Abstract
Oleogels, a semi-solid fat-like material, have emerged as a promising alternative to traditional saturated fats in food products. This study aimed to develop and characterize novel oleogels using starch and extracts from cassava (Manihot esculenta) to be used as a fat replacement in cookies, addressing the growing demand for healthier baked goods. Cassava starch was used as the structuring agent, while extracts provided functional properties to the oleogels. The oleogels were prepared and then incorporated into a cookie formulation, fully replacing the conventional fat. The resulting cookies were analyzed for their physicochemical properties, including texture, moisture content, and color. Rheological and microscopy analyses were also subjected to a sensory evaluation panel. The results demonstrated that the cassava-based oleogels effectively mimicked the functional role of fat, producing cookies with a significantly lower fat content. The cookies made with the oleogels exhibited comparable textural attributes and sensory acceptance to the full-fat control group, with no significant differences in flavor or mouthfeel reported by the panelists. These findings suggest that oleogels derived from cassava are a highly effective and innovative solution for producing healthier cookies without compromising quality, representing a viable strategy for fat reduction in the food industry.
1. Introduction
A primary reason why baked products are often considered unhealthy is their significant fat content, specifically the high levels of saturated and trans fatty acids [1]. Ironically, saturated fatty acids (SFA) are indispensable in the bakery and confectionery industry because of their functional properties, which include establishing structure, texture, and flavor. SFA is vital for achieving desirable attributes such as creaminess, lubrication, mouthfeel, and overall product acceptability [2]. In response to health concerns, numerous scientific studies propose the reformulation of these products with ingredients that offer health benefits [3,4], notably using oils that are rich in unsaturated fatty acids [5,6]. Still, the technological hurdle of substituting the solid fats typically used in baking is considerable [7]. The primary issue is that the liquid consistency and absence of a solid phase in alternative oils cause the oil to migrate out of the product matrix, consequently jeopardizing the proper structure of the finished biscuit [8].
In recent years, there are more and more publications reporting the possibility of using gelling oil through the use of appropriate substances. Oleogels are structured systems formed by three-dimensional networks of gelators capable of structuring liquid oils to create semi-solid fats [9]. Oleogelling agents are widely used in the oleogelation process. They allow the gelling of large quantities of liquid oil (generally more than 90% by weight) [10], using a relatively low amount of oleogelling molecules. This oleogelation technique is efficient in preserving the nutritional value of unsaturated liquid oils [11]. Oleogelling agents encompass a variety of compounds, both lipid and polymeric in origin; carbohydrates are widely used as structuring agents in the production of oleogels, monoglycerides, cellulose derivatives, xanthan gum, κ-carragenine, and starch [12]. Also, the deterioration of fats via oxidation is commonly counteracted through the addition of antioxidants. However, the demand for natural antioxidants is increasing, driven by both consumer desire and the recommendations of nutrition professionals. In response, the scientific literature highlights the successful use of plant materials rich in polyphenols for bakery applications. These sources include extracts or other forms derived from olive leaves [13], chokeberry [14], and various herbal sources [15].
Different oleogels havr been developed with the addition of extracts; e.g., Qiu et al. [16], prepared oleogels using soybean and peanut oil as base oils, crude blueberry extract, and resveratrol; Laszczyk et al. [17] developed oleogels with the addition of a triterpene extract; and Salamma et al. [18] produced sunflower oil–beeswax oleogels with and without the addition of Moringa oleifera leaf phenolic extract.
Cassava leaves are a source of protein, minerals (potassium, magnesium, and calcium), vitamins (B1, B2, and C), and essential amino acids [19]. The presence of phenolic compounds in cassava leaves, which have recognized antioxidant capacity, has been reported. These secondary metabolites, when incorporated into a food matrix, confer bioactive properties, contributing to the inhibition of oxidative processes and the potential development of functional foods [20]. Furthermore, the incorporation of bioactive compounds from plant matrices, such as natural plant extracts, confers natural antimicrobial and free radical-scavenging properties. This means that they provide antioxidant properties to the food matrix, generating significant benefits for food, including inhibiting phenomena such as enzymatic browning and prolonging food shelf life. This is why they are increasingly becoming important food additives in the food industry [21]. Oleogels are an alternative solution to replace saturated fats in foods without disturbing the rheological and textural properties of the original product and have gained importance and attention in recent years. Therefore, this work aims to develop oleogels formulated from cassava starch and cassava leaf extract, characterize their structural and rheological properties, and evaluate their application as a fat replacement in cookies.
2. Materials and Methods
2.1. Materials
Solvents and acids used in this study included ethanol (99.5% purity), sourced from Panreac (Barcelona, Spain); Orthophosphoric acid (85% purity), purchased from Scharlab S.L. (Sentmenat, Spain); and acetonitrile (99.8% purity), which was supplied by Macron (Poland). Acetic acid (≥99.5% FCC, FG) was also obtained. The antioxidant standards and reagents were procured from Sigma–Aldrich (St. Louis, MO, USA) and included gallic acid standard (>98% purity), Trolox (97% purity), Folin–Ciocalteu’s reagent, DPPH (95% purity), and ABTS (≥95% purity). Sorbitan monostearate (Span® 60) was also supplied by this vendor. Extra virgin oil, virgin flax, and argan oils (all food-grade) were sourced from local markets. Xanthan gum and starch were purchased from Tecnas (Medellin, Colombia). Olive oil, butter, flour, sugar, salt, baking powder, eggs, and citrosan were purchased in local markets. The sweet cassava leaves were collected in Magangué (9°14′31.3″ N, 74°45′16.8″ W), Bolívar, Colombia, between September and December 2023. The region has a warm tropical climate, with an average annual temperature ranging from 27 to 29 °C and relative humidity between 75% and 85%, providing optimal environmental conditions for cassava cultivation. The sweet cassava leaves exhibited a remarkable proximate composition due to their nutritional value. Their moisture content ranged from 64.57% to 73.43%, while crude protein content varied between 23.12% and 29.78%, highlighting their potential as a plant-based protein source. Lipids were present in low proportions (1.21% to 2.87%), and the leaves contained significant amounts of minerals such as calcium (144.21–212.67 mg/100 g), potassium (2211.11–3123.33 mg/100 g), and iron (5.67–9.78 mg/100 g). Additionally, cassava leaves were a good source of vitamins, particularly vitamin A (13,267–19,833 IU/100 g) and vitamin C (27.11–39.44 mg/100 g), reinforcing their functional value and potential use in the formulation of nutritionally enriched food products [22].
2.2. Methods
2.2.1. Extraction Procedure
Cassava leaves were washed in a solution of water with 0.3% of citrosan, and then dried using a hot-air tray dryer (Food Dehydrator Machine, Cercker, Shenzhen (China)) at 40 °C for 2 h. Dried leaves were ground and sieved to obtain a powder with a particle size < 200 μm.
Microwave-assisted extraction of was performed in a microwave oven (WMWO-25DSILVER, Wurden, Zhejiang, China), following the procedures described by Ramirez-Brewer et al. [23], with some modifications. Briefly, cassava leaves powder was dissolved in ethanol as a solvent and subjected to magnetic stirring for 2 min; then, the suspensions were treated with a microwave at 180 W for 3 min. The mixture was then filtered and rotavaporized to remove ethanol, resulting in a viscous paste extract. The cassava leaf extracts were stored at −5 °C for further analysis.
2.2.2. Development of Oleogels
The oleogels were prepared following the methodology described by Zhu et al. [24], by mixing and homogenizing oil solution (OS) with the cassava starch and xanthan solution (CS-XG), evaluating the starch and extract content (Table 1). To prepare OS, olive oil was mixed with Span 60 (10%wt) on a hot plate at 58 °C for 45 min; after that, the temperature was lowered to 40 °C, and cassava extract was added with constant stirring at 400 rpm. CS-XG was prepared by mixing cassava starch, xanthan gum (0.5%wt), and deionized water at 350 rpm for 30 min at 25 °C; then, the temperature was raised to 62 °C for 5 min to achieve complete gelatinization of the starch granules. In total, 30 g of CS-XG was mixed with 70 g of OS and homogenized at 8000 rpm for 7 min using an Ultra Turrax rotor–stator (IKA digital T20 Ultra Turrax, Staufen, Germany) and freeze-dried at −40 °C for 4 h using a lyophilizer (Labconco™ FreeZone™ 2.5 L −50 °C Benchtop Freeze Dryer, 230 V model, Kansas City, MO, USA).

Table 1.
Formulation of oleogels based on starch and an extract from cassava.
The concentrations of cassava leaf extract (0.25% and 0.5%) were selected based on preliminary assays and scientific literature reports indicating that low inclusion levels of plant extracts are sufficient to provide significant antioxidant activity without compromising the structural or rheological properties of oleogels. These concentrations were chosen to evaluate the effect of the extract on the physical stability and textural integrity of the oleogel system, while also incorporating bioactive compounds with functional potential. The addition of this extract to food matrices may help reduce the formation of free radicals in fats, thereby improving oxidative stability and extending the shelf life of processed products such as cookies, as reported in various studies [25].
2.2.3. Development of Cookies
To prepare the control cookie dough, 44% of butter and 11% of sugar were initially mixed for 5 min; subsequently, 5.5% of egg and 37.18% of wheat flour were added to this mixture, which was gradually added along with the rest of the dry ingredients (salt and baking powder). They were then mixed until a homogeneous dough was obtained, from which small portions of approximately 3 mm thickness were taken; subsequently, the portions were refrigerated for one hour at 4 °C. After the time had elapsed, and with the oven preheated to 180 °C, they were baked for 10 min in convection mode. Finally, the cookies were cooled at room temperature for 2 h and then refrigerated for use in subsequent analysis [26]. A total of seven formulation were obtained (Table 2).

Table 2.
Formulation of cookies prepared with oleogels based on starch and an extract from cassava.
2.2.4. Determination of Total Phenolic Content and Antioxidant Capacity
To determine the Total Phenolic Content (TPC) and antioxidant capacity, 1 g of cookies was ground and mixed with 5 mL of methanol, and then the mixture was sonicated at 25 Hz at 35 °C for 15 min, kept at 4 °C for 30 min, and centrifuged at 10,000× g for 10 min. Finally, the supernatant was collected and stored at −20 °C until its analysis.
TPC was determined using the Folin–Ciocalteu method [27]. For this assay, a solution of 0.05 mL of extract was prepared with 3 mL of distilled water and 0.25 mL of the Folin–Ciocalteu reagent. Then 0.75 mL of a 20% by mass sodium carbonate solution and 0.95 mL of distilled water were added. The mixture was stored in the dark for 2 h, and the absorbance was measured at 760 nm using a Genesys 10S UV-vis spectrophotometer (Thermo Fischer Scientific Inc., Waltham, MA, USA). The results were expressed as gallic acid equivalents (GAEs) in mg per gram of extract.
The antioxidant capacity of extracts was determined using the DPPH assay method described by Brand-Williams et al. [28], with minor modifications. A volume of 0.025 mL of sample was added to 0.975 mL of DPPH radical in ethanol; the reaction took place at room temperature in the dark, considering the stabilization curve (3 h). The absorbance was then measured at 515 nm using a UV-Vis spectrophotometer (Genesys 10S, Thermo Fisher Scientific, Waltham, MA, USA). The DPPH concentration within the reaction medium was quantified using a calibration curve established via linear regression. To determine the maximum absorbance of the DPPH radical, a control sample was measured, containing the same volume of solvent used during the extract preparation. Trolox served as the reference standard for this assay, and the results are therefore reported as TEAC values (μmol Trolox equivalent/g of extract).
Antioxidant capacity was determined using an ABTS assay, following the methodology described by Re et al. [29], with some modifications. For the ABTS assay, 0.01 mL of sample was first added to 0.99 mL of ABTS radical in ethanol. The ABTS reaction was carried out at room temperature in a dark place, considering the stabilization curve (4 h). Subsequently, the absorbance at 734 nm was determined using a Genesys 10S UV-vis spectrophotometer. The results were expressed as Trolox equivalents (TEAC) (μmol Trolox/g of extract).
2.2.5. Determination of Physicochemical and Proximal Properties
Acidity, pH, moisture, fat, ash, protein, and carbohydrate were determined following the procedures described by AOAC [30]. The color of the oleogel and cookie samples was recorded using a CIT colorimeter (Portable Color Analyzer Digital Precise Colorimeter Color Difference Meter Tester 8 mm), which determines color parameters such as lightness (L*), red–green color (a*), yellow–blue color (b*), and change in color () [31]; the change in color (∆E) was calculated using Equation (1):
∆E = [(∆L*)2 + (∆a*)2 + (∆b*)2]0.5
2.2.6. Rheological Analysis
Rheological properties were evaluated following the procedures described by Barraza et al. [32], with some modifications.
For the steady-state viscous flow test, a shear rate ranging from 0.001 to 1000 s−1 was used to analyze how the apparent viscosity varies. The experimental data of the oleogels were fitted to the Carreau–Yasuda model (Equation (2)), presenting a fitting coefficient R2 > 0.99:
In this rheological model, η0 represents the zero-shear viscosity [Pa·s] and η∞ is the infinite-shear viscosity [Pa·s]. The shear rate is denoted by γ [s−1], while λ is a time constant indicating the relaxation time [s]. The flow behavior index is given by n, and a describes the width of the transition region between Newtonian and power law behaviors.
The linear viscoelastic zone (LVZ) was first established by conducting a stress sweep test. This involved applying stress from 0.01 Pa up to 1000 Pa, while maintaining a constant frequency of 1 Hz at a temperature of 25 °C. Following this, the material’s mechanical spectrum was recorded through a frequency sweep test. This test utilized a stress value selected from within the LVZ and covered a frequency range spanning 10−2 and 102 rad·s−1.
2.2.7. Microscopy Analysis
For this test, a Primo Star optical microscope (Carl Zeiss Primo Star Microscopy GmbH, Jena, Germany) with a 100X objective was used. A magnifying lens was used to study the droplet size and internal distribution of the emulsions produced. A digital camera with live ZEN 2.3 lite software was used to capture the images, giving us a clear view of what was observed through the microscope.
2.2.8. Sensorial Analysis
For the sensory evaluation of the cookies, a nine-point hedonic test was conducted according to the technical guide GTC 165 [33] to assess consumer acceptability of the oleogel-formulated samples. The panel consisted of 30 untrained participants (15 women and 15 men) aged between 18 and 45 years, comprising students and professors from the Faculty of Engineering. Panelists evaluated the attributes of color, aroma (instead of smell), flavor, texture, and overall acceptability using the nine-point hedonic scale ranging from 1 (dislike extremely) to 9 (like extremely). The evaluation was carried out in a standardized tasting room equipped with individual booths, and samples were presented on white plastic plates labeled with randomly assigned three-digit codes to ensure blinding.
2.2.9. Statistical Analysis
Statistical analysis was executed using Statgraphics Centurion XVIII. Data are presented descriptively, showing the mean ± standard deviation (SD) for three independent replicates of each experiment. Significant variations across the sample means were assessed via Analysis of Variance (ANOVA), setting the level of significance at 0.05. Where differences were found, Tukey’s multiple range test was employed for post hoc mean comparisons.
3. Results and Discussion
3.1. Cassava Leaf Extracts—CLE
Natural extracts from cassava leaves were obtained with extraction yields of 8.00 ± 2.03%, total phenolic compounds of 4.92 ± 0.03 g GAE/g extract, and antioxidant capacity of 93.27 ± 3.95 µmol Trolox/g extract to the ABTS radical and 1.08 ± 0.04 µmol Trolox/g extract to DPPH. Cassava extracts presented higher extraction yields than the extracts reported by Tao et al. [34], who reported extraction yields of 6.9% using ultrasound-assisted extraction. The total phenolic compound (TPC) presented higher values than those reported by Laya & Koubala [20] for cassava leaf extracts (CLEs), obtained with various solvents such as methanol, ethanol, water, and ethyl acetate by applying ultrasound-assisted extraction, with values around 0.019 g GAE/g extract, but lower than those reported by Chahyadi & Elfahmi [35], for CLEs, obtained by ultrasound-assisted and microwave extraction, with a value of 8.961 g GAE/g extract [35]. The antioxidant activity presented values similar to those reported by other authors, such as Laya & Koubala [20], who evaluated the polyphenols in cassava leaves and their stability in antioxidant potential after in vitro gastrointestinal digestion, obtaining DPPH values of 176.67 ± 2.83 µmol Trolox/g dry weight of leaves. Phenolic compounds contribute significantly to the antioxidant activity of plant extracts, meaning that the higher the TPC value, the greater the antioxidant activity.
3.2. Oleogels Based on Starch and Extracts
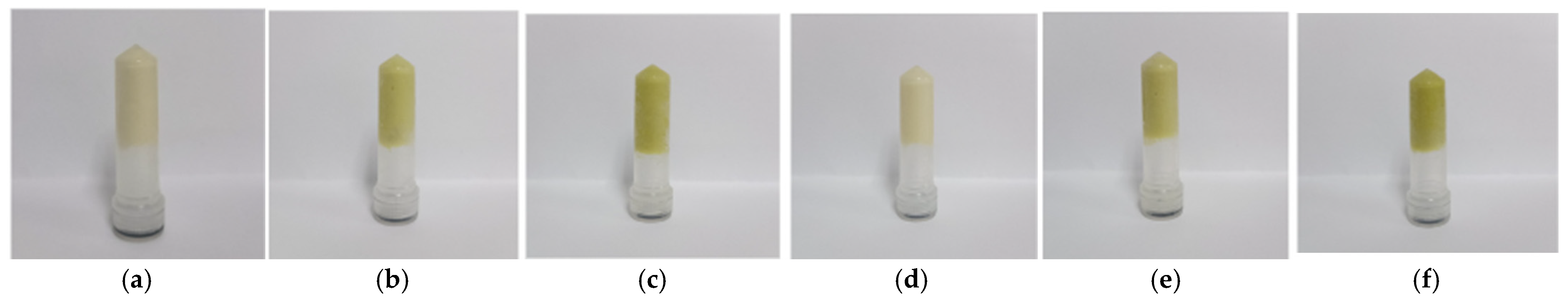
Different oleogels were developed with 0.5 and 1.0% of starch and 0.25 and 0.50% of extract, obtaining six (6) formulations (Table 1), where starch played a crucial role as a thickening agent, increasing the viscosity of the aqueous phase and preventing phase separation of the emulsion. During lyophilization, this starch forms a gel-like network that encapsulates both the oil and water components, ensuring the structural integrity of the oleogel [36]. Furthermore, the starch concentration directly influences the texture and rheological properties, resulting in firmer oleogels that displayed firm structural properties, highlighted by their ability to maintain integrity without flow upon inversion, indicating the formation of firm and stable emulsions (Figure 1). This behavior is essential for applications requiring structure and stability retention.

Figure 1.
Photographs of oleogels based on starch and extracts of cassava. Samples (a) OG_S0.5_E0.00, (b) OG_S0.5_E0.25, (c) OG_S0.5_E0.50, (d) OG_S1.0_E0.00, (e) OG_S1.0_E0.25 and (f) OG_S1.0_E0.50.
3.2.1. Physicochemical Properties of Oleogels
The physicochemical properties of the oleogels are presented in Table 3. The pH of oleogels decreases (p < 0.05) with the content of cassava starch and CLE due to the acidity of extract, which contributes to the decrease in the pH of the oleogels. These results are in accordance with the data reported by Abou-Elsoud et al. [37], who showed a decrease in pH in these colloidal systems when adding bioactive compounds. This suggests that a higher percentage of cassava starch and CLE correlates with a decrease in pH in the oleogels.

Table 3.
Physicochemical properties of oleogels based on starch and extracts of cassava.
Regarding the color parameters, significant differences were found (p < 0.05). a* (+, red; −, green) presents positive values for samples without extract (OG_S0.5_E0.00 and OG_S1.0_E0.00), indicating reddish colors that can be attributed to compounds present in butter and olive oil such as β carotene, and negative value for samples with extract, indicating a green coloration; also, a decrease in a* with the percentage of starch and extract was observed. b* (+, yellow; −, blue) increases with the addition of extract, but no differences were found with the addition of starch (p > 0.05). The luminosity L* (p < 0.05) decreases significantly with the addition of extract and the increase in starch; this is because the samples with the lowest amount of starch were more translucent. Similar results were reported by Miranda et al. [38], who evaluated CLE as a material to produce biopolymer-based films, and by Lastra Ripoll et al. [39], where the addition of extracts obtained from mango peel influences the green color of the edible coatings produced.
3.2.2. Viscous Properties of Oleogels
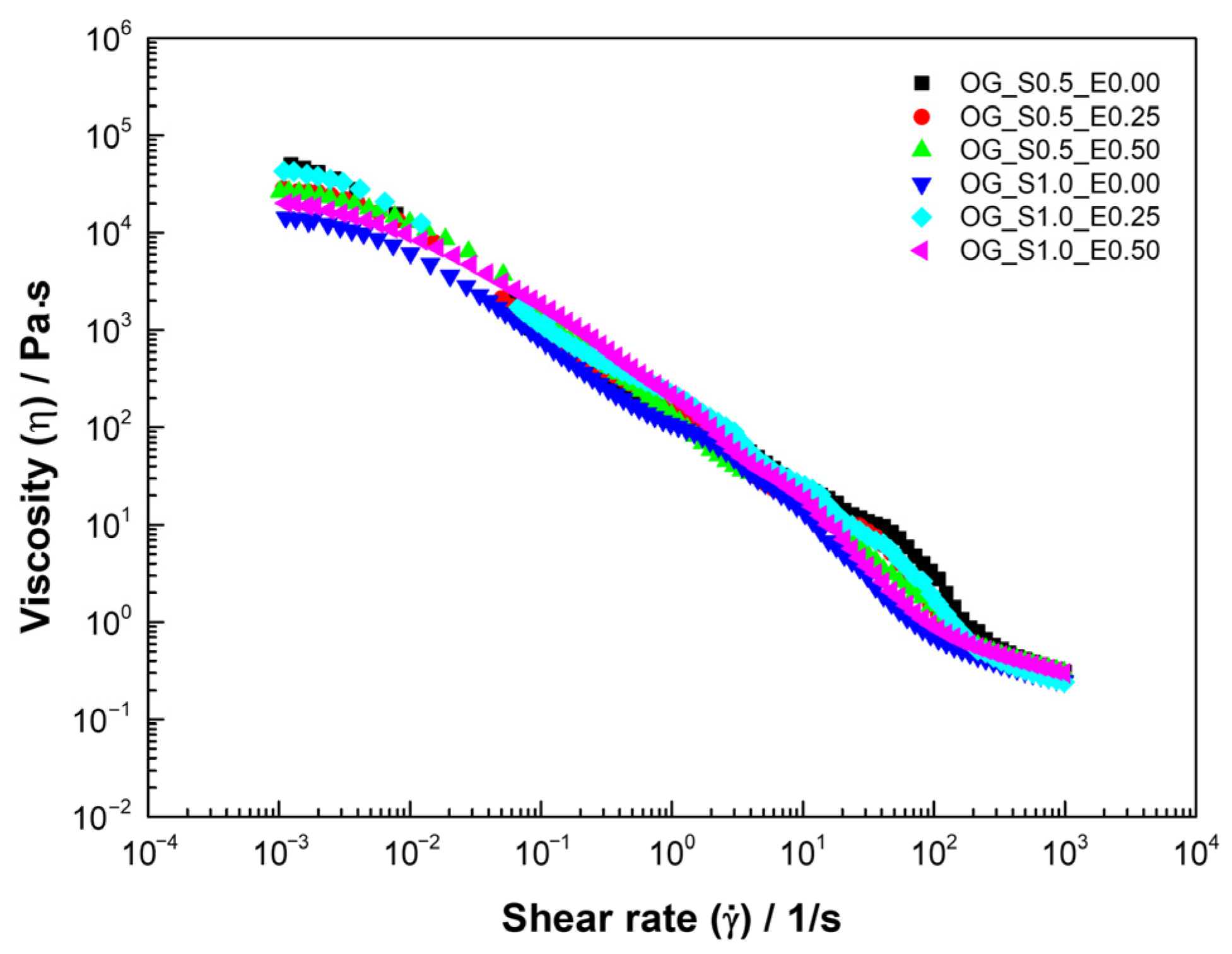
The viscous flow curve of oleogels (Figure 2) evidences a decrease in apparent viscosity with the shear rate; this behavior is consistent with structured systems where molecules or particles align in the direction of flow under shear. The viscosity of the fluid decreased as the shear rate increased, which is characteristic of shear-thinning in a non-Newtonian fluid. This reduction is attributed to the disaggregation of the dispersed phase structure. As the shear forces intensify, the aggregated particles are broken down, effectively reducing the dispersed phase concentration and, consequently, the effective viscosity. Viscosity then becomes independent of the shear rate once all aggregates are fully separated into individual droplets [40].
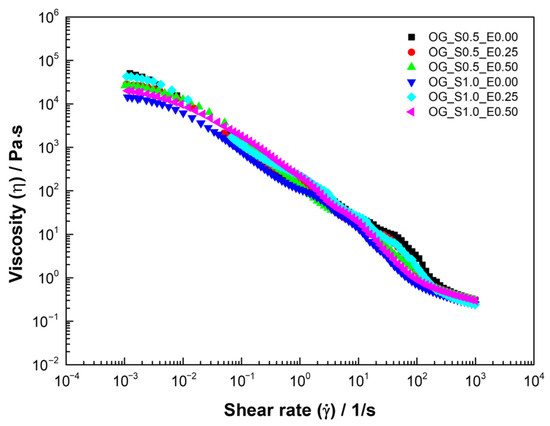
Figure 2.
Viscous flow curve of oleogels based on starch and extracts of cassava.
Then, experimental data were adjusted to the Carreau–Yasuda model (Table 4), presenting R2 > 0.99, which varied depending on the percentage of starch and CLE used. decreases with the percentage of extracts, and starch formulations with 1% starch exhibit lower residual viscosity () and lower relaxation times (), indicating less elastic but more cohesive networks compared to samples with 0.5% starch, because indicates the resistance to flow under high-shear-stress conditions. This phenomenon could be attributed to the fact that the phenolic compounds present in the extract, due to their acidic characteristics, interfere with the molecular interactions of starch, altering the density of the structural network and facilitating deformation under shear [37].

Table 4.
Rheological properties of oleogels based on starch and extracts of cassava adjusted to the Carreau–Yasuda model.
Regarding the initial viscosity (), representing the resistance to flow under low-stress conditions, the OG_S0.5_E0.00 sample presents the highest value (60,341.06), indicating greater resistance, while OG_S1.0_E0.00 exhibits the lowest value (16,277.37), reflecting a lower initial resistance to flow. With respect to the relaxation time (λ), OG_S0.5_E0.00 takes longer to reach equilibrium after deformation, suggesting that this oleogel responds slowly to changes in stress, compared to OG_S1.0_E0.50, which requires less time to reach equilibrium after deformation.
The power law index (n) reflects the pseudoplasticity of the material; this does not present significant differences, since it shows similar behavior across all samples, confirming uniform stress thinning behavior. On the other hand, parameter (a), which describes the transition between flow regimes, presents significant differences, since it varies notably across the six oleogels formulations, being higher in the OG_S1.0_E0.25 sample and lower in the OG_S1.0_E0.50 sample. Then, it is possible to demonstrate how the cassava extract influences the elasticity and cohesion of the oleogels, reducing the elasticity at higher concentrations, probably due to a decrease in molecular interactions within it.
The rheological properties of oleogels were evaluated by evaluating their behavior in a stationary and oscillatory state. Figure 2 shows a decrease in viscosity with the shear rate, typical of shear thinning behavior.
3.2.3. Viscoelastic Properties of Oleogels
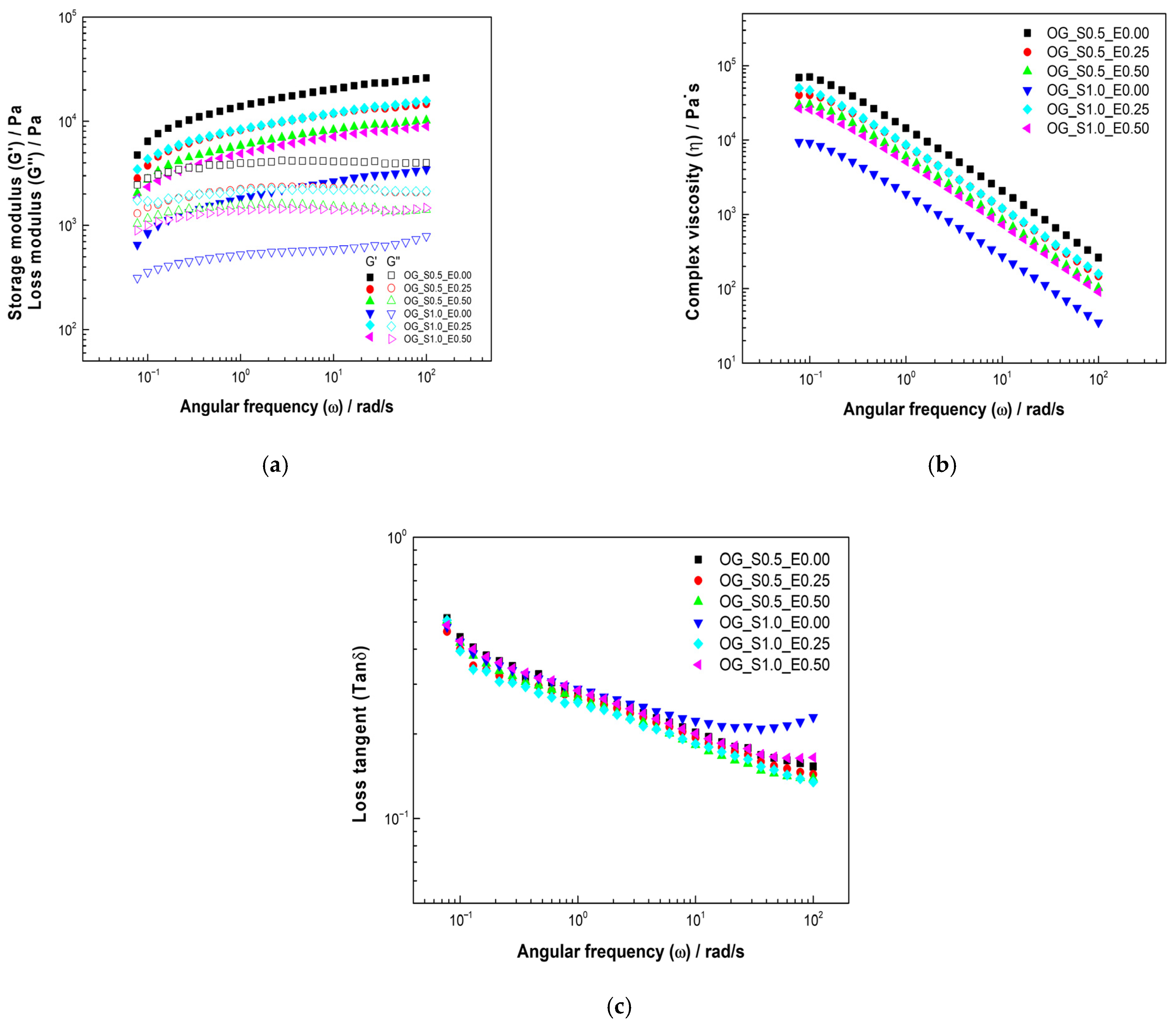
The rheological analysis of the oleogels (Figure 3a) reveals that all formulations consistently exhibited across the measured angular frequency range. This modulus relationship confirms the formation of a true gel structure with predominantly elastic (solid-like) properties, which is characteristic of a stable oleogel matrix. The starch concentration emerges as the most significant factor determining overall structural rigidity. Formulations incorporating 1.0% starch (OG_S1.0 series) generally displayed higher values compared to the 0.5% starch formulations (OG_S0.5 series). This is mechanistically attributed to starch acting as the primary structuring agent; a higher concentration facilitates a more extensive and robust three-dimensional network through gelatinization or an increased number of hydrogen bonding and particle-to-particle interactions within the oil phase, thus conferring greater resistance to deformation. The cassava leaf extract (CLE) functions as a modulating agent, notably impacting the stiffness, particularly within the 1.0% starch series. The OG_S1.0_E0.50 sample exhibited a significantly higher than its across the entire frequency spectrum, suggesting a highly rigid internal structure. This indicates a synergistic effect between the high starch (1.0%) and CLE (0.5%) concentrations. The polyphenolic compounds in the CLE likely interact with the starch matrix—via hydrogen bonding and/or hydrophobic interactions—effectively increasing the number or strength of the network’s cross-linking points. This reinforcement yields a more solid-like viscoelastic response where both and increase with frequency, a signature of solid viscoelastic materials, as was also observed for OG_S1.0_E0.00 and OG_S1.0_E0.25. Conversely, the OG_S0.5 series formulations (OG_S0.5_E0.25 and OG_S0.5_E0.50) presented intermediate modulus values, reflecting less rigidity than the most concentrated formulation (OG_S1.0_E0.50) but a more structured character relative to the base 1.0% starch oleogels without extract (OG_S1.0_E0.00). This demonstrates that while the lower starch content limits the maximum potential rigidity, the extract still provides a degree of network reinforcement. Similar findings regarding viscoelastic behavior have been reported by Carrillo-Zurita et al. [41] in whey protein isolate oleogels and by Qu et al. [41] for oleogels structured with waxes and ethylcellulose.
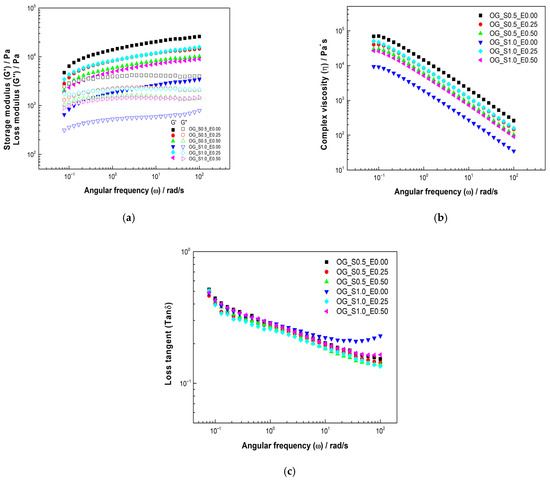
Figure 3.
Viscoelastic properties of oleogels based on starch and extracts of cassava: (a) storage and loss modulus, (b) complex viscosity, and (c) loss tangent.
Figure 3b illustrates the dependence of complex viscosity on angular frequency . In all cases, a decrease in is observed with increasing . This shear-thinning behavior is characteristic of structured, non-Newtonian materials and signifies that the internal interactions of the oleogels matrix weaken under high shear rates, reflecting the structure’s inability to rapidly reorient in response to the applied deformation [42]. Correspondingly, the starch content has a highly significant effect on the magnitude of . The 1.0% starch formulations consistently maintain higher viscosity values than their 0.5% counterparts, confirming starch as the dominant component defining the network’s resistance to flow. The CLE addition modulates the viscosity in a concentration-dependent manner. At the low concentration (0.25%), the extract appears to slightly reinforce the network, resulting in a minor increase in complex viscosity. However, at the higher concentration (0.5%), the effect is less pronounced, showing either stabilization or a relative decrease in . This complex phenomenon is consistent with reports that the incorporation of polyphenols into starch systems may reach a critical concentration [43]. Beyond this threshold, the increased polyphenol load may lead to the saturation of binding sites or self-association of extract components that fail to contribute effectively to network reinforcement, potentially acting as a plasticizer and resulting in a stabilization or decrease in viscosity. This type of shear-thinning response, where values gradually decrease with increasing applied force, has been previously reported in vegetable wax oleogels by Yılmaz et al. [44].
The loss tangent describes the relationship between energy lost and energy stored in each cycle [42] and serves as a key indicator of structural stability and viscoelastic dominance (Figure 3c). All oleogels exhibited values below 1, unequivocally confirming the predominant elastic (gel-like) behavior of the samples.
The influence of both components is clearly highlighted by the changes in : starch oleogels without extract (OG_S1.0_E0.00) showed a structured matrix with decreasing as the angular frequency increased, characteristic of elastic systems. Incorporating 0.25% CLE (OG-S1-E0.25) stabilized the structure further, evidenced by a more gradual reduction in . The most significant stabilizing effect was seen upon increasing the extract concentration to 0.5% (OG-S1-E0.5), where decreased substantially, revealing a more rigid and highly resistant system. Mechanistically, a lower is consistent with the enhanced cross-linking provided by the polyphenols, which increases the proportion of stored elastic energy relative to dissipated viscous energy.
Conversely, reducing the starch concentration to 0.5% (OG-S0.5-E0) initially resulted in a higher , indicating less elasticity and structural stability due to the decrease in the primary polysaccharide builder. However, the subsequent addition of 0.25% extract (OG-S0.5-E0.25) immediately improved the elastic properties. Crucially, combining 0.5% starch with the higher 0.5% extract (OG-S0.5-E0.5) led to a significant recovery of elasticity, marked by a substantial reduction in . This demonstrates that the cassava leaf extract actively functions as a structural reinforcer, partially compensating for the lower starch content by stabilizing the matrix through non-covalent interactions. These findings are consistent with those reported by Tanislav et al. [45], where decreased with increasing polysaccharide concentration, confirming that the incorporation of hydrocolloids contributes to a more pronounced elastic behavior in oleogels.
3.2.4. Microstructural Properties of Oleogels
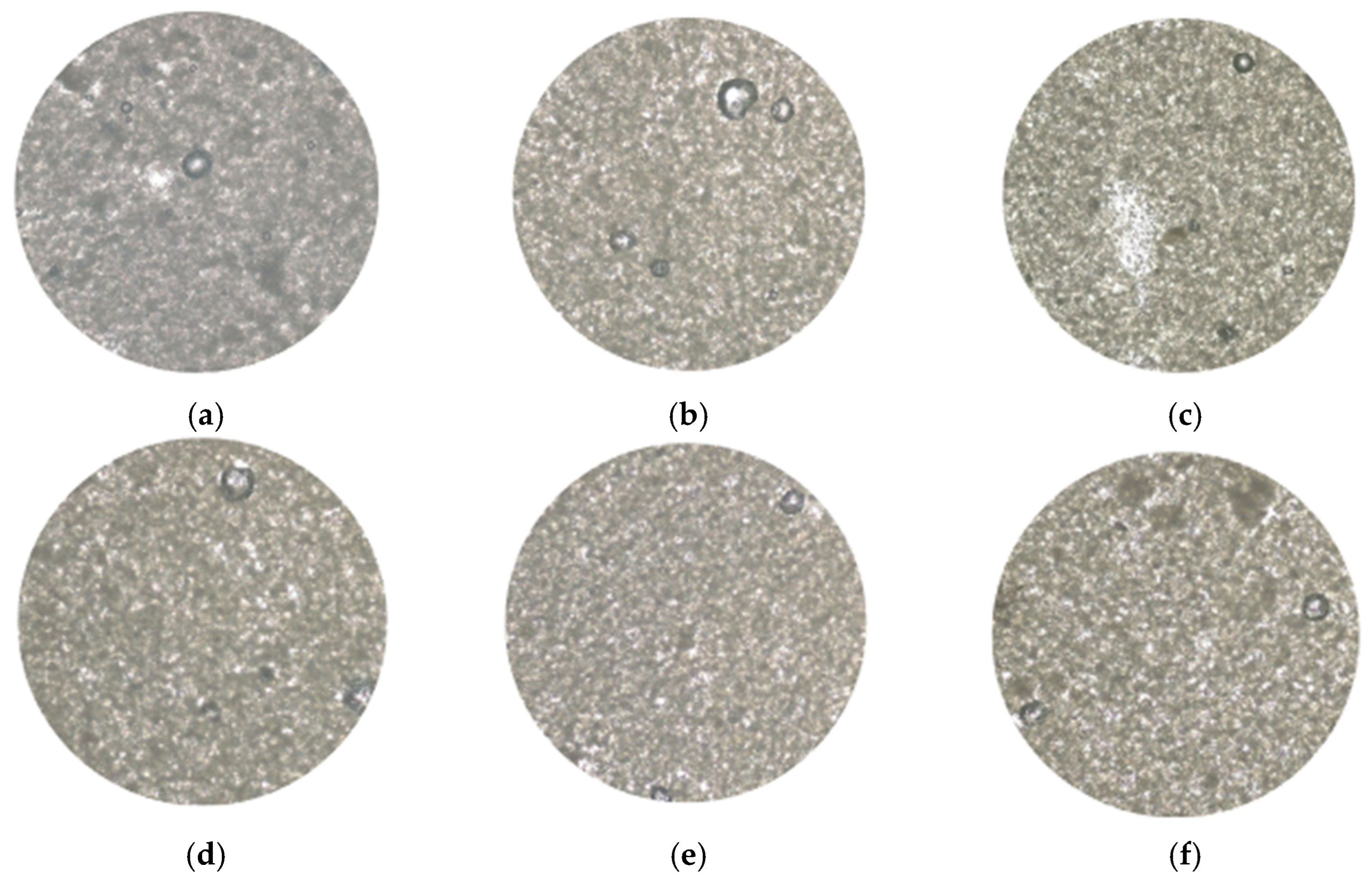
Figure 4 shows the results obtained when analyzing the oleogels with an optical microscope, demonstrating the stability of the system after lyophilization. Formulations with a lower amount of starch (0.5%) present a more dispersed microstructure, highlighting the importance of starch in the formation of a more compact network. In turn, when analyzing the formulations containing extract, it is evident that its incorporation significantly improves the organization and cohesion of the oleogel network.
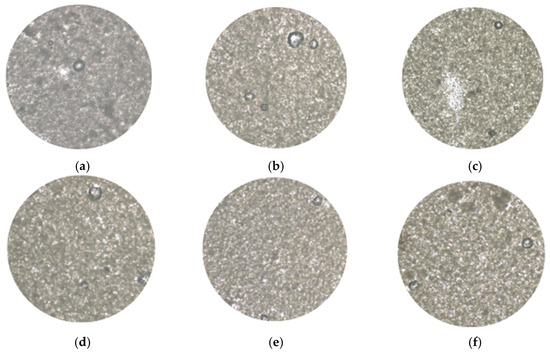
Figure 4.
Micrograph of oleogels based on starch and extracts of cassava: (a) OG_S0.5_E0.00, (b) OG_S0.5_E0.25, (c) OG_S0.5_E0.50, (d) OG_S1.0_E0.00, (e) OG_S1.0_E0.25, and (f) OG_S1.0_E0.50.
As shown, in formulations with 0.25% extract (OG-S1-E0.25 and OG-S0.5-E0.25), the particle distribution is more uniform, suggesting that this concentration enhances the interactions between the components of the system. Indicating that cassava extracts, along with span 60, act as an efficient structuring agent, likely due to their emulsifying properties and ability to interact with starch and lipids, similar results were observed in the study conducted by Trujillo-Ramírez et al. [46], who evaluated the stability of oleogels made from chia seed using sorbitan monostearate as a stabilizer.
The relationship between starch and cassava leaf extract formulations with 1% starch (OG-S1-E0, OG-S1-E0.25, and OG-S1-E0.5) exhibited a more compact and stable network compared to those with 0.5% starch. This suggests that starch contributes significantly to the viscosity of the matrix. These results, in turn, are consistent with those reported by Zhu et al. [47], who highlighted that oleogels made with polysaccharides have a high oil retention capacity, greater hardness, viscoelasticity, and a more compact internal structure, essential characteristics for the stability and functionality of this type of system.
3.3. Low-Fat Cookies
3.3.1. Physicochemical Properties of Cookies
As shown in Table 5, the proximate analysis of cookie formulations revealed a direct relationship between the partial substitution of butter with oleogels and the final product composition, characterized by a significant reduction in fat content and a concomitant increase in carbohydrate (CHO) and protein fractions. Statistically significant differences were observed in acidity, strongly influenced by the addition of cassava starch and cassava leaf extract (CLE). The control formulation (100% butter) exhibited the lowest acidity (0.008), indicating that the absence of oleogels contributed to a minimal acid profile. In contrast, oleogel-containing formulations showed higher acidity values, dependent on starch and CLE proportions. The formulation with starch only (C_OG_S1.0_E0.00) reached 0.015, while samples containing CLE—particularly C_OG_S1.0_E0.25 and C_OG_S0.5_E0.25—recorded the highest acidity levels (0.019 and 0.012, respectively). This trend suggests that CLE increases acidity due to free acid groups from phenolic compounds concentrated in the extract. However, a decrease in acidity at 0.5% CLE indicates potential volatilization or thermal degradation of sensitive bioactive compounds during baking, consistent with previous findings on plant powder incorporation in baked products [47].

Table 5.
Physicochemical properties of cookies prepared with oleogels based on starch and CLE.
Moisture content also varied significantly. The control cookies presented 3.70 ± 0.16%, whereas formulation C_OG_S0.5_E0.50 increased to 5.00 ± 0.11%, suggesting improved water retention due to the hydrophilic nature of CLE proteins and polysaccharides, which enhance the matrix’s hydration capacity. Similar behavior was reported by Zulfiqar et al. [48], confirming the positive influence of oleogels on moisture retention. Ash content ranged from 0.44% to 1.28% across formulations, showing a positive correlation with CLE concentration and peaking in the 0.5% extract samples. This confirms CLE’s effectiveness as a mineral fortification source, consistent with reports highlighting its high potassium and calcium content [37].
Cookies with partial butter replacement and CLE addition showed up to 80% fat reduction, the main evidence of oleogel efficiency. This notable decrease is attributed to cassava starch’s polysaccharide nature, acting as a bulking and binding agent that forms a structured matrix capable of physically entrapping oil and limiting its migration during baking. Such structural efficiency aligns with reports indicating that gelled emulsions can reduce saturated fat by up to 70% [48]. Similarly, Ahsan et al. [49] observed fat reduction when replacing butter with banana pectin, reinforcing the role of polysaccharides in lipid minimization. Moreover, the oleogel structure efficiently traps and encapsulates oil [50]. The protein increase (up to 9.52 ± 0.02) is attributed to the cassava leaf’s protein contribution. These insoluble proteins enhance both nutritional value and technological performance by synergistically interacting with carbohydrates [51], stabilizing the oleogel network and promoting effective fat encapsulation [37].
In comparison, other studies [48] that replaced 100% of butter with olive oil–alginate gelled emulsions reported up to 40% less fat, 70% less saturated fat, higher hardness, and good overall sensory acceptance, supporting the feasibility of total butter replacement using oleogel systems. Although the present study evaluated partial substitution, the results indicate that cassava starch–xanthan oleogels enriched with CLE can serve as functional fat replacers, improving nutritional quality while maintaining acceptable texture and sensory characteristics. Future research should explore complete butter replacement (100%) and assess oxidative stability and shelf life to validate the long-term functionality of cassava-based oleogels in bakery products.
3.3.2. Total Phenolic Content and Antioxidant Capacity
Table 6 presents the results obtained from the Folin and ABTS tests performed on the six cookie extracts. Significant differences were found in the phenolic compound content. In the Folin test, which measures the total phenolic compound content, significant differences were evident in all cookie samples. The control sample presented the lowest GAE/g value (1.01 ± 0.03), indicating a low phenolic content and, therefore, a lower antioxidant capacity. On the other hand, C_OG_S1.0_E0.25, with 3.520 ± 0.021 GAE/g, showed the highest phenolic content, suggesting that this extract has the highest antioxidant potential in terms of phenolic compounds. Likewise, the cookie extract formulations C_OG_S0.5_E0.25 and C_OG_S1.0_E0.50, with 2.795 ± 0.084 and 2.305 ± 0.017 GAE/g, respectively, presented high values, although lower than those of C_OG_S1.0_E0.25. The results expressed in this study are lower than those reported in the literature when evaluating the total phenolic content of cookies fortified with bioactive compounds. Authors such as Gattuso et al. [49] obtained TPC results of 29.63 ± 1 mg GAE/100 g, when studying the fortification of vegetable fat with natural antioxidants from bergamot pomace for use as an ingredient in cookie making.

Table 6.
Total phenolic content and antioxidant capacity of cookies prepared with oleogels based on starch and extracts of cassava.
Regarding the ABTS assay, it should be noted that it evaluates the ability of a substance to inhibit the formation of ABTS free radicals. The % inhibited ABTS was calculated as the difference between the absorbance of the sample without the antioxidant and the absorbance of the sample with the antioxidant, divided by the absorbance of the sample without the antioxidant, and multiplied by 100. A high % inhibited ABTS indicates that the substance has high antioxidant activity; i.e., it can effectively neutralize free radicals and prevent cell damage. Considering the results obtained, the cookie extract C_OG_S1.0_E0.25 showed the best results, with an inhibition of 66.566%, reinforcing the relationship between high phenolic content and antioxidant capacity. In comparison, the control sample presented a free radical inhibition of 44.64%, the lowest inhibition percentage among the extracts analyzed, which coincides with its low phenolic content. The extracts C_OG_S1.0_E0.50 (56.26%) and C_OG_S0.5_E0.25 (48.15%) showed good free radical inhibition values, indicating good antioxidant capacity. Finally, formulation C_OG_S1.0_E0.00 and formulation C_OG_S0.5_E0.50 presented values of 37.856% and 40.642%, respectively, representing the formulations with the lowest antioxidant activity in this assay.
The cookie extract C_OG_S1.0_E0.25 showed the highest phenolic content, along with favorable antioxidant capacity. In contrast, the control cookie extract GM-0 exhibited the lowest antioxidant potential in both assays, indicating that its low phenolic content limits its ability to inhibit free radicals. These results are consistent with those reported by Zhao et al. [50], who evaluated the effect of antioxidants on the oxidative stability of expeller-pressed high-oleic soybean oil oleogels and cookies.
3.3.3. Color Properties
The appearance and color of cookies are strongly related to the type of fat used in their preparation. Fatty components, such as natural pigments present in butter or butter substitutes, can significantly influence the color of the resulting cookies [51]. Likewise, their color is determined by non-enzymatic browning reactions, specifically Maillard reactions. The occurrence and intensity of these reactions depend on several variables, such as temperature, pH, and others. The results of the color analysis are presented in Table 7.

Table 7.
Color properties of low-fat cookies prepared with oleogels based on starch and extracts.
Significant differences were observed in all the color parameters. Parameter (a*) showed positive values in all formulations, indicating a reddish color. C_OG_S0.5_E0.00 showed the highest a* (redness) values (3.983 ± 0.105). Likewise, the (b*) value remained positive across all formulations due to the predominance of yellow hues, being highest in the C_OG_S1.0_E0.50 formulation (34.116 ± 0.265). The yellow color can be attributed to additives that enrich the composition with colorants such as β-carotene, given that this is the most abundant carotenoid in olive oil [52], which was used in the production of the oleogels. Authors such as Grzelczyk et al. [53] have reported similar results when evaluating the color of cookies with the addition of plant extracts.
Luminosity (L*) was affected by the increase in cassava starch and cassava leaf extract, with C_OG_S0.5_E0.50 showing the greatest opacity (59.753 ± 1.377).
Finally, it was observed that formulation C_OG_S1.0_E0.50 (1–0.5%) presented the highest total color difference (ΔE) (10.601 ± 0.174), while formulation C_OG_S0.5_E0.00 showed the lowest differences with respect to the control cookie (2.139 ± 0.271). These results are similar to those described by Onacik-Gür & Zbikowska [54], which evaluated the effect of green tea extract and oleogels on the physicochemical properties and oxidative stability of shortcrust pastry cookies during storage.
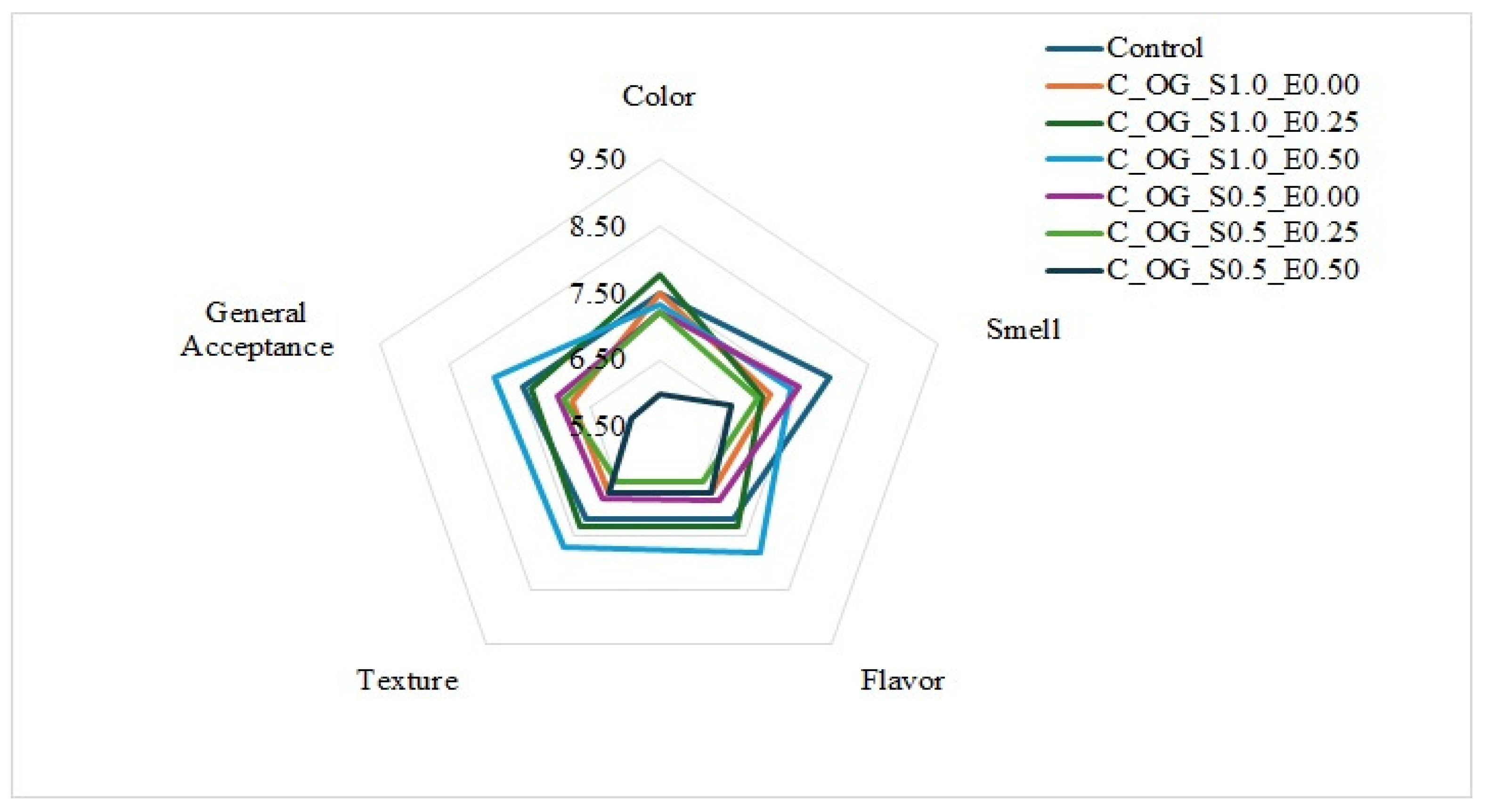
3.3.4. Sensorial Properties
The development of food products with partial fat replacement that preserves the desirable sensory profile is a challenge in the food industry today. The analysis of the results of the hedonic sensory evaluation carried out on the seven cookie formulations (Figure 5) shows significant differences in the formulations with partial replacement of butter by oleogels in relation to the control cookie. Regarding color, the C_OG_S1.0_E0.50 formulation (1–0.5%) obtained a score of 7.33, surpassed by the C_OG_S1.0_E0.25 formulation (1–0.25%) with a result of 7.77, a result similar to the control cookie. Regarding odor, the control cookie obtained a value of 7.93, being the formulation with the greatest acceptance by the panelists, followed by the C_OG_S0.5_E0.00 and C_OG_S1.0_E0.50 formulation, which in turn obtained favorable results with a score of 7.37, outperforming most formulations.
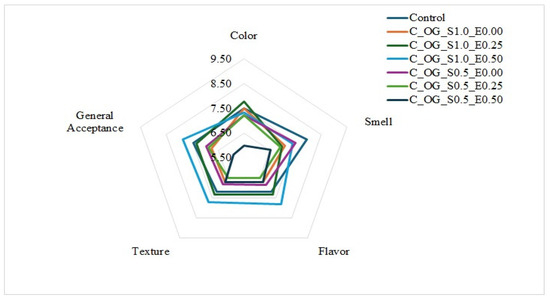
Figure 5.
Sensorial properties of low-fat cookies prepared with oleogels based on starch and extracts of cassava.
Regarding the flavor attribute, C_OG_S1.0_E0.50 presented a favorable score of 7.83, similar to that obtained by C_OG_S1.0_E0.25, with a score of 7.33, which contained 0.25% extract. It should be noted that the formulations containing extracts generated interest among the panelists, due to their softer texture and higher moisture content. Similarly, regarding texture, the C_OG_S1.0_E0.50 formulation stood out (7.70) compared to the other cookie formulations, which presented lower scores.
Finally, regarding the attribute of general acceptance, the formulation with the highest score was the C_OG_S1.0_E0.50 formulation (7.87), being generally preferred. In contrast, C_OG_S0.5_E0.50 presented the lowest values across the evaluated attributes, standing out negatively in color (6.00), odor (6.53), and general acceptance (5.90), which suggests an unfavorable impact of its composition; that is, reducing the percentage of starch in the oleogels provides undesirable characteristics in the cookies. These results coincide with those obtained by Chen & Wang [52], where cookies were made with the addition of candelilla wax-based oleogels, and other authors, such as Gutiérrez-Luna et al. [48], who made reduced-fat cookies made with a gelled emulsion of olive oil and alginate, where it was observed that cookies made with a gelled emulsion of olive oil as a substitute for butter showed good sensory attributes. Likewise, results similar to those reported by Sereti et al. [55] were obtained. Finally, the results reflect that C_OG_S1.0_E0.50 has the greatest acceptance by the panelists, which indicates that partial replacement of butter with oleogels is possible, meeting consumer expectations; however, the total replacement of butter in bakery products should continue to be studied.
4. Conclusions
This study demonstrates that cassava extract provides antioxidant properties and improves oleogel organization and cohesion, especially at 0.5% concentrations, acting as an efficient structuring agent alongside Span 60 and cassava starch. Formulations with 1% starch, meanwhile, generate more compact, viscous, and rigid networks, reinforcing the system’s stability and functionality.
These characteristics position the oleogels developed in this study as a versatile tool for developing healthier foods, demonstrating that it is possible to improve the nutritional profile of baked goods without sacrificing their sensory quality.
The use of oleogels made from cassava starch and cassava leaf extracts as a substitute for 50% of the butter in cookies represents a promising alternative for reducing saturated fat content and improving the product’s functional profile. The results obtained show that the formulations with oleogels present acceptable sensory properties, standing out for their smooth texture and increased moisture content, with the formulation C_OG_S1.0_E0.50 standing out, both for its general acceptance by the panelists and for its moisture content (3.01 ± 0.17%). It is worth noting that this formulation presents favorable results by incorporating bioactive compounds with antioxidant capacity derived from cassava extracts (2.30 ± 0.017 g GAE/g extract). This formulation does not present an increase in acidity content compared to other formulations, such as C_OG,_S1.0_E0.00, which does not present favorable characteristics with respect to sensory evaluation. This opens new opportunities for innovation in the food industry, focusing on sustainable and functional solutions that promote consumer health.
Future research should prioritize in vitro digestion studies to accurately evaluate the cookies’ behavior during the gastrointestinal process. This is crucial for obtaining a better understanding of the release kinetics and bioavailability of key nutrients, particularly the bioactive compounds contributed by the cassava leaf extract. Additionally, we strongly recommend implementing detailed texture analyses to structurally assess the influence of the oleogels on final product quality, which will facilitate the exploration of using oleogels for higher percentages of butter replacement. These complementary analyses are essential for optimizing the final product design and providing robust data for future commercial applications.
Author Contributions
S.E.Q. and L.A.G.-Z.: conceptualization. V.M.M.-C., S.E.Q. and L.A.G.-Z.: formal analysis, data curation, investigation, methodology, validation, visualization, and writing—original draft. V.M.M.-C. and S.E.Q.: writing—review and editing—and supervision. L.A.G.-Z.: Resources and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universidad de Cartagena, grant number Minute 00470/2022 of Universidad de Cartagena.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Dataset available on request from the authors.
Acknowledgments
This work was supported by the Universidad de Cartagena, under research project Minute 00470/2022. The authors gratefully acknowledge this financial support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Żbikowska, A.; Onacik-Gür, S.; Kowalska, M.; Rutkowska, J. Trans Fatty Acids in Polish Pastry. J. Food Prot. 2019, 82, 1028–1033. [Google Scholar] [CrossRef]
- Vieira, S.A.; McClements, D.J.; Decker, E.A. Challenges of Utilizing Healthy Fats in Foods. Adv. Nutr. 2015, 6, 309S–317S. [Google Scholar] [CrossRef]
- Marak, N.R.; Malemnganbi, C.C.; Marak, C.R.; Mishra, L.K. Functional and Antioxidant Properties of Cookies Incorporated with Foxtail Millet and Ginger Powder. J. Food Sci. Technol. 2019, 56, 5087–5096. [Google Scholar] [CrossRef] [PubMed]
- Najjar, Z.; Kizhakkayil, J.; Shakoor, H.; Platat, C.; Stathopoulos, C.; Ranasinghe, M. Antioxidant Potential of Cookies Formulated with Date Seed Powder. Foods 2022, 11, 448. [Google Scholar] [CrossRef] [PubMed]
- Onacik-Gür, S.; Żbikowska, A. Effect of High-Oleic Rapeseed Oil Oleogels on the Quality of Short-Dough Biscuits and Fat Migration. J. Food Sci. Technol. 2020, 57, 1609–1618. [Google Scholar] [CrossRef] [PubMed]
- Kupiec, M.; Zbikowska, A.; Marciniak-Lukasiak, K.; Zbikowska, K.; Kowalska, M.; Kowalska, H.; Rutkowska, J. Study on the Introduction of Solid Fat with a High Content of Unsaturated Fatty Acids to Gluten-Free Muffins as a Basis for Designing Food with Higher Health Value. Int. J. Mol. Sci. 2021, 22, 9220. [Google Scholar] [CrossRef]
- Dassanayake, L.S.K.; Kodali, D.R.; Ueno, S. Formation of Oleogels Based on Edible Lipid Materials. Curr. Opin. Colloid. Interface Sci. 2011, 16, 432–439. [Google Scholar] [CrossRef]
- Calligaris, S.; Manzocco, L.; Valoppi, F.; Nicoli, M.C. Effect of Palm Oil Replacement with Monoglyceride Organogel and Hydrogel on Sweet Bread Properties. Food Res. Int. 2013, 51, 596–602. [Google Scholar] [CrossRef]
- Sivakanthan, S.; Fawzia, S.; Mundree, S.; Madhujith, T.; Karim, A. Investigation of the Influence of Minor Components and Fatty Acid Profile of Oil on Properties of Beeswax and Stearic Acid-Based Oleogels. Food Res. Int. 2024, 184, 114213. [Google Scholar] [CrossRef]
- Shuai, X.; Li, Y.; Zhang, M.; Wei, C.; Du, L.; Liu, C.; Chen, J.; Dai, T. Effect of Different Oleogelation Mechanisms on Physical Properties and Oxidative Stability of Macadamia Oil-Based Oleogels and Its Application. LWT 2024, 198, 115978. [Google Scholar] [CrossRef]
- Li, L.; Liu, G. Engineering Effect of Oleogels with Different Structuring Mechanisms on the Crystallization Behavior of Cocoa Butter. Food Chem. 2023, 422, 136292. [Google Scholar] [CrossRef]
- Palla, C.; Giacomozzi, A.; Genovese, D.B.; Carrín, M.E. Multi–Objective Optimization of High Oleic Sunflower Oil and Monoglycerides Oleogels: Searching for Rheological and Textural Properties Similar to Margarine. Food Struct. 2017, 12, 1–14. [Google Scholar] [CrossRef]
- Difonzo, G.; Pasqualone, A.; Silletti, R.; Cosmai, L.; Summo, C.; Paradiso, V.M.; Caponio, F. Use of Olive Leaf Extract to Reduce Lipid Oxidation of Baked Snacks. Food Res. Int. 2018, 108, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Bialek, M.; Rutkowska, J.; Bialek, A.; Adamska, A. Oxidative Stability of Lipid Fraction of Cookies Enriched with Chokeberry Polyphenols Extract. Pol. J. Food Nutr. Sci. 2016, 66, 77–84. [Google Scholar] [CrossRef]
- Mišan, A.; Mimica-Dukić, N.; Sakač, M.; Mandić, A.; Sedej, I.; Šimurina, O.; Tumbas, V. Antioxidant Activity of Medicinal Plant Extracts in Cookies. J. Food Sci. 2011, 76, C1239–C1244. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Qiu, Z.; Chen, Z.; Liu, L.; Wang, J.; Jiang, H.; Zhang, H.; Liu, G. qin Antioxidant Properties of Blueberry Extract in Different Oleogel Systems. LWT 2021, 137, 110364. [Google Scholar] [CrossRef]
- Laszczyk, M.; Jäger, S.; Simon-Haarhaus, B.; Scheffler, A.; Schempp, C.M. Physical, Chemical and Pharmacological Characterization of a New Oleogel-Forming Triterpene Extract from the Outer Bark of Birch (Betulae Cortex). Planta Med. 2006, 72, 1389–1395. [Google Scholar] [CrossRef]
- Salama, M.A.; Abdin, M.E.; Saleh, M.N.; Abd El-Baset, W.S.; Hendawy, E.A.A.; Ibrahim, A.I. Preparation and Evaluation of Oleogels Incorporated with Moringa Oleifera Leaves Extract in Biscuits Production. Food Technol. Res. J. 2024, 4, 1–15. [Google Scholar] [CrossRef]
- Tao, H.T.; Qiu, B.; Du, F.L.; Xu, T.C.; Liu, L.N.; Lü, F.; Li, K.M.; Liu, W. The Protective Effects of Cassava (Manihot esculenta Crantz) Leaf Flavonoid Extracts on Liver Damage of Carbon Tetrachloride Injured Mice. Afr. J. Tradit. Complement. Altern. Med. 2015, 12, 52–56. [Google Scholar] [CrossRef]
- Laya, A.; Koubala, B.B. Polyphenols in Cassava Leaves (Manihot esculenta Crantz) and Their Stability in Antioxidant Potential after in Vitro Gastrointestinal Digestion. Heliyon 2020, 6, e03567. [Google Scholar] [CrossRef]
- Sharma, N.; Gulati, A. Natural Vitamins as Food Antimicrobials in Stem and Thorn Extracts of Hippophae Species Studied by HPLC-ESI-MS. Food Humanit. 2023, 1, 415–420. [Google Scholar] [CrossRef]
- Makieu, P.; Kanu, M.S.; Sillah, A.; Sheriff, A. Nutritional Values of Cassava Leaves in Three Districts, Kenema, Kailahun, and Bo, Sierra Leone. Food Humanit. 2025, 4, 100592. [Google Scholar] [CrossRef]
- Ramírez-Brewer, D.; Quintana, S.E.; García-Zapateiro, L.A. Modeling and Optimization of Microwave-Assisted Extraction of Total Phenolics Content from Mango (Mangifera indica) Peel Using Response Surface Methodology (RSM) and Artificial Neural Networks (ANN). Food Chem. X 2024, 22, 101420. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Liu, L.; Li, X.; Zhang, Q.; Wang, Z.; Chen, N.; Wang, H.; Xie, F.; Qi, B.; Jiang, L. Construction of Soybean Oil Bodies–Xanthan Gum Composite Oleogels by Emulsion-Templated Method: Preparation, Characterization, and Stability Analysis. Food Hydrocoll. 2024, 149, 109526. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Li, S.; Qin, Y.; Wu, Z. Oleogels Based on Millet Prolamin and Basil Essential Oil: Fabrication, Characterization, and Application in Puff Pastry and 3D Printing. Int. J. Biol. Macromol. 2025, 329, 147871. [Google Scholar] [CrossRef]
- Pradhan, A.; Anis, A.; Alam, M.A.; Al-Zahrani, S.M.; Jarzebski, M.; Pal, K. Effect of Soy Wax/Rice Bran Oil Oleogel Replacement on the Properties of Whole Wheat Cookie Dough and Cookies. Foods 2023, 12, 3650. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; AOAC: Gaithesburg, MD, USA, 1995. [Google Scholar]
- Mieles-Gómez, L.; Lastra-Ripoll, S.E.; Torregroza-Fuentes, E.; Quintana, S.E.; García-Zapateiro, L.A. Rheological and Microstructural Properties of Oil-in-Water Emulsion Gels Containing Natural Plant Extracts Stabilized with Carboxymethyl Cellulose/Mango (Mangiferaindica) Starch. Fluids 2021, 6, 312. [Google Scholar] [CrossRef]
- López-Barraza, D.; Ortega-Ramos, A.; Torregroza-Fuentes, E.; Quintana, S.E.; García-Zapateiro, L.A. Rheological and Functional Properties of Hydrocolloids from Pereskia Bleo Leaves. Fluids 2021, 6, 349. [Google Scholar] [CrossRef]
- ICONTEC Analisis Sensorial. Guia General-GTC 165; ICONTEC Analisis Sensorial: Bogotá, Colombia, 1999. [Google Scholar]
- Tao, H.; Cui, B.; Zhang, H.; Bekhit, A.E.D.; Lu, F. Identification and Characterization of Flavonoids Compounds in Cassava Leaves (Manihot esculenta Crantz) by HPLC/FTICR-MS. Int. J. Food Prop. 2019, 22, 1134–1145. [Google Scholar] [CrossRef]
- Chahyadi, A.; Elfahmi. The Influence of Extraction Methods on Rutin Yield of Cassava Leaves (Manihot esculenta Crantz). Saudi Pharm. J. SPJ 2020, 28, 1466. [Google Scholar] [CrossRef] [PubMed]
- Sriprablom, J.; Winuprasith, T.; Suphantharika, M.; Wongsagonsup, R. Physical Properties and In-Vitro Gastrointestinal Digestion of Oil-in-Water Emulsions Stabilized by Single- and Dual-Modified Cassava Starches with Cross-Linking and Octenylsuccinylation. Int. J. Biol. Macromol. 2024, 262, 129965. [Google Scholar] [CrossRef] [PubMed]
- Abou-Elsoud, M.; Salama, M.; Li, Z.; Wang, S.; Cai, Z.; Ahn, D.U.; Huang, X. Structuring Low-Density Lipoprotein-Based Oleogels with Pectin via an Emulsion-Templated Approach: Formation and Characterization. J. Food Eng. 2025, 387, 112340. [Google Scholar] [CrossRef]
- Miranda, C.G.; Speranza, P.; Sato, A.C.K. Evaluation of Cassava Leaves Extract as a Material to Produce Biopolymer-Based Films. Food Hydrocoll. 2023, 144, 108944. [Google Scholar] [CrossRef]
- Lastra-Ripoll, S.E.; Quintana, S.E.; García-Zapateiro, L.A. Chemical, Technological, and Rheological Properties of Hydrocolloids from Sesame (Sesamum indicum) with Potential Food Applications. Arab. J. Chem. 2022, 15, 104146. [Google Scholar] [CrossRef]
- Campanella, O.H.; Dorward, N.M.; Singh, H. A Study of the Rheological Properties of Concentrated Food Emulsions. J. Food Eng. 1995, 25, 427–440. [Google Scholar] [CrossRef]
- Carrillo-Zurita, R.J.; Pierre, K.; Culler, M.; Rousseau, D. Microstructure and Rheology of Cellulose Bead-Filled Whey Protein Isolate Oleogels. Food Chem. 2025, 470, 142563. [Google Scholar] [CrossRef]
- Tao, X.; Hu, Y.; Liu, Z.; Jiang, K.; Li, J.; Guo, X.; Zhu, B. Marine Sulfated Polysaccharide Affects the Formation Mechanism of Gelatin Emulsion-Based Oleogels. Food Hydrocoll. 2025, 159, 110706. [Google Scholar] [CrossRef]
- Ramírez-Brewer, D.; Quintana-Martinez, S.E.; García-Zapateiro, L.A. Obtaining and Characterization of Natural Extracts from Mango (Mangifera indica) Peel and Its Effect on the Rheological Behavior in New Mango Kernel Starch Hydrogels. Food Chem. 2025, 462, 140949. [Google Scholar] [CrossRef] [PubMed]
- Sarraf, M.; Naji-Tabasi, S.; Beig-Babaei, A.; Moros, J.E.; Carrillo, M.C.S.; Tenorio-Alfonso, A. Developing Edible Oleogels Structure Prepared with Emulsion-Template Approach Based on Soluble Biopolymer Complex. Food Chem. X 2024, 24, 101917. [Google Scholar] [CrossRef]
- Tanislav, A.E.; Pușcaș, A.; Păucean, A.; Mureșan, A.E.; Semeniuc, C.A.; Mureșan, V.; Mudura, E. Evaluation of Structural Behavior in the Process Dynamics of Oleogel-Based Tender Dough Products. Gels 2022, 8, 317. [Google Scholar] [CrossRef]
- Trujillo-Ramírez, D.; Lobato-Calleros, C.; Jaime Vernon-Carter, E.; Alvarez-Ramirez, J. Cooling Rate, Sorbitan and Glyceryl Monostearate Gelators Elicit Different Microstructural, Viscoelastic and Textural Properties in Chia Seed Oleogels. Food Res. Int. 2019, 119, 829–838. [Google Scholar] [CrossRef]
- Zhu, T.; Cui, W.; Jiang, H.; Zhang, T.; Zhang, L.; Yang, C.; Guo, X.; Chen, F. Characterization of Soybean Protein Isolate-Chitosan-Based Emulsion Template-Oleogel as Fast-Frozen Special Fat Substitute and Its Mechanism on the Quality Improvement of Fast-Frozen Food. LWT 2024, 205, 116485. [Google Scholar] [CrossRef]
- Gutiérrez-Luna, K.; Astiasaran, I.; Ansorena, D. Fat Reduced Cookies Using an Olive Oil-Alginate Gelled Emulsion: Sensory Properties, Storage Stability and in Vitro Digestion. Food Res. Int. 2023, 167, 112714. [Google Scholar] [CrossRef] [PubMed]
- Gattuso, A.; Piscopo, A.; Santacaterina, S.; Imeneo, E.; De Bruno, A.; Poiana, M. Fortification of Vegetable Fat with Natural Antioxidants Recovered by Bergamot Pomace for Use as an Ingredient for the Production of Biscuits. Sustain. Food Technol. 2023, 1, 951–961. [Google Scholar] [CrossRef]
- Zhao, M.; Yang, Z.; Rao, J.; Chen, B. Effects of Antioxidants on the Oxidative Stability of Expeller-Pressed High Oleic Soybean Oil (EPHOSO) Oleogel and Cookie. Food Chem. 2025, 470, 142613. [Google Scholar] [CrossRef]
- Feng, Z.; He, D.; Zhang, L.; Li, Q.; Xue, C.; Yi, X.; Liao, L.; Pei, Z.; Shen, X. Preparation of Myofibrillar Protein Oleogels by Emulsion Template Method: Application of Fat Substitute for Sponge Cakes. LWT 2025, 216, 117350. [Google Scholar] [CrossRef]
- Chen, X.; Wang, W. The Lipid-Amylose Complexes Enhance Resistant Starch Content in Candelilla Wax-Based Oleogels Cookies. Int. J. Biol. Macromol. 2024, 278, 134804. [Google Scholar] [CrossRef]
- Grzelczyk, J.; Drożdżyński, P.; Budryn, G.; Czarnecki, A.; Paprocka, Z.; Gałązka-Czarnecka, I. High-Fiber Cookies with Bamboo Flour and Edible Flowers: Evaluation of Structural Properties, Phenolic Content, Antioxidant Activity and Nutritional Value. LWT 2025, 216, 117321. [Google Scholar] [CrossRef]
- Onacik-Gür, S.; Zbikowska, A. The Effect of Green Tea Extract and Oleogels on the Physico-Chemical Properties and Oxidative Stability of Short-Dough Biscuits during Storage. LWT 2022, 172, 114197. [Google Scholar] [CrossRef]
- Sereti, V.; Kotsiou, K.; Biliaderis, C.G.; Lazaridou, A. Emulsion Gel Enriched with a Barley β-Glucan Concentrate for Reducing Saturated Fat in Biscuits. Food Hydrocoll. 2023, 145, 109163. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).