Nanosecond Laser-Fabricated Titanium Meshes and Their Chemical Modification for Photocatalytic and SERS Applications
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Mesh Preparation
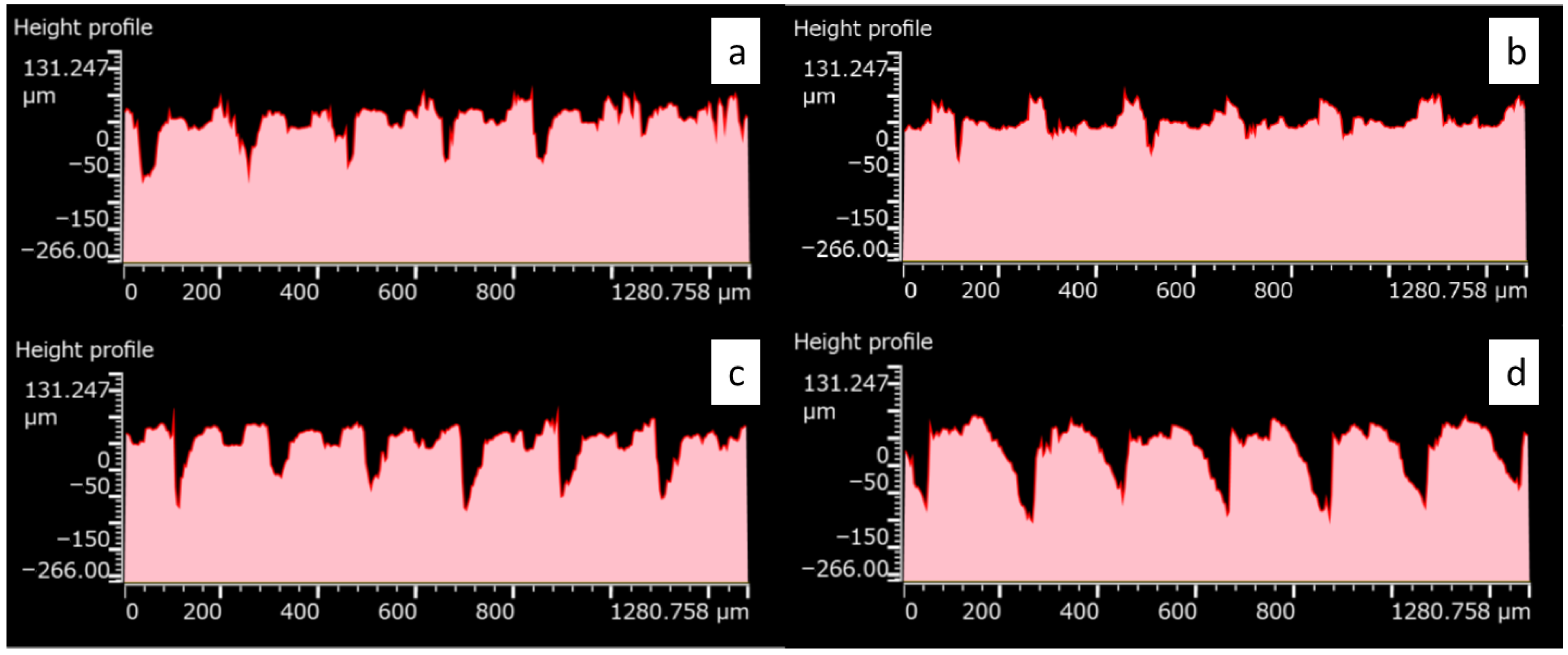
2.2. Morphological and Structural Surface Characterization
2.3. Modification of Titanium Mesh
2.4. Photocatalytic Properties
3. Results and Discussion
3.1. Morphological Analysis
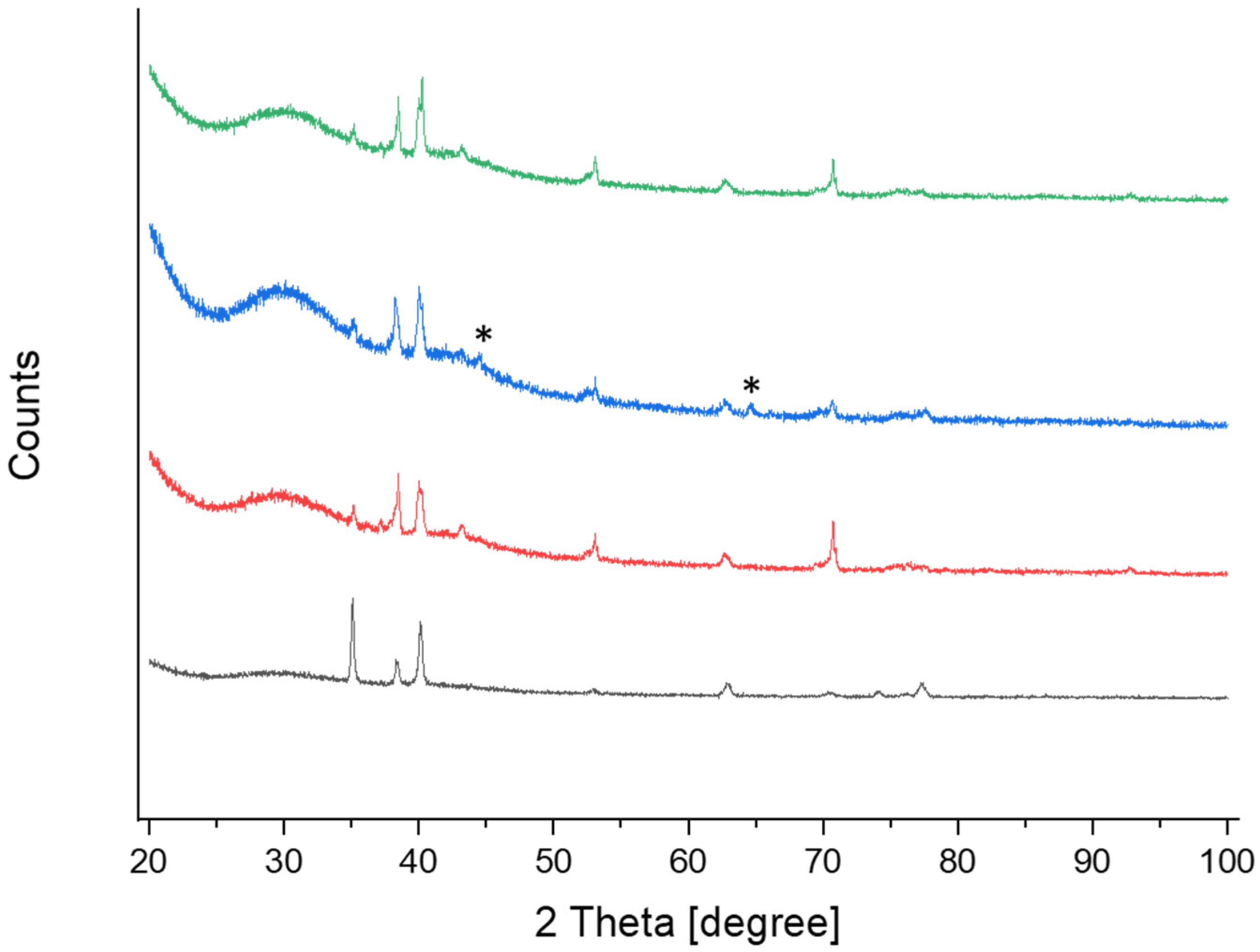
3.2. Surface Composition
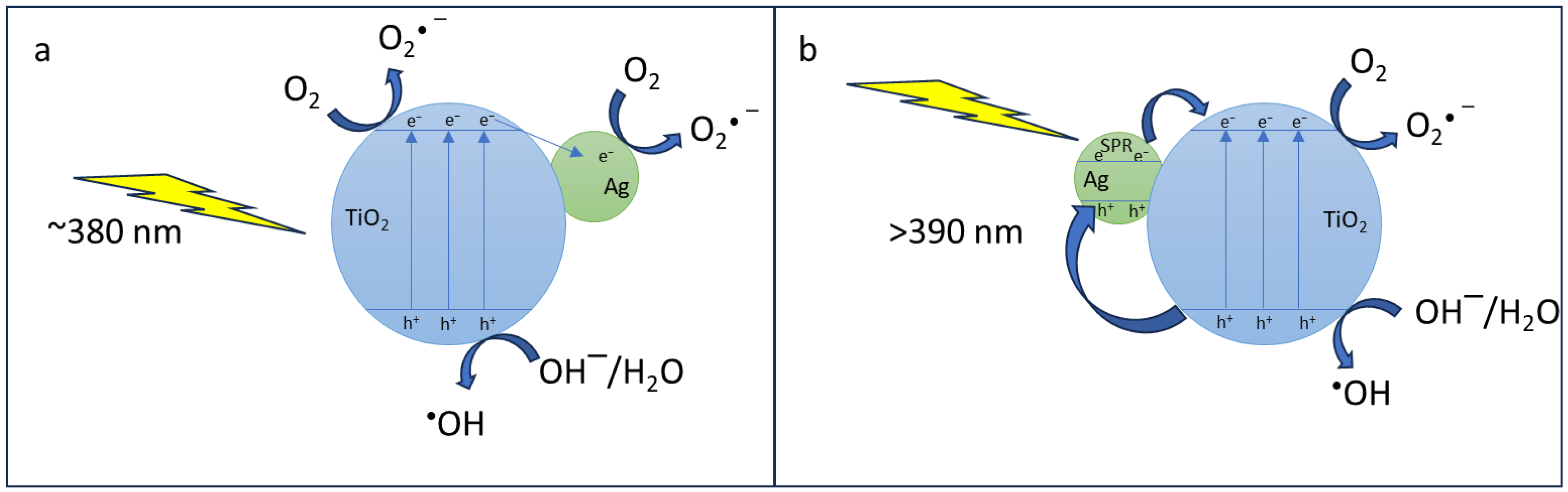
3.3. Photocatalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SERS | Surface-enhanced Raman scattering |
| FESEM | Field emission scanning electron microscope |
| EDS | Energy-dispersive X-ray spectroscopy |
| XRD | X-ray diffractometer |
| EDTA | Ethylenediaminetetraacetic acid |
| MB | Methylene blue |
References
- Amparo-Salcedo, M.; Pérez-Gimeno, A.; Navarro-Pedreño, J. Water Security Under Climate Change: Challenges and Solutions Across 43 Countries. Water 2025, 17, 633. [Google Scholar] [CrossRef]
- Chan, E.Y.Y.; Tong, K.H.Y.; Dubois, C.; Mc Donnell, K.; Kim, J.H.; Hung, K.K.C.; Kwok, K.O. Narrative Review of Primary Preventive Interventions against Water-Borne Diseases: Scientific Evidence of Health-EDRM in Contexts with Inadequate Safe Drinking Water. Int. J. Environ. Res. Public Health 2021, 18, 12268. [Google Scholar] [CrossRef]
- Ejiohuo, O.; Onyeaka, H.; Akinsemolu, A.; Nwabor, O.F.; Siyanbola, K.F.; Tamasiga, P.; Al-Sharify, Z.T. Ensuring water purity: Mitigating environmental risks and safeguarding human health. Water Biol. Secur. 2025, 4, 100341. [Google Scholar] [CrossRef]
- Han, X.X.; Rodriguez, R.S.; Haynes, C.L.; Ozaki, Y.; Zhao, B. Surface-enhanced Raman spectroscopy. Nat. Rev. Methods Primers 2021, 1, 87. [Google Scholar] [CrossRef]
- Langer, J.; de Aberasturi, D.J.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef]
- Roguska, A.; Kudelski, A.; Pisarek, M.; Opara, M.; Janik-Czachor, M. Surface-enhanced Raman scattering (SERS) activity of Ag, Au and Cu nanoclasters on TiO2-nanotubes/Ti substrate. Appl. Surf. Sci. 2011, 257, 8182–8189. [Google Scholar] [CrossRef]
- Song, J.-M.; Yang, W.-R.; Hsu, P.-K.; Chen, S.-Y.; Yasuda, K. Magnetically-manipulatable and self-cleaning lab-on-a-bubble Ag@TiO2@Fe3O4 hollow spheres and their application in SERS detection and photocatalytic degradation. Appl. Surf. Sci. 2024, 673, 160897. [Google Scholar] [CrossRef]
- Ye, S.; Cao, Q.; Wang, Q.; Wang, T.; Peng, Q. A highly efficient, stable, durable, and recyclable filter fabricated by femtosecond laser drilling of a titanium foil for oil-water separation. Sci. Rep. 2016, 6, 37591. [Google Scholar] [CrossRef]
- Lei, S.; Zhao, X.; Yu, X.; Hu, A.; Vukelic, S.; Jun, M.B.G.; Joe, H.; Yao, Y.L.; Shin, Y.C. Ultrafast Laser Applications in Manufacturing Processes: A State-of-the-Art Review. ASME. J. Manuf. Sci. Eng. 2020, 142, 031005. [Google Scholar] [CrossRef]
- Karkantonis, Y.; Pelletier, E.; Barnard, T.; Tuohy, S.; Karnakis, D. Laser drilling of high-density micro-holes on metals using a1064 nm nanosecond pulsed fibre laser. In Proceedings of the LiM 2023 Proceedings: Lasers in Manufacturing Conference 2023, Munich, Germany, 26–29 June 2023; Wissenschaftliche Gesellschaft Lasertechnik und Photonik: Munich, Germany, 2023. [Google Scholar]
- Kabekkodu, S. PDF-4+ (Database); International Centre for Diffraction Data: Newtown Square, PA, USA, 2015. [Google Scholar]
- Gražulis, S.; Daškevič, A.; Merkys, A.; Chateigner, D.; Lutterotti, L.; Quirós, M.; Serebryanaya, N.R.; Moeck, P.; Downs, R.T.; LeBail, A. Crystallography Open Database (COD): An open-access collection of crystal structures and platform for world-wide collaboration. Nucleic Acids Res. 2012, 40, D420–D427. [Google Scholar] [CrossRef]
- Barylyak, A.; Wojnarowska-Nowak, R.; Kus-Liśkiewicz, M.; Krzemiński, P.; Płoch, D.; Cieniek, B.; Bobitski, Y.; Kisała, J. Photocatalytic and antibacterial activity properties of Ti surface treated by femtosecond laser–a prospective solution to peri-implant disease. Sci. Rep. 2024, 14, 20926. [Google Scholar] [CrossRef]
- Challagulla, S.; Tarafder, K.; Ganesan, R.; Roy, S. Structure sensitive photocatalytic reduction of nitroarenes over TiO2. Sci. Rep. 2017, 7, 8783. [Google Scholar] [CrossRef]
- Liu, G.; Yang, H.G.; Sun, C.; Cheng, L.; Wang, L.; Qing, G.; Cheng, H.-M. Titania polymorphs derived from crystalline titanium diboride. Cryst. Eng. Comm. 2009, 11, 2677–2682. [Google Scholar]
- Jin, H.; Cai, Y.; Song, C.; Jin, S.; Lin, Q. Advances in single-molecule surface-enhanced Raman spectroscopy (SERS) for biosensing. Vib. Spectrosc. 2025, 38, 103784. [Google Scholar] [CrossRef]
- Ding, S.Y.; Yi, J.; Li, J.F.; Ren, B.; Wu, D.Y.; Panneerselvam, R.; Tian, Z.Q. Nanostructure-based plasmon-enhanced Raman spectroscopy for surface analysis of materials. Nat. Rev. Mater. 2016, 1, 16021. [Google Scholar] [CrossRef]
- Xiao, G.N.; Man, S.Q. Surface-enhanced Raman scattering of methylene blue adsorbed on cap-shaped silver nanoparticles. Chem. Phys. Lett. 2007, 447, 305–309. [Google Scholar] [CrossRef]
- Del Pilar Rodríguez-Torres, M.; Díaz-Torres, L.A.; Romero-Servin, S. Heparin Assisted Photochemical Synthesis of Gold Nanoparticles and Their Performance as SERS Substrates. Int. J. Mol. Sci. 2014, 15, 19239–19252. [Google Scholar] [CrossRef]
- Atkins, P.W.; De Paula, J. Atkins’ Physical Chemistry, 8th ed.; Oxford University Press: Oxford, NY, USA, 2006. [Google Scholar]
- Hosseini, Z.S.; Haghparast, F.; Masoudi, A.A.; Mortezaali, A. Enhanced visible photocatalytic performance of un-doped TiO2 nanoparticles thin films through modifying the substrate surface roughness. Mat. Chem. Phys. 2022, 279, 125530. [Google Scholar] [CrossRef]
- Carreon, M.A.; Choi, S.Y.; Mamak, M.; Chopra, N.; Ozin, G.A. Pore architecture affects photocatalytic activity of periodic mesoporous nanocrystalline anatase thin films. J. Mater. Chem. 2007, 17, 82–89. [Google Scholar] [CrossRef]
- Sopha, H.; Kashimbetova, A.; Hromadko, L.; Saldan, I.; Celko, L.; Montufar, E.B.; Macak, J.M. Anodic TiO2 nanotubes on 3D-printed titanium meshes for photocatalytic application. Nano Lett. 2021, 21, 8701–8706. [Google Scholar] [CrossRef] [PubMed]
- Awazu, K.; Fujimaki, M.; Rockstuhl, C.; Tominaga, J.; Murakami, H.; Ohki, Y.; Yoshida, N.; Watanabe, T. A plasmonic photocatalyst consisting of silver nanoparticles embedded in titanium dioxide. J. Am. Chem. Soc. 2008, 130, 1676–1680. [Google Scholar] [CrossRef] [PubMed]
- Yussuf, N.A.M.; Li, J.; Jung, Y.J.; Huang, H. Design of high-SERS sensitive substrates based on branched Ti nanorods coated with Ag nanoparticles. Sci. Rep. 2022, 12, 11631. [Google Scholar] [CrossRef] [PubMed]

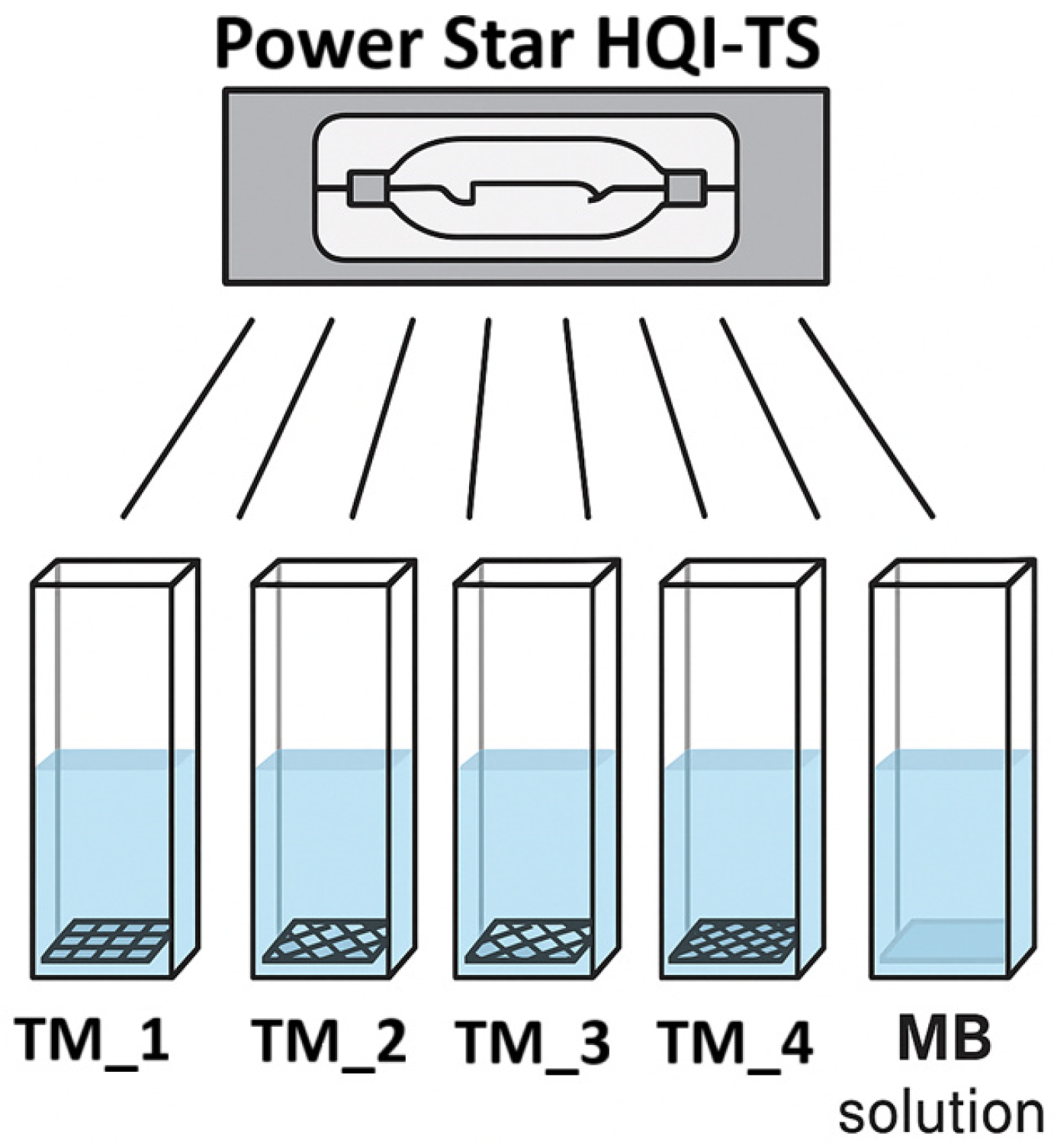
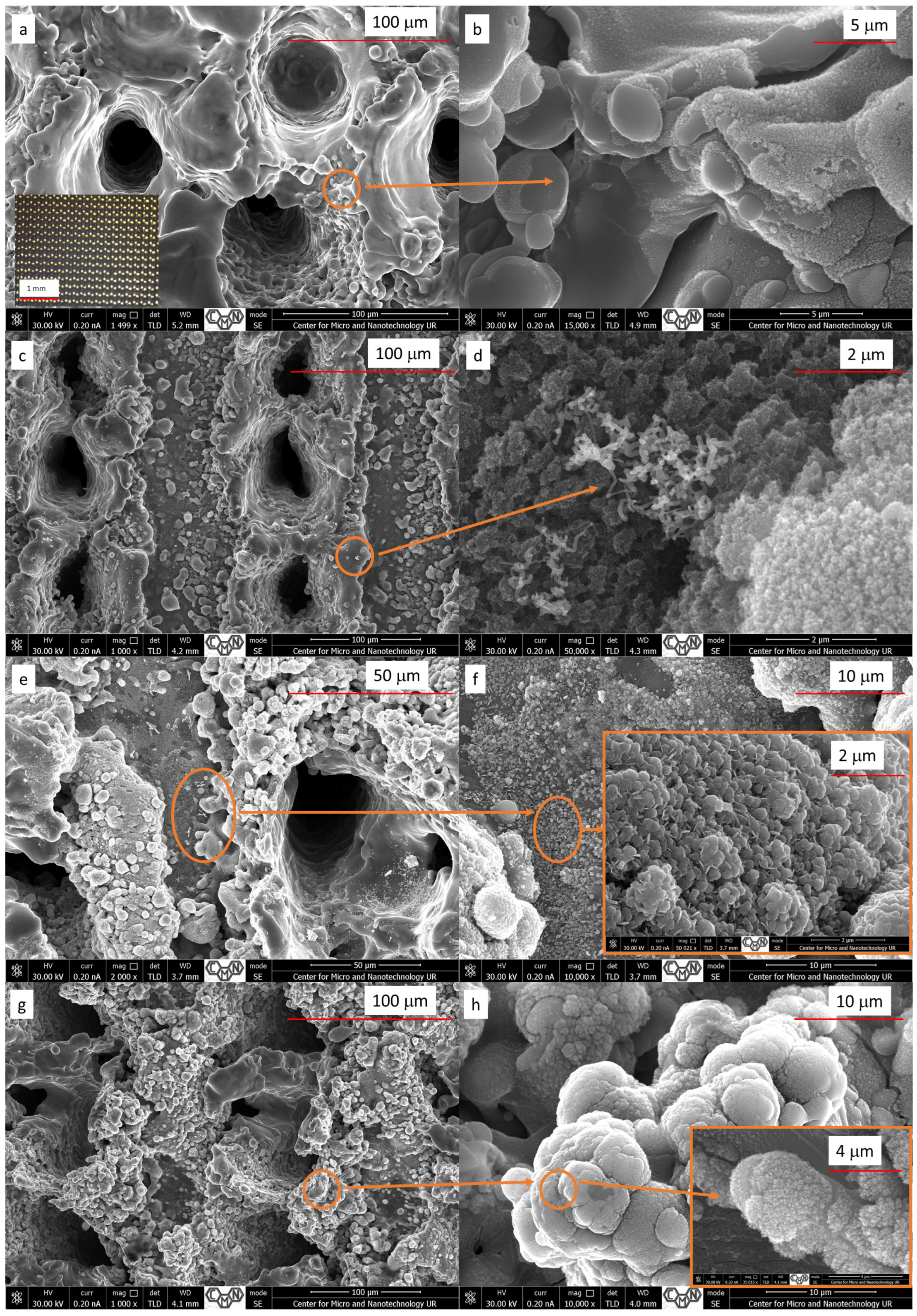
| Specimen | Reagent Composition | Volume and Concentrations | Activation Method | Duration | Post-Treatment |
|---|---|---|---|---|---|
| TM_1 | n.a. | n.a. | n.a. | n.a. | n.a. |
| TM_2 | EDTA + Ascorbic acid (AA) + AgNO3 | 18 mL of mixed solution containing 4 mM EDTA, 3.6 mM AA, and 250 μL of 50 mM AgNO3 | Blue light irradiation (450 nm) | 30 min | Washed with distilled water and blotted dry |
| TM_3 | EDTA + Ascorbic acid (AA) + AgNO3 | Same as TM_2 | Ultrasonic bath (40 kHz) | 15 min | Same as TM_2 |
| TM_4 | H2O2 50% | 100 μL of 50% H2O2 | - | 30 min | Same as TM_2 |
| Specimen | Sa [μm] | Sdq [-] | Sdr [%] |
|---|---|---|---|
| TM_1 | 19.61 | 27.38 | 311.20 |
| TM_2 | 20.16 | 29.42 | 204.13 |
| TM_3 | 19.20 | 25.92 | 203.18 |
| TM_4 | 21.80 | 29.67 | 311.62 |
| Specimen | Ti [% at.] | O [% at.] | Ag [% at.] |
|---|---|---|---|
| TM_1 | 68.90 | 31.10 | 0 |
| TM_2 | 74.38 | 24.22 | 1.4 |
| TM_3 | 73.47 | 21.22 | 5.31 |
| TM_4 | 62.58 | 37.42 | 0 |
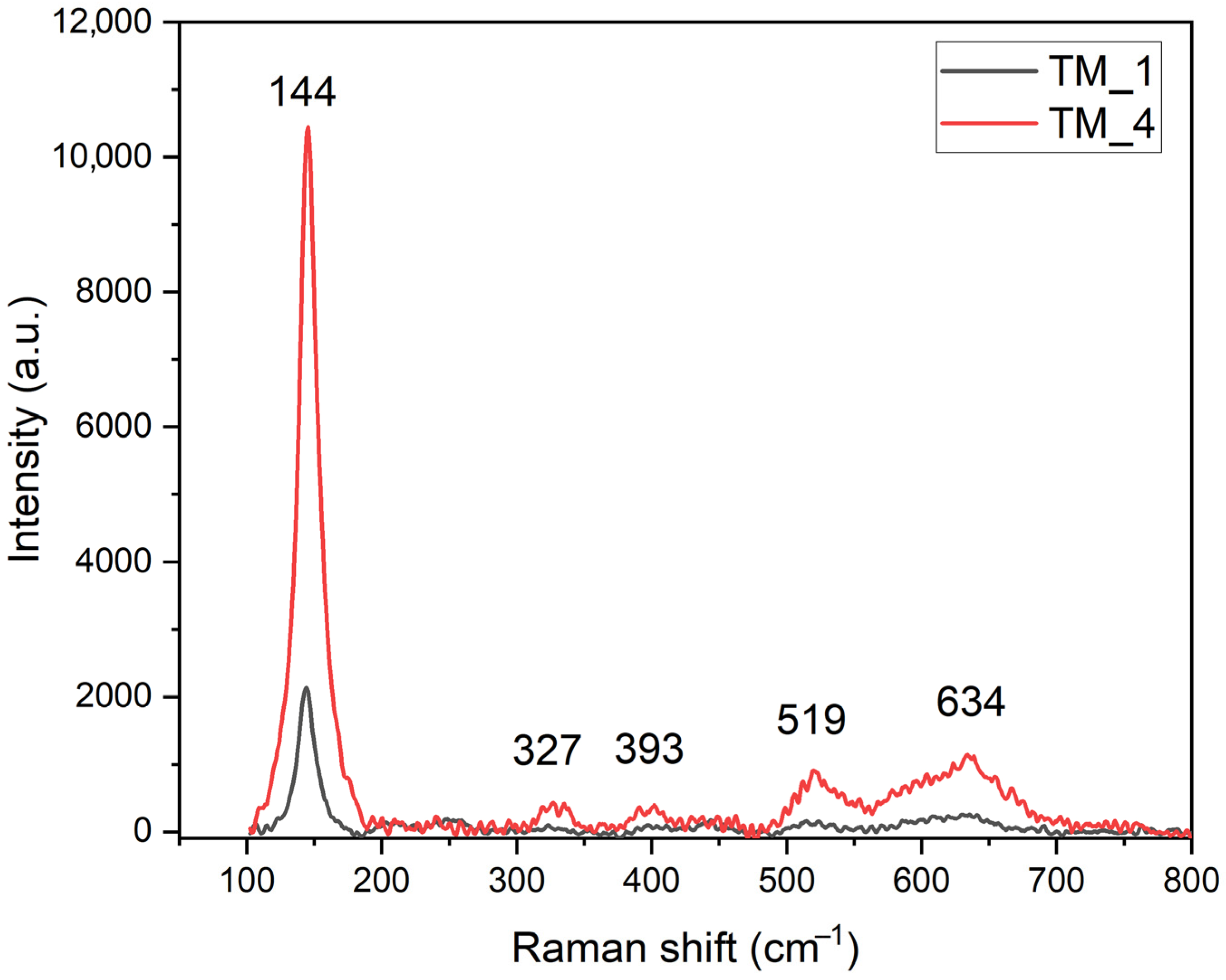
| TM_1 Band Position (cm−1) | TM_4 Band Position (cm−1) | Band Identification | Crystal Structure |
|---|---|---|---|
| 144 | 144 | Eg | Anatase |
| 252 | ---- | Eg | Rutile |
| 330 | 327 | Bg | Anatase |
| 398 | 393 | B1g | Anatase |
| 442 | ---- | Eg | Rutile |
| 516 | 519 | A1g | Anatase |
| 610 | --- | A1g | Rutile |
| 633 | 634 | Eg | Anatase |
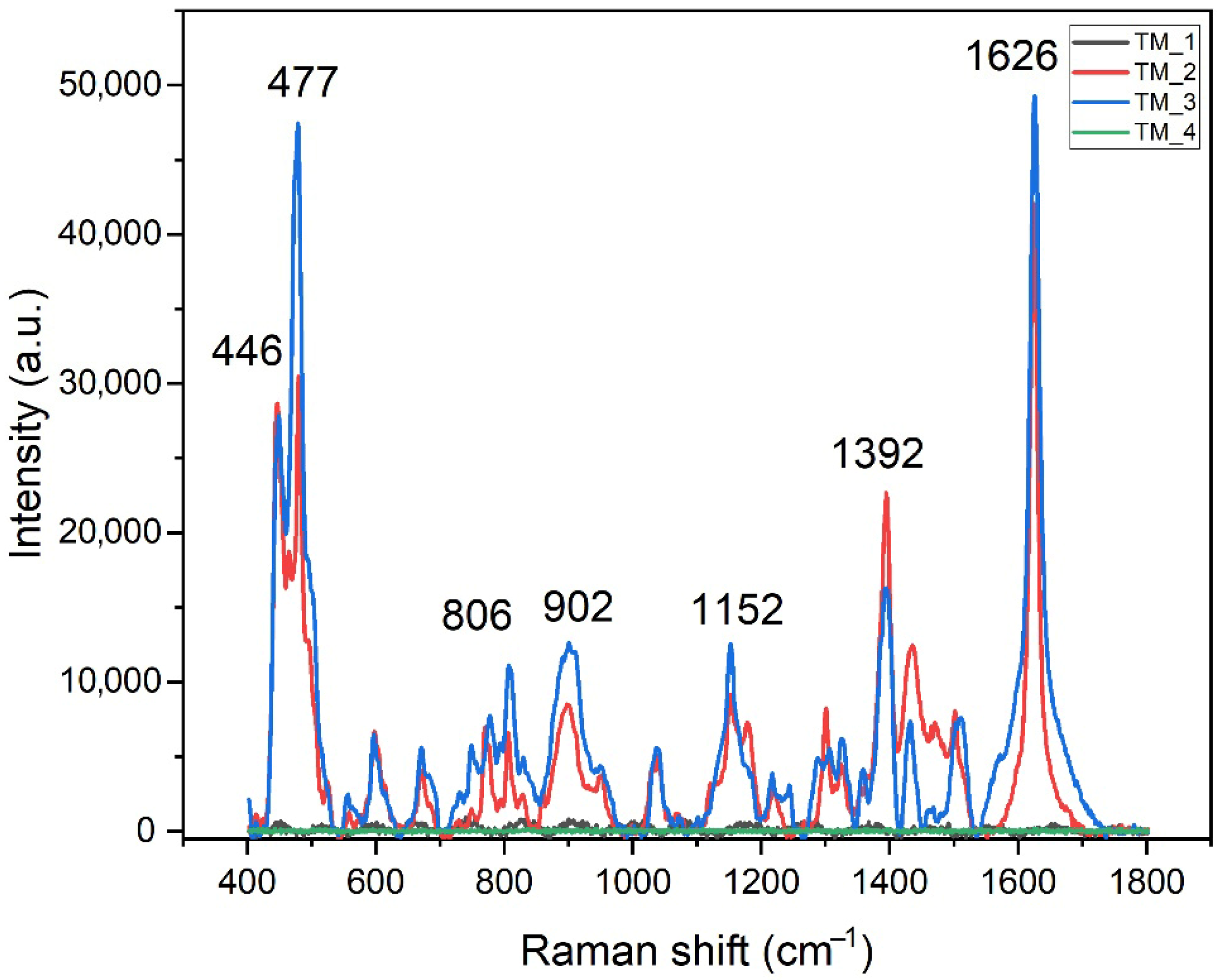
| MB on TM_2 Band Position (cm−1) | MB on TM_3 Band Position (cm−1) | Band Identification |
|---|---|---|
| 1625 | 1626 | ν(C–C) ring |
| 1503 | 1508 | ν(C–C) |
| 1469 | 1463 | ν(C–N) |
| 1435 | 1431 | ν(C–N) |
| 1394 | 1392 | α(C–H) |
| 1325 | 1324 | ν(C–C) in ring |
| 1302 | 1306 | δ(C–C–C) in ring δ(C–H) |
| 1218 | 1217 | ν(C–N) |
| 1152 | 1152 | γ(C–H) |
| 1039 | 1037 | β(C–H) |
| 896 | 902 | C–N/C–S in ring |
| 800 | 806 | γ(C–H) |
| 769 | 776 | γ(C–H) |
| 673 | 670 | γ(C–H) |
| 597 | 597 | δ(C–S–C) |
| 480 | 477 | δ(C–N–C) |
| 446 | 446 | δ(C-N-C) |
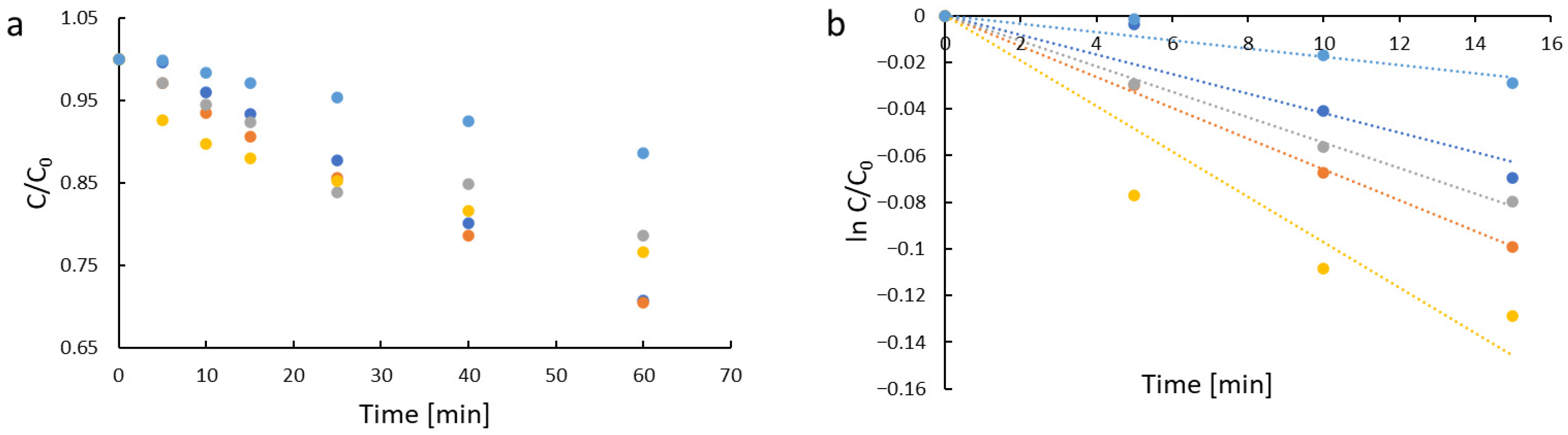
| Sample | kapp [min−1] | R2 |
|---|---|---|
| TM_1 | 4.2 × 10−3 | 0.95 |
| TM_2 | 6.6 × 10−3 | 0.99 |
| TM_3 | 5.4 × 10−3 | 0.99 |
| TM_4 | 9.7 × 10−3 | 0.96 |
| Control (photolysis) | 1.8 × 10−3 | 0.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzemiński, P.; Lazaukas, A.; Meskinis, S.; Wojnarowska-Nowak, R.; Cieniek, B.; Bobitski, Y.; Kisała, J. Nanosecond Laser-Fabricated Titanium Meshes and Their Chemical Modification for Photocatalytic and SERS Applications. Appl. Sci. 2025, 15, 11579. https://doi.org/10.3390/app152111579
Krzemiński P, Lazaukas A, Meskinis S, Wojnarowska-Nowak R, Cieniek B, Bobitski Y, Kisała J. Nanosecond Laser-Fabricated Titanium Meshes and Their Chemical Modification for Photocatalytic and SERS Applications. Applied Sciences. 2025; 15(21):11579. https://doi.org/10.3390/app152111579
Chicago/Turabian StyleKrzemiński, Piotr, Algirdas Lazaukas, Sarunas Meskinis, Renata Wojnarowska-Nowak, Bogumił Cieniek, Yaroslav Bobitski, and Joanna Kisała. 2025. "Nanosecond Laser-Fabricated Titanium Meshes and Their Chemical Modification for Photocatalytic and SERS Applications" Applied Sciences 15, no. 21: 11579. https://doi.org/10.3390/app152111579
APA StyleKrzemiński, P., Lazaukas, A., Meskinis, S., Wojnarowska-Nowak, R., Cieniek, B., Bobitski, Y., & Kisała, J. (2025). Nanosecond Laser-Fabricated Titanium Meshes and Their Chemical Modification for Photocatalytic and SERS Applications. Applied Sciences, 15(21), 11579. https://doi.org/10.3390/app152111579









