of colds and respiratory infections |
| [63] |
| Ashwagandha | Withania somnifera |
| adaptogenic properties and stress-reducing effects |
| [64] |
| Hawthorn | Crataegus spp. |
| improving heart health and function through vasodilatory effects |
| [65] |
| Nettle | Urtica dioica |
| anti-inflammatory and antihistamine properties |
| [66] |
| Valerian | Valeriana officinalis |
| improved sleep quality and managing anxiety |
| [67] |
| Aloe Vera | Aloe barbadensis |
| antimicrobial and healing properties due to its potential role in blood glucose regulation |
| [68] |
| Milk Thistle | Silybum marianum |
| hepatoprotective properties through active constituents |
| [69] |
| Garlic | Allium sativum |
| lipid-lowering effects and antimicrobial properties through active components |
| [57] |
3.3. Fortification of Food Supplements Using Medicinal Plants
3.4. Medicinal Plant Supplements in the European Regulatory Context
3.5. Regulatory Enforcement Challenges
3.6. European Consumer Preferences and Behavior
3.7. Demographic Profiles and Determinants of Food Supplement Use
3.8. Psychographic Factors and Cultural Influences
3.9. Market Trends and Challenges for Fortified Dietary Supplements with Medicinal Plants
3.10. The Farm to Fork Concept and Strategy and Its Implications for the Herbal Medicinal Plant Food Supplement Value Chain
4. Conclusions, Limitations, and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bilia, A.R. Herbal medicinal products versus botanical-food supplements in the European market: State of art and perspectives. Nat. Prod. Commun. 2015, 10, 125–131. [Google Scholar] [CrossRef]
- Costa-Font, J.; Sato, A. Cultural persistence and the ‘herbal medicine paradox’: Evidence from European data. J. Health Psychol. 2025, 30, 171–185. [Google Scholar] [CrossRef]
- Hoshino, T.; Muto, N.; Tsukada, S.; Nakamura, T.; Maegawa, H. European Ethnopharmaceuticals for Self-Medication in Japan: Review Experience of Vitis vinifera L., Folium Extract and Vitex agnus-castus L., Fructus Extract as OTC Drugs. Medicines 2018, 5, 3. [Google Scholar] [CrossRef]
- Rausch, H.; Schröder, S.; Friedemann, T.; Cameron, S.; Kuchta, K.; Konrad, M. History and Present of European Traditional Herbal Medicine (Phytotherapy). In History, Present and Prospect of World Traditional Medicine; World Scientific: Singapore, 2023; pp. 131–234. [Google Scholar]
- Chhabra, N.; Shiriskar, J.; Srinivasan, G. Current and Future Market of the Dietary Supplements and Nutraceuticals in the Global Economy. In Dietary Supplements and Nutraceuticals; Springer: Berlin/Heidelberg, Germany, 2025; pp. 1–48. [Google Scholar]
- Muilu-Mäkelä, R.; Brännström, H.; Weckroth, M.; Kohl, J.; Da Silva Viana, G.; Diaz, M.; Ghalibaf, M.; Hagner, M.; Hiidenhovi, J.; Ilvesniemi, J. Valuable Biochemicals of the Future: The Outlook for Bio-Based Value-Added Chemicals and Their Growing Markets; 2024. Available online: https://jukuri.luke.fi/items/35a030c3-b16a-407a-8272-b335095281d4 (accessed on 26 October 2025).
- Ababneh, M.A.; Halloush, S.; Altawalbeh, S.; Mardini, A. Knowledge and attitudes towards herbal and dietary products use during the COVID-19 pandemic. Clin. Nutr. Open Sci. 2023, 49, 118–129. [Google Scholar] [CrossRef]
- Hijazi, M.A.; Shatila, H.; Abu Qiyas, S.; Aboul-Ela, M.; El-Lakany, A.; Naja, F. Complementary and alternative medicine use during the COVID-19 pandemic: Community pharmacists’ knowledge, attitudes, and practices. Res. Social. Adm. Pharm. 2023, 19, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Nisticò, G.; Roth-Behrendt, D. The European legislation (directive 2004/24/EC) brings clarification and recognition to herbal medicinal products. In Herbal Medicines: Development and Validation of Plant-Derived Medicines for Human Health; CRC Press: Boca Raton, FL, USA, 2012; pp. 1–5. [Google Scholar]
- Parliament, E. Union tCotE: Directive 2004/24/EC of the European Parliament and of the Council of 31 March 2004 amending, as regards traditional herbal medicinal products, Directive 2001/83/EC on the Community code relating to medicinal products for human use. Off. J. Eur. Union 2004, 136, 85–90. [Google Scholar]
- Zovi, A.; Vitiello, A.; Sabbatucci, M.; Musazzi, U.M.; Sagratini, G.; Cifani, C.; Vittori, S. Food Supplements Marketed Worldwide: A Comparative Analysis Between the European and the U.S. Regulatory Frameworks. J. Diet. Suppl. 2025, 22, 25–40. [Google Scholar] [CrossRef]
- Irshad, M.; Ahmad, M.S.; Malik, O. Understanding consumers’ trust in social media marketing environment. Int. J. Retail. Distrib. Manag. 2020, 48, 1195–1212. [Google Scholar] [CrossRef]
- Mesias, F.J.; Martin, A.; Hernandez, A. Consumers’ growing appetite for natural foods: Perceptions towards the use of natural preservatives in fresh fruit. Food Res. Int. 2021, 150, 110749. [Google Scholar] [CrossRef] [PubMed]
- Obahiagbon, E.G.; Ogwu, M.C. Consumer perception and demand for sustainable herbal medicine products and market. In Herbal Medicine Phytochemistry: Applications and Trends; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1919–1952. [Google Scholar]
- Restani, P.; Di Lorenzo, C.; Garcia-Alvarez, A.; Frigerio, G.; Colombo, F.; Maggi, F.M.; Mila-Villarroel, R.; Serra-Majem, L. The PlantLIBRA consumer survey: Findings on the use of plant food supplements in Italy. PLoS ONE 2018, 13, e0190915. [Google Scholar] [CrossRef]
- Singar, S.; Nagpal, R.; Arjmandi, B.H.; Akhavan, N.S. Personalized Nutrition: Tailoring Dietary Recommendations through Genetic Insights. Nutrients 2024, 16, 2673. [Google Scholar] [CrossRef]
- Sovold, L.E.; Naslund, J.A.; Kousoulis, A.A.; Saxena, S.; Qoronfleh, M.W.; Grobler, C.; Munter, L. Prioritizing the Mental Health and Well-Being of Healthcare Workers: An Urgent Global Public Health Priority. Front. Public Health 2021, 9, 679397. [Google Scholar] [CrossRef]
- Ndugbu, K.U. Mental Health as a Public Health Priority: A National Strategy for Addressing the Mental Health Crisis in the Post-Pandemic Era. Univ. Libr. Med. Health Sci. 2025, 3. [Google Scholar] [CrossRef]
- Kiran, B.R.; Prasad, M.N.V.; Mohan, S.V. Farm to fork: Sustainable agrifood systems. In Sustainable and Circular Management of Resources and Waste Towards a Green Deal; Elsevier: Amsterdam, The Netherlands, 2023; pp. 25–38. [Google Scholar]
- Fiore, M.; Sauvée, L.; Wiśniewska-Paluszak, J. Opportunities and challenges of EU farm-to-fork strategy. Int. Food Agribus. Manag. Rev. 2022, 25, 703–707. [Google Scholar]
- de Almeida, C.; da Silva, M.L.; Junior, W.S.F.; da Silva, T.C. Can socioeconomic factors and the availability of medicinal plant resources influence people’s perception of risk in relation to diseases? J. Ethnobiol. Ethnomed. 2025, 21, 35. [Google Scholar] [CrossRef]
- Giannenas, I.; Sidiropoulou, E.; Bonos, E.; Christaki, E.; Florou-Paneri, P. The history of herbs, medicinal and aromatic plants, and their extracts: Past, current situation and future perspectives. In Feed Additives; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–18. [Google Scholar]
- Reque, P.M.; Brandelli, A.; Miyazaki, C.M.S.; Pessoa, A.F.M. Medicinal Plants and Herbal Medicines for Managing Anxiety and Depression via Gut Microbiota Modulation. Adv. Gut Microbiome Res. 2025, 2025, 3675425. [Google Scholar] [CrossRef]
- Sanchez, M.; Gonzalez-Burgos, E.; Iglesias, I.; Lozano, R.; Gomez-Serranillos, M.P. Current uses and knowledge of medicinal plants in the Autonomous Community of Madrid (Spain): A descriptive cross-sectional study. BMC Complement. Med. Ther. 2020, 20, 306. [Google Scholar] [CrossRef] [PubMed]
- Ntakirutimana, G.K. The Impact of Urbanization on Traditional Medicinal Plant Use in Disease Management. Res. Invent. J. Biol. Appl. Sci. 2025, 5, 47–50. [Google Scholar] [CrossRef]
- Khalil, M.; Abdallah, H.; Calasso, M.; Khalil, N.; Daher, A.; Missaoui, J.; Diab, F.; Zeaiter, L.; Vergani, L.; Di Ciaula, A.; et al. Herbal Medicine in Three Different Mediterranean Living Areas During the COVID-19 Pandemic: The Role of Polyphenolic-Rich Thyme-like Plants. Plants 2024, 13, 3340. [Google Scholar] [CrossRef]
- Koleva, V.; Dragoeva, A.; Nanova, Z.; Koynova, T.; Dashev, G. An ethnobotanical study on current status of some medicinal plants used in Bulgaria. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 297–305. [Google Scholar]
- Sendker, J.; Sheridan, H. History and current status of herbal medicines. In Toxicology of Herbal Products; Springer: Berlin/Heidelberg, Germany, 2017; pp. 11–27. [Google Scholar]
- Blanchemain, T. Healing the Sick: An Archaeobotanical Approach to Medieval Nordic Monasteries and Manuscripts. Master’s Thesis, Lund University, Lund, Sweden, 2024. [Google Scholar]
- Mendes, M.C.; Navalho, S.; Ferreira, A.; Paulino, C.; Figueiredo, D.; Silva, D.; Gao, F.; Gama, F.; Bombo, G.; Jacinto, R. Algae as food in Europe: An overview of species diversity and their application. Foods 2022, 11, 1871. [Google Scholar] [CrossRef] [PubMed]
- Nova, P.; Martins, A.P.; Teixeira, C.; Abreu, H.; Silva, J.G.; Silva, A.M.; Freitas, A.C.; Gomes, A.M. Foods with microalgae and seaweeds fostering consumers health: A review on scientific and market innovations. J. Appl. Phycol. 2020, 32, 1789–1802. [Google Scholar] [CrossRef]
- Csatlos, N.I.; Simon, E.; Teleky, B.E.; Szabo, K.; Diaconeasa, Z.M.; Vodnar, D.C.; Ciont Nagy, C.; Pop, O.L. Development of a Fermented Beverage with Chlorella vulgaris Powder on Soybean-Based Fermented Beverage. Biomolecules 2023, 13, 245. [Google Scholar] [CrossRef] [PubMed]
- Molin, T.R.D.; Leal, G.C.; Muller, L.S.; Muratt, D.T.; Marcon, G.Z.; Carvalho, L.M.; Viana, C. Regulatory framework for dietary supplements and the public health challenge. Rev. Saude Publica 2019, 53, 90. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.; Huang, L.; Rha, J.Y.; Suh, B.; Yoon, J. Trends in cross-border shopping for dietary supplements in South Korea with a focus on major types of health functional foods. Nutr. Res. Pract. 2025, 19, 241–256. [Google Scholar] [CrossRef]
- Rahman, S.U.; Shafique, E.; Tajummal, A.; Nageen, M.; Zahoor, A.; Ali, N.; Javed, F. Regulatory Considerations for Herbal Products. In Herbal Pharmacopeia; CRC Press: Boca Raton, FL, USA, 2025; pp. 440–457. [Google Scholar]
- Bagheri, H.; Akhavan-Mahdavi, S.; Sarabi-Aghdam, V.; Mirarab Razi, S.; Singh Beniwal, A.; Rashidinejad, A. Targeted dairy fortification: Leveraging bioactive compounds to enhance nutritional value. Crit. Rev. Food Sci. Nutr. 2025, 65, 1–25. [Google Scholar] [CrossRef]
- Habib, M.; Qureshi, I.; Singh, S.; Jan, S.; Bashir, K. Phytochemical Fortification. In Food Fortification; CRC Press: Boca Raton, FL, USA, 2024; pp. 253–283. [Google Scholar]
- Durmus, N.; Gulsunoglu-Konuskan, Z.; Kilic-Akyilmaz, M. Recovery, Bioactivity, and Utilization of Bioactive Phenolic Compounds in Citrus Peel. Food Sci. Nutr. 2024, 12, 9974–9997. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.; Barba, F.J. Sustainable Production Technology in Food; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Ruben, R.; Cavatassi, R.; Lipper, L.; Smaling, E.; Winters, P. Towards food systems transformation-five paradigm shifts for healthy, inclusive and sustainable food systems. Food Secur. 2021, 13, 1423–1430. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Bokelmann, J.M. Medicinal Herbs in Primary Care-E-Book: An Evidence-Guided Reference for Healthcare Providers; Elsevier Health Sciences: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Sarkar, R.; Nayak, S.L.; Suthar, M.K.; Das, M. Nutraceutical Formulations from Medicinal Plants: A Potential Therapeutic Agent. In Ethnopharmacology and OMICS Advances in Medicinal Plants Volume 1: Uncovering Diversity and Ethnopharmacological Aspects; Springer: Berlin/Heidelberg, Germany, 2024; pp. 391–417. [Google Scholar]
- Dzeparoski, M.; Trajkovic-Jolevska, S. Impact of regulation on advertising and promotion of traditional herbal medicines and food supplements. Int. J. Pharm. Healthc. Mark. 2018, 12, 77–90. [Google Scholar] [CrossRef]
- Radovanovic, K.; Gavaric, N.; Acimovic, M. Anti-Inflammatory Properties of Plants from Serbian Traditional Medicine. Life 2023, 13, 874. [Google Scholar] [CrossRef] [PubMed]
- Sathvika, V.P.; Subhas, P.G.; Bhattacharjee, D.; Koppad, V.N.; Samrat, U.; Karibasappa, S.B.; Sagar, K.M. Review of Case Study Results: Assessing the Effectiveness of Curcumin, St. John’s Wort, Valerian Root, Milk Thistle, and Ashwagandha in the Intervention for Obsessive-Compulsive Disorder. Drugs Drug Candidates 2024, 3, 838–859. [Google Scholar] [CrossRef]
- Das, D.; Khatun, R.; Sengupta, S.; Bhattacharya, M. Bioactive Compounds—A New Era of Therapeutic Medicines. J. Plant Sci. 2025, 13, 112–121. [Google Scholar] [CrossRef]
- Pop, O.L.; Kerezsi, A.D.; Ciont, C. A Comprehensive Review of Moringa oleifera Bioactive Compounds—Cytotoxicity Evaluation and Their Encapsulation. Foods 2022, 11, 3787. [Google Scholar] [CrossRef]
- Ciont, C.; Difonzo, G.; Pasqualone, A.; Chis, M.S.; Ranga, F.; Szabo, K.; Simon, E.; Naghiu, A.; Barbu-Tudoran, L.; Caponio, F.; et al. Phenolic profile of micro- and nano-encapsulated olive leaf extract in biscuits during in vitro gastrointestinal digestion. Food Chem. 2023, 428, 136778. [Google Scholar] [CrossRef]
- Aziez, M.; Suharoschi, R.; Merakeb, M.S.; Pop, O.L.; Ciont, C.; Ranga, F.; Ferhat, R.; Affenai, S.; Vodnar, D.C.; Cozma, A.; et al. Phenolic Profiling and Bioactive Properties of Arthrospira platensis Extract in Alleviating Acute and Sub-Chronic Colitis. Int. J. Mol. Sci. 2025, 26, 5692. [Google Scholar] [CrossRef]
- Ng, Q.X.; Venkatanarayanan, N.; Ho, C.Y. Clinical use of Hypericum perforatum (St John’s wort) in depression: A meta-analysis. J. Affect. Disord. 2017, 210, 211–221. [Google Scholar] [CrossRef]
- Rahimi, E.; Minoei, K.; Yazdanpanah, K.; Roshani, D.; Farhadi, L. Evaluating the effect of Glycyrrhiza glabra, Matricaria chamomilla, and Achillea millefolium on the symptoms of irritable bowel syndrome: Clinical trial: Effect of three plants on IBS. Chronic Dis. J. 2023, 11, 215–222. [Google Scholar]
- Elmaidomy, A.H.; Mohyeldin, M.M.; Ibrahim, M.M.; Hassan, H.M.; Amin, E.; Rateb, M.E.; Hetta, M.H.; El Sayed, K.A. Acylated Iridoids and Rhamnopyranoses from Premna odorata (Lamiaceae) as Novel Mesenchymal-Epithelial Transition Factor Receptor Inhibitors for the Control of Breast Cancer. Phytother. Res. 2017, 31, 1546–1556. [Google Scholar] [CrossRef] [PubMed]
- Mirhashemi, S.H.; Hakakzadeh, A.; Yeganeh, F.E.; Oshidari, B.; Rezaee, S.P. Effect of 8 Weeks milk thistle powder (silymarin extract) supplementation on fatty liver disease in patients candidates for bariatric surgery. Metabol. Open 2022, 14, 100190. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Piñero, S.; Muñoz-Carrillo, J.C.; Victoria-Montesinos, D.; García-Muñoz, A.M.; Andreu-Caravaca, L.; Gómez, M.; Schölzel, M.; García-Guillén, A.I.; López-Román, F.J. Efficacy of Boswellia serrata Extract and/or an Omega-3-Based Product for Improving Pain and Function in People Older Than 40 Years with Persistent Knee Pain: A Randomized Double-Blind Controlled Clinical Trial. Nutrients 2023, 15, 3848. [Google Scholar] [CrossRef]
- Salutondok, W.; Weubun, A.N.B.; Silaban, H. An Overview of Public Knowledge and Attitudes towards the Use of Herbs during the Covid-19 Pandemic. J. Drug Deliv. Ther. 2022, 12, 147–153. [Google Scholar] [CrossRef]
- Minghetti, P.; Franze, S.; Zaccara, V.; Raso, F.; Morazzoni, P. Innovation in Phytotherapy: Is a New Regulation the Feasible Perspective in Europe? Planta Medica 2016, 82, 591–595. [Google Scholar] [CrossRef]
- Mihai, M.; Ciont, C.; Marchiș, C.; Olar-Pop, L.; Pop, O.L. Food Fortification and Nutrition Enhancement Strategies in the Agri-food Sector in Support of the Farm-To-Fork Initiative of the European Union. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Food Sci. Technol. 2025, 82, 44–76. [Google Scholar] [CrossRef]
- Thakkar, S.; Anklam, E.; Xu, A.; Ulberth, F.; Li, J.; Li, B.; Hugas, M.; Sarma, N.; Crerar, S.; Swift, S.; et al. Regulatory landscape of dietary supplements and herbal medicines from a global perspective. Regul. Toxicol. Pharmacol. 2020, 114, 104647. [Google Scholar] [CrossRef]
- Abdel-Tawab, M. Do we need plant food supplements? A critical examination of quality, safety, efficacy, and necessity for a new regulatory framework. Planta Medica 2018, 84, 372–393. [Google Scholar] [CrossRef] [PubMed]
- Gatt, A.R.; Vella Bonanno, P.; Zammit, R. Ethical considerations in the regulation and use of herbal medicines in the European Union. Front. Med. Technol. 2024, 6, 1358956. [Google Scholar] [CrossRef] [PubMed]
- Routledge, P.A.; Bracchi, R. Adverse drug reactions to herbal medicines. Advers. Drug React. Bull. 2021, 331, 1283–1286. [Google Scholar] [CrossRef]
- Gandhi, S.; Sigh, O.; Tiwari, A.; Apshingekar, P.; Jain, S.; Jain, V.; Pal, P.; Vyas, A. Regulatory Frameworks for Integrated Medicine Management in USA, Europe, Japan, and China. Int. J. Drug Regul. Aff. 2024, 12, 37–45. [Google Scholar] [CrossRef]
- Biagi, M.; Pecorari, R.; Appendino, G.; Miraldi, E.; Magnano, A.R.; Governa, P.; Cettolin, G.; Giachetti, D. Herbal Products in Italy: The Thin Line between Phytotherapy, Nutrition and Parapharmaceuticals; A Normative Overview of the Fastest Growing Market in Europe. Pharmaceuticals 2016, 9, 65. [Google Scholar] [CrossRef]
- Frenzel, C.; Teschke, R. Herbal Hepatotoxicity: Clinical Characteristics and Listing Compilation. Int. J. Mol. Sci. 2016, 17, 588. [Google Scholar] [CrossRef]
- Bhat, M.D.A.; Malik, R. Challenges in monitoring the safety of herbal medicines-an appraisal. J. Pharmacovigil. Drug Res. 2022, 3, 3–8. [Google Scholar] [CrossRef]
- Altyn, I.; Twaruzek, M. Mycotoxin Contamination Concerns of Herbs and Medicinal Plants. Toxins 2020, 12, 182. [Google Scholar] [CrossRef] [PubMed]
- Habs, M.; Koller, M. Material Risks of Homeopathic Medicinal Products: Regulatory Frameworks, Results of Preclinical Toxicology, and Clinical Meta-Analyses and Their Implications. Complement. Med. Res. 2021, 28, 64–84. [Google Scholar] [CrossRef]
- van Hunsel, F.; van der Kooi, D.; van de Koppel, S.; Kroes, B.H.; Woerdenbag, H.J. Analysis of Reports on Adverse Drug Reactions Related to Herbal Medicinal Products and Herbal Supplements in the Netherlands Received by the National Pharmacovigilance Centre Lareb. Drug Saf. 2022, 45, 651–661. [Google Scholar] [CrossRef]
- Knez, M.; Ranić, M.; Gurinović, M. Underutilized plants increase biodiversity, improve food and nutrition security, reduce malnutrition, and enhance human health and well-being. Let’s put them back on the plate! Nutr. Rev. 2024, 82, 1111–1124. [Google Scholar] [CrossRef]
- Maietti, A.; Tedeschi, P.; Catani, M.; Stevanin, C.; Pasti, L.; Cavazzini, A.; Marchetti, N. Nutrient Composition and Antioxidant Performances of Bread-Making Products Enriched with Stinging Nettle (Urtica dioica) Leaves. Foods 2021, 10, 938. [Google Scholar] [CrossRef]
- Chaubey, P.S.; Somani, G.; Kanchan, D.; Sathaye, S.; Varakumar, S.; Singhal, R.S. Evaluation of debittered and germinated fenugreek (Trigonella foenum graecum L.) seed flour on the chemical characteristics, biological activities, and sensory profile of fortified bread. J. Food Process. Preserv. 2018, 42, e13395. [Google Scholar] [CrossRef]
- Phillips, A.; Sen, M. Biobased Antimicrobial Food Packaging Coatings. In Food Coatings and Preservation Technologies; John Wiley & Sons: Hoboken, NJ, USA, 2024; pp. 515–549. [Google Scholar]
- Kolonas, A.; Vareltzis, P.; Kiroglou, S.; Goutzourelas, N.; Stagos, D.; Trachana, V.; Tsadila, C.; Mossialos, D.; Mourtakos, S.; Gortzi, O. Antioxidant and Antibacterial Properties of a Functional Sports Beverage Formulation. Int. J. Mol. Sci. 2023, 24, 3558. [Google Scholar] [CrossRef]
- Pekacar, S.; Bulut, S.; Ozupek, B.; Orhan, D.D. Anti-Inflammatory and Analgesic Effects of Rosehip in Inflammatory Musculoskeletal Disorders and Its Active Molecules. Curr. Mol. Pharmacol. 2021, 14, 731–745. [Google Scholar] [CrossRef]
- Michalska-Ciechanowska, A.; Brzezowska, J.; Nicolet, N.; Haladyn, K.; Bruck, W.M.; Hendrysiak, A.; Andlauer, W. Valorization of Rosehip (Rosa canina L.) Pomace Using Unconventional Carbohydrate Carriers for Beverage Obtainment. Molecules 2025, 30, 141. [Google Scholar] [CrossRef]
- Kandyliari, A.; Potsaki, P.; Bousdouni, P.; Kaloteraki, C.; Christofilea, M.; Almpounioti, K.; Moutsou, A.; Fasoulis, C.K.; Polychronis, L.V.; Gkalpinos, V.K.; et al. Development of Dairy Products Fortified with Plant Extracts: Antioxidant and Phenolic Content Characterization. Antioxidants 2023, 12, 500. [Google Scholar] [CrossRef]
- Wani, S.A.; Elshikh, M.S.; Al-Wahaibi, M.S.; Naik, H.R. Functional Foods: Technological Challenges and Advancement in Health Promotion; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
- Umesha, S.S.; Manohar, R.S.; Indiramma, A.R.; Akshitha, S.; Naidu, K.A. Enrichment of biscuits with microencapsulated omega-3 fatty acid (Alpha-linolenic acid) rich Garden cress (Lepidium sativum) seed oil: Physical, sensory and storage quality characteristics of biscuits. LWT—Food Sci. Technol. 2015, 62, 654–661. [Google Scholar] [CrossRef]
- Tarhan, O.; Spotti, M.J. Nutraceutical delivery through nano-emulsions: General aspects, recent applications and patented inventions. Colloids Surf. B Biointerfaces 2021, 200, 111526. [Google Scholar] [CrossRef] [PubMed]
- Székely-Szentmiklósi, I.; Rédai, E.M.; Szabó, Z.-I.; Kovács, B.; Albert, C.; Gergely, A.-L.; Székely-Szentmiklósi, B.; Sipos, E. Microencapsulation by Complex Coacervation of Lavender Oil Obtained by Steam Distillation at Semi-Industrial Scale. Foods 2024, 13, 2935. [Google Scholar] [CrossRef]
- Ardestani, L.; Hosseini, M.; Jahanshahi, M.; Amiri, A. Melissa officinalis extraction with nanoencapsulation By chitosan as an ecofriendly compound. J. Water Environ. Nanotechnol. 2022, 7, 380–388. [Google Scholar]
- Napiórkowska, A.; Kurek, M. Coacervation as a Novel Method of Microencapsulation of Essential Oils—A Review. Molecules 2022, 27, 5142. [Google Scholar] [CrossRef] [PubMed]
- Giordano Maffioly, N.; Sosa, A.M.; Martinez, L.M.; Alonso, S.d.V. Solid Lipid Microparticles As Carriers of Vaccinium Myrtillus And Schinus Molle Linn Additives for Food Application. J. Food Sci. Nutr. 2023, 9, 162. [Google Scholar] [CrossRef]
- Ichim, M.C. The DNA-Based Authentication of Commercial Herbal Products Reveals Their Globally Widespread Adulteration. Front. Pharmacol. 2019, 10, 1227. [Google Scholar] [CrossRef] [PubMed]
- Pop, O.L.; Ciont Nagy, C.; Gabianelli, R.; Coldea, T.E.; Pop, C.R.; Mudura, E.; Min, T.; Rangnoi, K.; Yamabhai, M.; Vlaic, R.; et al. Deciphering contaminants and toxins in fermented food for enhanced human health safeguarding. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13428. [Google Scholar] [CrossRef]
- Kosalec, I.; Cvek, J.; Tomic, S. Contaminants of medicinal herbs and herbal products. Arh. Hig. Rada Toksikol. 2009, 60, 485–501. [Google Scholar] [CrossRef] [PubMed]
- Letsyo, E.; Jerz, G.; Winterhalter, P.; Lindigkeit, R.; Beuerle, T. Incidence of Pyrrolizidine Alkaloids in Herbal Medicines from German Retail Markets: Risk Assessments and Implications to Consumers. Phytother. Res. 2017, 31, 1903–1909. [Google Scholar] [CrossRef] [PubMed]
- Raclariu-Manolica, A.C.; Mauvisseau, Q.; de Boer, H.J. Horizon scan of DNA-based methods for quality control and monitoring of herbal preparations. Front. Pharmacol. 2023, 14, 1179099. [Google Scholar] [CrossRef] [PubMed]
- Awodele, O.; Daniel, A.; Popoola, T.D.; Salami, E.F. A study on pharmacovigilance of herbal medicines in Lagos West Senatorial District, Nigeria. Int. J. Risk Saf. Med. 2013, 25, 205–217. [Google Scholar] [CrossRef]
- Qu, L.; Zou, W.; Zhou, Z.; Zhang, T.; Greef, J.; Wang, M. Non-European traditional herbal medicines in Europe: A community herbal monograph perspective. J. Ethnopharmacol. 2014, 156, 107–114. [Google Scholar] [CrossRef]
- Knoess, W.; Wiesner, J. The globalization of traditional medicines: Perspectives related to the European Union regulatory environment. Engineering 2019, 5, 22–31. [Google Scholar] [CrossRef]
- Ekstrand, S.S. The implementation of the food supplement directive 2002/46/EC. Eur. Food Feed. Law Rev. 2006, 1, 49–52. [Google Scholar]
- McGuffin, M.; Hobbs, C.; Upton, R.; Goldberg, A. Botanical Safety Handbook; American Herbal Products Association: Silver Spring, MD, USA; CRC Press, LLC: Boca Raton, FL, USA, 1997. [Google Scholar]
- Schwitters, B.; Achanta, G.; van der Vlies, D.; Bast, A.; Hanekamp, J.C. The European regulation of food supplements and food fortification. Environ. Law Manag. 2007, 19, 19. [Google Scholar]
- Vas, J.; Hegyi, G. A Systematic Literature Review of Complementary and Alternative Medicine Prevalence in EU. Forsch. Komplementärmedizin/Res. Complement. Med. 2012, 19 (Suppl. S2), 18–28. [Google Scholar]
- Kürzel, A. Impact of the Implementation of Directive 2004/24/EC: Development of Marketing Authorisations for Herbal Medicinal Products and Registrations for Traditional Herbal Medicinal Products in Germany in the European Regulatory Environment. Master’s Thesis, University of Bonn, Bonn, Germany, 2013. [Google Scholar]
- Klaus, B.; Corini, A. Recent Developments in Italy in the Field of Food Supplements Containing Botanicals. Eur. Food Feed. L. Rev. 2015, 10, 266. [Google Scholar]
- Wróbel, K.; Milewska, A.J.; Marczak, M.; Kozłowski, R. Dietary supplements questioned in the Polish notification procedure upon the basis of data from the national register of functional foods and the European system of the RASFF. Int. J. Environ. Res. Public Health 2022, 19, 8161. [Google Scholar] [CrossRef] [PubMed]
- Sultana, J.; Zaccaria, C.; de Lisa, R.; Rossi, F.; Capuano, A.; Ferrajolo, C. Good Pharmacovigilance Practice in Paediatrics: An Overview of the Updated European Medicines Agency Guidelines. Paediatr. Drugs 2019, 21, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Anastassiadou, M.; Devos, Y.; Dujardin, B.; Eskes, C.; Kouloura, E.; López-Gálvez, G.; Rizzi, V.; Gilsenan, M.B. Translating data into policy informing decisions: Current and future perspectives from the European Food Safety Authority (EFSA). In Proceedings of the Nutrition Society; Cambridge University Press: Cambridge, UK, 2025; pp. 1–22. [Google Scholar]
- EFSA Scientific Committee. Scientific Opinion on a Qualified Presumption of Safety (QPS) approach for the safety assessment of botanicals and botanical preparations. EFSA J. 2014, 12, 3593. [Google Scholar] [CrossRef]
- Agency, E.M. Guideline on Quality of Herbal Medicinal Products/Traditional Herbal Medicinal Products; European Medicines Agency: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Directive, E. Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the approximation of the laws of the Member States relating to food supplements. Off. J. Eur. Communities Legis. 2002, 45, 51–57. [Google Scholar]
- Bampidis, V.; Azimonti, G.; Bastos, M.d.L.; Christensen, H.; Durjava, M.F.; Kouba, M.; López-Alonso, M.; Puente, S.L.; Marcon, F.; Mayo, B.; et al. Safety and efficacy of feed additives consisting of dried extracts from Echinacea angustifolia DC. or Echinacea purpurea (L.) Moench for use in cats and dogs (CIAM). EFSA J. 2021, 19, e06446. [Google Scholar] [CrossRef]
- Kumar, S.; Walia, R.; Saxena, S.; Dey, P.; Madaan, R. Regulatory considerations of herbal bioactive–based formulations. In Herbal bioactive-Based Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2022; pp. 419–436. [Google Scholar]
- Krishna, P.D.; Gowrav, M.; Bhaskaran, M.; Kruthika, M.R. Current regulations of herbal medicines in the US and EU. Curr. Tradit. Med. 2024, 10, 141–151. [Google Scholar] [CrossRef]
- European Parliament. Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutrition and health claims made on foods. Off. J. Eur. Union 2006, 404, 9–25. [Google Scholar]
- Cohen, P.A.; Ernst, E. Safety of herbal supplements: A guide for cardiologists. Cardiovasc. Ther. 2010, 28, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Lindenmaier, M.P.; Bernart, M.W.; Brinckmann, J.A. Advanced Methodologies for the Quality Control of Herbal Supplements and Regulatory Considerations. In Phytochemical Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2025. [Google Scholar] [CrossRef]
- Pradhan, N.; Gavali, J.; Waghmare, N. WHO (World Health Organization) guidelines for standardization of herbal drugs. Int. Ayurvedic Med. J. 2015, 3, 2238–2243. [Google Scholar]
- Trimble, M. The EU Geo-Blocking Regulation: A Commentary; Edward Elgar Publishing: Cheltenham, UK, 2024. [Google Scholar]
- Chatfield, K.; Salehi, B.; Sharifi-Rad, J.; Afshar, L. Applying an Ethical Framework to Herbal Medicine. Evidence-Based Complement. Altern. Med. 2018, 2018, 1903629. [Google Scholar] [CrossRef]
- Lapenna, S.; Gemen, R.; Wollgast, J.; Worth, A.; Maragkoudakis, P.; Caldeira, S. Assessing herbal products with health claims. Crit. Rev. Food Sci. Nutr. 2015, 55, 1918–1928. [Google Scholar] [CrossRef]
- Michael Alurame, E.; Esther Oleiye, I.; James Tabat, B. Exploring herbal medicine regulation in Nigeria: Balancing traditional practices with modern standards. GSC Adv. Res. Rev. 2024, 18, 83–90. [Google Scholar] [CrossRef]
- Hossain, C.M.; Gera, M.; Ali, K.A. Current Status and Challenges of Herbal Drug Development and Regulatory Aspect: A Global Perspective. Asian J. Pharm. Clin. Res. 2022, 15, 31–41. [Google Scholar] [CrossRef]
- International Trade in Medicinal and Pharmaceutical Products. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=International_trade_in_medicinal_and_pharmaceutical_products (accessed on 23 September 2025).
- Committee on Herbal Medicinal Products (HMPC): Annual Report 2024. Available online: https://www.ema.europa.eu/en/committees/committee-herbal-medicinal-products-hmpc?utm_source=chatgpt.com (accessed on 23 September 2025).
- RASFF—The Rapid Alert System for Food and Feed: Annual Report 2024. Available online: https://webgate.ec.europa.eu/rasff-window/screen/search (accessed on 23 September 2025).
- Asmelashe Gelayee, D.; Binega Mekonnen, G.; Asrade Atnafe, S.; Birarra, M.K.; Asrie, A.B. Herbal Medicines: Personal Use, Knowledge, Attitude, Dispensing Practice, and the Barriers among Community Pharmacists in Gondar, Northwest Ethiopia. Evidence-Based Complement. Altern. Med. 2017, 2017, 6480142. [Google Scholar] [CrossRef]
- Geronimo, A.C.R.; Melo, E.S.P.; Silva, K.R.N.; Pereira, H.S.; Nascimento, V.A.; Machate, D.J.; do Nascimento, V.A. Human Health Risk Assessment of Heavy Metals and Metalloids in Herbal Medicines Used to Treat Anxiety: Monitoring of Safety. Front. Pharmacol. 2021, 12, 772928. [Google Scholar] [CrossRef]
- Wang, J.; Shi, Y.; Chen, G. Post-market technical evaluation of dietary supplements based on domestic and foreign experiences. Shanghai J. Prev. Med. 2022, 34, 1261–1266. [Google Scholar]
- Cencic, A.; Chingwaru, W. The role of functional foods, nutraceuticals, and food supplements in intestinal health. Nutrients 2010, 2, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Steel, A.; Gallego-Perez, D.F.; Ijaz, N.; Gall, A.; Bangpan, M.; Dos Santos Boeira, L.; Schveitzer, M.C.; Chutaputti, A.; Mahlanza-Langer, L.; Pillai, G.K.G.; et al. Integration of Traditional, Complementary, and Integrative Medicine in the Institutionalization of Evidence-Informed Decision-Making: The World Health Organization Meeting Report. J. Integr. Complement. Med. 2025, 31, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Hajnal, Z. Current challenges of European market surveillance regarding products sold online. Public Goods Gov. 2020, 5, 1–8. [Google Scholar] [CrossRef]
- Popescu, G.G. Food fraud in the European Union. Int. J. Leg. Soc. Order 2023, 3, 348–360. [Google Scholar] [CrossRef]
- Borghetto, M.V. Towards an EU Sustainable Food-Labelling Framework: Enhancing the Complexity of the Food System Through a Public and Private Mixed Approach. 2025. Available online: https://hdl.handle.net/20.500.14242/209310 (accessed on 26 October 2025).
- Castanheira, M.; Perilleux, G.; Talukder, D.; Bandelow, N. Sustainable Governance Indicators 2024: Belgium Report; Bertelsmann Stiftung: Gütersloh, Germany, 2024. [Google Scholar]
- Dores, A.R.; Peixoto, M.; Castro, M.; Sá, C.; Carvalho, I.P.; Martins, A.; Maia, E.; Praça, I.; Marques, A. Knowledge and Beliefs about Herb/Supplement Consumption and Herb/Supplement–Drug Interactions among the General Population, including Healthcare Professionals and Pharmacists: A Systematic Review and Guidelines for a Smart Decision System. Nutrients 2023, 15, 2298. [Google Scholar] [CrossRef]
- Obahiagbon, E.G.; Ogwu, M.C. The nexus of business, sustainability, and herbal medicine. In Herbal Medicine Phytochemistry: Applications and Trends; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–42. [Google Scholar]
- Arora, P.; Arora, N.; Mehta, S. Survey and Statistical Analysis for the Consumption Scenario of Nutritional Supplements; Apple Academic Press: Burlington, ON, Canada, 2026; p. 77. [Google Scholar]
- Ventura, I.; Hunt, T.; Mayer, J.G. Plants and Medicine. In A Cultural History of Plants in the Post-Classical Era; Bloomsbury Publishing: Bloomsbury, UK, 2023; Volume 2, p. 101. [Google Scholar]
- Embling, R.; Neilson, L.; Mellor, C.; Durodola, M.; Rouse, N.; Haselgrove, A.; Shipley, K.; Tales, A.; Wilkinson, L. Exploring consumer beliefs about novel fortified foods: A focus group study with UK-based older and younger adult consumers. Appetite 2024, 193, 107139. [Google Scholar] [CrossRef]
- Schunko, C.; Lechthaler, S.; Vogl, C.R. Conceptualising the factors that influence the commercialisation of non-timber forest products: The case of wild plant gathering by organic herb farmers in South Tyrol (Italy). Sustainability 2019, 11, 2028. [Google Scholar] [CrossRef]
- Kemppainen, L.M.; Kemppainen, T.T.; Reippainen, J.A.; Salmenniemi, S.T.; Vuolanto, P.H. Use of complementary and alternative medicine in Europe: Health-related and sociodemographic determinants. Scand. J. Public Health 2018, 46, 448–455. [Google Scholar] [CrossRef]
- Meiselman, H.L.; Kuesten, C.; Bi, J. The Use of Demographics and Psychographics to Study Product Effects with Nutrient Supplements: Exploratory Multi-Country Data. Foods 2021, 10, 1918. [Google Scholar] [CrossRef]
- Aranceta-Bartrina, J.; Partearroyo, T.; Lopez-Sobaler, A.M.; Ortega, R.M.; Varela-Moreiras, G.; Serra-Majem, L.; Perez-Rodrigo, C.; Collaborative Group for the Dietary Guidelines for the Spanish Population. Updating the Food-Based Dietary Guidelines for the Spanish Population: The Spanish Society of Community Nutrition (SENC) Proposal. Nutrients 2019, 11, 2675. [Google Scholar] [CrossRef]
- Welz, A.N.; Emberger-Klein, A.; Menrad, K. The importance of herbal medicine use in the German health-care system: Prevalence, usage pattern, and influencing factors. BMC Health Serv. Res. 2019, 19, 952. [Google Scholar] [CrossRef] [PubMed]
- Pushpa, N.D.; Didwania, N. Cryopreservation Technique: A Powerful Tool for Long-term Preservation of Endangered Medicinal Plants. Ambient. Sci. 2019, 6. [Google Scholar] [CrossRef]
- Gasparini, M.; Aurilia, C.; Lubian, D.; Testa, M. Herbal remedies and the self-treatment of stress: An Italian survey. Eur. J. Integr. Med. 2016, 8, 465–470. [Google Scholar] [CrossRef]
- Dadfar, F.; Bamdad, K. The effect of Saliva officinalis extract on the menopausal symptoms in postmenopausal women: An RCT. Int. J. Reprod. Biomed. 2019, 17, 287–292. [Google Scholar] [CrossRef]
- Rahayu, Y.Y.S.; Araki, T.; Rosleine, D. Factors affecting the use of herbal medicines in the universal health coverage system in Indonesia. J. Ethnopharmacol. 2020, 260, 112974. [Google Scholar] [CrossRef]
- Krsnik, S.; Erjavec, K. Factors Influencing Use of Medicinal Herbs. J. Patient Exp. 2024, 11, 23743735241241181. [Google Scholar] [CrossRef]
- Cadar, R.-L.; Amuza, A.; Dumitraş, D.E.; Pocol, C.B. Consumer behaviour of products obtained from medicinal and aromatic plants: A segmentation based on frequency and purpose of their use. Sci. Pap. Ser. Manag. Econ. Eng. Agric. Rural. Dev. 2021, 21, 127–136. [Google Scholar]
- Cadar, R.-L.; Amuza, A.; Dumitras, D.E.; Mihai, M.; Pocol, C.B. Analysing clusters of consumers who use medicinal and aromatic plant products. Sustainability 2021, 13, 8648. [Google Scholar] [CrossRef]
- Alrhmoun, M.; Sulaiman, N.; Pieroni, A. What Drives Herbal Traditions? The Influence of Ecology and Cultural Exchanges on Wild Plant Teas in the Balkan Mountains. Land 2024, 13, 2146. [Google Scholar] [CrossRef]
- Ivanova, T.; Bosseva, Y.; Chervenkov, M.; Dimitrova, D. Lamiaceae Plants in Bulgarian Rural Livelihoods—Diversity, Utilization, and Traditional Knowledge. Agronomy 2022, 12, 1631. [Google Scholar] [CrossRef]
- Solarov, S.; Eljuga, G.; Ilić, M.; Živković, J.; Jovanović-Lješković, N.; Šavikin, K. Traditional use of medicinal plants in rural areas of Osijek-Baranja county, Republic of Croatia. Lek. Sirovine 2022, 42, 10–23. [Google Scholar] [CrossRef]
- Arjona-Garcia, C.; Blancas, J.; Beltran-Rodriguez, L.; Lopez Binnquist, C.; Colin Bahena, H.; Moreno-Calles, A.I.; Sierra-Huelsz, J.A.; Lopez-Medellin, X. How does urbanization affect perceptions and traditional knowledge of medicinal plants? J. Ethnobiol. Ethnomed. 2021, 17, 48. [Google Scholar] [CrossRef]
- Kallman, M.; Bergstrom, S.; Holgersson, G.; Jaras, J.; Randen Engqvist, R.; Bergqvist, M. Regional Perspectives on Complementary and Alternative Medicine: Results of a Regional Survey. Complement. Med. Res. 2024, 31, 497–505. [Google Scholar] [CrossRef]
- Chaloupkova, P.; Petrtyl, M.; Verner, V.; Kokoska, L. Dietary supplements versus functional foods: Consumers’ attitudes to their consumption. Br. Food J. 2020, 122, 3853–3868. [Google Scholar] [CrossRef]
- Muck, F.; Scotti, F.; Mauvisseau, Q.; Thorbek, B.L.G.; Wangensteen, H.; de Boer, H.J. Three-tiered authentication of herbal traditional Chinese medicine ingredients used in women’s health provides progressive qualitative and quantitative insight. Front. Pharmacol. 2024, 15, 1353434. [Google Scholar] [CrossRef]
- Garcia-Alvarez, A.; Egan, B.; de Klein, S.; Dima, L.; Maggi, F.M.; Isoniemi, M.; Ribas-Barba, L.; Raats, M.M.; Meissner, E.M.; Badea, M.; et al. Usage of plant food supplements across six European countries: Findings from the PlantLIBRA consumer survey. PLoS ONE 2014, 9, e92265. [Google Scholar] [CrossRef]
- Willis, E.; Royne Stafford, M. Health consciousness or familiarity with supplement advertising. Int. J. Pharm. Healthc. Mark. 2016, 10, 130–147. [Google Scholar] [CrossRef]
- Escobar-Farfan, M.; Garcia-Salirrosas, E.E.; Guerra-Velasquez, M.; Veas-Gonzalez, I.; Gomez-Bayona, L.; Gallardo-Canales, R. Psychological Determinants of Healthy Food Purchase Intention: An Integrative Model Based on Health Consciousness. Nutrients 2025, 17, 1140. [Google Scholar] [CrossRef]
- Lee, E.; Jung, H.S.; Jang, J.A. Consumers’ perceptions of dietary supplements before and after the COVID-19 pandemic based on big data. J. Nutr. Health 2023, 56, 330–347. [Google Scholar]
- Rangel, J.M.L.; do Nascimento, A.L.B.; Ramos, M.A. The influence of urbanization on local ecological knowledge: A systematic review. J. Ethnobiol. Ethnomed. 2024, 20, 106. [Google Scholar] [CrossRef]
- Baker, M.T.; Lu, P.; Parrella, J.A.; Leggette, H.R. Consumer Acceptance toward Functional Foods: A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 1217. [Google Scholar] [CrossRef]
- Bhattacharjee, B.; Sandhanam, K.; Ghose, S.; Barman, D.; Sahu, R.K. Market overview of herbal medicines for lifestyle diseases. In Role of Herbal Medicines: Management of Lifestyle Diseases; Springer: Berlin/Heidelberg, Germany, 2024; pp. 597–614. [Google Scholar]
- Seo, Y.; Jin, C.; Cho, S.Y.; Park, S.U.; Jung, W.S.; Moon, S.K.; Park, J.M.; Ko, C.N.; Cho, K.H.; Kwon, S. Current Utilization and Research Status of Traditional East Asian Herbal Medicine Treatment for Multiple Sclerosis: A Scoping Review. Front. Neurol. 2021, 12, 710769. [Google Scholar] [CrossRef]
- Manuti, A.; Martiradonna, V.; Panniello, U.; Gorgoglione, M. To be or not to be confident in medicine: That’s the question! An explorative study on consumer information inferences toward food supplements consumption. Br. Food J. 2023, 125, 2931–2948. [Google Scholar] [CrossRef]
- El-Dahiyat, F.; Rashrash, M.; Abuhamdah, S.; Abu Farha, R.; Babar, Z.U. Herbal medicines: A cross-sectional study to evaluate the prevalence and predictors of use among Jordanian adults. J. Pharm. Policy Pract. 2020, 13, 2. [Google Scholar] [CrossRef]
- Galman, A.; Chikhaoui, M.; Bouhrim, M.; Eto, B.; Shahat, A.A.; Herqash, R.N.; Lotfi, R.; Belamgharia, H.; Daoudi, D.; Kaddouri, M.; et al. Fitness and Dietary Supplements: A Cross-Sectional Study on Food Practices and Nutrivigilance. Nutrients 2024, 16, 3928. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S. Introduction to Ethnobotany and Traditional Medicine. In Traditional Resources and Tools for Modern Drug Discovery: Ethnomedicine and Pharmacology; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–30. [Google Scholar]
- Mssusa, A.K.; Holst, L.; Maregesi, S.; Kagashe, G. Pharmacovigilance systems for safety monitoring of herbal medicines: Assessment of the national regulatory authority, manufacturers and marketing authorisation holders in Tanzania. J. Pharm. Policy Pract. 2025, 18, 2438223. [Google Scholar] [CrossRef]
- Schindler, C.; Heral, E.; Drinkwater, E.; Timoshyna, A.; Muir, G.; Walter, S.; Leaman, D.; Schippmann, U. Wildcheck–Assessing the Risks and Opportunities of Trade in Wild Plant Ingredients; Food & Agriculture Organization: Rome, Italy, 2022. [Google Scholar]
- Rocha-Miranda, F.; Venâncio, A. Mycotoxigenic fungi in plant-based supplements and medicines. Curr. Opin. Food Sci. 2019, 30, 27–31. [Google Scholar] [CrossRef]
- Raclariu, A.C.; Mocan, A.; Popa, M.O.; Vlase, L.; Ichim, M.C.; Crisan, G.; Brysting, A.K.; de Boer, H. Veronica officinalis Product Authentication Using DNA Metabarcoding and HPLC-MS Reveals Widespread Adulteration with Veronica chamaedrys. Front. Pharmacol. 2017, 8, 378. [Google Scholar] [CrossRef]
- Heller, T.; Kloos, C.; Mueller, N.; Roemelt, J.; Keinki, C.; Wolf, G.; Mueller, U.A.; Huebner, J. Complementary and alternative medicine is positively associated with religiousness/spirituality. J. Complement. Integr. Med. 2020, 18, 185–192. [Google Scholar] [CrossRef]
- Dafni, A.; Petanidou, T.; Vallianatou, I.; Kozhuharova, E.; Blanche, C.; Pacini, E.; Peyman, M.; Dajić Stevanovic, Z.; Franchi, G.G.; Benitez, G. Myrtle, basil, Rosemary, and three-lobed sage as ritual plants in the monotheistic religions: An historical–ethnobotanical comparison. Econ. Bot. 2020, 74, 330–355. [Google Scholar]
- Storl, W.-D. The Heart and Its Healing Plants: Traditional Herbal Remedies and Modern Heart Conditions; Simon and Schuster: New York, NY, USA, 2024. [Google Scholar]
- Siddiqui, S.; Khatoon, A.; Ahmad, K.; Upadhyay, S.; Srivastava, A.; Trivedi, A.; Husain, I.; Ahmad, R.; Ali Khan, M.; Arshad, M. Traditional Islamic Herbal Medicine and Complementary Therapies. In Complementary Therapies; Bernardo-Filho, M., Taiar, R., de Sá-Caputo, D.d.C., Seixas, A., Eds.; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar]
- Usmani, A.; Almoselhy, R.I. Current trends in Nigella sativa L.(Black seed) from traditional to modern medicine with advances in extraction, formulation, quality control, regulatory status, and pharmacology. Int. J. Pharm. Chem. Anal. 2024, 11, 11–24. [Google Scholar] [CrossRef]
- Dabeer, S.; Rather, M.A.; Rasool, S.; Rehman, M.U.; Alshahrani, S.; Jahan, S.; Rashid, H.; Halawi, M.; Khan, A. History and traditional uses of black seeds (Nigella sativa). In Black Seeds (Nigella sativa); Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–28. [Google Scholar]
- Abd Al Rahman, M. Content Marketing Is a Strategic Marketing Approach Focused on Creating and Distributing Valuable, Relevant, and Consistent Content to Attract and Retain a Clearly Defined Audience, Ultimately Aiming to Drive profitable Customer Action; University of Roehampton: London, UK, 2024. [Google Scholar]
- Mahmoodi-Eshkaftaki, M.; Mohtashami, S.; Parseh, M.J.; Ghani, A. Integrated of Hyperspectral Imaging and Machine Learning Algorithms for Nondestructive Detection of Therapeutic Properties of Plants. Chem. Biodivers. 2025, 22, e202401942. [Google Scholar] [CrossRef]
- Muela-Molina, C.; Perello-Oliver, S.; Garcia-Arranz, A. False and misleading health-related claims in food supplements on Spanish radio: An analysis from a European Regulatory Framework. Public. Health Nutr. 2021, 24, 5156–5165. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, D.; Tsekouropoulos, G.; Chatzigeorgiou, C.; Kokkinis, G. Empirical Categorization of Factors Affecting Online Consumer Behavior of Gen Z Regarding Newly Launched Technological Products and Moderating Impact of Perceived Risk. Behav. Sci. 2025, 15, 371. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, Y.K.; Ismagilova, E.; Hughes, D.L.; Carlson, J.; Filieri, R.; Jacobson, J.; Jain, V.; Karjaluoto, H.; Kefi, H.; Krishen, A.S.; et al. Setting the future of digital and social media marketing research: Perspectives and research propositions. Int. J. Inf. Manag. 2021, 59, 102168. [Google Scholar] [CrossRef]
- Czigle, S.; Toth, J.; Jedlinszki, N.; Haznagy-Radnai, E.; Csupor, D.; Tekelova, D. Ginkgo biloba Food Supplements on the European Market—Adulteration Patterns Revealed by Quality Control of Selected Samples. Planta Medica 2018, 84, 475–482. [Google Scholar] [CrossRef]
- Ng, J.Y.; Tahir, U.; Dhaliwal, S. Barriers, knowledge, and training related to pharmacists’ counselling on dietary and herbal supplements: A systematic review of qualitative studies. BMC Health Serv. Res. 2021, 21, 499. [Google Scholar] [CrossRef]
- Heinrich, M.; Sharma, S.K.; Suetterle, U.; Bhamra, S.K. Herbal medicine use in the UK and Germany and pharmacy practice—A commentary. Res. Social. Adm. Pharm. 2023, 19, 535–540. [Google Scholar] [CrossRef]
- Brunelli, L.; Arnoldo, L.; Mazzilis, G.; d’Angelo, M.; Colautti, L.; Cojutti, P.G.; Parpinel, M. The knowledge and attitudes of pharmacists related to the use of dietary supplements: An observational study in northeastern Italy. Prev. Med. Rep. 2022, 30, 101986. [Google Scholar] [CrossRef]
- Stayduhar, J.M.; Covvey, J.R.; Schreiber, J.B.; Witt-Enderby, P.A. Pharmacist and Student Knowledge and Perceptions of Herbal Supplements and Natural Products. Pharmacy 2023, 11, 96. [Google Scholar] [CrossRef]
- Innovation Union. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions; European Commission: Brussels, Belgium, 2014. [Google Scholar]
- Goulas, A.; Papachatzis, A. Farm to Fork Strategy: A sustainable solution for rural communities. In Annals of the University of Craiova, Biology, Horticulture, Food Products Processing Technology, Environmental Engineering; Universitatea din Craiova: Craiova, Romania, 2024; Volume 29. [Google Scholar]
- Kontele, I. Farm-to-Fork Strategy and other EU and WHO policies on sustainability. Public Health Toxicol. 2022, 2 (Suppl. S1), A12. [Google Scholar]
- Elshaer, I.A.; Azazz, A.M.; Hassan, S.S.; Fayyad, S. Farm-to-fork and sustainable agriculture practices: Perceived economic benefit as a moderator and environmental sustainability as a mediator. Sustainability 2023, 15, 11462. [Google Scholar]
- Marcelino, S.; Hamdane, S.; Gaspar, P.D.; Paço, A. Sustainable agricultural practices for the production of medicinal and aromatic plants: Evidence and recommendations. Sustainability 2023, 15, 14095. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines on Good Agricultural and Collection Practices [GACP] for Medicinal Plants; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Grigore, A.; Pirvu, L.; Bubueanu, C.; Colceru-Mihul, S.; Ionita, C.; Ionita, L. Medicinal plant crops-important source of high value-added products. Sci. Pap. Ser. A Agron. 2016, 59, 298–307. [Google Scholar]
- Petrescu-Mag, R.M.; Vermeir, I.; Roba, C.; Petrescu, D.C.; Bican-Brisan, N.; Martonos, I.M. Is “wild” a food quality attribute? Heavy metal content in wild and cultivated sea buckthorn and consumers’ risk perception. Int. J. Environ. Res. Public Health 2021, 18, 9463. [Google Scholar] [CrossRef] [PubMed]
- Lenssen, K.G.; Bast, A.; de Boer, A. International perspectives on substantiating the efficacy of herbal dietary supplements and herbal medicines through evidence on traditional use. Compr. Rev. Food Sci. Food Saf. 2019, 18, 910–922. [Google Scholar] [CrossRef]
- Cadar, R.L.; šedík, P.; Predanócyová, K.; Pocol, C.B. From Field to Consumer: A Comprehensive Analysis of Medicinal and Aromatic Plant Product Preferences Through Generations. Sci. Pap. Ser. Manag. Econ. Eng. Agric. Rural. Dev. 2024, 24, 229–238. [Google Scholar]
- Aftab, T.; Khan, M.M.A.; Naeem, M. Overexploitation and conservation strategies for medicinal and aromatic plants. In Essential Oil-Bearing Plants; Elsevier: Amsterdam, The Netherlands, 2025; pp. 95–105. [Google Scholar]
- Krochmal-Marczak, B.; Sawicka, B.; Barbaś, P.; Pszczółkowski, P.; Ziarati, P. Medicinal Plants: Uses, Cultivation, and Preservation. In Biotechnology and Phytochemical Prospects in Drug Discovery; Springer: Berlin/Heidelberg, Germany, 2025; pp. 7–81. [Google Scholar]
- Antosch, L.; Morgan, B. Trading FairWild. Traffic Bull. 2017, 29, 9–14. [Google Scholar]
- Brinckmann, J. In-Depth: The FairWild Standard-A Q&A with Josef Brinckmann. HerbalGram 2019, 123, 49. [Google Scholar]
- Tiradritti, M. The Environmental Impact Generated by a Natural Healthcare Product Measured Through the Life Cycle Assessment Approach; Sapienza Università di Roma: Rome, Italy, 2024. [Google Scholar]
- Alshirah, M.H.; Abdul Rahman, A.; Mustapa, I.R. Board of directors’ characteristics and corporate risk disclosure: The moderating role of family ownership. EuroMed J. Bus. 2020, 15, 219–252. [Google Scholar] [CrossRef]
- Stamboulakis, D.; Sanderson, J. Certifying biodiversity: The union for ethical biotrade and the search for ethical sourcing. J. Environ. Law 2020, 32, 503–528. [Google Scholar] [CrossRef]
- Sanderson, J.; Wiseman, L.; Stamboulakis, D. Certified ABS: The Union for Ethical Biotrade and the use of trade and certification marks to encourage and facilitate behaviour change. In Biodiversity, Genetic Resources and Intellectual Property; Routledge: London, UK, 2018; pp. 220–250. [Google Scholar]
- Shariatzadeh, M.; Bijani, M.; Bahadori, F. Sustainable agriculture and export development of Damask Rose products: New evidence from Iran’s situation. In Environment, Development and Sustainability; Springer: Berlin/Heidelberg, Germany, 2025; pp. 1–17. [Google Scholar]
- Takahashi, N. Rose (Rosa sp.) More Than Just Beautiful: Exploring the Therapeutic Properties of the Rose Species. In Advances in Medicinal and Aromatic Plants; Apple Academic Press: Burlington, ON, Canada, 2024; Volume 2, pp. 263–297. [Google Scholar]

| Botanical Name | AT | BE | BG | HR | CY | CZ | DK | EE | FI | FR | DE | GR | HU | IE | IT | LV | LT | MT | NL | PL | PT | RO | SK | SI | ES | SE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arnica montana | ✓ | – | – | – | – | – | – | ✓ | – | – | ✓ | – | – | – | – | – | – | ✓ | – | – | – | – | – | – | – | – |
| Melissa officinalis | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓ | ✓✓ | ✓✓ | ✓✓ | ✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ |
| Salvia officinalis | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Hypericum perforatum | ✓ | ✓✓ | ✓ | ✓ | – | ✓ | ✓ | ✓ | ✓ | ✓ | ✓✓ | ✓ | ✓ | ✓ | ✓✓ | ✓ | ✓ | ✓ | – | ✓✓ | ✓ | ✓ | ✓✓ | ✓ | ✓✓ | ✓✓ |
| Echinacea purpurea | ✓✓ | ✓✓ | ✓ | ✓✓ | ✓ | ✓✓ | ✓✓ | ✓ | ✓✓ | ✓✓ | ✓✓ | ✓ | ✓ | ✓✓ | ✓✓ | ✓ | ✓ | ✓✓ | ✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ |
| Thymus vulgaris | ✓✓ | ✓✓ | ✓✓ | ✓ | ✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓ | ✓✓ | ✓✓ | ✓✓ | ✓ | ✓ | ✓✓ | ✓ | ✓✓ | ✓✓ | ✓ | ✓✓ | ✓ | ✓✓ | ✓✓ |
| Urtica dioica | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | – | ✓ | – | ✓ | ✓ | – | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Matricaria chamomilla | ✓✓ | ✓✓ | ✓ | ✓ | ✓ | ✓✓ | ✓✓ | ✓ | ✓✓ | ✓ | ✓✓ | ✓ | ✓ | ✓ | ✓✓ | ✓ | ✓ | ✓✓ | ✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ |
| Gentiana lutea | ✓ | – | – | – | – | ✓ | – | – | – | ✓ | ✓ | – | – | – | ✓ | – | – | ✓ | – | ✓ | ✓ | – | ✓ | ✓ | ✓ | ✓ |
| Regulatory Aspect | FS | THMPs | Source |
|---|---|---|---|
| Regulatory Framework | Governed under Directive 2002/46/EC on food supplements and Regulation (EC) No 178/2002 (General Food Law). | Governed under Directive 2004/24/EC, amending Directive 2001/83/EC on medicinal products. | [10,104] |
| Proof of Use/Efficacy | No requirement for clinical or traditional-use evidence; must not claim to treat or prevent disease. | Requires at least 30 years of traditional use, including 15 years within the EU, demonstrating plausible efficacy based on long-standing use and experience. | [10,103,105] |
| Quality/CMC Requirements | Must comply with general food safety and hygiene legislation; limited testing for active compounds unless specified by EFSA or national law. | Must meet full CMC requirements consistent with the European Pharmacopoeia and EMA Guideline on Quality of Herbal Medicinal Products (Rev. 3, 2022). | [105,106] |
| Safety Assessment | Safety evaluation based on ingredient-level data (toxicology, contaminants, ADIs); no pre-market efficacy review. | Safety assessed through toxicological, pharmacological, and bibliographic data supporting traditional use; may require additional non-clinical data. | [106] |
| Claims Permitted | May only carry nutritional or functional claims approved under Regulation (EC) No 1924/2006; no therapeutic claims allowed. | Can make therapeutic indications consistent with traditional use (e.g., “for relief of mild stress”); claims must align with EMA Community Herbal Monographs. | [103] |
| Labeling Requirements | Must include dosage, list of ingredients, and warnings if relevant; claims must comply with EFSA approval. | Must display dosage, contraindications, side effects, and “Traditional herbal medicinal product for use in…” statement. | [103] |
| Pharmacovigilance | No formal EU-wide pharmacovigilance requirement; adverse event monitoring varies by Member State (voluntary or food-safety based). | Full pharmacovigilance obligations under Good Pharmacovigilance Practices (GVP), including ADR reporting to EudraVigilance. | [107,108] |
| Authorization/Notification | Simple notification procedure to national authorities (e.g., Italy’s Registro degli Integratori Alimentari, Poland’s GIS register). | Requires registration or marketing authorization with EMA or national competent authority following THMPD criteria. | [97,99] |
| Supervising Authority | National Food Safety Authorities or Ministries of Health. | National Medicines Agencies (e.g., BfArM, MHRA, AIFA) under EMA coordination. | [97,105,107,108] |
| Sourcing Practice | Description | Alignment with Farm to Fork Pillars |
|---|---|---|
| Ethical sourcing | Fair wages, equal opportunities, reinvestment in local communities; cooperatives over large-scale farming |
|
| Local sourcing | Site selection by ecological/geographic factors; traditional methods; supports biodiversity and local economy |
|
| Organic production | Minimized chemicals; use of compost, green manure, crop rotation; organic certification on labels |
|
| Sustainable harvesting | Quotas, seasonal restrictions, continuous monitoring to avoid over-exploitation |
|
| Circular economy valorization | Residues (e.g., rosemary, sage, basil, lavender) turned into compost, biopesticides, dietary fibers, bioactive compounds |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihai, M.; Ciont, C.; Pop, O.-L.; Dumitras, D.E.; Mihai, V.C.; Morariu, I.D.; Pocol, C.B. European Consumer and Regulatory Trends in Medicinal Plant Food Supplements and Their Functional Properties: The Road from Farm to Fork. Appl. Sci. 2025, 15, 11605. https://doi.org/10.3390/app152111605
Mihai M, Ciont C, Pop O-L, Dumitras DE, Mihai VC, Morariu ID, Pocol CB. European Consumer and Regulatory Trends in Medicinal Plant Food Supplements and Their Functional Properties: The Road from Farm to Fork. Applied Sciences. 2025; 15(21):11605. https://doi.org/10.3390/app152111605
Chicago/Turabian StyleMihai, Mihaela, Călina Ciont, Oana-Lelia Pop, Diana E. Dumitras, Valentin C. Mihai, Ionela Daniela Morariu, and Cristina Bianca Pocol. 2025. "European Consumer and Regulatory Trends in Medicinal Plant Food Supplements and Their Functional Properties: The Road from Farm to Fork" Applied Sciences 15, no. 21: 11605. https://doi.org/10.3390/app152111605
APA StyleMihai, M., Ciont, C., Pop, O.-L., Dumitras, D. E., Mihai, V. C., Morariu, I. D., & Pocol, C. B. (2025). European Consumer and Regulatory Trends in Medicinal Plant Food Supplements and Their Functional Properties: The Road from Farm to Fork. Applied Sciences, 15(21), 11605. https://doi.org/10.3390/app152111605









