Abstract
Electronic stethoscopes address limitations of auscultation with analog stethoscopes, such as the dependency on the physicians’ hearing ability, their experience, and their subjective interpretation. However, electronic stethoscopes currently found on the commercial market fail to exploit the full potential of cutting-edge microphone technology and innovative multi-sensor approaches. Our novel device, called Open-source Modular Electronic Stethoscope (OMES), proposes a modular upgrade to an analog stethoscope that incorporates multiple sensor types and features microphone array capabilities. OMES has been tested for its performance in detecting heart beats but is designed to be applied to other auscultation sites as well. Above that, it can be employed as an educational and potential research platform to promote the development of revolutionary signal processing techniques and artificial intelligence algorithms.
1. Introduction
The human body emits internal sounds that can propagate through tissue to the surface of the skin. These sounds contain crucial information about a patient’s health condition and are an essential tool to diagnose a disease or monitor its progression. Listening to body sounds is called auscultation and is a non-invasive and easy-to-use technique. A doctor performs auscultation by placing the chestpiece of a stethoscope at specific auscultation points on the skin of a patient. The diaphragm of the chestpiece captures skin vibrations induced by internal body sounds, and the stethoscope tubes transmit the sound to the ears of a physician [1]. However, this analog auscultation procedure is strongly affected by the doctor’s hearing abilities and experience as well as his/her subjective interpretation.
Electronic stethoscopes, available on the commercial market since the early 1990s [1], address these issues by recording body sounds with acoustic sensors and relaying them to the user in an amplified fashion. Today, commercial electronic stethoscopes offer a wide range of functionalities in addition to measuring and amplifying body sounds. The user can specify the target organ to auscultate using pre-configured filters, store recordings to replay them at another time, or share them with specialists. In addition, some commercial stethoscopes offer artificial intelligence (AI) classifiers to automatically identify and categorize a medical condition.
The conventional stethoscope is a frequently used instrument in medicine. However, most electronic stethoscopes are manufactured as a fully stand-alone device, and there hardly exist any add-on modules to digitalize the auscultation process of a conventional stethoscope. Paradoxically, state-of-the-art electronic stethoscopes try to mimic conventional stethoscopes and often filter for the human hearing range [2,3], presenting the same limiting frequency range. By disregarding valuable diagnostic information beyond this range, the potential of cutting-edge microphone technology is not fully exploited. Additionally, the stethoscopes often employ only one acoustic sensor to detect body sounds and thereby omit options for sensor fusion techniques that could promote the accuracy of disease diagnosis.
We propose an electronic upgrade to widespread conventional stethoscopes that enables simultaneous analog and digital auscultation. In comparison to other designs on the commercial market, e.g., [3,4,5], and in the scientific literature, e.g., [6,7,8,9], our prototype can embed a traditional stethoscope without interfering with its physical appearance or mechanically modifying the natural sound propagation path. This approach gives fully functional analog stethoscopes a new purpose and keeps them from being discarded in the replacement of a fully electronic stethoscope. To our knowledge, the presented prototype is the first one converting traditional stethoscopes into electronic stethoscopes without intrusive modifications. Furthermore, the design exploits multiple sensor technologies that offer heterogeneous responses and can thus potentially be suitable for different auscultation sites.
We propose an electronic upgrade to widespread conventional stethoscopes that enables simultaneous analog and digital auscultation. In comparison to other designs on the commercial market, e.g., [3,4,5], and in the scientific literature, e.g., [6,7,8,9], our prototype can embed a traditional stethoscope without interfering with its physical appearance or mechanically modifying the natural sound propagation path. This approach gives fully functional analog stethoscopes a new purpose and keeps them from being discarded in the replacement of a fully electronic stethoscope. To our knowledge, the presented prototype is the first one converting traditional stethoscopes into electronic stethoscopes without intrusive modifications. Furthermore, the design exploits multiple sensor technologies that offer heterogeneous responses and can thus potentially be suitable for different auscultation sites.
Above that, our multi-sensor modular electronic stethoscope, called OMES, aims to meet the following three scientific goals:
- Modularity: The prototype offers multiple acoustic sensor types that can be exchanged or combined in a modular fashion, thus allowing for technology comparison. It does not physically interfere with the conventional stethoscope to which it is attached, and therefore the original condition of the stethoscope can be easily restored. The modular approach additionally facilitates the repair or replacement of damaged components and thereby promotes the device’s sustainability factor.
- Microphone array capability: The prototype features a microphone array configuration that enables advanced signal processing algorithms for sensor fusion and potentially drive AI algorithms. In future works, this arrangement can help to locate the sound source and might provide an added value to the diagnosis of diseases.
- Research and Educational Platform: Our electronic stethoscope aspires to be used as an educational and potential research platform. The prototype is compatible with multiple computational platforms, making its application spectrum versatile. In future works, it can enable verifying the performance of signal processing techniques or newly emerging AI algorithms for disease classification with sounds from real-life auscultation scenarios. Furthermore, it can serve as an educational tool to teach students and professionals about existing microphone technologies and auscultation techniques, thereby potentially reinforcing multi-disciplinary collaboration.
This paper is structured as follows. Section 2 provides valuable information about body sounds and how they can be detected with acoustic sensors. Advancements of electronic stethoscopes currently available on the market and in the scientific field are reviewed in Section 3. Our electronic stethoscope is explained in Section 4, and its performance is experimentally evaluated in Section 5. Finally, Section 6 draws a conclusion about OMES and gives an outlook on future works.
2. Background
The stethoscope is an instrument used to collect internal body sounds and serves as a diagnostic tool to detect medical conditions [1]. The major body sounds detectable with a stethoscope originate from movements of the heart, lungs, joints, or abdomen [1]. Today, the stethoscope consists of a chestpiece that detects vibrations of the patient’s skin and a tubing that directs the sound waves to a headset [10]. A sketch of a typical acoustic stethoscope is depicted in Figure 1.
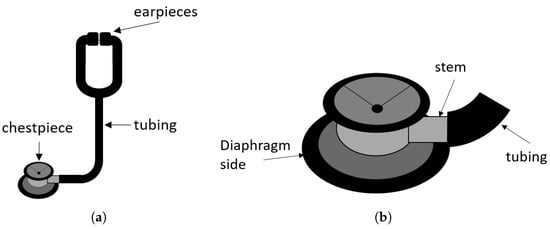
Figure 1.
(a) Sketch of an acoustic stethoscope. (b) Drawing of a typical chestpiece.
2.1. Body Sounds
The electronic stethoscope presented in this work is designed for multiple auscultation sites, but its performance is evaluated on detecting heart sounds. This section therefore has a special focus on the typical heart beat profile and its standard auscultation process, but it also provides information on other body sounds.
2.1.1. Heart Sounds
There are four heart sounds in a cardiac cycle: The first two heart sounds, S1 and S2, can be heard in all healthy hearts. The third and fourth heart sounds, S3 and S4, can be pathologic and are also called ventricular or atrial gallop, respectively [11]. The S1 sound is the highest in amplitude and duration and generates a “crescendo-decrescendo” signal [12]. It is caused by the closure of the tricuspid and mitral valve which is delayed by 20–30 ms (“the split”) [13]. The S2 sound results from the closure of the aortic and pulmonary valve likewise separated by a split of approximately 30 ms. The S3 heart sound arises from rapid ventricular filling during early diastole and can be a sign of a diseased heart in adults. The fourth heart sound S4 comes from the contraction of the atria prior to the onset of a systole. Other sounds detected during a cardiac cycle are called murmurs and suggest a heart malfunction [13]. A visualization of cardiac sounds over time is called a phonocardiogram (PCG), and an example can be seen in Figure 2a.
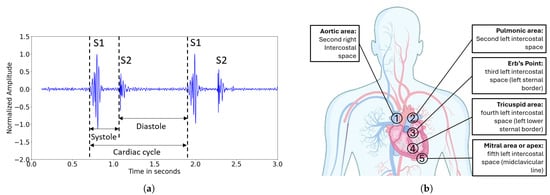
Figure 2.
(a) Visualization of a typical PCG showing the first two heart sounds, S1 and S2, recorded with the Littmann CORE Digital Stethoscope from auscultation point 2. (b) Auscultation scheme of the heart. There are five points for listening to sound originating from the aortic valve (point 1), the pulmonic valve (point 2), the tricuspid valve (point 4), and the mitral valve (point 5). The Erb’s point serves to listen to both semilunar valves similarly.
To make those heart sounds audible, the stethoscope is placed at five dedicated auscultation points [14]. The arrangement of these points on the chest is illustrated in Figure 2b. Auscultation points 1, 2, 4 and 5 allow for detailed listening to sounds originating from the four heart valves individually. Auscultation point 3, the so-called Erb’s point, is the spot where the opening and closing of the aortic and the pulmonary valve can be heard similarly [14]. Table 1 summarizes the features of different heart sounds and gives an overview of their frequency distributions. The S1 and S2 heart sounds contain higher frequency components than the S3 and S4 heart sounds, but in general, cardiac sounds do not exceed 500 Hz [1].

Table 1.
Comparison of features of the four major heart sounds S1, S2, S3, S4, and pathologic heart murmurs. S3 and S4 may be caused by a diseased heart as well and are then referred to as ventricular or atrial gallop, respectively [11]. The auscultation points refer to the auscultation scheme illustrated in Figure 2b.
2.1.2. Lung, Bowel and Knee Joint Sounds
The stethoscope is a versatile instrument and can auscultate a wide range of organs besides the heart. For example, analysis of lung sounds can reveal obstructions in the pulmonary tracts as well as other lung diseases. The breath sounds are a superposition of normal breath sounds during in- and expiration as well as adventitious sounds that are caused by pulmonary disorder. The latter can include crackles that appear as explosive sounds during inspiration, wheezes with an almost musical character throughout the whole breath cycle, or cough sounds, among others [16]. Typically, lung sounds have frequency components between 60 and 1200 Hz [1].
Auscultation of bowel sounds can be a patient-friendly, economic, and time-efficient alternative to other diagnostic tools to detect abdominal diseases. The sounds can be separated into clicks that are short-time, high-pitched sounds, and bursts that take more time. The main frequencies of intestinal sounds are between 50 and 1500 Hz [17].
Knee joints consist of three articulating bones, namely the femur, tibia, and patella. Frictions in the joint induce vibrations that propagate to the surface of the skin and can be picked up by a stethoscope. This examination is ideal for assessing the rehabilitation progress after joint injuries. Frequencies emitted by the knee joints lie between 15 and 21,000 Hz [18].
Table 2 summarizes the typical frequency ranges for the main body sounds detectable with a stethoscope.

Table 2.
Overview of auscultation sites and their corresponding frequencies.
2.2. Acoustic Sensors
Electronic stethoscopes enable digital auscultation and allow the detection of body sounds with acoustic sensors. This section gives an overview of various sensor types that can be considered in an electronic stethoscope design.
2.2.1. Electret Condenser Microphones
Electret condenser microphones are capacitive microphones [19]. They consist of a fixed backplate and a flexible diaphragm that form a capacitor [19]. Any movement of the membrane results in a change in capacitance relative to the deflection of the membrane and, ultimately, in a change in output voltage [20].
This type of microphone can be purchased at low cost [20] in the range of a few cents and is typically a few millimeters to centimeters large. It shows low sensitivity, and therefore its output voltage must be amplified and filtered before it can be digitized with an analog-to-digital converter (ADC) [20].
2.2.2. Piezoelectric Transducers
These kinds of sensors exploit the piezoelectric effect. This phenomenon occurs when a piezoelectric crystal is deformed and generates charges on conductive plates surrounding the piezoelectric material. The evolving potential is proportional to the input deformation and is measured as an output signal [19].
An advantage of piezoelectric transducers is that they do not need to be actively driven by a power source due to their intrinsic voltage creation capability. Their analog output signals have to be digitized with an ADC. The authors of [21] point out that sounds captured with a piezoelectric transducer are different in tonality than sounds heard with an acoustic stethoscope, and [22] states that piezoelectric elements require careful stabilization to operate effectively. In conventional auscultation scenarios, the chestpiece is placed in direct contact with the skin to pick up vibrations induced by internal body sounds. In a similar manner, piezoelectric microphones can detect skin vibrations, making them particularly appropriate for use in electronic stethoscopes [1].
2.2.3. MEMS Microphones
Microphone engineering has been revolutionized by the introduction of microelectromechanical system (MEMS) technology, miniaturizing devices and therefore making them light-weight and of a compact size [19]. Nowadays, MEMS microphones can be found in manifold configurations exploiting various working principles such as piezoelectric, piezoresistive, capacitive, and optical MEMS microphones [19].
MEMS microphones come with several advantages over previously discussed acoustic sensors, ranging from their compact size to low power consumption, in particular, due to optimized current consumption [23]. The output of MEMS microphones can be analog or digital. Digital MEMS microphones provide the output immediately in a digitized form and therefore require a minimum number of components to which they need to be connected. This ultimately leads to a smaller size of manufactured printed circuit boards (PCBs) and an increased ease of usage. Furthermore, the authors of [24] suggest that digital outputs might be less susceptible to interferences from surrounding devices than analog outputs.
3. Related Work
3.1. Commercial Electronic Stethoscopes
One of the first electronic stethoscopes introduced to the industrial market was the E-Scope II presented by Cardionics (Houston, TX, USA) in 1991. Its chestpiece recorded electrocardiography (ECG) signals that were converted to PCG signals by a computer embedded in a housing adjacent to the tubing [25]. Today, Cardionics (Houston, TX, USA) additionally incorporates a microphone in their device that acquires sounds directly.
In the following years, other manufacturers such as JABES by GST (Seoul, South Korea) or Thinklabs’ ONE (Thinkslabs, CO, USA) entered the market. Their system designs did not contain any internal wireless transmission module, and therefore computation, sound storage, and acoustic output were performed on the device. The inventor of Thinklabs’ ONE (Thinkslabs, CO, USA), incorporated all necessary components in a chestpiece that can be connected to ordinary auxiliary headphones.
Today, all devices come with the option to transmit data wirelessly and some abandon stethoscope tubings, such as eKuore’s Pro stethoscope (eKuore Medical Devices, Burjassot, Spain). Others offer a modular system that allows the user to remove the tubing and listen to the sound remotely. This approach is taken, for instance, by Eko Health Inc. (Emeryville, CA, USA) with their Eko Core 500. An additional feature of the Eko Core 500 is a built-in ECG that is displayed on the chestpiece during auscultation. Another noteworthy device is the Littmann CORE published by 3M (Maplewood, MN, USA) that offers to switch between amplified and analog listening modes.
Furthermore, manufacturers are beginning to incorporate diagnostic AI into their stethoscopes. For example, Eko Health Inc. (Emeryville, CA, USA) published an app that is capable of detecting heart diseases, such as atrial fibrillation or brady- and tachycardia. The Eko app can be connected to the Eko Core 500 as well as the Littmann CORE.
Table 3 summarizes the electronic stethoscopes currently available on the market with regard to their technical specifications, and Figure 3 shows an example of commercially available electronic stethoscopes, i.e., the Littmann CORE Digital Stethoscope by (3M, Maplewood, MN, USA). Most companies do not publish the sensor type or the amount of sensors they employ. This lack in information limits the degree of comparison between the commercial electronic stethoscopes. The table reveals that all considered manufacturers filter for the human hearing range. However, there is no common standard among manufacturers for the filtering bandwidths.

Table 3.
Comparison of the state-of-the-art electronic stethoscopes on the commercial market sorted by their release dates: NM: not mentioned, PCG: phonocardiogram, ECG: electrocardiogram.
Figure 3.
Photograph of the Littmann CORE Digital Stethoscope by 3M, Maplewood, (MN), USA.
3.2. Scientific Electronic Stethoscopes
While commercial manufacturers of electronic stethoscopes primarily focus on recording heart and lung sounds, researchers in the scientific domain also consider sounds originating from the joints and the abdomen. This section is dedicated to providing an overview of the recent scientific advances made in recording body sounds, and Table 4 summarizes their key features.
Teague et al. [18] proposed a wearable knee joint sound acquisition unit to continuously evaluate the rehabilitation process after a musculoskeletal injury. They collect sound using three microphone types simultaneously: namely a piezoelectric film, an electret microphone, and a MEMS microphone. Wang et al. [36] developed a flexible patch for continuous real-time monitoring bowel sounds. The patch holds a MEMS microphone to sense bowel sounds and a Bluetooth chip to send them to an external mobile phone for inspection and analysis. Another wearable prototype for continuous breath monitoring is being developed by Yilmaz et al. [22]. The authors claim that with a wearable device, the limitations of stethoscopes, such as user-dependent improper positioning of the sensor or insufficient pressure on the skin, can be overcome. As a sound transducer, they opted for a piezoelectric film embedded in a silicone rubber that is in direct contact with the skin to improve the transducer’s stability and reduce ambient noise. In 2023, Soo Hyun Lee et al. [37] developed a wearable, flexible patch that continuously monitors respiratory function in real time. The patch can be glued to the patient’s back with medical adhesive. A flexible PCB holds all relevant components for capturing lung sounds: a battery powering a microcontroller unit (MCU) and a MEMS microphone. The electronic circuit is protected by a skin-colored cover that additionally functions as a barrier against surrounding noise.
Inspired by the COVID19 pandemic that required medical personnel to wear protective suits and consequently impaired the auscultation process, Yang et al. [9] developed a contactless auscultation device for heart and lung sounds. The authors found a simple and cost-effective way to electrify a regular stethoscope chestpiece by placing a collar microphone behind its stem. Cowdhury et al. [6] proposed a portable stethoscope by mounting an electret microphone in a regular stethoscope tubing close to the chestpiece. The acoustic signals are being filtered, pre-amplfied and transmitted to a heart sound classification algorithm. In [38], Chuchnowska et. al published an electronic stethoscope specifically designed for remote auscultations with virtual medical supervision. The prototype consists of a digital MEMS microphone breakout board glued to a stethoscope chestpiece that is incorporated in a modular 3D-printed casing. Duggan et al. [39] employ an optical transducer that captures vibrations from a reflective membrane. With this approach, they claim to reduce the impact of ambient noise and are able to capture low-frequency components that might improve medical diagnosis.
Further advances in electronic auscultation can be achieved by deploying multiple microphones simultaneously. Tian Wang et al. [8] arranged 16 electret condenser microphones in a 4 × 4 array to record heart sounds. The authors state that choosing microphone arrays increases the amplitude of the total signal output, enhances sensitivity, and further enlarges the auscultation area, therefore providing more accurate heart sound recordings. Han et al. [40] also adopted a sensor array approach by fabricating a 2 × 2 array with stretchable piezoelectric transducers. They state that the stretchability enhances cardiac and respiratory long-term monitoring. In [41], McDonald et al. propose a novel design approach that features six piezoelectric transducers that increase the sensing area and can guide non-skilled users in the optimal placement of the device. During auscultation, the device is held by the patients themselves and can be applied over clothing, making it especially suitable for telemedicine.
Some researchers combined their devices with other biosignals that can be collected from the same auscultation site. For example, Park et al. [7] presented a light-weight, ergonomic prototype that can simultaneously measure heart sounds and pulse. The front side of the device is placed on the chest, and heart sounds are being collected with a digital MEMS microphone mounted behind a stethoscope bell. The back of the device is equipped with a photoplethysmography (PPG) sensor that simultaneously takes pulse measurements. Lee et al. [42] combined heart sound monitoring with ECG measurements. An electret microphone collects heart sounds, while an ECG patch simultaneously measures electrical activity. After signal synchronization, the aligned signals are displayed on a mobile phone and can be analyzed by a heart murmur classification algorithm. A multi-modal, multi-sensor approach has been taken by Giordano et al. [43] who designed a device consisting of 48 electret condenser microphones that detect heart sounds from all auscultation sites simultaneously and three electrodes to additionally sample ECG. They embedded all sensors in a flexible PCB to enable conformity with the human chest and allow untrained users to perform auscultation, e.g., in a homecare setting. Another multi-modal approach has been taken by Klum et al. [44] who suggested a patch for long-term auscultation by measuring body sounds and ambient noise, and performing ECG, impedance pneumography and actigraphy in parallel. Other researches propose universal electronic stethoscopes that can be applied to various auscultation sites by switching between dedicated frequency filters. This approach has, for example, been taken by Wu et al. [45], who presented a prototype that comprises interchangeable analog filters for lung and heart sounds.

Table 4.
Electronic stethoscopes presented in the scientific literature sorted by year, ECM: electret condenser microphone; and NM: not mentioned.
Table 4.
Electronic stethoscopes presented in the scientific literature sorted by year, ECM: electret condenser microphone; and NM: not mentioned.
| Ref. | Release Year | Application | Sensor Type | Number of Sensors | Sample Rate [kHz] | Frequency Bandwidth [Hz] |
|---|---|---|---|---|---|---|
| [18] | 2016 | knee | piezoelectric film, ECM, analog MEMS | 1 | piezo and MEMS: 50 electret: 44.1 | piezo: 15k–21k electret: 7k–16k |
| [6] | 2019 | heart | ECM | 1 | 2 | 20–600 |
| [36] | 2019 | abdomen | analog MEMS | 1 | 2 | <=600 |
| [44] | 2019 | heart and lung | digital MEMS | 2 | 10,000 | heart: 20–150, lung: 75–2500 |
| [22] | 2020 | lung | piezoelectric film | 1 | 5 | 100–1600 |
| [8] | 2021 | heart | ECM | 4 × 4 | 8 | <=4000 |
| [9] | 2021 | lung | collar microphone | 1 | NM | NM |
| [45] | 2022 | heart and lung | ECM | 2 | 44.1 | heart: <=400, lung: 100–2000 |
| [7] | 2022 | heart | digital MEMS | 1 | NM | 20–400 |
| [42] | 2023 | heart | ECM | 1 | 2 | 20–300 |
| [43] | 2023 | heart | ECM | 48 | 1 | >=2 |
| [37] | 2023 | lung | digital MEMS | 1 | NM | heart: 20–200 lung: 50–500 |
| [40] | 2024 | heart and lung | piezoelectric sensor | 2 × 2 | NM | NM |
| [39] | 2024 | heart | optical sensor | 1 | 2.9 | NM |
| [38] | 2025 | heart | digital MEMS | 1 | NM | NM |
| [41] | 2025 | heart | piezoelectric sensors | 6 | 5.120 | >=20 |
3.3. The Need for a Multi-Sensor Modular Approach
Table 3 and Table 4 summarize the features of commercial and scientific state-of-the-art electronic stethoscopes proposed in the preceding decades. While a high variety of devices have been proposed ranging from modifications of analog stethoscopes to wearable sound acquisition modules, developers of electronic stethoscopes often disregard the full potential of cutting-edge microphone technology and innovative multi-sensor approaches. Specifically, they tend to employ only one acoustic sensor instead of microphone arrays, precluding any sensor fusion techniques and failing to optimize the design for sensitivity. Secondly, the captured signals are often filtered for the human hearing range, although frequencies outside this range can also contain diagnostically important details and could potentially be relevant for decision-support algorithms. Thirdly, most developers opt for a monolithic design approach, making it impossible to easily repair or exchange damaged components, thereby contributing to electronic waste and reducing the design’s flexibility. Lastly, most systems are battery driven and become fully inoperable in the event of energy depletion, which can be extremely inconvenient in emergency situations.
Our multi-sensor modular electronic stethoscope OMES seeks to address these shortcomings of previously published electronic stethoscope design approaches. Traditional stethoscopes are a widely spread instrument in medicine, and medical personnel are trained for their use. For this reason, the prototype presented in this paper is an add-on device that enables an easy electrification of conventional stethoscopes. Furthermore, this approach prevents the device from becoming fully non-functional in the event of energy depletion, as it can always be used in analog mode. The prototype offers microphone array arrangements and is compatible with three different acoustic sensor types that can be arbitrarily interchanged due to the prototype’s modular design. As every type of acoustic transducer comes with different acoustic properties, this interchangeability between different acoustic sensors enables an auscultation process tailored to the target body sound. In addition, the device is modular-compatible with multiple computational platforms, offering a versatile application spectrum and making it an optimal choice to be employed in education or research.
4. OMES: An Open-Source Multi-Sensor Modular Electronic Stethoscope
An illustrative sketch of OMES is depicted in Figure 4a. The add-on device is attached to a conventional stethoscope chestpiece and offers four connectors configured in an array arrangement to mount acoustic sensors. At this stage of the device, the user can freely choose from three types of transducers, namely an electret condenser microphone, a piezoelectric transducer, and a digital MEMS microphone. Detected body sounds can be transmitted wirelessly, for example, to a personal computer (PC). The whole setup is encapsulated by a casing that consolidates the components and protects the device from mechanical damage. As shown in Figure 4b, the proposed device consists of three highly flexible layers:
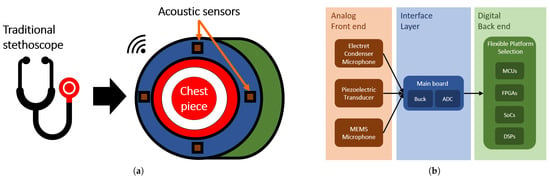
Figure 4.
(a) Schematic of the multi-sensor modular stethoscope design in form of a chestpiece add-on device. The device surrounds the conventional chestpiece highlighted in red. Acoustic sensors arranged in an array of four are shown in brown. (b) System design of the developed electronic stethoscope. The analog front end consists of three acoustic sensors that transmit data to the interface layer represented by a main board. The device is powered by a buck converter and features an ADC that digitizes the signals received from the analog front end. The back end offers a flexible platform selection, and the user of the device is free to choose the system most appropriate for her or his application.
- Analog front end: comprises the three acoustic sensors deployed.
- Interface layer: consists of a main board that collects measured data and converts analog signals into digital signals using an ADC.
- Digital back end: is compatible with a wide range of computational platforms.
In accordance with the signal flow path illustrated in Figure 4b, the following subsections detail the analog front end, the interface layer, and the digital back end with special emphasis on their modularity.
4.1. Analog Front End
The prototype features three acoustic sensor types: namely, an electret condenser microphone, a piezoelectric transducer, and a digital MEMS microphone. In compliance with the modular design approach, each of these transducers along with their electronic circuit is installed on a dedicated PCB, here also called a breakout board, to allow deliberate interchange between them. In the following text, the front end PCBs are explained in detail by reasoning about the electronic components used and describing their interfacing circuits.
4.1.1. Electret Condenser Microphone Board
Condenser microphones have been used frequently in scientific literature and have been proven to accurately capture heart sounds, see, e.g., [6,8,42,45], among others. Consequently, these microphones can be used as a reference to compare the performance of other microphone types. In addition, electret condenser microphones are very cost-effective. Therefore, the CMA-4544PF-W electret condenser microphone is one of the three acoustic sensors deployed in the design. It is an omnidirectional electret condenser microphone and promises a flat frequency response curve between 50 Hz and 2 kHz that covers the typical body sound frequencies. It offers a sensitivity of −44 dBV at 94 dB sound pressure level (SPL) and a signal-to-noise ratio (SNR) of 60 dBA at 1 kHz. Its power consumption is determined by a current consumption of maximally 0.5 mA and an operating voltage between 3 and 10 V.
A block diagram of the circuit designed to operate the electret condenser microphone is shown in Figure 5. The electret condenser microphone is connected through a resistor of 2.2 k to a 5 V voltage supply. The DC component of the output signal is removed when passing the coupling resistor of 10 µF. Subsequently, the signal is amplified and filtered by two active low-pass-filter (LPF) stages with cutoff frequency of 2 kHz, in which the second offers a customizable amplification factor through the means of a trimmer potentiometer. Subsequently, a 50 Hz notch filter removes mains hum frequency components, and the signal is filtered another time by a second order Sallen-Key LPF. Furthermore, the signal is referenced to be around 1.25 V to stay within the voltage range that the ADC in the interface layer expects.
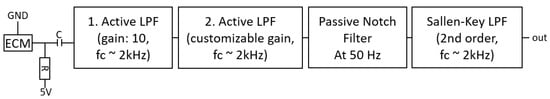
Figure 5.
Electrical circuit that interfaces the electret condenser microphone (CMA-4544PF-W). The microphone is powered with the typical application circuit for electret condenser microphones (ECMs). The output signal is amplified and filtered by two active LPF stages, and then it is subsequently filtered by a notch filter with a target frequency of 50 Hz mains hum and another LPF.
Figure 6 shows the breakout board produced that holds the electret condenser microphone. The board is 30.4 mm in length and 17.1 mm in width.

Figure 6.
Photograph showing the front and back sides of the developed electret microphone board with a one-euro coin for scale reference. The breakout board is 30.4 mm long and 17.1 mm wide.
4.1.2. Piezoelectric Transducer Board
Body sounds propagate to the skin’s surface and induce skin vibrations. Piezoelectric transducers can convert these vibrations into electrical signals and ultimately into audio signals, thereby mimicking the behavior of a traditional stethoscope diaphragm. For this reason, the second acoustic sensor employed is a piezoelectric element, namely the CEB-20D64 manufactured by Same Sky (Lake Oswego, OR, USA). This sensor is a typical piezoelectric disc with two wire leads carrying the voltage changes when the transducer is deformed. It is 20 mm in diameter and has a resonance frequency of 6.5 kHz.
Figure 7 shows a block diagram of the electrical circuit that interfaces the piezoelectric transducer. The acoustic signals are fed into a differential amplifier to obtain the voltage differences between the backplate of the transducer and the piezoelectric material. This stage features passive LPFs for both differential inputs at a cutoff frequency of 1.6 kHz and an amplification factor of 1000. Similarly as for the electret condenser microphones, the sensed signals are referenced around 1.25 V to stay within voltage ranges expected by the ADC in the interface layer. The first stage is followed by a buffer amplifier with a unity gain of 1. This stage has shown to stabilize the circuit and offers the possibility to be bypassed. Finally, the signal is filtered by an active Sallen-Key LPF with a cutoff frequency of 2 kHz.
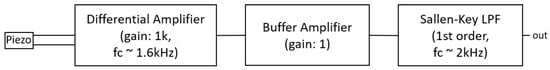
Figure 7.
Electrical circuit that interfaces the piezoelectric transducer (CEB-20D64). The signal is captured with a differential amplifier that provides the difference between the backplate of the transducer and the piezoelement. Then a buffer amplifier with gain 1 follows. There is a bypassing possibility to this stage. Lastly, the signals are filtered with a Sallen-Key LPF.
The piezoelectric transducer needs to be encapsulated in a casing due to two reasons. First, the transducer consists of a conductive material, and the measured signals get distorted when in direct contact with the electrically conductive human skin. Second, the piezoelectric effect is based on the compression of the piezoelectric material, hence the transducer’s backside must be fixed. Figure 8 shows an image of the encapsulated piezoelectric transducer. The transducer is glued with superglue to the lower part and the upper part of the casing. The lower part fixes the outer surface of the transducer while leaving a cavity below the transducer. In case of mechanical stress, the top part presses the transducer into the cavity. In response to this deformation, the wire leads of the piezoelectric element experience a voltage change.
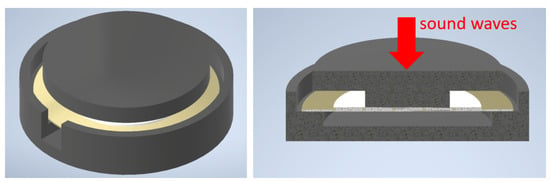
Figure 8.
Piezoelectric transducer (gold–white) encapsulated by a casing consisting of two black parts. (Left): isometric view on the assembly; a notch is left open for the wires of the transducer. (Right): cross-section of the setup, sound waves exert forces on the center of the transducer, leading to its deformation and, ultimately, to an electric signal.
The final physical appearance of the encapsulated piezoelectric transducer, along with its PCB, is shown in Figure 9. The developed PCB has a width of 17.0 mm and a length of 20.4 mm.

Figure 9.
Photograph showing the front and back sides of the developed PCB and the casing of the piezoelectric transducer with a one-euro coin for scale reference. The breakout board is 20.4 mm long and 17.0 mm wide.
4.1.3. Digital MEMS Microphone Board
MEMS microphones offer a light-weight and compact alternative to the previously presented transducers. They generally operate on low power consumption and are easy to install. Digital MEMS microphones, in particular, convert analog audio signals internally into a digital form, thereby making audio data immediately available without the need for intermediate filtering, amplification, or conversion steps. This property makes them less susceptible to noise and interference from surrounding devices. Therefore, a digital MEMS microphone, namely the DMM-4026-B-I2S-R, is selected as the third type of acoustic transducer to capture body sounds. The output of the chosen microphone is formatted with the I2S standard. It was specifically opted for this standard, as it transmits data over a dedicated data line, making audio data directly available to access without any complex decoding steps, as opposed to the PDM standard. The DMM-4026-B-I2S-R promises a flat frequency response ranging from 20 Hz to 20 kHz, thus covering all audible frequencies. The microphone provides an 18-bit precision resolution, a sensitivity of −26 dBV at 94 dB SPL, and an SNR of 64 dBA at 1 kHz. Its power consumption is composed of a voltage supply of 1.8 V and a current consumption of 820–1000 µA.
The digital MEMS microphone is electronically interfaced as suggested in its datasheet. As all microphone breakout boards are supplied with 5 V, a low-dropout regulator (LDO) scales down the input voltage to the required level of 1.8 V for the MEMS microphones. The PCB holding the MEMS microphone is 13.02 mm long and 17.1 mm wide and can be inspected in Figure 10.
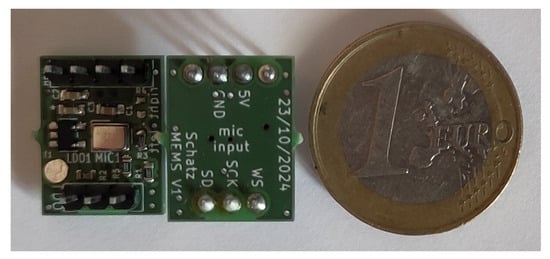
Figure 10.
Photograph showing the front and back sides of the developed digital MEMS board with a one-euro coin for scale reference. The breakout board is 13.02 mm long and 17.1 mm wide.
4.2. Interface Layer
The second layer in the signal transmission path shown in Figure 4b is represented by a main board that acts as an interface between the analog front end and the digital back end. A buck converter powers the circuitry of the main board with 3.3 V and the microphone boards with 5 V. Once again, to promote modularity of the design, the buck converter is manufactured on an individual PCB and can be easily replaced in case of damage.
Figure 11a shows the front side of the developed main board PCB. Four female pin headers offer a connection to the individual microphone breakout boards. Each of them is represented by a 1 × 4 pin header and a 1 × 3 pin header. The 1 × 4 pin header supplies the microphone boards with voltage and manages the communication with the analog microphones, while the 1 × 3 pin header establishes the I2S interface between the digital microphones and the back end. This arrangement serves two main purposes: first, it avoids accidentally switching the orientation of the microphone boards when plugging them onto the main board, and second, this design allows for keeping analog and digital voltage planes galvanically isolated, thereby reducing noise and interferences. A circular ground (GND) trace additionally acts as a barrier to current flow between these voltage domains. Lastly, this configuration guarantees a secure connection of the microphone boards to the main board. The main board’s back side, shown in Figure 11b, offers pin headers in the Arduino UNO R3 standard, which allows every back end device with this format to be used. The diameter of the PCB is 96 mm, leaving enough room in its center to mount a traditional stethoscope chestpiece with a diameter of 47 mm.
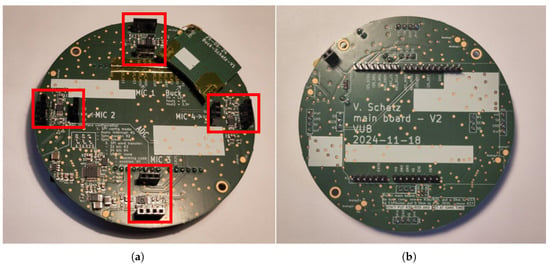
Figure 11.
Photograph showing the front and back sides of the developed main board. (a) The front side shows the headers, highlighted by red rectangles, to which the microphone boards can be attached, as well as the PCB of the buck converter that powers the whole setup. (b) The back side shows the physical interface to the back end with the Arduino UNO R3 standard.
The principal task of the main board is to receive audio data from the individual microphone breakout boards and convert it to a format that the back end can interpret. The outputs of the digital MEMS microphones are immediately provided in the I2S format and can therefore be forwarded without any further processing steps to the back end. For analog signals from the electret microphone and the piezoelectric transducer, the main board contains an ADC, namely the ADS131A04, that digitizes the signals and sends them over Serial Peripheral Interface (SPI) to the back end. The ADS131A04 is a four-channel delta-sigma ADC with a data rate of up to 128 kSPS and a high resolution of 24 bits ensuring superior accuracy, which is crucial for audio applications. This ADC was explicitly chosen for three main reasons. First, it features the delta-sigma method that oversamples the analog signal at a much higher frequency than the Nyquist rate, consequently reducing the risk for aliasing and promising little presence of noise in the converted signal. Secondly, it offers a wide range of programmable features such as the oversampling ratio (OSR) or the digital gain setting for individual ADC channels. And finally, because it runs on a low power consumption of 7.2 mW, the ADC can be configured in multiple SPI modes, such as asynchronous interrupt and synchronous master or slave mode. The PCB of the main board is designed in a flexible configuration that allows for switching between SPI modes through minor changes, in essence resistor replacements, on the PCB.
Figure 12 shows the interface diagram using the example of the asynchronous SPI interface. This communication includes a DRDY line that indicates the time point when data are ready to be sampled, a CS line that starts the data read out, a clock line SCK, and two lines MISO and MOSI for data exchange between the ADC and the back end device. The digital MEMS microphones are immediately interfaced to the back end device.
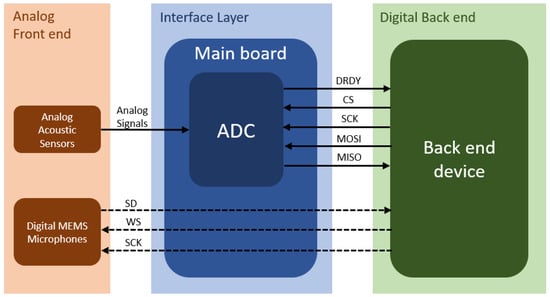
Figure 12.
Interface diagram between analog front end, main board and digital back end. The analog signal conversion is shown in solid lines, and the digital signal transmission is shown in dashed lines. The analog signals from the electret condenser microphone and piezoelectric transducer are converted with an ADC to digital data. The ADC communicates over asynchronous SPI with the digital back end. The digital audio data of the MEMS microphones are immediately sent over an I2S interface to the digital back end.
4.3. Digital Back End
The final stage of the signal transmission path shown in Figure 4b is the back end to which all computational platforms with Arduino UNO R3 compatibility can connect. In the following, some compatible digital back ends are presented, emphasizing the prototype’s flexibility. Thereafter, the back end realized in this work is described, highlighting the MCU deployed and explaining the firmware developed.
4.3.1. Flexible Platform Selection
The options for back end devices are manifold and range from typical MCUs to high-performing FPGAs. Commercially available platforms with suitable header configurations exist on the market. The following text briefly summarizes the advantages of suitable systems.
- Microcontroller Units: MCUs are general-purpose integrated circuits (ICs) that incorporate CPU, memory, and I/O pins in one compact chip. They are suited for simple general-purpose applications and are easy to operate. MCUs are available at low cost and are energy-efficient. The Adafruit METRO 328, for example, incorporates the ATmega328P, is interoperable with the Arduino IDE, and can be used for simple projects. Adafruit also provides the ESP32-S3 with Arduino-compatible output headers. This powerful chip supports both WiFi and Bluetooth, making it most suitable for Internet-of-Things (IoT) projects. Another example is the ESPDuino that features an ESP32-WROOM-32.
- Digital Signal Processors: DSPs are low-cost microprocessors designed to perform efficient signal processing that is crucial for audio applications [46]. The prototype presented herein could serve as a framework for testing and engineering novel audio processing technologies.
- System-on-Chips: SoCs are a combination of multiple computer components in a single IC and thus can execute diverse and more complex tasks. When a desired system with Arduino header compatibility is unavailable, it can be easily mounted on a dedicated bridging board, as has been shown, for example, by [47]. With this approach, any possible platform can be connected to the electronic stethoscope developed in this work.
- Field-Programmable Gate Arrays: FPGAs are reprogrammable and reconfigurable ICs that offer a parallel processing architecture [46]. This makes them a powerful tool to execute the most complex algorithms, such as advanced signal processing or even machine learning algorithms [47]. Combining the developed electronic stethoscope with such a system enables for sophisticated and versatile engineering applications.
4.3.2. Firmware
The firmware establishes the software communication between the analog front end and the digital back end, as shown in the interface diagram in Figure 12. In this work, an ESPDuino was chosen to demonstrate the functionality of the developed prototype. This Arduino compatible MCU contains an ESP32-WROOM-32 chip and features a wide range of wired and wireless communication types, including I2S, SPI, and WiFi. For both I2S and SPI communication, a double-buffering structure was chosen that alternates between filling and wirelessly sending two buffers with audio data from the analog front end. These two tasks run in parallel on the two cores offered by the MCU, thereby enabling real-time data streaming without delays. An interrupt service routine (ISR) initializes the readout of audio samples from the analog front end. In asynchronous SPI communication, the ISR is triggered externally by the DRDY pin that pulsates at a sample rate of 16 kHz. In I2S mode, the ISR is triggered by an internal interrupt that is repeatedly executed at a frequency of 48 kHz. Whenever a buffer is full, it is transmitted in the form of UDP packages to the IP address of a PC for further processing and analysis.
4.4. Prototype Demonstration
The previous sections described the design of the developed electronic stethoscope and its three essential layers. An objective of this work is to enable simultaneous analog and digital auscultation. For this purpose, a casing encloses a conventional chestpiece at the center of the prototype. Figure 13 shows a photograph of the final assembly.
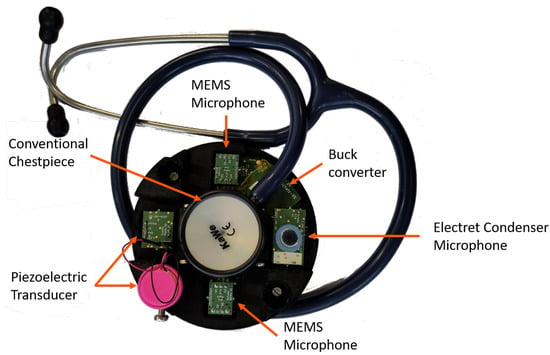
Figure 13.
Setup of OMES featuring digital MEMS microphones, piezoelectric transducers, and electret condenser microphones. The microphones are arranged in an array of four surrounding a conventional stethoscope chestpiece, thereby enabling simultaneous digital and analog auscultation.
The housing was designed to meet three requirements:
- All acoustic sensors and the diaphragm of the conventional stethoscope had to be flush to ensure equal detection of heart sounds.
- The design had to be modular and easily exchangeable in case of damage. Therefore, the construction is tied together with screws and can thus be quickly assembled and disassembled. The casing of the piezoelement is mounted with screws as well.
- The casing offers access to the microphone boards to enable for a quick exchange between sensor types.
The prototype can also be used as a stand-alone device without a conventional stethoscope attached to it. Figure 14 shows photographs of the device’s three configurations with, from left to right, the piezoelectric transducer encapsulated in a magenta-colored casing, the electret condenser microphone boards, and the MEMS microphone boards. The latter two microphones can be operated without housing the prototype. For the piezoelectric transducer, the casing is necessary in order to achieve adequate stabilization of the sensor. However, the main board and the buck converter provide space for no more than three piezoelectric transducers. Nevertheless, the setup’s microphone array configurations enable advanced technologies, like sensor fusion, beamforming, or multi-sensor body sound acquisition.

Figure 14.
Photographs of the three microphone configurations from left to right: the piezoelectric transducer with its magenta-colored casing, the electret condenser microphone, and the MEMS microphone boards.
5. Experimental Evaluation
The experiments can be separated into two kinds of measurements: reproducible tests conducted in an anechoic box and examinations performed on a human heart. The measurements carried out in the anechoic box include a noise floor analysis, an SNR analysis, a frequency response determination, and a sensitivity measurement for each microphone board individually. To assess the performance of the developed microphone boards in sensing heart sounds, the developed prototype was applied to all five heart auscultation points typically investigated in medical examinations. All tests were also conducted with the commercial 3M Littmann CORE digital stethoscope to obtain a reference measurement. This commercial stethoscope incorporates multiple signal enhancement steps such as filtering and amplification stages, and the user does not have access to raw audio data. Therefore, the 3M Littmann CORE stethoscope was treated as a “black box” in the experiments.
5.1. Microphone Characterization in the Anechoic Box
The image on the left in Figure 15 shows the inside of the anechoic box with the used loudspeaker. On the right, the measurement setup is depicted. The distance between the loudspeaker and each microphone was set to 3 cm for all measurements except the sensitivity experiment, in which the distance was increased to 50 cm in order to increase the number of measurable data points.
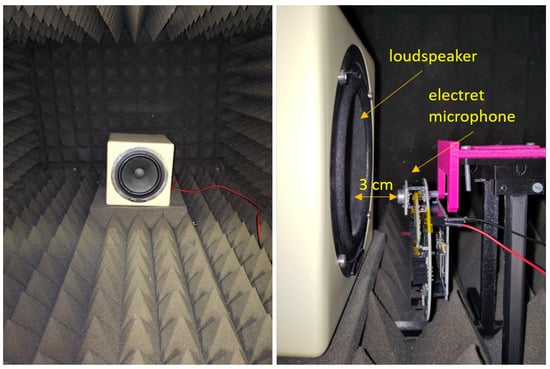
Figure 15.
Measurement setup in the anechoic box. (Left): Inside of the anechoic box with the employed loudspeaker. (Right): Exemplary measurement setup with the electret condenser microphone for measuring the noise floor, the SNR, and the frequency response. The same setup was used for every other microphone type and for the 3M Littmann CORE digital stethoscope. For the sensitivity test, the distance between the loudspeaker and microphone was increased to approximately 50 cm.
For the noise floor, the SNR and the sensitivity test, the root-mean-square (RMS) value was chosen as a metric to quantify the effective energy present in the recorded signal. The RMS value for N data points in a signal was calculated with formula (1):
and expressed in dB with formula (2):
The 3M Littmann CORE digital stethoscope was chosen as a commercial reference to compare the experimental results of the three microphones boards. However, the extent of comparison is limited by two aspects: First, in contrast to OMES, the 3M Littmann CORE digital stethoscope does not offer access to raw unfiltered audio data. And second, the commercial stethoscope comes with a generic chestpiece that mechanically increases the sound intensity at the location of the microphone and therefore contributes to the overall sensitivity of the device.
Furthermore, both the 3M Littmann CORE digital stethoscope and the piezoelectric transducer are meant to be used in direct contact with a pulsating surface and could therefore show a weaker performance when exposed to sound waves transmitted through air. However, for the experiments conducted, they were treated as if their behavior was equal to a generic microphone to establish a comparability between the test results.
5.1.1. Sensitivity and Noise Floor Measurement
Sensitivity measurements serve to evaluate how changing sound volumes influence the effective energy of recorded signals. The acoustic sensors were placed approximately 50 cm in front of the loudspeaker that emitted a sine wave of 300 Hz. This frequency was chosen because it lies in the range of typical heart sounds (compare Section 2). At first, the loudspeaker was configured to output its maximum sound level. Then, a 30 s recording was performed with every microphone board and the commercial stethoscope individually. At the same time, a decibel meter measured the sound volume in dB. Subsequently, the volume of the loudspeaker was successively reduced between recordings until it reached its minimal volume.
The captured signals were filtered by a digital Butterworth bandpass filter with cutoff frequencies 270 and 330 Hz, respectively, to isolate the relevant frequency components. Next, the RMS value of the signals was calculated and plotted over the sound volume measured with the decibel meter.
In a last step, the noise floor was determined in order to obtain the minimum level of noise that the microphones perceive in the measurement setup. This measured noise can, for example, originate from acoustic noise emitted by the loudspeaker, the ESP32 module, or from mechanical vibrations inside the anechoic box. But also electric distortions, like 50 Hz main hum, can be considered noise. For this purpose, the whole measurement setup was switched on but the loudspeaker did not emit sound. Again, a recording of 30 s was made for each microphone and the 3M Littmann CORE digital stethoscope individually. Finally, the RMS value of the noise floor signals was added as a green line to the sensitivity curves shown in Figure 16.
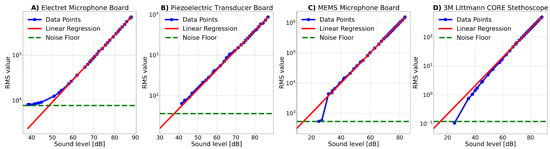
Figure 16.
Response curves for the developed microphone boards and the commercial reference stethoscope for different sound volumes at a frequency of 300 Hz. The RMS values were calculated from the recorded signals after applying a bandpass filter with cutoff frequencies 270 Hz and 330 Hz. A regression line was fit to fifteen data points in the linear regions of each curve and is shown in red. Additionally, the RMS value of the noise floor measurement was inserted as a green horizontal line.
All graphs show linear growth for increasing sound levels. To emphasize this trend, a regression model was applied to fifteen data points inside the linear region of the sensitivity curves. The regression line follows the structure of the equation:
and the coefficients for every microphone board and the commercial stethoscope are given in Table 5. The exponential component in the equation comes from the fact that x is the sound level in dB, and thus it is given in a logarithmic scale.

Table 5.
Coefficients of the linear regression model from Equation (3) for the three microphone boards and the commercial reference stethoscope and their noise floor levels in dB deduced from the intersection point of the measured RMS value of the noise floor level and the regression line in Figure 16.
The sensitivity curves given in Figure 16 show the recorded data points in blue, the fitted linear regression line in red, and the measured noise floor in green. The noise floor in dB corresponds to the intersection point of the red regression line and the green noise floor as the RMS value and is also given in Table 5. In the following, the graphs are explained in detail for each microphone type individually:
- Electret Microphone Board: The sensitivity curve of the electret microphone board is visualized in Figure 16A. The graph rises smoothly for increasing sound volumes until it meets the fitted regression line at around 59 dB. The noise floor of the electret microphone board is 48.5 dB and represents thus the highest noise floor among all sensors tested. The high noise floor of the electret microphone board makes it less suitable for detecting low-amplitude signals.
- Piezoelectric Transducer Board: As can be seen in Figure 16B, the recordings made with the piezoelectric transducer board demonstrate a linear sensitivity curve for all sound volumes. The noise floor lies below all measured data points, indicating that the noise floor has never been reached during the measurements. The theoretical noise floor is 37.5 dB.
- MEMS Microphone Board:Figure 16C shows the sensitivity curve recorded with the MEMS microphone board. The graph starts with two data points measured at the noise floor level. At a sound level of approximately 30 dB, the graph suddenly jumps and then follows a linear trend for increasing sound intensity. The rapid increase in RMS value at the beginning of the curve might occur because the output of the MEMS microphone is digital and might therefore intrinsically truncate sound volumes of low intensity, e.g., due to internal sound volume thresholds that intend to reduce noise sensitivity. However, this truncation can imply a reduced applicability of the MEMS microphone board to detect low-intensity body sounds. The intersection of the red regression curve and the green noise floor line indicates a theoretical noise floor level of 14.5 dB when neglecting this truncation.
- 3M Littmann Core Stethoscope: The sensitivity curve of the commercial stethoscope is shown in Figure 16D and starts with a data point close to the noise floor level. For increasing sound levels, the curve approximates the regression line. It is noteworthy that the measured data points are located below the regression line, demonstrating a non-linear nature for sound levels of low intensity and indicating a lower signal strength than predicted by the regression model. However, it should be kept in mind that the stethoscope is not indented to be used for these kinds of experimental characterizations, and internal signal processing might explain this behavior. The theoretical noise floor of the 3M Littmann CORE stethoscope is 14.75 dB.
5.1.2. Signal-to-Noise Ratio Analysis
The SNR is the signal over the noise floor and is a good indicator to evaluate the clarity of a signal. This section provides the maximal SNR () and a theoretical SNR for a typical heart beat () per microphone board. The is calculated via the following formula (4):
Here, represents the theoretical maximum RMS value that a microphone board can output and is the RMS value of the noise floor determined in the previous Section 5.1.1.
To obtain values for , the subsequent Equation (5) was used:
This equation is composed of two parts. Firstly, the maximum data value that can be transmitted from the developed main board to the ESP is because the data resolution is 24 bit. Secondly, the RMS value of a sine wave with amplitude = 1 can be estimated by . Therefore, multiplying these two terms approximates the theoretical maximal RMS value.
To determine the microphone’s SNR for a typical heart sound, the following procedure was applied: First, an auscultation of a healthy 26-year-old woman with a body-mass-index (BMI) of 21.1 was performed at auscultation point 1 with the commercial stethoscope. Then, the RMS value of the resulting heart beat signal was determined to be 122.12. The corresponding sound level was calculated with the means of the regression model for the commercial stethoscope provided in the previous Section 5.1.1 and yields 75.8 dB. Inserting this sound volume into the regression models for the developed microphone boards delivers the corresponding RMS value for every developed microphone board.
It should be noted that only one single person’s heart beat was auscultated to determine . In reality, the sound pressure level of a heart is unique for each person. However, this approach approximates the orders of volume magnitude that can be expected from a healthy heart sound. The is determined with the following formula (6):
The SNRs obtained are summarized in Table 6. The highest theoretical is achieved by the piezoelectric transducer board with 110.4 dB followed by the MEMS microphone board with 87.0 dB. The lowest is reached by the electret microphone board with 63.7 dB. The theoretical maximal RMS of the 3M Littmann CORE cannot be determined, as 3M, Maplewood, MN, USA does not provide any information about the transfer word size deployed.

Table 6.
SNRs for the developed microphone boards and the commercial reference stethoscope. represents the maximal SNR that a sensor can achieve and is the SNR that can be expected for a typical heart sound. The of the 3M Littmann CORE stethoscope is not available as 3M (Maplewood, MN, USA) does not provide the specifications necessary to calculate the SNR.
Regarding , the two analog sensors offer acceptable values: 27.0 dB for the electret microphone board and 36.9 dB for the piezoelectric transducer. The MEMS microphone board and the commercial stethoscope yield comparable SNRs of 61.4 dB and 60.3 dB, respectively, and are therefore superior in terms of signal strength over noise.
For every developed microphone board, the is considerably larger than the . In particular, for the electret microphone board, the latter is so small that detecting low-amplitude signals might become difficult, especially in a noisy environment. In future work, the SNRs of all microphone boards for typical body sound volumes need to be optimized, e.g., by reducing their intrinsic noise floors and enhancing their sensitivity.
5.1.3. Frequency Response
The frequency response reveals how the output of a system changes with varying input signal frequency, and it is hence another crucial aspect of microphone characterization. In pursuit of obtaining the frequency response, the microphones were exposed to a chirp signal that covers frequencies from 2 Hz to 20 kHz. This experiment was repeated three times, and the recorded signals were averaged in the time domain to compensate for any potential disturbances. Subsequently, the DC offset was removed from the averaged signal by subtracting its mean value. Then, the zero-centered signal was normalized by dividing through its maximal value to achieve a comparability between the sensors’ signals. In the next step, the signal was converted into the frequency domain by means of a Fast Fourier Transform (FFT). Finally, the spectrum was smoothed out in order to remove noise and fluctuations that obscure the genuine frequency response. For smoothing, an averaging window of 32 Hz was slid across the spectrum.
The spectra obtained for all the sensors can be seen in Figure 17. Hereafter, a comprehensive analysis of the frequency responses subdivided by the microphone types is provided:
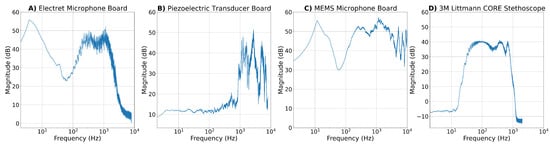
Figure 17.
Frequency responses for all developed microphone boards and the commercial reference stethoscope after exposing them to a chirp signal from 2 Hz to 20 kHz. The recorded signals were post-processed by averaging over three recordings, removing the DC components and normalizing the signal. The frequency response was obtained by applying an FFT to the time-domain signals and subsequently smoothing the acquired spectrum by shifting an averaging window of 32 Hz over the spectrum.
- Electret Microphone Board: The ADC sampling frequency was configured to 16 kHz. Consequently, consistent with the Nyquist criterion, the electret microphone cannot detect frequencies beyond 8 kHz. The frequency response of the electret condenser breakout board shown in Figure 17A is akin to its circuitry described in Section 4.1.1. A pronounced dip at 50 Hz is caused by the notch filter, and frequencies above 2 kHz are being attenuated due to the LPFs applied in the circuit.
- Piezoelectric Transducer Board: Similarly to the electret microphone, the maximum perceptible frequency of the piezoelectric transducer is 8 kHz. Its frequency response is depicted in Figure 17B and shows a nearly flat behavior for frequencies below 200 Hz. For larger frequencies, the spectrum is highly irregular and shows noticeable peaks at 2.7 kHz, 5.1 kHz and 6.7 kHz. The latter could potentially represent the resonance frequency of the CEB-20D64 piezoelectric element. The LPF designed in the circuitry seems to be insufficient at attenuating frequencies above 2 kHz because high frequencies are more perceptible than those of the cardiac frequency range below 500 Hz. However, it has to be kept in mind that the piezoelectric element should be placed against a vibrating surface, unlike in this experiment, where sound waves were transmitted through air. This could lead to a deformation of the true frequency response.
- MEMS Microphone Board: The MEMS microphones are sampled at a frequency of 48 kHz and can therefore theoretically sense frequencies up to 24 kHz. Figure 17C reveals that similarly to the frequency response of the electret condenser microphone, the spectrum of the MEMS microphone shows an evident dip at 50 Hz, suggesting that a notch filter was incorporated into its internal circuitry. Apart from that, the frequency response of the MEMS microphone appears non-uniform and shows similar sharp peaks like the piezoelectric transducer at 5.1 kHz, 6.9 kHz and 12.5 kHz.
- 3M Littmann Core Stethoscope: The Fourier transform of signals recorded with the commmercial stethoscope covers frequencies up to 2 kHz. As can be seen in Figure 17D, frequencies below 10 Hz are being suppressed. The frequency response is fairly flat in the range of 60 Hz to 750 Hz with peaks at 100 Hz, 400 Hz, and 670 Hz. The spectrum begins to decline at frequencies beyond 750 Hz.
5.1.4. Summary of Experimental Results
OMES has been tested for its performance regarding sensitivity, noise floor, SNR and frequency response and was compared to the 3M Littmann CORE digital stethoscope. A summary of the findings can be seen in the comparative Table 7. The electret microphone board has the highest noise floor among all tested sensors and hence performs the worse in SNR and in detecting low-amplitude signals. This drawback needs to be addressed in future work in order to fully exploit the benefits of this type of acoustic sensor. In contrast to that, the MEMS microphone board performs similarly to the commercial reference stethoscope with regard to the noise floor and .

Table 7.
Summary of the performance of OMES’ microphone boards in comparison with the Littmann CORE digital stethoscope as a commercial reference. The frequency bandwidth represents the theoretical Nyquist limit according to the corresponding sample rates. For a detailed insight on the frequency response, it is referred to Section 5.1.3.
5.2. Microphone Performance on Recording Heart Sounds
After characterizing the microphone boards in the anechoic box, they were applied to real-life scenarios capturing cardiac sounds from the five heart auscultation points. Each microphone was placed precisely at the auscultation points and in direct contact with the skin. All recordings were made in a silent environment. As some microphone boards do not show sufficient sensitivity, for some measurements, the subject was instructed to perform a short exercise of approximately 30 s to increase the intensity of the heart sound. This is indicated in the captions of Figure 18, Figure 19 and Figure 20 that show the zero-centered, normalized heart sound signal per auscultation point along with the corresponding spectrogram. The resulting signals were analyzed in terms of shape and frequency components and compared to the profile of a reference signal found in the literature.
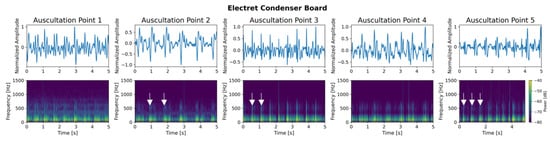
Figure 18.
Heart sound of a healthy 26-year-old woman with BMI 21.1 performed with the electret condenser breakout board at all five auscultation points. (Top): recorded signals of 5 s with normalized amplitude for each auscultation point. (Bottom): corresponding spectrogram showing the frequency distribution over time. For all auscultation points, the subject performed 30 s of exercise to increase heart beat intensity. White arrows indicate heart sounds S1 or S2.
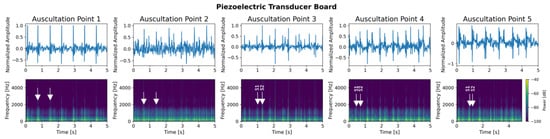
Figure 19.
Heart sound of a healthy 26-year old woman with BMI 21.1 performed with the piezoelectric transducer breakout board at all five auscultation points. (Top): recorded signals of 5 s with normalized amplitude for each auscultation point. (Bottom): corresponding spectrogram showing the frequency distribution over time. For auscultation point 1, the subject performed 30 s of exercise to increase the heart beat intensity. White arrows indicate heart sounds S1 or S2.
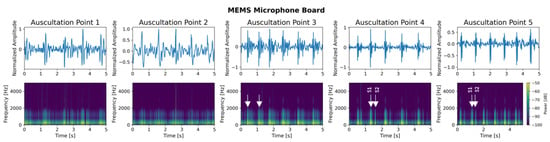
Figure 20.
Heart sound of a healthy 26-year old woman with BMI 21.1 performed with the MEMS microphone breakout board at all five auscultation points. (Top): recorded signals of 5 s with normalized amplitude for each auscultation point. (Bottom): corresponding spectrogram showing the frequency distribution over time. All recordings were performed at resting heart beat. White arrows indicate heart sounds S1 or S2.
Hereafter, the recordings per microphone board are described in detail:
- Electret Microphone Board: Figure 18 shows the signals recorded with an electret microphone board for each auscultation point. The test person performed 30 s of exercise to increase the intensity of the heart beat as no heart sounds were detected for a resting pulse. This might be due to the prominent noise floor and the weak SNR of the microphone board (compare Section 5.1.1 and Section 5.1.2).In general, the signals recorded with the electret microphone suffer from noise and it is hard to adequately distinguish heart beats from each other.
- Piezoelectric Transducer Board: Heart sounds recorded with the piezoelectric transducer board and their corresponding spectrograms are shown in Figure 19. For auscultating points 1 and 2, the test person performed 30 s of exercise, as the intensity of the resting heart beat was too weak to detect a valuable signal. For auscultation points 3–5, one can distinguish the first and the second heart sound in the spectrograms.
- MEMS Microphone Board: The signals recorded with the MEMS microphone board are depicted in Figure 20. For this microphone type, no physical activity was necessary as the microphone has a sufficiently high SNR. In the signals collected from auscultation points 3–5, both major heart sounds S1 and S2 can be visibly distinct with the S1 heart sound generating a higher amplitude in the signals than the S2 heart sound. This is reflected in the spectrograms for these auscultation points. In contrast, the signals recorded at auscultation points 1 and 2 suffer from more distortions and noise and show a blurring between the first and the second heart sound in both the signal plot and the corresponding spectrograms.
6. Conclusions and Future Work
Stethoscopes are an essential instrument for evaluating body sounds and are prevalent in medical practice. In the early 1990s, electronic stethoscopes emerged on the market, striving for an enhanced sound quality and compensating for the varying hearing abilities of physicians. However, they often target the human hearing range and thereby ignore the potential of nowadays affordable advanced microphone technology.
This work proposes an upgrade of analog stethoscopes that allows the recording of body sounds during the traditional auscultation process. This gives fully functional conventional stethoscopes an added value and prevents them from being discarded in the replacement of a new electronic stethoscope. Our multi-sensor modular electronic stethoscope, called OMES, offers high modular flexibility regarding microphone selection, features microphone array arrangements, and is compatible with multiple computation platforms. This versatility enables the prototyping of algorithms ranging from signal processing techniques to AI models, making the device suitable for research and education.
The experimental evaluation of OMES, detailed in Section 5, showed the inferior performance of the electret microphone board and the piezoelectric transducer board compared to the commercial reference stethoscope. In particular, their noise floors need to be reduced to increase their SNR and improve the detection of low-amplitude signals. The MEMS microphone board showed similar results as the commercial stethoscope but demonstrated an internal truncation of low-intensity sound volumes.
In this work, the prototype has been experimentally characterized and evaluated for its performance in detecting heart sounds. However, OMES is engineered to sense other body sounds as well. Its sensors offer heterogeneous responses and can potentially be qualified for different auscultation sites. Comprehensive studies are needed to assess the performance per microphone type and body sound. OMES can be easily assembled with a generic stethoscope chestpiece through a 3D-printed casing. However, the casing has not yet been tested or optimized for its influence on detecting body sounds. Furthermore, the arrangement of the microphone array has not yet been exhausted for signal processing and analysis. In upcoming projects, techniques like sensor fusion or beamforming can be employed, for example, to determine the exact location of the sound source. This could potentially reveal new diagnostic details and eventually lead to more accurate disease identification and characterization, as signals from multiple locations surrounding an auscultation point could be a valuable feature for machine or deep learning algorithms.
Author Contributions
Conceptualization, V.C.S., J.V.V., L.S. and B.d.S.; methodology, V.C.S., J.V.V., L.S. and B.d.S.; software, V.C.S., J.V.V. and L.S.; validation, J.V.V., L.S. and B.d.S.; resources, B.d.S.; writing—original draft preparation, V.C.S.; writing—review and editing, J.V.V., L.S. and B.d.S.; supervision, J.V.V., L.S. and B.d.S.; project administration, B.d.S.; funding acquisition, B.d.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was exempted by the Ethics Committee for Human Sciences (ECHW) of the Vrije Universiteit Brussel (VUB). Dossier ECHW_W_019, date of approval: 13 October 2025.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data are available with an open-source license in: https://gitlab.com/etrovub/embedded-systems/publications/omes (accessed on 22 October 2025).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lee, S.H.; Kim, Y.S.; Yeo, W.H. Advances in microsensors and wearable bioelectronics for digital stethoscopes in health monitoring and disease diagnosis. Adv. Healthc. Mater. 2021, 10, 2101400. [Google Scholar] [CrossRef]
- Thinklabs. Thinklabs One User’s Manual. Available online: https://www.oaktreeproducts.com/img/product/description/TL-One%20user%20manual.pdf (accessed on 8 September 2024).
- TelehealthTechnology. Electronic Stethoscopes—JABES. Available online: https://telehealthtechnology.org/toolkit/electronic-stethoscopes-jabes/ (accessed on 8 September 2024).
- Cardionics. Clinical E-Scope® Electronic Stethoscope. Available online: https://cardionics.com/en/product/clinical-e-scope-electronic-stethoscope/ (accessed on 8 September 2024).
- Thinklabs. Thinklabs ONE—Digital Stethoscope. Available online: https://www.thinklabs.com/ (accessed on 7 November 2024).
- Chowdhury, M.E.H.; Khandakar, A.; Alzoubi, K.; Mansoor, S.; Tahir, A.M.; Reaz, M.B.I.; Al-Emadi, N. Real-time smart-digital stethoscope system for heart diseases monitoring. Sensors 2019, 19, 2781. [Google Scholar] [CrossRef]
- Park, H.; Wei, Q.; Lee, S.; Lee, M. Novel Design of a Multimodal Technology-Based Smart Stethoscope for Personal Cardiovascular Health Monitoring. Sensors 2022, 22, 6465. [Google Scholar] [CrossRef]
- Wang, T.; Gong, M.; Yu, X.; Lan, G.; Shi, Y. Acoustic-pressure sensor array system for cardiac-sound acquisition. Biomed. Signal Process. Control 2021, 69, 102836. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, W.; Pang, Z.; Zhang, J.; Zou, D.; Zhang, X.; Guo, S.; Wan, J.; Wang, K.; Pang, W.; et al. A low-cost, ear-contactless electronic stethoscope powered by Raspberry Pi for auscultation of patients with COVID-19: Prototype development and feasibility study. JMIR Med. Inform. 2021, 9, e22753. [Google Scholar] [CrossRef] [PubMed]
- Nussbaumer, M.; Agarwal, A. Stethoscope acoustics. J. Sound Vib. 2022, 539, 117194. [Google Scholar] [CrossRef]
- McGee, S. Evidence-Based Physical Diagnosis, 2nd ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2007; pp. 411–471. [Google Scholar]
- Pappano, A.J.; Wier, W.G. Cardiovascular Physiology; Elsevier: Amsterdam, The Netherlands, 2013; Chapter 4—The Cardiac Pump; pp. 55–90. [Google Scholar]
- Abbas, A.K.; Bassam, R. Phonocardiography Signal Processing; Morgan & Claypool Publishers: San Rafael, CA, USA, 2009; Volume 31, Chapter 1; pp. 1–27. [Google Scholar]
- Sherazi, M.H. The Objective Structured Clinical Examination Review; Springer: Berlin/Heidelberg, Germany, 2019; Chapter 4—The Cardiovascular System; pp. 111–130. [Google Scholar]
- Jeong, Y.; Kim, J.; Kim, D.; Kim, J.; Lee, K. Methods for improving deep learning-based cardiac auscultation accuracy: Data augmentation and data generalization. Appl. Sci. 2021, 11, 4544. [Google Scholar] [CrossRef]
- Reichert, S.; Gass, R.; Brandt, C.; Andrès, E. Analysis of respiratory sounds: State of the art. Clin. Med. Circ. Respir. Pulm. Med. 2008, 2, CCRPM–S530. [Google Scholar] [CrossRef]
- Kölle, K.; Aftab, M.F.; Andersson, L.E.; Fougner, A.L.; Stavdahl, Ø. Data driven filtering of bowel sounds using multivariate empirical mode decomposition. Biomed. Eng. Online 2019, 18, 28. [Google Scholar] [CrossRef]
- Teague, C.N.; Hersek, S.; Töreyin, H.; Millard-Stafford, M.L.; Jones, M.L.; Kogler, G.F.; Sawka, M.N.; Inan, O.T. Novel methods for sensing acoustical emissions from the knee for wearable joint health assessment. IEEE Trans. Biomed. Eng. 2016, 63, 1581–1590. [Google Scholar] [CrossRef]
- Shah, M.A.; Shah, I.A.; Lee, D.G.; Hur, S. Design approaches of MEMS microphones for enhanced performance. J. Sens. 2019, 2019, 9294528. [Google Scholar] [CrossRef]
- Van Rhijn, A. Integrated circuits for high performance electret microphones. In Proceedings of the Audio Engineering Society Convention 114, Amsterdam, The Netherlands, 22–25 March 2003; Audio Engineering Society: New York, NY, USA, 2003. [Google Scholar]
- Leng, S.; Tan, R.S.; Chai, K.T.C.; Wang, C.; Ghista, D.; Zhong, L. The electronic stethoscope. Biomed. Eng. Online 2015, 14, 66. [Google Scholar] [CrossRef]
- Yilmaz, G.; Rapin, M.; Pessoa, D.; Rocha, B.M.; de Sousa, A.M.; Rusconi, R.; Carvalho, P.; Wacker, J.; Paiva, R.P.; Chételat, O. A wearable stethoscope for long-term ambulatory respiratory health monitoring. Sensors 2020, 20, 5124. [Google Scholar] [CrossRef]
- Ottoy, G.; Thoen, B.; De Strycker, L. A low-power MEMS microphone array for wireless acoustic sensors. In Proceedings of the 2016 IEEE Sensors Applications Symposium (SAS), Catania, Italy, 20–22 April 2016; IEEE: Piscataway, NJ, USA, 2016; pp. 1–6. [Google Scholar]
- Kusainov, R.K.; Makukha, V.K. Evaluation of the applicability of MEMS microphone for auscultation. In Proceedings of the 2015 16th International Conference of Young Specialists on Micro/Nanotechnologies and Electron Devices, Erlagol, Russia, 29 June–3 July 2015; IEEE: Piscataway, NJ, USA, 2015; pp. 595–597. [Google Scholar]
- Johnson, K.H.; Underwood, D.A. Recording, Digital Stethoscope for Identifying PCG Signatures. U.S. Patent 5,025,809, 25 June 1991. [Google Scholar]
- Cardionics. Operator’s Manual E-Scope® II Electronic Stethoscope. Available online: https://www.mystethoscope.com/PDF/escopeii.pdf?srsltid=AfmBOoqdh901PmsrY34ywaigVO7-yPkJzSKeqzVwhVMtTE1-Ja3rNUhA (accessed on 9 November 2023).
- Smith, C. Transducer for Sensing Body Sounds. U.S. Patent 6661897, 9 December 2003. [Google Scholar]
- eKuore. eKuore Pro Electronic Stethoscope. Available online: https://ekuore.com/product/ekuorepro-electronic-stethoscope/ (accessed on 9 November 2023).
- eKuore. eKuore Trajectory. Available online: https://ekuore.com/about/ (accessed on 9 November 2023).
- eKuore. eKuore Pro User Manual. Available online: https://www.manualslib.com/manual/2449084/Ekuore-Pro.html (accessed on 16 October 2025).
- 3M. 3M™ Littmann® CORE Digital Stethoscope. Available online: https://www.littmann.com/3M/en_US/p/d/b5005222000/ (accessed on 7 November 2024).
- Eko Health Inc. Eko’s Story. Available online: https://www.ekohealth.com/pages/about-us (accessed on 12 November 2024).
- 3M. 3M™ Littmann® CORE Digital Stethoscope FAQs. Available online: https://www.littmann.com/3M/en_US/littmann-stethoscopes/advantages/core-digital-stethoscope/ (accessed on 7 November 2024).
- Eko Health Inc. Eko Core 500™ Digital Stethoscope. Available online: https://www.ekohealth.com/products/core-500-digital-stethoscope?variant=39662120304736 (accessed on 6 November 2024).
- Eko Health. Instructions for Use CORE 500™ Digital Stethoscope. Available online: https://support.ekohealth.com/hc/en-us/articles/8890312188443-User-manuals (accessed on 16 October 2025).
- Wang, F.; Wu, D.; Jin, P.; Zhang, Y.; Yang, Y.; Ma, Y.; Yang, A.; Fu, J.; Feng, X. A flexible skin-mounted wireless acoustic device for bowel sounds monitoring and evaluation. Sci. China Inf. Sci. 2019, 62, 202402. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, K.R.; Kim, T.; Im, S.; Lee, Y.J.; Jeong, S.; Shin, H.; Kim, M.; Lee, J.; Kim, D.; et al. A wearable stethoscope for accurate real-time lung sound monitoring and automatic wheezing detection based on an AI algorithm. Engineering 2025, in press. [CrossRef]
- Chuchnowska, I.; Białas, K. Prototype of Self-Service Electronic Stethoscope to Be Used by Patients During Online Medical Consultations. Sensors 2025, 25, 226. [Google Scholar] [CrossRef] [PubMed]
- Duggan, D.; Sarana, V.; Factor, A.; Shelevytska, V.; Temko, A.; Popovici, E. Towards a Wearable, High Precision, Multi-Functional Stethoscope. In Proceedings of the 2024 46th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 15–19 July 2024; IEEE: Piscataway, NJ, USA, 2024; pp. 1–4. [Google Scholar]
- Han, L.; Liang, W.; Liu, Y.; Zeng, W.; Wang, J.; Yang, Z.; Zhou, Q.; Dong, Y.; Wang, X. Stretchable piezoelectret electronic stethoscope for phonocardiography and lung sound detection in motion and noise conditions. Appl. Mater. Today 2024, 36, 102077. [Google Scholar] [CrossRef]
- McDonald, A.; Nussbaumer, M.; Rathnayake, N.; Steeds, R.; Agarwal, A. A flexible multi-sensor device enabling handheld sensing of heart sounds by untrained users. IEEE J. Biomed. Health Inform. 2025, 29, 5575–5584. [Google Scholar] [CrossRef]
- Lee, S.Y.; Su, P.H.; Hsieh, Y.T.; Huang, S.H.; Lee, I.P.; Chen, J.Y. Intelligent Stethoscope System and Diagnosis Platform with Synchronized Heart Sound and Electrocardiogram Signals. IEEE Access 2023, 11, 47420–47431. [Google Scholar] [CrossRef]
- Giordano, N.; Rosati, S.; Balestra, G.; Knaflitz, M. A wearable multi-sensor array enables the recording of heart sounds in homecare. Sensors 2023, 23, 6241. [Google Scholar] [CrossRef] [PubMed]
- Klum, M.; Leib, F.; Oberschelp, C.; Martens, D.; Pielmus, A.G.; Tigges, T.; Penzel, T.; Orglmeister, R. Wearable multimodal stethoscope patch for wireless biosignal acquisition and long-term auscultation. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 5781–5785. [Google Scholar]
- Wu, Y.C.; Han, C.C.; Chang, C.S.; Chang, F.L.; Chen, S.F.; Shieh, T.Y.; Chen, H.M.; Lin, J.Y. Development of an electronic stethoscope and a classification algorithm for cardiopulmonary sounds. Sensors 2022, 22, 4263. [Google Scholar] [CrossRef] [PubMed]
- Diouri, O.; Gaga, A.; Ouanan, H.; Senhaji, S.; Faquir, S.; Jamil, M.O. Comparison study of hardware architectures performance between FPGA and DSP processors for implementing digital signal processing algorithms: Application of FIR digital filter. Results Eng. 2022, 16, 100639. [Google Scholar] [CrossRef]
- Solé Morillo, Á.; Lambert Cause, J.; Baciu, V.E.; da Silva, B.; Garcia-Naranjo, J.C.; Stiens, J. Ppg edukit: An adjustable photoplethysmography evaluation system for educational activities. Sensors 2022, 22, 1389. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).