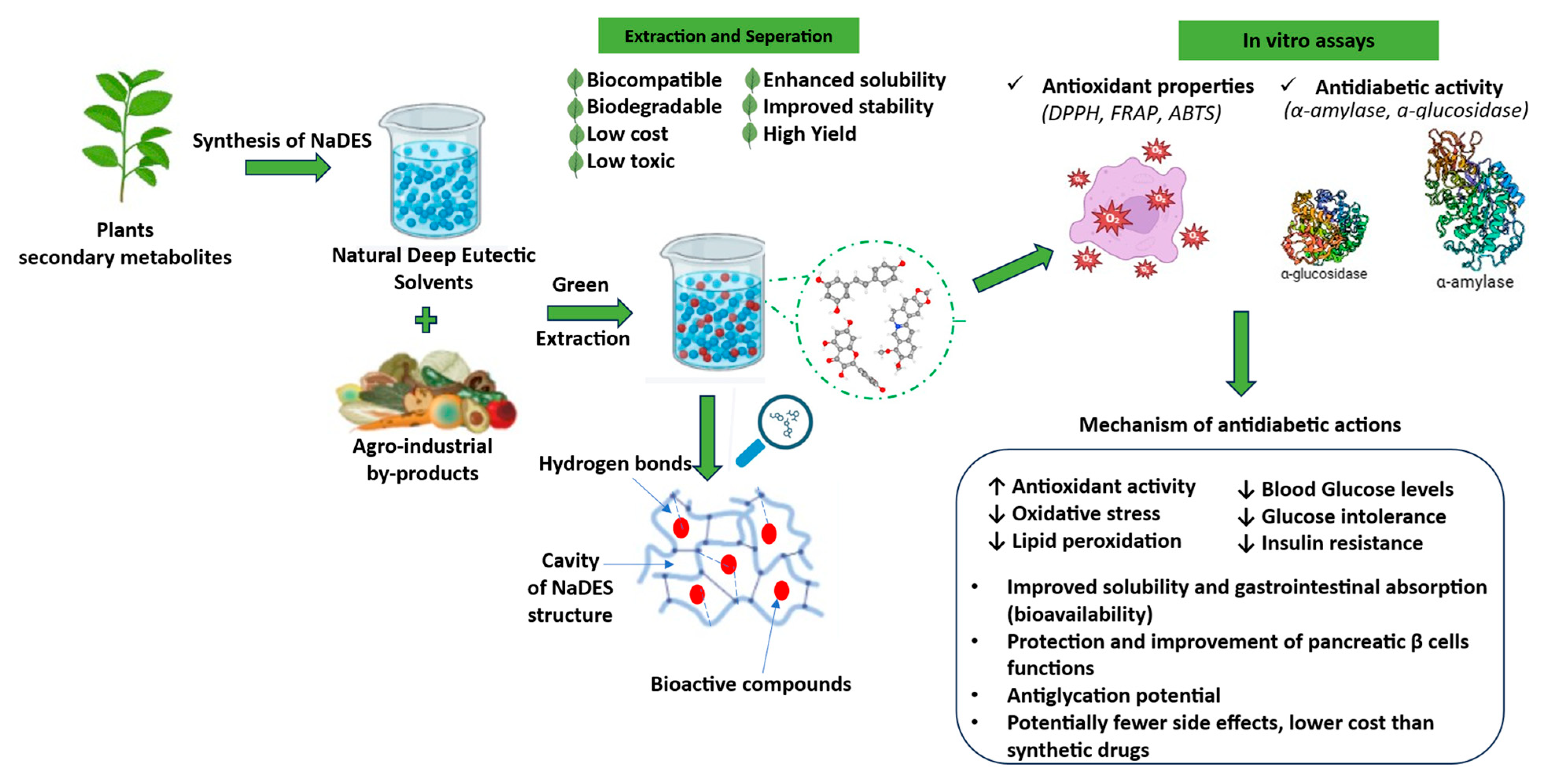
Natural Deep Eutectic Solvents for Agro-Industrial By-Product Valorization: Emerging Strategies for the Development of Functional Foods Targeting Diabetes
Abstract
1. Introduction
2. Natural Deep Eutectic Solvents: Characteristics and Properties
2.1. Solubility and Stability Considerations of Plant-Derived Bioactive Compounds
2.2. The Role of the Water Content in Natural Deep Eutectic Solvents
3. Extraction of Bioactive Compounds from Food and Agro-Industrial Waste Using Natural Deep Eutectic Solvents
3.1. Phytochemicals
3.1.1. Polyphenols
3.1.2. Flavonoids
3.1.3. Anthocyanins
3.1.4. Carotenoids
3.2. Proteins
3.3. Polysaccharides
4. NADES-Based Extracts in the Management of Diabetes Mellitus (DM)
5. Mechanistic Perspectives on Bioactive Compound Extraction
6. Limitations and Challenges
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, M.; Fakayode, O.A.; Li, H. Green Extraction of Polyphenols via Deep Eutectic Solvents and Assisted Technologies from Agri-Food By-Products. Molecules 2023, 28, 6852. [Google Scholar] [CrossRef]
- Bairaktari, M. Reduction Food Loss and Waste: The Role of Deep Eutectic Solvents (DESs). Ann. Chem. Sci. Res. 2024, 4. [Google Scholar] [CrossRef]
- Procentese, A.; Raganati, F.; Olivieri, G.; Russo, M.E.; Rehmann, L.; Marzocchella, A. Deep Eutectic Solvents Pretreatment of Agro-Industrial Food Waste. Biotechnol. Biofuels 2018, 11, 37. [Google Scholar] [CrossRef]
- Molnar, M.; Gašo-Sokač, D.; Komar, M.; Jakovljević Kovač, M.; Bušić, V. Potential of Deep Eutectic Solvents in the Extraction of Organic Compounds from Food Industry By-Products and Agro-Industrial Waste. Separations 2024, 11, 35. [Google Scholar] [CrossRef]
- de los Ángeles Fernández, M.; Espino, M.; Gomez, F.J.V.; Silva, M.F. Novel Approaches Mediated by Tailor-Made Green Solvents for the Extraction of Phenolic Compounds from Agro-Food Industrial by-Products. Food Chem. 2018, 239, 671–678. [Google Scholar] [CrossRef]
- Murador, D.C.; de Souza Mesquita, L.M.; Vannuchi, N.; Braga, A.R.C.; de Rosso, V.V. Bioavailability and Biological Effects of Bioactive Compounds Extracted with Natural Deep Eutectic Solvents and Ionic Liquids: Advantages over Conventional Organic Solvents. Curr. Opin. Food Sci. 2019, 26, 25–34. [Google Scholar] [CrossRef]
- Negi, T.; Kumar, A.; Sharma, S.K.; Rawat, N.; Saini, D.; Sirohi, R.; Prakash, O.; Dubey, A.; Dutta, A.; Shahi, N.C. Deep Eutectic Solvents: Preparation, Properties, and Food Applications. Heliyon 2024, 10, e28784. [Google Scholar] [CrossRef]
- El Achkar, T.; Greige-Gerges, H.; Fourmentin, S. Basics and Properties of Deep Eutectic Solvents: A Review. Environ. Chem. Lett. 2021, 19, 3397–3408. [Google Scholar] [CrossRef]
- Sánchez, P.B.; González, B.; Salgado, J.; José Parajó, J.; Domínguez, Á. Physical Properties of Seven Deep Eutectic Solvents Based on L-Proline or Betaine. J. Chem. Thermodyn. 2019, 131, 517–523. [Google Scholar] [CrossRef]
- Kivelä, H.; Salomäki, M.; Vainikka, P.; Mäkilä, E.; Poletti, F.; Ruggeri, S.; Terzi, F.; Lukkari, J. Effect of Water on a Hydrophobic Deep Eutectic Solvent. J. Phys. Chem. B 2022, 126, 513–527. [Google Scholar] [CrossRef]
- Liang, X.; Huang, Z.; Zhang, J.; Guo, Y. Ionic Liquid Recovery and Recycling via Electrodialysis in Biomass Processing: An Economical Assessment. Biores. Technol. 2023, 384, 129332. [Google Scholar] [CrossRef] [PubMed]
- Kalhor, P.; Ghandi, K. Deep Eutectic Solvents for Pretreatment, Extraction, and Catalysis of Biomass and Food Waste. Molecules 2019, 24, 4012. [Google Scholar] [CrossRef]
- Tham, P.E.; Ng, Y.J.; Sankaran, R.; Khoo, K.S.; Chew, K.W.; Yap, Y.J.; Malahubban, M.; Zakry, F.A.A.; Show, P.L. Recovery of Protein from Dairy Milk Waste Product Using Alcohol-Salt Liquid Biphasic Flotation. Processes 2019, 7, 875. [Google Scholar] [CrossRef]
- Emami, S.; Shayanfar, A. Deep Eutectic Solvents for Pharmaceutical Formulation and Drug Delivery Applications. Pharm. Dev. Technol. 2020, 25, 779–796. [Google Scholar] [CrossRef]
- Ferreira, C.; Sarraguça, M. A Comprehensive Review on Deep Eutectic Solvents and Its Use to Extract Bioactive Compounds of Pharmaceutical Interest. Pharmaceuticals 2024, 17, 124. [Google Scholar] [CrossRef]
- Santos-Martín, M.; Cubero-Cardoso, J.; González-Domínguez, R.; Cortés-Triviño, E.; Sayago, A.; Urbano, J.; Fernández-Recamales, Á. Ultrasound-Assisted Extraction of Phenolic Compounds from Blueberry Leaves Using Natural Deep Eutectic Solvents (NADES) for the Valorization of Agrifood Wastes. Biomass Bioenergy 2023, 175, 106882. [Google Scholar] [CrossRef]
- Silva, M.D.O.; Honfoga, J.N.B.; de Medeiros, L.L.; Madruga, M.S.; Bezerra, T.K.A. Obtaining Bioactive Compounds from the Coffee Husk (Coffea arabica L.) Using Different Extraction Methods. Molecules 2020, 26, 46. [Google Scholar] [CrossRef]
- Morgana, N.M.; Magdalena, E.; de los Angeles Fernandez, M.; Fernanda, S.M. NADES for Food Industry Innovation: Novel Bioadditives Based on Olive Oil Byproducts. Food Bioprod. Process. 2022, 134, 193–201. [Google Scholar] [CrossRef]
- Benítez-Correa, E.; Bastías-Montes, J.M.; Acuña-Nelson, S.; Muñoz-Fariña, O. Effect of Choline Chloride-Based Deep Eutectic Solvents on Polyphenols Extraction from Cocoa (Theobroma cacao L.) Bean Shells and Antioxidant Activity of Extracts. Curr. Res. Food Sci. 2023, 7, 100614. [Google Scholar] [CrossRef]
- Frontini, A.; Luvisi, A.; Negro, C.; Apollonio, M.; Accogli, R.; De Pascali, M.; De Bellis, L. Polyphenols Extraction from Different Grape Pomaces Using Natural Deep Eutectic Solvents. Separations 2024, 11, 241. [Google Scholar] [CrossRef]
- Panić, M.; Damjanović, A.; Radošević, K.; Cvjetko Bubalo, M.; Dujmić, F.; Škegro, M.; Radojčić Redovniković, I.; Brnčić, M. Enhanced Preparative-Scale Extraction from Graševina Grape Pomace Using Ultrasound-Assisted Extraction and Natural Deep Eutectic Solvents. Appl. Sci. 2024, 14, 6185. [Google Scholar] [CrossRef]
- Turan, O.; Isci, A.; Yılmaz, M.S.; Tolun, A.; Sakiyan, O. Microwave-Assisted Extraction of Pectin from Orange Peel Using Deep Eutectic Solvents. Sustain. Chem. Pharm. 2024, 37, 101352. [Google Scholar] [CrossRef]
- Zhang, S.; Niu, L.; Si, X.; Li, L.; Sheng, Z. Microwave-Assisted Extraction of Luteolin from Peanut Shells Using Natural Deep Eutectic Solvents and Its Molecular Mechanism. Ind. Crops Prod. 2025, 225, 120578. [Google Scholar] [CrossRef]
- Vo, T.P.; Tran, T.Q.D.; Vo, L.T.V.; Nguyen, T.H.P.; Nguyen, V.K.; Dang, T.C.T.; Nguyen, L.G.K. Optimization of Ultrasonic-Assisted Extraction to Attain Tannins and Flavonoids from Spent Tea Leaves Using Natural Deep Eutectic Solvents. Chem. Eng. Commun. 2024, 211, 974–985. [Google Scholar] [CrossRef]
- Sun, P.; Yang, W.; Sun, T.; Tang, Y.; Li, M.; Cheng, S.; Chen, G. Effects of Ultrasonic-Assisted Natural Deep Eutectic Solvent on the Extraction Rate, Stability and Antifungal Ability of Polyphenols from Cabernet Sauvignon Seeds. Food Res. Int. 2024, 191, 114674. [Google Scholar] [CrossRef]
- Benvenutti, L.; Zielinski, A.A.F.; Ferreira, S.R.S. Subcritical Water Extraction (SWE) Modified by Deep Eutectic Solvent (DES) for Pectin Recovery from a Brazilian Berry by-Product. J. Supercrit. Fluids 2022, 189, 105729. [Google Scholar] [CrossRef]
- Benvenutti, L.; Zielinski, A.A.F.; Ferreira, S.R.S. Pressurized Aqueous Solutions of Deep Eutectic Solvent (DES): A Green Emergent Extraction of Anthocyanins from a Brazilian Berry Processing by-Product. Food Chem. X 2022, 13, 100236. [Google Scholar] [CrossRef]
- Bairaktari, M. Deep Eutectic Solvents (DESs) in Waste Reduction, By-Product Valorization, and Advancing the Circular Economy. Sustain. Chem. Eng. 2025, 218–228. [Google Scholar] [CrossRef]
- Dias, M.; Romaní-Pérez, M.; Romaní, A.; de la Cruz, A.; Pastrana, L.; Fuciños, P.; Amado, I.R. Recent Technological Advances in Phenolic Compounds Recovery and Applications: Source of Nutraceuticals for the Management of Diabetes. Appl. Sci. 2022, 12, 9271. [Google Scholar] [CrossRef]
- El Ghouizi, A.; Ousaaid, D.; Laaroussi, H.; Bakour, M.; Aboulghazi, A.; Soutien, R.S.; Hano, C.; Lyoussi, B. Ficus carica (Linn.) Leaf and Bud Extracts and Their Combination Attenuates Type-1 Diabetes and Its Complications via the Inhibition of Oxidative Stress. Foods 2023, 12, 759. [Google Scholar] [CrossRef]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep Eutectic Solvents: Syntheses, Properties and Applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- Socas-Rodríguez, B.; Torres-Cornejo, M.V.; Álvarez-Rivera, G.; Mendiola, J.A. Deep Eutectic Solvents for the Extraction of Bioactive Compounds from Natural Sources and Agricultural By-Products. Appl. Sci. 2021, 11, 4897. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef]
- Panzella, L.; Moccia, F.; Nasti, R.; Marzorati, S.; Verotta, L.; Napolitano, A. Bioactive Phenolic Compounds from Agri-Food Wastes: An Update on Green and Sustainable Extraction Methodologies. Front. Nutr. 2020, 7, 60. [Google Scholar] [CrossRef]
- Cysewski, P.; Jeliński, T. Eutectic Solvents. Crystals 2021, reprint. [Google Scholar] [CrossRef]
- Lu, W.; Liu, S. Choline Chloride–Based Deep Eutectic Solvents (Ch-DESs) as Promising Green Solvents for Phenolic Compounds Extraction from Bioresources: State-of-the-Art, Prospects, and Challenges. Biomass Convers. Biorefin. 2022, 12, 2949–2962. [Google Scholar] [CrossRef]
- Benvenutti, L.; Zielinski, A.A.F.; Ferreira, S.R.S. Which Is the Best Food Emerging Solvent: IL, DES or NADES? Trends Food Sci. Technol. 2019, 90, 133–146. [Google Scholar] [CrossRef]
- Palos-Hernández, A.; Gutiérrez Fernández, M.Y.; Escuadra Burrieza, J.; Pérez-Iglesias, J.L.; González-Paramás, A.M. Obtaining Green Extracts Rich in Phenolic Compounds from Underexploited Food By-Products Using Natural Deep Eutectic Solvents. Opportunities and Challenges. Sustain. Chem. Pharm. 2022, 29, 100773. [Google Scholar] [CrossRef]
- Martínez, R.; Berbegal, L.; Guillena, G.; Ramón, D.J. Natural Deep Eutectic Solvents. Green Chem. 2016, 18, 1724–1730. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents—Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.C.E.; Witkamp, G.J.; Verpoorte, R. Are Natural Deep Eutectic Solvents the Missing Link in Understanding Cellular Metabolism and Physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef]
- Dai, Y.; Varypataki, E.M.; Golovina, E.A.; Jiskoot, W.; Witkamp, G.J.; Choi, Y.H.; Verpoorte, R. Natural Deep Eutectic Solvents in Plants and Plant Cells: In Vitro Evidence for Their Possible Functions. Adv. Bot. Res. 2021, 97, 159–184. [Google Scholar] [CrossRef]
- Kovács, A.; Neyts, E.C.; Cornet, I.; Wijnants, M.; Billen, P. Modeling the Physicochemical Properties of Natural Deep Eutectic Solvents. ChemSusChem 2020, 13, 3789–3804. [Google Scholar] [CrossRef]
- Wawoczny, A.; Gillner, D. The Most Potent Natural Pharmaceuticals, Cosmetics, and Food Ingredients Isolated from Plants with Deep Eutectic Solvents. J. Agric. Food Chem. 2023, 71, 10877–10900. [Google Scholar] [CrossRef]
- Ristivojević, P.; Krstić Ristivojević, M.; Stanković, D.; Cvijetić, I. Advances in Extracting Bioactive Compounds from Food and Agricultural Waste and By-Products Using Natural Deep Eutectic Solvents: A Circular Economy Perspective. Molecules 2024, 29, 4717. [Google Scholar] [CrossRef]
- Aroso, I.M.; Paiva, A.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents from Choline Chloride and Betaine—Physicochemical Properties. J. Mol. Liq. 2017, 241, 654–661. [Google Scholar] [CrossRef]
- Hikmawanti, N.P.E.; Ramadon, D.; Jantan, I.; Mun’im, A. Natural Deep Eutectic Solvents (Nades): Phytochemical Extraction Performance Enhancer for Pharmaceutical and Nutraceutical Product Development. Plants 2021, 10, 2091. [Google Scholar] [CrossRef]
- Jeliński, T.; Przybyłek, M.; Cysewski, P. Natural Deep Eutectic Solvents as Agents for Improving Solubility, Stability and Delivery of Curcumin. Pharm. Res. 2019, 36, 116. [Google Scholar] [CrossRef]
- Mustafa, N.R.; Spelbos, V.S.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Solubility and Stability of Some Pharmaceuticals in Natural Deep Eutectic Solvents-Based Formulations. Molecules 2021, 26, 2645. [Google Scholar] [CrossRef]
- Cao, J.; Cao, J.; Wang, H.; Chen, L.; Cao, F.; Su, E. Solubility Improvement of Phytochemicals Using (Natural) Deep Eutectic Solvents and Their Bioactivity Evaluation. J. Mol. Liq. 2020, 318, 113997. [Google Scholar] [CrossRef]
- Popović, B.M.; Uka, D.; Boublia, A.; Agić, D.; Kukrić, T.; Albrahim, M.; Elboughdiri, N.; Benguerba, Y. Solubility and Extractability Enhancement of the Main Food Flavonoids by Using Choline Chloride-Based Natural Deep Eutectic Solvents. J. Mol. Liq. 2024, 415, 126333. [Google Scholar] [CrossRef]
- Torres-Vega, J.; Gómez-Alonso, S.; Pastene-Navarrete, E.; Pérez-Navarro, J. Green Extraction of Alkaloids and Polyphenols from Peumus Boldus Leaves with Natural Deep Eutectic Solvents and Profiling by HPLC-PDA-IT-MS/MS and HPLC-QTOF-MS/MS. Plants 2020, 9, 242. [Google Scholar] [CrossRef]
- Amphlett, J.T.M.; Choi, S. The Effect of Increasing Water Content on Transition Metal Speciation in Deep Eutectic Solvents. J. Mol. Liq. 2021, 332, 115845. [Google Scholar] [CrossRef]
- Jančíková, V.; Jablonský, M.; Voleková, K.; Šurina, I. Summarizing the Effect of Acidity and Water Content of Deep Eutectic Solvent-like Mixtures—A Review. Energies 2022, 15, 9333. [Google Scholar] [CrossRef]
- Mero, A.; Moody, N.R.; Husanu, E.; Mezzetta, A.; D’Andrea, F.; Pomelli, C.S.; Bernaert, N.; Paradisi, F.; Guazzelli, L. Challenging DESs and ILs in the Valorization of Food Waste: A Case Study. Front. Chem. 2023, 11, 1270221. [Google Scholar] [CrossRef]
- Lazzarini, C.; Casadei, E.; Valli, E.; Tura, M.; Ragni, L.; Bendini, A.; Toschi, T.G. Sustainable Drying and Green Deep Eutectic Extraction of Carotenoids from Tomato Pomace. Foods 2022, 11, 405. [Google Scholar] [CrossRef]
- Lanjekar, K.J.; Gokhale, S.; Rathod, V.K. Utilization of Waste Mango Peels for Extraction of Polyphenolic Antioxidants by Ultrasound-Assisted Natural Deep Eutectic Solvent. Biores. Technol. Rep. 2022, 18, 101074. [Google Scholar] [CrossRef]
- Singh, N.; Panwar, D.; Kumar, G.; Kashyap, P. New Horizons for the Enhanced Recovery of Phenolic Compounds by Integration of Natural Deep Eutectic Solvents and Microwave-Assisted Extraction. Food Biosci. 2024, 60, 104375. [Google Scholar] [CrossRef]
- Pojić, M.; Teslić, N.; Banjac, V.; Mrkonjić, Ž.; Stupar, A.; Mandić, A.; Mišan, A.; Pavlić, B. Natural Deep Eutectic Solvents for Efficient Recovery of Bioactive Compounds from By-Product of Industrial Hemp Processing: Pretreatment, Modeling and Optimization. Ind. Crops Prod. 2024, 222, 119617. [Google Scholar] [CrossRef]
- Mavai, S.; Bains, A.; Sridhar, K.; Chawla, P.; Sharma, M. Emerging Deep Eutectic Solvents for Food Waste Valorization to Achieve Sustainable Development Goals: Bioactive Extractions and Food Applications. Food Chem. 2025, 462, 141000. [Google Scholar] [CrossRef]
- Siamandoura, P.; Tzia, C. Comparative Study of Novel Methods for Olive Leaf Phenolic Compound Extraction Using NADES as Solvents. Molecules 2023, 28, 353. [Google Scholar] [CrossRef]
- Chaves, J.O.; de Souza Mesquita, L.M.; Strieder, M.M.; Contieri, L.S.; Pizani, R.S.; Sanches, V.L.; Viganó, J.; Neves Bezerra, R.M.; Rostagno, M.A. Eco-Friendly and High-Performance Extraction of Flavonoids from Lemon Peel Wastes by Applying Ultrasound-Assisted Extraction and Eutectic Solvents. Sustain. Chem. Pharm. 2024, 39, 101558. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Bhattacharya, D.; Pati, S.; Sarkar, T. (Eds.) Bioactive Ingredients for Healthcare Industry Volume 2; Springer Nature: Singapore, 2025. [Google Scholar] [CrossRef]
- Wang, T.; Jiao, J.; Gai, Q.Y.; Wang, P.; Guo, N.; Niu, L.L.; Fu, Y.J. Enhanced and Green Extraction Polyphenols and Furanocoumarins from Fig (Ficus carica L.) Leaves Using Deep Eutectic Solvents. J. Pharm. Biomed. Anal. 2017, 145, 339–345. [Google Scholar] [CrossRef]
- Saini, A.; Kumar, A.; Panesar, P.S.; Thakur, A. Potential of Deep Eutectic Solvents in the Extraction of Value-added Compounds from Agro-industrial By-products. Appl. Food Res. 2022, 2, 100211. [Google Scholar] [CrossRef]
- Aryal, D.; Joshi, S.; Thapa, N.K.; Chaudhary, P.; Basaula, S.; Joshi, U.; Bhandari, D.; Rogers, H.M.; Bhattarai, S.; Sharma, K.R.; et al. Dietary Phenolic Compounds as Promising Therapeutic Agents for Diabetes and Its Complications: A Comprehensive Review. Food Sci. Nutr. 2024, 12, 3025–3045. [Google Scholar] [CrossRef]
- Cao, Y.; Song, Z.; Dong, C.; Ni, W.; Xin, K.; Yu, Q.; Han, L. Green Ultrasound-Assisted Natural Deep Eutectic Solvent Extraction of Phenolic Compounds from Waste Broccoli Leaves: Optimization, Identification, Biological Activity, and Structural Characterization. LWT 2023, 190, 115407. [Google Scholar] [CrossRef]
- Wang, B.; Chen, P.; Zhang, H.; Chen, Y.; Chen, L. Optimization of Polyphenols Extraction by Deep Eutectic Solvent from Broccoli Stem and Characterization of Their Composition and Antioxidative Effects. Sci. Rep. 2025, 15, 16066. [Google Scholar] [CrossRef]
- Lee, S.Y.; Liang, Y.N.; Stuckey, D.C.; Hu, X. Single-Step Extraction of Bioactive Compounds from Cruciferous Vegetable (Kale) Waste Using Natural Deep Eutectic Solvents. Sep. Purif. Technol. 2023, 317, 123677. [Google Scholar] [CrossRef]
- de Lima, N.D.; da Silva Monteiro Wanderley, B.R.; Andrade Ferreira, A.L.; Pereira-Coelho, M.; da Silva Haas, I.C.; Vitali, L.; dos Santos Madureira, L.A.; Müller, J.M.; Fritzen-Freire, C.B.; de Mello Castanho Amboni, R.D. Green Extraction of Phenolic Compounds from the By-Product of Purple Araçá (Psidium myrtoides) with Natural Deep Eutectic Solvents Assisted by Ultrasound: Optimization, Comparison, and Bioactivity. Food Res. Int. 2024, 191, 114731. [Google Scholar] [CrossRef]
- Rashid, R.; Mohd Wani, S.; Manzoor, S.; Masoodi, F.A.; Masarat Dar, M. Green Extraction of Bioactive Compounds from Apple Pomace by Ultrasound Assisted Natural Deep Eutectic Solvent Extraction: Optimisation, Comparison and Bioactivity. Food Chem. 2023, 398, 133871. [Google Scholar] [CrossRef]
- Han, M.; Hou, M.; Gao, Z. Extraction of Apple Pomace Polyphenols Using Natural Deep Eutectic Solvents: A Sustainable Approach. Food Biosci. 2024, 62, 105083. [Google Scholar] [CrossRef]
- Gómez-Urios, C.; Viñas-Ospino, A.; Puchades-Colera, P.; López-Malo, D.; Frígola, A.; Esteve, M.J.; Blesa, J. Sustainable Development and Storage Stability of Orange By-Products Extract Using Natural Deep Eutectic Solvents. Foods 2022, 11, 2457. [Google Scholar] [CrossRef]
- Viñas-Ospino, A.; Panić, M.; Bagović, M.; Radošević, K.; Esteve, M.J.; Radojčić Redovniković, I. Green Approach to Extract Bioactive Compounds from Orange Peel Employing Hydrophilic and Hydrophobic Deep Eutectic Solvents. Sustain. Chem. Pharm. 2023, 31, 100942. [Google Scholar] [CrossRef]
- de Oliveira, J.A.R.; de Paula Menezes Barbosa, P.; Macêdo, G.A. High Concentrate Flavonoids Extract from Citrus Pomace Using Enzymatic and Deep Eutectic Solvents Extraction. Foods 2022, 11, 3205. [Google Scholar] [CrossRef]
- Shilpa, V.S.; Shams, R.; Dash, K.K.; Pandey, V.K.; Dar, A.H.; Ayaz Mukarram, S.; Harsányi, E.; Kovács, B. Phytochemical Properties, Extraction, and Pharmacological Benefits of Naringin: A Review. Molecules 2023, 28, 5623. [Google Scholar] [CrossRef]
- Ojeda, G.A.; Vallejos, M.M.; Sgroppo, S.C.; Sánchez-Moreno, C.; de Ancos, B. Enhanced Extraction of Phenolic Compounds from Mango By-Products Using Deep Eutectic Solvents. Heliyon 2023, 9, e16912. [Google Scholar] [CrossRef]
- Della Posta, S.; Cutè, E.; De Gara, L.; Fanali, C. Deep Eutectic Solvents Based Green Approach for Bioactive Molecules Recovery from Spirulina. J. Chromatogr. A 2025, 1743, 465695. [Google Scholar] [CrossRef]
- Bener, M.; Şen, F.B.; Önem, A.N.; Bekdeşer, B.; Çelik, S.E.; Lalikoglu, M.; Aşçı, Y.S.; Capanoglu, E.; Apak, R. Microwave-Assisted Extraction of Antioxidant Compounds from by-Products of Turkish Hazelnut (Corylus avellana L.) Using Natural Deep Eutectic Solvents: Modeling, Optimization and Phenolic Characterization. Food Chem. 2022, 385, 132633. [Google Scholar] [CrossRef]
- Cerdá-Bernad, D.; Pitterou, I.; Tzani, A.; Detsi, A.; Frutos, M.J. Novel Chitosan/Alginate Hydrogels as Carriers of Phenolic-Enriched Extracts from Saffron Floral by-Products Using Natural Deep Eutectic Solvents as Green Extraction Media. Curr. Res. Food Sci. 2023, 6, 100469. [Google Scholar] [CrossRef]
- Zurob, E.; Cabezas, R.; Villarroel, E.; Rosas, N.; Merlet, G.; Quijada-Maldonado, E.; Romero, J.; Plaza, A. Design of Natural Deep Eutectic Solvents for the Ultrasound-Assisted Extraction of Hydroxytyrosol from Olive Leaves Supported by COSMO-RS. Sep. Purif. Technol. 2020, 248, 117054. [Google Scholar] [CrossRef]
- de Almeida Pontes, P.V.; Ayumi Shiwaku, I.; Maximo, G.J.; Caldas Batista, E.A. Choline Chloride-Based Deep Eutectic Solvents as Potential Solvent for Extraction of Phenolic Compounds from Olive Leaves: Extraction Optimization and Solvent Characterization. Food Chem. 2021, 352, 129346. [Google Scholar] [CrossRef]
- de Almeida Pontes, P.V.; Czaikoski, A.; Almeida, N.A.; Fraga, S.; Rocha, L.d.O.; Cunha, R.L.; Maximo, G.J.; Batista, E.A.C. Extraction Optimization, Biological Activities, and Application in O/W Emulsion of Deep Eutectic Solvents-Based Phenolic Extracts from Olive Pomace. Food Res. Int. 2022, 161, 111753. [Google Scholar] [CrossRef]
- Carmona, I.; Aguirre, I.; Griffith, D.M.; García-Borrego, A. Towards a Circular Economy in Virgin Olive Oil Production: Valorization of the Olive Mill Waste (OMW) “Alpeorujo” through Polyphenol Recovery with Natural Deep Eutectic Solvents (NADESs) and Vermicomposting. Sci. Total Environ. 2023, 872, 162198. [Google Scholar] [CrossRef]
- Maimulyanti, A.; Nurhidayati, I.; Mellisani, B.; Amelia Rachmawati Putri, F.; Puspita, F.; Restu Prihadi, A. Development of Natural Deep Eutectic Solvent (NADES) Based on Choline Chloride as a Green Solvent to Extract Phenolic Compound from Coffee Husk Waste. Arab. J. Chem. 2023, 16, 104634. [Google Scholar] [CrossRef]
- Tzani, A.; Lymperopoulou, T.; Pitterou, I.; Karetta, I.; Belfquih, F.; Detsi, A. Development and Optimization of Green Extraction Process of Spent Coffee Grounds Using Natural Deep Eutectic Solvents. Sustain. Chem. Pharm. 2023, 34, 101144. [Google Scholar] [CrossRef]
- Bassani, A.; García-Roldán, A.; Spigno, G.; Jauregi, P. Extraction of Phenolic Compounds from Spent Coffee Ground Using Natural Deep Eutectic Solvents: New Modeling Approach. J. Food Process. Eng. 2024, 47, e14584. [Google Scholar] [CrossRef]
- García-Roldán, A.; Piriou, L.; Jauregi, P. Natural Deep Eutectic Solvents as a Green Extraction of Polyphenols from Spent Coffee Ground with Enhanced Bioactivities. Front. Plant Sci. 2023, 13, 1072592. [Google Scholar] [CrossRef]
- Loarce, L.; Oliver-Simancas, R.; Marchante, L.; Díaz-Maroto, M.C.; Alañón, M.E. Implementation of Subcritical Water Extraction with Natural Deep Eutectic Solvents for Sustainable Extraction of Phenolic Compounds from Winemaking By-Products. Food Res. Int. 2020, 137, 109728. [Google Scholar] [CrossRef]
- Moni Bottu, H.; Mero, A.; Husanu, E.; Tavernier, S.; Pomelli, C.S.; Dewaele, A.; Bernaert, N.; Guazzelli, L.; Brennan, L. The Ability of Deep Eutectic Solvent Systems to Extract Bioactive Compounds from Apple Pomace. Food Chem. 2022, 386, 132717. [Google Scholar] [CrossRef]
- Nunes da Silva, C.; Pina de Almeida, L.M.; Lemes, A.C.; Ribeiro, B.D. Citrus By-Products Valorization Using Deep Eutectic Solvents—A Review. Food Biosci. 2024, 60, 104446. [Google Scholar] [CrossRef]
- Ramírez-Sucre, M.O.; Avilés-Betanzos, K.A.; López-Martínez, A.; Rodríguez-Buenfil, I.M. Evaluation of Polyphenol Profile from Citrus Peel Obtained by Natural Deep Eutectic Solvent/Ultrasound Extraction. Processes 2024, 12, 2072. [Google Scholar] [CrossRef]
- Yang, Y.; Trevethan, M.; Wang, S.; Zhao, L. Beneficial Effects of Citrus Flavanones Naringin and Naringenin and Their Food Sources on Lipid Metabolism: An Update on Bioavailability, Pharmacokinetics, and Mechanisms. J. Nutr. Biochem. 2022, 104, 108967. [Google Scholar] [CrossRef]
- Tzani, A.; Kalafateli, S.; Tatsis, G.; Bairaktari, M.; Kostopoulou, I.; Pontillo, A.R.N.; Detsi, A. Natural Deep Eutectic Solvents (NaDESs) as Alternative Green Extraction Media for Ginger (Zingiber officinale Roscoe). Sustain. Chem. 2021, 2, 576–599. [Google Scholar] [CrossRef]
- Al-Ishaq, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Büsselberg, D. Flavonoids and Their Anti-Diabetic Effects: Cellular Mechanisms and Effects to Improve Blood Sugar Levels. Biomolecules 2019, 9, 430. [Google Scholar] [CrossRef]
- Bouyahya, A.; Balahbib, A.; Khalid, A.; Makeen, H.A.; Alhazmi, H.A.; Albratty, M.; Hermansyah, A.; Ming, L.C.; Goh, K.W.; El Omari, N. Clinical Applications and Mechanism Insights of Natural Flavonoids against Type 2 Diabetes Mellitus. Heliyon 2024, 10, e29718. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhao, H.; Zheng, Y.; Cui, L.; Zhang, Z.; Hussain, H.; Sun, N.; Li, X.; Luan, J.; Zou, Y.; et al. Effects of Deep Eutectic Solvent Systems on the Extraction of Antioxidant Components from Peanut (Arachis hypogaea L.) Hulls. LWT 2024, 208, 116712. [Google Scholar] [CrossRef]
- Ngoc Nhon, H.T.; Diem My, N.T.; Tuong Vi, V.N.; Kim Lien, P.T.; Thao Minh, N.T.; Doan Duy, L.N.; Hong Anh, L.T.; Anh Dao, D.T. Enhancement of Extraction Effectiveness and Stability of Anthocyanin from Hibiscus sabdariffa L. J. Agric. Food Res. 2022, 10, 100408. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Osamede Airouyuwa, J.; Sivapragasam, N.; Ali Redha, A.; Maqsood, S. Sustainable Green Extraction of Anthocyanins and Carotenoids Using Deep Eutectic Solvents (DES): A Review of Recent Developments. Food Chem. 2024, 448, 139061. [Google Scholar] [CrossRef]
- Dai, Y.; Rozema, E.; Verpoorte, R.; Choi, Y.H. Application of Natural Deep Eutectic Solvents to the Extraction of Anthocyanins from Catharanthus roseus with High Extractability and Stability Replacing Conventional Organic Solvents. J. Chromatogr. A 2016, 1434, 50–56. [Google Scholar] [CrossRef]
- Frontini, A.; Tarentini, G.; Negro, C.; Luvisi, A.; Apollonio, M.; De Bellis, L. Effective Liquid–Liquid Extraction for the Recovery of Grape Pomace Polyphenols from Natural Deep Eutectic Solvents (NaDES). Separations 2025, 12, 148. [Google Scholar] [CrossRef]
- Sportiello, L.; Tolve, R.; Grassi, F.; Zanoni, M.; Simonato, B.; Favati, F. Eco-Friendly Extraction of Anthocyanins Compounds from Red Radicchio by-Products with Natural Deep Eutectic Solvents. Eur. Food Res. Technol. 2025, 251, 179–191. [Google Scholar] [CrossRef]
- Sportiello, L.; Tolve, R.; Galgano, F.; Giarola, M.; Musollini, S.; Favati, F. Optimizing Carotenoids NaHDES Extraction for Enhancing Spreadable Chocolate’s Nutritional Value. Food Biosci. 2024, 62, 105109. [Google Scholar] [CrossRef]
- Krgović, N.; Jovanović, M.S.; Nedeljković, S.K.; Šavikin, K.; Lješković, N.J.; Ilić, M.; Živković, J.; Menković, N. Natural Deep Eutectic Solvents Extraction of Anthocyanins—Effective Method for Valorisation of Black Raspberry (Rubus occidentalis L.) Pomace. Ind. Crops Prod. 2025, 223, 120237. [Google Scholar] [CrossRef]
- Jin, Y.; Arroo, R. The Protective Effects of Flavonoids and Carotenoids against Diabetic Complications—A Review of in Vivo Evidence. Front. Nutr. 2023, 10, 1020950. [Google Scholar] [CrossRef]
- Buarque, F.S.; Soares, M.A.; Ribeiro, B.D.; Marrucho, I.M. Development of Hydrophobic Eutectic Solvents Composed of DL-Menthol and Fatty Acids/Alcohols: Application in the Extraction of Capsaicinoids and Carotenoids from Capsicum Frutescens. J. Mol. Liq. 2025, 417, 126591. [Google Scholar] [CrossRef]
- Vlachoudi, D.; Chatzimitakos, T.; Athanasiadis, V.; Bozinou, E.; Lalas, S.I. Enhanced Extraction of Carotenoids from Tomato Industry Waste Using Menthol/Fatty Acid Deep Eutectic Solvent. Waste 2023, 1, 977–992. [Google Scholar] [CrossRef]
- Marinaccio, L.; Zengin, G.; Bender, O.; Cichelli, A.; Novellino, E.; Stefanucci, A.; Mollica, A. Ultrasound Assisted Lycopene Extraction from Tomato Skin Waste by Volatile Natural Deep Eutectic Solvent. Food Chem. Adv. 2024, 4, 100656. [Google Scholar] [CrossRef]
- Vasyliev, G.; Lyudmyla, K.; Hladun, K.; Skiba, M.; Vorobyova, V. Valorization of Tomato Pomace: Extraction of Value-Added Components by Deep Eutectic Solvents and Their Application in the Formulation of Cosmetic Emulsions. Biomass Convers. Biorefin. 2022, 12, 95–111. [Google Scholar] [CrossRef]
- Plaza, M.; Marina, M.L. Hydrophobic Natural Deep Eutectic Solvents for the Sustainable Extraction of Carotenoids from Persimmon Peels. Microchem. J. 2025, 208, 112421. [Google Scholar] [CrossRef]
- Karabulut, G.; Köroğlu, D.G.; Feng, H.; Karabulut, Z. Sustainable Fungi-Based Protein Extraction from Agro-Waste Mushroom Stem Using Deep Eutectic Solvents. Food Chem. X 2024, 24, 101931. [Google Scholar] [CrossRef]
- Roy, V.C.; Park, J.S.; Haque, A.R.; Ali, M.S.; Lee, H.J.; Chun, B.S. Bio-Refinery of Brewery Spent Grain Utilizing Natural Deep Eutectic Solvent-Induced Subcritical Water. J. Supercrit. Fluids 2024, 204, 106108. [Google Scholar] [CrossRef]
- Hernández-Corroto, E.; Olivares-Galván, S.; Marina, M.L.; García, M.C. Approaching the Extraction of Proteins from Brewing Wastes Using Deep Eutectic Solvents. LWT 2023, 189, 115470. [Google Scholar] [CrossRef]
- Hadinoto, K.; Ling, J.K.U.; Park, J.W.; Tran, T.T.; Pu, S. Extraction of Brewer’s Spent Grain Protein Using Natural Deep Eutectic Solvent Compared to Alkaline Extraction: Evaluating Their Yields, Physicochemical Characteristics, and Functionalities. Food Bioprod. Process. 2025, 150, 12–24. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Wilpiszewska, K.; Spychaj, T. Deep Eutectic Solvents for Polysaccharides Processing. A Review. Carbohydr. Polym. 2018, 200, 361–380. [Google Scholar] [CrossRef]
- Riyamol; Jeevitha, G.C. Microwave and Ultrasound-Assisted Natural Deep Eutectic Solvents-Based Extraction of Pectin from Onion Peel Wastes. CYTA-J. Food 2024, 22, 2311215. [Google Scholar] [CrossRef]
- Chen, S.; Xiao, L.; Li, S.; Meng, T.; Wang, L.; Zhang, W. The Effect of Sonication-Synergistic Natural Deep Eutectic Solvents on Extraction Yield, Structural and Physicochemical Properties of Pectins Extracted from Mango Peels. Ultrason. Sonochem. 2022, 86, 106045. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, R.; Jeevitha, G.C. Sustainable Approach for Utilization of Mango Peel Wastes for Pectin Extraction by Natural Deep Eutectic Solvents. J. Food Process. Preserv. 2024, 2024, 4540390. [Google Scholar] [CrossRef]
- Gómez Vargas, C.; Ponce, N.M.A.; Stortz, C.A.; Fissore, E.N.; Bonelli, P.; Otálora González, C.M.; Gerschenson, L.N. Pectin Obtention from Agroindustrial Wastes of Malus Domestica Using Green Solvents (Citric Acid and Natural Deep Eutectic Solvents). Chemical, Thermal, and Rheological Characterization. Front. Chem. 2024, 12, 1504582. [Google Scholar] [CrossRef]
- Riyamol; Kamaraj, V.; Jeevitha, G.C.; Mittal, A.; Arya, R.K. Jackfruit Waste Utilization for Production of Pectin Using Natural Deep Eutectic Solvents: A Comparative Study. Biomass Convers. Biorefin. 2025, 1–15. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. Prevention or Delay of Diabetes and Associated Comorbidities: Standards of Care in Diabetes—2025. Diabetes Care 2025, 48, S50–S58. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2025. Diabetes Care 2025, 48, S27–S49. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wu, Y.; Ni, Y.; Xu, H.; He, Y. Global, Regional, and National Burden of Type 2 Diabetes Mellitus Caused by High BMI from 1990 to 2021, and Forecasts to 2045: Analysis from the Global Burden of Disease Study 2021. Front. Public Health 2025, 13, 1515797. [Google Scholar] [CrossRef]
- Zanzabil, K.Z.; Hossain, M.S.; Hasan, M.K. Diabetes Mellitus Management: An Extensive Review of 37 Medicinal Plants. Diabetology 2023, 4, 186–234. [Google Scholar] [CrossRef]
- Tebbi, S.O.; Trapali, M.; Letsiou, S. Exploring the Anti-Diabetic, Antioxidant and Anti-Microbial Properties of Clematis flammula L. Leaves and Pistacia lentiscus L. Fruits Using Choline Chloride-Based Deep Eutectic Solvent. Waste Biomass Valorization 2024, 15, 2869–2879. [Google Scholar] [CrossRef]
- Roy, S.; Ghosh, A.; Majie, A.; Karmakar, V.; Das, S.; Dinda, S.C.; Bose, A.; Gorain, B. Terpenoids as Potential Phytoconstituent in the Treatment of Diabetes: From Preclinical to Clinical Advancement. Phytomedicine 2024, 129, 155638. [Google Scholar] [CrossRef]
- Coscarella, M.; Nardi, M.; Alipieva, K.; Bonacci, S.; Popova, M.; Procopio, A.; Scarpelli, R.; Simeonov, S. Alternative Assisted Extraction Methods of Phenolic Compounds Using NaDESs. Antioxidants 2024, 13, 62. [Google Scholar] [CrossRef]
- Yang, Y.; Guan, M.; Fang, M.; Gu, Y.; Yi, L.; Ren, D. Simultaneous Extraction and Selective Separation of Catechins, Caffeine and Theanine from Waste Tea Residue Facilitated by Citric Acid-Based Deep Eutectic Solvent. Sep. Purif. Technol. 2025, 360, 130918. [Google Scholar] [CrossRef]
- Gómez-Urios, C.; Viñas-Ospino, A.; Puchades-Colera, P.; Blesa, J.; López-Malo, D.; Frígola, A.; Esteve, M.J. Choline Chloride-Based Natural Deep Eutectic Solvents for the Extraction and Stability of Phenolic Compounds, Ascorbic Acid, and Antioxidant Capacity from Citrus sinensis Peel. LWT 2023, 177, 114595. [Google Scholar] [CrossRef]
- Yulion, R.; Aliyah, S.H.; Perawati, S.; Andriani, L.; Eryunita, P.; Arfiah, B.; Atmaja, A.S. Novel Natural Deep Eutectic Solvent (NaDES) Yellow Choline Chloride and Molecular Docking Soybean Extract (Glycine Max) as Diabetes Drugs Candidate. Indones. J. Chem. Stud. 2024, 3, 46–51. [Google Scholar] [CrossRef]
- Zengin, G.; Cádiz-Gurrea, M.d.l.L.; Fernández-Ochoa, Á.; Leyva-Jiménez, F.J.; Carretero, A.S.; Momotko, M.; Yildiztugay, E.; Karatas, R.; Jugreet, S.; Mahomoodally, M.F.; et al. Selectivity Tuning by Natural Deep Eutectic Solvents (NADESs) for Extraction of Bioactive Compounds from Cytinus hypocistis—Studies of Antioxidative, Enzyme-Inhibitive Properties and LC-MS Profiles. Molecules 2022, 27, 5788. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, I.P. Phytometabolomics of Wedelia Chinensis Leaves Extracted with Natural Deep Eutectic Solvents: Bioactivities and Anti-Diabetic Mechanisms. J. Mol. Liq. 2025, 428, 127516. [Google Scholar] [CrossRef]
- Wang, W.; Yang, X.; Tang, Y.; Zhang, Y.; Yue, S. The Total Tannins of Geum japonicum Thunb. var. chinense Extracted by Green and Natural Deep Eutectic Solvent: Extraction Optimization, Component Identification and Hypoglycemic Activity. Ind. Crops Prod. 2024, 222, 119679. [Google Scholar] [CrossRef]
- Olfat, A.; Mostaghim, T.; Shahriari, S.; Salehifar, M. Extraction of Bioactive Compounds of Hypnea flagelliformis by Ultrasound-Assisted Extraction Coupled with Natural Deep Eutectic Solvent and Enzyme Inhibitory Activity. Algal Res. 2024, 78, 103388. [Google Scholar] [CrossRef]
- Subhash, A.J.; Bamigbade, G.B.; Tarique, M.; Al-Ramadi, B.; Abu-Jdayil, B.; Kamal-Eldin, A.; Nyström, L.; Ayyash, M. Bioactive Properties and Gut Microbiota Modulation by Date Seed Polysaccharides Extracted Using Ultrasound-Assisted Deep Eutectic Solvent. Food Chem. X 2024, 22, 101354. [Google Scholar] [CrossRef]
- Djaoudene, O.; Bachir-Bey, M.; Schisano, C.; Djebari, S.; Tenore, G.C.; Romano, A. A Sustainable Extraction Approach of Phytochemicals from Date (Phoenix dactylifera L.) Fruit Cultivars Using Ultrasound-Assisted Deep Eutectic Solvent: A Comprehensive Study on Bioactivity and Phenolic Variability. Antioxidants 2024, 13, 181. [Google Scholar] [CrossRef]
- Iftikhar, H.; Akram, S.; Khalid, N.u.A.; Ahmed, D.; Khan, M.H.; Ashraf, R.; Mushtaq, M. Deep Eutectic Solvent-Based Green Extraction of Strychnos Potatorum Seed Phenolics: Process Optimization via Response Surface Methodology and Artificial Neural Network. Talanta 2025, 286, 127443. [Google Scholar] [CrossRef]
- Qi, H.; Fu, W.; Liu, Y.; Bai, J.; Wang, R.; Zou, G.; Shen, H.; Cai, Y.; Luo, A. Electron Beam Irradiation Coupled Ultrasound-Assisted Natural Deep Eutectic Solvents Extraction: A Green and Efficient Extraction Strategy for Proanthocyanidin from Walnut Green Husk. Food Chem. 2025, 463, 141279. [Google Scholar] [CrossRef]
- Ahmad, I.; Arifianti, A.E.; Sakti, A.S.; Saputri, F.C.; Mun’im, A. Simultaneous Natural Deep Eutectic Solvent-Based Ultrasonic-Assisted Extraction of Bioactive Compounds of Cinnamon Bark and Sappan Wood as a Dipeptidyl Peptidase IV Inhibitor. Molecules 2020, 25, 3832. [Google Scholar] [CrossRef]
- Kartini, S.; Abu Bakar, M.F.; Abu Bakar, F.I.; Hendrika, Y.; Juariah, S.; Endrini, S. Antioxidant and Antidiabetic Activities of Zingiber officinale Var. Rubrum Extracted with Natural Deep Eutectic Solvents. Food Res. 2024, 8, 1–10. [Google Scholar] [CrossRef]
- Jovanović, J.; Jović, M.; Trifković, J.; Smiljanić, K.; Gašić, U.; Krstić Ristivojević, M.; Ristivojević, P. Green Extraction of Bioactives from Curcuma Longa Using Natural Deep Eutectic Solvents: Unlocking Antioxidative, Antimicrobial, Antidiabetic, and Skin Depigmentation Potentials. Plants 2025, 14, 163. [Google Scholar] [CrossRef] [PubMed]
- Taco, V.; Almachi, D.; Bonilla, P.; Gijón-Arreortúa, I.; Benali, S.; Raquez, J.M.; Duez, P.; Nachtergael, A. Natural Deep Eutectic Solvents (NADESs) for the Extraction of Bioactive Compounds from Quinoa (Chenopodium quinoa Willd.) Leaves: A Semi-Quantitative Analysis Using High Performance Thin-Layer Chromatography. Molecules 2025, 30, 2620. [Google Scholar] [CrossRef]
- Ren, T.; Xiao, M.; Zhou, Q.; Li, H.; Zhou, L.; Wu, M.; Gao, Z.; Cao, Y.; Wang, J.; Zhao, W. Extraction of Polysaccharides from Quinoa (Chenopodium quinoa Willd) Bran Using Deep Eutectic Solvent: Isolation, Structural Characterization, and Hypoglycemic Activity. Ind. Crops Prod. 2025, 230, 121111. [Google Scholar] [CrossRef]
- Tian, Y.; Ou, Z.; Xiong, W.; Fan, W.; Yang, W.; Zhang, B.; Pan, L.; Ren, H. Extraction and Optimization of Polyphenols from Morchella Spp. Using Ultrasound-assisted Deep Eutectic Solvents: Potential Intervention for Type 2 Diabetes Mellitus. J. Food Sci. 2025, 90, e70145. [Google Scholar] [CrossRef]
- Dai, Y.; Row, K.H. Application of Natural Deep Eutectic Solvents in the Extraction of Quercetin from Vegetables. Molecules 2019, 24, 2300. [Google Scholar] [CrossRef]
- Ciardi, M.; Ianni, F.; Sardella, R.; Di Bona, S.; Cossignani, L.; Germani, R.; Tiecco, M.; Clementi, C. Effective and Selective Extraction of Quercetin from Onion (Allium cepa L.) Skin Waste Using Water Dilutions of Acid-Based Deep Eutectic Solvents. Materials 2021, 14, 6465. [Google Scholar] [CrossRef]
- Eid, H.M.; Haddad, P.S. The Antidiabetic Potential of Quercetin: Underlying Mechanisms. Curr. Med. Chem. 2017, 24, 355–364. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Xiao, H.; Ye, X.; Pan, H.; Chen, S. Revisiting Dietary Proanthocyanidins on Blood Glucose Homeostasis from a Multi-Scale Structural Perspective. Curr. Res. Food Sci. 2024, 9, 100926. [Google Scholar] [CrossRef]
- Singh, A.K.; Rana, H.K.; Singh, V.; Chand Yadav, T.; Varadwaj, P.; Pandey, A.K. Evaluation of Antidiabetic Activity of Dietary Phenolic Compound Chlorogenic Acid in Streptozotocin Induced Diabetic Rats: Molecular Docking, Molecular Dynamics, In Silico Toxicity, In Vitro and In Vivo Studies. Comput. Biol. Med. 2021, 134, 104462. [Google Scholar] [CrossRef]
- Wu, K.; Ren, J.; Wang, Q.; Nuerjiang, M.; Xia, X.; Bian, C. Research Progress on the Preparation and Action Mechanism of Natural Deep Eutectic Solvents and Their Application in Food. Foods 2022, 11, 3528. [Google Scholar] [CrossRef]
- Ruesgas Ramon, M.; Durand, E.; Garcia-Sosa, K.; Peña-Rodríguez, L.M. Exploring the Potential of Deep Eutectic Solvents (DES) in Bioactive Natural Product Research: From DES to NaDES, THEDES, and Beyond. PeerJ. Anal. Chem. 2023, 5, e28. [Google Scholar] [CrossRef]
- Şen, F.B.; Nemli, E.; Bekdeşer, B.; Çelik, S.E.; Lalikoglu, M.; Aşçı, Y.S.; Capanoglu, E.; Bener, M.; Apak, R. Microwave-Assisted Extraction of Valuable Phenolics from Sunflower Pomace with Natural Deep Eutectic Solvents and Food Applications of the Extracts. Biomass Convers. Biorefin. 2025, 15, 9915–9930. [Google Scholar] [CrossRef]
- Cajnko, M.M.; Vicente, F.A.; Novak, U.; Likozar, B. Natural Deep Eutectic Solvents (NaDES): Translating Cell Biology to Processing. Green Chem. 2023, 25, 9045–9062. [Google Scholar] [CrossRef]
- Faggian, M.; Sut, S.; Perissutti, B.; Baldan, V.; Grabnar, I.; Dall’Acqua, S. Natural Deep Eutectic Solvents (NADES) as a Tool for Bioavailability Improvement: Pharmacokinetics of Rutin Dissolved in Proline/Glycine after Oral Administration in Rats: Possible Application in Nutraceuticals. Molecules 2016, 21, 1531. [Google Scholar] [CrossRef]
- da Silva, D.T.; Smaniotto, F.A.; Costa, I.F.; Baranzelli, J.; Muller, A.; Somacal, S.; Monteiro, C.S.; Vizzotto, M.; Rodrigues, E.; Barcia, M.T.; et al. Natural Deep Eutectic Solvent (NADES): A Strategy to Improve the Bioavailability of Blueberry Phenolic Compounds in a Ready-to-Use Extract. Food Chem. 2021, 364, 130370. [Google Scholar] [CrossRef]
- Popović, B.M.; Gligorijević, N.; Aranđelović, S.; Macedo, A.C.; Jurić, T.; Uka, D.; Mocko-Blažek, K.; Serra, A.T. Cytotoxicity Profiling of Choline Chloride-Based Natural Deep Eutectic Solvents. RSC Adv. 2023, 13, 3520–3527. [Google Scholar] [CrossRef]
- Sánchez-Argüello, P.; Martín-Esteban, A. Ecotoxicological Assessment of Different Choline Chloride-Based Natural Deep Eutectic Solvents: In Vitro and in Vivo Approaches. Env. Res. 2025, 284, 122202. [Google Scholar] [CrossRef]
- Hayyan, M.; Mbous, Y.P.; Looi, C.Y.; Wong, W.F.; Hayyan, A.; Salleh, Z.; Mohd-Ali, O. Natural Deep Eutectic Solvents: Cytotoxic Profile. Springerplus 2016, 5, 913. [Google Scholar] [CrossRef]
- Benlebna, M.; Ruesgas-Ramón, M.; Bonafos, B.; Fouret, G.; Casas, F.; Coudray, C.; Durand, E.; Figueroa-Espinoza, M.C.; Feillet-Coudray, C. Toxicity of Natural Deep Eutectic Solvent Betaine:Glycerol in Rats. J. Agric. Food Chem. 2018, 66, 6205–6212. [Google Scholar] [CrossRef]
- Mbous, Y.; Hayyan, M.; Wong, W.; Looi, C.Y.; Hashim, M.A. Unraveling the Cytotoxicity and Metabolic Pathways of Binary Natural Deep Eutectic Solvent Systems. Sci. Rep. 2017, 7, 41257. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.J.; Oliveira, F.; Jesus, A.R.; Paiva, A.; Duarte, A.R.C. Current Methodologies for the Assessment of Deep Eutectic Systems Toxicology: Challenges and Perspectives. J. Mol. Liq. 2022, 362, 119675. [Google Scholar] [CrossRef]
- Truzzi, E.; Bertelli, D.; Catellani, B.; Jazi, D.D.; Benvenuti, S. Recovery of Bioactive Compounds from the Biomass of Aromatic Plants After Distillation Using NADES: A Sustainable Alternative Extraction Method. Molecules 2025, 30, 1120. [Google Scholar] [CrossRef]
- Panić, M.; Drakula, S.; Cravotto, G.; Verpoorte, R.; Hruškar, M.; Radojčić Redovniković, I.; Radošević, K. Biological Activity and Sensory Evaluation of Cocoa By-Products NADES Extracts Used in Food Fortification. Innov. Food Sci. Emerg. Technol. 2020, 66, 102514. [Google Scholar] [CrossRef]
- Salazar-Bermeo, J.; Moreno-Chamba, B.; Heredia-Hortigüela, R.; Lizama, V.; Martínez-Madrid, M.C.; Saura, D.; Valero, M.; Neacsu, M.; Martí, N. Green Technologies for Persimmon By-Products Revalorisation as Sustainable Sources of Dietary Fibre and Antioxidants for Functional Beverages Development. Antioxidants 2023, 12, 1085. [Google Scholar] [CrossRef]
- Gomez-Urios, C.; Siroli, L.; Gottardi, D.; Benedetti, S.; Lanciotti, R.; Frigola, A.; Buratti, S.; Blesa, J.; Di Nunzio, M.; Patrignani, F.; et al. Improving the Shelf-Life and Functionality of Orange Juice by the Addition of Polyphenol-Enriched Extracts Obtained Using Natural Deep Eutectic Solvents. Appl. Food Res. 2025, 5, 101074. [Google Scholar] [CrossRef]
- Pontillo, N.R.N.; Koutsoukos, S.; Welton, T.; Detsi, A. Investigation of the Influence of Natural Deep Eutectic Solvents (NaDES) in the Properties of Chitosan-Stabilised Films. Mater. Adv. 2021, 2, 3954–3964. [Google Scholar] [CrossRef]
- Velásquez, P.; Bustos, D.; Montenegro, G.; Giordano, A. Ultrasound-Assisted Extraction of Anthocyanins Using Natural Deep Eutectic Solvents and Their Incorporation in Edible Films. Molecules 2021, 26, 984. [Google Scholar] [CrossRef]
| Source/ By-Product | Bioactive Compound | DES (Molar Ratio; Water Content) | Extraction Method | Yields mg GAE g−1 | Year | Ref. |
|---|---|---|---|---|---|---|
| broccoli leaf waste | phenolic compounds | ChCl/1,2-propylene glycerol (1:2) | Ultrasound-assisted extraction | TPC: 4.91 | 2023 | [67] |
| broccoli stems | polyphenols trans-cinnamic acid (88.8%), sinapinic acid (5.32%), quercetin (3.06%), isochlorogenic acid (2.88%) | ChCl/urea (1:3; 60%) | conventional heating | 5.10 ± 0.04 | 2025 | [68] |
| kale waste | polyphenols | Betaine/glycerol (1:3) | conventional heating | TPC: 16.80 | 2023 | [69] |
| Psidium myrtoides purple araçá | phenolic compounds | ChCl/glycerol (1:2) | ultrasound -assisted extraction | 59.29 ± 4.80 | 2024 | [70] |
| apple pomace | polyphenol compounds, quercetin, chlorogenic acid, gallic acid, phloretin, phloridizin, rutin, procyanidin | ChCl/glycerol (1:3) | ultrasound -assisted extraction | 5.80 | 2023 | [71] |
| Betaine/urea (1:1) | 5.245 ± 0.124 | 2024 | [72] | |||
| Betaine/malic Acid (1:1) | 5.157 ± 0.164 | |||||
| orange peels | polyphenols | ChCl/malic acid (1:1) | solid–liquid extraction and hot solvent extraction | 10.53 AA = 4.30 | 2022 | [73] |
| Proline/malic acid (1:1) | conventional heating | 2.83 ± 0.07 | 2023 | [74] | ||
| lactic acid/glucose | ||||||
| citrus pomace | naringin, narirutin, hesperidin, ellagic acid, naringenin, hesperitin, diosmetin, gallic acid | Betaine/lactic acid (1:1) | conventional heating | 6.15 ± 0.03 | 2022 | [75] |
| ChCl/glycerol (1:2) | 5.45 ± 0.02 | |||||
| citrus peel waste | neohesperidin | ChCl/glucose (1:1) | ultrasound-assisted extraction | 10.45 ± 0.01 | 2024 | [76] |
| mango peel waste | polyphenols | lactic acid/glucose (5:1, 20%) | Ultrasound-assisted extraction | 69.85 | 2022 | [57] |
| mango by-products | polyphenols, mangiferin | ChCl/ethylene glycol (1:2) | conventional extraction and ultrasound-assisted extraction | 60.01 ± 1.40 | 2023 | [77] |
| ChCl/glycerol | ||||||
| β-alanine/DL-malic acid: H2O (1:1:3) | 50.61 ± 2.51 | |||||
| β-alanine/DL-malic acid: H2O (1:1:3) | ||||||
| spirulina | phenolic compounds, caffeic acid, chlorogenic acid, salicylic acid, quinic acid, gallic acid, catechin | Betaine/glucose (1:2, 30%) | Freezing–thawing cycle extraction | 11.77 ± 0.12 | 2025 | [78] |
| Corylus avellana L. by products | D-(−)-quinic acid, gallic acid, protocatechuic acid, hydroxybenzoic acid, catechin, caffeic acid, vanillic acid, epicatechin gallate, ferulic acid, sinapic acid; 16: rutin, quercetin-3-O-glucoside, salicylic acid, quercetin-3-O-rhamnoside, quercetin | ChCl/1,2-propylene glycol (1:4) | Microwave-assisted extraction | 5.60 | 2022 | [79] |
| Crocus sativus L. floral by-products | polyphenols | L-proline/glycerol (1:2) | Ultrasound-assisted extraction | 35.15 ± 1.99 | 2023 | [80] |
| olive oil industry by-products | hydroxytyrosol | citric acid/glycine:H2O (2:1:1) | Ultrasound-assisted extraction | - | 2020 | [81] |
| phenolic compounds oleuropein | ChCl/acetic acid (1:2) | solid–liquid equilibrium | 0.47 | 2021 | [82] | |
| ChCl/malic acid (1:1) | ||||||
| ChCl/malonic acid (1:1) | ||||||
| olive pomace | phenolic compounds | ChCl/malonic acid (1:1) | conventional extraction | 19.80 | 2022 | [83] |
| olive mill waste | phenolic compounds | citric acid/fructose (1:1) | conventional extraction | 3.99 | 2023 | [84] |
| coffee husk | phenolic compounds | ChCl/citric acid (1:1) | conventional extraction | 5.88 | 2023 | [85] |
| ChCl/proline (1:1) | ||||||
| spent coffee grounds | phenolic compounds, caffeine | Betaine/glycerol (1:3) | Ultrasound-assisted extraction, microwave-assisted extraction | 22.67 | 2023 | [86] |
| phenolic compounds | Betaine/triethylene glycol (1:2, 30–60%) | conventional heating | 1.27 ± 0.13 | 2024 | [87] | |
| gallic acid, 3-O-caffeoylquinic acid, caffeic acid | ChCl/1,2-propanediol (1:2) | agitation-assisted water | 138.5 ± 1.09 131.34 5 ± 0.75 63.17 ± 0.53 | 2023 | [88] | |
| Wine-making by-products | Catechins, tannins | ChCl/urea (1:2) | subcritical water extraction | 118.77 ± 5.83 14.24 ± 0.86 | 2020 | [89] |
| grape pomace | phenolic compounds | ChCl/lactic acid (1:1, 50%) | Solid–liquid extraction | 2024 | [20] | |
| ChCl/tartaric acid (1:1, 50%) | 127.8 | |||||
| ChCl/glycerol (1:1, 50%) | ||||||
| Graševina grape pomace | Epigallocatechin, Catechin, Epicatechin, gallic acid | Betaine/glucose (1:1) | Ultrasound-assisted extraction | 0.24 ± 0.01 0.74 ± 0.006 0.75 ± 0.005 0.55 ± 0.008 | 2024 | [21] |
| Source/ By-Product | Bioactive Compound | DES (Molar Ratio; Water Content) | Extraction Method | Yields mg g−1 | Year | Ref. |
|---|---|---|---|---|---|---|
| orange peels | flavonoids | ChCl/Malic Acid (1:1) | SLE/HSE | TFC = 0.95 | 2022 | [78] |
| lemon peel waste | hesperidin, narirutin | ChCl/Acetic Acid (1:2) | ultrasound-assisted extraction | 5.60 | 2024 | [64] |
| Magnifera indica L. by-products | flavonoids | ChCl/Ethylene Glycol | conventional extraction and ultrasound-assisted extraction | 7.60 ± 0.72 | 2023 | [80] |
| ChCl/Glycerol | ||||||
| β-alanine/D-Malic acid/H2O (1:1:3) | ||||||
| Arachis hypogaea L. hulls | flavonoids | L-proline/acetic acid (1:4) | ultrasound-assisted extraction | 2.51 | 2024 | [97] |
| peanut shells | luteolin | ChCl/ethylene glycol (1:2, 1:3, 1:4) | microwave-assisted extraction | 2.90 ± 0.02 | 2025 | [23] |
| Crocus sativus floral by-products | flavonols: kaempferol, quercetin, myricetin, and isorhamnetin glycosides | L-proline/glycerol (1:2) | ultrasound-assisted extraction | 8.04 ± 0.49 | 2023 | [83] |
| Camellia sinensis waste | epigallocatechin gallate (EGCG), epicatechin gallate (ECG) | citric acid/polypropylene glycol (1:1) | microwave-assisted extraction | (EGCG, 15.58), (ECG, 12.85) | 2025 | [98] |
| spent tea leaves | flavonoids | acetic acid/glycerol (2:1, 20%) | ultrasound-assisted extraction | 20.4 | 2024 | [24] |
| spend coffee grounds | flavonoids | betaine/glycerol (1:3) | ultrasound-assisted extraction, microwave-assisted extraction | 24.05 | 2023 | [89] |
| Source/ By-Product | Bioactive Compound | DES (Molar Ratio; Water Content) | Extraction Method | Yields | Year | Ref. |
|---|---|---|---|---|---|---|
| Myrciaria cauliflora by-product | cyanidin-3-glucoside | ChCl/malic acid (1:1) | pressurized liquid extraction | 1.60 ± 0.09 mgCGE g−1 DW | 2022 | [27] |
| ChCl/propylene glycol (1:2) | 1.7 ± 0.06 mgCGE g−1 DW | |||||
| Rubus occidentalis L. pomace | cyanidin-3-Orutinoside | citric acid/H2O (1:1:3) | ultrasound-assisted extraction | 7.60 mg g−1 DW and TAC 6.88 mg CGE g−1 DW | 2025 | [102] |
| Cichorium intybus by-products | cyanidin-3-glucoside | citric acid/ChCl (3:2, 25%) | ultrasound-assisted extraction | 11.35 ± 0.06 mg CGE g−1 DW | 2025 | [103] |
| Primitivo pomace | delphinidin 3-O-glucoside, cyanidin 3-O-glucoside, petunidin 3-O-glucoside | ChCl/lactic acid (1:1) ChCl/tartaric acid (1:1) ChCl/gycerol (1:1) | liquid–liquid extraction with 2-MeTHF | >80% | 2025 | [104] |
| Source/ By-Product | Bioactive Compound | DES (Molar Ratio; Water Content) | Extraction Method | Yields mg g−1 | Year | Ref. |
|---|---|---|---|---|---|---|
| tomato industry waste | carotenoids | menthol/hexanoic acid (2:1) | solid–liquid extraction | 94.5 | 2023 | [108] |
| tomato pomace | lycopene, β-carotene | ethyl acetate/ethyl lactate | ultrasound-assisted extraction | lycopene = 0.07 β-carotene = 3.95 | 2022 | [56] |
| tomato skin waste | lycopene | thymol/menthol (1:1) | ultrasound-assisted extraction | 358.7 ± 1.2 | 2024 | [109] |
| tomato processing by-products | lycopene | ChCL/lactic acid (1:2) | ultrasound-assisted extraction | 2024 | [110] | |
| persimmon peels | carotenoids | thymol/menthol (2:1) | ultrasound-assisted extraction | 3.12 ± 0.04 | 2025 | [111] |
| orange peels | carotenoids | DL-menthol/camphor (1:1) | conventional extraction | 1.64 | 2023 | [74] |
| DL-menthol/eucalyptol (1:1) | 1.69 | |||||
| lauric acid/octanoic acid (1:3) | 1.53 | |||||
| Proline/malic acid (1:1) | 2.83 |
| Source/ By-Product | Bioactive Compound | DES (Molar Ratio; Water Content) | Extraction Method | Total Protein mg g−1 | Year | Ref. |
|---|---|---|---|---|---|---|
| mushroom agro-waste | fungi-based protein | ChCl/glycerol (1:2) | ultrasound-assisted extraction | 22.04 ± 1.90 21.47 ± 1.05 | 2024 | [112] |
| ChCl/lactic acid (1:2) | ||||||
| brewery spent grain | leucine, isoleucine, phenylalanine, and histidine | ChCl/tartaric acid (1:2) | subcritical water hydrolysis | - | 2024 | [113] |
| brewing wastes | Protein | Guanidium/chloride (1:2) | high-intensity focused ultrasound | 0.13 ± 0.02 | 2023 | [114] |
| brewer’s spent grain | Protein | ChCl/trehalose (3:1) | conventional extraction | 42 ± 0.7 | 2025 | [115] |
| olive pomace | Protein | ChCl/malonic acid (1:1) | conventional extraction | 6.67 ± 0.31 | 2022 | [83] |
| Source/ By-Product | Bioactive Compound | DES (Molar Ratio; Water Content) | Extraction Method | Yield mg g−1/% | Year | Ref. |
|---|---|---|---|---|---|---|
| orange peels | pectin | ChCl/formic acid (1:2) | microwave-assisted extraction | 40.00 ± 0.3 | 2024 | [22] |
| Brazilian berry by-product | pectin | citric acid/glucose/H2O (1:2:3) | subcritical water extraction | 15.9 ± 0.6 | 2022 | [26] |
| onion peel waste | pectin | ChCl/tartaric acid (1:50) | ultrasound-assisted extraction and microwave-assisted extraction | 36 ± 0.85 | 2024 | [117] |
| mango peels | pectin | Betaine/citric acid (2:1) ChCl/malic acid (1:2) | conventional extraction | 36.76 ± 6.23 38.72 ± 5.61 | 2022 | [118] |
| mango peel wastes | pectin | Proline/malonic Acid (1:1) ChCl/tartaric acid (1:1) | microwave-assisted extraction | 88.8 ± 0 21 90.82 ± 0 11 | 2024 | [119] |
| Malus Domestica (pulp and peel) | pectin | citric acid/glucose/H2O (1:1:3) lactic acid/glucose/H2O (5:1:3) | conventional extraction | 13–18 | 2025 | [120] |
| jackfruit waste | pectin | ChCl/maleic acid (1:1) | microwave-assisted extraction | 33.18 | 2025 | [121] |
| Plant Source/ By-Product | DES (Molar Ratio; Water Content) | Bioactive Compounds | Biological Activity | Type of Study | Ref. |
|---|---|---|---|---|---|
| Glycine max | choline chloride/lactic acid (1:1) | genistein | docking studies alphaglucosidase PBD ID: 5nn8 and SGLT-2 inhibitor PDB ID: 7vsi | in silico | [131] |
| Cytinus hypocistis L. | choline chloride/urea (1:2) | total phenolics | α-glucosidase (2.20 mmol ACAE/g) | in vitro | [132] |
| total flavonoids | α-amylase (2.54 mmol ACAE/g) | ||||
| Wedelia chinensis leaves | citric acid/arginine | total phenolics | α-amylase (IC50: 0.4 ± 0.1 mg/mL) and α-glucosidase | in vitro in silico | [133] |
| total flavonoids | |||||
| Clematis flammula L. leaves Pistacia lentiscus L. black fruits | choline chloride/acetic acid (1:2) | antioxidants TBARS assay (72.80 ± 9.67%) | α -amylase (64.03 ± 1.21%) | in vitro | [126] |
| choline chloride/acetic acid (1:2) | antioxidants TBARS assay (81.32 ± 1.27%) | α-amylase (44.08 ± 2.61%) | |||
| Geum japonicum Thunb. var. chinense | L-proline/lactic acid (1:2) | total tannins | α-glucosidase (IC50: 1.401–4.801 μg/mL) | in vitro Caco-2 model system in vivo (animal) | [134] |
| Hypnea flagelliformis | choline chloride/lactic acid (1:2) | total phenol content | α-amylase IC50 (1.21 ± 0.03 mg/mL) | in vitro | [135] |
| α -glucosidase IC50 (0.94 ± 0.04 mg/mL) | |||||
| Phoenix dactylifera L. seed | choline chloride/ethylene glycol (1:1) | polysaccharides | α-amylase (1000 μg/mL, 82%) | in vitro | [136] |
| α-glycosidase (1000 μg/mL, 86%) | |||||
| Phoenix dactylifera L. fruit | lactic acid/sucrose (3:1) | total phenolics (1.29 mg GAE/g) | α-amylase (IC50: 45%) acetylcholinesterase (IC50: 37%) | in vitro | [137] |
| total flavonoids (53.8 mg QE/100 g) | |||||
| proanthocyanidins (179.5 mg CE/g) | |||||
| total triterpenoids (12.88 mg OAE/100 g) | |||||
| Psidium myrtoides by-product | choline chloride/glycerol (1:2) | phenolic compounds | α-amylase (12.00 ± 0.62%) | in vitro | [70] |
| α-glycosidase (17.17 ± 1.75%) | |||||
| Strychnos potatorum L. seed | glycerol/sodium acetate (3:1) | total phenolic content | α-amylase (46.95%) | in vitro | [138] |
| total flavonoid content | |||||
| walnut green husk | choline chloride/ethylene glycol (1:1) | proanthocyanidin (56.34 mg/g) antioxidant activity | α-amylase (3.91 μg/mL, IC50: 60%) | in vitro | [139] |
| α-glucosidase (125 μg/mL, IC50: 75%) | |||||
| anti-glycation capacity (IC50: 86.49 μg/mL) | |||||
| Cinnamomum burmannii Caesalpinia sappan | choline chloride/glycerol (2:1) | trans-cinnamaldehyde, coumarin, and trans-cinnamic acid | dipeptidyl peptidase IV, DPP IV (205.0 g/mL) dipeptidyl peptidase IV, DPP IV (1254.0 g/mL) | in vitro in silico | [140] |
| Zingiber officinale var. Rubrum | citric acid/sucrose (1:1) NADES1 | total phenolic content | in vitro | [141] | |
| sucrose:glucose/fructose (1:1:1) NADES2 | total flavonoid content | α-amylase (19.62 ± 0.20 µg/mL) from NADES1 | |||
| choline chloride/glycerol (1:2) NADES3 | α-glucosidase (IC50: 57.36 ± 6.08 µg/mL) from NADES1 | ||||
| Glycerol/urea (1:1) NADES4 | antioxidants (DPPH and FRAP methods) | ||||
| Curcuma longa L. | ChCl/lactic acid/water (1:2:5) | total phenolic content antioxidants (DPPH and ABTS methods) | α-amylase (90.0%) | in vitro | [142] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bairaktari, M.; Konstantopoulou, S.M.; Malisova, O.; Gioxari, A.; Stratakos, A.C.; Panoutsopoulos, G.I.; Argyri, K. Natural Deep Eutectic Solvents for Agro-Industrial By-Product Valorization: Emerging Strategies for the Development of Functional Foods Targeting Diabetes. Appl. Sci. 2025, 15, 11596. https://doi.org/10.3390/app152111596
Bairaktari M, Konstantopoulou SM, Malisova O, Gioxari A, Stratakos AC, Panoutsopoulos GI, Argyri K. Natural Deep Eutectic Solvents for Agro-Industrial By-Product Valorization: Emerging Strategies for the Development of Functional Foods Targeting Diabetes. Applied Sciences. 2025; 15(21):11596. https://doi.org/10.3390/app152111596
Chicago/Turabian StyleBairaktari, Maria, Stavroula Maria Konstantopoulou, Olga Malisova, Aristea Gioxari, Alexandros Ch. Stratakos, Georgios I. Panoutsopoulos, and Konstantina Argyri. 2025. "Natural Deep Eutectic Solvents for Agro-Industrial By-Product Valorization: Emerging Strategies for the Development of Functional Foods Targeting Diabetes" Applied Sciences 15, no. 21: 11596. https://doi.org/10.3390/app152111596
APA StyleBairaktari, M., Konstantopoulou, S. M., Malisova, O., Gioxari, A., Stratakos, A. C., Panoutsopoulos, G. I., & Argyri, K. (2025). Natural Deep Eutectic Solvents for Agro-Industrial By-Product Valorization: Emerging Strategies for the Development of Functional Foods Targeting Diabetes. Applied Sciences, 15(21), 11596. https://doi.org/10.3390/app152111596







