Preparation and Evaluation of CuMnOx-Modified Activated Carbon Fibers for Indoor VOCs Removals
Abstract
1. Introduction
2. Materials and Methods
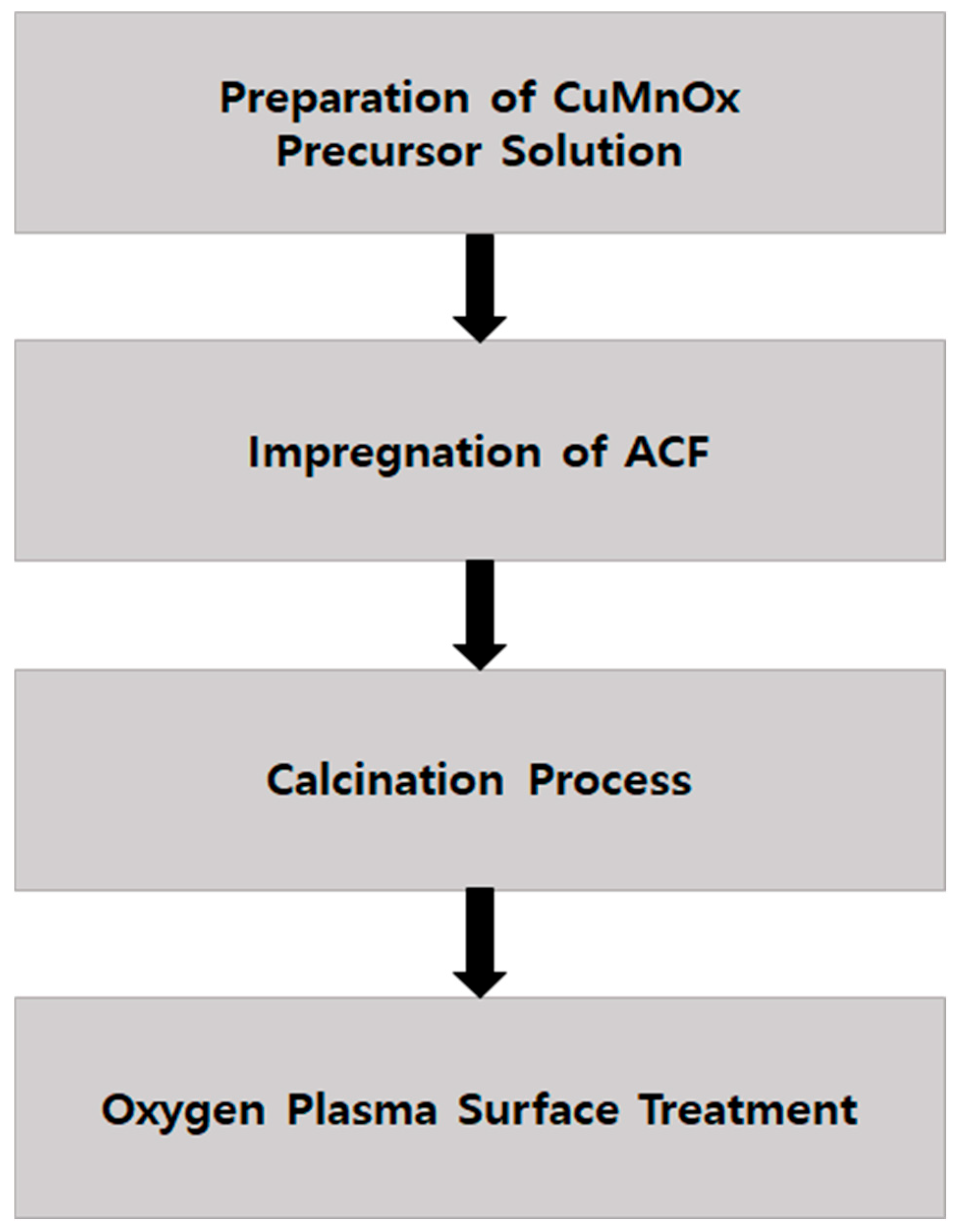
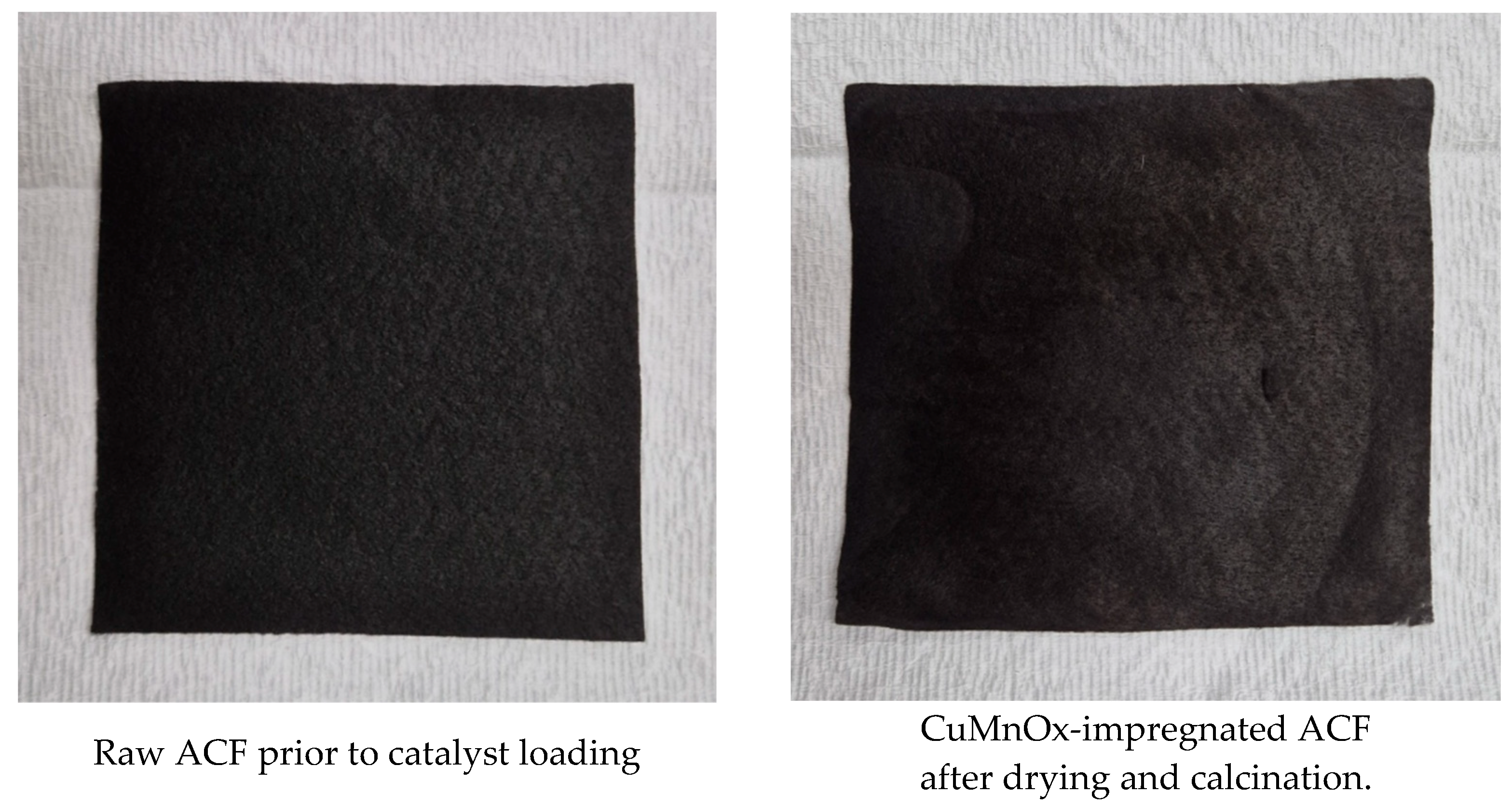
2.1. Synthesis of CuMnOx Catalysts and Incorporation into ACF via Impregnation
2.2. O2 Plasma Surface Treatment and Final Composite Preparation
2.3. Surface Characterization of ACF
2.4. Evaluation of VOC Removal Performance
3. Results and Discussion
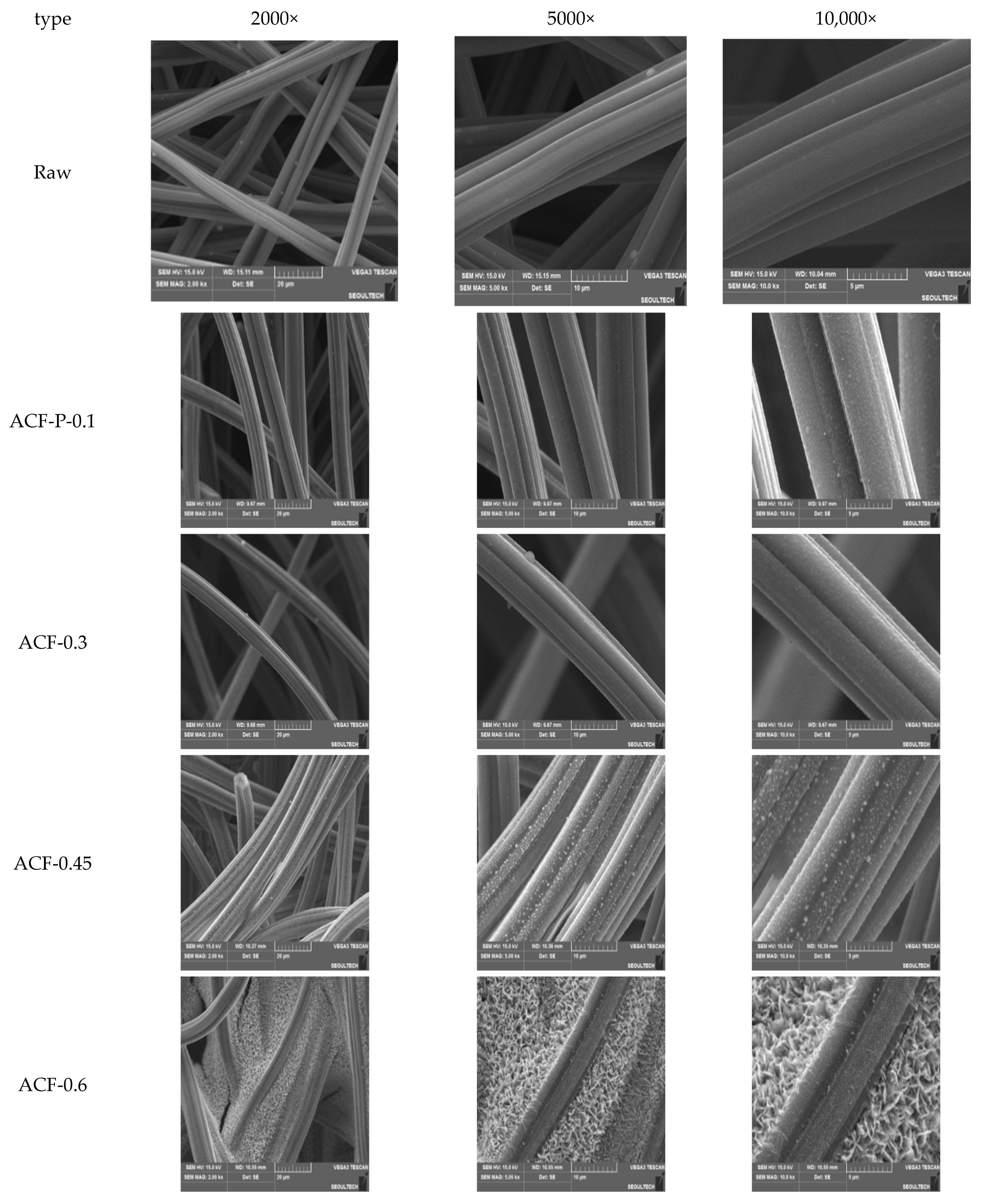
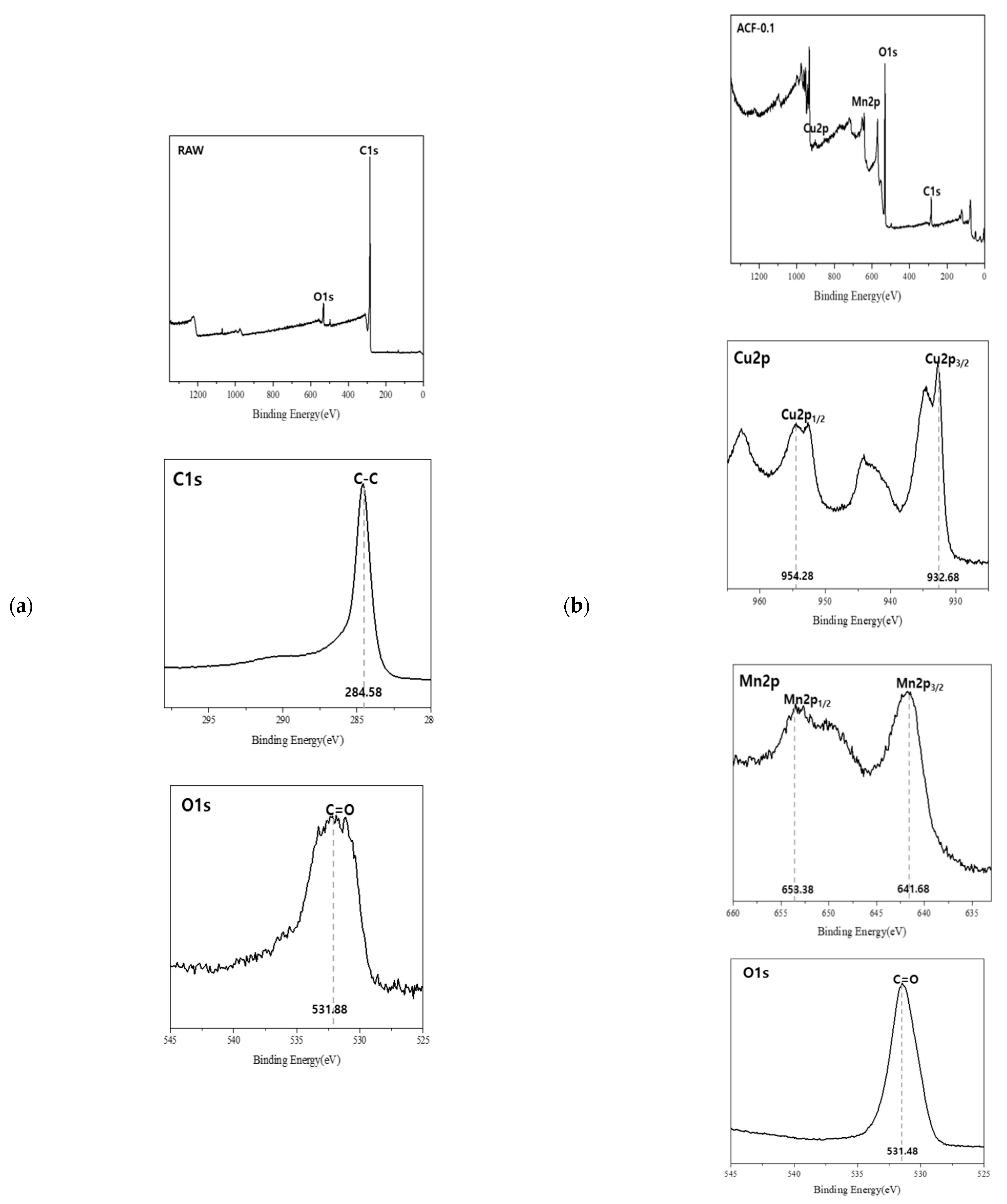
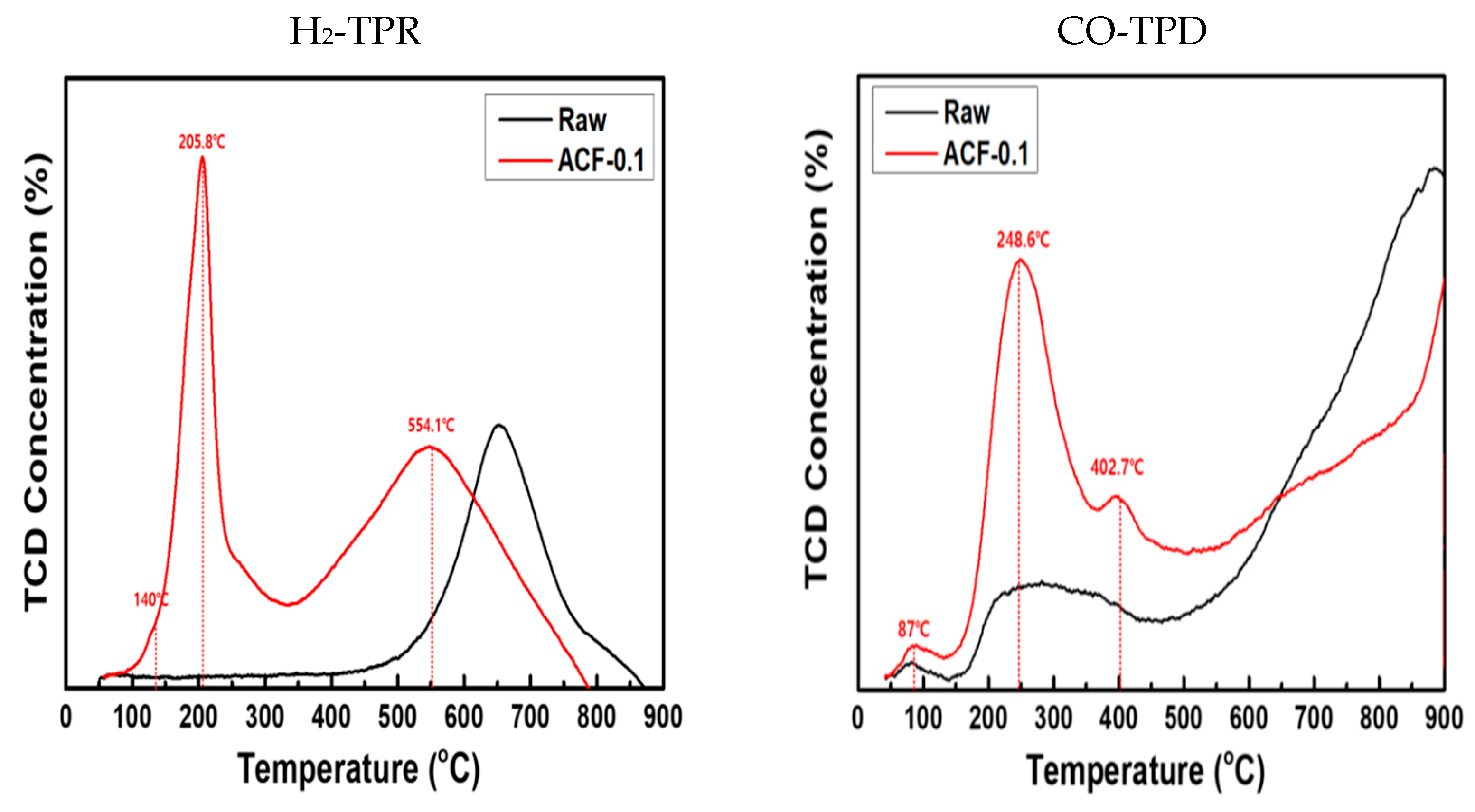
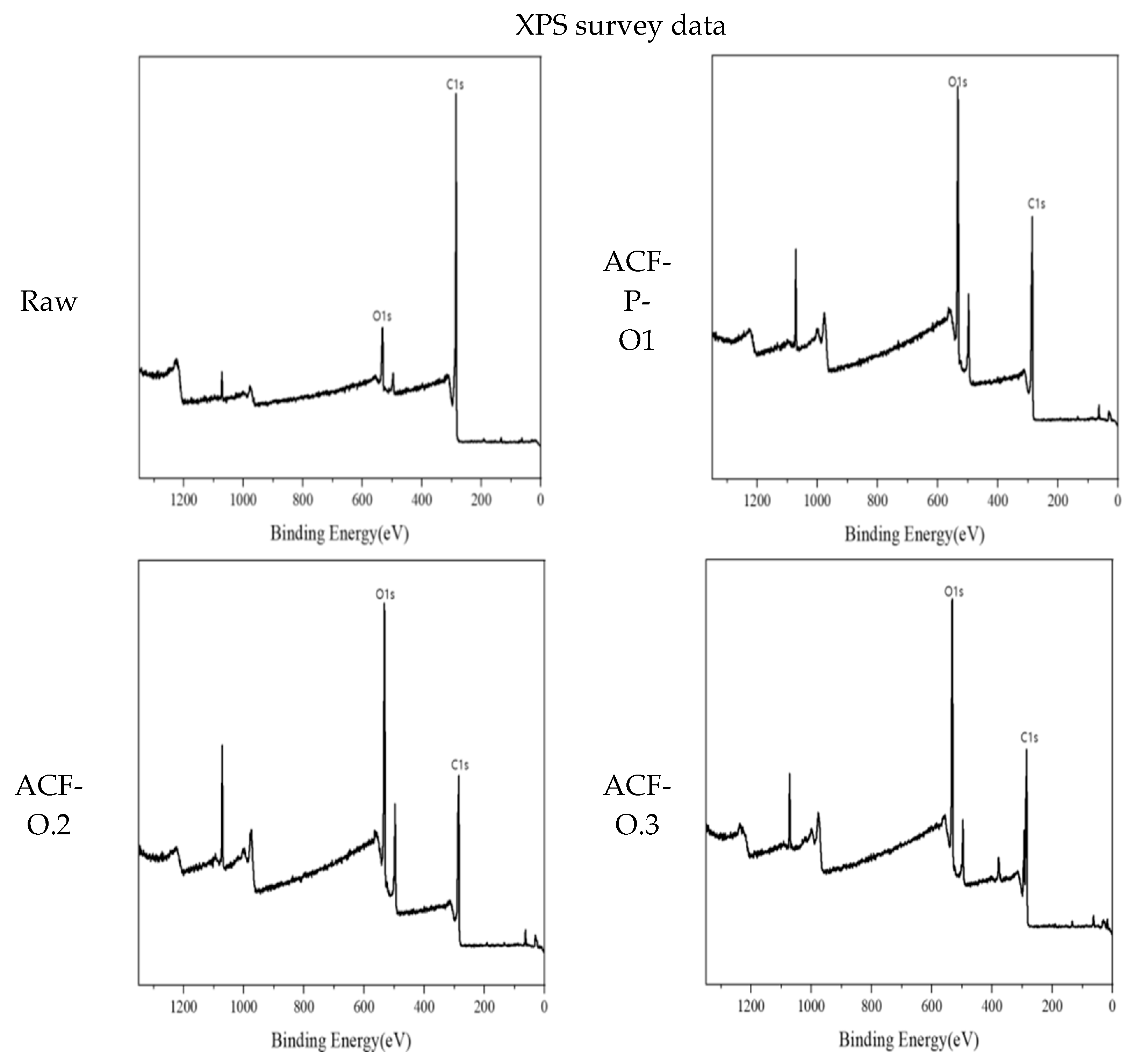
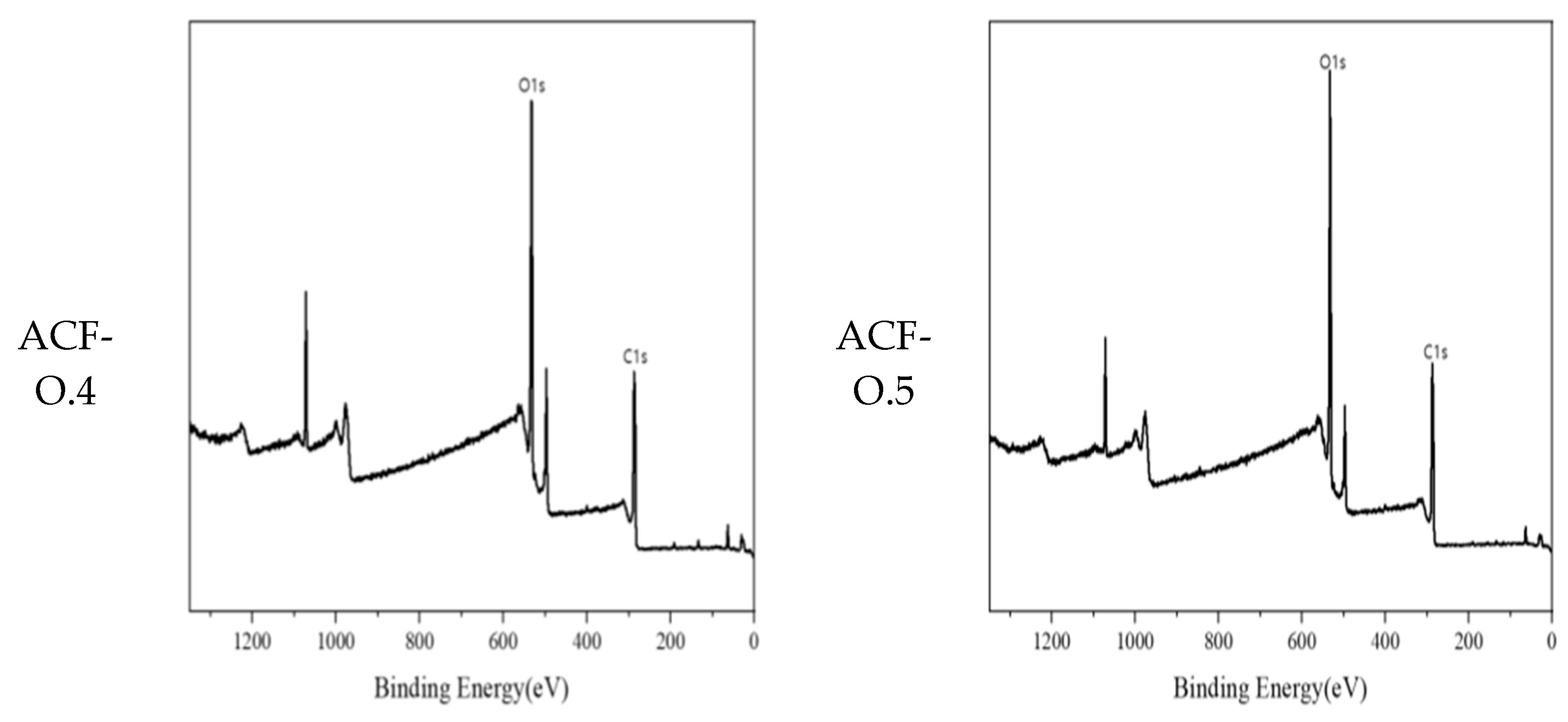
3.1. Physicochemical Characteristics of ACF After CuMnOx Incorporation
3.2. VOC Removal Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACF | Activated Carbon Fiber |
| BET | Brunauer–Emmett–Teller surface area |
| CO-TPD | Carbon Monoxide Temperature-Programmed Desorption |
| CuMnOx | Copper–Manganese Mixed Oxide |
| EDX | Energy-Dispersive X-ray Spectroscopy |
| EL | Eco-Label Certification Standard |
| H2-TPR | Hydrogen Temperature-Programmed Reduction |
| SEM | Scanning Electron Microscopy |
| VOC | Volatile Organic Compound |
| XPS | X-ray Photoelectron Spectroscopy |
References
- Montero-Montoya, R.; López-Vargas, R.; Arellano-Aguilar, O. Volatile Organic Compounds in Air: Sources, Distribution, Exposure and Associated Illnesses in Children. Ann. Glob. Health 2018, 84, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Horvat, T.; Pehnec, G.; Jakovljević, I. Volatile Organic Compounds in Indoor Air: Sampling, Determination, Sources, Health Risk, and Regulatory Insights. Toxics 2025, 13, 344. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Zhang, S.; Zhang, X.; Verma, P.; Wen, M. Advances in Catalytic Oxidation of Volatile Organic Compounds over Pd-Supported Catalysts: Recent Trends and Challenges. Front. Mater. 2020, 7, 595667. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Volatile Organic Compounds’ Impact on Indoor Air Quality. Available online: https://www.epa.gov/indoor-air-quality-iaq/volatile-organic-compounds-impact-indoor-air-quality (accessed on 23 October 2025).
- Bai, B.C.; Eom, K.M.; Lee, S.G. Lyocell-based Activated Carbon Fibers Improved the Adsorption Characteristics for Harmful Gases Generated by Coal or Oil Combustion. J. Korean Soc. Atmos. Environ. 2017, 33, 401–408. [Google Scholar] [CrossRef]
- Heo, Y.-J.; Le, M.-U.T.; Park, S.-J. Investigation of Carbon Dioxide Adsorption by Nitrogen-Doped Carbons Synthesized from Cubic MCM-48 Mesoporous Silica. Carbon Lett. 2016, 18, 62–66. [Google Scholar] [CrossRef]
- Rashidi, N.A.; Yusup, S. Characterization and Mechanisms of H2S and SO2 Adsorption by Activated Carbon. Energy Fuels 2015, 29, 6390–6398. [Google Scholar] [CrossRef]
- Hu, S.; Li, C.; Li, K.; Teng, W.; Li, F.; Zhang, P.; Wang, H. Advanced mesoporous adsorbents and catalysts for CO2, NOx, and VOC removal: Mechanisms and applications. Environ. Sci. Nano 2024, 11, 4666–4691. [Google Scholar] [CrossRef]
- Dey, S.; Dhal, G.C. Synthesis of CuMnOx catalysts by novel routes for selective catalytic oxidation of carbon monoxide. Mater. Today Chem. 2020, 17, 100342. [Google Scholar] [CrossRef]
- Li, M.; Wang, R. Combined Catalytic Conversion of NOx and VOCs: Present Status and Prospects. Materials 2025, 18, 39. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, S.; Cui, R.; Song, Z.; Zhang, X. Enhancement of catalytic combustion of toluene over CuMnOx hollow spheres prepared by oxidation method. Microporous Mesoporous Mater. 2021, 326, 111370. [Google Scholar] [CrossRef]
- Wu, L.; Zhu, Y.; Yuan, J.; Guo, X.; Zhang, Q. Advances in Adsorption, Absorption, and Catalytic Materials for VOCs Generated in Typical Industries. Energies 2024, 17, 1861. [Google Scholar] [CrossRef]
- Dey, S.; Dhal, G.C.; Mohan, D.; Prasad, R. Effect of Preparation Conditions on the Catalytic Activity of CuMnOx Catalysts for CO Oxidation. Bull. Chem. React. Eng. Catal. 2017, 12, 437–451. [Google Scholar] [CrossRef]
- Yi, F.Y.; Lin, X.D.; Chen, S.X.; Wei, X.Q. Adsorption of VOC on Modified Activated Carbon Fiber. J. Porous Mater. 2009, 16, 521–526. [Google Scholar] [CrossRef]
- Fu, Y.; Xin, Q.; Zhang, S.; Yang, Y. Simultaneous Catalytic Removal of VOCs and NOx with the Dual-layered Catalyst of CoCeOx and V2O5/TiO2. Aerosol Air Qual. Res. 2021, 21, 210214. [Google Scholar] [CrossRef]
- ISO 9277:2010; Determination of the Specific Surface Area of Solids by Gas Adsorption—BET Method. International Organization for Standardization (ISO): Geneva, Switzerland, 2010.
- Osterbind, M.S.; Oh, J.; Lungu, C.T. Estimation of Activated Carbon Fiber (ACF) Adsorption Capacity and Breakthrough Times Using a Predictive Model. Chem. Eng. Process Tech. 2023, 8, 1073. [Google Scholar]
- EL.608:2022; Korean Eco-Label Certification—Indoor Air Purification Filter Performance Test Method. Korea Environmental Industry & Technology Institute (KEITI): Seoul, Republic of Korea, 2022.
- Das, D.; Gaur, V.; Verma, N. Removal of Volatile Organic Compound by Activated Carbon Fiber. Carbon 2004, 42, 2949–2962. [Google Scholar] [CrossRef]
- Balanay, J.A.G.; Oh, J. Adsorption Characteristics of Activated Carbon Fibers in Respirator Cartridges for Toluene. Int. J. Environ. Res. Public Health 2021, 18, 8505. [Google Scholar] [CrossRef] [PubMed]
- Jeon, D.-H.; Park, H.-M.; Yoon, D.-H. Development and Performance Evaluation of Adsorption-Type Activated Carbon Fiber Filter for VOC Removal. J. Inst. Korean Electr. Electron. Eng. 2023, 27, 151–158. [Google Scholar] [CrossRef]
- ISO 22309:2011; Quantitative Energy-Dispersive X-Ray Spectrometry in the Scanning Electron Microscope. International Organization for Standardization (ISO): Geneva, Switzerland, 2011.
- ISO 11885:2007; Water Quality—Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES). International Organization for Standardization (ISO): Geneva, Switzerland, 2007.
- ISO 15472:2010; X-Ray Photoelectron Spectrometers—Calibration of Energy Scales. International Organization for Standardization (ISO): Geneva, Switzerland, 2010.
- Wen, T.; Wang, J.; Zhang, J.; Long, C. Regulating oxygen vacancies and hydroxyl groups of α-MnO2 nanorods for enhancing post-plasma catalytic removal of toluene. Environ. Res. 2023, 231, 117176. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Environment (Republic of Korea). Enforcement Rule of the Indoor Air Quality Control Act (실내공기질 관리법) Enforcement Rule of the Indoor Air Quality Control Act. Environment Ministry Ordinance No. 728, 27 December 2017 (Effective 1 January 2020). Available online: https://law.go.kr/LSW/lsInfoP.do?lsiSeq=200338 (accessed on 23 October 2025).

| C (Atom%) | O (Atom%) | Cu (Atom%) | Mn (Atom%) | Al (Atom%) | |
|---|---|---|---|---|---|
| Raw | 91.31 | 8.69 | - | - | - |
| ACF-P-0.1 | 81.40 | 13.18 | 2.77 | 1.37 | 1.28 |
| ACF-0.3 | 75.29 | 14.39 | 4.15 | 3.76 | 2.41 |
| ACF-0.45 | 68.65 | 15.29 | 7.12 | 6.10 | 2.83 |
| ACF-0.6 | 36.82 | 20.92 | 26.11 | 11.47 | 4.69 |
| Sample ID | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Sample name | Raw | ACF-P-0.1 | ACF-0.3 | ACF-0.45 | ACF-0.6 |
| BET surface area (m2/g) | 1740.1 | 1342.7 | 559.6 | 645.4 | 484.3 |
| Total pore volume (cm3/g) | 0.749 | 0.579 | 0.245 | 0.272 | 0.229 |
| Micropore volume (cm3/g) | 0.693 | 0.534 | 0.225 | 0.255 | 0.197 |
| Micropore (%) | 92.52 | 92.23 | 91.84 | 93.75 | 86.03 |
| Samples | C1s (Atom%) | O1s (Atom%) |
|---|---|---|
| Raw | 87.24 | 10.39 |
| ACF-P-O1 | 62.51 | 31.26 |
| ACF-O2 | 58.54 | 35.19 |
| ACF-O3 | 55.70 | 36.75 |
| ACF-O4 | 52.93 | 38.63 |
| ACF-O5 | 54.03 | 39.23 |
| Elapsed Time (min) | Removal (%) | |||||
|---|---|---|---|---|---|---|
| Formaldehyde | Acetaldehyde | Benzene | ||||
| Raw | ACF-P- 0.1 | Raw | ACF-P- 0.1 | Raw | ACF-P- 0.1 | |
| 30 | 81.6 | 85.0 | 85.7 | 85.7 | 96.2 | 97.5 |
| 60 | 96.6 | 96.6 | 85.7 | 85.7 | 97.5 | 97.5 |
| 90 | 96.6 | 96.6 | 85.7 | 89.2 | 97.5 | 97.5 |
| 120 | 96.6 | 96.6 | 87.5 | 89.2 | 97.5 | 97.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youn, H.C.; Kim, B.-k.; Jung, Y.-H.; Shin, H.-S. Preparation and Evaluation of CuMnOx-Modified Activated Carbon Fibers for Indoor VOCs Removals. Appl. Sci. 2025, 15, 11527. https://doi.org/10.3390/app152111527
Youn HC, Kim B-k, Jung Y-H, Shin H-S. Preparation and Evaluation of CuMnOx-Modified Activated Carbon Fibers for Indoor VOCs Removals. Applied Sciences. 2025; 15(21):11527. https://doi.org/10.3390/app152111527
Chicago/Turabian StyleYoun, Hun Chul, Bo-kyung Kim, Yeon-Hoon Jung, and Hyun-Sang Shin. 2025. "Preparation and Evaluation of CuMnOx-Modified Activated Carbon Fibers for Indoor VOCs Removals" Applied Sciences 15, no. 21: 11527. https://doi.org/10.3390/app152111527
APA StyleYoun, H. C., Kim, B.-k., Jung, Y.-H., & Shin, H.-S. (2025). Preparation and Evaluation of CuMnOx-Modified Activated Carbon Fibers for Indoor VOCs Removals. Applied Sciences, 15(21), 11527. https://doi.org/10.3390/app152111527







